Abstract
Chemical pruritogens and algogens evoke primarily itch and pain, respectively, when administered to the skin of healthy human subjects. However, the dominant sensory quality elicited by an algesic chemical stimulus may change in patients with chronic itch where bradykinin, elicits itch in addition to pain. Here we tested whether normally pruritic and algesic chemicals evoked abnormal itch- or pain-like behaviors in the mouse after the development of contact hypersensitivity (CHS), an animal model of allergic contact dermatitis. Mice previously sensitized to a hapten (squaric acid dibutylester) applied to the abdomen, exhibited spontaneous itch-like scratching and pain-like wiping directed to the site on the cheek of the CHS elicited by a subsequent challenge with the same hapten. In comparison with responses of control mice, CHS mice exhibited a significant increase in the scratching evoked by bovine adrenal medulla 8–22, a peptide that elicits a histamine-independent itch, but did not alter the scratching to histamine. Bradykinin, an algogen that elicited only wiping in control mice, additionally evoked significant scratching in CHS mice. Thus, within an area of CHS, histamine-independent itch is enhanced and chemically evoked pain is accompanied by itch.
Keywords: Contact, hypersensitivity, Itch, Pain, Pruritogen, Algogen
1Introduction
Pruritic chemicals normally elicit a dominant sensation of itch and algesic chemical, pain when applied to the skin of humans and, itch-like and pain-like behaviors when applied to the cheek of the mouse [1–4]. However, these sensations and sensory behaviors are not fully consistent with the observations in patients with chronic itch or chronic neuropathic pain [5–8]. For example, bradykinin, an algogen that is normally painful and not itchy, elicited itch as well as pain when administered to lesional skin of patients with atopic dermatitis [7]. And the pruritogen, histamine, evoked pain but not itch when delivered to an area of hyperalgesia in patients with post-herpetic neuralgia [8]. These alterations in sensory qualities may result from the sensitization of neurons mediating itch or pain in the peripheral and/or central nervous system [5–10].
Allergic contact dermatitis (ACD) is often accompanied by spontaneous itch and pain [11,12]. In a recent study, human subjects, previously sensitized to the contact sensitizer squaric acid dibutylester (SADBE), reported spontaneous itch and nociceptive sensations within an area of ACD produced by a subsequent application of the chemical [11]. Within this area, heat stimuli that in normal skin elicit only pain sensation, elicited the additional sensation of itch and intradermal injection of certain pruritic chemicals evoked an enhanced itch [11].
SADBE was used in a similar fashion in the mouse to produce an area of CHS (model of ACD in humans) on the leg or cheek [13]. Analogous to humans reporting spontaneous itch and nociceptive sensations with ACD [11], the mouse exhibited spontaneous itch- and pain-like behaviors directed to the site of CHS [13]. Moreover, one type of cutaneous nociceptor expressing Mas-related G-protein-coupled receptor A3 (MrgprA3) exhibited electrophysiological signs of hyperexcitability in response to noxious heat or mechanical stimuli applied to their receptive fields within the area of CHS [13]. In other studies, most neurons expressing MrgprA3 were shown to respond to multiple pruritogens, including histamine and to bovine adrenal medulla 8–22 (BAM8-22), a peptide cleaved from proenkephalin A [14,15]. To our knowledge, there is little information available on the behavioral responses to pruritic or algesic chemical stimuli delivered to an area of CHS in the mouse. Our purpose was to test whether the itch-and pain-like behaviors normally elicited by an algesic or pruritic chemical are altered when the same stimuli are delivered to the site of SADBE-induced CHS on the cheek of the mouse.
2Materials and methods
2.1Animals
C57BL/6 mice (Charles River, Wilmington, MA), 64 males, were tested, each weighing between 20 and 25 g. Mice were housed in groups of four under a 12 h light/dark cycle. During brief anesthesia with isoflurane (2% in 100% oxygen), each cheek and the abdomen were shaved at least two days before the application of a chemical to the skin. The experimental procedures were approved by the Institutional Animal Care and Use Committee of Yale University School of Medicine and were in accordance with the guidelines provided by the National Institute of Health and the International Association for the Study of Pain.
2.2Chemicals
BAM8-22 was obtained from Tocris Bioscience (Ellisville, MO, USA). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA). SADBE was dissolved in a vehicle of acetone. Histamine dihydrochloride, BAM8-22 and bradykinin were each dissolved in a vehicle of sterile, normal saline. The doses of all chemicals used in this study were based on the results of pilot studies or published findings [3,4,16].
2.3Induction of contact hypersensitivity in the mouse
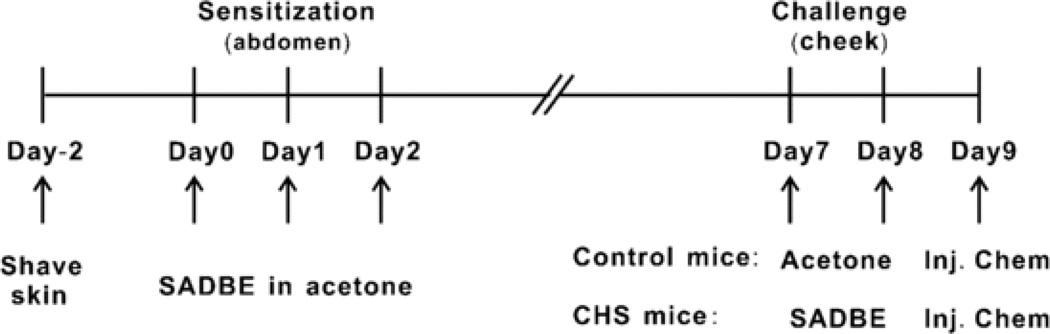
CHS was produced on the mouse cheek as described [13] and schematically summarized in Fig. 1. The mice were sensitized by the topical application of 25 µL of 1% SADBE in acetone to abdominal skin once daily for three consecutive days. Five days later, the right cheek was challenged either with a topical application of 25 µL of 1% SADBE in acetone (“CHS mice”) or only the acetone vehicle (“control mice”). A second challenge was similarly delivered 24 hrs later.
Figure 1.
The schematic experimental schedule for the induction of CHS. The mice were sensitized by the topical application of 25 µL of 1% SADBE in acetone to abdominal skin once daily for three consecutive days. Five days later, the right cheek was challenged either with a topical application of 25 µL of 1% SADBE in acetone (“CHS mice”) or the acetone vehicle alone (“control mice”) once a day for 2 consecutive days. Twenty-four hours later after the second challenge, a pruritic or algesic chemical was intradermally injected into the right cheek.
2.4Behavioral testing
All behaviors were assessed 24 hrs after the second challenge of SADBE or acetone. Each of two mice was placed in a separate, clear, plastic container, each 9 × 9 × 13 cm. A small amount of bedding was placed in each container to absorb any urine voided by the mouse. A camcorder (Sony Model DCR-DVD 300) was positioned above the mice to record the behavior of the two mice at the same time. There were four angled mirrors, one on each side of each container, affording the camera a four-sided view in addition to the view from the top. Experiments were conducted inside a sound proof room. Pseudo-white noise was delivered from a radio to mask extraneous laboratory noises. The experimenter was present briefly to start the video recording, and 30 min later, to inject a chemical stimulus.
2.5Experimental protocol
At 24 hrs after the second challenge, each mouse was placed in the test container and its spontaneous behavior was recorded for 30 min. Then each mouse received an intradermal injection into the previously challenged cheek of 5 µL of a chemical solution (via a 0.3 mlL insulin syringe with a 31 gauge needle) and returned to the container to have its behavior recorded for another 30 min. For different groups of 8 mice each, the solution consisted of a normal saline vehicle alone or the vehicle containing either histamine (5 µg), BAM8-22 (1 µg) or bradykinin (2.65 µg).
2.6Behavioral analyses
The video recording was played back on a Blu-ray player connected to a HDTV screen. The number of bouts of scratching and the number of wipes directed to the mouse cheek were scored in bins of one minute. A scratching bout was defined as one or more rapid back-and-forth motions of the ipsilateral hind paw directed toward the injected cheek, and ending with placement of the hind paw on the floor and/or to the mouth. A wiping was defined as a motion of the ipsilateral forelimb beginning at the back of the cheek, and moving forward in a caudal to rostral direction [3]. Wiping and scratching behaviors were included only if directed to the site of the intradermal injection on the cheek. Simultaneous wiping with both forelimbs (grooming) or a unilateral wiping or scratching directed to loci other than the cheek such as the bridge of the nose, the eye, ear, snout, or neck were identified and then excluded.
2.7Statistical analysis
The significance of differences in the mean numbers of a particular behavior obtained for two different experimental conditions was tested using Student's t-test. The criterion for significance was P < 0.05. In cases where the assumption of an equality of variances was unmet as determined by Levene's test, the nonparametric Mann-Whitney U–Whitney U test was used instead. The data in bar graphs in the figures are the means and standard errors of the mean (SEM).
3Results
3.1Behavioral effects of each chemical injected into vehicle challenged skin
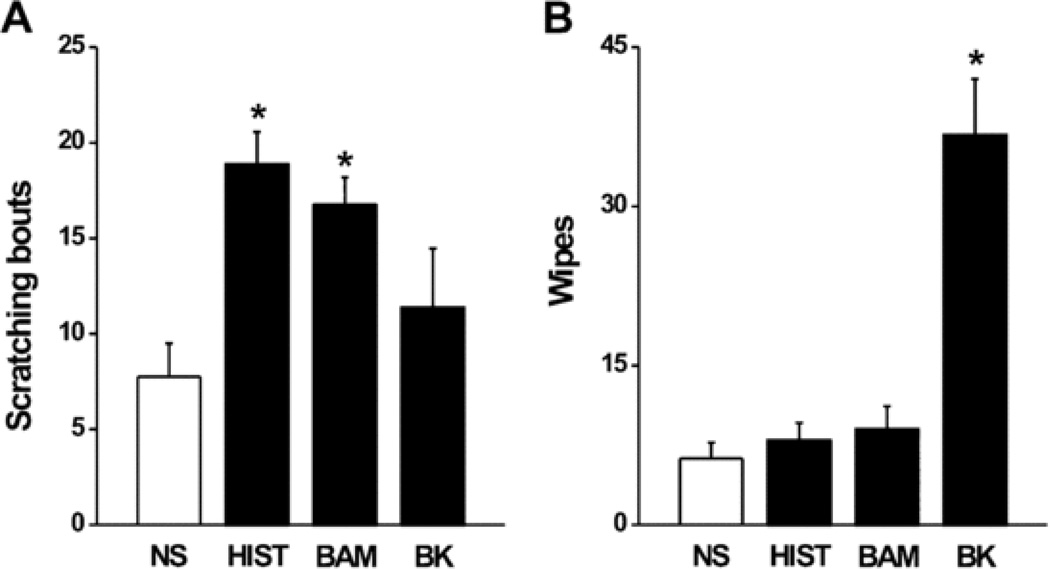
For the control mice, the behaviors elicited by the injection of the algogen, bradykinin, and the pruritogens, histamine and BAM8-22 were similar to those previously reported for normal, healthy skin [3,4,16]. In comparison with the mean numbers of site-directed behaviors evoked by the injection of normal saline vehicle, bradykinin evoked significantly more wipes but not more bouts of scratching whereas histamine and BAM8-22 each elicited significantly more bouts of scratching but not more wipes (Fig. 2).
Figure 2.
Behavioral effects of each chemical injected into vehicle challenged skin (“control mice”). The mean number of bouts of scratching (A) and mean number of wipes (B) evoked by an injection of each chemical into the area of skin previously challenged with acetone alone. *P*P < 0.05, compared to normal saline injection, n = 8/group. Mann-Whitney Un = 8/group. Mann–Whitney U test was used to compare the differences between BAM- and NS-evoked scratching and HIST and NS-induced wiping. Abbreviations: NS, normal saline; HIST, histamine; BAM, BAM8-22; BK, bradykinin.
3.2Behavioral effects of each chemical injected into SADBE challenged skin
In comparison with acetone treated controls, the CHS mice displayed a significantly greater number of bouts of spontaneous site-directed scratching and wiping to the SADBE challenged skin after the second challenge (scratching: 1.2 ± 0.67 vs. 16.4 ± 3.40, wiping: 1.0 ± 0.75 vs 27.8 ± 5.96, respectively, P < 0.05), consistent with our previous findings [13]. For the 30 min period following an injection of the saline vehicle in CHS mice, there were no significant differences in the mean numbers of bouts of scratching or wipes than occurred spontaneously during the previous half an hour (scratching: 17.6 ± 3.48 vs 16.4 ± 3.40, wiping: 20.9 ± 4.09 vs 27.8 ± 5.96, respectively, P > 0.05). Thus, the injection of normal saline alone did not increase the number of itch- or pain-like behaviors directed to the area of CHS beyond what were already occurring spontaneously.
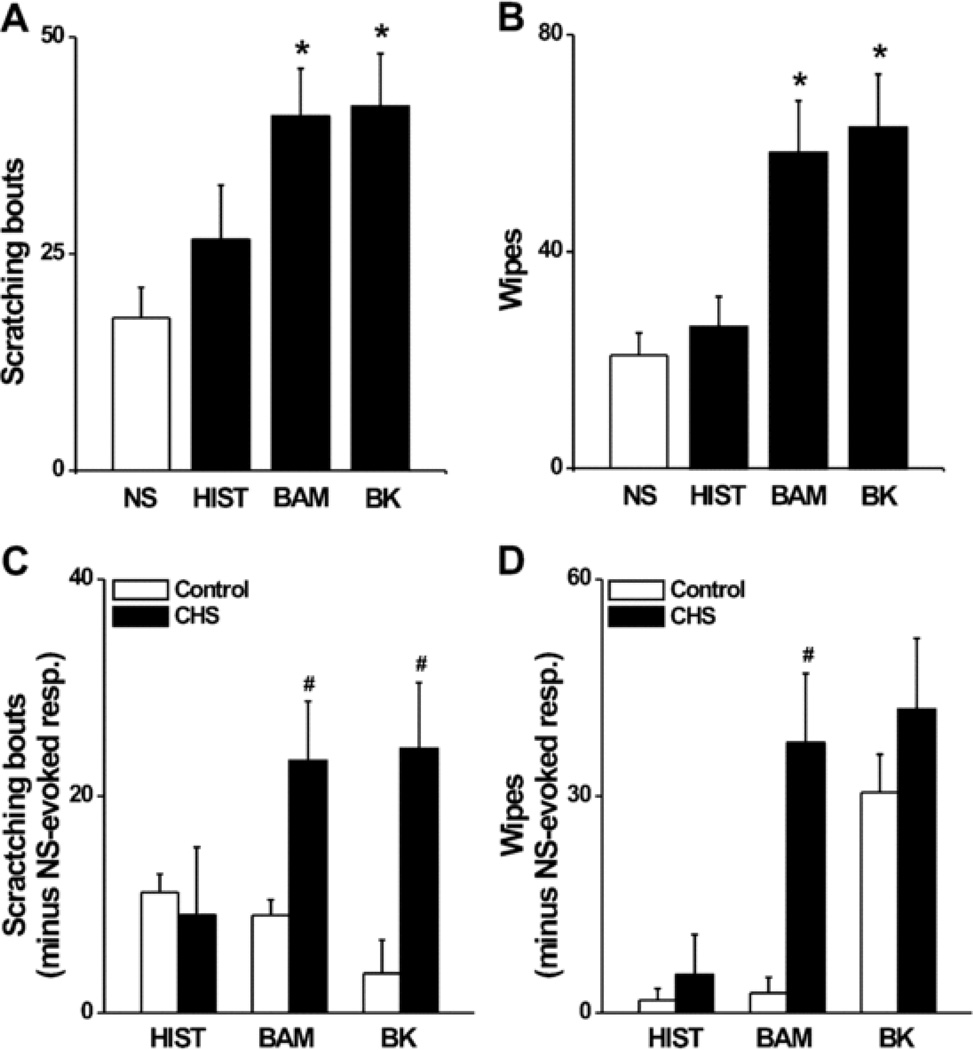
Statistical comparisons were made, for injections into SADBE challenged skin, between the behaviors evoked by each chemical and the saline vehicle alone (Fig. 3AB) and B). BAM8-22 and bradykinin, but not histamine, each evoked a significantly greater mean number of scratching bouts and wipes.
Figure 3.
Behavioral effects of each chemical injected into SADBE challenged skin (“CHS mice”). The mean number of bouts of scratching (A) and mean number of wipes (B) evoked by an injection of each chemical in the area of SADBE-induced CHS. Same format as in Fig. 2. *P*P < 0.05, compared with saline injection in CHS mice, n = 8/group. (C,n = 8/group. (C and D) Comparison between CHS and control mice of the mean numbers of bouts of scratching (C) and mean number of wipes (D) where the mean response to the saline vehicle in each group was subtracted from the responses to each chemical obtained from each animal (see RESULTS). #P Section 3). #P < 0.05, compared with control mice, n = 8/group. Mann-Whitney Un = 8/group. Mann–Whitney U test was used for comparing the differences between CHS and control mice for BAM-evoked scratching and HIST-induced wiping. Abbreviations: NS, normal saline; HIST, histamine; BAM, BAM8-22; BK, bradykinin.
Because injections into CHS mice included spontaneous behaviors produced by the SADBE treatment, we wanted to control for that effect. Therefore, we determined whether the chemically induced itch and pain-like behaviors in CHS mice were significantly different from those in control mice when the effects of normal saline vehicle injection per se were eliminated. We assumed the same number of spontaneous behaviors occurred during normal saline injection as during chemical injections. For each of these treatments, the mean number of bouts of scratching (or wipes) in response to saline injection, was subtracted from the respective number of responses to each chemical. The means of these numbers were then statistically compared between CHS and control mice for each chemical. BAM8-22 and bradykinin, but not histamine, each evoked a significantly greater number of scratching bouts in CHS mice than that in control mice (Fig. 3 C). There were no significant differences in the mean number of wipes evoked by these chemicals except for BAM8-22 which elicited a greater number in CHS mice (Fig. 3D).
4Discussion
There have been extensive studies of the immunological mechanisms of CHS in murine models of ACD in humans [17,18] but relatively few studies of the troublesome sensory symptoms of the disease. The local inflammation (dermatitis), including swelling, redness, blistering, is a source of spontaneous itch and nociceptive sensations [11,12] and the site of behavioral responses such as scratching [13,19]. Our present purpose was to test whether chemically evoked itch- or pain-like behaviors are altered in an area of CHS produced by application of the hapten, SADBE.
In normal (vehicle treated) skin and in confirmation with previous findings [3,16], histamine or BAM8-22, which are primarily pruritic to humans [2,20], evoked more site-directed scratching with the hind limb than wiping with the forelimb. In contrast, bradykinin, which is painful to humans [21,22], evoked more wiping than scratching [4]. Thus, the mouse provided quantifiable behaviors that were analogous to psychophysically measured itch and nociceptive (pain-like) sensations obtained from humans in response to these algesic or pruritic chemicals.
The effects of CHS in the present study were different for each of the two pruritogens having no specific effect on the itch- or pain-like behaviors evoked by histamine while enhancing both types of behavior in response to BAM8-22. In contrast, when each chemical was injected into an area of ACD experimentally produced with SADBE in human subjects, histamine and BAM8-22 each evoked a greater than normal itch without any increase in nociceptive sensations such as pricking/stinging or burning [11]. One factor that was different for the two species was that mice were allowed to scratch the area of dermatitis whereas humans were not. In the future, the amount of chronic scratching allowed in the mice could be manipulated to determine its effect on chemically evoked itch- and pain-like behavior.
The absence of enhanced behavioral responses to histamine in an area of CHS in the mouse is consistent with the lack of evidence for a major role of histamine as the cause of the itch of ACD or atopic dermatitis and the ineffectiveness of antihistamines in treating the itch [23,24]. Histamine also does not elicit enhanced scratching behavior or enhance the responses of neurons in the dorsal root ganglion or dorsal horn in a murine model of dry skin [10,25]. There is evidence that hapten-induced scratching in some strains of mice might be reduced by an H4-receptor antagonist [26]. However, histamine acts on H1 receptors to excite primary sensory neurons via intracellular signaling that activates transient receptor potential (TRP) cation channel, subfamily V, member 1 (TRPV1) ion channels [27]. And CHS induced inflammation and scratching behavior in the mouse persist after pharmacological inhibition or genetic deletion of TRPV1 [19].
In contrast, BAM 8–22 produces a histamine-independent itch in humans [20] and itch-like behavior in mouse [16], and is an agonist for MrgprC11 receptors that excite a subset of pruriceptive neurons in the mouse [15] through downstream activation of TRP cation channel, subfamily A, member 1 (TRPA1) ion channels [16]. When TRPA1 was genetically ablated or inhibited by selective antagonists, spontaneous scratching behavior in addition to the inflammation of CHS produced by the hapten, oxazolone, was reduced [19]. Although these findings indicate that TRPA1 is required for the pruritus of hapten induced CHS in the mouse, the cellular mechanisms of the enhanced behavioral responses to BAM8-22 that we observed after SADBE induced CHS are unknown.
Neurons that express the MrgprC11 receptor constitute the majority of neurons that also express MrgprA3, the receptor for chloroquine, and these MrgprA3 neurons also express the H1 receptor [14]. MrgprA3 expressing neurons that innervated an area of SADBE induced CHS became spontaneously active and their cell bodies more excitable [13]. But whether these neurons might nevertheless respond normally to histamine yet exhibit an increased response to BAM8-22, analogous to our behavioral findings was not tested. Other possibilities to be tested are whether there is an upregulation of MrgprC11 signaling in neurons other than those expressing MrgprA3, for example, nociceptive neurons that are normally non-pruriceptive but might then contribute to the increased pain-like behaviors elicited by BAM8-22 after the development of CHS.
Bradykinin is released in a wide range of inflammatory conditions and is generally known to be a potent pain mediator [28]. We found that bradykinin, injected into the area of SADBE induced CHS on the cheek, elicited the same pain-like behavior of wiping as in normal skin. But unlike the case for normal skin, the bradykinin-evoked wiping in an area of CHS was accompanied by robust scratching behavior. A similar phenomenon was also observed in the lesional skin of patients with atopic dermatitis and in mouse skin inflamed with complete Freund's adjuvant [7,29]. There are a number of potential neural mechanisms that could be explored. For example, certain pruriceptive primary sensory neurons may become more responsive to bradykinin after CHS. In addition or alternatively, CHS generated activity in peripheral pruriceptive neurons might induce a sensitization of central pruriceptive neurons that receive a convergent input from bradykinin-responsive pain-mediating nociceptors. This could result in a de novo de novo itch from a normally painful input.
5Conclusions
CHS enhanced the itch-like scratching behavior evoked by a histamine-independent pruritic chemical, BAM8-22, but not the itch of histamine. Bradykinin, which evoked only pain-like behavior of wiping in normal skin, evoked scratching in addition to wiping in an area of CHS. It is speculated that these alterations in sensory behaviors may result from the selective sensitization of peripheral and/or central pruriceptive neurons.
Acknowledgements
The work was supported by a grant from the US National Institutes of Health to R.H.L. (NS014624NS014624) and a grant from the Chinese National Natural Science Foundation to H.N. (No. 81373993). K.F. to H.N. (No. 81373993). K.F. is the recipient of a scholarship from Jinan University Jinan University, China. L.Q. is the recipient of a fellowship from the Canadian Institutes of Health Research (CIHR).
Footnotes
Author contributions
K.F. collected and analyzed the data. K.F., S.G.S, H.N. and R.H.L. designed the experiment. K.F. wrote and L.Q., S.G.S., H.N. and R.H.L edited the manuscript. The project was supervised by S.G.S., H.N. and R.H.L.
Conflict of Interest statement
All authors declare that there are no conflicts of interest.
Contributor Information
Hong Nie, Email: hongnie1970@163.com.
Robert H. LaMotte, Email: robert.lamotte@yale.edu.
References
- 1.Sikand P, Shimada SG, Green BG, LaMotte RH. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain. 2009;144:66–75. doi: 10.1016/j.pain.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sikand P, Shimada SG, Green BG, LaMotte RH. Sensory responses to injection and punctate application of capsaicin and histamine to the skin. Pain. 2011;152:2485–2494. doi: 10.1016/j.pain.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimada SG, LaMotte RH. Behavioral differentiation between itch and pain in mouse. Pain. 2008;139:681–687. doi: 10.1016/j.pain.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akiyama T, Carstens MI, Carstens E. Differential itch- and pain-related behavioral responses and micro-opioid modulation in mice. Acta Derm Venereol. 2010;90:575–581. doi: 10.2340/00015555-0962. [DOI] [PubMed] [Google Scholar]
- 5.Ikoma A, Rukwied R, Stander S, Steinhoff M, Miyachi Y, Schmelz M. Neuronal sensitization for histamine-induced itch in lesional skin of patients with atopic dermatitis. Arch. Dermatol. 2003;139:1455–1458. doi: 10.1001/archderm.139.11.1455. [DOI] [PubMed] [Google Scholar]
- 6.Ikoma A, Fartasch M, Heyer G, Miyachi Y, Handwerker H, Schmelz M. Painful stimuli evoke itch in patients with chronic pruritus: central sensitization for itch. Neurology. 2004;62:212–217. doi: 10.1212/wnl.62.2.212. [DOI] [PubMed] [Google Scholar]
- 7.Hosogi M, Schmelz M, Miyachi Y, Ikoma A. Bradykinin is a potent pruritogen in atopic dermatitis: a switch from pain to itch. Pain. 2006;126:16–23. doi: 10.1016/j.pain.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Baron R, Schwarz K, Kleinert A, Schattschneider J, Wasner G. Histamine-induced itch converts into pain in neuropathic hyperalgesia. Neuroreport. 2001;12:3475–3478. doi: 10.1097/00001756-200111160-00020. [DOI] [PubMed] [Google Scholar]
- 9.Schmelz M, Hilliges M, Schmidt R, Orstavik K, Vahlquist C, Weidner C, Handwerker HO, Torebjork HE. Active "itch fibers" in chronic pruritus. Neurology. 2003;61:564–566. doi: 10.1212/01.wnl.0000078193.64949.08. [DOI] [PubMed] [Google Scholar]
- 10.Akiyama T, Carstens MI, Carstens E. Enhanced scratching evoked by PAR-2 agonist and 5-HT but not histamine in a mouse model of chronic dry skin itch. Pain. 2010;151:378–383. doi: 10.1016/j.pain.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sikand P, King BA, LaMotte RH. Psychophysical measurements of itch and nociceptive sensations in an experimental model of allergic dermatitis in humans. Soc. Neurosci. 2012 doi: 10.1016/j.jpain.2015.04.009. Abstract 675.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruckner AL, Weston WL. Allergic contact dermatitis in children: a practical approach to management. Skin Therapy Lett. 2002;7:3–5. [PubMed] [Google Scholar]
- 13.Qu L, Fan N, Ma C, Wang T, Han L, Fu K, Wang Y, Shimada SG, Dong X, Lamotte RH. Enhanced excitability of MRGPRA3- and MRGPRD-positive nociceptors in a model of inflammatory itch and pain. Brain. 2014;137:1039–1050. doi: 10.1093/brain/awu007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, Undem BJ, Kollarik M, Chen ZF, Anderson DJ, Dong X. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, Li Z, McNeil B, He S, Guan Y, Xiao B, Lamotte RH, Dong X. A subpopulation of nociceptors specifically linked to itch. Nat. Neurosci. 2013;16:174–182. doi: 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat. Neurosci. 2011;14:595–602. doi: 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gober MD, Gaspari AA. Allergic contact dermatitis. Curr. Dir. Autoimmun. 2008;10:1–26. doi: 10.1159/000131410. [DOI] [PubMed] [Google Scholar]
- 18.Christensen AD, Haase C. Immunological mechanisms of contact hypersensitivity in mice. APMIS. 2012;120:1–27. doi: 10.1111/j.1600-0463.2011.02832.x. [DOI] [PubMed] [Google Scholar]
- 19.Liu B, Escalera J, Balakrishna S, Fan L, Caceres AI, Robinson E, Sui A, McKay MC, McAlexander MA, Herrick CA, Jordt SE. TRPA1 controls inflammation and pruritogen responses in allergic contact dermatitis. FASEB J. 2013;27:3549–3563. doi: 10.1096/fj.13-229948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sikand P, Dong X, LaMotte RH. BAM8-22 peptide produces itch and nociceptive sensations in humans independent of histamine release. J. Neurosci. 2011;31:7563–7567. doi: 10.1523/JNEUROSCI.1192-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manning DC, Raja SN, Meyer RA, Campbell JN. Pain and hyperalgesia after intradermal injection of bradykinin in humans. Clin. Pharmacol. Ther. 1991;50:721–729. doi: 10.1038/clpt.1991.212. [DOI] [PubMed] [Google Scholar]
- 22.Koppert W, Reeh PW, Handwerker HO. Conditioning of histamine by bradykinin alters responses of rat nociceptor and human itch sensation. Neurosci. Lett. 1993;152:117–120. doi: 10.1016/0304-3940(93)90497-9. [DOI] [PubMed] [Google Scholar]
- 23.Funk JO, Maibach HI. Horizons in pharmacologic intervention in allergic contact dermatitis. J. Am. Acad. Dermatol. 1994;31:999–1014. doi: 10.1016/s0190-9622(94)70272-1. [DOI] [PubMed] [Google Scholar]
- 24.Thurmond RL, Gelfand EW, Dunford PJ. The role of histamine H1 and H4 receptors in allergic inflammation: the search for new antihistamines. Nat. Rev. Drug Discov. 2008;7:41–53. doi: 10.1038/nrd2465. [DOI] [PubMed] [Google Scholar]
- 25.Akiyama T, Carstens MI, Carstens E. Enhanced responses of lumbar superficial dorsal horn neurons to intradermal PAR-2 agonist but not histamine in a mouse hindpaw dry skin itch model. J. Neurophysiol. 2011;105:2811–2817. doi: 10.1152/jn.01124.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossbach K, Wendorff S, Sander K, Stark H, Gutzmer R, Werfel T, Kietzmann M, Baumer W. Histamine H4 receptor antagonism reduces hapten-induced scratching behaviour but not inflammation. Exp. Dermatol. 2009;18:57–63. doi: 10.1111/j.1600-0625.2008.00762.x. [DOI] [PubMed] [Google Scholar]
- 27.Shim WS, Tak MH, Lee MH, Kim M, Kim M, Koo JY, Lee CH, Kim M, Oh U. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J. Neurosci. 2007;27:2331–2337. doi: 10.1523/JNEUROSCI.4643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petho G, Reeh PW. Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol. Rev. 2012;92:1699–1775. doi: 10.1152/physrev.00048.2010. [DOI] [PubMed] [Google Scholar]
- 29.Liang J, He Y, Ji W. Bradykinin-evoked scratching responses in complete Freund's adjuvant-inflamed skin through activation of B1 receptor. Exp. Biol. Med. 2012;237:318–326. doi: 10.1258/ebm.2011.011308. [DOI] [PubMed] [Google Scholar]