Abstract
Obesity and osteoporosis are two of the most common chronic disorders of the 21st century. Both are accompanied by significant morbidity. The only place in the mammalian organism where bone and fat lie adjacent to each other is in the bone marrow. Marrow adipose tissue is a dynamic depot that likely exists as both constitutive and regulated compartments. Adipocytes secrete cytokines and adipokines that either stimulate or inhibit adjacent osteoblasts. The relationship of marrow adipose to other fat depots is complex and may play very distinct roles in modulating metabolic homeostasis, hematopoiesis and osteogenesis. Understanding the relationship between bone and fat cells that arise from the same progenitor within the ‘niche’ provides insight into the pathophysiology of age-related osteoporosis, diabetes mellitus and obesity.
Introduction
Obesity and osteoporosis are chronic disorders with extremely high prevalence rates and secondary disorders that lead to significant morbidity and mortality. Once thought to be mutually exclusive diseases, it is now clear that both frequently coexist and may have a causal relationship. Indeed, in the Osteoporotic Fractures in Men (MrOS) Study, a prospective cohort study of the determinants of fracture in older men, obesity is a major risk factor for osteoporotic fractures.(1) In addition, Type II diabetes mellitus that is characterized by obesity and insulin resistance is associated with normal or high bone mass but a greater risk of fractures.(2) And in Type I diabetes mellitus, fractures are a major co-morbidity, and often associated with marrow adiposity.(3) The pathophysiology of these disorders has provided us with critical links between adipocyte dysfunction and osteoblastic differentiation. A closer examination of their origins also reveals shared pathophysiologic pathways within the bone marrow microenvironment.
A common mesenchymal progenitor found in the marrow, in the stromal vascular fraction of adipose depots and adjacent to vascular structures in other tissues, can give rise to osteoblasts, adipocytes, and myocytes.(4, 5) There is widespread support for the hypothesis that these mesenchymal stromal cells enter only one lineage in a mutually exclusive manner and that this ‘choice’ is determined by an orderly array of transcription factors and hormones.(6) However, with newer technologies for lineage tracing and dynamic imaging, this tenet is probably too simple and does not consider the potential plasticity of progenitors that are already presumed to be committed to one lineage (see Figure 1). Furthermore, there are now animal models demonstrating the positive ‘coexistence’ of marrow adipocytes and osteoblasts in mice with a high bone mass phenotype.(7) Notwithstanding the complexities of numerous differentiation factors and the variable phenotypes of genetic models, the bone marrow still provides our best window into the process of mesenchymal differentiation and ultimately lineage allocation. As such, it is also likely that therapeutic interventions for both disorders will ultimately be directed at this bone-marrow interface.
Figure 1. Bone–fat interactions in the bone marrow microenvironment.
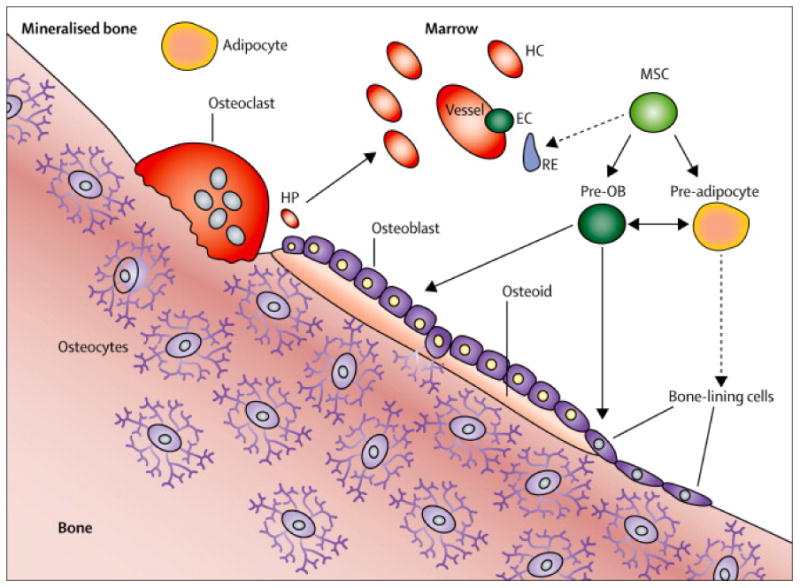
The bone marrow microenvironment includes osteoblasts, bone lining cells, pre-osteoblasts, pre-adipocytes, endothelial cells, reticuloendothelial cells that might be the earliest progenitor for mesenchymal stromal cells, osteoclasts that resorb bone, haematopoietic cells, haematopoietic progenitor cells, and, within the bone matrix, osteocytes. The communication between osteocytes and bone lining cells and osteoblasts organises the bone remodelling process. Mesenchymal stromal cells can differentiate into pre-osteoblasts or pre-adipocytes and these early cells can be plastic. The haematopoietic progenitors are intimately associated with the endosteal osteoblasts, and both are involved in blood cell differentiation. Bone lining cells are fibroblast-like cells; their function is not known, although it is likely these cells become osteoblasts, and express markers of osteoblasts and osteocytes. These cells could become adipocytes in response to injury. Dotted lines indicate hypothesised pathways; solid lines indicate known pathways. Pre-OB=pre-osteoblasts. EC=endothelial cells. RE=reticuloendothelial cells. MSCs=mesenchymal stromal cells. HC=haematopoietic cell. HP=haematopoietic progenitor. Adapted from DiGirolamo and colleagues,7 by permission of Macmillan Publishing Ltd.
Overview and Fundamental Questions
Adipocytes in bone marrow have been noted since the 19th century,(8) but these cells were thought to be quiescent and metabolically inert. Recent studies have revealed that marrow adipose tissue (MAT) has, in some instances, high metabolic activity (whereas in others, low), is responsive to physiological stimuli such as calorie restriction and cold temperature, and actively interacts with osteoblasts to mediate skeletal homeostasis.(7, 9-12)Endosteal adipocytes are rare in neonates, accumulating steadily thereafter throughout the lifespan,(13) and comprising a greater proportion of the marrow cavity of long bones in aging, osteoporosis, anorexia nervosa, diabetes, and skeletal unloading (Table 1). (14-20)
Table 1. Relationships of marrow adipose and bone mass in humans.
| Condition | Marrow adipose | BMD | Ameliorated by treatment? | References |
|---|---|---|---|---|
| Puberty | Increased | Increased | N/A | Kricun 1985,(21) Moore 1990(13) |
| Aging | Increased | Decreased | N/A | Tuljapurkar 2011(15) |
| Starvation | Increased | Decreased | Yes | Abella 2002,(22)Bredella 2009,(14)Ecklund 2010,(16)Fazeli 2012(23) |
| Osteoporosis | Increased | Decreased | Yes | Cohen 2012,(24) Duque 2011,(25) Schwartz 2013,(18)Yeung 2005(20) |
| Unloading | Increased | Decreased | No | Dudley-Javorski 2008,(26)Minaire 1984,(27) Qin 2010,(12)Trudel 2009(19) |
| Type 1 diabetes | Increased | Decreased | No | Moyer-Mileur 2008,(28) McCabe 2007, 2011(3, 29) |
In the axial skeleton of most mammals, marrow adiposity is only evident with advanced age. The increase in MAT in metabolic disorders of low bone mass and the observation that osteoblasts and adipocytes derive from a common pool of mesenchymal progenitors suggested a simple tradeoff between bone and fat mass, with the greater formation of adipocytes at the expense of osteoblasts thereby leading to lower bone mass. However, despite over a decade of active investigations into marrow adipose tissue, fundamental questions remain about how bone-fat interactions influence skeletal homeostasis and vice versa.
First, the function(s) of MAT is unclear, and may indeed be context specific. Marrow adipocytes are found in the long bones of healthy individuals, and increase most rapidly around peak skeletal acquisition,(13, 21) suggesting these cells could be normal residents of the endosteal niche. Moreover, MAT is found across virtually all skeletal sites in humans (i.e. particularly in the appendicular skeleton post puberty) and is thought to comprise up to 15% of total fat stores in adults. (personal communication, Will Cawthorn) Yet there is clear evidence that marrow adiposity is also negatively associated with hematopoiesis, both in normal states and in blood disorders such as aplastic anemia and multiple myeloma.(30) These observations have raised the question of whether marrow adipocytes prolong the status of hematopoietic stem cells and inhibit their differentiation. As such, adipocytes could act as ‘place holders’ to maintain stemness until other factors override the secretory factors that may regulate hematopoietic differentiation. In support of that hypothesis, it is well recognized that after radiation +/- chemotherapy for bone marrow transplantation, marrow adipogenesis is florid and only subsides at the appearance of hematopoietic precursors.(30) On the other hand, the negative association of high marrow adiposity and low bone mass is more heterogeneous, and is found in a diverse set of metabolic diseases. Based on those observations, marrow adipose tissue has been considered deleterious to the skeleton. Yet we know that during puberty the appendicular skeleton converts from red to yellow marrow even as bone formation is at its peak.(13, 21) Moreover, several animal models have high bone mass and increased marrow adipose tissue.(9, 31) So, an essential question is whether the MAT of healthy individuals differs from the fat that accumulates in disease.(32) This might suggest there are two types of marrow adipose, one that is present throughout life (i.e. constitutive), and one that is regulated by local and systemic factors.(7, 10)
Second, the relationships between the bone marrow adipose tissue and white adipose depots are complex. High body mass has been thought to maintain bone mass via greater mechanical loading, but white fat may contribute to osteoporosis by disrupting bone maintenance through the release of inflammatory cytokines such as IL6 and TNF.(33) Bone marrow adipocytes tend to accumulate when white fat depots are depleted, but the precise effects of white fat vs. marrow adipose on bone remain a subject of active investigation.(14, 16, 34) In contrast, feeding high fat diets to rodents leads to marked increases in visceral fat depots, but only marginal increases in marrow adipose tissue.(35) (Figure 2)
Figure 2.
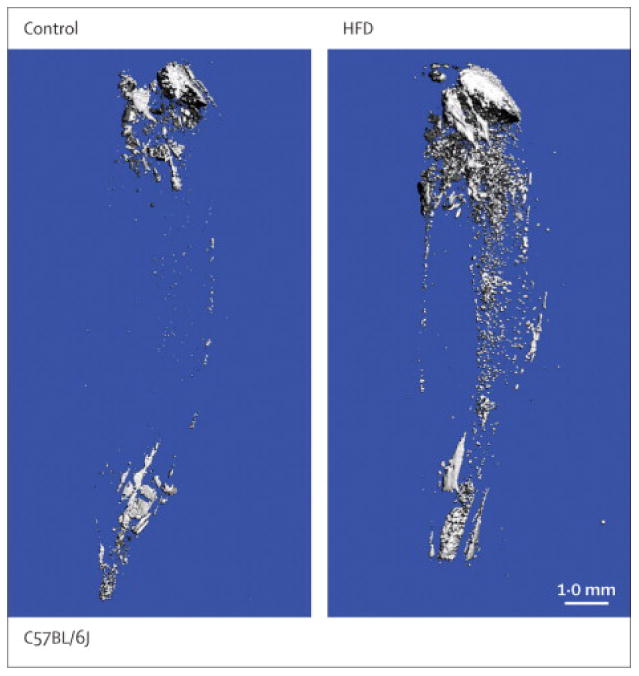
Osmium tetroxide (OsO4) staining of bone marrow fat visualized by microcomputed tomography. Representative images from the tibia show that compared to normal diet (Control, left panel), marrow adiposity is increased only marginally by high fat diet (HFD, right panel).
Third, the origin of marrow adipocytes remains unknown. Although an essential part of the marrow ‘niche’, it is uncertain how these cells arise, particularly over extended periods such as aging, and how these cells relate to osteoblasts and hematopoietic elements. There is some support for the tenet that marrow adipocytes may have unique properties that distinguish them from brown or white adipocytes.(32) For example, after irradiation, even distant from the direct exposure site, or chemotherapy, marrow adiposity is observed and can persist for months or years.(35) Injury of any kind to the marrow provides the stimulus for infiltration of adipocytes, but are these cells derived from bone lining cells, hematopoietic-like precursors, stromal elements such as reticulo-endothelial cells, or a unique adipocyte progenitor that arises de novo? Once again, animal and human models have provided some insights but questions remain.
Fourth, the mechanical implications of extensive marrow adiposity have not been well delineated. Does the presence of fat in the marrow confer an increased risk of fracture by changing the skeletal response to particular stresses? During the aging process and with diabetes mellitus, cortical porosity is a prominent feature whereby the cortical bone surrounds elements of the marrow cavity.(36, 37) Does the presence of MAT in these “pores” lead to weaker bones, particularly in response to stress on the periosteum?
The goal of this review is to describe recent progress in understanding bone-fat interactions both inside and outside the marrow compartment, and to highlight areas for further investigation. Specifically, we focus on whether there is evidence for a final common pathway leading to marrow adipocyte formation in physiologic states, and whether there are disease-specific pathways to greater marrow adiposity. More importantly, we raise some important clinical examples from the study of this important component of the marrow niche, to underlie the relative translational significance of marrow adipose tissue.
Although it is well known that bone marrow adipocytes accumulate with time in normal, healthy individuals,(24) it is less clear whether more marrow adipose always means less bone. MAT accumulation peaks at puberty in the appendicular skeleton, coinciding with peak bone mass acquisition,(13, 21) implying that simultaneous rapid accumulation of bone mass and MAT is possible. In support of this notion, a recent study of girls in early puberty found that bone mineral content is positively related to bone marrow adipose, although this association may be mediated via a positive association of both factors with total body fat.(38) However, other recent studies have found that bone mineral density (BMD) and bone marrow adiposity are inversely correlated even in young, healthy individuals.(39-42) While it remains to be determined whether there are tradeoffs between MAT and bone mass in young, healthy individuals, such tradeoffs are more evident in metabolic disease, including anorexia nervosa, diabetes, obesity, osteoporosis, and mechanical unloading.
Anorexia nervosa
Increased marrow adiposity at both axial and appendicular sites is widely seen in anorexia nervosa, along with depletion of visceral and subcutaneous adipose depots, hypoleptinemia, and suppressed bone acquisition.(14, 16, 22) Recent studies demonstrated that accumulation of MAT in anorexia is associated with circulating levels of DLK1 (also known as preadipocyte factor-1, Pref-1), a regulator of adipocyte and osteoblast differentiation that is higher in women with anorexia vs. control women.(43) Consistent with this concept, DLK1 levels fall, bone marrow adipose fraction decreases, and bone mass increases as women recover from anorexia.(23)
Experiments in model organisms are an essential complement to human studies and continue to provide key insights into the mechanisms linking MAT and bone. Rodent models of anorexia nervosa consistently support an inverse association of leptin and marrow adiposity, although the link between leptin and bone mass is more complex.(27) Hypoleptinemia secondary to caloric restriction leads to high MAT and lower trabecular bone mass in young but not older rodents.(44-46)Interestingly, β-adrenergic blockade during caloric restriction in rats was recently shown not only to mitigate both low bone mass and high MAT, but also to blunt the starvation-induced decrease in serum leptin,(47) implying central regulation of bone mass and MAT in caloric restriction. Hypoleptinemia and leptin insensitivity are also associated with high marrow adiposity in ob/ob and db/db mice, which lack leptin and the leptin receptor, respectively. In the ob/ob mouse, Hamrick et al. showed that leptin treatment both decreases marrow adiposity and increases bone formation, suggesting a possible role for leptin in mediating the balance between bone and fat.(48) However, a subsequent study found that leptin stimulation of VMH receptors reduced both peripheral white adipocytes and marrow adipocytes without affecting osteoblast surface area, implying leptin affects bone formation and marrow adiposity via distinct pathways.(49)
Diabetes mellitus
High marrow adipose has also been shown in Type 1 diabetes (T1D), along with impaired cortical bone geometry.(3, 28, 29) In contrast, high MAT is not seen in Type 2 diabetes (T2D), in which fracture risk is elevated despite normal to high BMD.(17, 50) However, a difference in MAT composition in T2D was recently revealed by magnetic resonance spectroscopy (MRS): the marrow cavity contains relatively more saturated lipid in women with T2D, and less unsaturated lipid in the subset of women with T2D who also have prior fractures.(51) While these findings are intriguing, it is not yet known how marrow saturation or unsaturation might be related to the overall amount of fat, or to fracture risk.
Animal models of Type 1 diabetes are consistent with human data, exhibiting hypoleptinemia, high MAT, and low bone mass. This would be consistent with the paradoxical response of the marrow to global substrate deficiency, i.e. enhanced marrow adiposity. Studies of streptozotocin-induced diabetes in BALB/c and non-obese diabetic (NOD) mice found that in both models, the proximal tibia exhibited marked increases in marrow adipocyte number and levels of peroxisome proliferator-activated receptor gamma (PPARG) and FABP4 mRNA, along with decreases in trabecular and cortical bone mineral density and bone volume fraction, as well as in osteocalcin mRNA.(52, 53) Treatment with leptin or with a PPARG antagonist, bisphenol A diglycidyl ether (BADGE), prevented accumulation of marrow adiposity but not bone loss, suggesting these phenomena result from partially independent mechanisms in diabetes.(54, 55) In contrast, BADGE treatment in wildtype adult mice, with or without concurrent vitamin D, increased bone formation and decreased marrow adipogenesis.(56) The implication is that there is a PPARG-mediated tradeoff between bone formation and marrow adipocyte formation, but that this is not the only mechanism responsible for low bone mass in type I diabetes.
In Type II diabetes mellitus, insulin resistance and obesity are well recognized features. Bone mineral density is usually normal or slightly elevated, but fracture risk is increased. (57, 58) The mechanisms responsible for skeletal fragility in this disorder are still being investigated, although cortical porosity is one feature that may impair bone strength, particularly in the appendicular skeleton where fractures are most prevalent.(37, 59) Marrow adiposity is not a feature of generalized insulin resistance, although B6 mice given a high fat diet for 12 weeks after wean show a modest increase in marrow adipocytes in the tibia as measured by osmium micro CT (personal communication, Casey Doucette). On the other hand, the thiazolidinediones (TZDs), such as rosiglitazone and pioglitazone, are very strong inducers of MAT and bone loss in the appendicular skeleton of rodents. (60, 61) In vitro, the TZDs are extremely potent stimulators of adipogenesis in mesenchymal progenitor cells, and are inhibitors of osteogenesis.(62) In humans, some studies suggest that marrow adiposity occurs in response to TZDs, although in others this was not found.(63, 64)
Unloading
Accumulation of bone marrow adipose is associated with skeletal unloading in humans, e.g. paralysis.(12, 26, 27) Recently, the Women International Space Simulation for Exploration study showed that 60 days of bedrest increased bone marrow adiposity, and that the increase persisted for a year after reambulation.(19) A subsequent study in men found that resistive exercise, alone or in combination with low magnitude total body vibration, inhibited MAT accumulation, although effects on bone mass were not reported and the sample size was modest.(65)
In support of the notion that mechanical loading plays a role in bone-fat interactions, skeletal unloading is associated with high marrow adiposity and low bone mass in animal models. Early studies of rats exposed to microgravity in spaceflight or terrestrial hindlimb unloading showed that impaired bone mineral acquisition and greater marrow adiposity reverted to normal upon reloading in both models.(66-69) A recent study of hindlimb unloading sufficient to induce bone loss and high MAT found that treatment with sclerostin antibody reduced bone loss, but had no effect on marrow adipose,(21) suggesting bone loss and high MAT are mediated independently in this model.
On a mechanistic level, unloaded rats exhibited reduced bone formation and lower levels of RUNX2, osteocalcin, and type 1 collagen mRNA, but more marrow adipocytes and higher levels of PPARG, lipoprotein lipase, and FABP4 (fatty acid binding protein 4, also known as adipocyte protein 2 (aP2)). Both gene expression and bone formation were normalized following transforming growth factor-beta2 (TGF-B2) treatment.(70) Subsequent experiments showed that unloading increased CCAAT/enhancer-binding protein alpha and beta (CEBPA and CEBPB) expression, which in turn increased PPARG2 expression, but that TGF-B2 prevented unloading-induced changes in gene expression and activity.(71)
Osteoporosis
As first noted by Meunier,(72) increased marrow adipose tissue is seen in osteoporosis. Transiliac biopsies of premenopausal women with idiopathic osteoporosis or osteopenia revealed more numerous, voluminous marrow adipocytes compared to controls, although only controls had the expected positive correlation of MAT and age and negative correlations of MAT, bone formation and bone volume.(25) A recent study in the AGES-Reykjavik cohort found that higher MAT was negatively related to trabecular BMD measured by QCT in women, and positively related to existing vertebral fractures in men.(18)Both osteoporosis and osteopenia were associated with a lower proportion of unsaturated lipid, as assessed by proton magnetic resonance spectroscopy ((1)H-MRS).(20)
One of the lingering questions about MAT and bone is whether MAT accumulation precedes, follows, or parallels bone loss. In most rodent models, aging is associated with significant marrow adiposity, and this has been linked with low trabecular bone mineral density in rodents(73) as well as in humans,(18) suggesting this is a mutually exclusive process. On the other hand, female C57BL6 mice start losing trabecular bone as early as eight weeks of age and yet increased marrow adiposity does not become apparent until much later in life.(10, 74, 75) In a model of glucocorticoid-induced osteoporosis in rabbits, longitudinal assessment of marrow lipid fraction and bone mineral density in the proximal femur indicated the increase in MAT preceded the decrease in bone mineral density, and quantitative histology showed that an initial increase in the number of marrow adipocytes was followed by an increase in adipocyte diameter.(76) Interestingly, while concurrent zoledronic acid treatment was sufficient to eliminate the glucocorticoid-induced increases in adipocyte number and diameter, bone mineral density was only partially restored.(77)
Summary and Potential Therapeutic Implications
To summarize, in humans, there are conflicting data about whether marrow adipose and bone mass are positively or negatively correlated. While adolescents gain both simultaneously,(13, 21) MAT and bone seem to be inversely related later in adulthood.(39-42) Data from animal models generally support the idea that marrow adiposity is inversely correlated with bone mass in certain metabolic diseases, although there are exceptions: the ob/ob mouse phenotype includes high MAT and high axial but low appendicular bone mass, despite the total absence of leptin,(78) and comparison of the C3H and C57Bl/6J strains demonstrates that bone mass and MAT are frequently but not always inversely correlated, particularly in the latter strain.(9) In the former, high bone mass (trabecular and cortical) coexists with increased marrow adipose tissue across the lifespan.
Both human and animal data also indicate that high marrow adiposity arises via multiple mechanisms. Accumulation of marrow adipose appears to result at least in part from hypoleptinemia in anorexia nervosa and Type 1 diabetes, but mechanical unloading is a likely candidate in bedrest and perhaps in age-related osteoporosis. Ghrelin levels, which are increased in anorexia, can stimulate marrow adiposity.(79) The resulting marrow adipocytes also exhibit differing degrees of plasticity in response to metabolic signals. Anorexia-induced marrow adiposity ameliorates with weight gain, whereas MAT in unloading and diabetes appears more persistent despite re-ambulation or insulin treatment, respectively. Part of this may be related to how MAT is defined, since it is possible that there are two types of marrow adipose tissue, constitutive (i.e. seen consistently in the distal femur) and ‘regulated’ in the diaphysis and proximal femur and tibia (i.e. variably present in response to metabolic perturbations).(80) Thus in addition to identifying the specific pathways underlying marrow adipocyte formation in healthy individuals and in metabolic disease, more work is needed to understand the mechanisms that lead to regression of different marrow adipose compartments and its ultimate distribution following recovery.
What are the potential diagnostic and therapeutic implications from our knowledge of marrow adiposity and its interaction with the niche elements? There are several intriguing possibilities. First, a major clinical sequel from the treatment of malignant diseases with chemotherapy and radiation is osteoporotic fractures.(35) Most patients treated for hematopoietic and solid tumors (e.g. lymphoma, leukemia) require these therapeutic approaches for potential cures. But the resulting osteopenia and increased skeletal fragility later in life, particularly for children treated early, is a major co-morbidity.(81) Many of these patients have persistent marrow adiposity on bone biopsy or by MRI, and their bone density is usually well below age-matched controls. Lifelong treatment with anti-osteoporosis drugs is not a palatable or cost effective option. However, if an agent could early on alter the lineage allocation in the irradiated marrow to favor osteogenesis, this could have huge therapeutic implications downstream and tremendous cost savings. Second, Type 1 diabetes mellitus is associated with skeletal fragility particularly in individuals with the disease for more than two decades. The fundamental concern in these patients is the low bone mass and the reduced bone formation. But an increase in marrow adiposity is another characteristic feature. Once again, developing drugs that would enhance osteogenesis and reduce marrow adipose infiltration should have a major impact on skeletal morbidity in this chronic disease. Finally, if MAT has a specific spectroscopic signal of greater fat saturation on MRI in high risk patients with age related osteoporosis or Type 2 diabetes mellitus, then screening affected individuals, particularly those with normal bone density, may prove a cost effective means of identifying patients to treat with osteoporosis medications.(20, 51)
In sum, it is critical to understand cell fate in the marrow, and its implications for physiologic and pathologic states. Marrow adiposity provides a window of opportunity to understand the remodeling of the marrow niche and its potential manipulation for treating chronic diseases.
Acknowledgments
This work was supported by USPHS R24 NIDDK 092759.
Footnotes
Conflicts of Interest: We declare that we have no conflicts of interest.
Contributions: MD and CR designed the search criteria, performed the literature search, and wrote the manuscript. CR created the figures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Maureen Devlin, The University of Michigan, Ann Arbor, Michigan 48109.
Clifford J Rosen, Maine Medical Center Research Institute, Scarborough, Maine 04074.
References
- 1.Nielson CM, Marshall LM, Adams AL, LeBlanc ES, Cawthon PM, Ensrud K, et al. BMI and fracture risk in older men: the osteoporotic fractures in men study (MrOS) Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011 Mar;26(3):496–502. doi: 10.1002/jbmr.235. Epub 2010/09/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leslie WD, Rubin MR, Schwartz AV, Kanis JA. Type 2 diabetes and bone. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2012 Nov;27(11):2231–7. doi: 10.1002/jbmr.1759. Epub 2012/10/02. eng. [DOI] [PubMed] [Google Scholar]
- 3.McCabe LR. Understanding the pathology and mechanisms of type I diabetic bone loss. Journal of cellular biochemistry. 2007 Dec 15;102(6):1343–57. doi: 10.1002/jcb.21573. Epub 2007/11/03. eng. [DOI] [PubMed] [Google Scholar]
- 4.Bianco P, Robey PG, Saggio I, Riminucci M. “Mesenchymal” stem cells in human bone marrow (skeletal stem cells): a critical discussion of their nature, identity, and significance in incurable skeletal disease. Human gene therapy. 2010 Sep;21(9):1057–66. doi: 10.1089/hum.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell stem cell. 2008 Apr 10;2(4):313–9. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muruganandan S, Roman AA, Sinal CJ. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cell Mol Life Sci. 2009 Jan;66(2):236–53. doi: 10.1007/s00018-008-8429-z. Epub 2008/10/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahman S, Lu Y, Czernik PJ, Rosen CJ, Enerback S, Lecka-Czernik B. Inducible brown adipose tissue, or beige fat, is anabolic for the skeleton. Endocrinology. 2013 Aug;154(8):2687–701. doi: 10.1210/en.2012-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neumann E. Das Gesetz Verbreitung des gelben und roten Markes in den Extremitatenknochen. Centralblatt für die medicinischen Wissenschaft. 1882;20:321–3. [Google Scholar]
- 9.Fazeli PK, Horowitz MC, MacDougald OA, Scheller EL, Rodeheffer MS, Rosen CJ, et al. Marrow fat and bone--new perspectives. The Journal of clinical endocrinology and metabolism. 2013 Mar;98(3):935–45. doi: 10.1210/jc.2012-3634. Epub 2013/02/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone. 2012 Feb;50(2):546–52. doi: 10.1016/j.bone.2011.06.016. Epub 2011/07/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheu Y, Cauley JA. The Role of Bone Marrow and Visceral Fat on Bone Metabolism. Current osteoporosis reports. 2011 Mar 5; doi: 10.1007/s11914-011-0051-6. Epub 2011/03/05. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin W, Bauman WA, Cardozo C. Bone and muscle loss after spinal cord injury: organ interactions. Annals of the New York Academy of Sciences. 2010 Nov;1211:66–84. doi: 10.1111/j.1749-6632.2010.05806.x. Epub 2010/11/11. eng. [DOI] [PubMed] [Google Scholar]
- 13.Moore SG, Dawson KL. Red and yellow marrow in the femur: age-related changes in appearance at MR imaging. Radiology. 1990 Apr;175(1):219–23. doi: 10.1148/radiology.175.1.2315484. Epub 1990/04/01. eng. [DOI] [PubMed] [Google Scholar]
- 14.Bredella MA, Fazeli PK, Miller KK, Misra M, Torriani M, Thomas BJ, et al. Increased bone marrow fat in anorexia nervosa. The Journal of clinical endocrinology and metabolism. 2009 Jun;94(6):2129–36. doi: 10.1210/jc.2008-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuljapurkar SR, McGuire TR, Brusnahan SK, Jackson JD, Garvin KL, Kessinger MA, et al. Changes in human bone marrow fat content associated with changes in hematopoietic stem cell numbers and cytokine levels with aging. Journal of anatomy. 2011 Nov;219(5):574–81. doi: 10.1111/j.1469-7580.2011.01423.x. Epub 2011/09/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ecklund K, Vajapeyam S, Feldman HA, Buzney CD, Mulkern RV, Kleinman PK, et al. Bone Marrow Changes in Adolescent Girls with Anorexia Nervosa. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010 Aug 4;25(2):298–304. doi: 10.1359/jbmr.090805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baum T, Yap SP, Karampinos DC, Nardo L, Kuo D, Burghardt AJ, et al. Does vertebral bone marrow fat content correlate with abdominal adipose tissue, lumbar spine bone mineral density, and blood biomarkers in women with type 2 diabetes mellitus? Journal of magnetic resonance imaging : JMRI. 2012 Jan;35(1):117–24. doi: 10.1002/jmri.22757. Epub 2011/12/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz AV, Sigurdsson S, Hue TF, Lang TF, Harris TB, Rosen CJ, et al. Vertebral bone marrow fat associated with lower trabecular BMD and prevalent vertebral fracture in older adults. The Journal of clinical endocrinology and metabolism. 2013 Apr 3; doi: 10.1210/jc.2012-3949. Epub 2013/04/05. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trudel G, Payne M, Madler B, Ramachandran N, Lecompte M, Wade C, et al. Bone marrow fat accumulation after 60 days of bed rest persisted 1 year after activities were resumed along with hemopoietic stimulation: the Women International Space Simulation for Exploration study. J Appl Physiol. 2009 Aug;107(2):540–8. doi: 10.1152/japplphysiol.91530.2008. Epub 2009/05/30. eng. [DOI] [PubMed] [Google Scholar]
- 20.Yeung DK, Griffith JF, Antonio GE, Lee FK, Woo J, Leung PC. Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: a proton MR spectroscopy study. Journal of magnetic resonance imaging : JMRI. 2005 Aug;22(2):279–85. doi: 10.1002/jmri.20367. Epub 2005/07/20. eng. [DOI] [PubMed] [Google Scholar]
- 21.Kricun ME. Red-yellow marrow conversion: its effect on the location of some solitary bone lesions. Skeletal radiology. 1985;14(1):10–9. doi: 10.1007/BF00361188. Epub 1985/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 22.Abella E, Feliu E, Granada I, Milla F, Oriol A, Ribera JM, et al. Bone marrow changes in anorexia nervosa are correlated with the amount of weight loss and not with other clinical findings. American journal of clinical pathology. 2002 Oct;118(4):582–8. doi: 10.1309/2Y7X-YDXK-006B-XLT2. [DOI] [PubMed] [Google Scholar]
- 23.Fazeli PK, Bredella MA, Freedman L, Thomas BJ, Breggia A, Meenaghan E, et al. Marrow fat and preadipocyte factor-1 levels decrease with recovery in women with anorexia nervosa. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2012 Sep;27(9):1864–71. doi: 10.1002/jbmr.1640. Epub 2012/04/18. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen A, Dempster DW, Stein EM, Nickolas TL, Zhou H, McMahon DJ, et al. Increased marrow adiposity in premenopausal women with idiopathic osteoporosis. The Journal of clinical endocrinology and metabolism. 2012 Aug;97(8):2782–91. doi: 10.1210/jc.2012-1477. Epub 2012/06/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duque G, Li W, Adams M, Xu S, Phipps R. Effects of risedronate on bone marrow adipocytes in postmenopausal women. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2011 May;22(5):1547–53. doi: 10.1007/s00198-010-1353-8. Epub 2010/07/28. eng. [DOI] [PubMed] [Google Scholar]
- 26.Dudley-Javoroski S, Shields RK. Dose estimation and surveillance of mechanical loading interventions for bone loss after spinal cord injury. Phys Ther. 2008 Mar;88(3):387–96. doi: 10.2522/ptj.20070224. Epub 2008/01/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Minaire P, Edouard C, Arlot M, Meunier PJ. Marrow changes in paraplegic patients. Calcified tissue international. 1984 May;36(3):338–40. doi: 10.1007/BF02405340. Epub 1984/05/01. eng. [DOI] [PubMed] [Google Scholar]
- 28.Moyer-Mileur LJ, Slater H, Jordan KC, Murray MA. IGF-1 and IGF-binding proteins and bone mass, geometry, and strength: relation to metabolic control in adolescent girls with type 1 diabetes. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2008 Dec;23(12):1884–91. doi: 10.1359/jbmr.080713. Epub 2008/07/31. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCabe L, Zhang J, Raehtz S. Understanding the skeletal pathology of type 1 and 2 diabetes mellitus. Crit Rev Eukaryot Gene Expr. 2011;21(2):187–206. doi: 10.1615/critreveukargeneexpr.v21.i2.70. Epub 2011/11/15. eng. [DOI] [PubMed] [Google Scholar]
- 30.Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009 Jul 9;460(7252):259–63. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ackert-Bicknell CL, Shockley KR, Horton LG, Lecka-Czernik B, Churchill GA, Rosen CJ. Strain-specific effects of rosiglitazone on bone mass, body composition, and serum insulin-like growth factor-I. Endocrinology. 2009 Mar;150(3):1330–40. doi: 10.1210/en.2008-0936. Epub 2008/10/25. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lecka-Czernik B. Marrow fat metabolism is linked to the systemic energy metabolism. Bone. 2012 Feb;50(2):534–9. doi: 10.1016/j.bone.2011.06.032. Epub 2011/07/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao JJ. Effects of obesity on bone metabolism. J Orthop Surg Res. 2011;6:30. doi: 10.1186/1749-799X-6-30. Epub 2011/06/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosen CJ, Klibanski A. Bone, fat, and body composition: evolving concepts in the pathogenesis of osteoporosis. Am J Med. 2009 May;122(5):409–14. doi: 10.1016/j.amjmed.2008.11.027. Epub 2009/04/21. eng. [DOI] [PubMed] [Google Scholar]
- 35.Georgiou KR, Hui SK, Xian CJ. Regulatory pathways associated with bone loss and bone marrow adiposity caused by aging, chemotherapy, glucocorticoid therapy and radiotherapy. American journal of stem cells. 2012;1(3):205–24. [PMC free article] [PubMed] [Google Scholar]
- 36.Zebaze RM, Ghasem-Zadeh A, Bohte A, Iuliano-Burns S, Mirams M, Price RI, et al. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet. 2010 May 15;375(9727):1729–36. doi: 10.1016/S0140-6736(10)60320-0. Epub 2010/05/18. eng. [DOI] [PubMed] [Google Scholar]
- 37.Patsch JM, Burghardt AJ, Yap SP, Baum T, Schwartz AV, Joseph GB, et al. Increased cortical porosity in type 2 diabetic postmenopausal women with fragility fractures. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2013 Feb;28(2):313–24. doi: 10.1002/jbmr.1763. Epub 2012/09/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newton AL, Hanks LJ, Davis M, Casazza K. The relationships among total body fat, bone mineral content and bone marrow adipose tissue in early-pubertal girls. BoneKEy Rep. 2013;2(4) doi: 10.1038/bonekey.2013.49. 04/10/online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Iorgi N, Mo AO, Grimm K, Wren TA, Dorey F, Gilsanz V. Bone acquisition in healthy young females is reciprocally related to marrow adiposity. The Journal of clinical endocrinology and metabolism. 2010 Jun;95(6):2977–82. doi: 10.1210/jc.2009-2336. Epub 2010/04/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Iorgi N, Rosol M, Mittelman SD, Gilsanz V. Reciprocal relation between marrow adiposity and the amount of bone in the axial and appendicular skeleton of young adults. The Journal of clinical endocrinology and metabolism. 2008 Jun;93(6):2281–6. doi: 10.1210/jc.2007-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wren TA, Chung SA, Dorey FJ, Bluml S, Adams GB, Gilsanz V. Bone marrow fat is inversely related to cortical bone in young and old subjects. The Journal of clinical endocrinology and metabolism. 2011 Mar;96(3):782–6. doi: 10.1210/jc.2010-1922. Epub 2010/12/24. eng. [DOI] [PubMed] [Google Scholar]
- 42.Shen W, Chen J, Gantz M, Punyanitya M, Heymsfield SB, Gallagher D, et al. MRI-measured pelvic bone marrow adipose tissue is inversely related to DXA-measured bone mineral in younger and older adults. European journal of clinical nutrition. 2012 Sep;66(9):983–8. doi: 10.1038/ejcn.2012.35. Epub 2012/04/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fazeli PK, Bredella MA, Misra M, Meenaghan E, Rosen CJ, Clemmons DR, et al. Preadipocyte factor-1 is associated with marrow adiposity and bone mineral density in women with anorexia nervosa. The Journal of clinical endocrinology and metabolism. 2010 Jan;95(1):407–13. doi: 10.1210/jc.2009-1152. Epub 2009/10/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tatsumi S, Ito M, Asaba Y, Tsutsumi K, Ikeda K. Life-long caloric restriction reveals biphasic and dimorphic effects on bone metabolism in rodents. Endocrinology. 2008 Feb;149(2):634–41. doi: 10.1210/en.2007-1089. [DOI] [PubMed] [Google Scholar]
- 45.Hamrick MW, Ding KH, Ponnala S, Ferrari SL, Isales CM. Caloric restriction decreases cortical bone mass but spares trabecular bone in the mouse skeleton: implications for the regulation of bone mass by body weight. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2008 Jun;23(6):870–8. doi: 10.1359/jbmr.080213. [DOI] [PubMed] [Google Scholar]
- 46.Devlin MJ, Cloutier AM, Thomas NA, Panus DA, Lotinun S, Pinz I, et al. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010 Sep;25(9):2078–88. doi: 10.1002/jbmr.82. Epub 2010/03/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baek K, Bloomfield SA. Blocking beta-adrenergic signaling attenuates reductions in circulating leptin, cancellous bone mass, and marrow adiposity seen with dietary energy restriction. J Appl Physiol. 2012 Dec 1;113(11):1792–801. doi: 10.1152/japplphysiol.00187.2012. Epub 2012/09/22. eng. [DOI] [PubMed] [Google Scholar]
- 48.Hamrick MW, Della-Fera MA, Choi YH, Pennington C, Hartzell D, Baile CA. Leptin treatment induces loss of bone marrow adipocytes and increases bone formation in leptin-deficient ob/ob mice. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2005 Jun;20(6):994–1001. doi: 10.1359/JBMR.050103. [DOI] [PubMed] [Google Scholar]
- 49.Hamrick MW, Della Fera MA, Choi YH, Hartzell D, Pennington C, Baile CA. Injections of leptin into rat ventromedial hypothalamus increase adipocyte apoptosis in peripheral fat and in bone marrow. Cell Tissue Res. 2007 Jan;327(1):133–41. doi: 10.1007/s00441-006-0312-3. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz AV, Vittinghoff E, Bauer DC, Hillier TA, Strotmeyer ES, Ensrud KE, et al. Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA : the journal of the American Medical Association. 2011 Jun 1;305(21):2184–92. doi: 10.1001/jama.2011.715. Epub 2011/06/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patsch JM, Li X, Baum T, Yap SP, Karampinos DC, Schwartz AV, et al. Bone marrow fat composition as a novel imaging biomarker in postmenopausal women with prevalent fragility fractures. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2013 Apr 4; doi: 10.1002/jbmr.1950. Epub 2013/04/06. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Botolin S, McCabe LR. Bone loss and increased bone adiposity in spontaneous and pharmacologically induced diabetic mice. Endocrinology. 2007 Jan;148(1):198–205. doi: 10.1210/en.2006-1006. Epub 2006/10/21. eng. [DOI] [PubMed] [Google Scholar]
- 53.Botolin S, Faugere MC, Malluche H, Orth M, Meyer R, McCabe LR. Increased bone adiposity and peroxisomal proliferator-activated receptor-gamma2 expression in type I diabetic mice. Endocrinology. 2005 Aug;146(8):3622–31. doi: 10.1210/en.2004-1677. Epub 2005/05/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Botolin S, McCabe LR. Inhibition of PPARgamma prevents type I diabetic bone marrow adiposity but not bone loss. Journal of cellular physiology. 2006 Dec;209(3):967–76. doi: 10.1002/jcp.20804. Epub 2006/09/15. eng. [DOI] [PubMed] [Google Scholar]
- 55.Motyl KJ, McCabe LR. Leptin treatment prevents type I diabetic marrow adiposity but not bone loss in mice. Journal of cellular physiology. 2009 Feb;218(2):376–84. doi: 10.1002/jcp.21608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duque G, Li W, Vidal C, Bermeo S, Rivas D, Henderson J. Pharmacological inhibition of PPARgamma increases osteoblastogenesis and bone mass in male C57BL/6 mice. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2013 Mar;28(3):639–48. doi: 10.1002/jbmr.1782. Epub 2012/10/10. eng. [DOI] [PubMed] [Google Scholar]
- 57.Yamamoto M, Yamaguchi T, Yamauchi M, Kaji H, Sugimoto T. Diabetic patients have an increased risk of vertebral fractures independent of BMD or diabetic complications. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2009 Apr;24(4):702–9. doi: 10.1359/jbmr.081207. Epub 2008/12/04. eng. [DOI] [PubMed] [Google Scholar]
- 58.Schwartz AV, Vittinghoff E, Bauer DC, Hillier TA, Strotmeyer ES, Ensrud KE, et al. Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA : the journal of the American Medical Association. 2011 Jun 1;305(21):2184–92. doi: 10.1001/jama.2011.715. Epub 2011/06/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burghardt AJ, Issever AS, Schwartz AV, Davis KA, Masharani U, Majumdar S, et al. High-resolution peripheral quantitative computed tomographic imaging of cortical and trabecular bone microarchitecture in patients with type 2 diabetes mellitus. The Journal of clinical endocrinology and metabolism. 2010 Nov;95(11):5045–55. doi: 10.1210/jc.2010-0226. Epub 2010/08/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rzonca SO, Suva LJ, Gaddy D, Montague DC, Lecka-Czernik B. Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology. 2004 Jan;145(1):401–6. doi: 10.1210/en.2003-0746. Epub 2003/09/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Syversen U, Stunes AK, Gustafsson BI, Obrant KJ, Nordsletten L, Berge R, et al. Different skeletal effects of the peroxisome proliferator activated receptor (PPAR)alpha agonist fenofibrate and the PPARgamma agonist pioglitazone. BMC endocrine disorders. 2009;9:10. doi: 10.1186/1472-6823-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lecka-Czernik B, Ackert-Bicknell C, Adamo ML, Marmolejos V, Churchill GA, Shockley KR, et al. Activation of peroxisome proliferator-activated receptor gamma (PPARgamma) by rosiglitazone suppresses components of the insulin-like growth factor regulatory system in vitro and in vivo. Endocrinology. 2007 Feb;148(2):903–11. doi: 10.1210/en.2006-1121. Epub 2006/11/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grey A, Beckley V, Doyle A, Fenwick S, Horne A, Gamble G, et al. Pioglitazone increases bone marrow fat in type 2 diabetes: results from a randomized controlled trial. European journal of endocrinology / European Federation of Endocrine Societies. 2012 Jun;166(6):1087–91. doi: 10.1530/EJE-11-1075. Epub 2012/03/13. eng. [DOI] [PubMed] [Google Scholar]
- 64.Harslof T, Wamberg L, Moller L, Stodkilde-Jorgensen H, Ringgaard S, Pedersen SB, et al. Rosiglitazone decreases bone mass and bone marrow fat. The Journal of clinical endocrinology and metabolism. 2011 May;96(5):1541–8. doi: 10.1210/jc.2010-2077. [DOI] [PubMed] [Google Scholar]
- 65.Trudel G, Coletta E, Cameron I, Belavy DL, Lecompte M, Armbrecht G, et al. Resistive exercises, with or without whole body vibration, prevent vertebral marrow fat accumulation during 60 days of head-down tilt bed rest in men. Journal of applied physiology. 2012 Jun;112(11):1824–31. doi: 10.1152/japplphysiol.00029.2012. [DOI] [PubMed] [Google Scholar]
- 66.Wronski TJ, Morey-Holton E, Jee WS. Cosmos 1129: spaceflight and bone changes. The Physiologist. 1980 Dec;23(Suppl 6):S79–82. Epub 1980/12/01. eng. [PubMed] [Google Scholar]
- 67.Jee WS, Wronski TJ, Morey ER, Kimmel DB. Effects of spaceflight on trabecular bone in rats. The American journal of physiology. 1983 Mar;244(3):R310–4. doi: 10.1152/ajpregu.1983.244.3.R310. Epub 1983/03/01. eng. [DOI] [PubMed] [Google Scholar]
- 68.Wronski TJ, Morey ER. Skeletal abnormalities in rats induced by simulated weightlessness. Metabolic bone disease & related research. 1982;4(1):69–75. doi: 10.1016/0221-8747(82)90011-x. Epub 1982/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 69.Wronski TJ, Morey ER. Recovery of the rat skeleton from the adverse effects of simulated weightlessness. Metabolic bone disease & related research. 1983;4(6):347–52. Epub 1983/01/01. eng. [PubMed] [Google Scholar]
- 70.Ahdjoudj S, Lasmoles F, Holy X, Zerath E, Marie PJ. Transforming growth factor beta2 inhibits adipocyte differentiation induced by skeletal unloading in rat bone marrow stroma. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2002 Apr;17(4):668–77. doi: 10.1359/jbmr.2002.17.4.668. Epub 2002/03/29. eng. [DOI] [PubMed] [Google Scholar]
- 71.Ahdjoudj S, Kaabeche K, Holy X, Fromigue O, Modrowski D, Zerath E, et al. Transforming growth factor-beta inhibits CCAAT/enhancer-binding protein expression and PPARgamma activity in unloaded bone marrow stromal cells. Experimental cell research. 2005 Feb 1;303(1):138–47. doi: 10.1016/j.yexcr.2004.09.013. Epub 2004/12/02. eng. [DOI] [PubMed] [Google Scholar]
- 72.Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clinical orthopaedics and related research. 1971 Oct;80:147–54. doi: 10.1097/00003086-197110000-00021. Epub 1971/10/01. eng. [DOI] [PubMed] [Google Scholar]
- 73.Duque G, Rivas D, Li W, Li A, Henderson JE, Ferland G, et al. Age-related bone loss in the LOU/c rat model of healthy ageing. Experimental gerontology. 2009 Mar;44(3):183–9. doi: 10.1016/j.exger.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 74.Glatt V, Canalis E, Stadmeyer L, Bouxsein ML. Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2007 Aug;22(8):1197–207. doi: 10.1359/jbmr.070507. [DOI] [PubMed] [Google Scholar]
- 75.Lazarenko OP, Rzonca SO, Hogue WR, Swain FL, Suva LJ, Lecka-Czernik B. Rosiglitazone induces decreases in bone mass and strength that are reminiscent of aged bone. Endocrinology. 2007 Jun;148(6):2669–80. doi: 10.1210/en.2006-1587. Epub 2007/03/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li GW, Xu Z, Chen QW, Chang SX, Tian YN, Fan JZ. The temporal characterization of marrow lipids and adipocytes in a rabbit model of glucocorticoid-induced osteoporosis. Skeletal radiology. 2013 Sep;42(9):1235–44. doi: 10.1007/s00256-013-1659-7. Epub 2013/06/12. eng. [DOI] [PubMed] [Google Scholar]
- 77.Li GW, Chang SX, Fan JZ, Tian YN, Xu Z, He YM. Marrow adiposity recovery after early zoledronic acid treatment of glucocorticoid-induced bone loss in rabbits assessed by magnetic resonance spectroscopy. Bone. 2013 Feb;52(2):668–75. doi: 10.1016/j.bone.2012.11.002. Epub 2012/11/13. eng. [DOI] [PubMed] [Google Scholar]
- 78.Hamrick MW. Leptin, bone mass, and the thrifty phenotype. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2004 Oct;19(10):1607–11. doi: 10.1359/JBMR.040712. [DOI] [PubMed] [Google Scholar]
- 79.Thompson NM, Gill DA, Davies R, Loveridge N, Houston PA, Robinson IC, et al. Ghrelin and des-octanoyl ghrelin promote adipogenesis directly in vivo by a mechanism independent of the type 1a growth hormone secretagogue receptor. Endocrinology. 2004 Jan;145(1):234–42. doi: 10.1210/en.2003-0899. [DOI] [PubMed] [Google Scholar]
- 80.Scheller EL, Rosen CJ. What's the matter with MAT? – Marrow adipose tissue, metabolism and skeletal health. Annals of the New York Academy of Sciences. 2013 doi: 10.1111/nyas.12327. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kang MJ, Lim JS. Bone mineral density deficits in childhood cancer survivors: Pathophysiology, prevalence, screening, and management. Korean journal of pediatrics. 2013 Feb;56(2):60–7. doi: 10.3345/kjp.2013.56.2.60. [DOI] [PMC free article] [PubMed] [Google Scholar]


