Abstract
This study examined whether level of exposure to Stimulant Abuser Groups to Engage in 12-Step (STAGE-12), a 12-Step facilitative therapy, is related to treatment outcome. Data were from a large National Drug Abuse Treatment Clinical Trials Network (CTN) study comparing STAGE-12 combined with Treatment-as-Usual (TAU) to TAU alone. These analyses include only those randomized to STAGE-12 (n = 234). Assessments occurred at baseline and 30, 60, 90, and 180 days following randomization. High-exposure patients (n = 158; attended at least 2 of 3 individual, and 3 of 5 group, sessions), compared to those with less exposure (n = 76), demonstrated: (1) higher odds of self-reported abstinence from, and lower rates of, stimulant and non-stimulant drug use; (2) lower probabilities of stimulant-positive urines; (3) more days of attending and lower odds of not attending 12-Step meetings; (4) greater likelihood of reporting no drug problems; (5) more days of duties at meetings; and (6) more types of 12-Step activities. Many of these differences declined over time, but several were still significant by the last follow-up. Treatment and research implications are discussed.
Keywords: 12-Step, 12-Step facilitation, Treatment exposure, Intensive referral, Cocaine, Methamphetamine
1 Introduction
Twelve-Step facilitative therapy refers to a form of treatment that seeks to increase attendance and involvement with 12-Step mutual support groups. There is strong research support for the ability of these therapies to increase attendance and active involvement in 12-Step fellowships, improve drinking or other substance use outcomes, and have long-lasting effects (Carroll, Nich, Ball, McCance, & Rounsaville, 1998; Carroll et al., 2000; Donovan & Floyd, 2008; Project Match Research Group, 1997; Timko DeBenedetti, & Billow; 2006; Timko & DeBenedetti, 2007). This is also true for studies focusing specifically on stimulant users (cocaine or methamphetamine). In the Cocaine Collaborative Treatment Study (Crits-Christoph et al., 1999) Individual Drug Counseling (IDC) plus Group Drug Counseling (GDC), both of which have a strong 12-Step focus, was superior to GDC-plus brief case management and to individual cognitive or supportive-expressive therapy combined with GDC. Among alcohol and cocaine dependent adults, Carroll et al. (1998) found both Twelve-Step Facilitation (TSF) (adapted from Project Match; Project Match Research Group, 1997) and Cognitive Behavioral Therapy (CBT) superior to a clinical management condition at reducing during-treatment cocaine use, but this difference was not maintained at one-year follow-up (Carroll et al., 2000).
In the parent study for the current analyses, Donovan and colleagues (2013) compared Stimulant Abuser Groups to Engage in 12-Step (STAGE-12), an intervention consisting of 5 group sessions adapted from TSF combined with 3 individual sessions adapted from the Intensive Referral Program intervention (Timko et al., 2006), combined with treatment as usual (TAU) to TAU-alone among stimulant users in intensive outpatient treatment. Results revealed that those receiving STAGE-12 were more likely to be abstinent during treatment than those in TAU-alone, but among those who were not abstinent, STAGE-12 participants used stimulants on more during-treatment days (Donovan et al., 2013). The current study examines whether level of exposure to STAGE-12 was related to outcome.
A number of studies have found that greater doses of 12-Step oriented treatments are associated with more involvement in 12-Step or other mutual support groups as well as improved substance use outcomes. Kaskutas et al. (Kaskutas, Subbaraman, Witbrodt & Zemore, 2009), found a dose response for “Making Alcoholics Anonymous Easier” MAAEZ treatment at the 12-month follow-up point – the group that completed 0–1 sessions had a self-reported abstinence rate of 70% from both alcohol and drugs, whereas those completing 3 sessions had a rate of almost 80%, and those completing all 6 sessions had a rate of 90%. In a population with substance use disorder (SUD) co-occurring with a psychiatric disorder, Timko, et al. (Timko, Sutkowi, Cronkite, Makin-Byrd, & Moos, 2011) found that a higher dose of Intensive Referral services was associated with attending at least one dually-focused mutual help group (DFGs), greater readiness to attend DFGs, attending more substance-focused groups (SFGs), and being more involved in SFGs. In a study of TSF with dually diagnosed alcohol dependent patients, Bogenschutz et al. (2014) found number of TSF sessions was associated with increased abstinence and decreased drinking intensity both during and following treatment. Brown et al. (Brown, Seraganian, Tremblay & Annis, 2002) found that a higher dose of TSF was associated with increased confidence in high-risk situations but not with improved substance abuse outcomes. Findings related to readiness (Timko et al., 2011) and confidence (Brown et al., 2002) and their association with 12-Step treatment dose raise the possibility of a “third factor” explanation of dose-outcome associations. Greater motivation or self-efficacy at the outset of 12-Step treatments might lead to both better attendance and improved outcomes. The present study examines both dose-outcome associations and this possible explanation for them.
Twelve-step intervention studies vary in the way they have measured “treatment exposure” or “dose.” Exposure may be measured using the number of sessions attended or the number of weeks in treatment (Timko et al., 2011; Kaskutas, 2009; Kaskutas et al., 2009; Fiorentine & Hillhouse, 2000; Maude-Griffin, et al. 1998; Project MATCH Research group, 1997; Wells, Peterson, Gainey, Hawkins, & Catalano, 1994). Fiorentine & Hillhouse (2000) measured treatment exposure as the number of weeks in treatment, but also included a “treatment completion” variable in their study of substance abusers who most frequently identified stimulants as their primary drug of choice. In that study, treatment providers determined at the end of 24 weeks of treatment whether participants had successfully completed treatment, dropped out, or needed additional treatment.
The purpose of the present study was to examine whether level of exposure to a 12-Step oriented therapy is related to stimulant users’ treatment outcomes such as self-reported stimulant use, self-reported non-stimulant use, biological measures of drug use, or self-reported attendance and active involvement in 12-Step meetings. The parent study, a large National Drug Abuse Treatment Clinical Trials Network (CTN) efficacy/effectiveness trial (Donovan et al., 2013) provided the opportunity to examine whether those with a high level of exposure to the STAGE-12 intervention differed in their treatment outcomes from those with less exposure.
2 Methods
The University of Washington’s Institutional Review Board (IRB) approved the study’s procedures, as did the IRBs of universities with which each study site was affiliated. The National Institute on Drug Abuse convened a Data and Safety Monitoring Board that reviewed study design, progress and results.
2.1 Design
The parent study employed a two-group randomized repeated measures design. The two study conditions were: the Stimulant Abuser Groups to Engage in 12-Step (STAGE-12) intervention integrated into treatment as usual (TAU) (henceforth referred to as “STAGE-12”) or TAU alone. Because intervention exposure was measured only for the STAGE-12 participants, these analyses are confined to the 234 participants randomly assigned to that condition. Assessments took place at baseline and at 30 (mid-treatment), 60 (end-of-treatment) 90 (first follow-up), and 180 days (last follow-up) following randomization. Assessments were primarily in-person, with phone interviews done in rare cases in which direct contact was impractical. Participants received $30 for each assessment and a $40 bonus if they completed all assessments. Additional details about the STAGE-12 intervention, study methods, and primary substance use outcomes appear elsewhere (Daley, Baker, Donovan, Hodgkins, & Perl, 2011; Donovan, Daley, Brigham, Hodgkins, Perl, & Floyd, 2011; Donovan et al., 2013).
2.2 Participants
2.2.1 Treatment sites and providers
Participant recruitment, assessment, and treatment took place at 10 community-based treatment programs (CTPs) within the CTN that provided outpatient psychosocial SUD treatment. Interested CTP clinicians who volunteered were randomly assigned to provide the STAGE-12 intervention or continue to provide TAU.
2.2.2 Patients
To be included, patients: (1) were at least 18 years old; (2) were enrolled in outpatient treatment at the participating site; (3) reported stimulant use in the past 60 days (or in the past 90 days if incarcerated during the past 60); (4) met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for current stimulant abuse or dependence with a stimulant as primary or secondary drug of abuse; and (5) were able to provide consent and were willing to agree to study procedures. They were excluded if they: (1) needed opiate detoxification; (2) were seeking detoxification only, opiate substitution, or inpatient/residential treatment; (3) had a medical or psychiatric condition that could be worsened by participation; (4) reported being incarcerated for more than 60 of the previous 90 days; or (5) had pending legal action that would interfere with study participation.
2.3 STAGE-12 Intervention
Descriptions of the STAGE-12 intervention can be found elsewhere (Daley, et al., 2011; Donovan et al., 2013) and the intervention manual (Baker, Daley, Donovan & Floyd, 2009) is available at http://ctndisseminationlibrary.org/display/888.htm. Designed to fit well into standard outpatient SUD treatment (Donovan et al., 2011), STAGE-12 replaced 5 group and 3 individual TAU counseling sessions. The five 90-minute group sessions were based on TSF Therapy for Drug Abuse and Dependence (Baker, 1998; Carroll et al., 1998).
The three individual sessions were used to both introduce and draw to a close the group intervention. In addition, a second purpose of the individual sessions was to initiate an intensive referral process modeled after Timko et al. (2006) and AA’s Bridging the Gap program (AA, 1991). This included linking the participant to a 12-Step volunteer who would attend a meeting with the participant. The combined group and individual sessions were designed to be delivered over a period of 5–8 weeks, with group treatment delivered weekly and individual sessions delivered before, during, and after the 5 group sessions.
STAGE-12 was integrated into TAU; that is, STAGE-12 sessions replaced standard treatment sessions that would otherwise have been received (see Donovan et al., 2011). However, STAGE-12 participants did receive portions of the standard TAU that were not replaced. “The 10 selected CTPs provided a mean of 9.6 hours of treatment per week (standard deviation [SD] = 2.85, ranging from 6 to 15 hours) for an average of 13.5 weeks (SD = 5.12, ranging from 7.5 to 24 weeks). During the course of the study patients in participating CTPs typically attended three group sessions and one individual session per week (Donovan et al., 2013, pp. 105).”
2.4 Measures
2.4.1 Demographics and diagnosis
The parent study used a standard CTN-developed form and the Addiction Severity Index – Lite (ASI-Lite) (Cacciola, Alterman, McLellan, Lin, & Lynch, 2007) to collect demographic information. The DSM-IV Criteria Checklist (DSM-IV Checklist) interview (Hudziak et al., 1993) was used to determine substance use disorder (SUD) diagnosis.
2.4.2 Baseline motivation and self-efficacy
The 15-item Survey of Readiness for Alcoholics Anonymous Participation (SYRAAP; Kingree, Simpson, Thompson, McCrady & Tonigan, 2007; Kingree, et al. 2006) measures ambivalence toward and readiness to engage in 12-step activities. It includes three subscales (5 items each) with each item rated on a 5-point Likert scale (1 = strongly disagree, 5 = strongly agree). Items query self-perceptions about the severity of substance use problems, Perceived Benefits of involvement in 12-step groups, and Perceived Barriers to participating in 12-step groups. Among those assigned to STAGE-12, reliability coefficients were alpha = .80 for Perceived Severity, alpha = .84 for Perceived Benefit, and alpha = .71 for Perceived Barriers.
The SOCRATES-8D (Stages of Change Readiness and Treatment Eagerness Scale, version 8 Drugs; Miller & Tonigan, 1996) is a 19-item measure of readiness to change drug use. The baseline reliability coefficient for the SOCRATES was alpha = .85. The Drug Taking Confidence Scale − 8 (DTCQ8) measures confidence in one’s ability to cope with high risk situations for drug use (Sklar & Turner, 1999). Reliability at baseline for the 8-item DTCQ-8 was alpha = .89.
Whether or not a patient reported being mandated to participate in this episode of SUD treatment was also considered as a proxy for internal versus external motivation.
2.4.3 Level of exposure
The counselor recorded participant attendance at individual and group sessions. While designing the STAGE-12 intervention, the investigators identified an adequate dose of the intervention a priori as having attended 2 or more individual sessions plus 3 or more group sessions. Selection of this criterion was based on the experience of the investigators and treatment program representatives with interventions of this length and type and the desire to identify a minimal therapeutic dose. In addition, the shorter 12-Step oriented interventions that had previously been evaluated (Kaskutas, et al., 2009; Timko & DeBenedetti, 2007; Timko, et al., 2006; 2011) were 6 and 4 sessions in length, respectively. For this study, this level of attendance was judged to provide sufficient exposure to STAGE-12 content and to the intensive referral process to be therapeutic.
2.4.4 Participant satisfaction
Stage-12 participants completed the Participant Satisfaction Survey (PSS) at 60 days, the end of the Stage-12 treatment phase. Seven of the 8 PSS items (e.g., “Overall, how satisfied are you with the STAGE-12 treatment you received for your drug problem?”) were based on a 7-point Likert scale, with endpoints such as 1 = extremely dissatisfied and 7 = extremely satisfied; whereas, one item (“If you were to seek treatment in the future, would you want to be involved in Stage-12?”) was based on a 5-point Likert scale (1 = definitely not and 5 = definitely yes). Two subscale scores were calculated: Total Satisfaction Score (6 items, alpha = .88) and Total Benefit Score (2 items, alpha = .78). In addition, participants rated 8 parts of the STAGE-12 program on a 5-point Likert scale of 1 = extremely unhelpful to 5 = extremely helpful. For each of these 8 helpfulness ratings, participants could also indicate whether the STAGE-12 component was not applicable (NA) or did not occur.
2.4.5 Substance use outcome
Number of days of self-reported stimulant drug use and non-stimulant drug use were computed based on a timeline follow-back procedure, the Substance Use Calendar (SUC) (Fals-Stewart, O'Farrell, Freitas, McFarlin, & Rutigliano, 2000; Sobell & Sobell, 1992; Walitzer, Dermen, and Barrick, 2009), collected at baseline, mid-treatment (30-day), end-of-treatment (60-day), first follow-up (90-day), and last (180-day) follow-up. At baseline, participants were asked about the prior 90 days and at all other time points they were asked about the days since their last SUC report, thus providing continuous SUC data from 90 days prior to baseline to the final follow-up (180-day). For the purpose of analyses, these data were grouped in 30-day increments. The Addiction Severity Index (ASI) Drug Use Composite and Alcohol Use Composite scores (ranging from 0–1) were used to measure substance use problems. These scores were available at baseline and at first and last follow-ups.
At each assessment point, participants provided a urine sample. The variable analyzed for this report was whether or not a participant submitted a urine drug screen positive for a stimulant drug (cocaine, amphetamines, or methamphetamine).
2.4.6 Mutual Support Meeting Attendance and 12-Step Activities
Participants’ self-reported attendance at 12-Step mutual support meetings was recorded on the SUC for the 90-day period prior to baseline and the period between assessments at the mid- and end-of-treatment visits and at the final two follow-up visits. As with substance use data, if an assessment was missed, data were collected for the period since the last visit attended. Three variables derived from the Self-Help Activities Questionnaire (SHAQ) (Weiss et al., 1996) provided information about participant involvement in a variety of activities. The first variable was the maximum number of days of self-reported speaking at AA, NA, CA or CMA meetings, based on how many of the past 30 days participants reported they attended and spoke at each of the 4 kinds of meetings. The second variable was the maximum number of days of self-reported duties at AA, NA, CA or CMA meetings within a 30-day window of assessment. This maximum was computed in the same way; greatest number of days of service reported among the 4 types. The third variable was the number of other self-help activities in which the participant engaged within the 30-day assessment window. It ranged in value from 0 to 6, indicating how many of the following were reported: (1) met with one or more AA/NA/CA/CMA members outside a meeting; (2) met with sponsor(s) outside meeting; (3) phoned sponsor(s); (4) received at least one phone call from sponsor(s); (5) received at least one phone call from other members, and (6) read 12-Step literature for at least 5 minutes.
2.5 Analysis Approach
Six of the outcomes are count data, including days of self-reported stimulant use, days of self-reported non-stimulant use, days of self-help attendance, days of speaking at meetings, days of self-reported duties, and number of self-help activities. To account for the excess of zeroes and over-dispersion with three SUC outcomes of count data, a zero-inflated negative binomial random-effects regression model was utilized. This is a mixture regression model with two components: a logistic part for zero-inflation (always zero versus not always zero) and a negative binomial part to assess the full range of count values including some zeroes which are over-dispersed at each time point. This model was used to evaluate differences between “high exposure” and “lower exposure” participants on stimulant use (cocaine, amphetamines or methamphetamines), non-stimulant use (cannabis, opiates or benzodiazepines), and the number of days of self-reported Self-Help meeting attendance. The maximum percent of zeroes across 6 post-baseline time points was 77.3%, 73.0%, and 37.1% for these 3 variables, respectively.
Zero-inflated negative binomial random-effects regression models assume linearity. For three SUC variables, (number of days of stimulant use, non-stimulant use or number of days of Self-Help meeting attendance) there was deviation from linearity between baseline and the first, mid-treatment assessment. To deal with this non-linearity and to avoid more complex modeling, such as the inclusion of orthogonal polynomials with linear and quadratic time components, data from 3 pre-treatment periods (60+ to 90 days pre-baseline, 30+ to 60 days pre-baseline, and 30 days pre-baseline) were averaged. This created one averaged pre-treatment covariate for each SUC outcome, while allowing for the linearity assumption to be met on the variables across the remaining time points modeled. Although data from the three pre-treatment time periods are count data and tend to be somewhat positively skewed, they were not zero-inflated and their average provided more normally-distributed data suitable for a model covariate. There were no statistically significant differences between high and low exposure participants on the average pre-treatment covariates for stimulant use (p = .53), non-stimulant use (p = .40) or the number of days of Self-Help meeting attendance (p = .99).
The exposure status by time interaction results for the zero-inflated models were interpreted by considering both the odds ratios (OR) for the logistic part of the model (zero-inflation and abstinence assessment or no Self-Help meeting attendance) and the incidence rate ratio (RR) for the negative binomial part (count of substance use days or days of Self-Help meeting attendance) of the model at each of the 6 post-baseline time points, along with their 95% confidence intervals (CI).
In the analyses with the other three variables of count data without zero-inflation, a regular negative binomial with over-dispersion data (for the number of self-reported days of speaking or duties at meetings from the SHAQ) or Poisson with data displaying equidispersion (for the number of other self-help activities from the SHAQ) random-effects regression model was used. With these three variables, a single baseline measure was included within the time effect for the model.
The statistical significance of the exposure status by time interaction effect with the zero-inflated and regular negative binomial and Poisson regression models was conducted with algorithms for the OR or RR provided by Hilbe (2008; 2011). With all count data and the repeated measures aspect of the assessment, random effects models were considered appropriate to account for the correlation within participants across time.
Analysis of urine screens was conducted with a logistic random effects regression model, with a baseline urine screen measure covariate. Due to the distributional characteristics of the ASI data, Alcohol Use and Drug Use composite scores were considered as both a binary variable (0 versus > 0) and as a continuous variable for scores above 0, utilizing simple logistic regression or a t-test for the type of variable, respectively, at baseline, 90- and 180-day follow-up. Given the negative skewness of all Likert-scaled item level and subscale PSS variables, the non-parametric Wilcoxon two-sample test was used to evaluate differences between high exposure and low-exposure participants. Differences in proportions for helpfulness were assessed with the chi-square statistic.
The repeated measures analyses generally required at least one post-baseline value, and due to missing data across all time assessments for some cases, the regression models are based on available information and subsamples of the original 234 STAGE-12 participants. Statistical results were obtained with the SAS (2010) program, and the logistic, negative binomial and Poisson random-effects regression models were estimated with the NLMIXED procedure.
3 Results
3.1 Participant characteristics
A complete description of participants for the full study appears in Donovan et al. (2013). Of the 234 participants randomly assigned to the Stage-12 intervention, 62% were female, 6.4% reported Hispanic/Latino ethnicity, 46.2% reported Caucasian race, and 37.6% reported Black/African American race. They averaged 38.2 years of age (SD = 10.0) and 12.2 years of education (SD = 1.7). Thirty-four percent were unemployed, and only 15.5% were currently married. A majority (72.7%) met DSM-IV criteria for current cocaine, 33.8% for methamphetamine, 6.8% for amphetamine, and 44.9% for alcohol dependence. About one fifth (22.2%) were legally mandated to attend treatment. The STAGE-12 participants were not different demographically from those in the TAU condition not included in these analyses.
3.2 Study retention
Participants assigned to STAGE-12 had the following completion rates of research visits: baseline n = 233 (99.6%); mid-treatment (30 days post-baseline) n = 190 (81.2%); end-of-treatment (60 days) n = 168 (71.8%); first follow-up (90 days) n = 153 (65.4%); and last follow-up (180 days) n = 158 (67.5%). To be included in the analyses presented below, participants had to have at least one post-baseline visit. Of the 234 assigned to STAGE-12, 30 (12.8%) did not meet this criterion, leaving an analyzable sample of 204. Those included in the analysis did not differ significantly from those excluded on gender, age, education, baseline self-reported stimulant or non-stimulant use, or baseline urine drug screen stimulant use results.
3.3 Individual and group session attendance
Of the 234 people assigned to STAGE-12, attendance at individual sessions was as follows: 20 (8.5%) attended no sessions; 105 (44.9%) attended 2; and 109 (46.6%) attended all three. Group session attendance was also negatively skewed: 34 (14.5%) attended no group sessions; 20 (8.5%) attended 1; 22 (9.4%) attended 2; 32 (13.7%) attended 3 and the number was the same for 4 sessions; and 94 (40.2%) attended all 5 sessions. The number meeting the previously defined criterion for therapeutic (high) exposure, attending at least 2 individual and 3 group sessions was 158 (67.5%). Of these, 88 individuals (56% of those with high exposure) attended all 8 intervention sessions. The distribution of attendance data supported the decision to use a binary independent variable; examining “dose” of the STAGE-12 intervention as a continuous variable with these skewed data was not advisable.
Within the analyzable sample of 204 STAGE-12 participants having post-baseline data, 157 (77%) met criteria for high exposure to STAGE −12. This indicates that high-exposure participants are over-represented and those with low exposure under-represented in the analysis sample. Of the 30 participants who are not included in this analysis, 97% also did not meet criteria for high exposure to the STAGE-12 intervention.
3.4 Participant satisfaction
Results from the PSS indicated that high exposure participants scored higher both on the Total Satisfaction (low exposure M = 27.96, SD = 9.11; high exposure M = 33.85, SD = 4.65; Z = −3.3720, p = .0007) and Benefit (low exposure M = 9.77, SD = 2.59; high exposure M = 11.62, SD = 1.79; Z = −3.5710, p = .0004) subscales. Additionally, participants with high exposure rated all 8 parts of the STAGE-12 program as significantly more helpful than those with low exposure (p < .05). Concerning whether or not each intervention component occurred (Rated 1- 5 versus not applicable or did not occur), the proportions of high versus low-exposure respondents did not differ.
3.5 Substance use outcomes
3.5.1 Self-reported stimulant use
Table 1 presents odds ratios for self-reported stimulant abstinence and rate ratios for days of use among those who were not abstinent. Each of these ratios provides different information and each is necessary for a full understanding, given the zero-inflated distribution of the data (See section 2.5 for rationale). For the 30 day window from baseline to mid-treatment, the odds of self-reported abstinence from stimulant use were 41.31 times greater for high- than for low-exposure participants. The odds ratios declined over time but remained statistically significant at the 30-day windows between mid-treatment and end-of-treatment, from end-of-treatment to first follow-up (90 days), and from 91 to 120 days. The rate ratios in Table 1 show that the rate of stimulant use for non-abstinent high exposure participants was less than half that of non-abstinent low exposure individuals (RR = 0.42) during the 30-day window between baseline and mid-treatment and about half by end-of-treatment. Although the rate of stimulant use among those with high exposure did not catch up to that of their low-exposure counterparts until the last 30- day window collected at the last follow-up (days 151–180), the differences were not significant after the end of treatment.
Table 1.
Interaction ORs (abstinence) and incidence RRs (days of use) with primary outcome of self-reported days of stimulant use within a 30-day window of assessment, Stage-12 high exposure versus low exposure. a
| Assessment time point (days from baseline)b |
Logistic portion of model (abstinence vs. non-abstinence) |
Model-based average predicted probabilities of abstinence from stimulant use within each 30-day window |
|||
|---|---|---|---|---|---|
| OR | 95% CI for OR | Low Exposure N = 47 |
High Exposure N = 157 |
Total N = 204 |
|
| Mid-treatment (30 days) | 41.31 | 6.55, 260.46 | 0.43 | 0.80 | 0.71 |
| End of treatment (60 days) | 20.38 | 4.07, 102.05 | 0.49 | 0.78 | 0.72 |
| First-follow-up (90 days) |
10.05 | 2.32, 43.54 | 0.55 | 0.77 | 0.72 |
| Last follow-up | |||||
| 91 – 120 days | 4.96 | 1.18, 20.76 | 0.60 | 0.76 | 0.73 |
| 121 – 150 days | 2.45 | 0.54, 11.15 | 0.66 | 0.75 | 0.73 |
| 151 – 180 days | 1.21 | 0.22, 6.63 | 0.71 | 0.74 | 0.73 |
| Assessment time point (days from baseline)b |
Negative binomial portion of model (count of non-zero use) |
Model-based average predicted values of number of days of stimulant substance use within each 30-day window |
|||
|---|---|---|---|---|---|
| RR | 95% CI for RR | Low Exposure N = 47 |
High Exposure N = 157 |
Total N = 204 |
|
| Mid-treatment (30 days) | 0.42 | 0.22, 0.81 | 3.09 | 0.64 | 1.20 |
| End of treatment (60 days) | 0.51 | 0.28, 0.93 | 2.99 | 0.83 | 1.32 |
| First follow-up (90 days) | 0.62 | 0.36, 1.10 | 2.86 | 1.07 | 1.48 |
| Last follow-up | |||||
| 91 – 120 days | 0.76 | 0.43, 1.34 | 2.70 | 1.39 | 1.69 |
| 121– 150 days | 0.93 | 0.51, 1.70 | 2.50 | 1.79 | 1.96 |
| 151–180 days | 1.14 | 0.58, 2.22 | 2.26 | 2.31 | 2.30 |
There was a single covariate, average number of days self-reported stimulant use across three 30-day windows (a 90 day period) prior to baseline.
The Substance Use Calendar timeline follow-back was administered for 30 day windows covering the time from the previously completed assessment.
3.5.2 Self-reported non-stimulant use
High exposure to STAGE-12 was associated with 4.28 the odds of self-reported abstinence from non-stimulant in use in the 30 days between baseline and mid-treatment than low exposure (Table 2). At the end-of-treatment interview, the difference approached significance, but differences were not significant at follow-up time points. Results were similar for the rate of non-stimulant use among those who were not abstinent (shown in the rate ratios), with rate of use for those with high exposure being half that of the low-exposure participants at mid-treatment, and the end-of-treatment results approaching significance.
Table 2.
Interaction ORs (abstinence) and incidence RRs (days of use) with days of self-reported non-stimulant use within a 30-day window of assessment, Stage-12 high exposure versus low exposure. a
| Assessment time point (days from baseline)b |
Logistic portion of model (abstinence vs. non-abstinence) |
Model-based average predicted probabilities of abstinence from non- stimulant use within each 30-day window |
|||
|---|---|---|---|---|---|
| OR | 95% CI for OR | Low Exposure N = 47 |
High Exposure N = 157 |
Total N = 204 |
|
| Mid-treatment (30 days) | 4.28 | 1.14, 16.04 | 0.50 | 0.71 | 0.66 |
| End of treatment (60 days) | 3.20 | 1.02, 9.98 | 0.52 | 0.69 | 0.65 |
| First follow-up (90 days) | 2.39 | 0.85, 6.71 | 0.53 | 0.66 | 0.63 |
| Last follow-up | |||||
| 91 – 120 days | 1.79 | 0.64, 5.00 | 0.55 | 0.63 | 0.61 |
| 121 – 150 days | 1.34 | 0.43, 4.13 | 0.56 | 0.60 | 0.59 |
| 151 – 180 days | 1.0 | 0.27, 3.70 | 0.58 | 0.57 | 0.57 |
| Assessment time point (days from baseline)b |
Negative binomial portion of model (count of non-zero use) |
Model-based average predicted values of number of days of non-stimulant substance use within each 30-day window |
|||
|---|---|---|---|---|---|
| RR | 95% CI for RR | Low Exposure N = 47 |
High Exposure N = 157 |
Total N = 204 |
|
| Mid-treatment (30 days) | 0.50 | 0.25, 0.98 | 3.83 | 1.17 | 1.78 |
| End of treatment (60 days) | 0.57 | 0.30, 1.09 | 3.97 | 1.48 | 2.06 |
| First follow-up (90 days) | 0.66 | 0.35, 1.23 | 4.12 | 1.88 | 2.40 |
| Last follow-up | |||||
| 91 – 120 days | 0.76 | 0.41, 1.41 | 4.27 | 2.38 | 2.81 |
| 121 – 150 days | 0.87 | 0.46, 1.65 | 4.42 | 2.99 | 3.32 |
| 151 – 180 days | 1.00 | 0.51, 1.96 | 4.58 | 3.76 | 3.94 |
There was a single covariate, average number of days self-reported non-stimulant use across three 30-day windows (a 90 day period) prior to baseline.
The Substance Use Calendar timeline follow-back was administered for 30 day windows covering the time from the previously completed assessment.
3.5.3 Urine drug screens
STAGE-12 participants with high exposure were significantly less likely at mid-treatment, end-of-treatment and at the first (90-day) follow-up to provide a urine drug screen (UDS) positive for stimulants. By the time of the last (180-day) follow-up the difference was not significant, largely due to an increased probability of a positive UDS among the high exposure individuals. These results are shown in Table 3.
Table 3.
Interaction odds ratios (OR) and model-based average predicted probabilities of having a positive urine drug screen (UDS) for stimulants, Stage-12 high exposure versus low exposurea
| Interaction Odds Ratios | Model-based average predicted probabilities of having a stimulant- positive UDS |
||||
|---|---|---|---|---|---|
| Assessment time points (days from baseline) |
OR | 95% CI for OR | Low Exposure N = 44 |
High Exposure N = 154 |
Total N = 198 |
| Mid-treatment (30 days) | 0.19 | 0.05, 0.69 | 0.38 | 0.14 | 0.19 |
| End-of-treatment (60 days) | 0.24 | 0.08, 0.76 | 0.38 | 0.16 | 0.21 |
| First follow-up (90 days) | 0.30 | 0.10, 0.89 | 0.39 | 0.19 | 0.23 |
| Last follow-up (180 days) | 0.59 | 0.11, 3.00 | 0.41 | 0.27 | 0.30 |
There was a single dichotomous covariate derived from the baseline urine screen for stimulant drugs.
3.5.4 Motivation and Self-Efficacy as Alternate Explanations
Because participants self-selected into high- and low-exposure groups, there are a number of possible other-than-causal explanations of the relationship between exposure and substance use outcomes. One likely explanation is that individuals who begin with greater motivation to change their drug use are both more likely to remain involved in the intervention and less likely to use stimulants. Confidence in ability to remain abstinent could be hypothesized to have either a negative or positive relationship with treatment attendance. High confidence could lead patients to believe they can stop use without treatment or it could increase optimism about the potential outcome of treatment. Baseline SOCRATES and SYRAAP scores were not related to STAGE-12 exposure. Having been mandated to treatment was also unrelated to exposure. However, the DTCQ total score was significantly positively associated with STAGE-12 exposure, such that, for a one standard deviation increase in confidence score (SD = 24.75), the odds of having high exposure to treatment were over 1 1/2 times that of having low exposure (odds ratio = 1.64; 95% CI = [1.23, 2.19]; χ2 = 11.32, p = 0.0008). Baseline DTCQ score was, therefore, entered as a covariate into the models for self-reported stimulant and non-stimulant use and stimulant-positive UDS results. The DTCQ was not a significant covariate, and the odds and rate ratios were such that their interpretation would remain the same (close values and significance and direction the same as without the covariate). Results shown in the tables are without the addition of this covariate.
3.5.5 ASI Drug and Alcohol use Composite scores
Odds of having a non-zero ASI Drug Composite Score were compared at baseline, first (90-day) and last (180-day) follow-up. At baseline there was no significant difference, 71 of 75 low exposure (95%), and 136 of 156 high exposure participants (87%) had scores greater than zero (χ2 = 3.05, p = 0.08). High-exposure participants (74 of 124; 60%) were significantly less likely to have a non-zero Drug Composite score at the 90-day follow-up than were their low-exposure counterparts (24 of 30; 80%) (χ2 =4.31, p = 0.04). By the time of the last (180-day) follow-up, the two groups were again not different from each other. Seventy seven of 123 with high exposure (63%) had a non-zero score compared with 29 of 37 (78%) with low exposure (χ2 = 3.17, p = 0.08). Comparison of numerical Drug Composite scores among those who had a non-zero score revealed no differences at any of the three time points (baseline, first and last follow-up) and no differential change from baseline to 90 days, baseline to 180 days, or 90 to 180 days between the two groups.
ASI Alcohol Composite Scores differed by exposure status only at baseline. Proportionately fewer of those with high exposure (81 of 149; 54%) had non-zero Alcohol Composites than did the others (50 of 64; 78%) (χ2 = 10.68, p = 0.001), indicating that those who had a less than optimal dose of the Stage-12 intervention entered the study with more alcohol problems. The proportions with non-zero scores did not differ at follow-up, and the numeric Composite Scores among those with non-zero scores did not differ at any of the three time points nor change differently for the two exposure groups across the three time points.
3.6 12-Step participation
3.6.1 Meeting attendance
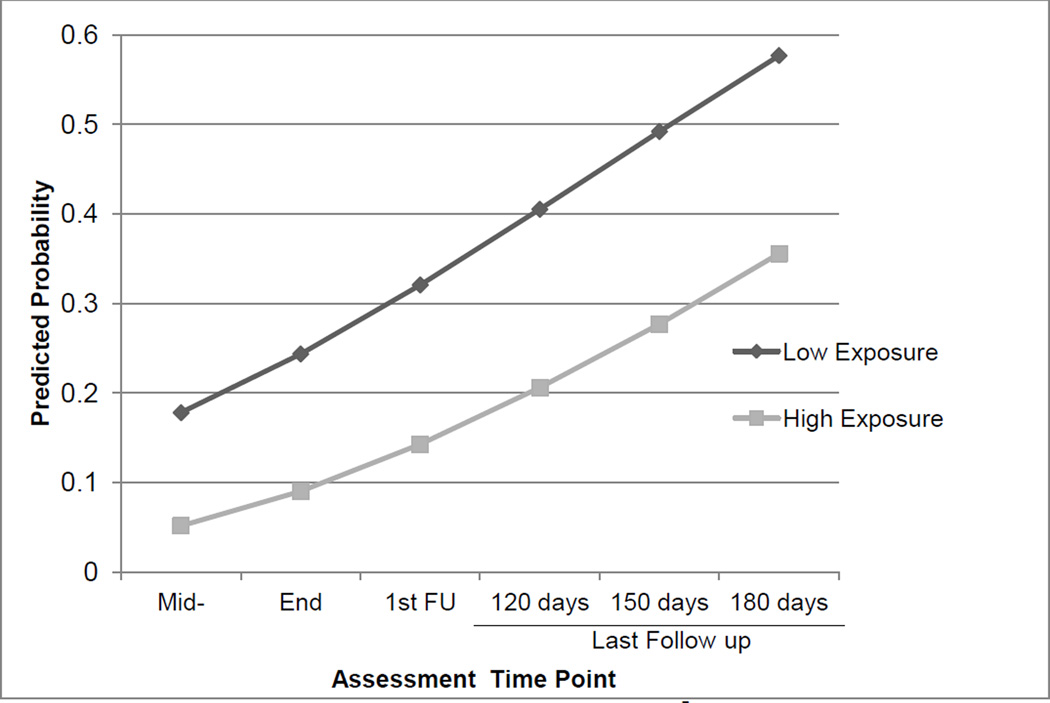
Figure 1 shows the probability of not attending self-help meetings as reported on the Substance use Calendar during each 30-day period for the two exposure groups. Presenting the probability of not attending may seem confusing, but recall that this analysis is carried out in the same way as analysis of days of self-reported substance use. The distribution is zero-inflated, but in this case the zero represents the undesirable outcome (no days of attending), whereas with substance use outcomes, the zero represents abstinence (no days of use). The odds of not attending any self-help meetings were significantly less for high- than for low-exposure participants at all six assessments. The rate of attending (number of days of meeting attendance among those with non-zero attendance) differed significantly between the two groups at mid-treatment (average predicted days for high exposure = 14.44, for low exposure = 7.91), end-of-treatment (high = 12.80, low = 7.68), and the first (90-day) follow-up (high = 11.22, low = 7.33) but was not significant at later 30-day intervals.
Figure 1.
Model-based average predicted probability of NOT attending self-help meetings, STAGE-12 high exposure versus low exposure.
3.6.2 Mutual support activities
The number of days speaking at a 12-Step meeting did not differ at any time point between exposure groups. However, those with high exposure reported more days of performing duties at meetings. This difference was significant from baseline through the first (90-day) follow-up but not at the 180-day follow-up. There was a significant time effect (t191 = 2.66, p = .008) but no significant Time by Exposure Status interaction. This indicates that both exposure groups increased performing duties over time but the two groups did not increase at a differential rate.
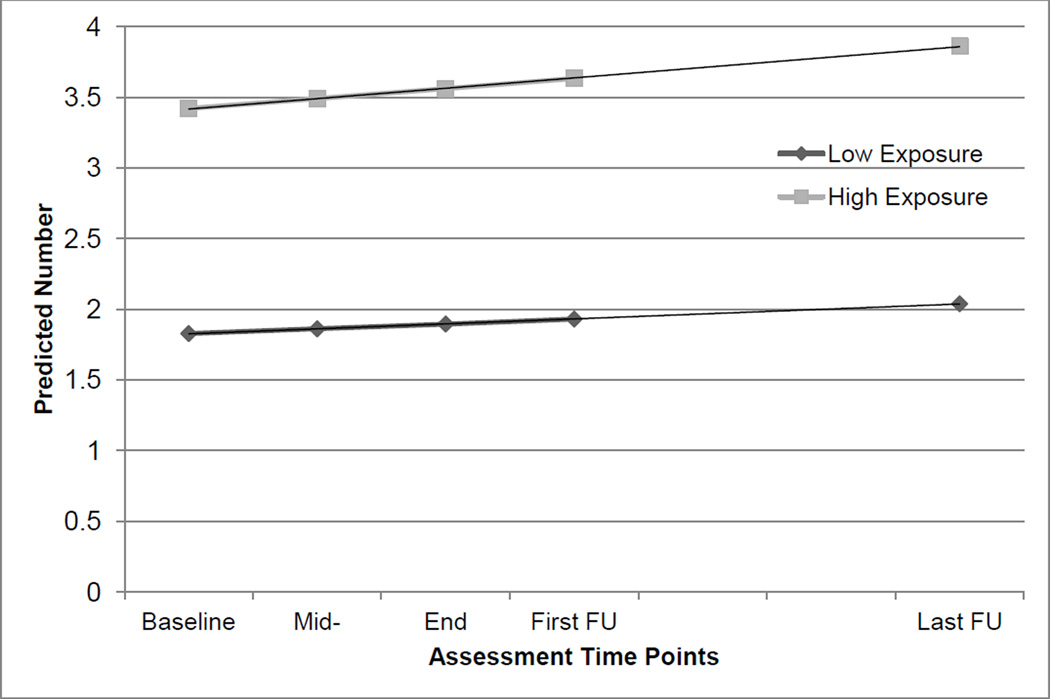
The model-based average predicted number of other 12-Step-related activities is shown in Figure 2. The two exposure groups differed significantly at every time point, including baseline, indicating that those with high exposure began the study with greater meeting involvement. There was not a significant time effect, nor was there a significant time by exposure interaction. The initial difference between those who met optimal exposure criteria and those who did not was simply maintained.
Figure 2.
Model-based average predicted number of other self-help activities, STAGE-12 high exposure versus low exposure.
4 Discussion
4.1 Results Summary
Although outpatient SUD treatment is thought to be characterized by high drop-out rates and low rates of treatment completion, exposure to STAGE-12 treatment was relatively high for this 8-session intervention embedded within intensive outpatient treatment as usual. Over two thirds of patients met criteria for high exposure to the STAGE-12 intervention, and there was relatively low early attrition; only 8 percent failed to attend any sessions. These results compare favorably with other studies of short-term outpatient treatment with stimulant users. For example, Aharonovich (2006) and colleagues reported that 28% of cocaine users completed treatment (in treatment at least 12 weeks and missing no more than 2 consecutive weeks). Similarly, Covi and colleagues (Covi, Hess, Schroeder & Preston, 2002) found that 33.8% of cocaine users completed 12 weeks of individually delivered CBT, regardless of whether they were assigned to attend twice weekly, weekly, or bi-weekly sessions. However, the experimental intervention in this study required somewhat less of participants than was required in those studies. In some CTPs concurrent TAU was about the same length as STAGE-12, but in others it was quite a bit longer. Because the current study did not gather data on attendance at concurrent TAU, it is not possible to determine how either length or attendance at TAU correlated with STAGE-12 exposure or how TAU attendance affected outcome.
Patient satisfaction with treatment is one possible basis for high levels of exposure. Indeed, in this study, high exposure participants rated the STAGE-12 intervention as more beneficial and they reported greater overall satisfaction with it than did those who attended less. A particular source of this satisfaction was not identified, as high-exposure participants rated all 8 program elements (counselor, group meetings, individual sessions, better understanding of 12-Step programs, assignments/recovery tasks, encouragement to get involved in 12-Step, arrange to have outside 12-Step member help, and attend 12-Step meetings in the community) as helpful. Greater exposure to the treatment could result in patients’ perceiving more benefit. For clinicians, early patient dissatisfaction with treatment might serve as a warning signal for drop-out, and addressing it could lead to improved treatment retention and outcome.
Regarding both primary and secondary outcomes, those achieving high exposure to STAGE-12 compared with those with less exposure, demonstrated: (1) higher odds of self-reported abstinence from and lower rates of stimulant drug use; (2) lower probabilities of stimulant positive urines; (3) higher odds of self-reported abstinence from and lower rates of non-stimulant drug use; (4) lower odds of not attending and higher rates (days) of attending 12-Step self-help groups; (5) greater likelihood of reporting no drug problems; (6) a greater maximum number of days of self-reported duties at meetings; and (7) more types of other 12-Step activities engaged in during 30 day assessment windows. Most of these differences declined over time from early treatment to 180-day follow-up, but two (attending any versus no 12-Step meetings, and active involvement in 12-Step activities) were still significant by the last follow-up visit. The latter difference represented maintenance of a disparity present at baseline. It is of note in this context that the MAAEZ 12-step facilitative intervention had its greatest effect in the subgroup of individuals with more prior 12-Step meeting exposure and the only factor found to mediate positive outcomes for the entire sample was doing service across the 6- and 12-month post-treatment follow-up period (Subbaraman, Kaskutas & Zemore, 2011).
As discussed under “limitations” below, it cannot be concluded that STAGE-12 exposure produced these better short-term outcomes, as the reverse could very well be true. Individuals struggling with abstinence during treatment are known by clinicians to be less likely to comply with treatment. In addition, continuing to use drugs after entering treatment is likely to influence the degree to which a person is a reliable attender or active participant in self-help meetings. This latter point is also consistent with previous findings that lower levels of patient satisfaction are associated with and may be a marker of greater substance use within the active phase of a number of different psychosocial therapies, including TSF (Donovan, Kadden, DiClemente, & Carroll, 2002).
As with the findings regarding treatment satisfaction, one or more additional factors could account for both compliance and outcome. Measures of pretreatment readiness to change and confidence in ability to avoid using drugs were examined as possible alternate explanations. Readiness to change was not associated with exposure to STAGE-12, and confidence did not account for the association between exposure level and substance use outcomes.
Participants who attended the STAGE-12 intervention at a high level showed evidence at the study outset of greater 12-Step involvement, and to a great extent, they maintained a higher rate of involvement over time. From the primary outcome analysis for this study (Donovan et al., 2013), as a group, STAGE-12 participants had greater odds of self-reported abstinence but also higher rates of during-treatment drug use among those not abstinent than among those not randomized to STAGE-12. The current analysis indicates that the greater degree of abstinence is likely accounted for by high exposure STAGE-12 participants. Together, both analyses suggest that STAGE-12 influences treatment outcome by supporting already existing 12-Step meeting involvement over time. Unlike the STAGE-12 – TAU comparison in the primary outcome paper, STAGE-12 high attenders who were not abstinent did not show higher rates of drug use than those with lower attendance. This suggests that perhaps the higher rates of during-treatment drug use for STAGE-12 versus TAU found in the primary outcome paper may be driven by those with lower exposure to the intervention.
4.2 Comparison of Findings to Previous Studies
This study is consistent with findings of previous trials of interventions seeking to increase utilization of 12-Step meetings, demonstrating that higher “doses” of 12-Step-oriented interventions are related to improved 12-Step attendance and participation. For example, Timko and colleagues (2011) reported that the number of intensive referral sessions attended was associated with attending mutual support meetings, greater readiness to attend dual-focus (for co-occurring disorders) meetings, and greater involvement in substance-focus meetings over six months following intervention. Timko et al. (2011) did not report the association between intervention dose and substance use outcome; however STAGE-12 findings are consistent with those of other studies that have found associations between dose of 12-Step oriented therapies and substance use outcome. For example, Kaskutas and colleagues (2009) found a positive linear association between dose of MAAEZ and self-reported abstinence at 12 month follow-up. The current study replicates the finding of an association between 12-Step oriented treatment exposure and both 12-Step involvement and substance use outcome; but the associations were longer-lasting in both the Timko et al. (2011) and Kaskutas et al. (2009) studies. This difference could be a function of differences in setting, clinicians, patients, or the interventions.
4.3 Limitations
This analysis examined only individuals initiating treatment in CTN community treatment programs (CTPs) who volunteered to participate in a randomized study of STAGE-12 versus TAU. These CTPs may represent a select set of programs in that they have agreed to ongoing involvement in research and may have more readiness to implement treatment innovations than do other programs. However, as recounted in Potter et al. (2011), the selection process for the STAGE-12 study involved purposefully recruiting three research-naïve sites from among the many programs with membership in the CTN. “The inclusion of such research naïve sites…increased the generalizability of findings to ‘real life’ community programs rather than only to large, research intensive sites.” (Potter et al., 2011, p. 406). Likewise, patients who volunteer for such a study may be more interested in facilitation of their involvement in 12-Step programs or may be generally more motivated to do well in treatment. Thus, the results likely do not apply equally to all patients in all types of programs. However, similar limitations can be applied to most SUD treatment studies, and every effort was made in these clinics to recruit all potentially eligible patients. The proportion of women in the sample, 62%, is not typical of SUD treatment. The gender distribution has varied considerably across CTN studies, and, within studies, across sites. Korte and colleagues (Korte, Rosa, Wakim, & Perl, 2011) examined gender differences in treatment exposure across 24 completed CTN trials, including the STAGE-12 study, and found no effect of gender on exposure. Within the STAGE-12 trial, there was also no gender difference.
Only those assigned to the STAGE-12 arm are included in these analyses, so differences between levels of exposure can only be generalized to this or very similar interventions. Within the STAGE-12 condition, participants self-selected into exposure groups, and more of those with low exposure failed to return for follow-up assessment. This resulted in underrepresentation of low exposure patients in these analyses. It is not known what effect this might have on the results. In general, loss to follow-up may have biased these findings.
Because of a lack of comparable attendance data for those assigned to TAU in this study, it is not possible to examine interactions between condition assignment and intervention exposure, and we cannot make causal attributions about the association between exposure and outcome. The association must be investigated further, using different designs.
Two additional limitations, common to a number of SUD treatment studies, are a reliance on self-report for most outcomes and limited use of urine drug screens to validate this report. These screens were done at a limited number of assessment points, known in advance to the participants. Regarding these two limitations, all self-report and urine screen data were kept confidential; they were not reported to staff at the treatment programs, and there were no negative consequences for reporting substance use or providing a positive UDS.
4.4 Implications for Treatment
Beginning 12-Step involvement early in treatment has been found to be beneficial (Moos & Moos, 2004). Although a majority of community-based treatment programs either encourage or require 12-Step attendance, few have a standard program for systematically orienting patients to 12-Step involvement and promoting an understanding of 12-Step principles and philosophy as well as attendance and active involvement in meetings (Caldwell, 1999; Humphreys & Moos, 2007; Humphreys et al., 2004). Manual-based interventions, such as STAGE-12, provide programs with a method of standardizing this type of orientation and encouragement. The intervention highlights the potentially powerful benefits of 12-Step involvement, particularly for patients with initial reservations. Furthermore, for those who are hesitant to go to 12 Step meetings, it provides active support for attending a first meeting, in the person of a community volunteer.
The current study demonstrates that it is feasible to interest people entering intensive outpatient treatment in a 12-Step oriented intervention and that individuals who agree to participate can be retained in the intervention at relatively high rates. Note that this is not meant to suggest a higher rate of attendance than TAU. It is important to note, however, in this particular study, the STAGE-12 intervention was embedded within standard TAU for those agreeing to participate and assigned to STAGE-12, mirroring what would likely be the case in programs adopting STAGE-12. Thus, attending STAGE-12 was part of meeting requirements for completing treatment. About 20% of participants were court-mandated to treatment (Donovan et al., 2013), but others may also have had external pressures related to satisfying program requirements. In addition, no association between being mandated to treatment and level of STAGE-12 exposure was found. Under these conditions, the current study demonstrates feasibility in that when this intervention is part of TAU, patients do attend.
The design of this secondary analysis cannot demonstrate a causal relationship between STAGE-12 exposure and positive outcomes. Studies that experimentally examine differing levels of exposure to treatments of the same type are rare, most likely due to difficulty differentiating non-specific effects of time and attention from specific effects of different doses of a given treatment. In studies employing experimental designs that manipulate psychosocial treatment exposure, the majority have failed to find an effect of exposure (Covi, Hess, Schroeder, & Preston, 2002; Dennis et al., 2004; Kidorf et al., 2013). However, one study that manipulated counseling attendance through contingency management (Brooner et al., 2004) found both better counseling attendance and better treatment response among methadone patients to whom contingencies were applied. What does it mean, then, that treatment exposure and outcomes are associated but that this is generally not reproduced experimentally and is not, in this study, accounted for by patient motivation or abstinence self-efficacy? One interpretation shared by both clinicians and researchers is that both exposure (e.g., sessions attended or weeks retained) and patient behavior outside of treatment (e.g., substance use or 12-Step meeting attendance) are related indications of outcome, or how a patient is doing in treatment. Will simply working to increase attendance result in greater abstinence? Perhaps not, but early missed sessions may signal a patient who is in need of adjustments to the treatment plan. In this analysis, more satisfaction with STAGE-12 was associated with higher exposure. In a separate analysis of STAGE-12 data (Campbell et al., in press), both therapeutic alliance and counselor competence were significantly associated with treatment retention. These results point to a need for attention to counselor training and to ongoing monitoring of the counselor-patient relationship and patient satisfaction.
Among STAGE-12 participants, there was a baseline difference in ASI Alcohol Composite scores, with low attenders more likely to begin treatment with more severe alcohol problems. There is evidence that stimulant users in treatment who have concurrent alcohol problems have a number of other problems that may make them more likely to fail to remain in treatment (Hartzler, Donovan, & Huang, 2011). Assuming again, that retention is one indicator of patient progress, clinicians may find it beneficial to focus greater efforts on those struggling most with alcohol. The declining effects of STAGE-12 exposure across follow-up time points suggest a possible need for booster group or individual sessions that would reinforce lessons learned in the earlier sessions. This clinical approach fits within a framework of chronic disease management in which single episodes of treatment are not expected to have long-lasting effects. These clinical implications are also topics for further research.
4.5 Implications for Future Research
The present analyses demonstrate a clinical advantage associated with attending at least 2 individual and 3 group STAGE-12 sessions. It does not contribute to an understanding of the essential components of the intervention. A two-group randomized trial cannot address this question, but it could be investigated using a multi-phase optimization strategy (MOST) (Collins et al., 2011; Collins, Murphy & Strecher, 2007), a framework for employing factorial designs to identify the most effective intervention modules.
The current study focused specifically on facilitating involvement in 12-Step oriented mutual aid groups (AA, CA, NA, or CMA). It is not clear whether the 12-Step philosophy is an essential component of mutual aid or programs that facilitate involvement in mutual aid. This warrants more research, perhaps examining involvement in alternative mutual aid groups, such as SMART Recovery and Rational Recovery.
A number of programs involved in the CTN trial of STAGE-12 chose to incorporate all or parts of the STAGE-12 intervention into their programs at the conclusion of the trial. Some programs incorporated only the group sessions, while retaining their standard treatment for the individual sessions. Several program directors indicated that implementation of the intensive referral posed the greatest challenge; it necessitated additional coordination with 12-Step volunteers in the community. Research on implementation of 12-Step-oriented treatments would help to identify organizational, staffing, intervention, and policy context variables that act as facilitators or barriers to adoption.
Highlights.
This study examined whether exposure to a 12-Step facilitative therapy is related to treatment outcome among stimulant users.
High exposure patients reported greater rates of abstinence, had fewer days of stimulant use, and lower rates of stimulant-positive urines compare with low-exposure patients.
High-exposure patients were more likely to attend 12-Step meetings and were more active in these meetings than were low-exposure patients.
Acknowledgements
This report was supported by a series of grants from NIDA as part of the Cooperative Agreement on National Drug Abuse Treatment CTN: Appalachian/Tri-States Node (U10DA20036), Florida Node Alliance (U10DA13720), Ohio Valley Node (U10DA13732), Oregon Node (U10DA13036), Pacific Region Node (U10DA13045), Pacific Northwest Node (U10DA13714), Southern Consortium Node (U10DA13727), and Texas Node (U10DA20024). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIDA.
We would like to express our appreciation to the patients and the administrative, clinical, and research staff of the 10 CTPs that participated in the present study: Center for Psychiatric & Chemical Dependency Services, Pittsburgh, PA; ChangePoint, Inc. Portland, OR; Dorchester Alcohol & Drug Commission, Summerville, SC; Evergreen Manor, Everett, WA; Gateway Community Services, Jacksonville, FL; HinaMauka, Kaneohe, HI; Maryhaven, Columbus, OH; Nexus Recovery Center, Dallas, TX; Recovery Centers of King County, Seattle, WA; and Willamette Family Treatment Services, Eugene, OR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug and Alcohol Dependence. 2006;81:313–322. doi: 10.1016/j.drugalcdep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Alcoholics Anonymous. Bridging the gap between treatment and AA through temporary contact programs. New York, NY: Alcoholics Anonymous World Services, Inc; 1991. [Google Scholar]
- Baker S. Twelve step facilitation for drug dependence. New Haven, CT: Psychotherapy Development Center, Department of Psychiatry, Yale University; 1998. [Google Scholar]
- Baker S, Daley DC, Donovan DM, Floyd AS. STAGE-12: Stimulant Abuser Groups to Engage in 12-Step programs: A combined group and individual treatment program, version 3. Unpublished therapy manual. Bethesda, MD: Center for the Clinical Trials Network, National Institute on Drug Abuse; 2009. [Google Scholar]
- Bogenschutz MP, Rice SL, Tonigan JS, Vogel HS, Nowinski J, Hume D, Arenella PB. 12-Step facilitation for the dually diagnosed: A randomized clinical trial. Journal of Substance Abuse Treatment. 2014;46:403–411. doi: 10.1016/j.jsat.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooner RK, Kidorf MS, King VL, Stoller KB, Peirce JM, Bigelow GE, Kolodner K. Behavioral contingencies improve counseling attendance in an adaptive treatment model. Journal of Substance Abuse Treatment. 2004;27:223–232. doi: 10.1016/j.jsat.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Brown TG, Seraganian P, Tremblay J, Annis H. Process and outcome changes with relapse prevention versus 12-step aftercare programs for substance abusers. Addiction. 2002;97:677–689. doi: 10.1046/j.1360-0443.2002.00101.x. [DOI] [PubMed] [Google Scholar]
- Cacciola JS, Alterman AI, McLellan AT, Lin YT, Lynch KG. Initial evidence for the reliability and validity of a “Lite” version of the Addiction Severity Index. Drug and Alcohol Dependence. 2007;87:297–302. doi: 10.1016/j.drugalcdep.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Caldwell PE. Fostering client connections with Alcoholics Anonymous: A framework for social workers in various practice settings. Social Work in Health Care. 1999;28:45–61. doi: 10.1300/J010v28n04_04. [DOI] [PubMed] [Google Scholar]
- Campbell BK, Guydish J, Le T, Wells EA, McCarty D. The relationship of therapeutic alliance and treatment delivery fidelity with treatment retention in a multisite trial of Twelve-Step Facilitation. Psychology of Addictive Behaviors. doi: 10.1037/adb0000008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Ball SA, McCance E, Rounsaville BJ. Treatment of cocaine and alcohol dependence with psychotherapy and disulfiram. Addiction. 1998;93:713–728. doi: 10.1046/j.1360-0443.1998.9357137.x. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Ball SA, McCance E, Frankforter TL, Rounsaville BJ. One-year follow-up of disulfiram and psychotherapy for cocaine-alcohol users: sustained effects of treatment. Addiction. 2000;95:1335–1349. doi: 10.1046/j.1360-0443.2000.95913355.x. [DOI] [PubMed] [Google Scholar]
- Collins LM, Baker TB, Mermelstein RJ, Piper ME, Jorenby DE, Smith SS, Schlam TR, Cook JW, Fiore MC. The multiphase optimization strategy for engineering effective tobacco use interventions. Annals of Behavioral Medicine. 2011;41:208–226. doi: 10.1007/s12160-010-9253-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins LM, Murphy SA, Strecher V. The Multiphase Optimization Strategy (MOST) and the Sequential Multiple Assignment Randomized Trial (SMART): New methods for more potent ehealth interventions. American Journal of Preventive Medicine. 2007;32(5s):S112–S118. doi: 10.1016/j.amepre.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covi L, Hess JM, Schroeder JR, Preston KL. A dose response study of cognitive behavioral therapy in cocaine abusers. Journal of Substance Abuse Treatment. 2002;23:191–197. doi: 10.1016/s0740-5472(02)00247-7. [DOI] [PubMed] [Google Scholar]
- Crits-Christoph P, Siqueland L, Blaine J, Frank A, Luborsky L, Onken LS, Muenz LR, Thase ME, Weiss RD, Gastfriend DR, Woody GE, Barber JP, Butler SF, Daley D, Salloum I, Bishop S, Najavits LM, Lis J, Mercer D, Griffin ML, Moras K, Beck AT. Psychosocial treatments for cocaine dependence: Results of the NIDA Cocaine Collaborative Study. Archives of General Psychiatry. 1999;56:493–502. doi: 10.1001/archpsyc.56.6.493. [DOI] [PubMed] [Google Scholar]
- Daley DC, Baker S, Donovan DM, Hodgkins CG, Perl H. A combined group and Individual 12-Step facilitative intervention targeting stimulant abuse in the NIDA Clinical Trials Network: STAGE-12. Journal of Groups in Addiction & Recovery. 2011;6:228–244. doi: 10.1080/1556035X.2011.597196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Godley SJ, Diamond G, Tims FM, Funk R. The Cannabis Youth Treatment (CYT) Study: Main findings from two randomized trials. Journal of Substance Abuse Treatment. 2004;27:197–213. doi: 10.1016/j.jsat.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Donovan DM, Daley DC, Brigham GS, Hodgkins CC, Perl HI, Floyd AS. How practice and science are balanced and blended in the NIDA Clinical Trials Network: The bidirectional process in the development of the STAGE-12 protocol as an example. American Journal of Drug and Alcohol Abuse. 2011;37:408–416. doi: 10.3109/00952990.2011.596970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan DM, Daley DC, Brigham GS, Hodgkins CC, Perl HI, Garrett SB, Doyle SR, Floyd AS, Knox PC, Botero C, Kelly TM, Killeen TK, Hayes C, Kau’iBaumhofer N, Seamans C, Zammarelli L. Stimulant abuser groups to engage in 12-Step: A multisite trial in the National Institute on Drug Abuse Clinical Trials Network. Journal of Substance Abuse Treatment. 2013;44:103–114. doi: 10.1016/j.jsat.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan DM, Floyd AS. Facilitating involvement in Twelve-Step programs. In: Galanter M, Kaskutas LA, editors. Recent Developments in Alcoholism. Vol. 18. New York: Springer; 2008. pp. 303–320. [DOI] [PubMed] [Google Scholar]
- Donovan DM, Kadden RM, DiClemente CC, Carroll KM. Client satisfaction with three therapies in the treatment of alcohol dependence: Results from Project MATCH. American Journal on Addictions. 2002;11(4):291–307. doi: 10.1080/10550490290088090. [DOI] [PubMed] [Google Scholar]
- Fals-Stewart W, O’Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: Psychometric properties. Journal of Consulting and Clinical Psychology. 2000;68:134–144. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- Fiorentine R, Hillhouse MP. Exploring the additive effects of drug misuse treatment and Twelve Step involvement: does Twelve-Step ideology matter? Substance Use and Misuse. 2000;35:367–397. doi: 10.3109/10826080009147702. [DOI] [PubMed] [Google Scholar]
- Hartzler B, Donovan DM, Huang Z. Rates and influences of alcohol use disorder comorbidity among primary stimulant misusing treatment-seekers: Meta-analytic findings across eight NIDA CTN trials. American Journal of Drug and Alcohol Abuse. 2011;37:460–471. doi: 10.3109/00952990.2011.602995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbe JM. Negative binomial regression. 2nd Ed. New York: Cambridge University Press; 2011. [Google Scholar]
- Hilbe JM. Brief overview on interpreting count model risk ratios: An addendum to Negative Binomial Regression, Cambridge University Press (2008) 2007 See http://courses.statistics.com/count/HILBE_NBR_OVERVIEW_ON_INTERPRETING_RISK_RATIOS.pdf.
- Hudziak JJ, Helzer JE, Wetzel WW, Kessel KB, McGee B, Janca A, Przybeck T. The use of the DSM-III-R Checklist for initial diagnostic assessment. Comprehensive Psychiatry. 1993;34:375–383. doi: 10.1016/0010-440x(93)90061-8. [DOI] [PubMed] [Google Scholar]
- Humphreys K, Moos RH. Encouraging posttreatment self-help group involvement to reduce demand for continuing care services: Two-year clinical and utilization outcomes. Alcoholism: Clinical & Experimental Research. 2007;31:64–68. doi: 10.1111/j.1530-0277.2006.00273.x. [DOI] [PubMed] [Google Scholar]
- Humphreys K, Wing S, McCarty D, Chappel J, Gallant L, Haberle B, Horvath AT, Kaskutas LA, Kirk T, Kivlahan D, Laudet A, McCrady BS, McLellan AT, Morgenstern J, Townsend M, Weiss R. Self-help organizations for alcohol and drug problems: Toward evidence-based practice and policy. Journal of Substance Abuse Treatment. 2004;26:151–158. doi: 10.1016/S0740-5472(03)00212-5. (SAMHSA/Veterans Health Administration Workgroup on Substance Abuse Self-Help Organizations) [DOI] [PubMed] [Google Scholar]
- Kaskutas LA. Alcoholics anonymous effectiveness: Faith meets science. Journal of Addictive Diseases. 2009;28:145–57. doi: 10.1080/10550880902772464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaskutas LA, Subbaraman MS, Witbrodt J, Zemore SE. Effectiveness of making Alcoholics Anonymous easier: A group format 12-step facilitation approach. Journal of Substance Abuse Treatment. 2009;37:228–339. doi: 10.1016/j.jsat.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidorf M, Brooner RK, Gandotra N, Antoine D, King VL, Peirce J, Ghazarian S. Reinforcing integrated psychiatric service attendance in an opioid-agonist program: A randomized and controlled trial. Drug and Alcohol Dependence. 2013;133:30–36. doi: 10.1016/j.drugalcdep.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingree JB, Simpson A, Thompson M, McCrady BS, Tonigan JS. The predictive validity of the Survey of Readiness for Alcoholics Anonymous participation. Journal of Studies on Alcohol and Drugs. 2007;68:141–148. doi: 10.15288/jsad.2007.68.141. [DOI] [PubMed] [Google Scholar]
- Kingree JB, Simpson A, Thompson M, McCrady BS, Tonigan JS, Lautenshlager G. The development and initial evaluation of the Survey of Readiness for Alcoholics Anonymous Participation. Psychology of Addictive Behaviors. 2006;20:453–462. doi: 10.1037/0893-164X.20.4.453. [DOI] [PubMed] [Google Scholar]
- Korte JE, Rosa CL, Wakim PG, Perl HI. Addiction treatment trials: How gender, race/ethnicity, and age relate to ongoing participation and retention in clinical trials. Substance Abuse and Rehabilitation. 2011;2:205–218. doi: 10.2147/SAR.S23796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude-Griffin PM, Hohenstein JM, Humfleet GL, Reilly PM, Tusel DJ, Hall SM. Superior efficacy of cognitive-behavioral therapy for urban crack cocaine abusers: Main and matching effects. Journal of Consulting and Clinical Psychology. 1998;66:832–837. doi: 10.1037//0022-006x.66.5.832. [DOI] [PubMed] [Google Scholar]
- Miller WR, Tonigan JS. Assessing drinkers’ motivation for change: The Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES) Psychology of Addictive Behaviors. 1996;10(2):81–89. [Google Scholar]
- Moos RH, Moos BS. Long-term influence of duration and frequency of participation in alcoholics anonymous on inidividuals with alcohol use disorders. Journal of Consulting and Clinical Psychology. 2004;72:81–90. doi: 10.1037/0022-006X.72.1.81. [DOI] [PubMed] [Google Scholar]
- Potter JS, Donovan DM, Weiss RD, Gardin J, Lindblad R, Wakim P, Dodd D. Site selection in community-based clinical trials for substance use disorders: Strategies for effective site selection. The American Journal of Drug and Alcohol Abuse. 2011;37:400–407. doi: 10.3109/00952990.2011.596975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Project Match Research Group. Matching alcoholism treatments to client heterogeneity: Project MATCH posttreatment drinking outcomes. Journal of Studies on Alcohol. 1997;58:7–29. [PubMed] [Google Scholar]
- SAS Institute, Inc. SAS/STAT 9.3 User’s Guide. Cary, NC: SAS Institute Inc; 2010. [Google Scholar]
- Sklar SM, Turner NE. A brief measure for the assessment of coping self-efficacy among alcohol and other drug users. Addiction. 1999;94:723–729. doi: 10.1046/j.1360-0443.1999.94572310.x. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline followback: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: Psychosocial and biological methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Subbaraman MS, Kaskutas LA, Zemore S. Sponsorship and service as mediators of the effects of Making Alcoholics Anonymous Easier (MAAEZ), a 12-step facilitation intervention. Drug and Alcohol Dependence. 2011;116(1–3):117–124. doi: 10.1016/j.drugalcdep.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timko C, DeBenedetti A. A randomized controlled trial of intensive referral to 12-step self-help groups: One-year outcomes. Drug and Alcohol Dependence. 2007;90:270–279. doi: 10.1016/j.drugalcdep.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Timko C, DeBenedetti A, Billow R. Intensive referral to 12-Step self-help groups and 6-month substance use disorder outcomes. Addiction. 2006;101:678–688. doi: 10.1111/j.1360-0443.2006.01391.x. [DOI] [PubMed] [Google Scholar]
- Timko C, Sutkowi A, Cronkite RC, Makin-Byrd K, Moos RH. Intensive referral to 12-step dual-focused mutual-help groups. Drug and Alcohol Dependence. 2011;118:194–201. doi: 10.1016/j.drugalcdep.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Walitzer KS, Dermen KH, Barrick C. Facilitating involvement in Alcoholics Anonymous during out-patient treatment: A randomized clinical trial. Addiction. 2009;104:391–401. doi: 10.1111/j.1360-0443.2008.02467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Griffin ML, Najavits LM, Hufford C, Kogan J, Thompson HJ, Siqueland L. Self-help activities in cocaine dependent patients entering treatment: Results from NIDA Collaborative Cocaine Treatment Study. Drug and Alcohol Dependence. 1996;43:79–86. doi: 10.1016/s0376-8716(96)01292-6. [DOI] [PubMed] [Google Scholar]
- Wells EA, Peterson PL, Gainey RR, Hawkins JD, Catalano RF. Outpatient treatment for cocaine abuse: A controlled comparison of relapse prevention and twelve step approaches. American Journal of Drug and Alcohol Abuse. 1994;20:1–17. doi: 10.3109/00952999409084053. [DOI] [PubMed] [Google Scholar]