Abstract
Cardiosphere-derived cells (CDCs) effect therapeutic regeneration after myocardial infarction (MI) both in animal models and in humans. Here we test the hypothesis that cell-cell contact plays a role in mediating the observed therapeutic benefits of CDCs, above and beyond conventional paracrine effects. Human CDCs or vehicle were injected into immunodeficient (SCID) mouse hearts during acute MI. CDC transplantation augmented the proportion of cycling (Ki67+) cardiomyocytes and improved ventricular function. CDC-conditioned media only modestly augmented the percentage of Ki67+ cardiomyocytes (>control but <CDCs), but did not improve pump function. When neonatal rat ventricular myocytes (NRVMs) were co-cultured with human CDCs in vitro, the percentage of cycling NRVMs (Ki67+ or BrdU+ nuclei) increased relative to solitary NRVM culture. To further dissect the relative contributions of soluble factors versus contact-dependent mechanisms, we compared CDCs grown with NRVMs in a transwell contact-free system versus admixed co-culture. The percentage of cycling NRVMs was higher in admixed co-culture than in the contact-free system. Pre-treatment with inhibitors of MEK and PI3K, or with β1 integrin neutralizing antibody, blocked the ability of CDCs to promote myocyte cycling. While conditioned media is not inert, direct apposition of CDCs to cardiomyocytes produces greater enhancement of cardiomyocyte proliferation in vitro and in vivo, and improves function post-MI. Intact cardiomyocyte β1 integrin signaling is necessary for the contact-dependent cardioproliferative effects of CDCs.
Keywords: cardiosphere-derived cells, cardiomyocyte, proliferation, paracrine factors, cell-cell communication, β1 integrin
Introduction
Human cardiomyocytes turn over at a rate of ~1% per year in early adulthood1, a number which is comparable to the basal turnover rate in mice2,3. Such a small physiologic renewal capacity does not suffice to offset the catastrophic injury of MI, where up to 40% of cardiomyocytes can die within hours4. Cell therapy may be able to increase regenerative capacity by attracting endogenous precursors5,6 and/or by stimulating the proliferation of preformed cardiomyocytes3,7,8. Cardiosphere-derived cells (CDCs) have been shown to boost the number of cycling cardiomyocytes in animal models of MI9,10, a finding consistent with the increase in viable myocardium observed in CDC-treated patients (but not controls) in the CADUCEUS clinical trial11. Work in bitransgenic mice further reveals that the proliferation of fate-mapped cardiomyocytes is upregulated several-fold by CDCs post-MI2. However, the mechanisms underlying the enhanced cardiomyocyte proliferation are unknown. We now recognize that the favorable effects of CDCs on cardiac structure and function persist at least 6 months in rodents and one year in humans, despite the fact that transplanted cells are cleared from the heart by 4 weeks12. This paradox points to indirect effects, but does not necessarily imply that soluble factors are solely responsible: it is additionally possible that transplanted CDCs may trigger a cascade of benefit by interacting physically with surrounding cardiomyocytes, even if CDC survival is evanescent.
The present work, therefore, seeks to determine the relative roles of classical paracrine mechanisms versus cell-cell contact in triggering the beneficial effects of CDCs. We report that the proliferation of cardiomyocytes is enhanced by in vitro co-culture with CDCs, and by in vivo transplantation of CDCs. While exposure to CDC-conditioned media partially reproduces the phenotype, cell-cell contact is required for the full effect, which in turn can be blunted by blocking β1 integrin on cardiomyocytes. Downstream signaling pathways involve MEK and PI3K, but not MAPK. This is the first report of a role for cell-cell interaction through β1 integrin in mediating therapeutic regeneration.
Methods
Cell culture and co-culture
Human CDCs were prepared and cultured as described13. Neonatal rat ventricular myocytes (NRVMs) were isolated from 2–3-day-old rat hearts as described14. Briefly, hearts were minced and digested with Trypsin-EDTA (GIBCO) solution. To purify the cardiomyocytes, the cells were pre-plated for 2 hours to remove non-myocytes. NRVMs were cultured in M199 medium containing 10% FBS. For direct contact co-culture, human CDCs, treated with mitomycin C, were plated and freshly-isolated NRVMs were added 24 hours later and cultured for 3 days. To investigate paracrine effects, we used a transwell contact-free system (BD Falcon™ Multiwell 24-well insert). Anti-β1 integrin antibody or isotype control (10 µg/ml, BD Pharmingen), RGDS peptide (Arg-Gly-Asp-Ser, Calbiochem) or scrambled control peptides (Gly-Arg-Gly-Asp-Thr-Pro, 100 µM, Sigma), PD98059 (5 µM), SB203580 (5 µM), or LY294002 (10 µM, Calbiochem) were added in some co-culture experiments as noted15.
Flow cytometry
Adult cardiomyocytes were isolated from 8–12-week-old mouse hearts by enzymatic digestion as described16. Ki67+ was detected using a BD Biosciences kit (following the manufacturer’s protocol). For the detection of BrdU incorporation in cultured cells, NRVMs were harvested and stained following the manufacturer’s protocol (BD Biosciences). Mouse anti-troponin T (0.25µg/ml, Clone 13-11, Lab Vision) was used to label cardiomyocytes. Isotype antibody served as the negative control. Quantitative flow cytometric assays were performed using a Cyan flow cytometer with Summit software (Beckman Coulter). Data were analyzed with FlowJo software.
Myocardial infarction model and cell and media injection
Male SCID-beige mice (8–12 weeks old) underwent open-chest permanent ligation of the left anterior descending coronary artery. Immediately afterwards, 1×105 human CDCs, or CDC-conditioned media or vehicle were injected at four sites in the infarct border zone for a total injection volume of 40 µl. Injection of cells or conditioned media was performed in a randomized and blinded fashion by a single investigator (B.S.). The conditioned media was serum-free IMDM bathing 1×106 cells/mL for 3 days, concentrated 2.5 times using 10-kDa MW cut-off ultrafiltration membranes. Thus, the injected 40 µL media was conditioned by 105 cells, equivalent to the number of injected CDCs. Sham animals underwent thoracotomy without coronary artery ligation. The surgeons were blinded as to group assignment.
Immunocytochemistry
Adult single myocytes were isolated by Langendorff perfusion16 and spun down onto slides. Co-cultured NRVMs and hCDCs were fixed by 4% PFA after 3 days culture. For BrdU labeling, cells were incubated with 10 µM BrdU (BD Pharmingen) for the last 12 hours unless otherwise stated. The cells were stained with the following antibodies: rabbit anti-Ki67 (Abcam), mouse anti-α sarcomeric actinin (Sigma-Aldrich), and sheep anti-BrdU (Abcam); secondary antibodies were obtained from Invitrogen. Images were taken using Leica TCS SP5 confocal microscopy.
Histology
Mice were sacrificed and excised hearts were snap-frozen and cryosectioned into 10 µm sections. Heart cryosections were fixed with 4% PFA and then stained with rabbit anti-Ki67 (Abcam), mouse anti-α sarcomeric actinin (Sigma-Aldrich), and wheat germ agglutinin-FITC (Sigma-Aldrich). Images were taken using Leica TCS SP5 confocal microscopy. The infarct zone was defined as the sectors including MI. The border zone was identified as the sectors immediately adjacent to the infarct zone, and the remote zone was identified as the sectors furthest removed from the border zone17. For each section, cells positive for each antigen were counted in 7 to 10 random high-power fields18. To define individual cardiomyocytes, wheat germ agglutinin (WGA) was used to highlight cell borders.
Time-lapse imaging
Human CDCs stained with CellTrace™ Far Red DDAO-SE (Invitrogen) were plated first, and freshly-isolated NRVMs stained with CellTracker™ green CMFDA (Invitrogen) were added 24 hours later. Time-lapse images were taken using Leica TCS SP5 two-photon confocal microscopy19.
Cardiac function assessment
Mice underwent echocardiography 2 hours (baseline) and 3 weeks post-MI using Vevo 770™ Imaging System (VISUALSONICS™, Toronto, Canada). Hearts were imaged two-dimensionally in long-axis views at the level of the greatest left ventricular (LV) diameter. LV ejection fraction (LVEF) was measured with VisualSonics V1.3.8 software from views taken through the infarcted area. Echocardiographic data were analyzed by a reader (D.S.) who was blinded to group assignment.
Statistical analysis
Results are presented as mean±S.D. unless specified otherwise. Comparisons between any two groups were performed using two-tailed unpaired Student’s t-test. Comparisons among more than two groups were performed using one-way ANOVA. Each experiment was performed at least three times and the differences were considered statistically significant when p<0.05.
Results
CDCs increase cardiomyocyte cycling in vitro
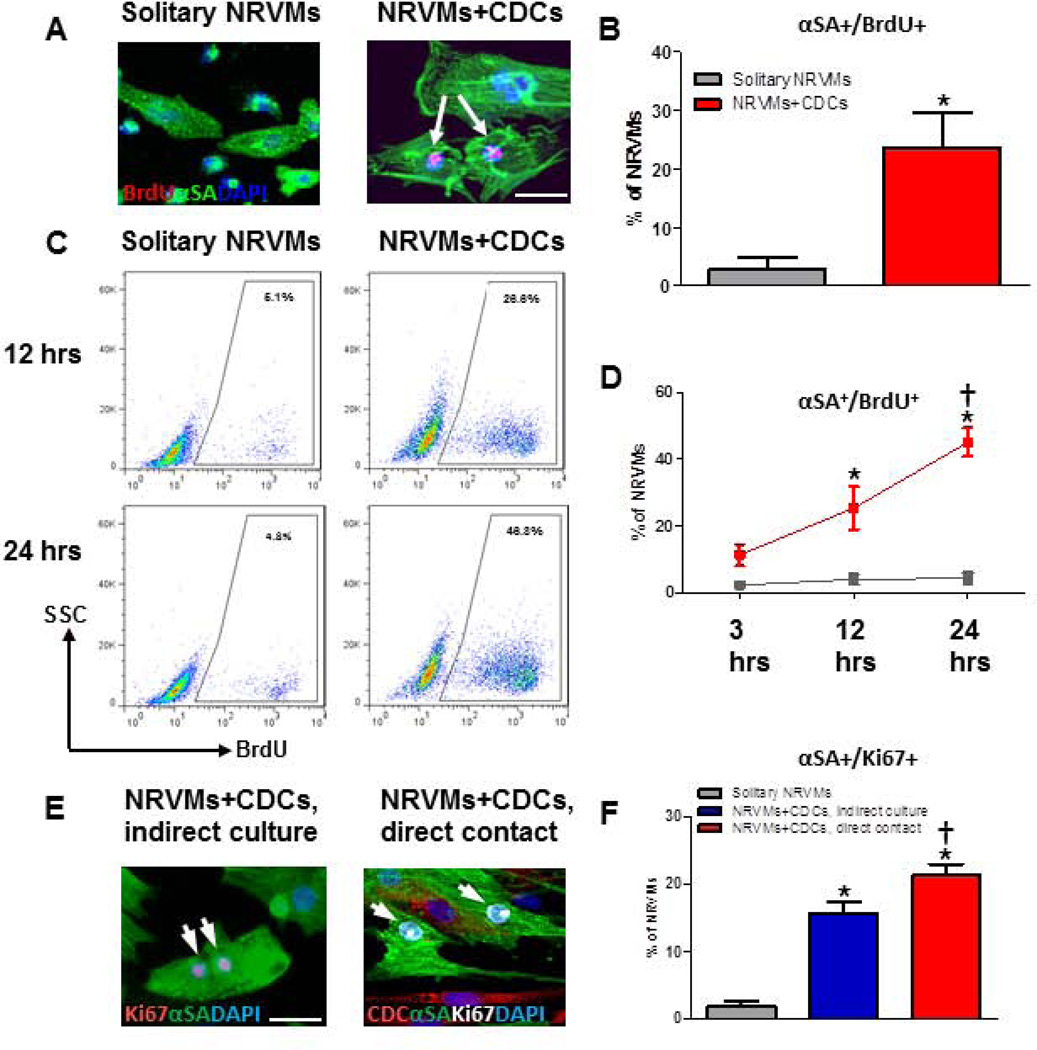
To identify cycling cardiomyocytes, cells were co-stained for α-sarcomeric actinin (αSA) and one of the three proliferation markers: Ki67 (a marker of late G1, S, G2, and M phase), BrdU (a nucleoside analog incorporated into DNA during S phase), or H3P (a marker for karyokinesis). We examined the proliferation of NRVMs co-cultured with hCDCs. The percentage of NRVMs that have undergone DNA replication (BrdU+) is indeed higher when NRVMs are co-cultured with human CDCs than in solitary NRVM culture (Figs. 1A, B). Furthermore, NRVMs at various stages of the cell cycle were observed in NRVM-CDC co-cultures (Supplemental Fig. 1). Flow cytometry analysis of NRVMs, using pre-staining to exclude co-cultured CDCs (Supplemental Fig.2), revealed a higher proportion of BrdU+ NRVMs in co-culture with CDCs as compared to solitary NRVM culture (Figs. 1C, D). Furthermore, the percentage of proliferating NRVMs rose rapidly with time in CDC co-culture, reaching 45% at 24 hours (Fig. 1D).
Figure 1. Admixed co-culture CDCs and CDC-conditioned media stimulate NRVM cycling in vitro.
A, Representative confocal images of BrdU+ NRVMs, with αSA shown as green, BrdU as red, and DAPI as blue. B, Quantification of BrdU+ NRVMs admixed co-cultured with CDCs, or cultured alone (solitary NRVMs, n=4). C, Representative flow cytometry analysis of BrdU+ NRVMs at 3, 12 and 24 hours after incubation with BrdU. D, Percentage, as reported by flow cytometry, of BrdU+ NRVMs admixed co-cultured with CDCs, or cultured alone (solitary NRVMs) (n=3 per group). E, Representative confocal images of NRVMs cultured indirectly with CDCs (left panel) or cultured with direct contact with CDCs (right panel). F, The quantification of Ki67+ NRVM co-cultured with CDCs or cultured alone (solitary NRVMs, n=5 to 6). Arrows point to BrdU+/Ki67+ NRVMs. * indicates p<0.05 when compared to solitary NRVM culture group or 3-hour group; † indicates p<0.05 compared to all other groups. Scale bars indicate 50 µm.
To visualize the dynamic cell cycle progression in NRVMs over time, NRVMs and CDCs were pre-labeled with a green and a far-red flurorescent dye, respectively, and co-cultured under time-lapse live-cell imaging. NRVMs undergoing cell division were frequently evident when co-cultured with CDCs, while such events were rare in solitary NRVM culture (Supplemental movies 1 and 2). Taken together, the data demonstrate that CDCs markedly enhance the number of cycling cardiomyocytes in vitro.
Stimulation of cardiomyocyte proliferation by CDCs is more efficient with direct cell-cell contact in vitro and with cell transplantation after MI in vivo
We next asked if the cardioproliferative effects of CDCs require direct cell-cell contact or if soluble factors secreted from CDCs suffice. We compared NRVMs in direct contact co-culture with those grown in a culture system that physically separates the two cell types but shares the same medium in close adjacency. The percentage of Ki67+ NRVMs was higher when CDCs and NRVMs were co-cultured with physical cell-cell contact (21.2±4.0 vs. 15.6±3.8 %; n=5–6; p<0.05, Figs. 1E, F). Paracrine effects, while less prominent than in admixed co-culture, were nevertheless substantial (Fig. 1F). Thus, direct CDC-cardiomyocyte interaction, in addition to the soluble factors released from CDCs, contributes to cardiomyocyte proliferation.
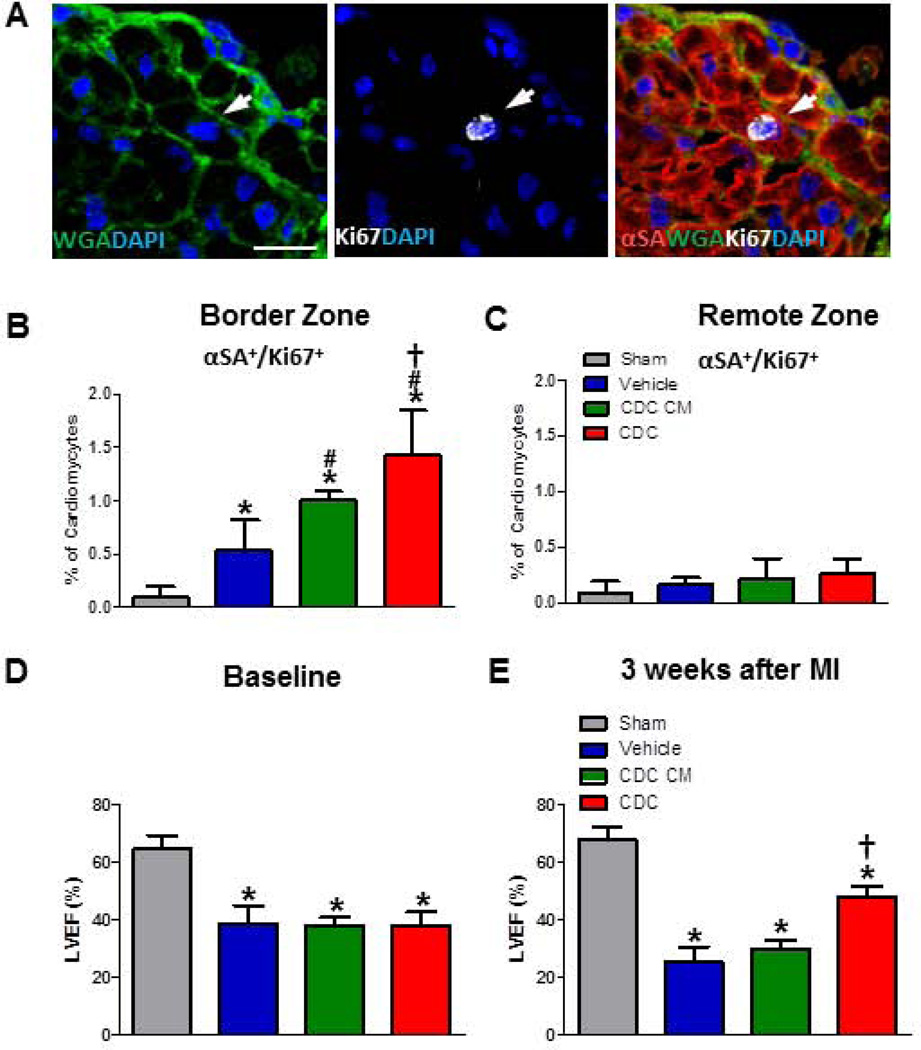
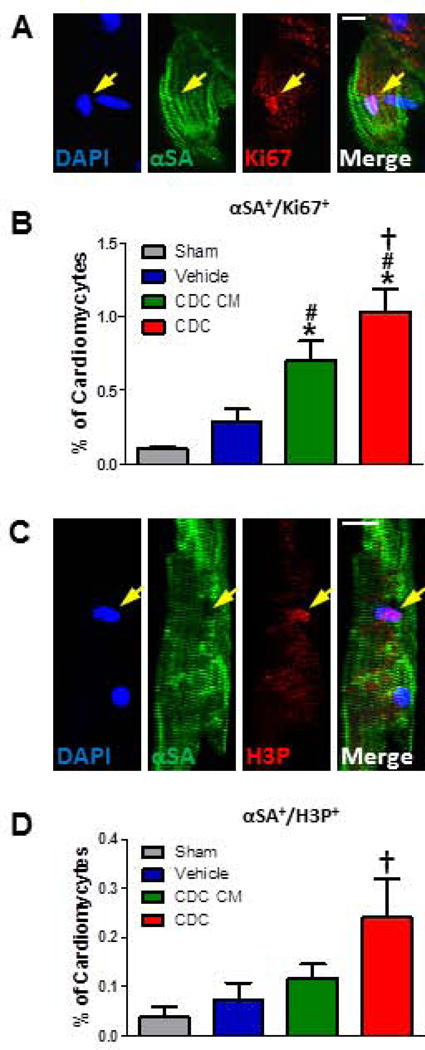
To confirm this novel finding in vivo, 105 human CDCs, CDC-conditioned media (CDC CM), or vehicle were injected into SCID mouse hearts after LAD ligation. Hearts were sectioned for immunohistochemistry, and wheat germ agglutinin (WGA) was used to outline cell borders. Actively-cycling cardiomyocytes (αSA+/Ki67+) are more frequent post-MI in the border zone treated with CDC CM than vehicle (or sham; Fig. 2). Hearts transplanted with CDCs demonstrated ~30% more cycling cardiomyocytes compared to CDC CM group in the border zone (Fig. 2B), but not in remote myocardium (Fig. 2C). Similar results were obtained with Ki67 staining of isolated single cardiomyocytes (Fig. 3A and B), which were isolated by Langendoff perfusion and then spun down onto slides (Supplemental Fig. 3). Quantification of H3P (a marker for karyokinesis)-positive myocytes (Fig. 3C and D) also indicate greater proliferation in CDCs than in CDC CM. These data support the co-culture findings. A consistent indicator of therapeutic potency in this model is the ability to produce cardiac functional benefit. LVEF values at baseline (i.e., two hours post-infarction) were comparable except for the Sham group (gray bar), indicating similar degrees of ischemic injury among groups (Fig. 2D). Over the 3 week time course of follow-up, LVEF deteriorated in the vehicle group (blue bar, Fig. 2E), but CDC injection (red bar) preserved LVEF, which ended up being higher than in any other treatment group (p<0.05; Fig. 2E). The functional benefit from CDC CM injection (green bar) is minimal and statistically insignificant.
Figure 2. CDC transplantation increases the percentage of cycling cardiomyocytes and improves cardiac function compared to conditioned media after MI in vivo.
A, Representative confocal images of Ki67+ cardiomyocyte from CDC group. (αSA: red; WGA: green; Ki67: white; DAPI: blue) in the heart injected with CDCs. B and C, Quantification of cycling (Ki67+) cardiomyocytes from the infarct border (B) or remote zone (C) at days 7 after MI (n=6–7 per group). D and E, Left ventricular ejection fraction (LVEF) in baseline (D) and three weeks after MI (E) with different treatment. Arrows point to Ki67+ cardiomyocytes. * indicates p<0.05 when compared to sham operation groups; # indicates p<0.05 compared to vehicle groups; † indicates p<0.05 compared to vehicle groups or CDC condition media (CDC CM) groups. Scale bars indicate 10 µm.
Figure 3. CDCs promote more resident cycling cardiomyocyte turnover in the adult MI mouse heart than conditioned media assessed by immunocytochemistry.
A and B, Representative confocal images (A) and quantification (B) of Ki67+ single enzymatically-isolated cardiomyocyte from the sham or MI heart injected with vehicle, CDC CM or CDCs. C and D, The representative single H3P+ cardiomyocyte (C) and the quantitative data (D) from four groups after MI (n=3–4 per group). Arrows point to Ki67+/H3P+ cardiomyocytes. * indicates p<0.05 when compared to sham operation groups; # indicates p<0.05 compared to vehicle groups; † indicates p<0.05 compared to vehicle groups or CDC CM groups. Scale bars indicate 10 µm.
β1 integrin is required for cell-cell contact-mediated cardiomyocyte proliferation
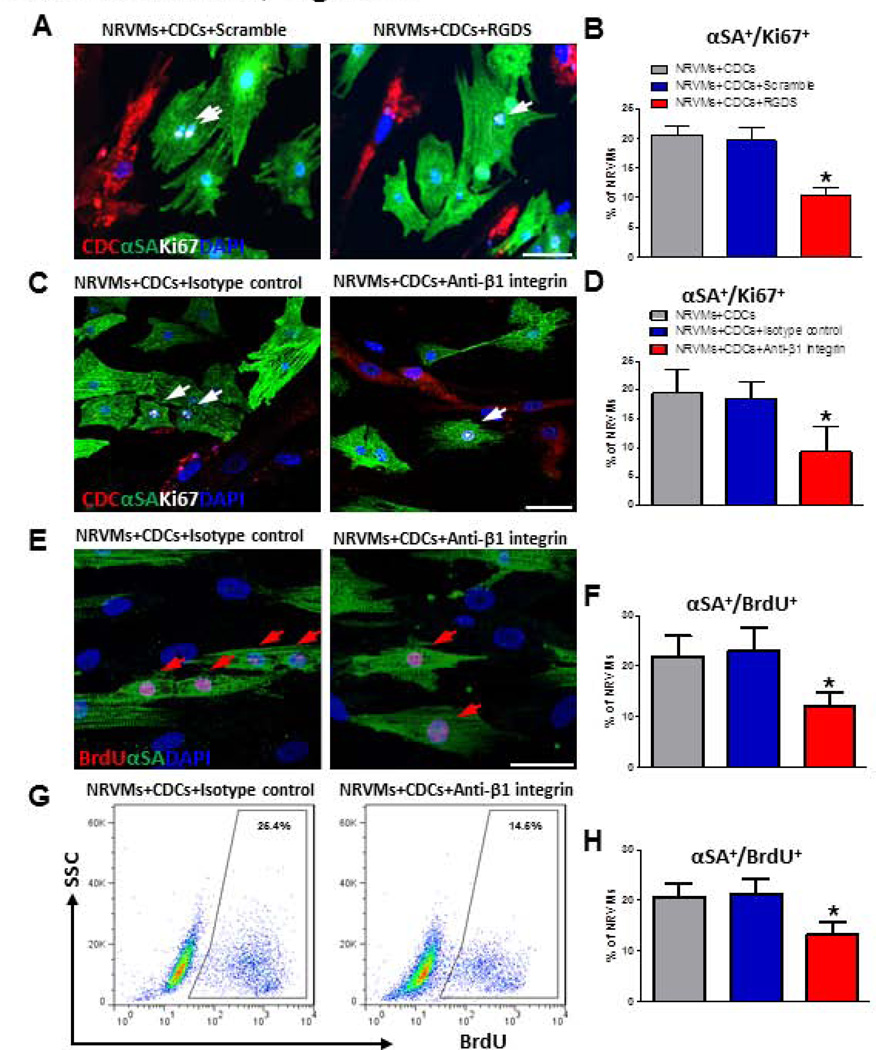
Integrins are cell surface receptors that mediate adhesion, signal transduction and cell cycle regulation20. To investigate if integrin signaling is involved in CDC-mediated cardiomyocyte proliferation, we blocked integrin signaling in the co-culture system. First, we introduced the broad-spectrum integrin-blocking peptide RGDS into CDC-NRVM co-cultures. Three days later, cells were fixed and stained for α-SA and Ki67. Addition of RGDS attenuated the number of Ki67+ NRVMs in co-culture (Figs. 4A, B). RGDS blocks all integrins; we further probed β1 integrin specifically, given its key roles in myocardial growth and development15,21. Addition of anti-β1 integrin antibody into admixed co-cultures of CDCs and NRVMs attenuated the percentages of Ki67+ NRVMs (Figs. 4C, D) and BrdU+ NRVMs (Figs. 4E, F). A similar reduction in BrdU+ NRVMs was observed by flow cytometry (Figs. 4G, H).
Figure 4. Integrin blockade abrogates the effect of CDCs on NRVM proliferation.
A and B, Ki67+ NRVMs co-cultured with CDCs in the presence of a pan-integrin blocker, RGDS or a control peptide (GRGDTP, “scramble”). n=4 for the average values. C and D, Ki67+ NRVMs co-cultured with CDCs in the presence of an anti-β1 integrin antibody or an isotype control. n=5 for each group. E and F, Same experiment as C and D, but immunostained for BrdU rather than Ki67. G and H, BrdU+ NRVMs detected by flow cytometry in CDC co-culture with or without anti-β1 integrin antibody. Representative flow cytometry plots (G) and the quantitative data (H) of BrdU+ NRVMs (n=4 to 5 per group). Arrows point to Ki67+ or BrdU+ cardiomyocytes. * indicates p<0.05 when compared to control groups. Scale bars indicate 50 µm.
To examine whether CDC CM induced cardiomyocyte proliferation also involves β1 integrin, NRVMs were co-cultured in CDC or CDC CM with or without anti-β1 integrin antibody (Methods). The cycling NRVMs were reduced in admixed direct contact co-culture, but not in conditioned media as observed by immunocytochemistry (Supplemental Fig. 4) and flow cytometry (Supplemental Fig. 5). Thus, β1 integrin signaling is important for the cardioproliferative effects of cell-cell contact, but not those elicited solely by conditioned media.
MEK and PI3K pathways are involved for cardiomyocyte proliferation
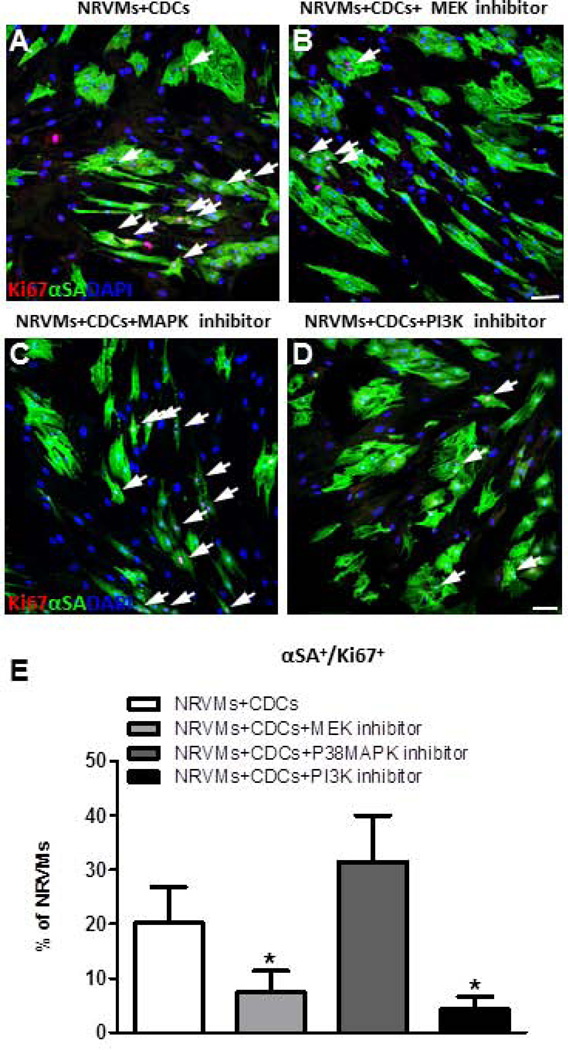
Beta-1 integrin coordinates with growth factors through receptor tyrosine kinases (RTKs) to activate various signaling pathways: phosphatidylinositol-3-OH kinase (PI3K)/Akt, mitogen-activated protein kinases (MAPK) and extracellular-signal-regulated kinase (ERK) or MAPK/ERK kinase (MEK)15,20,22,23. To determine whether these downstream pathways are involved in CDC-induced upregulation of NRVM cycling, relevant inhibitors were added into the CDC co-culture system (Fig. 5A–D). The percentage of Ki67+ NRVMs was decreased by treatment with MEK or PI3K inhibitor, but not with a P38MAPK inhibitor (Fig. 5E). These observations suggest that, MEK and PI3K, but not P38MAPK, appear to be involved in NRVM proliferation in the co-culture system.
Figure 5. Inhibition of MEK or PI3K signaling abolishes pro-proliferative effects of CDCs on NRVMs.
A–D, Representative confocal images of NRVMs co-cultured with human CDC (A), with a MEK inhibitor (B), with a P38MAPK inhibitor (C), or a PI3K inhibitor (D). E, Averaged percentages of Ki67+ NRVMs with different inhibitors (n=4 per group). Arrows point to Ki67+ cardiomyocytes. * indicates p<0.05 compared to no inhibitor control. Scale bars indicate 50 µm.
CDC-mediated cardiomyocyte proliferation requires intact β1 integrin on cardiomyocytes, not on CDCs
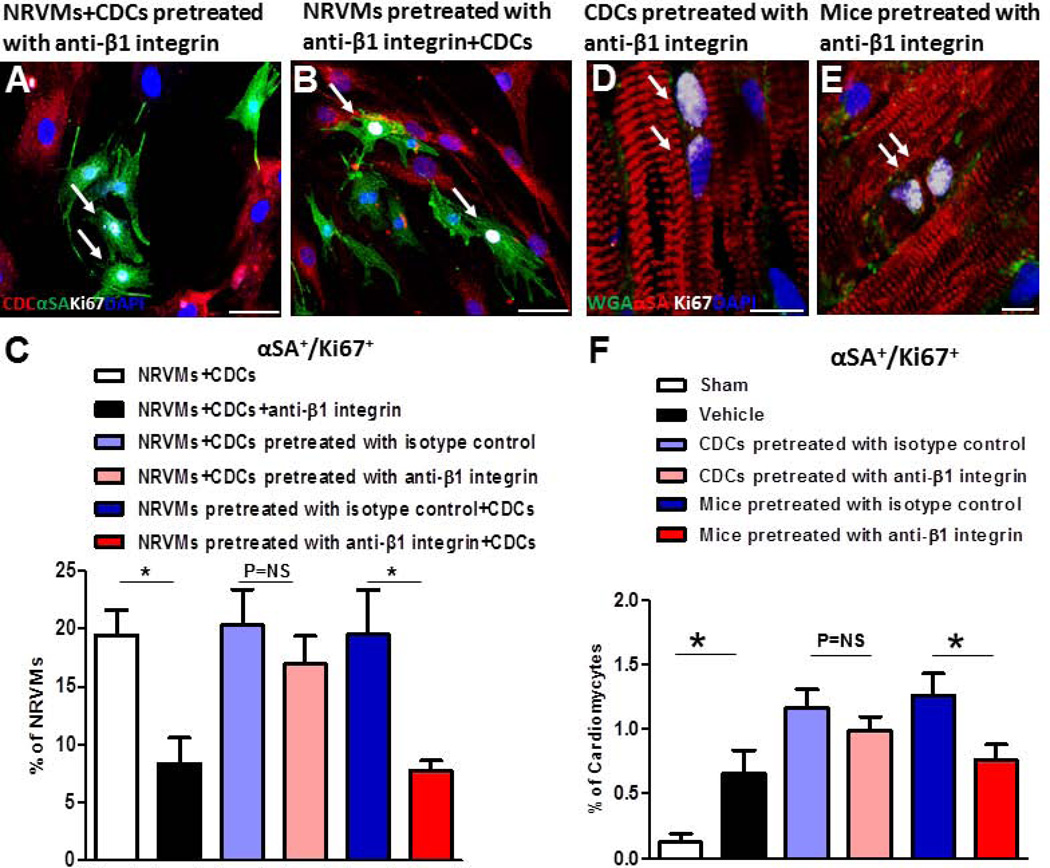
Both CDCs and NRVMs express β1 integrin23. We set out to determine whether β1 integrin is needed in both cell types for CDC-mediated cardiomyocyte proliferation. CDCs or NRVMs were incubated with anti-β1 integrin antibody, washed to remove unbound antibody from the media, and directly co-cultured with non-blocked NRVMs or CDCs, respectively (Figs. 6 A, B). Co-culture of β1 integrin-blocked CDCs with NRVMs did not attenuate CDC-induced NRVM proliferation (Fig. 6C, pink and light blue bar). Conversely, blocking β1 integrin on NRVMs reduced CDC-mediated NRVM proliferation to levels equivalent to those achieved with nonselective exposure to β1 integrin-blocking antibody (Fig. 6C, red and blue bar compared to black and white bar). These findings indicate that intact β1 integrin on cardiomyocytes, but not on CDCs, is required for CDC-induced cardiomyocyte proliferation.
Figure 6. Blockage of β1 integrin on cardiomyocytes, but not on CDCs, eliminates the pro-proliferative effects of CDCs.
A and B, Representative confocal images of Ki67+ NRVMs co-cultured with CDCs which were pretreated with anti-β1 integrin antibody (A) and CDCs co-cultured with NRVMs which were pretreated with anti-β1 integrin antibody (B). C. Average percentages of cycling NRVMs (n=3 to 5). D and E, Representative confocal images of Ki67+ cardiomyocytes, αSA (red), WGA (green), Ki67 (white) and DAPI (blue), from mouse hearts transplanted with CDCs that were pretreated with anti-β1 integrin antibody (D) or mouse hearts injected with anti-β1 integrin antibody before CDC transplantation (E). F, Quantification of Ki67+ cardiomyocytes from border zone 7 days after MI (n=3 to 5 per group). *p<0.05. Scale bar indicates 50 µm in A and B and 10 µm in D and E. Arrows point to Ki67+ cardiomyocytes.
To verify this finding in vivo, we transplanted β1 integrin-blocked CDCs into post-MI mice, and harvested the hearts one week later (Fig. 6D–F). The percentage of cycling Ki67+ cardiomyocytes was not different between control CDCs (pretreated with isotype control) and CDCs pretreated with β1 integrin antibody; both groups enhanced the number of cycling cardiomyocytes to a similar extent as observed in untreated CDCs (Fig. 6F, pink and light blue bar; cf. Fig. 3B). These findings indicate that β1 integrin on CDCs is not important for CDC-enhanced cardiomyocyte proliferation.
On the other hand, we injected anti-β1 integrin antibody or isotype control directly into SCID mouse hearts, which effectively blocked β1 integrin in vivo (Supplemental Fig. 6) before CDC transplantation. One week post-MI, we collected heart tissue for immunohistochemistry and flow cytometry (Fig. 6E, Supplemental Fig. 7; cf. Fig 2A). To exclude the possibility that cardiomyocytes may have been derived from cardiomyogenic differentiation of CDCs, we labeled CDCs with a membrane-impermeable, far red-fluorescent dye, DiD, prior to transplantation, and purified DiD−/TnT+ cardiomyocytes for further analysis (Supplemental Fig. 3B). Hearts from animals with endogenous β1 integrin blocked before CDC transplantation exhibit fewer Ki67+/αSA+ cardiomyocytes compared to isotype control (Fig. 6F, red and blue bars; Supplemental Fig. 7). Taken together, the data indicate that intact β1 integrin on cardiomyocytes, but not on CDCs, is required for CDC-induced cardiomyocyte proliferation.
Discussion
When it comes to indirect mechanisms of stem cell-mediated regeneration, previous studies have focused on soluble factors secreted by the therapeutic cells6,24,25. However, the effects of cell-cell contact have been overlooked. We investigated the roles of cell-cell contact and integrin signaling in cell therapy for heart repair. There are biological precedents for the importance of integrin-mediated cell-cell contact in triggering various processes of relevance to regeneration. Hematopoietic stem cell proliferation is known to require interactions with the mesenchymal/stromal cell surface: blocking β1 integrin reduces hematopoietic stem cell differentiation from 70% to 41%26. This and other lines of evidence support the notion that integrin-mediated cell-cell interactions play an important role in the proliferation of hematopoietic stem cells27. Likewise, when induced pluripotent stem cells are cultured without feeder layers on Matrigel™, β1 integrin is required for adhesion and proliferation, as shown by immunological blockade experiments28. Irradiated embryonic stem cells improve cardiac function after acute MI, a paradoxical finding seemingly related to cell-cell interactions, paracrine factors, and a scaffolding effect25. Our findings show that soluble factors are important (they appear to account for at least 70% of the benefit; Figs. 1–3), but direct contact between CDCs and cardiomyocytes, as visualized in Fig. 1E, is necessary to fully recapitulate the overall benefit of cell therapy (Figs. 1F, 2B).
Although we highlight the importance of cell-cell contact in therapeutic regeneration of CDCs, <1% of transplanted CDCs persist in the heart 3 weeks later29. How can such a diminutive number of CDCs, present only transiently, produce durable benefits? The mechanisms of this “amplifing effect” are not yet fully elucidated10. We speculate that soluble factors and transient physical contact initiate changes in intracellular signaling that trigger myocyte survival and cycling. One plausible mechanism would involve epigenetic changes in cardiomyocytes induced by CDCs; we are presently investigating that possibility. Nevertheless, prolonging the opportunity for physical contact may augment benefit, such that bioengineering approaches to enhance cell engraftment and prolong cell-cell contact warrant further investigation31.
Cell cycling requires a combination of growth factor receptor tyrosine kinases (RTK or growth factor receptors), integrin-RTK and direct integrin signals to ensure passage through the G0/G1 restriction point and entry into S-phase20,22. The classical signaling for cell proliferation is via the PI3K, ERK/MAPK or MEK pathways15,20,22. Here, we have found that cardiomyocyte contact-dependent proliferation depends on intact β1 integrin signaling in myocytes, with downstream involvement of PI3K and MEK, but not MAPK (Fig. 5). Cooperating with integrin signaling are growth factors that are necessary for cell cycling20. CDCs produce a cocktail of angiopoietin-2, bFGF, HGF, IGF-1, SDF-1, and VEGF, in a more balanced pattern as compared to mesenchymal stem cells or bone marrow mononuclear cells23. Interestingly, myocardial proliferation during heart development is regulated by cardiac fibroblasts, also through β1 integrin signaling. Embryonic cardiac fibroblasts secrete fibronectin and collagen, and coordinate with heparin-binding EGF-like growth factor via β1 integrin signaling to promote myocardial proliferation15. Although CDCs are not fibroblasts (they are stromal cells of intrinsic cardiac origin32, with a subpopulation of genuine cardiac progenitors13,33,34), there are interesting parallels between CDC-induced adult cardiomyocyte regeneration and mechanisms in play during heart development.
There are at least two possible sources for endogenous cardiomyocyte regeneration, namely differentiation of progenitor populations5,35,36, and cardiomyocyte proliferation7,8 (with or without dedifferentiation37). By combining genetic fate-mapping with multi-isotope imaging mass spectrometry, cardiomyocytes were found to be regenerated by the division of pre-existing cardiomyocytes with normal aging and myocardial injury3. Here, we show that the majority of cycling cardiomyocytes in the adult heart are smaller than their non-cycling counterparts (Supplemental Fig. 8), consistent with previous findings in fate-mapped mice2. Cardiomyocytes from neonatal animals show robust proliferative ability when co-cultured with CDCs, but not in solitary culture (Fig. 1). These results hint that the cycling myocytes in adult mice may derive from an immature cardiomyocyte subpopulation, as previously suggested2, although the characteristics of that subpopulation remain to be fully defined.
Our study has several limitations. First, we have not conclusively excluded the possibility a role for recruitment of cardiac progenitors. The rapidity of the effect (with evident stimulation of cardiomyocyte proliferation as soon as 12 hours after CDCs are added in vitro, Fig. 2D, and by 1 week in vivo [Fig. 3B]) argues against recruitment, activation and differentiation of cardiac progenitors, which takes longer5. Fate-mapping experiments further support our interpretation that preformed myocytes are being stimulated to divide2,3. Second, an in vivo comparison between CDCs and conditioned media produced by the same number of CDCs is difficult to interpret. Proteins in the conditioned media may undergo rapid proteolysis in vivo, a factor which would be less operative when CDCs are in the immediate vicinity of the cells. Also, the secretome of injected CDCs may well be different from that of CDCs in culture. Third, we have noted that CDCs secrete integrin ligand(s) which may activate β1 integrin signaling in cardiomyocytes, as supported by proteomic analysis of the CDC secretome38, but we have not yet pinpointed the identity of the putative ligand(s). Further studies are warranted to flesh out these as-yet-unexplored aspects of the integrin-dependent CDC-cardiomyocyte interactions identified here.
Conclusions
We demonstrate that cardiomyocyte cycling is boosted by CDCs. Indirect mechanisms account for most of the benefit; however, cell-cell contact also plays a role. Blocking β1 integrin on cardiomyocytes negates CDCs’ cardioproliferative ability. Downstream signaling pathways involve MEK and PI3K, but not MAPK. We conclude that direct cell-cell contact plays a role in therapeutic cardiomyocyte regeneration.
Supplementary Material
Acknowledgments
The work was conceived, and predominantly executed, at the Cedars-Sinai Heart Institute. A number of additional experiments were performed in Shanghai Rui Jin Hospital in the process of revision. We thank K. Wawrowsky for help with confocal microscopy experiments, P. Lin for flow cytometry analysis, E. Tseliou, R. Smith, R. Zhang, E. Cingolani and Y. Zhang for helpful discussions. This work was supported by the National Institutes of Health (R01HL083109 to E.M.) and the National Natural Science Foundation of China (81170122 to Y.X.) K. Cheng is supported by American Heart Association Beginning Grant-In-Aid 12BGIA12040477.
Footnotes
Author contributions: Y.X.: conception and design, collection and/or assembly of data, data analysis and interpretation, financial support, manuscript writing; A.I., K.C., Z.W., L.L. and G.L.: collection and/or assembly of data, data analysis and interpretation; B.S., D.S.: collection of data; K.C., W.L., K.M., H.C.C. and T.L.: data analysis and interpretation, manuscript writing; E.M.: conception and design, data analysis and interpretation, financial support, manuscript writing, final approval of manuscript.
Disclosure of potential conflicts of interest
Eduardo Marbán holds equity in Capricor Therapeutics, Inc. K. Malliaras receives a consulting fee from Capricor Therapeutics, Inc. Capricor Therapeutics provided no funding for the present study. The remaining authors report no conflicts.
References
- 1.Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malliaras K, Zhang Y, Seinfeld J, et al. Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart. EMBO Mol Med. 2013;5:191–209. doi: 10.1002/emmm.201201737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Senyo SE, Steinhauser ML, Pizzimenti CL, et al. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–436. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robey TE, Saiget MK, Reinecke H, Murry CE. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol. 2008;45:567–581. doi: 10.1016/j.yjmcc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loffredo FS, Steinhauser ML, Gannon J, Lee RT. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell. 2011;8:389–398. doi: 10.1016/j.stem.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatzistergos KE, Quevedo H, Oskouei BN, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 8.Kuhn B, del Monte F, Hajjar RJ, et al. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med. 2007;13:962–969. doi: 10.1038/nm1619. [DOI] [PubMed] [Google Scholar]
- 9.Cheng K, Shen D, Smith J, et al. Transplantation of platelet gel spiked with cardiosphere-derived cells boosts structural and functional benefits relative to gel transplantation alone in rats with myocardial infarction. Biomaterials. 2012;33:2872–2879. doi: 10.1016/j.biomaterials.2011.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malliaras K, Li TS, Luthringer D, et al. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells. Circulation. 2012;125:100–112. doi: 10.1161/CIRCULATIONAHA.111.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J, Narsinh KH, Lan F, et al. Early stem cell engraftment predicts late cardiac functional recovery: preclinical insights from molecular imaging. Circ Cardiovasc Imaging. 2012;5:481–790. doi: 10.1161/CIRCIMAGING.111.969329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith RR, Barile L, Cho HC, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 14.Kapoor N, Galang G, Marban E, Cho HC. Transcriptional suppression of connexin43 by TBX18 undermines cell-cell electrical coupling in postnatal cardiomyocytes. J Biol Chem. 2011;286:14073–14079. doi: 10.1074/jbc.M110.185298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ieda M, Tsuchihashi T, Ivey KN, et al. Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Dev Cell. 2009;16:233–244. doi: 10.1016/j.devcel.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang W, Oudit GY, Patel MM, et al. Role of phosphoinositide 3-kinase {alpha}, protein kinase C, and L-type Ca2+ channels in mediating the complex actions of angiotensin II on mouse cardiac contractility. Hypertension. 2010;56:422–429. doi: 10.1161/HYPERTENSIONAHA.109.149344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashikaga H, Mickelsen SR, Ennis DB, et al. Electromechanical analysis of infarct border zone in chronic myocardial infarction. Am J Physiol Heart Circ Physiol. 2005;289:H1099–H1105. doi: 10.1152/ajpheart.00423.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tseliou E, Pollan S, Malliaras K, et al. Allogeneic cardiospheres safely boost cardiac function and attenuate adverse remodeling after myocardial infarction in immunologically mismatched rat strains. J Am Coll Cardiol. 2013;61:1108–1119. doi: 10.1016/j.jacc.2012.10.052. [DOI] [PubMed] [Google Scholar]
- 19.Xie Y, Yin T, Wiegraebe W, et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature. 2009;457:97–101. doi: 10.1038/nature07639. [DOI] [PubMed] [Google Scholar]
- 20.Streuli CH, Akhtar N. Signal co-operation between integrins and other receptor systems. Biochem J. 2009;418:491–506. doi: 10.1042/BJ20081948. [DOI] [PubMed] [Google Scholar]
- 21.Shai SY, Harpf AE, Babbitt CJ, et al. Cardiac myocyte-specific excision of the beta1 integrin gene results in myocardial fibrosis and cardiac failure. Circ Res. 2002;90:458–464. doi: 10.1161/hh0402.105790. [DOI] [PubMed] [Google Scholar]
- 22.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 23.Li TS, Cheng K, Malliaras K, et al. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol. 2012;59:942–953. doi: 10.1016/j.jacc.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chimenti I, Smith RR, Li TS, et al. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971–980. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burt RK, Chen YH, Verda L, et al. Mitotically inactivated embryonic stem cells can be used as an in vivo feeder layer to nurse damaged myocardium after acute myocardial infarction: a preclinical study. Circ Res. 2012;111:1286–1296. doi: 10.1161/CIRCRESAHA.111.262584. [DOI] [PubMed] [Google Scholar]
- 26.Jing D, Fonseca AV, Alakel N, et al. Hematopoietic stem cells in co-culture with mesenchymal stromal cells--modeling the niche compartments in vitro. Haematologica. 2010;95:542–550. doi: 10.3324/haematol.2009.010736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schreiber TD, Steinl C, Essl M, et al. The integrin alpha9beta1 on hematopoietic stem and progenitor cells: involvement in cell adhesion, proliferation and differentiation. Haematologica. 2009;94:1493–1501. doi: 10.3324/haematol.2009.006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rowland TJ, Miller LM, Blaschke AJ, et al. Roles of integrins in human induced pluripotent stem cell growth on Matrigel and vitronectin. Stem Cells Dev. 2010;19:1231–1240. doi: 10.1089/scd.2009.0328. [DOI] [PubMed] [Google Scholar]
- 29.Li TS, Cheng K, Lee ST, et al. Cardiospheres recapitulate a niche-like microenvironment rich in stemness and cell-matrix interactions, rationalizing their enhanced functional potency for myocardial repair. Stem Cells. 2010;28:2088–2098. doi: 10.1002/stem.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang KL, Wang YS, Chang CC, et al. Reciprocal complementation of the tumoricidal effects of radiation and natural killer cells. PLoS One. 2013;8:e61797. doi: 10.1371/journal.pone.0061797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng K, Li TS, Malliaras K, Davis DR, Zhang Y, Marban E. Magnetic targeting enhances engraftment and functional benefit of iron-labeled cardiosphere-derived cells in myocardial infarction. Circ Res. 2010;106:1570–1581. doi: 10.1161/CIRCRESAHA.109.212589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White AJ, Smith RR, Matsushita S, et al. Intrinsic cardiac origin of human cardiosphere-derived cells. Eur Heart J. 2013;34:68–75. doi: 10.1093/eurheartj/ehr172. [DOI] [PubMed] [Google Scholar]
- 33.Davis DR, Kizana E, Terrovitis J, et al. Isolation and expansion of functionally-competent cardiac progenitor cells directly from heart biopsies. J Mol Cell Cardiol. 2010;49:312–321. doi: 10.1016/j.yjmcc.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis DR, Zhang Y, Smith RR, et al. Validation of the cardiosphere method to culture cardiac progenitor cells from myocardial tissue. PLoS One. 2009;4:e7195. doi: 10.1371/journal.pone.0007195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smart N, Bollini S, Dube KN, et al. De novo cardiomyocytes from within the activated adult heart after injury. Nature. 2011;474:640–644. doi: 10.1038/nature10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Li TS, Lee ST, et al. Dedifferentiation and proliferation of mammalian cardiomyocytes. PLoS One. 2010;5:e12559. doi: 10.1371/journal.pone.0012559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stastna M, Chimenti I, Marban E, Van Eyk JE. Identification and functionality of proteomes secreted by rat cardiac stem cells and neonatal cardiomyocytes. Proteomics. 2010;10:245–253. doi: 10.1002/pmic.200900515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.