Abstract
SB269652 (1) is the first drug-like allosteric modulator of the dopamine D2 receptor (D2R), but contains structural features associated with orthosteric D2R antagonists. Using a functional complementation system to control the identity of individual protomers within a dimeric D2R complex, we converted the pharmacology of the interaction between SB269652 and dopamine from allosteric to competitive by impairing ligand binding to one of the protomers, indicating that the allostery requires D2R dimers. Additional experiments identified a “bitopic” pose for SB269652 extending from the orthosteric site into a secondary pocket at the extracellular end of the transmembrane (TM) domain, involving TM2 and TM7. Engagement of this secondary pocket was a requirement for the allosteric pharmacology of SB269652. This suggests a novel mechanism whereby a bitopic ligand binds in an extended pose on one G protein-coupled receptor protomer to allosterically modulate the binding of a ligand to the orthosteric site of a second protomer.
Introduction
G protein-coupled receptors (GPCRs) are the largest superfamily of cell surface receptors, are involved in virtually all physiological processes1,2 and are targeted by approximately one third of current medications1,3. Over the last decade, the study of allosteric sites on GPCRs has emerged as an attractive means of expanding the chemical space associated with these drug targets3. More recently, “bitopic” ligands, i.e., molecules in which orthosteric and allosteric pharmacophores have been linked together, have emerged as a novel approach to developing selective GPCR ligands4,5. By concomitantly engaging both orthosteric and allosteric sites, bitopic ligands combine the advantages of selectivity that can result from engagement of an allosteric site with the high affinity and well-defined structure activity relationships (SAR) associated with targeting an orthosteric pocket4,5. Interestingly, existing GPCR ligands that display unprecedented modes of selectivity may do so via hitherto-unappreciated bitopic mechanisms5,6. Despite the presence of a secondary pharmacophore, a bitopic ligand should still display competitive behavior because the primary pharmacophore occupies the orthosteric site (essentially behaving as a more selective competitive agonist or antagonist); any deviation from such behavior requires a more complex mechanism of action6–8.
The dopamine D1-D5 receptors (D1–5Rs) mediate the physiological functions of the catecholamine neurotransmitter, dopamine, with the D2-like dopamine receptors (D2,3,4Rs) being particularly acknowledged as important targets for the treatment of numerous central nervous system disorders, including schizophrenia9. In an effort to develop novel antipsychotics, there has been considerable research into the design of more subtype-selective dopamine receptor ligands, albeit from an orthosteric ligand perspective10. The ligand, SB269652, emerged from one such series of studies11,12. As illustrated in Figure 1a, the tetrahydroisoquinoline (THIQ) core of SB269652 (derivatives of which are known to interact with dopamine receptors13) contains the key elements expected to interact with the orthosteric binding site of aminergic receptors. The molecule also contains a lipophilic appendage (an indole-2-carboxamide) attached by an appropriately spaced linker10, which is a feature of numerous subtype-selective D2R ligands. Recently, however, Maggio and co-workers made the surprising finding that SB269652 antagonizes the D2R through an allosteric, rather than an orthosteric, mechanism14, thus identifying this compound as the first drug-like allosteric small molecule at this highly important therapeutic target.
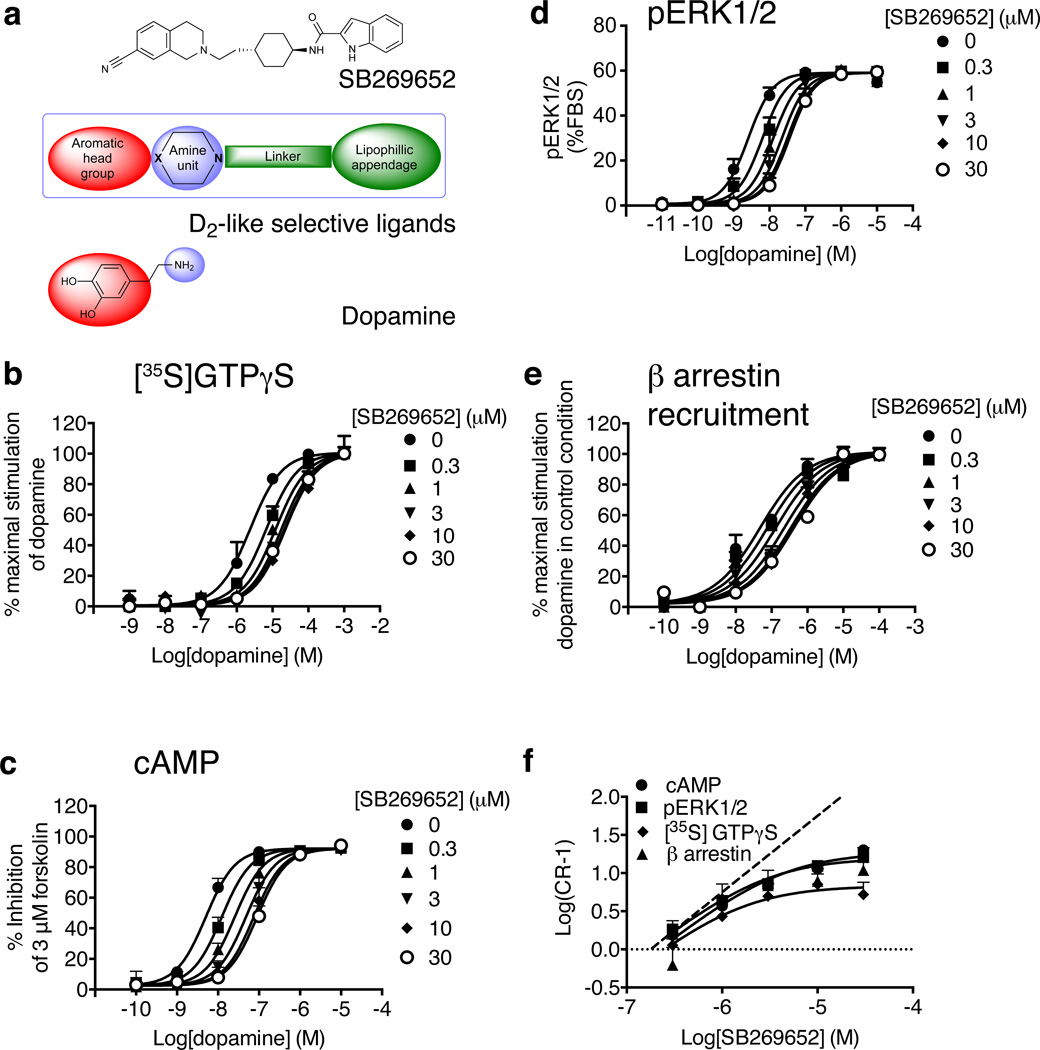
Figure 1. SB269652 is a negative allosteric modulator of the D2R.
a) SB269652 contains key structural features consistent with an orthosteric mode of interaction at D2-like dopamine receptors12,17. The action of increasing concentrations of SB269652 upon a dopamine concentration-response curve was measured at multiple signaling endpoints using whole cells expressing the hD2LR or membranes derived from these cells ([35S]GTPγS (b), cAMP (c), pERK1/2 (d) and β arrestin recruitment (e)). The allosteric behavior of SB269652 is illustrated in a Schild plot of the functional data (f), showing a clear deviation away from a line of unity (dashed). Data represent mean values plus S.E.M from three independent experiments.
A possible mechanism by which an orthosteric ligand can act allosterically is within a dimeric/oligomeric receptor complex. Prior studies have provided evidence that the D2R can exist as a homodimer, and that canonical orthosteric ligands can interact cooperatively in this complex15–17. However, in all these instances, the cooperativity is highly negative such that the pharmacology is virtually indistinguishable from classic competition and thus the physiological relevance, and pharmacological exploitation, of D2R homodimers remains to be definitively established. In contrast, the pharmacology of SB269652 is unambiguously allosteric, characterized by limited degrees of negative cooperativity with orthosteric agonists or antagonists14.
The purpose of the current study was to determine how a ligand with a presumed orthosteric mode of receptor engagement might act as a “classic” GPCR allosteric modulator. Herein, we confirm the allosteric effects of SB269652 but demonstrate that truncated derivatives containing a THIQ moiety act in a competitive manner with dopamine. To reconcile an orthosteric mode of receptor engagement with the purely allosteric effects mediated by the full length SB269652, we utilized a novel complementation assay in which we can control the components of the D2R signaling unit in a dimeric receptor complex15 to demonstrate that SB269652 engages one protomer of a D2R dimer and negatively modulates the binding of ligands to the second protomer. When this system is constrained to restrict ligand binding to only one protomer of the dimer, SB269652 acts competitively, thus identifying the molecule as a novel chemical probe that can differentiate D2R monomers from dimers/oligomers depending on the observed pharmacology. This property was exploited to demonstrate the presence of native D2R dimers in rat striatum. Finally, by combining molecular modeling with receptor mutagenesis and synthetic chemistry, we propose a mechanistic basis for this unique pharmacology that involves SB269652 adopting an extended binding pose as a bitopic ligand within a D2R protomer. The THIQ core binds within the orthosteric site while the indole moiety interacts with a secondary pocket involving TM2 and TM7, an interaction that is required for the transmission of the allosteric effect to the second protomer. This novel mechanism extends the repertoire of behaviors that can be expressed by multi-site-targeting bitopic GPCR ligands.
Results
SB269652 acts as a pure allosteric modulator at the D2R
We performed interaction studies between SB269652 and the endogenous ligand, dopamine, using multiple assays (D2R-mediated [35S]GTPγS binding (Figure 1b), inhibition of forskolin-stimulated cAMP production (Figure 1c), ERK1/2 phosphorylation (Figure 1d) and β-arrestin 2 recruitment (Figure 1e; Table 1). In all cases, SB269652 behaved allosterically at the D2R. Specifically, the SB269652-mediated reduction in dopamine potency approached a limit at the highest antagonist concentrations. This behavior is consistent with limited negative cooperativity upon saturation of an allosteric site, and is readily visualized in the form of a Schild regression (Figure 1f). In contrast to the theoretically limitless dextral displacement of an agonist concentration-response (C/R) curve mediated by an orthosteric antagonist (yielding a Schild regression of unit slope), the interaction between SB269652 and dopamine was characterized by a curvilinear Schild regression18. Application of an allosteric ternary complex model18 to the C/R data yielded an estimate of SB269652 affinity for the unoccupied receptor (KB = 145 – 416 nM across the different assays, Table 1) and its cooperativity with dopamine (αβ= 0.06 – 0.12). Thus, SB269652 reduces dopamine potency by a factor of approximately 8 – 16 fold, acting as a “partial” antagonist by allowing some dopamine tone to be retained at maximal modulator concentrations.
Table 1.
Characterization of SB269652 binding and function at the D2R. The ability of SB269652, or fragments of SB269652 containing the THIQ7C moiety, to modulate either the functional effect of agonists or their affinity at the D2R expressed in Flp-In CHO cells was tested at various signaling endpoints. For SB269652, functional data were fitted to either an allosteric ternary complex model (equation (10)) to derive values of affinity (KB) and cooperativity (αβ) with the orthosteric probe, whereas for fragments (THIQ7C, MIPS1071, MIPS1059) data were best fitted with a Gaddum-Schild model of competitive antagonism (equation 7). Radioligand binding data were best fitted with equation (3).
| Assay | Test Ligand |
Probe Ligand |
pKB (KB, nM) |
Logαβ (αβ) |
Schild Slope |
|---|---|---|---|---|---|
| pERK1/2 | SB269652 | dopamine | 6.84 ± 0.04 (145) | −1.22 ± 0.04 (0.06) | − |
| S-3PPP | 6.91 ± 0.10 (123) | −1.42 ± 0.03* (0.03) | − | ||
| aripiprazole | 6.81 ± 0.19 (155) | −0.92 ± 0.11 (0.12) | − | ||
| THIQ7C | dopamine | 5.60 ± 0.15 (2510) | 1.03 ± 0.18 | − | |
| MIPS1071 | dopamine | 6.42 ± 0.21 (380) | 1.00 ± 0.08 | − | |
| S-3PPP | 6.44 ± 0.10 (363) | − | 1.11 ± 0.18 | ||
| aripiprazole | 6.24 ± 0.23 (575) | − | 0.94 ± 0.18 | ||
| MIPS1059 | dopamine | 7.63 ± 0.12 (23.4) | − | 1.06 ± 0.04 | |
| cAMP | SB269652 | dopamine | 6.69 ± 0.06 (204) | −1.20 ± 0.02 (0.06) | − |
| [35S]GTPγS | SB269652 | dopamine | 6.57 ± 0.37 (269) | −0.89 ± 0.20 (0.12) | − |
| β-arrestin | SB269652 | dopamine | 6.38 ± 0.21 (416) | −0.98 ± 0.13 (0.10) | − |
| Logα(α) | |||||
| radioligand binding | SB269652 | dopamine | 6.38 ± 0.08 (416) | −0.85 ± 0.13 (0.14) | − |
| [3H]spiperone | −0.65 ± 0.03 (0.22) | − | |||
Analysis with one-way ANOVA followed by a Bonferroni post-test revealed that SB269652 displayed a significantly higher cooperativity with S-3PPP as compared to dopamine and aripiprazole in a pERK1/2 assay, * = P < 0.05. Estimated parameter values represent the mean ± SEM of at least three experiments performed in duplicate.
Because negative allosteric modulation can be exerted upon dopamine affinity (α) and/or signaling efficacy (β), we performed radioligand binding assays to monitor the effects of SB269652 directly on orthosteric ligand affinity. First, we performed saturation binding experiments with the orthosteric antagonist, [3H]spiperone, in the presence of SB269652 (Supplementary Results, Supplementary Fig. 1a & b), which caused a limited rightward shift in [3H]spiperone pKD with no significant decrease in Bmax. Analysis using an allosteric ternary complex model yielded an affinity of KB = 933 nM, and modest negative cooperativity with [3H]spiperone (α = 0.28). We then performed [3H]spiperone competition binding experiments with dopamine in the presence of SB269652. Analysis of these data yielded values of affinity (KB = 416 nM), and cooperativity with dopamine (α = 0.14), similar to those determined from the functional assays (Table 1, Supplementary Fig. 1 and Supplementary Table 1). This indicates that SB269652 behaves predominantly as a negative modulator of orthosteric ligand affinity.
SB269652 displays ‘probe dependence’
Probe dependence is a phenomenon whereby the allosteric effect (specifically, the extent of cooperativity) changes depending on the properties of the orthosteric ligand used to probe receptor function19. This is distinct from the behavior of an orthosteric antagonist, which does not discriminate between different orthosteric agonists. We thus chose two structurally distinct D2R agonists, namely the clinically effective antipsychotic, aripiprazole, and the partial agonist S-3PPP (preclamol)19, and investigated the effects of SB269652 using D2R-mediated phosphorylation of ERK1/2 as a functional assay. Although the estimated affinity (KB) determined for SB269652 did not differ significantly when tested against each agonist, the degree of negative cooperativity between SB269652 and S-3PPP (αβ = 0.03; equating to a maximal 33-fold decrease in dopamine potency) was significantly higher than that observed for either dopamine or aripiprazole (αβ = 0.06 and 0.12 respectively, Supplementary Fig. 2, Table 1). Thus SB269652 displays probe dependence.
The THIQ moiety of SB269652 occupies the orthosteric site
Another classic expectation of allosteric modulators is structural diversity from orthosteric ligands, since the modulators target a spatially distinct binding pocket20. In this regard, the action of SB269652 as a negative allosteric modulator of the dopamine D2R is surprising given that it was first generated as part of a series of orthosteric D2R antagonists and contains structural features consistent with an orthosteric mode of binding10,21. In particular, the THIQ moiety of SB269652 contains an aliphatic amine that is expected to form a salt bridge with the conserved aspartate (Asp3.32) of aminergic GPCRs.
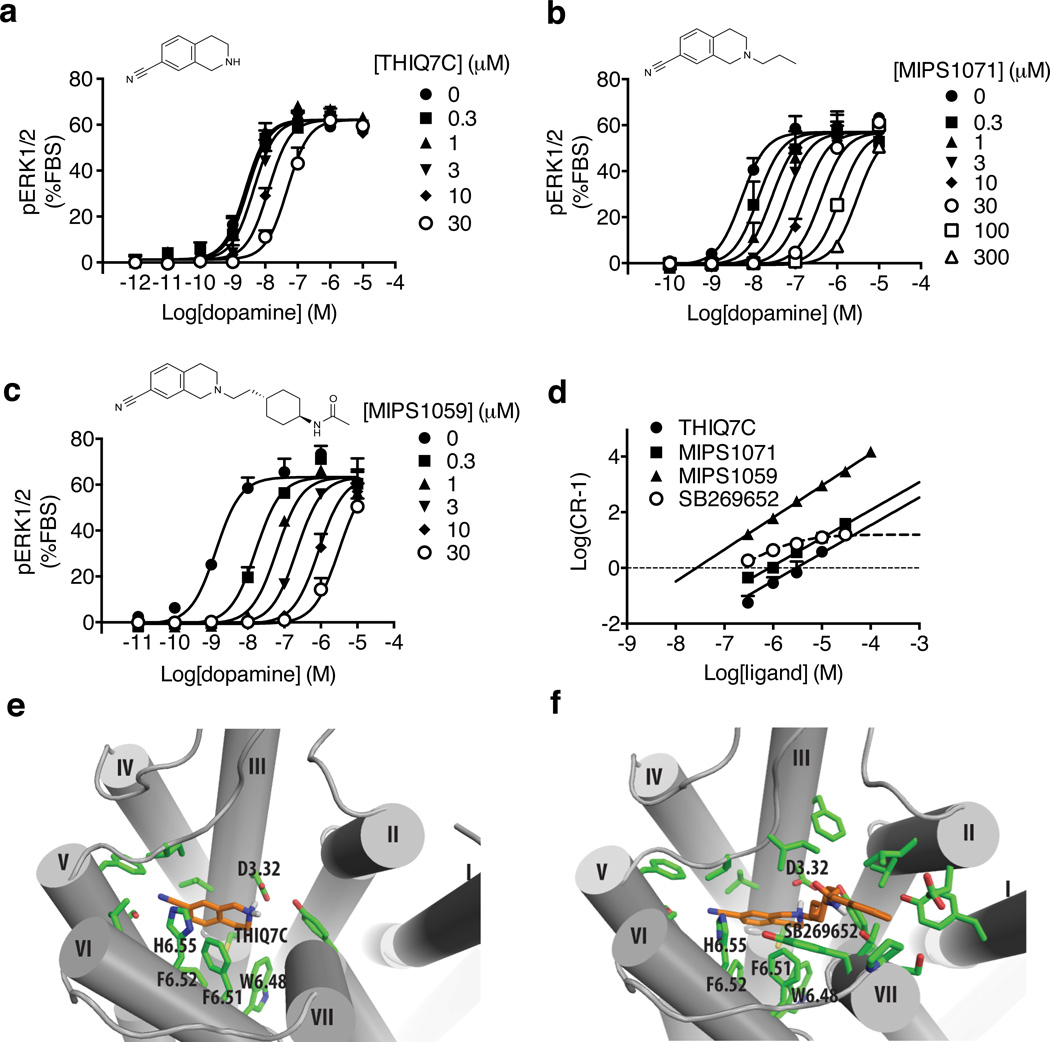
To confirm whether the binding of SB269652 involves occupancy of the orthosteric site of the D2R, we generated progressively truncated derivatives that retained the 1,2,3,4-tetrahydroisoquinoline-7-carbonitrile (THIQ7C) moiety. As illustrated in Figure 2, these SB269652 fragments acted competitively with dopamine in an ERK1/2 phosphorylation assay. The smallest fragment, THIQ7C (2) (Figure 2a), had a 17-fold lower affinity (KB = 2.51 µM, Table 1) than SB269652. When this fragment was extended to 2-propyl-1,2,3,4-tetrahydroisoquinoline-7-carbonitrile (3, MIPS1071, Figure 2b) the affinity increased (KB = 380 nM, Table 1) to the same level as SB269652, but the inhibition remained competitive. We also performed analogous experiments using either aripiprazole (Supplementary Fig. 3a & c) or S-3PPP (Supplementary Fig. 3b & c) as the orthosteric agonist and determined affinities for MIPS1071, which were not significantly different from those determined when dopamine was used (aripiprazole - KB = 575 nM, S-3PPP - KB = 363 nM, Table 1). The competitive behavior of MIPS1071 was confirmed in a second assay measuring inhibition of forskolin-stimulated cAMP production (Supplementary Fig. 3d & e). N-((trans)-4-(2-(7-cyano-3,4-dihydroisoquinolin-2(1H)yl)ethyl)cyclohexyl)acetamide (4, MIPS1059), which included the carboxamide but not the indole moiety of SB269652, also behaved competitively (KB = 23.4 nM, Figure 2c & Table 1). In all cases, Schild slopes were not significantly different from unity (Figure 2d).
Figure 2. Fragments of SB269652 containing the tetrahydroisoquinoline pharmacophore interact with the D2R in an orthosteric manner.
We generated progressively truncated fragments containing the tetrahydroisoquinoline moiety from the smallest 1,2,3,4-tetrahydroisoquinoline-7-carbonitrile (THIQ7C) (a), to MIPS1071 (b) and MIPS1059 (c). In an assay measuring D2R mediated phosphorylation of ERK1/2, all fragments caused parallel dextral shifts in dopamine potency. This is illustrated graphically by a Schild plot (d). Data represent mean values plus S.E.M from three independent experiments. Molecular modeling and ligand docking experiments, using a homology model of the D2R, reveal that both the smallest orthosteric fragment THIQ7C (e) and SB269652 (f) occupy the orthosteric binding site within the receptor with the protonated tertiary amine of the tetrahydrosioquinoline moiety. In addition the indole-2-carboxamide moiety of SB269652 extends out towards TM2 and TM7.
Molecular docking using a homology model of the D2R, based on the crystal structure of the D3R, located the smallest fragment, THIQ7C, in the orthosteric site. In this pose, the aromatic head group interacts with conserved aromatic residues of TM6, and the positively charged aliphatic amine forms a salt bridge with Asp1143.32, both characteristic interactions in the orthosteric binding site of aminergic GPCRs (Figure 2e). Interestingly, when the full length SB269652 was docked with the THIQ core in the orthosteric site, the linker and secondary pharmacophore could be accommodated in a potential bitopic manner by a secondary binding pocket towards the extracellular interface between TM2 and TM7 (Figure 2f), similar to a previous finding with the extended D2R antagonist R-2222,23. In subsequent MD simulations, the THIQ core of SB269652 was extremely stable, consistent with its binding to the orthosteric site in the context of the full length molecule. Based on these results, however, we would also expect the full-length SB269652 to act competitively (Figure 3a), in contrast to its observed allosteric behavior, suggesting that an additional element is required to account for this mechanism.
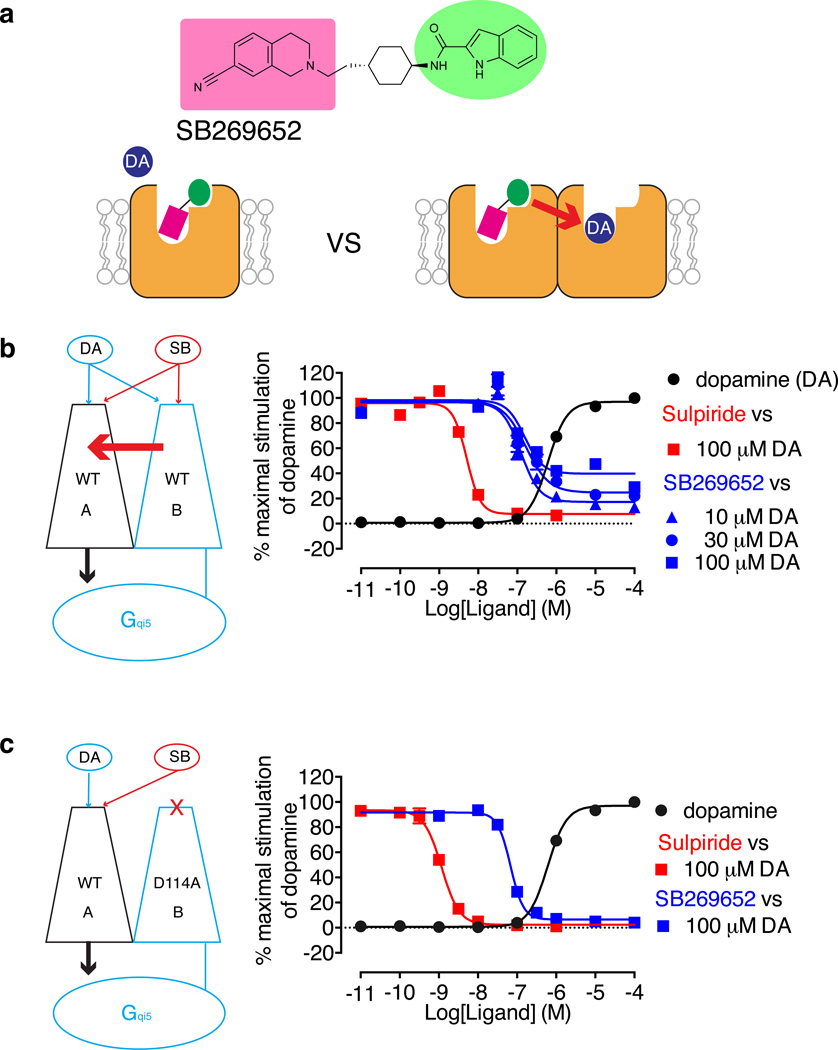
Figure 3. A functional complementation assay demonstrates that SB269652 acts as a negative allosteric modulator across a D2R dimer.
(a) The purely allosteric action of SB269652 cannot be reconciled with the dual orthosteric/allosteric mode of interaction predicted by both our ligand fragment and molecular modeling experiments. At a dimeric D2R, SB269652 can bind in a bitopic manner to one protomer and exert negative cooperativity across the dimer for the binding of dopamine to the other protomer in agreement with its purely allosteric pharmacology (b) We used a complementation assay to investigate the pharmacology of a D2R dimer. This system consists of a non-functional D2R-Gαqi5 fusion protein (protomer B) that can still bind dopamine or SB269652 and co-expression of a WT D2R (protomer A) to restore a functional unit that couples to an aequorin readout of receptor function. SB269652 is unable to completely antagonize the action of 100 µM dopamine at the functional D2R dimer, in contrast to the complete inhibition exerted by the competitive antagonist sulpiride. (c) The functional effect of dopamine at the dimer is retained even if the important Asp1143.32 of protomer B is mutated to alanine (D114A). At this complemented pair, SB269652 is able to completely inhibit the action of 100 µM dopamine. Data represent the mean ± S.E.M. of three independent experiments.
SB269652 exerts allostery across a D2R dimer
Family A GPCRs, including the D2R, may form dimers or higher order oligomers, although some debate remains as to both their transience and functional importance 16,24–30. An attractive hypothesis to reconcile the allosteric pharmacology of SB269652 with its apparent engagement of the orthosteric site is that SB269652 binds within one protomer of a dimeric or oligomeric D2R complex and allosterically modulates the binding of dopamine at the other protomer(s) (Figure 3a). If it is assumed that SB269652 exerts very high negative cooperativity with itself, which would prevent a second molecule of SB269652 binding to the other protomer within the complex, but limited negative cooperativity with dopamine and other canonical orthosteric ligands, then this can account for the observed pharmacology.
One obstacle to understanding the relevance of Family A GPCR oligomerization has been the relative paucity of robust experimental techniques to allow control of the identity of the components comprising the signaling unit. We recently addressed this by developing a functional complementation assay that enables such control15. In brief, a D2R-Gqi5 fusion construct (termed ‘protomer B’) was generated in which the G protein was fused directly to the short cytoplasmic tail of the D2R (Figure 3b). Although expressed at the cell surface, this construct is non-functional, as measured in an aequorin-based assay, due to steric constraint of the fused G protein. When coexpressed, wild-type (WT) D2R (‘protomer A’), which also cannot signal alone due to the absence of Gq coupling or free Gqi5, rescues function by signaling through the G protein provided by protomer B. Thus, coexpression of two ‘non-functional’ D2R protomers allows the study of a defined D2R dimer or higher order complex. We used this functional complementation assay to test our hypothesis that the modulation mediated by SB269652 occurs across a dimeric complex.
When the two D2R protomers (A and B) were stably coexpressed, a robust concentration-dependent response to dopamine was observed (pEC50 = 6.24 ± 0.11) (Figure 3b). We then tested the ability of increasing concentrations of the orthosteric antagonist, sulpiride, or SB269652 to inhibit the effect of different dopamine concentrations. As expected, sulpiride completely inhibited the effect of the highest concentration (100 µM) of dopamine (pIC50 = 8.30 ± 0.18). In contrast, SB269652 only partially antagonized dopamine (Figure 3b), consistent with the limited negative cooperative effects we had identified in our cell-based and radioligand binding assays.
We also previously demonstrated that mutation of the conserved D2R Asp1143.32 to alanine (D114A) in protomer B, which prevents ligand binding to the orthosteric site of that protomer, allows agonist binding to protomer A to engage the Gqi5 protein fused to protomer B, reflecting a receptor-G protein engagement mechanism in which only one protomer is competent to bind ligand15. If SB269652 interacts in a bitopic manner with the D2R in protomer B to transmit an allosteric effect to protomer A, then we predicted this effect would be lost upon coexpression of WT D2R as protomer A and D2-Asp1143.32Ala-Gqi5 as protomer B. Under this condition, the only mode of interaction available to both dopamine and SB269652 would be via protomer A, which would manifest as competition because both ligands would engage the orthosteric site upon binding this protomer. As shown in Figure 3c, dopamine retained the ability to stimulate a response with a potency not significantly different from that of the WT-WT complement pair (pEC50 = 6.27 ± 0.11, Student’s t-test P >0.05) but, importantly, the response to 100 µM dopamine was now completely antagonized by both sulpiride and SB269652 (pIC50 = 8.92 ± 0.10 and pIC50 = 7.17 ± 0.10, respectively) in a manner that is indistinguishable from a competitive interaction. Collectively, our functional complementation experiments are consistent with a model whereby SB269652 acts as an allosteric modulator only when able to transmit its cooperative effects across a D2R dimer interface; engagement of a single protomer results in competition with the cognate agonist.
Bitopic engagement by SB269652 of D2R is critical
SB269652 has an extended structure similar to many competitive D2R ligands10. However, in contrast to previous studies that have reported very high negative cooperativity between orthosteric ligands at the D2R15–17, the negative allosteric effect of SB269652 is clearly saturable. Consequently, we hypothesized that this unique pharmacology is related to SB269652’s ability to bind in a bitopic pose that extends from the orthosteric site into a secondary pocket between TM2 and TM7 identified in our molecular model. The interaction between the indole-2-carboxamide moiety and the secondary pocket may be a requirement for the cross-protomer allosteric action of SB269652.
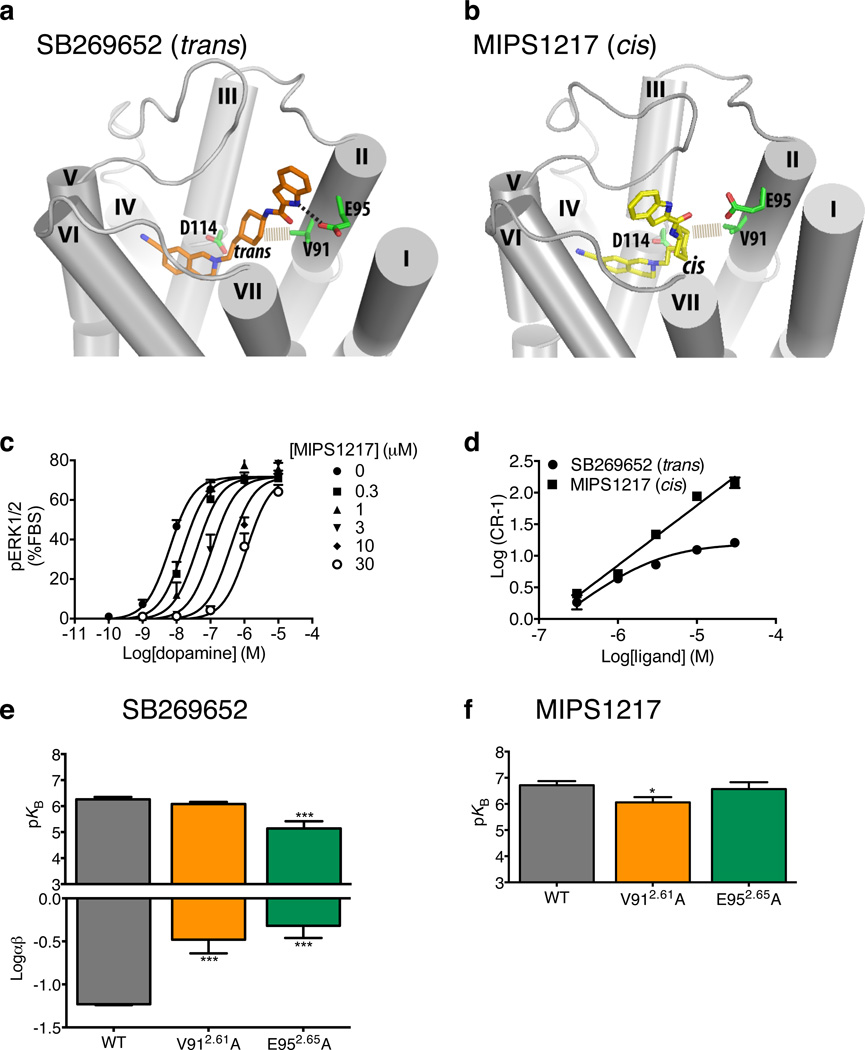
To investigate this, we first generated the cis-isomer of SB269652 (5, MIPS1217) with the hypothesis that the indole-2-carboxamide moiety would have a different orientation relative to the THIQ orthosteric core. In our molecular docking, in which the THIQ core is restrained in a similar pose as THIQ7C, the indole-2-carboxamide moiety of SB269652 is oriented towards a secondary pocket lined by the extracellular portions of TM2 and TM7, while that of its cis-isomer cannot reach this pocket. Based on these initial docking poses, we carried out molecular dynamics simulations to explore the best binding modes of SB269652 and MIPS1217, containing the trans- or cis-orientation of the cyclohexyl spacer, respectively. Similar to SB269652, the THIQ core of MIPS1217 is very stable in the orthosteric site. SB269652 remains in an extended conformation, and its cyclohexyl and indole-2-carboxamide moieties interact with Val912.61 and Glu952.65, respectively (Figure 4a). In contrast, the cyclohexyl group of the cis-isomer, MIPS1217, still contacts Val912.61 but the indole-2-carboxamide is oriented towards TM6 and not TM2 (Figure 4b). Thus, if the interaction of the secondary pharmacophore with TM2 is essential for the allosteric effect, one would expect MIPS1217 to act as a competitive antagonist. In agreement with this hypothesis, MIPS1217 antagonized dopamine at the D2R in a purely competitive manner in a pERK1/2 assay (KB = 195 nM, Figure 4c, 4d & Supplementary Table 2).
Figure 4. Interaction of the indole moiety with a secondary pocket between TMs 2 and 7 is required for the allosteric pharmacology of SB269652.
When docked into a homology model of the D2R the tetrahydroisoquinoline core of both SB258652 (a) and the cis-isomer (MIPS1217, b) occupy similar positions within the orthosteric core. In contrast while the indole amine moiety of SB269652 extends into a secondary pocket between TM2 and TM7, where the nitrogen of the indole heterocyle makes a hydrogen bond interaction with Glu952.65 and the cyclohexyl group makes a hydrophobic interaction with Val912.61, this moiety has a different orientation for MIPS1217 and extends towards the top of TM6. (c) In an assay measuring D2R mediated phosphorylation of ERK1/2, MIPS1217 behaved competitively (parallel dextral shifts in dopamine potency). This is illustrated graphically by a Schild plot (d). SB269652 affinity or negative cooperativity with dopamine (e) or affinity of the cis isomer MIPS1217 (e) at mutant D2R receptors was determined through interaction studies with dopamine in an ERK1/2 phosphorylation assay. Data represent mean values plus S.E.M from three independent experiments.
We then explored the interactions that might contribute to the allosteric effect of SB269652. We mutated Val912.61 & Glu952.65 to alanine and generated additional cell lines stably expressing WT or mutant D2Rs at similar levels (supplementary Table 3). Dopamine displayed similar potencies at all mutants compared to the WT, consistent with the mutated residues being outside the orthosteric site (Supplementary Table 2). At the WT D2R, SB269652’s affinity (KB = 549 nM) and negative cooperativity (αβ = 0.06) were very similar to the results presented above (Supplementary Fig. 4, Supplementary Tables 2 & 4). Unfortunately, the Val912.61Ala mutant displayed no detectable binding of [3H]spiperone up to a concentration of 10 nM, but saturation experiments with an alternative orthosteric ligand, [3H]raclopride, revealed that the receptor variant bound with an affinity and Bmax not significantly different from WT (Supplementary Table 3). Val912.61 was predicted to make a hydrophobic interaction with the cyclohexyl group of both SB269652 and MIPS1217. Mutation to alanine did not alter the affinity (KB = 831 nM) of SB269652 but led to a significant decrease in the negative cooperativity of with dopamine (αβ = 0.33) (Figure 4e, Supplementary Table 2). In contrast, a significant decrease in affinity was observed for MIPS1217 (KB = 891 nM, Supplementary Table 2 & Figure 4f). Glu952.65 was predicted to make a hydrogen bond interaction with the heterocyclic nitrogen within the indole moiety of SB269652 but not MIPS1217. Mutation of this residue to alanine caused both a significant decrease in both the negative cooperativity (αβ = 0.48, Supplementary Table 2) with dopamine and affinity of SB269652 for the D2R (KB = 724 nM, Figure 4e, Supplementary Table 2). In contrast Glu952.65Ala had no effect on the competitive profile of MIPS1217 (Figure 4f, Supplementary Table 2). Given that this mutation had the most significant effect on the allosteric pharmacology of SB269652, we extended our studies to a radioligand binding assay. These experiments revealed that, while Glu952.65Ala had no effect on dopamine affinity, significant decreases were observed in SB269652 affinity (WT, KB = 645 nM; Glu952.65Ala, KB = 2754 nM), as well as its negative cooperativity with dopamine (α = 0.60), and [3H]spiperone (α = 0.47) compared to the WT receptor (dopamine, α = 0.29; [3H]spiperone, α =0.29), consistent with the functional studies (Supplementary Fig. 5, Supplementary Table 4).
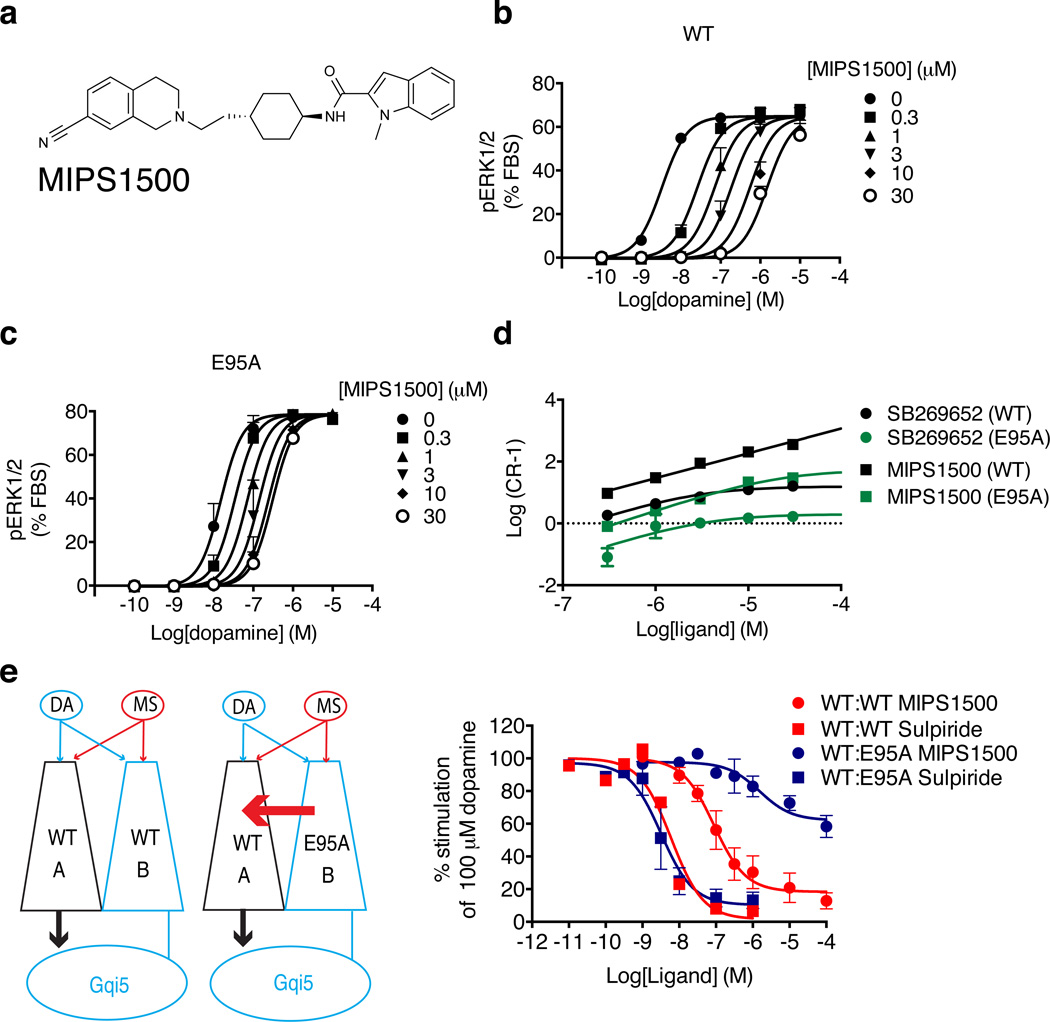
To further explore the importance of the predicted hydrogen bond interaction between the indole heterocyclic nitrogen of SB269652 and Glu952.65 at the top of TM2, we generated an N-methyl indole derivative of SB269652, MIPS1500 (6), which would be unable to form the hydrogen bond with Glu952.65 and thus unable to properly engage the secondary pocket (Figure 5a). In a pERK1/2 assay, MIPS1500 antagonized dopamine in a competitive manner (KB = 58 nM, Figure 5b & Supplementary Table 2). We also hypothesized that the N-methyl indole of MIPS1500 would interact with a hydrophobic alanine residue at position 2.65, functionally recapitulating the allosteric effect resulting from the interaction between the heterocyclic nitrogen of the indole of SB269652 and Glu952.65 in the WT receptor. Therefore, we repeated the experiment using the Glu952.65Ala mutant and MIPS1500. In contrast to our observations at the WT receptor, the reduction in dopamine potency mediated by MIPS1500 approached a limit, consistent with negative cooperativity (KB = 239 nM, logαβ = 0.03, Figure 5c, 5d & Supplementary Table 2); remarkably, MIPS1500 thus displayed both an affinity and negative cooperativity with dopamine at the Glu952.65Ala mutant that was not significantly different from SB269652 at the WT D2R (Student’s t-test, P > 0.05). This ‘rescue’ of allosteric pharmacology underlines the importance of the interaction of the indole-2-carboxamide with a secondary pocket between TM2 and TM7, and in particular with Glu952.65.
Figure 5. When the D2R Glu952.65 Ala mutant is expressed as protomer B, the orthosteric antagonist MIPS1500 acts as a negative allosteric modulator across a D2R dimer.
The action of MIPS1500 (a), which differs from SB269652 through the N-methylated of the nitrogen in the indole heterocycle, at the WT D2R (b) or the D2R Glu952.65Ala mutant (c) in an assay measuring D2R mediated phosphorylation of ERK1/2. These data are illustrated graphically by a Schild plot (d). (e) At a complement pair consisting of coexpression of a non-functional D2R-Gαqi5 fusion protein (protomer B) and a WT D2R (protomer A), MIPS1500 is able to antagonize the action of 100 µM dopamine at the functional D2R dimer. At a complement pair of a WT D2R (protomer A) and a D2R Glu952.65Ala (E95A) mutant (Protomer B), MIPS1500 displays incomplete antagonism of 100 µM dopamine. Data represent mean values plus S.E.M from three independent experiments. Data for sulpiride at the WT:WT complement pair are replotted from Figure 3.
Finally, we made use of the complementation system to investigate whether the allosteric action of MIPS1500 at the Glu952.65Ala mutant is analogous to our proposed mode of action for SB269652; namely allostery across a D2R dimer. Consistent with a competitive interaction, MIPS1500 antagonized the effect of 100 µM dopamine when protomers A and B were both WT D2R (Figure 5e, pIC50 = 7.06 ± 015). In contrast when a complemented pair was expressed consisting of the Glu952.65Ala mutant D2R as protomer B and a WT D2R as protomer A, MIPS1500 only partially antagonized the effect of dopamine (Figure 5e, pIC50 = 5.81 ± 0.30) due to limited negative cooperativity mediated by the D2R mutant across the dimer. Sulpiride competitively inhibited the action of 100 µM dopamine at both the WT:WT and WT:Glu952.65Ala with similar potency (WT:WT, pIC50 = 8.21 ± 0.14; WT:Glu95Ala, pIC50 = 8.49 ± 0.17, Figure 5e). Collectively, our data provided evidence that SB269652 binds to one protomer of a D2R dimer in a bitopic mode and modulates the action of dopamine at the other; the interaction of the indole-2-carboxamide moiety of SB269652 with a secondary pocket is essential for this allosteric pharmacology.
SB269652 is allosteric at D2Rs in native tissue
If the mode of action of SB269652 involves an obligate allosteric interaction in a dimeric context but a competitive interaction in a monomeric context, then the potential exists that the molecule can be used as a molecular ‘probe’ to investigate the presence of such complexes in native tissues and, potentially, in vivo. The D2R is highly expressed in the striatum9 and we thus performed a [35S]GTPγS binding assay using membranes from rat striatal tissue (Supplementary Figure 6). Dopamine stimulated [35S]GTPγS binding in a concentration-dependent manner with a pEC50 =5.85 ± 0.11. Increasing concentrations of SB269652 caused a limited dextral shift in the dopamine C/R curve, consistent with negative allosteric modulation (KB = 537 nM; αβ = 0.22). As illustrated by the Schild regression, this effect was not significantly different (Student’s t-test) from that observed when analogous experiments were performed in heterologous cells expressing the D2R (Supplementary Figure 6, Table 1). Thus, in the context of our currently proposed bitopic model of action, the pharmacology of SB269652 in rat striatal membranes as a negative allosteric modulator suggests the presence of native D2R dimers or higher order oligomers in this tissue.
Discussion
By using complementary approaches, we have identified a unique mechanism of action for SB269652, characterized by a “switch” in pharmacology from allosteric to competitive, depending on whether the interaction occurs at a functional dimeric (or higher order oligomer) versus monomeric Family A GPCR. Although prior studies have provided some evidence for cooperativity between orthosteric ligands at oligomeric GPCRs16,31–34, we propose that the unique allosteric/competitive switch described here within the same molecule is likely related to its binding in a bitopic pose that extends from the orthosteric site into a secondary pocket between TM2 and TM7, the latter pocket being essential for transmission of cooperativity across a D2R dimer. This finding extends the concept of the bitopic ligand from a novel means of engendering receptor selectivity within a monomeric receptor to one that can also yield chemical probes sensitive to GPCR dimerization status.
Many central nervous system diseases, including schizophrenia and Parkinson’s disease, are treated with drugs that bind D2-like dopamine receptors9. To date, however, drug discovery at these receptors has focused on targeting the orthosteric site, and such an approach is limited by challenges associated with lack of receptor subtype selectivity and unwanted side-effects. As such, allosteric targeting may hold several advantages, such as greater subtype selectivity and/or maintenance of spatiotemporal patterns associated with endogenous neurohumoral signalling3. This is particularly pertinent to schizophrenia, in which orthosteric blockade of the D2R, whilst effective for the treatment of the positive symptoms of the disease, is associated with extrapyramidal side-effects35. Partial blockade by a negative allosteric modulator with limited cooperativity represents a potentially safer therapeutic strategy. We demonstrate that SB269652 meets this mechanistic criterion at the D2R, with a modest negative cooperativity. However, because SB269652 binds in a bitopic manner occupying the orthosteric site, the mechanism behind the allostery mediated by SB269652 cannot readily be explained via the formation of a ternary complex comprising an orthosteric ligand, SB269652 and a single D2R protomer. Thus, the allosteric behavior of SB269652 differs from other prototypical allosteric modulators of aminergic GPCRs. For example, many allosteric modulators described for the muscarinic acetylcholine receptors have a pharmacology that can be theoretically accommodated within a monomeric receptor model36, as most recently demonstrated directly by the solution of the crystal structure of the M2 muscarinic receptor bound to both an agonist and a positive allosteric modulator37.
The crystal structure of the D3R bound to the antagonist eticlopride revealed a “secondary” pocket in the receptor structure that has been subsequently demonstrated to be accessed by extended orthosteric ligands, such as R-2221–23 to achieve subtype-selectivity. Recent studies have illustrated that the indole-2-carboxamide of R-22 also occupies a secondary pocket positioned at the interface between TMs 1, 2, and 7 in the highly homologous D2R22,23. It should be noted, however, that R-22, with the same indole-2-carboxamide moiety as SB269652, is a competitive antagonist23, while the interaction of the indole-2-carboxamide moiety with the secondary pocket is critical for the allosteric action of SB269652. As such, the exact nature of the interactions made within this pocket must be affected by the different orthosteric binding moieties and linker regions of R-22 and SB269652 to determine the differing pharmacological properties of these two ligands. Of interest, previous cysteine crosslinking studies identified residues at the extracellular end of TM1 that form a symmetrical interface between protomers of a D2R homo-oligomer25, and it is possible that this interface is involved in the communication of cooperativity from one SB269652-bound protomer to the other protomer in addition to the secondary pocket between TM2 and TM7.
To date, studies of bitopic ligands at GPCRs have been largely restricted to the muscarinic receptors. Such bitopic modes of interaction underlie the receptor subtype selectivity of these ligands or even confer biased agonism5,6,38. Our study suggests that ligands at other GPCRs, even those with allosteric pharmacology, may have an unappreciated bitopic mode of interaction. Furthermore, by demonstrating that SB269652 acts as an allosteric modulator at a D2R dimer we expand the novel pharmacology that can be conferred by a bitopic mode of interaction when placed in the context of a dimeric receptor.
With regards to the native functional signaling unit for Family A GPCRs, studies have shown that rhodopsin, the β2 adrenergic receptor and the µ opioid receptor can couple efficiently to G proteins when reconstituted into high density lipoprotein particles containing only a single receptor39–41. However, these observations do not rule out the ability of GPCRs to dimerize in their native environment, and there is accumulating evidence that Family A GPCRs, including the D2R, can form di/oligomeric complexes that modulate receptor function24,42. Indeed, negative cooperativity has been demonstrated for agonist and antagonist binding across a GPCR heterodimer, mediated by conformational changes within both protomers and/or at the dimer interface15,16,31,33,34,43,44. With the exception of our recent study, where we had to modify one protomer within a D2R dimer to observe positive modulation of agonist efficacy exerted by an antagonist binding at the unoccupied protomer15, the majority of prior work revealed high negative cooperativity of ligand binding across GPCR hetero or homo-oligomers such that ligand binding to one protomer precludes binding to the second protomer45. As such the exclusively weak negative allosteric modulation of agonist and antagonist binding exerted by SB269652 at the WT D2R is distinct. We have now made use of our functional complementation system to demonstrate that at a D2R dimer, SB269652 acts at one protomer to allosterically modulate the binding of dopamine at another protomer within the complex. Mutational impairment of the orthosteric site within one protomer to bias the interaction between ligands towards the other protomer revealed a mechanism consistent with competition. To our knowledge, such a switch between competitive and allosteric pharmacology for the same molecule depending on the oligomerization status of a GPCR has never been demonstrated before, and opens up a new avenue for chemical biology applications of GPCR ligands. It must be acknowledged, however, that our results do not completely rule out an alternative mechanism whereby SB269652 is distributed between two different binding orientations (orthosteric and allosteric) within the same protomer. However, such an interaction would require SB269652 to adopt a purely allosteric mode within the receptor with comparable affinity to its orthosteric mode. This type of interaction is not supported by our modeling, structure activity and mutagenesis studies, and is difficult to reconcile with the results of our complementation experiments or with the negative cooperativity of SB269652 with larger compounds such as aripiprazole.
There is increasing evidence that dimeric or oligomeric complexes of GPCRs may be transient in nature27–30. Thus, SB269652 may switch between orthosteric and allosteric pharmacology at the D2R depending on factors that might influence the formation of transient dimers, such as receptor number or membrane microdomains. Although many studies have demonstrated that D2R receptors can form homodimers in heterologous systems, evidence that such complexes exist in vivo remains elusive24,42. The allosteric action of SB269652 at D2Rs expressed in rat striatal tissue points towards the existence of D2R dimer/oligomers in this native tissue, assuming that our model is correct. This highlights the utility of ligands that have differential pharmacology at monomeric versus dimeric/oligomeric receptor complexes as potential probes for such complexes in vivo. In addition to homodimers, the D2R has been reported to form heteromeric complexes with other GPCRs and these complexes have been highlighted as attractive potential therapeutic targets42,46–49. Through the demonstration that the allosteric mechanism of action of SB269652 is consistent with a model involving a D2R dimer, our study provides a proof-of-concept for an approach to target such GPCR complexes with ligands that modulate receptor function specifically across GPCR heterodimers.
Online Methods
Materials
Dulbecco’s modified Eagle’s medium, Flp-In CHO cells, and hygromycin B were purchased from Invitrogen (Carlsbad, CA). Fetal bovine serum (FBS) was purchased from ThermoTrace (Melbourne, VIC, Australia). [3H]spiperone, [3H]raclopride, [35S]GTPγS (1000 Ci/mmol), AlphaScreen reagents and Ultima gold scintillation cocktail were from PerkinElmer (Boston, MA). pcDNA3L–His-CAMYEL was purchased from ATCC. All other reagents were purchased from Sigma Aldrich (St. Louis, MO).
Cell culture and membrane preparation
was performed as described previously50
Preparation of striatal membranes
Rats were decapitated, the whole brain removed from the skull. Striatal tissue was dissected and placed in 10 ml of 20 mM Tris–HCl buffer, pH 7.4, containing NaCl (100 mM), MgCl2 (6 mM) and EDTA (1 mM)] and a cocktail of protease inhibitors (Roche Diagnostics, Mannheim, Germany) at 4 °C. The tissue was homogenized using a Polytron homogeniser for 3 10-second intervals on the maximum setting with 30-second periods on ice between each burst. The homogenate was made up to 30 mL and centrifuged (1,000 g, 10 min, 4 °C), the pellet discarded and the supernatant recentrifuged at 30,000 g for 1 hour at 4 °C.
Molecular Biology
cDNA in pcDNA3.1+ encoding the long isoform of the wild-type human D2 dopamine receptor (D2LR) was obtained from Missouri University of Science and Technology (http://www.cdna.org). Oligonucleotides were purchased from GeneWorks (Hindmarsh, Australia). An N-terminal c-myc epitope tag (EQKLISEEDL) was introduced to the sequence of the D2LR and flanking AttB sites were introduced to the WT D2LR by overlap extension polymerase chain reaction to allow sub-cloning into the pDONR201™ vector. The WT or c-myc tagged wildtype (WT) D2LR receptor construct in pDONR201™ were subsequently transferred into the pEF5/frt/V5/dest vector using the LR clonase enzyme mix (Invitrogen). Desired mutations were introduced using the Quikchange™ site-directed mutagenesis kit (Agilent). Receptor constructs in pEF5/frt/V5/dest were used to transfect Flp-In CHO cells (Invitrogen). Cells were transfected using linear polyethyleneimine (PEI, Polysciences, Warrington, PA) as described previously51.
ERK1/2 phosphorylation assay
Experiments were performed as described previously50. Concentration-response stimulation or inhibition curves were generated by exposure of the cells to antagonist ligand for 30 min and then dopamine for 5 min. Data were normalized to the response generated by 10% fetal bovine serum.
BRET cAMP assay
D2LR-Flp-In CHO cells were transfected with 2µg of pcDNA3L–His-CAMYEL. The assay was performed as described previously with the following difference: 30 minutes prior to agonist addition appropriate concentrations of SB269652 or fragment ligand were added. 5 minutes following agonist addition 10 µL of forskolin was added to a final concentration of 3 µM. Data were normalized to the level of cAMP generated by 3 µM forskolin.
[35S]GTPγS Binding Assay
Cell membranes (5 µg D2L-Flp-In CHO or 20 µg rat striatal tissue) were equilibrated for 60 min at 30 °C with varying concentrations of ligands in binding buffer (20 mM HEPES, 10 mM MgCl2, 100 mM NaCl 1 mM EGTA, 1 mM EDTA, 0.1% ascorbic acid, 1 mM DTT; pH 7.4) containing 3 µM or 10 µM GDP (D2L-Flp-In CHO or rat striatum, respectively). [35S]GTPγS (0.1 nM) was added to a final volume of 0.2 mL (D2L-Flp-In CHO) or 1 mL (rat striatum) and membranes were incubated for further 60 min at 30 °C. For experiments using D2L-Flp-In CHO membranes 5 µg of saponin was added per assay point. For experiments using D2L-Flp-In CHO membranes termination of [35S]GTPγS binding was by rapid filtration with a Packard plate harvester onto 96-well GF/C filter plates followed by three washes with ice cold 0.9% NaCl. Bound radioactivity was measured in a Microbeta microplate counter (Perkin Elmer). For experiments using membranes of rat striatal tissue reactions were terminated by fast flow filtration over GF/B membranes using a Brandel Harvester followed by three washes with ice-cold 0.9% NaCl. Bound radioactivity was measured in a Tri-Carb 2900TR liquid scintillation counter (Perkin Elmer). Data were normalized to the maximal response of dopamine in the control condition.
[3H]spiperone binding assay
Experiments were performed using a methodology described previously50.
β-arrestin recruitment
HEK293T cells were transfected with a 2:2:4 ratio of cDNA coding for D2L-Rluc8, GRK2 and β-arrestin 2-YFP. Experiments were performed as described previously52. Antagonists were added 30 min prior to coelenterazine-h. Data were normalised to the maximal response of dopamine in the control condition.
Aequorin complementation assay
Experiments were performed as described previously15. Data was normalized to the maximal response of dopamine in the control condition.
Molecular Modeling
The binding modes of SB269652 and its tetrahydroisoquinoline core, THIQ7C, were investigated with a D2R model stabilized by eticlopride that we previously built based on the D3R structure and relaxed with MD simulations21,22. To acquire a reference binding mode for THIQ7C in the high-resolution structure of D3R (PDB: 3PBL)21, THIQ7C in the protonated form was first docked to the D3R structure with the induced-fit docking (IFD) protocol (Schrodinger, Inc.)53, and the lowest MM/GBSA energy pose from the largest pose cluster was selected as the reference pose. Assuming identical binding modes of THIQ7C in the near-identical orthosteric binding sites of D3R and D2R, the pose from the IFD trial with our D2R model that is closest to the reference pose in the D3R structure was selected. The full-length SB269652 was then docked to the D2R model by a core-constrained IFD protocol22 with restraints on the tetrahydroisoquinoline core (heavy-atom RMSD < 2.0 Å) to the selected pose of THIQ7C in D2R. The resulting 27 docked poses were grouped into three clusters with a ligand RMSD threshold of 5 Å, and a pose having the lowest IFDScore53 within the largest cluster was selected as the representative pose of SB269652. The representative binding pose of the cis-isomer of SB269652 (MIPS1217) in the D2R model was similarly acquired. The ligand partial charges were re-assigned using the QM-polarized ligand docking (QPLD) protocol54 (Schrödinger Suite 2012) for this representative pose. The D2R-SB269652 and D2R-MIPS1217 complexes were then relaxed by molecular dynamics simulations in the lipid bilayer environment using Desmond (version 3.0, D. E. Shaw Research, New York, NY, 2011)55.
Data analysis
GraphPad Prism 6.0b (San Diego, CA) was used for all statistical analysis, nonlinear regression, and simulations.
Analysis of radioligand binding experiments
For radioligand saturation binding data, the following equation was globally fitted to nonspecific and total binding data:
| (1) |
Where Y is radioligand binding, Bmax is the total receptor density, [A] is the free radioligand concentration, KA is the equilibrium dissociation constant of the radioligand, and NS is the fraction of nonspecific radioligand binding.
The interaction between [3H]spiperone and SB269652 in a saturation assay was best fit by the following equation derived from the allosteric ternary complex model:
| (2) |
Where Y is specific binding, A is the radioligand ([3H]spiperone), B is the allosteric modulator (SB269652), Bmax is the maximal concentration of receptors labelled by the radioligand, KA and KB the equilibrium dissociation constants for the orthosteric and allosteric drugs, respectively; and α defines the cooperativity between the radioligand and the allosteric modulator.
Competition-binding curves between [3H]spiperone and dopamine in the absence or presence of SB269652 were initially fitted to a one-site binding equation and two-site binding equation followed by F-test analysis for best fit56. Subsequently, data of experiments using membranes of WT D2R FlpIn CHO cells was fitted to the following allosteric ternary complex model57:
| (3) |
Where Y is percentage (vehicle control) binding, [A], [B], and [I] are the concentrations of [3H]spiperone, SB269652, and dopamine, respectively, KA and KB are the equilibrium dissociation constants of [3H]spiperone and SB269652, respectively, KHi and KLo are the equilibrium dissociation constants of dopamine for the high- and low-affinity receptor state, respectively, FracHi is the proportion of receptors in the high-affinity receptor state, and α and α′ are the cooperativities between SB269652 and [3H]spiperone or dopamine, respectively. Values of α (or α ′) >1 denote positive cooperativity; values <1 (but >0) denote negative cooperativity, and values = 1 denote neutral cooperativity.
Data from experiments using membranes of FlpIn CHO cells expressing the N terminal c-myc tagged WT or Glu952.65Ala mutant D2R were best fit to a one-site model58:
| (4) |
Where KI is the equilibrium dissociation constant of dopamine.
Competition-binding curves between [3H]spiperone and SB269652 could be fit to the allosteric ternary complex model using the following equation18:
| (5) |
Where Y is percentage (vehicle control) binding; [A] and [B] are the concentrations of [3H]spiperone and SB269652, respectively; KA and KB are the equilibrium dissociation constants of [3H]spiperone and SB269652, respectively; α is the cooperativity between SB269652 and [3H]spiperone Values of α >1 denote positive cooperativity; values <1 (but >0) denote negative cooperativity, and values = 1 denote neutral cooperativity.
Analysis of functional data
All concentration response (C/R) data were fitted to the following modified four-parameter Hill equation to derive potency estimates56:
| (6) |
Where E is the effect of the system, nH is the Hillslope, and EC50 is the concentration of agonist [A] that gives the midpoint response between basal and maximal effect of dopamine or other agonists (Emax), which are the lower and upper asymptotes of the response, respectively.
A logistic equation of competitive agonist-antagonist interaction was globally fitted to data from functional experiments measuring the interaction between dopamine and the various competitive antagonist fragments of SB269652, MIPS1217 or MIPS1500 at the WT D2R 56:
| (7) |
Where s represents the Schild slope for the antagonist, and pA2 represents the negative logarithm of the molar concentration of antagonist necessary to double the concentration of agonist needed to elicit the submaximal response obtained in the absence of antagonist.
For presentation purposes, we also used equation 3 values of EC50 for dopamine and other agonists in the presence (A′) and absence (A) of modulator or competitive antagonist (B) to generate concentration ratios (A′/A). Values of log(conc. ratio-1) on the Y-axis were then plotted against corresponding values of log[B]. For data of ligands with a competitive mode of interaction values of affinity (pKB) were obtained using the equation for interactions that gave a Schild slope not significantly different from unity:
| (8) |
In contrast for ligands with an allosteric mode of interaction, data were fit with the following equation, where αβ is the composite allosteric effect on orthosteric ligand function18:
| (9) |
Functional data describing the interaction between SB269652 and dopamine, S-3PPP or aripiprazole at the WT or mutant D2Rs used in this study or MIPS1500 at the Glu952.65Ala-D2R were globally analyzed according to the allosteric ternary complex model59.
| (10) |
Where Em is the maximum possible cellular response, [A] and [B] are the concentrations of orthosteric and allosteric ligands, respectively, and KB is the equilibrium dissociation constant of the allosteric ligand, αβ is a composite cooperativity parameter between the orthosteric and allosteric ligand that includes effects upon orthosteric ligand affinity and efficacy and nH is the Hill slope of the orthosteric agonist concentration-response curve. Values of α and/or β greater than 1 denote allosteric potentiation, whereas values less than 1 (but greater than 0) denote allosteric inhibition.
Statistical Analysis
All data points and values shown in the figures and tables are the means ± SEM of at least 3 separate experiments performed in duplicate unless otherwise stated. Statistically significant differences (taken at P < 0.05) between pKB or Logαβ values were determined by one-way ANOVA with a Bonferroni post-test or an unpaired Student’s t-test as appropriate.
Chemical Synthesis and characterization of compounds: performed as described in the Supplementary Note
Briefly, the synthesis of final compounds followed a general synthetic procedure previously reported in the literature for the synthesis of SB26965212. For the synthesis of MIPS1217, the commercially available 2-(cis-4-((tert-butoxycarbonyl)amino)cyclohexyl)acetic acid was esterified using Steglich conditions, then converted to the aldehyde following treatment with DIBAL-H. Reductive alkylation of the aldehyde and 7-cyano-1,2,3,4-tetrahydroisoquinoline in the presence of sodium triacetoxyborohydride afforded tert-butyl (cis-4-(2-(7-cyano-3,4-dihydroisoquinolin-2(1H)-yl)ethyl)cyclohexyl)carbamate, which was subsequently deprotected using trifluoroacetic acid (TFA) to give the free amine, following alkaline work-up. Finally, coupling of the free amine with indole-2-carboxylic acid in the presence of Castro’s reagent ((benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate) afforded MIPS1217.
The synthesis of MIPS1500 followed the same general synthetic procedure as for the synthesis of SB269652. However, in the final coupling step, indole-2-carboxylic acid was replaced with 1-methyl-1H–indole-2-carboxylic acid, and HCTU (O-(6-chlorobenzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate) used as the coupling reagent, to give MIPS1500.
Supplementary Material
Acknowledgements
We thank TGR BioSciences for generously providing the SureFire ERK1/2 kits. A.C. and P.M.S are Principal Research Fellows of the National Health and Medical Research Council (NHMRC) of Australia. J.R.L. is a Monash University Larkins Fellow and a R.D. Wright Biomedical Career Development Fellow of the NHMRC. This work was funded in part by NHMRC Program Grant No. APP1055134 (A.C., P.M.S.), Project Grant No. APP1011920 (J.R.L.), Project Grant APP1049564 (J.R.L., B.C) and Australian Research Council Discovery Grant No. DP110100687 (P.J.S., A.C.). J.R.L. acknowledges the financial support of the Netherlands Organization for Scientific Research [NWO VENI Grant 863.09.018]. This work was supported in part by NIH grants DA022413, MH54137 (J.A.J.) and DA023694 (L.S.) and the Lieber Center for Schizophrenia Research and Treatment (J.A.J.). Dr. Ann Stewart is thanked for technical assistance.
Footnotes
Author Contributions
J.R.L. conceived and supervised the project, generated receptor mutants and cell lines, performed data analysis, performed radioligand binding and functional assays, and wrote the manuscript. P.D performed complementation assay experiments, generated receptor constructs and wrote the manuscript. J.S. synthesized aripiprazole, SB269652 and its derivatives, and wrote the corresponding experimental section. C. D.-J. performed radioligand binding and functional assays, generated mutant receptors and cell lines, and performed data analysis. S.D. performed functional assays. M.M. conducted docking and homology modeling. L.S. conducted and supervised docking and homology modeling, and wrote the manuscript. L.L. planned mutagenesis experiments. P.J.S. planned and supervised chemical synthesis, and wrote the manuscript. B.C. planned and supervised chemical synthesis, and wrote the manuscript. P.M.S. supervised the project and wrote the manuscript. J.A.J. supervised the project and wrote the manuscript. A.C. conceived and supervised the project, performed data analysis and wrote the manuscript.
Competing financial interests
A.C. and P.M.S. have a research contract with Servier, France. A.C. has had recent consultancies with Johnson and Johnson (USA) and XOMA (USA), and is a Scientific Advisory Board member for Audeo, Australia.
References
- 1.Jacoby E, Bouhelal R, Gerspacher M, Seuwen K. The 7 TM G-protein-coupled receptor target family. ChemMedChem. 2006;1:761–782. doi: 10.1002/cmdc.200600134. [DOI] [PubMed] [Google Scholar]
- 2.Lagerström MC, Schiöth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat. Rev. Drug. Discov. 2008;7:339–357. doi: 10.1038/nrd2518. [DOI] [PubMed] [Google Scholar]
- 3.Kenakin T, Miller LJ. Seven transmembrane receptors as shapeshifting proteins: the impact of allosteric modulation and functional selectivity on new drug discovery. Pharmacol. Rev. 2010;62:265–304. doi: 10.1124/pr.108.000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohr K, et al. Rational design of dualsteric GPCR ligands: quests and promise. Br. J. Pharmacol. 2010;159:997–1008. doi: 10.1111/j.1476-5381.2009.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lane JR, Sexton PM, Christopoulos A. Bridging the gap: bitopic ligands of G-protein-coupled receptors. Trends Pharmacol. Sci. 2013;34:59–66. doi: 10.1016/j.tips.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Valant C, et al. A novel mechanism of G protein-coupled receptor functional selectivity. Muscarinic partial agonist McN-A-343 as a bitopic orthosteric/allosteric ligand. J. Biol. Chem. 2008;283:29312–29321. doi: 10.1074/jbc.M803801200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antony J, et al. Dualsteric GPCR targeting: a novel route to binding and signaling pathway selectivity. FASEB J. 2009;23:442–450. doi: 10.1096/fj.08-114751. [DOI] [PubMed] [Google Scholar]
- 8.Keov P, et al. Reverse Engineering of the Selective Agonist TBPB Unveils Both Orthosteric and Allosteric Modes of Action at the M1 Muscarinic Acetylcholine Receptor. Mol. Pharmaco.l. 2013;84:425–437. doi: 10.1124/mol.113.087320. [DOI] [PubMed] [Google Scholar]
- 9.Beaulieu J-M, Gainetdinov RR. The Physiology, Signaling, and Pharmacology of Dopamine Receptors. Pharmacol. Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- 10.Löber S, Hübner H, Tschammer N, Gmeiner P. Recent advances in the search for D3- and D4-selective drugs: probes, models and candidates. Trends Pharmacol. Sci. 2011;32:148–157. doi: 10.1016/j.tips.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Reavill C, et al. Pharmacological actions of a novel, high-affinity, and selective human dopamine D(3) receptor antagonist, SB-277011-A. J. Pharmacol. Exp. Ther. 2000;294:1154–1165. [PubMed] [Google Scholar]
- 12.Stemp G, et al. Design and Synthesis of trans- N-[4-[2-(6-Cyano-1,2,3,4-tetrahydroisoquinolin-2- yl)ethyl]cyclohexyl]-4-quinolinecarboxamide (SB-277011): A Potent and Selective Dopamine D 3Receptor Antagonist with High Oral Bioavailability and CNS Penetration in the Rat. J. Med. Chem. 2000;43:1878–1885. doi: 10.1021/jm000090i. [DOI] [PubMed] [Google Scholar]
- 13.Zhang A, Neumeyer JL, Baldessarini RJ. Recent progress in development of dopamine receptor subtype-selective agents: potential therapeutics for neurological and psychiatric disorders. Chem. Rev. 2007;107:274–302. doi: 10.1021/cr050263h. [DOI] [PubMed] [Google Scholar]
- 14.Silvano E, et al. The tetrahydroisoquinoline derivative SB269,652 is an allosteric antagonist at dopamine D3 and D2 receptors. Mol. Pharmacol. 2010;78:925–934. doi: 10.1124/mol.110.065755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han Y, Moreira IS, Urizar E, Weinstein H, Javitch JA. Allosteric communication between protomers of dopamine class A GPCR dimers modulates activation. Nat. Chem. Biol. 2009;5:688–695. doi: 10.1038/nchembio.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armstrong D, Strange PG. Dopamine D2 receptor dimer formation: evidence from ligand binding. J. Biol. Chem. 2001;276:22621–22629. doi: 10.1074/jbc.M006936200. [DOI] [PubMed] [Google Scholar]
- 17.Vivo M, Lin H, Strange PG. Investigation of cooperativity in the binding of ligands to the D(2) dopamine receptor. Mol. Pharmacol. 2006;69:226–235. doi: 10.1124/mol.105.012443. [DOI] [PubMed] [Google Scholar]
- 18.Christopoulos A, Kenakin T. G protein-coupled receptor allosterism and complexing. Pharmacol. Rev. 2002;54:323–374. doi: 10.1124/pr.54.2.323. [DOI] [PubMed] [Google Scholar]
- 19.Leach K, Sexton PM, Christopoulos A. Allosteric GPCR modulators: taking advantage of permissive receptor pharmacology. Trends Pharmacol. Sci. 2007;28:382–389. doi: 10.1016/j.tips.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Christopoulos A. Allosteric binding sites on cell-surface receptors: novel targets for drug discovery. Nat. Rev. Drug Discov. 2002;1:198–210. doi: 10.1038/nrd746. [DOI] [PubMed] [Google Scholar]
- 21.Chien EYT, et al. Structure of the Human Dopamine D3 Receptor in Complex with a D2/D3 Selective Antagonist. Science. 2010;330:1091–1095. doi: 10.1126/science.1197410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newman AH, et al. Molecular determinants of selectivity and efficacy at the dopamine D3 receptor. J. Med. Chem. 2012;55:6689–6699. doi: 10.1021/jm300482h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michino M, et al. A single glycine in extracellular loop 1 is the critical determinant for pharmacological specificity of dopamine D2 and D3 receptors. Mol. Pharmacol. 2013;84:854–864. doi: 10.1124/mol.113.087833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferré S, et al. Building a new conceptual framework for receptor heteromers. Nat. Chem. Biol. 2009;5:131–134. doi: 10.1038/nchembio0309-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo W, et al. Dopamine D2 receptors form higher order oligomers at physiological expression levels. EMBO J. 2008;27:2293–2304. doi: 10.1038/emboj.2008.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vilardaga J-P, et al. Conformational cross-talk between α2A–adrenergic and µ-opioid receptors controls cell signaling. Nat. Chem. Biol. 2008;4:126–131. doi: 10.1038/nchembio.64. [DOI] [PubMed] [Google Scholar]
- 27.Dorsch S, Klotz K-N, Engelhardt S, Lohse MJ, Bünemann M. Analysis of receptor oligomerization by FRAP microscopy. Nat. Methods. 2009;6:225–230. doi: 10.1038/nmeth.1304. [DOI] [PubMed] [Google Scholar]
- 28.Kasai RS, et al. Full characterization of GPCR monomer-dimer dynamic equilibrium by single molecule imaging. J. Cell. Biol. 2011;192:463–480. doi: 10.1083/jcb.201009128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hern JA, et al. Formation and dissociation of M1 muscarinic receptor dimers seen by total internal reflection fluorescence imaging of single molecules. Proc. Natl. Acad. Sci. USA. 2010;107:2693–2698. doi: 10.1073/pnas.0907915107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fonseca JM, Lambert NA. Instability of a Class A G Protein-Coupled Receptor Oligomer Interface. Mol. Pharmacol. 2009;75:1296–1299. doi: 10.1124/mol.108.053876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Damian M, Martin A, Mesnier D, Pin J-P, Banères J-L. Asymmetric conformational changes in a GPCR dimer controlled by G-proteins. EMBO J. 2006;25:5693–5702. doi: 10.1038/sj.emboj.7601449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albizu L, et al. Probing the existence of G protein-coupled receptor dimers by positive and negative ligand-dependent cooperative binding. Mol. Pharmacol. 2006;70:1783–1791. doi: 10.1124/mol.106.025684. [DOI] [PubMed] [Google Scholar]
- 33.Springael J-Y, et al. Allosteric modulation of binding properties between units of chemokine receptor homo- and hetero-oligomers. Mol. Pharmacol. 2006;69:1652–1661. doi: 10.1124/mol.105.019414. [DOI] [PubMed] [Google Scholar]
- 34.May LT, Bridge LJ, Stoddart LA, Briddon SJ, Hill SJ. Allosteric interactions across native adenosine-A3 receptor homodimers: quantification using single-cell ligand-binding kinetics. FASEB J. 2011;25:3465–3476. doi: 10.1096/fj.11-186296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seeman P. Targeting the dopamine D2 receptor in schizophrenia. Expert Opin. Ther. Targets. 2006;10:515–531. doi: 10.1517/14728222.10.4.515. [DOI] [PubMed] [Google Scholar]
- 36.Gregory KJ, Sexton PM, Christopoulos A. Allosteric modulation of muscarinic acetylcholine receptors. Curr. Neuropharmacol. 2007;5:157–167. doi: 10.2174/157015907781695946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kruse AC, et al. Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature. 2013;504:101–106. doi: 10.1038/nature12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bock A, et al. The allosteric vestibule of a seven transmembrane helical receptor controls G-protein coupling. Nat. Commun. 2012;3:1044. doi: 10.1038/ncomms2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuszak AJ, et al. Purification and functional reconstitution of monomeric mu-opioid receptors: allosteric modulation of agonist binding by Gi2. J. Biol. Chem. 2009;284:26732–26741. doi: 10.1074/jbc.M109.026922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whorton MR, et al. A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc. Natl. Acad. Sci. USA. 2007;104:7682–7687. doi: 10.1073/pnas.0611448104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bayburt TH, Leitz AJ, Xie G, Oprian DD, Sligar SG. Transducin activation by nanoscale lipid bilayers containing one and two rhodopsins. J. Biol. Chem. 2007;282:14875–14881. doi: 10.1074/jbc.M701433200. [DOI] [PubMed] [Google Scholar]
- 42.Milligan G. G protein-coupled receptor hetero-dimerization: contribution to pharmacology and function. Br. J. Pharmacol. 2009;158:5–14. doi: 10.1111/j.1476-5381.2009.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Albizu L, et al. Time-resolved FRET between GPCR ligands reveals oligomers in native tissues. Nat. Chem. Biol. 2010;6:587–594. doi: 10.1038/nchembio.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park PSH, Sum CS, Pawagi AB, Wells JW. Cooperativity and Oligomeric Status of Cardiac Muscarinic Cholinergic Receptors. Biochemistry. 2002;41:5588–5604. doi: 10.1021/bi011746s. [DOI] [PubMed] [Google Scholar]
- 45.Smith NJ, Milligan G. Allostery at G protein-coupled receptor homo- and heteromers: uncharted pharmacological landscapes. Pharmacol. Rev. 2010;62:701–725. doi: 10.1124/pr.110.002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Canals M, et al. Adenosine A2A–dopamine D2 receptor-receptor heteromerization: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J. Biol. Chem. 2003;278:46741–46749. doi: 10.1074/jbc.M306451200. [DOI] [PubMed] [Google Scholar]
- 47.Rashid AJ, et al. D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc. Natl. Acad. Sci. USA. 2007;104:654–659. doi: 10.1073/pnas.0604049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Navarro G, et al. Interactions between calmodulin, adenosine A2A, and dopamine D2 receptors. J. Biol. Chem. 2009;284:28058–28068. doi: 10.1074/jbc.M109.034231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carriba P, et al. Detection of heteromerization of more than two proteins by sequential BRET-FRET. Nat. Methods. 2008;5:727–733. doi: 10.1038/nmeth.1229. [DOI] [PubMed] [Google Scholar]
- 50.Shonberg J, et al. A Structure-Activity Analysis of Biased Agonism at the Dopamine D2 Receptor. J. Med. Chem. 2013;56:9199–9221. doi: 10.1021/jm401318w. [DOI] [PubMed] [Google Scholar]
- 51.Canals M, et al. A Monod-Wyman-Changeux Mechanism Can Explain G Protein-coupled Receptor (GPCR) Allosteric Modulation. J. Biol. Chem. 2012;287:650–659. doi: 10.1074/jbc.M111.314278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yeatman HR, et al. Allosteric Modulation of M1 Muscarinic Acetylcholine Receptor Internalization and Subcellular Trafficking. J. Biol. Chem. 2014;289:15856–15866. doi: 10.1074/jbc.M113.536672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sherman W, Day T, Jacobson MP, Friesner RA, Farid R. Novel Procedure for Modeling Ligand/Receptor Induced Fit Effects. J. Med. Chem. 2006;49:534–553. doi: 10.1021/jm050540c. [DOI] [PubMed] [Google Scholar]
- 54.Cho AE, Guallar V, Berne BJ, Friesner R. Importance of accurate charges in molecular docking: Quantum mechanical/molecular mechanical (QM/MM) approach. J. Comput. Chem. 2005;26:915–931. doi: 10.1002/jcc.20222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bowers KJ, et al. Scalable algorithms for molecular dynamics simulations on commodity clusters. SC 2006 Conference, Proceedings of the ACM/IEEE. 2006;1:43–43. [Google Scholar]
- 56.Motulsky HJ, Christopoulos A. Fitting Models to Biological Data Using Linear and Nonlinear Regression: A Practical guide to Curve Fitting. San Diego CA: GraphPad Software Inc; 2003. [Google Scholar]
- 57.Leach K, et al. Molecular mechanisms of action and in vivo validation of an M4 muscarinic acetylcholine receptor allosteric modulator with potential antipsychotic properties. Neuropsychopharmacol. 2010;35:855–869. doi: 10.1038/npp.2009.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nawaratne V, Leach K, Felder CC, Sexton PM, Christopoulos A. Structural determinants of allosteric agonism and modulation at the M4 muscarinic acetylcholine receptor: identification of ligand-specific and global activation mechanisms. J. Biol. Chem. 2010;285:19012–19021. doi: 10.1074/jbc.M110.125096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.May LT, Leach K, Sexton PM, Christopoulos A. Allosteric modulation of G protein-coupled receptors. Ann. Rev. Pharmacol. Toxicol. 2007;47:1–51. doi: 10.1146/annurev.pharmtox.47.120505.105159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.