Abstract
Background
Thirty percent of chronically transfused patients with sickle cell disease (SCD) become alloimmunized. Antigen-matching reduces alloimmunization. Matching may be prospective (for all patients) or based on history (only for patients with an alloimmunization history). We assessed the clinical and financial value of a potential assay to identify at-risk patients to guide antigen-matching.
Methods
A Markov-based model evaluated direct medical costs and alloimmunization events over 10 to 20 years for a cohort of initially transfusion-naïve patients with SCD receiving simple or exchange transfusion. Four strategies were evaluated: prospective matching, history-based matching, perfectly-informed matching (assay with 100% sensitivity, 100% specificity), and imperfectly-informed matching (reduced accuracy). RBCs were matched for C, E, K and any additional alloantibodies present. A hospital perspective was adopted, with costs (2012US$) and events discounted (3%).
Results
Perfectly-informed antigen-matching using a $1000 assay is expected to save $82,334 per patient over 10 years, as compared to prospective matching. Perfectly-informed antigen-matching is more costly than history-based matching, but reduces alloimmunization events by 45.6% over 10 years. Averting each alloimmunization event using perfectly-informed matching would cost an additional $10,934 per patient. Imperfectly-informed antigen-matching using an assay with 75% specificity and 75% sensitivity is less costly than prospective matching, but increases alloimmunization events. Compared to history-based matching, imperfectly-informed matching would decrease alloimmunization events by 32.61%, at an additional cost of $147,915 per patient over 10 years. Cost-effectiveness of informed antigen-matching is largely driven by assay specificity.
Conclusions
A sufficiently specific assay to inform antigen-matching may be cost-effective in reducing alloimmunization among transfused patients with SCD.
Keywords: sickle cell, alloimmunization, assay, economic evaluation, cost-effectiveness, antigen-matching
Introduction
Patients with sickle cell disease (SCD) frequently rely on chronic red blood cell (RBC) transfusion for disease management. Unfortunately, RBC transfusion among these patients may result in alloimmunization, defined by the development of alloantibodies directed against donor RBC antigens.1 This immune response may be partly explained by racial antigenic differences between patients with SCD and the blood donor population; patients with SCD are predominantly of African descent, while blood donors are often white.2,3 Transfusion using prophylactically antigen-matched blood has been shown to help avert alloimmunization and associated hemolytic transfusion reactions.4–7
Approximately 30% of transfused patients with SCD are likely to become alloimmunized,1,8–11 but there is no existing method to prospectively identify these patients.1,12 Thus, transfusion services are not currently able to determine which transfusion patients are at risk of alloimmunization and would benefit from receiving prophylactically-matched blood. Guidelines detailing the optimal methods to address alloimmunization among chronically transfused patients with SCD have not yet been established, but a preliminary report by an expert panel convened by the National Institutes of Health (NIH) recently identified knowledge gaps in the transfusion management of patients with SCD, highlighting the need for efficacy and cost-effectiveness evaluations of antigen-matching strategies to reduce alloimmunization among these patients.13 Currently, some transfusion services prophylactically match blood for all transfused patients with SCD, while others match blood only for those patients who have already developed alloantibodies.11 In addition, while some transfusion services consider a broad range of antigens when matching blood, others focus on a limited set, considering only the most commonly implicated antigens (C, E, K).
A recent cost-effectiveness analysis suggested that while prospectively providing C, E, K antigen-matched blood to all transfused patients is expected to yield fewer alloimmunization events than providing antigen-matched blood only to those patients with a history of alloimmunization, this prospective matching is extremely costly; averting a single alloimmunization event using prospective C, E, K matching is expected to cost between $369,482 and $769,284.14 Comparison of the health and financial impact of alternative antigen-matching strategies suggested that providing blood matched for a limited set of antigens only to those patients with a history of alloimmunization is likely to be the most valuable strategy for a transfusion service. If, however, a screening test were available to effectively identify those transfused patients with SCD likely to develop alloantibodies, the optimal strategy may be to use the results of such a test to determine which patients should receive prophylactically C, E, K antigen-matched blood. In the context of an available test, the most cost-effective strategy would depend upon the cost of the test, as well as its sensitivity and specificity. A strategy based upon a sufficiently inexpensive and relatively accurate test would likely be preferable to alternative strategies, when accounting for both financial and health impact.
Although such a screening test is not currently available, transfusion medicine has seen a variety of advancements within recent years, and it seems plausible that an appropriate screening test could be developed in the near future. It is important to understand the value for and necessary characteristics of a potential test,15–18 so we undertook an economic evaluation to assess the health and financial implications of an assay for identifying patients with SCD at risk of alloimmunization.
Methods
A Markov-based decision tree was constructed (TreeAge Pro, Williamstown, MA) to compare alloantibody formation and transfusion-related costs associated with alternative C, E, K antigen-matching strategies among chronically transfused patients with SCD (Figure 1A). This model is similar to a previous model constructed to evaluate the cost-effectiveness of antigen matching strategies.14 The evaluated antigen-matching strategies were based either on current hospital practice or on the use of hypothetical screening tests to identify patients likely to become alloimmunized. Strategies based on current hospital practice included prospective antigen-matching for all transfused patients with SCD and antigen-matching only for those patients who had previously developed alloantibodies. Strategies based on a screening test involved provision of prophylactically antigen-matched blood for patients identified by the assay as “responders” – individuals likely to develop alloantibodies to transfused blood. It has previously been reported that only about 30% of transfused patients with SCD are responders.1,9–11 Characteristics of the screening test, including cost, sensitivity, and specificity, were varied to evaluate the implications of a perfect test as well as with alternative imperfect assays.
Figure 1.
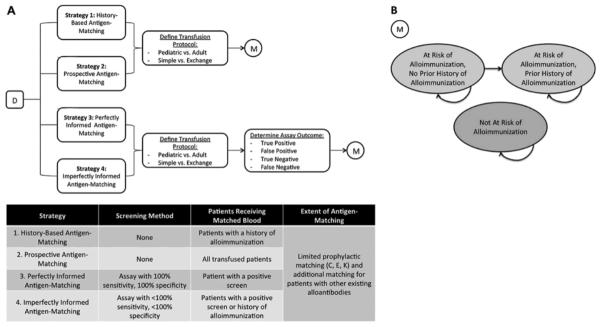
(A). A Markov-based decision tree (D) model was constructed to evaluate alloimmunization events and costs associated with four alternative strategies of antigen-matching among chronically transfusion patients with SCD. Strategy 1, “History-Based Antigen-Matching,” involved providing blood matched for CEK and any other developed alloantibodies only to patients with a history of alloimmunization. Strategy 2 characterized “Prospective Antigen-Matching,” where all transfused patients received prophylactically C, E, K matched blood. Strategy 3, “Perfectly Informed Antigen-Matching,” was based upon a hypothetical assay with 100% sensitivity and 100% specificity. Patients screening positive would prophylactically receive C, E, K antigen-matched blood. Strategy 4 characterized “Imperfectly Informed Antigen-Matching,” where a screening assay with <100% sensitivity and <100% specificity was used to determine which patients prophylactically received C, E, K matched blood. Patients who had not been identified by the imperfect test but developed alloantibodies would also receive antigen-matched blood after the initial alloimmunization event. Outcomes were evaluated for a cohort of initially transfusion-naïve patients with SCD. A series of chance nodes were used to distinguish between patients with different health-related and financial characteristics. For each strategy evaluated, possible transfusion protocols (Pediatric Simple, Pediatric Exchange, Adult Simple, or Adult Exchange) were distinguished. Each protocol was associated with a different number of units transfused and thus a different annual alloimmunization rate. For Strategies 3 and 4, chance nodes were used to distinguish between alternative assay outcomes (True Positive, False Positive, True Negative, or False Negative). (B) For each path along the decision tree, a Markov model (M) was used to track outcomes over a series of 1 year cycles of chronic transfusion therapy. The model consisted of three nodes, “At Risk of Alloimmunization, No Prior History of Alloimmunization,” “At Risk of Alloimmunization, Prior History of Alloimmunization,” and “Not At Risk of Alloimmunization.” At the beginning of the simulation, the patients identified as “at-risk” occupied the first node (At Risk of Alloimmunization, No Prior History of Alloimmunization), while all patients not at risk occupied the third node (Not At Risk of Alloimmunization). Transitions between nodes, as denoted by arrows, could occur between each cycle.
Model Structure and Transfusion Procedures
Markov models have been used extensively to simulate recurring processes,19 and are thus well-suited to describing transfusion therapy. This analysis utilized a simplified version of a previously described model (Figure 1B).14 In brief, we simulated a cohort of male and female patients with SCD undergoing either simple or exchange chronic transfusion therapy. Adult and pediatric (<18 years old) patients were included. At the beginning of the simulation, 30% of patients with SCD were randomly identified as responders. Only these “at-risk” patients had potential to develop alloantibodies and experience delayed hemolytic or serologic transfusion reactions (DHTR/DSTRs). All patients were assumed to begin the simulation without prior history of transfusion or alloimmunization. Rates of alloimmunization among “at-risk” patients were defined per unit transfused, and varied by the extent to which the transfused blood had been antigen-matched. While most at-risk patients formed only one alloantibody during a single transfusion, it was possible for patients to form multiple alloantibodies during a single transfusion. Transfusions, alloimmunization events, and associated costs were tracked over a series of 1-year cycles. Outcomes were evaluated over 10 and 20-year periods.
The simulated population assumed that the age distribution of patients with SCD was equivalent to the age distribution of the US population.20 For model simplicity, we assumed that all patients were transfused monthly (12 transfusion sessions per year). Among pediatric patients, 64.3% received simple transfusion,21 receiving 1 unit per transfusion session, while 35.7% received exchange transfusion,21 receiving 8 units per session. Half of adult patients receiving chronic transfusion underwent simple transfusion, receiving 2 units per session, while the other half of adult patients underwent exchange transfusion, receiving 10 units per session. The number of units transfused per exchange transfusion procedure is an estimation based upon conventional red cell exchanges at our institution, and not isovolemic hemodilution procedures. These values were varied in sensitivity analyses.
All transfused patients were assumed to undergo “initial testing” when beginning chronic transfusion therapy at the start of the simulation and “pre-transfusion testing” before each monthly transfusion. “Initial testing” involved ABO and Rh typing, antibody screening with subsequent antibody identification for positive screens, and 14-antigen phenotyping across all strategies. For strategies incorporating a screening test to identify patients likely to become alloimmunized, “initial testing” also included the screening assay. “Pre-transfusion testing” included an ABO and Rh type and an antibody screen. Patients with negative antibody screens were assumed to receive electronic compatibility testing, while patients with positive antibody screens underwent antibody identification, a direct antiglobulin test, and AHG compatibility testing. Furthermore, adsorption studies were performed if antibody screens suggested autoantibody formation, and an elution was conducted for positive direct antiglobulin tests. Similarly to a previous analysis,14 each of these testing procedures was associated with a cost (Table 1).
Table 1.
Model Input Parameters: Base Case Values and Ranges Used in Sensitivity Analyses
| Input Parameter | Base-Case Value (Range) | Source |
|---|---|---|
| Initial Testing | ||
| Cost: ABO Type | $7.71 (5.78, 9.64) | 25 |
| Cost: Rh Type | $7.71 (5.78, 9.64) | 25 |
| Cost: Antibody Screen | $14.95 (11.21, 18.69) | 25 |
| Cost: Antibody Identification (for patients with positive screen only) | $24.77 (18.58, 30.96) | 25 |
| Cost: Initial RBC Antigen Phenotyping (14-antigen)a | $364 (273, 455) | 35 |
| Cost: Perfect Screening Assay | $1000 (100,5000) | Assumed |
| Sensitivity: Perfect Screening Assay | 100% | Assumed |
| Specificity: Perfect Screening Assay | 100% | Assumed |
| Cost: Imperfect Screening Assay | $1000 (100,5000) | Assumed |
| Sensitivity: Imperfect Screening Assay | 75% (40–100) | Assumed |
| Specificity: Imperfect Screening Assay | 75% (40–100) | Assumed |
| Pre-transfusion Testing/Matching | ||
| Cost: Leukoreduced RBC unit | $198.87 (149.15, 248.59) | 24 |
| Cost: ABO Type | $7.71 (5.78, 9.64) | 25 |
| Cost: Rh Type | $7.71 (5.78, 9.64) | 25 |
| Cost: Antibody Screen | $14.95 (11.21, 18.69) | 25 |
| Cost: Antibody Identification (for patients with positive screen only) | $24.77 (18.58, 30.96) | 25 |
| Cost: Direct Antiglobulin Test (for patients with positive screen only) | $7.71 (5.78, 9.64) | 25 |
| Cost: Elution (for patients with positive DAT only) | $24.77 (18.58, 30.96) | 25 |
| Cost: Adsorption Study (for patients with positive screen indicating AutoAB only)b | $24.77 (18.58, 30.96) | 25 |
| Cost: Electronic Compatibility Testing (for patients with negative screen) | $14.95 (11.21, 18.69) | 25 |
| Cost: AHG Compatibility Testing (for patients with positive screen) | $24.77 (18.58, 30.96) | 25 |
| Cost: Negative Antigens (per antigen negative, per unit) | $80 (60, 100) | 35 |
| Post-transfusion Testing | ||
| Cost: DHTR Hospitalization | $1392.09 (1044.07, 1740.11) | 26 |
| Cost: Antibody Screen | $14.95 (11.21,18.69) | 25 |
| Cost: Antibody Identification (for patients with positive screen only) | $24.77 (18.58,30.96) | 25 |
| Cost: Direct Antiglobulin Test (for patients with positive screen only) | $7.71 (5.78,9.64) | 25 |
| Cost: Elution (for patients with positive DAT only) | $24.77 (18.58,30.96) | 25 |
| Cost: Adsorption Study (for patients with positive screen indicating AutoAB only) | $24.77 (18.58,30.96) | 25 |
| Alloimmunization Rate | ||
| Portion of Patients Experiencing Alloimmunization Risk | 30% (15, 45) | 1,9–11 |
| Matching ABO, D Only (among “responders”, per 100 units transfused) | 3.27 (1–5) | 7,36 |
| Percent Reduction in Alloimmunization Risk from Limited Matching (ABO, D, C, E, K) | 85% (75–95) | 4,6,7 |
| Portion of Patients with Positive DAT (among those with positive screen) | 25% (15–35) | 2 |
| DHTR | ||
| Portion of alloimmunization events leading to DHTRs (Pediatric/Adult) | 17.3/3.4(15–20/1–5) | 8 |
| Patient/Background Characteristics | ||
| Transfusion Sessions per Year for Chronic Therapy | 12 | Assumed |
| Units per Simple Transfusion Session (Pediatric Patients) | 1 (1–3) | Assumed |
| Units per Exchange Transfusion Session (Pediatric Patients) | 8 (6–12) | Assumed |
| Portion of Chronically Transfused Pediatric Patients Undergoing Exchange Transfusion | 64.3% (40–80) | 21 |
| Units per Simple Transfusion Session (Adult Patients) | 2 (2–4) | Assumed |
| Units per Exchange Transfusion Session (Adult Patients) | 10 (8–14) | Assumed |
| Portion of Chronically Transfused Adult Patients Undergoing Simple Transfusion | 50% (40–60) | 5 |
Antigen-Matching Strategies
Four antigen-matching strategies were defined. At a minimum, across all strategies, transfused RBC units were HbS-negative, leukocyte-reduced, and matched for ABO and D. Under all four strategies, when a unit was prophylactically antigen-matched, only antigens with the most frequently clinically-relevant antibodies (C, E, K) were considered. Any additional antigens (other than C, E, or K) against which alloantibodies had been developed were also considered.
The first strategy (Strategy 1) characterized “history-based antigen-matching”: only those patients who had previously developed alloantibodies would receive matched blood for C, E, K and any alloantibodies that had developed. The second strategy (Strategy 2) characterized “prospective antigen-matching”: all patients would receive C, E, K antigen-matched blood, regardless of alloimmunization history.
The second two strategies characterized informed antigen-matching. Under these strategies, antigen-matching would be based on results of a screening test performed before starting chronic transfusion therapy. Strategy 3 described “perfectly informed antigen-matching”: all patients would be screened using an assay with 100% sensitivity and 100% specificity, and patients identified as “at-risk” by the test would prophylactically receive C, E, K matched blood. Strategy 4 described “imperfectly informed antigen-matching,” where the screening assay had <100% sensitivity and <100% specificity. While using the perfect test would ensure that all patients at risk of alloimmunization prophylactically received C, E, K antigen-matched blood, using the imperfect test would unnecessarily provide C, E, K antigen-matched blood to individuals falsely testing positive and would miss the opportunity to avoid alloimmunization among some “at-risk” individuals who falsely tested negative. Under Strategy 4, patients who had not initially tested positive during the screen but who developed alloantibodies at some point during chronic transfusion therapy (false negative test) would also receive antigen-matched units after alloimmunization had been detected.
Distributions of red cell antigen phenotypes among patients with SCD were defined22 and used to determine the necessary characteristics and cost of appropriate antigen-matched units. Although C, E, and K would be considered in an antigen-match, costs of matching would be incurred only when the matched unit was selected to be negative for one of these antigens. Selected antigen-matched units would also be negative for any additional antigens against which alloantibodies had been formed. It was assumed that the appropriately matched units were available, and that the cost per negative antigen was $80.
Screening Test
Since no screening test currently exists, the analysis evaluated outcomes associated with a variety of assays, ranging in cost, sensitivity, and specificity. A perfect test (100% sensitivity and 100% specificity) was defined, along with alternative imperfect tests defined with suboptimal sensitivity and specificity. In the base-case scenario, the imperfect test was defined with 75% sensitivity and 75% specificity. Assays (both perfect and imperfect) were assumed to cost $1000 per test in the base-case scenario, since the assay would likely be a molecular test. Assay costs were varied from $100 to $5000 in sensitivity analyses. Sensitivity and specificity of the imperfect test were each varied from 40 to 100%.
Input Parameters
The cohort was tracked through the simulation, as individuals experienced transfusion-related events and accumulated associated expenses, which were discounted to the beginning of the simulation and expressed in 2012US$. As recommended by the Panel on Cost-effectiveness in Health and Medicine23, both costs and events were discounted at a rate of 3% per year. The analysis focused on the perspective of a hospital transfusion service, and included direct medical expenses only, estimated by 2012 Medicare reimbursement rates.24,25 The cost associated with a DHTR was approximated by previously reported hospital expenses for patients with SCD presenting with painful crises.26 Input parameters, with base-case values and ranges used in sensitivity analyses, are provided in Table 1.
Rates of alloimmunization among chronically transfused patients with SCD in the absence of antigen-matching were drawn from existing literature, and patients undergoing simple transfusion were assumed to face the same per-unit alloimmunization risk as patients undergoing exchange transfusion. The reported efficacy of antigen-matching in reducing alloimmunization and associated DHTRs/DSTRs varies widely. We assumed that when antigen-matched RBCs were not provided, 30% of patients with SCD (responders) would ultimately develop alloantibodies11 at a rate of 3.27 alloantibodies per 100 units transfused.7 Limited (3-antigen C, E, and K) matching was assumed to reduce alloimmunization events by 85%4,6,7 among these 30% of patients, but have no effect on alloimmunization among the remaining 70% of patients, as these patients are not responders and would not be at risk of developing alloantibodies (Table 1).
Analysis
Under the base-case scenario, strategies were analyzed over 10 and 20 year periods to estimate outcomes over a mid-range and a long-range time period. The portion of alloimmunized patients, in addition to transfusion costs and alloimmunization events per patient, were reported for each strategy. The cost to avert each alloimmunization event, using a common reference strategy of “history-based antigen-matching” (Strategy 1), was calculated. Extensive sensitivity analyses were performed to evaluate the impact of variation in test sensitivity and specificity, test cost, the efficacy of antigen-matching in reducing alloimmunization risk, and the portion of individuals likely to become alloimmunized. Costs were varied by 25% in either direction. Efficacy estimates and incidence rates were varied according to the 95% CI reported by the original data source wherever possible.
Results
Under base-case assumptions, using a perfect screening assay (Strategy 3) with a cost of $1000 per test to identify patients likely to become alloimmunized is expected to be cost-saving compared to prospective C, E, K matching for all patients (Strategy 2) (Table 2). Over a 10 year period, perfectly informed C, E, K antigen-matching (Strategy 3) is expected to cost $82,334 less per patient than prospective C, E, K matching for all patients (Strategy 2), but result in a similar quantity of alloimmunized patients, alloimmunization events, and DHTRs. Over a 20 year period, this cost savings increases to $144,343 per patient. One-way sensitivity analysis varying the cost of the assay suggested that a perfect test would remain cost saving over 20 years while the cost per test was $144,500 or less.
Table 2.
Base Case Results: Costs and Alloimmunization Events Associated with Alternative Antigen-Matching Strategies
| Patients Alloimmunized (%)a | Cost Per Patient ($) |
Alloimmunization Events Per Patient |
Cost Per Alloimmunization Event Averted ($, vs History-Based Matching)d | |||||
|---|---|---|---|---|---|---|---|---|
| Average Cost | Comparison vs History-Based Matchingb | Comparison vs Prospective Matchingc | Average No. Events | Comparison vs History-Based Matchingb | Comparison vs Prospective Matchingc | |||
| 10 Years | ||||||||
| Strategy 1: History-Based Antigen-Matching | 30 | 162,623 | -- | −84,578 | 0.46 | -- | 0.21 | -- |
| Strategy 2: Prospective Antigen-Matching | 20 | 247,201 | 84,578 | -- | 0.25 | −0.21 | -- | 412,132 |
| Strategy 3: Perfectly Informed Antigen-Matching | 20 | 164,866 | 2,244 | −82,334 | 0.25 | −0.21 | 0.00 | 10,934 |
| Strategy 4: Imperfectly Informed Antigen-Matching | ||||||||
| Test Sensitivity = 75%, Specificity = 75%, Cost = $1000 | 22 | 185,389 | 22,767 | −61,812 | 0.31 | −0.15 | 0.06 | 147,915 |
| Test Sensitivity = 75%, Specificity = 100%, Cost = $1000 | 22 | 164,556 | 1,933 | −82,645 | 0.31 | −0.15 | 0.06 | 12,558 |
| Test Sensitivity = 100%, Specificity = 75%, Cost = $1000 | 20 | 185,700 | 23,077 | −61,501 | 0.25 | −0.21 | 0.00 | 112,452 |
| 20 Years | ||||||||
| Strategy 1: History-Based Antigen-Matching | 30 | 287,313 | −145,752 | 0.60 | 0.19 | |||
| Strategy 2: Prospective Antigen-Matching | 26 | 433,064 | 145,752 | -- | 0.41 | −0.19 | -- | 759,799 |
| Strategy 3: Perfectly Informed Antigen-Matching | 26 | 288,721 | 1,409 | −144,343 | 0.41 | −0.19 | 0.00 | 7,344 |
| Strategy 4: Imperfectly Informed Antigen-Matching | ||||||||
| Test Sensitivity = 75%, Specificity = 75%, Cost = $1000 | 27 | 324,955 | 37,642 | −108,109 | 0.46 | −0.14 | 0.05 | 261,638 |
| Test Sensitivity = 75%, Specificity = 100%, Cost = $1000 | 27 | 288,619 | 1,307 | −144,445 | 0.46 | −0.14 | 0.05 | 9,082 |
| Test Sensitivity = 100%, Specificity = 75%, Cost = $1000 | 26 | 325,057 | 37,745 | −108,007 | 0.41 | −0.19 | 0.00 | 196,761 |
Portion of patients alloimmunized reflects the portion of patients in the cohort modeled who are expected to experience an alloimmunization event during the analysis period (10 or 20 years).
Comparison versus Strategy 1 (History-Based Antigen-Matching). Positive values indicate an increase in costs or alloimmunization events and negative values indicate a decrease in outcomes.
Comparison versus Strategy 2 (Prospective Antigen-Matching). Positive values indicate an increase in costs or alloimmunization events and negative values indicate a decrease in outcomes.
Cost per Alloimmunization Event Averted calculated using the incremental cost and incremental change in alloimmunization events, as compared to History-Based Antigen Matching
Compared to a strategy of history-based antigen-matching (Strategy 1), perfectly informed C, E, K antigen-matching (Strategy 3) is expected to increase per-patient costs over a 10 year period by $2,244 but result in a 10 percentage point reduction (20% vs. 30%) in the portion of alloimmunized patients and 45.65% (0.21/0.46) fewer alloimmunization events. Thus, perfectly informed C, E, K antigen-matching is expected to cost $10,934 per alloimmunization event averted, and remain cost-effective (at a threshold of $50,000 per alloimmunization event averted) while the cost of each test is $9,010 or lower. Over a 20 year period, perfectly informed C, E, K antigen-matching is cost-effective, with an incremental cost of $7,344 per additional alloimmunization event averted.
An C, E, K antigen-matching strategy informed by an imperfect screening assay (Strategy 4) with a sensitivity of 75%, a specificity of 75%, and a cost of $1000 per test is expected to be cost-saving compared to prospective matching for all patients (Strategy 2), but may not be cost-effective compared to a strategy of history-based antigen matching (Strategy 1). Over a 10 year period, imperfectly informed antigen-matching (Strategy 4) is expected to cost $61,812 less per patient than prospective matching for all patients (Strategy 2), but lead to a 2 percentage point increase in the portion of alloimmunized patients (22% vs. 20%) and a 24% (0.06/0.25) increase in alloimmunization events. Compared to history-based antigen-matching, a strategy of imperfectly informed antigen-matching is expected to cost $147,915 per additional alloimmunization event averted. Over a 20 year period, this cost increases to $261,638 per alloimmunization event averted. However, an alternative imperfectly informed antigen-matching strategy using an assay with 75% sensitivity but 100% specificity may be cost-effective, as compared to a strategy of history-based antigen-matching. Over a 10 year period, this strategy would cost $12,558 per alloimmunization event averted.
Varying the sensitivity, specificity, and cost of an imperfect test suggests that cost-effectiveness of imperfectly informed C, E, K antigen-matching is largely driven by specificity (Figure 2). Using a cost-effectiveness threshold of $50,000 per alloimmunization event averted, imperfectly informed antigen-matching using a $1000 assay of greater than 84% sensitivity would be cost-effective as long as specificity was greater than 90% (Table 3). An assay of 50% sensitivity would still be cost-effective, as long as specificity was greater than 94%. Decreasing the assay cost would further reduce these sensitivity and specificity thresholds.
Figure 2. One-Way Sensitivity Analyses: Cost-Effectiveness of Screening Assay to Identify Patients Likely to be Alloimmunized.
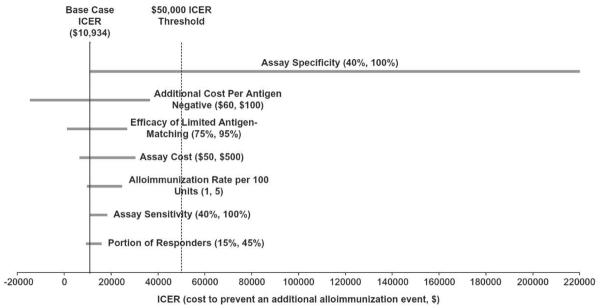
Tornado diagram shows the range of incremental cost-effectiveness ratios (ICERs) associated with variation in selected input variables. Ranges of input parameters are noted in parentheses. In the base case scenario, the ICER is calculated using a strategy of informed antigen-matching using a perfect assay (100% sensitivity, 100% specificity, and cost of $1000 per test), with history-based antigen-matching for C, E, and K as a reference strategy. Solid black line indicates the base-case ICER of $10,934 for each alloimmunization event averted. Variation in the ICER associated with varying a single input parameter is shown by each gray bar. Dashed black line reflects a threshold value of $50,000 per alloimmunization event averted. The extensive range of ICERs corresponding to variation in the assay specificity (40–100%) indicates that the cost-effectiveness of a potential assay is highly dependent on assay specificity.
Table 3.
Two-way Sensitivity Analysis: Incremental Cost-Effectiveness Ratio of Imperfectly Informed Antigen-Matching Varying Sensitivity and Specificity of Assay. Each value in the table reflects the incremental cost to prevent an additional alloimmunization event using a strategy of imperfectly informed antigen-matching with an assay of a particular sensitivity and specificity. History-based antigen-matching was used as a reference strategy. The shaded cells (dark background, white text) represent values less than $50,000 per alloimmunization event averted.
| Specificity (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 80 | 82 | 84 | 86 | 88 | 90 | 92 | 94 | 96 | 98 | 100 | ||
| Sensitivity (%) | 50 | 178,235 | 161,992 | 145,749 | 129,507 | 113,264 | 97,021 | 80,778 | 64,535 | 48,293 | 32,050 | 15,807 |
| 52 | 171,613 | 155,995 | 140,377 | 124,759 | 109,141 | 93,523 | 77,904 | 62,286 | 46,668 | 31,050 | 15,432 | |
| 54 | 165,481 | 150,442 | 135,402 | 120,363 | 105,323 | 90,283 | 75,244 | 60,204 | 45,164 | 30,125 | 15,085 | |
| 56 | 159,788 | 145,285 | 130,783 | 116,280 | 101,778 | 87,275 | 72,773 | 58,270 | 43,768 | 29,265 | 14,763 | |
| 58 | 154,487 | 140,485 | 126,482 | 112,480 | 98,477 | 84,475 | 70,472 | 56,470 | 42,468 | 28,465 | 14,463 | |
| 60 | 149,539 | 136,004 | 122,468 | 108,932 | 95,397 | 81,861 | 68,325 | 54,790 | 41,254 | 27,718 | 14,183 | |
| 62 | 144,911 | 131,812 | 118,713 | 105,614 | 92,515 | 79,416 | 66,317 | 53,218 | 40,119 | 27,020 | 13,921 | |
| 64 | 140,572 | 127,882 | 115,193 | 102,503 | 89,813 | 77,124 | 64,434 | 51,744 | 39,055 | 26,365 | 13,675 | |
| 66 | 136,496 | 124,191 | 111,886 | 99,581 | 87,275 | 74,970 | 62,665 | 50,360 | 38,055 | 25,750 | 13,444 | |
| 68 | 132,660 | 120,716 | 108,773 | 96,830 | 84,887 | 72,943 | 61,000 | 49,057 | 37,114 | 25,171 | 13,227 | |
| 70 | 129,043 | 117,441 | 105,839 | 94,237 | 82,635 | 71,033 | 59,431 | 47,829 | 36,227 | 24,625 | 13,023 | |
| 72 | 125,626 | 114,347 | 103,067 | 91,787 | 80,508 | 69,228 | 57,948 | 46,668 | 35,389 | 24,109 | 12,829 | |
| 74 | 122,395 | 111,420 | 100,445 | 89,470 | 78,495 | 67,521 | 56,546 | 45,571 | 34,596 | 23,621 | 12,646 | |
| 76 | 119,334 | 108,647 | 97,961 | 87,275 | 76,589 | 65,903 | 55,217 | 44,531 | 33,845 | 23,159 | 12,473 | |
| 78 | 116,429 | 106,017 | 95,605 | 85,193 | 74,781 | 64,369 | 53,957 | 43,545 | 33,133 | 22,721 | 12,309 | |
| 80 | 113,670 | 103,518 | 93,366 | 83,215 | 73,063 | 62,911 | 52,759 | 42,608 | 32,456 | 22,304 | 12,152 | |
| 82 | 111,045 | 101,141 | 91,237 | 81,333 | 71,429 | 61,525 | 51,620 | 41,716 | 31,812 | 21,908 | 12,004 | |
| 84 | 108,546 | 98,877 | 89,209 | 79,541 | 69,872 | 60,204 | 50,536 | 40,867 | 31,199 | 21,531 | 11,862 | |
| 86 | 106,162 | 96,719 | 87,275 | 77,832 | 68,388 | 58,945 | 49,501 | 40,058 | 30,614 | 21,171 | 11,727 | |
| 88 | 103,887 | 94,658 | 85,430 | 76,201 | 66,972 | 57,743 | 48,514 | 39,285 | 30,056 | 20,828 | 11,599 | |
| 90 | 101,713 | 92,690 | 83,666 | 74,642 | 65,618 | 56,595 | 47,571 | 38,547 | 29,523 | 20,499 | 11,476 | |
| 92 | 99,634 | 90,806 | 81,979 | 73,151 | 64,324 | 55,496 | 46,668 | 37,841 | 29,013 | 20,186 | 11,358 | |
| 94 | 97,643 | 89,003 | 80,364 | 71,724 | 63,084 | 54,444 | 45,804 | 37,165 | 28,525 | 19,885 | 11,245 | |
| 96 | 95,735 | 87,275 | 78,816 | 70,356 | 61,896 | 53,436 | 44,976 | 36,517 | 28,057 | 19,597 | 11,137 | |
| 98 | 93,905 | 85,618 | 77,331 | 69,044 | 60,757 | 52,469 | 44,182 | 35,895 | 27,608 | 19,321 | 11,034 | |
| 100 | 92,148 | 84,027 | 75,905 | 67,784 | 59,663 | 51,541 | 43,420 | 35,298 | 27 177 | 19,056 | 10,934 | |
In addition to being affected by the sensitivity, specificity, and cost of the assay, incremental cost-effectiveness ratios associated with informed antigen-matching strategies (Strategies 3 and 4) were sensitive to the portion of patients with SCD likely to become alloimmunized, the cost of selecting antigen-negative units, the efficacy of limited antigen-matching to prevent alloimmunization, and the per-unit alloimmunization rate (Figure 2)
Discussion
A recent analysis comparing currently implemented strategies to address alloimmunization among patients with SCD suggested that while prospectively providing C, E, K antigen-matched blood to all transfused patients is expected to reduce alloimmunization, prospective matching is extremely costly and may not clinically benefit the majority of patients with SCD.14 The current study has shown that if a screening assay were developed to identify patients with SCD likely to develop alloantibodies from transfusion, C, E, K antigen-matching informed by the results of this assay could be a valuable method to prevent alloimmunization without the excessively high costs of prospective matching. Under this informed antigen-matching strategy, all transfused patients with SCD would be tested for risk of alloimmunization before initiating chronic transfusion, and only those patients screening positive would receive antigen-matched blood. The cost-effectiveness of an antigen-matching strategy informed by an assay would be largely driven by the specificity of the assay. A sufficiently inexpensive and relatively accurate test would likely be preferable to alternative strategies, when considering both financial and health impact.
Although an assay to predict risk of alloimmunization has not yet been developed, we would anticipate that it would likely be a molecular assay. Using a genome-wide association study (GWAS), it was recently shown that single-nucleotide polymorphisms (SNPs) of three different genes are associated with HLA alloimmunization.27 Additional research is currently being conducted on the possible association of other SNPs and RBC alloimmunization.28
A perfect test with 100% sensitivity and 100% specificity would be cost-saving compared to prospective C, E, K matching while the cost of each test is less than $144,500. Additionally, a perfect screening test is expected to result in similar rates of alloimmunization events as prospective matching. Using a threshold of $50,000 per alloimmunization event averted, a perfect assay would be cost-effective in comparison to a strategy of history-based antigen-matching while the cost of each test is $9,010 or less. These data suggest that the development of assays to identify patients likely to become alloimmunized could be extremely valuable. Currently, a 14-antigen phenotype costs on the order of $300–400. Thus, even if a test were substantially more costly than these screens, use of the test would provide a much needed alternative to history-based or prospective antigen-matching.
This model suggests that an imperfect test may be less costly than prospective C, E, K antigen-matching, and cost-effective in comparison to history-based antigen-matching. A strategy of antigen-matching informed by the results of a $1000 imperfect assay with 100% specificity but only 75% sensitivity is expected to cost $12,558 per alloimmunization event averted over a 10 year period. At a sensitivity of 75%, a $1000 assay would need a specificity of greater than 92% to be cost-effective over a 10-year period. Further sensitivity analyses demonstrated that the cost-effectiveness of an assay is largely dependent on its specificity. Because the cost of selecting matched units is relatively high ($80 per negative antigen), any false positives identified by a screening test will lead to an increase in costs without any additional clinical benefit.
This analysis utilized a simplified model of alloimmunization among chronically transfused patients with SCD, and likely underestimates costs associated with antigen-matching. This model did not incorporate a dynamic population of patients with SCD, some of whom would be initiating or terminating chronic transfusion therapy each year. The data for the percentage of simple and exchange transfusions reflect practices in our tertiary care institution. However, these percentages may not be representative of all institutions. The model was defined assuming a per-unit alloimmunization rate. Thus, we assumed that increased RBC exposure associated with RBC exchanges would lead to increased alloantibody formation. 3,29 However, recent studies in children found alloimmunization rates may not increase for individuals undergoing RBC exchange compared to simple transfusion.30,31 Further research is needed in this area.
In addition, we did not account for some patients having a history of alloimmunization when entering a new clinical setting for chronic transfusion. Costs in this analysis focused exclusively on direct medical expenses and adopted a perspective of a transfusion service. However, patients and caregivers are likely to experience additional costs (e.g., extended hospital stays for individuals waiting for rare antigen-negative units), and we did not account for these types of non-medical or intangible expenses. In addition, the Medicare payments for hospital outpatient services may underestimate the true cost of alloimmunization.
In comparing the health and financial impact of alternative antigen-matching strategies, we utilized a threshold of $50,000 per additional alloimmunization event averted to define “cost-effectiveness.” To our knowledge, there is no previously published work translating alloimmunization into quality-adjusted life years or other more standard measures of effectiveness used in health-related economic evaluations. Variation of this threshold would have affected which strategies in this analysis appear preferable. Analyses focusing on clinical outcomes are likely to place high value on assay sensitivity, rather than specificity, since false negative results generally have more significant clinical implications than false positive results. The current analysis shows that cost-effectiveness is not particularly sensitive to variation in assay sensitivity. Therefore, determining the clinical utility of a test based on its sensitivity is a relatively cost-neutral decision.
While cost-effectiveness analyses are increasingly used to guide clinical policy, transfusion medicine practices and policy have not always adhered to general cost-effectiveness guidelines. It has previously been suggested that transfusion medicine is somehow different from other areas of healthcare, in that, as a society, we are willing to spend more for a given health improvement related to blood safety than we are willing to spend for a similar health improvement in another area of health.32 Thus, programs which would not otherwise appear cost-effective may still be implemented if they lead to a marginal improvement in blood safety. For example, expanded donor blood screening for HIV-1 using p24 antigen testing or nucleic acid testing have been shown to not be cost-effective in the context of most other medical interventions,33 but are still widely implemented. One study suggested that in the United States, nucleic acid testing for HIV, hepatitis C virus, and hepatitis B virus in whole-blood donations is expected to cost between $4.7 million and $11.2 million per quality-adjusted life-year saved.34 Thus, if an assay to identify patients likely to become alloimmunized were available but not cost-effective, it may still be implemented in an effort to improve health. In addition, this type of assay may be helpful for individuals with other hemoglobinopathies who receive chronic transfusion support, such as patients with thalassemia major. This analysis provides insight into the value of a screening test for alloimmunization risk, and suggests that such a test, even if imperfect, could provide clinical benefit to patients in a cost-effective manner.
Acknowledgements
A.A.R.T. was supported by the NIH 1K23AI093152-01A1 and Doris Duke Charitable Foundation Clinician Scientist Development Award (#22006.02). WJS was supported by an American Society of Hematology Scholar award and NIH R21HL107828-01A1.
Footnotes
Conflicts of Interest: All authors declare no conflicts of interest.
References
- 1.Yazdanbakhsh K, Ware RE, Noizat-Pirenne F. Red blood cell alloimmunization in sickle cell disease: pathophysiology, risk factors, and transfusion management. Blood. 2012;120:528–37. doi: 10.1182/blood-2011-11-327361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vichinsky EP, Earles A, Johnson RA, Hoag MS, Williams A, Lubin B. Alloimmunization in sickle cell anemia and transfusion of racially unmatched blood. N Engl J Med. 1990;322:1617–21. doi: 10.1056/NEJM199006073222301. [DOI] [PubMed] [Google Scholar]
- 3.Rosse WF, Gallagher D, Kinney TR, Castro O, Dosik H, Moohr J, Wang W, Levy PS. Transfusion and alloimmunization in sickle cell disease. The Cooperative Study of Sickle Cell Disease. Blood. 1990;76:1431–7. [PubMed] [Google Scholar]
- 4.Lasalle-Williams M, Nuss R, Le T, Cole L, Hassell K, Murphy JR, Ambruso DR. Extended red blood cell antigen matching for transfusions in sickle cell disease: a review of a 14-year experience from a single center (CME) Transfusion. 2011;51:1732–9. doi: 10.1111/j.1537-2995.2010.03045.x. [DOI] [PubMed] [Google Scholar]
- 5.Vichinsky EP. The prevention and management of alloimmunization in sickle cell disease: the benefit of extended phenotypic matching of red blood cells. Immunohematology. 2012;28:20–3. [PubMed] [Google Scholar]
- 6.Vichinsky EP, Luban NL, Wright E, Olivieri N, Driscoll C, Pegelow CH, Adams RJ. Prospective RBC phenotype matching in a stroke-prevention trial in sickle cell anemia: a multicenter transfusion trial. Transfusion. 2001;41:1086–92. doi: 10.1046/j.1537-2995.2001.41091086.x. [DOI] [PubMed] [Google Scholar]
- 7.Sakhalkar VS, Roberts K, Hawthorne LM, McCaskill DM, Veillon DM, Caldito GC, Cotelingam JD. Allosensitization in patients receiving multiple blood transfusions. Ann N Y Acad Sci. 2005;1054:495–9. doi: 10.1196/annals.1345.072. [DOI] [PubMed] [Google Scholar]
- 8.Aygun B, Padmanabhan S, Paley C, Chandrasekaran V. Clinical significance of RBC alloantibodies and autoantibodies in sickle cell patients who received transfusions. Transfusion. 2002;42:37–43. doi: 10.1046/j.1537-2995.2002.00007.x. [DOI] [PubMed] [Google Scholar]
- 9.Karafin MS, Shirey RS, Ness PM, King KE. Antigen-matched red blood cell transfusions for patients with sickle cell disease at The Johns Hopkins Hospital. Immunohematology. 2012;28:3–6. [PubMed] [Google Scholar]
- 10.King KE, Shirey RS. Transfusion management of patients with sickle cell disease: the continuing dilemma. Transfusion. 2010;50:2–4. doi: 10.1111/j.1537-2995.2009.02527.x. [DOI] [PubMed] [Google Scholar]
- 11.Osby M, Shulman IA. Phenotype matching of donor red blood cell units for nonalloimmunized sickle cell disease patients: a survey of 1182 North American laboratories. Arch Pathol Lab Med. 2005;129:190–3. doi: 10.5858/2005-129-190-PMODRB. [DOI] [PubMed] [Google Scholar]
- 12.Higgins JM, Sloan SR. Stochastic modeling of human RBC alloimmunization: evidence for a distinct population of immunologic responders. Blood. 2008;112:2546–53. doi: 10.1182/blood-2008-03-146415. [DOI] [PubMed] [Google Scholar]
- 13.National Heart, Lung, and Blood Institute Expert Panel Report on the Management of Sickle Cell Disease is Available for Public Comment Until August 31. [Accessed March 18, 2013];Office of the Director. . http://www.nhlbi.nih.gov/about/directorscorner/messages/2012-messages/august-2012/expert-panel-report-on-the-management-of-sickle-cell-disease-is-available-for-public-comment-until-august-31/index.html.
- 14.Kacker S, Ness PM, Savage WJ, Frick KD, Shirey RS, King K, Tobian A. Transfusion. 2013. Cost-effectiveness of Prospective Red Cell Antigen-Matching to Prevent Alloimmunization among Sickle Cell Patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kacker S, Frick KD, Tobian AA. The costs of transfusion: economic evaluations in transfusion medicine, Part 1. Transfusion. 2013;53:1383–5. doi: 10.1111/trf.12188. [DOI] [PubMed] [Google Scholar]
- 16.Kacker S, Frick KD, Tobian AA. Establishing a framework: economic evaluations in transfusion medicine, part 2. Transfusion. 2013;53:1634–6. doi: 10.1111/trf.12187. [DOI] [PubMed] [Google Scholar]
- 17.Kacker S, Frick KD, Tobian AA. Constructing a model: economic evaluations in transfusion medicine, Part 3. Transfusion. 2013;53:1885–7. doi: 10.1111/trf.12186. [DOI] [PubMed] [Google Scholar]
- 18.Kacker S, Frick KD, Tobian AA. Data and interpretation: economic evaluations in transfusion medicine, Part 4. Transfusion. 2013;53:2130–3. doi: 10.1111/trf.12185. [DOI] [PubMed] [Google Scholar]
- 19.Briggs A, Sculpher M. An introduction to Markov modelling for economic evaluation. Pharmacoeconomics. 1998;13:397–409. doi: 10.2165/00019053-199813040-00003. [DOI] [PubMed] [Google Scholar]
- 20.United States Census The 2012 Statistical Abstract: The National Data Book. [Accessed April 18, 2013];Resident Population by Sex and Age. 2013 http://www.census.gov/compendia/statab/cats/population.html.
- 21.Fasano RM, Paul W, Siegal E, Luban NL. Transfusion protocol for patients with sickle hemoglobinopathies at Children's National Medical Center. Immunohematology. 2012;28:13–6. [PubMed] [Google Scholar]
- 22.Reid ME, Lomas-Francis C. The blood group antigen factsbook. 2nd ed Elsevier/Academic Press; Amsterdam; Boston: 2004. [Google Scholar]
- 23.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276:1253–8. [PubMed] [Google Scholar]
- 24.Centers for Medicare & Medicaid Services Hospital Outpatient Prospective Payment System: Anndendum A Update. 2012 http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/Addendum-A-and-Addendum-B-Updates-Items/2012-July-Addendum-A.html.
- 25.AABB 2013 Proposed Medicare Payments for Hospital Outpatient Services [monograph on the internet] 2012 Available from: http://www.aabb.org/programs/reimbursementinitiatives/Pages/13hoppsruleprop.aspx.
- 26.Moore RD, Charache S, Terrin ML, Barton FB, Ballas SK. Cost-effectiveness of hydroxyurea in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. Am J Hematol. 2000;64:26–31. doi: 10.1002/(sici)1096-8652(200005)64:1<26::aid-ajh5>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 27.Seielstad M, Page G, Gaddis N, Deng X, Lee T, Lanteri MC, Wu Y, Kakaiya R, Busch MP. Genome-Wide Association Study of HLA Allo-Antibody Formation and Persistence. AABB Meeting 2013 Abstract S88-040B. Transfusion. 2013;53(Supplement) [Google Scholar]
- 28.Lee SC, Machado R, Ellis NA, Bataki K, Garcia J, Kittles RA. Percentage of West-African Ancestry Predicts Red Cell Alloimmunization in Transfused African American Sickle Cell Disease (SCD) Patients. AABB Meeting Abstract SP247. Transfusion. 2013;53(Supplement) [Google Scholar]
- 29.Vichinsky EP, Haberkern CM, Neumayr L, Earles AN, Black D, Koshy M, Pegelow C, Abboud M, Ohene-Frempong K, Iyer RV. A comparison of conservative and aggressive transfusion regimens in the perioperative management of sickle cell disease. The Preoperative Transfusion in Sickle Cell Disease Study Group. N Engl J Med. 1995;333:206–13. doi: 10.1056/NEJM199507273330402. [DOI] [PubMed] [Google Scholar]
- 30.Wahl SK, Garcia A, Hagar W, Gildengorin G, Quirolo K, Vichinsky E. Lower alloimmunization rates in pediatric sickle cell patients on chronic erythrocytapheresis compared to chronic simple transfusions. Transfusion. 2012;52:2671–6. doi: 10.1111/j.1537-2995.2012.03659.x. [DOI] [PubMed] [Google Scholar]
- 31.Venkateswaran L, Teruya J, Bustillos C, Mahoney D, Jr, Mueller BU. Red cell exchange does not appear to increase the rate of allo- and auto-immunization in chronically transfused children with sickle cell disease. Pediatr Blood Cancer. 2011;57:294–6. doi: 10.1002/pbc.22985. [DOI] [PubMed] [Google Scholar]
- 32.Custer B. Economic analyses of blood safety and transfusion medicine interventions: a systematic review. Transfus Med Rev. 2004;18:127–43. doi: 10.1016/j.tmrv.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 33.AuBuchon JP, Birkmeyer JD, Busch MP. Cost-effectiveness of expanded human immunodeficiency virus-testing protocols for donated blood. Transfusion. 1997;37:45–51. doi: 10.1046/j.1537-2995.1997.37197176950.x. [DOI] [PubMed] [Google Scholar]
- 34.Jackson BR, Busch MP, Stramer SL, AuBuchon JP. The cost-effectiveness of NAT for HIV, HCV, and HBV in whole-blood donations. Transfusion. 2003;43:721–9. doi: 10.1046/j.1537-2995.2003.00392.x. [DOI] [PubMed] [Google Scholar]
- 35.Winkler AM, Josephson CD. Transfusion practices for patients with sickle cell disease at major academic medical centers participating in the Atlanta Sickle Cell Consortium. Immunohematology. 2012;28:24–6. [PubMed] [Google Scholar]
- 36.Ambruso DR, Githens JH, Alcorn R, Dixon DJ, Brown LJ, Vaughn WM, Hays T. Experience with donors matched for minor blood group antigens in patients with sickle cell anemia who are receiving chronic transfusion therapy. Transfusion. 1987;27:94–8. doi: 10.1046/j.1537-2995.1987.27187121485.x. [DOI] [PubMed] [Google Scholar]


