Abstract
CYP19A1, or human aromatase catalyzes the conversion of androgens to estrogens in a three-step reaction through the formation of 19-hydroxy and 19-aldehyde intermediates. While the first two steps of hydroxylation are thought to proceed through a high-valent iron-oxo species, controversy exists surrounding the identity of the reaction intermediate that catalyzes the lyase and aromatization reaction. We investigated the kinetic isotope effect on the steady-state turnover of Nanodisc-incorporated human CYP19A1 to explore the mechanisms of this reaction. Our experiments reveal a significant (∼2.5) kinetic solvent isotope effect for the C10-C19 lyase reaction, similar to that of the first two hydroxylation steps (2.7 and 1.2). These data implicate the involvement of Compound 1 as a reactive intermediate in the final aromatization step of CYP19A1.
Keywords: Human aromatase, CYP19A1, steady-state kinetics, KSIE, C-C lyase
Introduction
The catalytic cycle of cytochrome P450 has evolved after the characterization of key intermediates involved in the hydrogen abstraction and substrate hydroxylation byP450cam [1,2] as depicted in Figure 1.
Figure 1. Cytochrome P450 Catalytic Cycle.
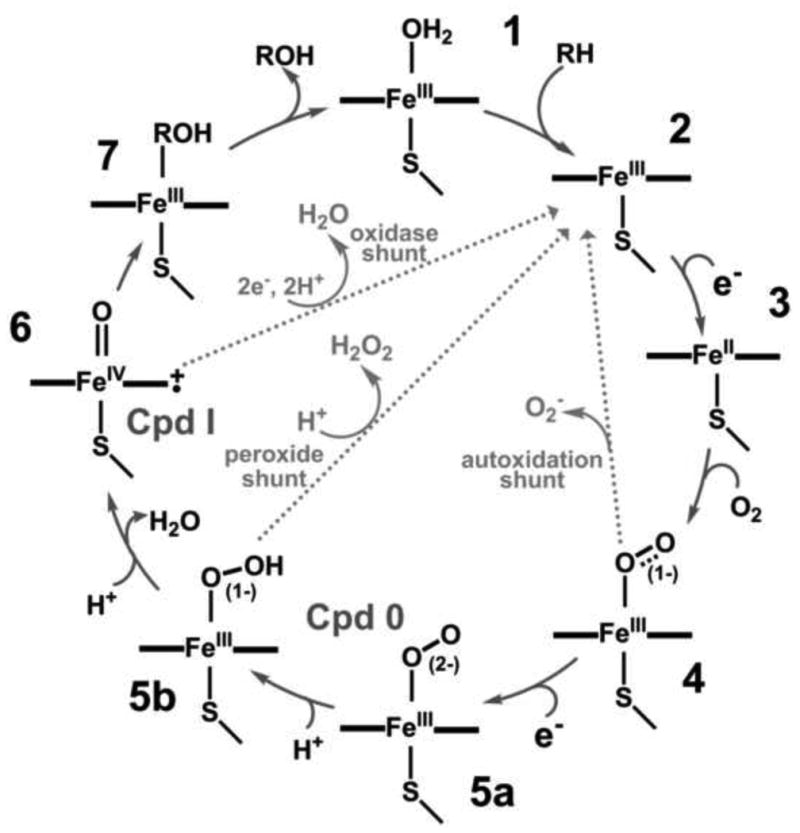
Binding of the substrate molecule to cytochrome P450 [1] partially or completely displaces the water molecule on the sixth coordination site of the heme iron and causes a shift from a hexa-coordinated low-spin complex to a penta-coordinated high-spin ferric state [2], thereby resulting in an increase in redox potential (by 100 - 130 mV) and facilitating the first electron transfer from its redox partners. Binding of molecular oxygen to the ferrous protein [3] generates the ferrous oxygenated (oxy-ferrous) state [4], a key intermediate in P450 catalysis. The availability of a second electron to the oxy-ferrous intermediate from the redox partner yields the “peroxo anion” state [5a]. Proton transfer to the distal oxygen atom orchestrated by the active site acid-alcohol pair and bound water molecules results in formation of the “hydroperoxo” state [5b]. Each of these peroxo states may undergo a non-productive release of peroxide, regenerating the ferric form of the enzyme (depicted as gray lines in Figure 1). Alternatively, a second protonation of the distal oxygen may occur which reduces the O-O bond order resulting in cleavage, release of a water molecule and generation of a higher valent metal-oxo species referred to as “Compound 1” (Cpd 1) [6] which then generates the hydroxylated substrate.
Human aromatase (CYP19A1) is a membrane-bound microsomal P450 that catalyzes the three-step conversion of androgens (androstenedione, testosterone and 16-α-hydroxytestosterone) to estrogens (estrone, 17-β-estradiol and estriol, respectively) utilizing three molecules each of NADPH and dioxygen per estrogen molecule. The reaction proceeds through two intermediates, 19-hydroxy (step I) and 19-aldehyde (step II) compounds, followed by the aromatization (step III) of ring A in the same active site (Figure 2) [3–8]. CYP19A1 also proceeds through the same typical P450 cycle as depicted in Figure 1, where the hydroxylation during the Step I and II reactions are believed to proceed through the conventional high-valent FeIV-O porphyrin cation radical, Cpd 1 [6], characteristic of most P450s. During these steps, the acid-alcohol pair, T310 and D309 conserved in the I-helix of CYP19A1 along with active site water molecules play an important role in protonating the distal oxygen atom of the peroxo anion to facilitate O-O bond cleavage and generate Cpd 1, as described in P450cam and human CYP17A1 [9–14]. However, there has been a considerable debate for the mechanism of the third step i.e. C10-C19 lyase reaction, in which two hypotheses have been proposed. In the first postulated mechanism, Cpd 1 is the key reactive species. Aromatization results from C10-C19 scission of 19-oxo-AD intermediate and subsequent release of C19 as formic acid, initiated by 1β-hydrogen abstraction of A-ring of the steroid molecule by Cpd1 [15–17]. Alternatively, the direct involvement of nucleophilic peroxo-ferric intermediate with the substrate's aldehyde functionality resulting in the decomposition of peroxo hemiacetal intermediate to yield the observed products has also been proposed [18].
Figure 2. Three-step sequential oxidation of steroid catalyzed by human CYP19A1.
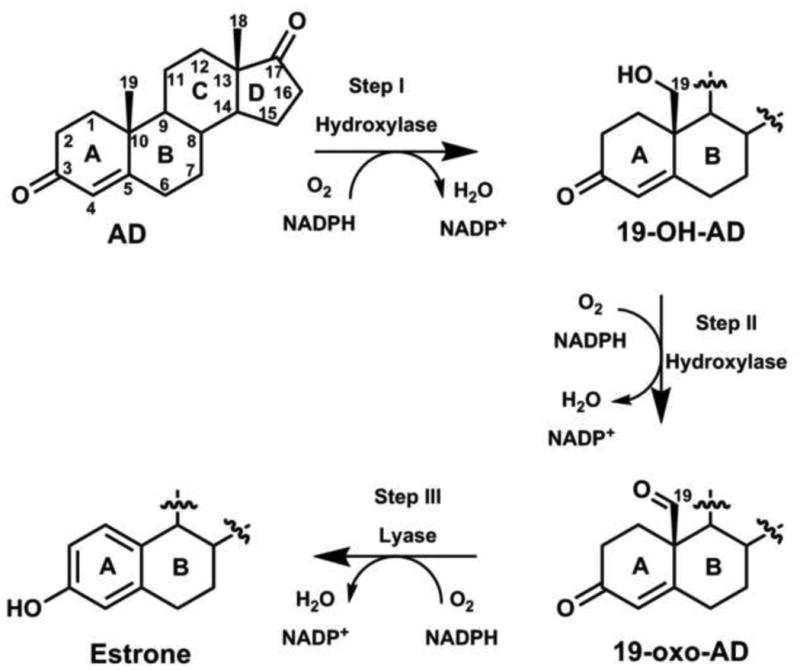
It was shown earlier that in AD-bound CYP19A1 the first intermediate that accumulates during the low temperature oxygenation and subsequent cryoreduction experiment is an unprotonated peroxo-ferric heme [19]. This suggested that the proton delivery pathway is more hindered in CYP19A1 in the presence of AD. AD, however, is the substrate for the hydroxylation step of CYP19A1. 19-oxo-AD, the substrate for the lyase step of CYP19A1 was not investigated in this study. The involvement of peroxo-ferric species as the reactive intermediate has also been suggested in CYP2B4, CYP51 and CYP17 for C–C cleavage reactions with aldehyde- or ketone-containing substrates [20–23], and by nitric oxide synthase [24]. Application of density functional theory on a minimal CYP19A1 active site showed an energetic preference for Cpd 1-mediated hydrogen abstraction from the C1 of 19-aldo AD leading to estrone formation rather than direct reaction of the peroxo-ferric species with 19-aldo AD [17]. Importantly, it has been hypothesized that the carbonyl pair (Thr310-Ala306) of CYP19A1 and the catalytic water molecule could also be responsible for the H2β abstraction of the 2,3-enolization processes during the aromatization step, in which the acid-alcohol pair Asp309 directly participate [7], unlike the indirect role of Asp251 and Glu244 in hydroxylation by P450cam and P450eryF, respectively [12]. Recently, Mak et al. characterized the oxy-complexes of AD- and 19-oxo-AD bound CYP19A1 using resonance Raman (rR) spectroscopy and found no difference in the rR signature of these two complexes. Their data also suggested the presence of a H-bonding interaction to the terminal oxygen atom of the Fe-O-O fragment for both cases, implying the involvement of Cpd 1 in both reactions [25]. Therefore, the involvement of reactive intermediates during the final stage of aromatization in the ring A of androgens by human CYP19A1 remains controversial and lacks an unambiguous conclusion for the C10-C19 lyase reaction.
Herein, we report investigation of kinetic solvent isotope effects (KSIEs) on the steady-state turnover of the hydroxylation (steps I and II) and lyase (step III) reactions by human CYP19A1. This technique has been shown to be useful in quantitating the consecutive proton-transfer processes, involved in generating the high-valent iron-oxo species [6] [26]. Therefore, the effect of deuterium substitution on the catalytic rates can provide an important indication of the number and identity of protons involved in O-O bond heterolysis [13]. As a result, either the involvement of Cpd 1, which depends on at least two protons to generate the oxoferryl intermediates ([5a]→[5b]→[6]), or nucleophilic reactivity of a ferric peroxoanion intermediate before involvement of the proton in O-O bond cleavage could be suggested, as was recently demonstrated for human CYP17A1 [23]. Although several steady-state parameters for the conversion of AD to estrone have been measured earlier either in human placental aromatase [6,27–29], recombinant human CYP19A1 expressed in insects [30,31] or bacteria [32–34], the transient-state kinetic studies during the conversion of AD to estrone have not been studied except by the Guengerich group [34]. However, all previous kinetic studies were performed in detergent solubilized-form and reconstituted with different stoichiometries of CPR, and the detailed mechanism of the lyase reaction was not addressed. In this study, we employed recombinant human CYP19A1 incorporated in Nanodiscs, which provides a soluble, homogenous, monodispersed protein with well controlled stoichiometry during reconstitution with its redox partner CPR and hence mimics the biological integrity in the membrane [35–37].
Materials and Methods
Human CYP19A1 expression, purification and Nanodisc incorporation
The expression and purification of CYP19A1 and cytochrome P450 oxidoreductase (CPR) was performed as described [19,38]. The self assembly of CYP19A1 Nanodiscs was performed as described [37].
NADPH oxidation
Incorporation of CPR into preformed and purified human CYP19A1 Nanodiscs was made by direct addition of oligomeric CPR at 1:4 CYP19A1 (200 pmol)/CPR (800 pmol) molar ratio, as described [39]. Briefly, 1 ml of CYP19A1 and CPR solution in 100 mM potassium phosphate buffer, pH 7.4, containing 50 mM NaCl and 50 μM substrate (AD, 19-OH-AD, or 19-Oxo-AD) was brought to 37 °C in a stirred quartz cuvette, path length of 0.4 cm. The sample was incubated for 3 min and the reaction was initiated by addition of 300 μM of NADPH. The consumption of NADPH was monitored by recording the absorbance at 340 nm for 10 min. The reaction was stopped by adding 50 μl of 9 M sulfuric acid to bring the pH below 4.0. The sample was removed from the cuvette, flash frozen in liquid nitrogen, and stored at −80 °C until product analysis. The optical measurements were performed on Hitachi U-3300 spectrophotometer supplied with temperature controller and built-in magnetic stirrer. The rate of NADPH oxidation was determined from the slope of absorption at 340 nm during the first three minutes using an extinction coefficient of 6.22 cm−1mM−1. A ratio of 1:4 of P450:CPR was chosen based on previously established optimal ratio of reductase to P450 for in vitro turnover experiments using the Nanodisc system [39]. In this approach the reductase, in a dynamic equilibrium with reductase molecules in solution, inserts into the P450 containing Nanodisc through its membrane anchoring tail thereby allowing the formation of an efficient electron transfer complex.
Catalytic turnover
The conversion of AD, 19-OH and 19-oxo-AD to estrone was analyzed by HPLC (Waters). Briefly, 1 μl of 18 mM progesterone solution in methanol was added to 1 ml each of the reaction sample, as an internal standard, and vortexed for 30 seconds. 2 ml of chloroform was added to each aliquots and vortexed for 30 seconds. The organic phase was removed and dried under the stream of nitrogen. The dried sample was dissolved in 100 μl of methanol and 30 μl was injected onto C18-HPLC column, using a 150 × 2.1 mm, 3 μm (ACE-111-1502) with the mobile phase of 45% each of methanol and acetonitrile in water and a flow rate of 0.2 ml/min. The 19-hydroxylated and 19-Oxo product of AD was separated in the linear gradient of methanol and acetonitrile from 20% to 80% in 30 min, and detected at 240 nm. The formation of estrogen was detected at 280 nm. Peak integration was performed with GRAM/32 software (Thermo Fischer Scientific).
Results and Discussion
Steady-state kinetic turnover by CYP19A1 in protiated and deuterated solvent systems
CYP19A1 and CPR self-assembled in Nanodiscs was used to quantitate product formation and NADPH oxidation rates in the presence of saturating concentrations of AD and 19-OH-AD for hydroxylation and 19-oxo-AD for the lyase reaction at 37°C and pH/pD 7.4. Deuterated samples were prepared by exhaustive exchange of the proteins in corresponding D2O buffers.
In order to determine the reaction time for the substrates AD, 19-OH-AD and 19-oxo-AD, firstly time-dependent substrate conversion was performed and the 10 min incubation time was chosen for the catalytic activity, during which the catalytic activity was in linear phase. (Figure 3) Using AD as the starting substrate, 19-OH-AD and 19-oxo-AD were detected in the reaction mixture under steady state conditions. Similarly, 19-oxo-AD and estrone were obtained when 19-OH-AD was used as the starting substrate for turnover experiments. Starting with AD, 19-OH-AD and 19-oxo-AD were obtained with the individual rates of 1.63 ± 0.04 min−1 and 4.06 ± 0.30 min−1, respectively, (total rate of 5.70 ± 0.34 min−1) when the reaction was carried out in a protiated buffer system. Rates of formation of both these products showed substantial slowing upon H/D substitution, yielding a KSIE for the step-I hydroxylation reaction of kH/kD = 2.7. In both the cases, the rate of second product formation was almost 2.5 times faster, suggesting a distributive nature of the enzyme. It is noteworthy that both the rates of formation of individual products (0.62 ± 0.07 min−1 and 1.52 ± 0.15 min−1 for 19-OH-AD and 19-oxo-AD, respectively) as well as total product (2.14 ± 0.22 min−1), displayed an isotope effect of ≥ 2.6. (Figure 4.A).
Figure 3.
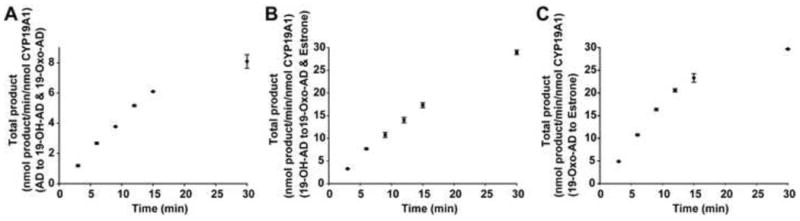
Time dependent total conversion of AD (A), 19-OH-AD (B) and 19-oxo-AD (C) by CYP19A1.
Figure 4.
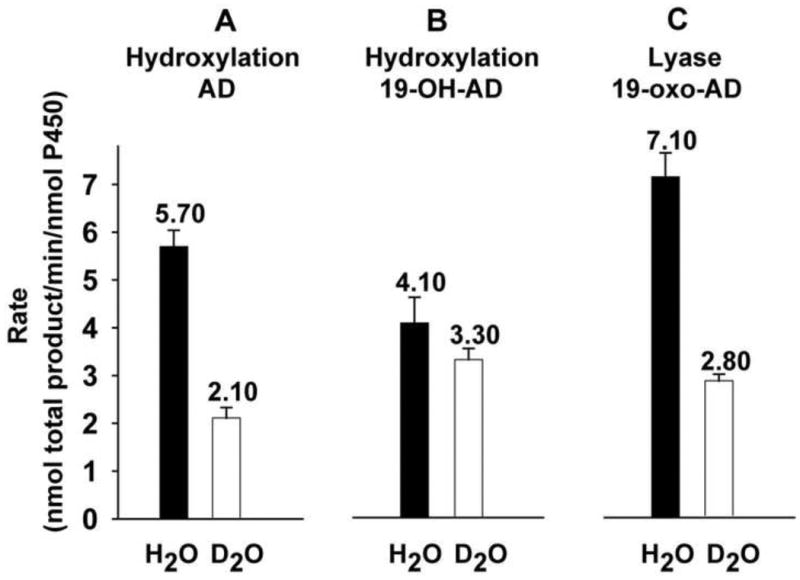
Steady-state kinetic solvent isotope effects observed for hydroxylase (A and B) and lyase (C) CYP19A1 catalysis. The number on the bar represents the value of the activity. The error bar represents the standard deviation of 3-5 independent measurements.
These KSIE data are very similar to the reported values for other well studied P450 systems catalyzing hydroxylation chemistry [13,40,41]. This was expected since the first two steps of CYP19A1 catalysis i.e. AD to 19-OH-AD and 19-OH-AD to 19-oxo-AD are standard P450 hydroxylations and are thought to go through the classical Cpd 1 mediated H-rebound mechanism [42].
When 19-oxo-AD was used as the C10-C19 lyase substrate, the rate of estrone formation was 2.5 times slower in D2O (i.e. 7.1 ± 0.8 min−1 in H2O versus 2.8 ± 0.8 min−1 in D2O) (Figure 4.C). This corresponds to the KSIE of kH/kD = 2.53, similar to that seen when AD was used as the starting substrate. This suggests the involvement of same reactive intermediate Cpd 1 during catalysis. In contrast, the involvement of unprotonated peroxo-ferric intermediate in the catalysis of C17-C20 lyase step by CYP17A1 resulted in a large inverse solvent isotope effect kH/kD = 0.4 arising from competition between catalysis through a proton-independent intermediate, viz. the peroxo anion, and uncoupling via proton-dependent uncoupling pathway(s), viz. the peroxide and the oxidase shunt [23].
The conversion of 19-OH-AD to its products by CYP19A1 is unusual. Use of 19-OH-AD, substrate for the second hydroxylation step, as the starting substrate also yielded two products. In H2O, 19-oxo-AD and estrone were obtained at a rate of 2.3 ± 0.2 min−1 and 1.8 ± 0.3 min−1 respectively giving a total rate of 4.1 ± 0.5 min−1. The second hydroxylation step of CYP19A1 also showed the same distributive nature of the enzyme and gave two products. However, the rate of second product formation was slower in this case. The rates of 19-oxo-AD and estrone decreased to 2.39 ± 0.14 min−1 and 0.91 ± 0.09 min−1, respectively (the total of 3.30 ± 0.23 min−1) in D2O, which gave an overall KSIE of kH/kD = 1.2 (Figure 4.B). Formation of estrone showed a KSIE of 2 (1.8 min−1 in H2O versus 0.91 min−1 in D2O). However, the formation of 19-oxo-AD showed no solvent isotope effect bringing the net KSIE of product formation down to 1.2. Conversion of 19-OH-AD to 19-oxo-AD goes through a hydroxylation at the C19 to produce a gem-diol that then undergoes dehydration to yield the 19-oxo-AD intermediate (Figure 5). It is plausible that the rate of dehydration of the gem-diol intermediate is partially rate limiting resulting in a diminished KSIE for 19-oxo-AD formation and giving rise to a smaller observed KSIE with respect to total product for step II as compared to steps I and III of CYP19A1 catalysis. It is noteworthy that the formation of estrone from 19-OH-AD shows a KSIE >2, which is consistent with other data. Table 1 summarizes the individual and total product formation rates using AD, 19-OH-AD and 19-oxo-AD as starting substrates in protiated and deuterated solvents.
Figure 5.
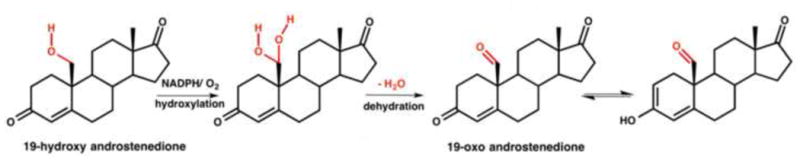
Conversion of 19-OH-AD to 19-oxo-AD by CYP19A1 going through dehydration of a gem-diol intermediate.
Table 1.
Rates of formation of CYP19 products and product intermediates in a reconstituted system under steady state turnover conditions.
| Substrate | 19-OH AD (min −1) |
19-oxo-AD (min −1) |
Estrone (min −1) |
Product 2/Product 1 | KSIE w.r.t Product 1 | KSIE w.r.t Product 2 | Total Product (min −1) |
Overall KSIE |
|---|---|---|---|---|---|---|---|---|
| Product | ||||||||
| AD | 1.63 | 4.06 | 2.49 | 2.6 | 2.7 | 5.69 | 2.7 | |
| 0.62 | 1.52 | 2.45 | 2.14 | |||||
| 19-OH AD | 2.30 | 1.80 | 0.78 | 1.0 | 2.0 | 4.10 | 1.2 | |
| 2.39 | 0.91 | 0.38 | 3.30 | |||||
| 19-oxo-AD | 7.10 | - | 2.5 | 7.10 | 2.5 | |||
| 2.80 | - | 2.80 | ||||||
H2O/D2O
NADPH oxidation rates
The overall rate of NADPH oxidation in the presence of various substrates of CYP19A1 was also studied. The total rates of conversion of AD and 19-OH in H2O are 37 ± 4.5 min−1 and 37 ± 4 min−1, and in D2O are 14 ± 1.5 min−1 and 8.00 ± 1.3 min−1, respectively. These data correspond to the “normal” KSIE observed in the product formation rate (Figure 6). Though both the conversions were highly uncoupled, the coupling efficiency was higher in D2O (16 % and 41%) compared to 15% and 11% in H2O for the substrates AD and 19-OH-AD, respectively. Likewise, when the lyase substrate 19-oxo-AD was used, the rate of NADPH oxidation in D2O was also decreased by 83%, from 35 ± 1 min−1 to 6 ± 1.2 min−1 (Figure 6). A higher level of coupling in deuterated solvents is due to slowing of the proton dependent uncoupling pathways, predominantly the peroxide and oxidase shunt pathways. The higher coupling efficiency observed in the presence of 19-OH-AD and 19-oxo-AD may be attributed to the polar nature of these substrates thereby making possible presence of additional water molecules and/or enhanced stabilization of the existing hydrogen-bonding network at the active site of the enzyme.
Figure 6.
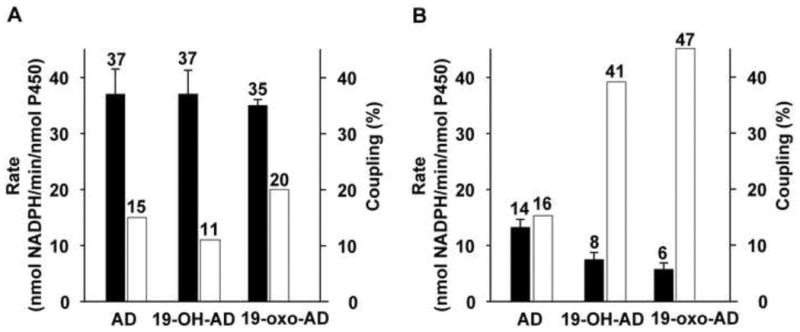
Comparison of the rates of NADPH oxidation (nmol NADPH/min/nmol p450) (solid bar) and coupling efficiency (%) (open bar) during CYP19A1 catalysis in H2O (A) and D2O (B). The error bar represents the standard deviation of 3-5 independent measurements.
Conclusion
We observe a normal slowing of Steps 1 and 2 of CYP19A1 catalysis (kH/kD of 2.7 and 1.2) consistent with the need for two protonation steps in concomitant formation of the hydroperoxo-ferric complex and Cpd 1 in D2O. These observations are also consistent with the previous reports of solvent isotope effects in P450cam and other P450s [10,41–43]. The presence of kinetic solvent isotope effect of a similar magnitude when 19-oxo-AD, the substrate for lyase step of CYP19A1, suggests the involvement of the same intermediate viz. Cpd 1 for the aromatization step of CYP19A1 as well. In addition, CYP19A1 also showed the highest coupling for the lyase substrate, 19-oxo-AD, compared with the hydroxylase substrates, AD and 19-OH-AD, in both protiated and deuterated solvent system.
Taken together, our results suggest that the involvement of the same high-valent iron-oxo species, Cpd 1, as the reactive intermediate during the conversion of AD, 19-OH-AD and 19-oxo-AD. This is based on the significant KSIE observed during the first two steps of hydroxylation as well as the last step of lyase reaction. Our conclusions are also are in line with the suggestion, albeit indirect, of Cpd1 mediated C-C scission by CYP19A1 provided by the rR, characterization of the oxy-complexes of AD- and 19-oxo-AD bound CYP19A1 in Nanodiscs. This suggestion of involvement of Cpd 1 during C10-C19 lyase reaction by CYP19A1 is in contrast to recent observation of an inverse KSIE in human CYP17A1 involved in androgen formation where the reactive peroxoanion intermediate is involved in the catalytic step [23].
In conclusion, our results highlight the involvement of Cpd 1 during the first two steps of aromatization by CYP19A1, a general mechanism known to be involved in other P450s. We also implicate the involvement of Cpd 1 as a reactive intermediate during the controversial C10-C19 lyase reaction by human aromatase.
Highlights.
The KSIE of the hydroxylation and lyase reactions by human CYP19A1 were investigated.
Compound 1 is involved in first two steps of hydroxylation.
The most controversial C-C lyase reaction (step III) also uses the Compound 1 intermediate.
Acknowledgments
This work was supported by the National Institutes of Health grant GM31756 and GM33775 to S.G.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sligar SG, Makris TM, Denisov IG. Thirty years of microbial P450 monooxygenase research: peroxo-heme intermediates--the central bus station in heme oxygenase catalysis. Biochem Biophys Res Commun. 2005;338:346–54. doi: 10.1016/j.bbrc.2005.08.094. [DOI] [PubMed] [Google Scholar]
- 2.Denisov IG, Makris TM, Sligar SG, Schlichting I. Structure and chemistry of cytochrome P450. Chem Rev. 2005;105:2253–77. doi: 10.1021/cr0307143. [DOI] [PubMed] [Google Scholar]
- 3.Akhtar M, Skinner SJ. The intermediary role of a 19-oxoandrogen in the biosynthesis of oestrogen. Biochem J. 1968;109:318–321. doi: 10.1042/bj1090318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akhtar M, Njar VC, Wright JN. Mechanistic studies on aromatase and related C-C bond cleaving P-450 enzymes. J Steroid Biochem Mol Biol. 1993;44:375–87. doi: 10.1016/0960-0760(93)90241-n. [DOI] [PubMed] [Google Scholar]
- 5.Braselton WE, Engel LL, Orr JC. The flux of intermediates and products in aromatizaton of C19 steroids by human placental microsomes. Eur J Biochem. 1974;48:35–43. doi: 10.1111/j.1432-1033.1974.tb03740.x. [DOI] [PubMed] [Google Scholar]
- 6.Thompson EA, Jr, Siiteri PK. The Involvement of Human Placental Microsomal Cytochrome P-450 in Aromatization. J Biol Chem. 1974;249:5373–5378. [PubMed] [Google Scholar]
- 7.Ghosh D, Griswold J, Erman M, Pangborn W. X-ray structure of human aromatase reveals an androgen-specific active site. J Steroid Biochem Mol Biol. 2010;118:197–202. doi: 10.1016/j.jsbmb.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lo J, Di Nardo G, Griswold J, Egbuta C, Jiang W, Gilardi G, Ghosh D. Structural basis for the functional roles of critical residues in human cytochrome p450 aromatase. Biochemistry. 2013;52:5821–9. doi: 10.1021/bi400669h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poulos TL, Finzel BC, Gunsalus IC, Wagner GC, Kraut J. The 2.6-A crystal structure of Pseudomonas putida cytochrome P-450. J Biol Chem. 1985;260:16122–30. [PubMed] [Google Scholar]
- 10.Raag R, Martinis SA, Sligar SG, Poulos TL. Crystal structure of the cytochrome P-450CAM active site mutant Thr252Ala. Biochemistry. 1991;30:11420–9. doi: 10.1021/bi00112a008. [DOI] [PubMed] [Google Scholar]
- 11.Imai Y, Nakamura M. Point mutations at threonine-301 modify substrate specificity of rabbit liver microsomal cytochromes P-450 (laurate (ω-1)-hydroxylase and testosterone 16α-hydroxylase) Biochem Biophys Res Commun. 1989;158:717–722. doi: 10.1016/0006-291x(89)92780-0. [DOI] [PubMed] [Google Scholar]
- 12.Nagano S, Poulos TL. Crystallographic study on the dioxygen complex of wild-type and mutant cytochrome P450cam. Implications for the dioxygen activation mechanism. J Biol Chem. 2005;280:31659–63. doi: 10.1074/jbc.M505261200. [DOI] [PubMed] [Google Scholar]
- 13.Vidakovic M, Sligar SG, Li H, Poulos TL. Understanding the Role of the Essential Asp251 in Cytochrome P450cam Using Site-Directed Mutagenesis, Crystallography, and Kinetic Solvent Isotope Effect. Biochemistry. 1998;37:9211–19. doi: 10.1021/bi980189f. [DOI] [PubMed] [Google Scholar]
- 14.Khatri Y, Gregory MC, Grinkova YV, Denisov IG, Sligar SG. Active site proton delivery and the lyase activity of human CYP17A1. Biochem Biophys Res Commun. 2014;443:179–84. doi: 10.1016/j.bbrc.2013.11.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korzekwa KR, Trager WF, Mancewicz J, Osawa Y. Studies on the mechanism of aromatase and other cytochrome P450 mediated deformylation reactions. J Steroid Biochem Mol Biol. 1993;44:367–373. doi: 10.1016/0960-0760(93)90240-w. [DOI] [PubMed] [Google Scholar]
- 16.Fishman J, Raju M. Mechanism of estrogen biosynthesis. Stereochemistry of C-1 hydrogen elimination in the aromatization of 2 beta-hydroxy-19- oxoandrostenedione. J Biol Chem. 1981;256:4472–4477. [PubMed] [Google Scholar]
- 17.Hackett JC, Brueggemeier RW, Hadad CM. The final catalytic step of cytochrome P450 aromatase: a density functional theory study. J Am Chem Soc. 2005;127:5224–37. doi: 10.1021/ja044716w. [DOI] [PubMed] [Google Scholar]
- 18.Akhtar M, Calder MR, Corina DL, Wright JN. Mechanistic studies C-19 demethylation in oestrogen biosynthesis. Biochem J. 1982;201:569. doi: 10.1042/bj2010569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gantt SL, Denisov IG, Grinkova YV, Sligar SG. The critical iron-oxygen intermediate in human aromatase. Biochem Biophys Res Commun. 2009;387:169–73. doi: 10.1016/j.bbrc.2009.06.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaz ADN, Pernecky SJ, Raner GM, Coon MJ. Peroxo-iron and oxenoid-iron species as alternative oxygenating agents in cytochrome P450-catalyzed reactions: switching by threonine-302 to alanine mutagenesis of cytochrome P450 2B4. Proc Natl Acad Sci. 1996;93:4644–8. doi: 10.1073/pnas.93.10.4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shyadehi AZ, Lamb DC, Kelly SL, Kelly DE, Schunck WH, Wright JN, Corina D, Akhtar M. The Mechanism of the Acyl-Carbon Bond Cleavage Reaction Catalyzed by Recombinant Sterol 14{alpha}-Demethylase of Candida albicans (Other Names Are: Lanosterol 14{alpha}-Demethylase, P-45014DM, and CYP51) J Biol Chem. 1996;271:12445–12450. doi: 10.1074/jbc.271.21.12445. [DOI] [PubMed] [Google Scholar]
- 22.Lee-Robichaud P, Shyadehi AZ, Wright JN, Akhtar M, Akhtar M. Mechanistic kinship between hydroxylation and desaturation reactions: acyl-carbon bond cleavage promoted by pig and human CYP17 (P-45017.alpha.; 17.alpha.-hydroxylase-17,20-lyase) Biochemistry. 1995;34:14104–14113. doi: 10.1021/bi00043a015. [DOI] [PubMed] [Google Scholar]
- 23.Gregory MC, Denisov IG, Grinkova YV, Khatri Y, Sligar SG. Kinetic solvent isotope effect in human P450 CYP17A1-mediated androgen formation: evidence for a reactive peroxoanion intermediate. J Am Chem Soc. 2013;135:16245–7. doi: 10.1021/ja4086403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woodward JJ, Chang MM, Martin NI, Marletta Ma. The second step of the nitric oxide synthase reaction: evidence for ferric-peroxo as the active oxidant. J Am Chem Soc. 2009;131:297–305. doi: 10.1021/ja807299t. [DOI] [PubMed] [Google Scholar]
- 25.Mak PJ, Luthra A, Sligar SG, Kincaid JR. Resonance Raman Spectroscopy of the Oxygenated Intermediates of Human CYP19A1 Implicates a Compound I Intermediate in the Final Lyase Step. J Am Chem Soc. 2014;136:4825–8. doi: 10.1021/ja500054c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aikens J, Sligar SG. Kinetic Solvent Isotope Effects during Oxygen Activation by Cytochrome P-450cam. J Am Chem Soc. 1994;116:1143–1144. [Google Scholar]
- 27.Kellis JTJ, Vickery LE. The active site of aromatase cytochrome P-450. Differential effects of cyanide provide evidence for proximity of heme-iron and carbon-19 in the enzyme-substrate complex. J Biol Chem. 1987;262:8840–8844. [PubMed] [Google Scholar]
- 28.Tan L, Muto N. Purification and reconstitution properties of human placental aromatase. A cytochrome P-450-type monooxygenase. Eur J Biochem. 1986;156:243–250. doi: 10.1111/j.1432-1033.1986.tb09574.x. [DOI] [PubMed] [Google Scholar]
- 29.Hagerman DD. Human placenta estrogen synthetase (aromatase) purified by affinity chromatography. J Biol Chem. 1987;262:2398–400. [PubMed] [Google Scholar]
- 30.Amarneh B, Simpson ER. Expression of a recombinant derivative of human aromatase P450 in insect cells utilizing the baculovirus vector system. Mol Cell Endocrinol. 1995;109:R1–5. doi: 10.1016/0303-7207(95)03524-b. [DOI] [PubMed] [Google Scholar]
- 31.Gartner CA, Thompson SJ, Rettie AE, Nelson SD. Human aromatase in high yield and purity by perfusion chromatography and its characterization by difference spectroscopy and mass spectrometry. Protein Expr Purif. 2001;22:443–54. doi: 10.1006/prep.2001.1464. [DOI] [PubMed] [Google Scholar]
- 32.Kagawa N, Cao Q, Kusano K. Expression of human aromatase (CYP19) in Escherichia coli by N-terminal replacement and induction of cold stress response. Steroids. 2003;68:205–9. doi: 10.1016/s0039-128x(02)00168-x. [DOI] [PubMed] [Google Scholar]
- 33.Kagawa N, Hori H, Waterman MR, Yoshioka S. Characterization of stable human aromatase expressed in E. coli. Steroids. 2004;69:235–43. doi: 10.1016/j.steroids.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Sohl CD, Guengerich FP. Kinetic analysis of the three-step steroid aromatase reaction of human cytochrome P450 19A1. J Biol Chem. 2010;285:17734–43. doi: 10.1074/jbc.M110.123711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Denisov IG, Sligar SG. Cytochromes P450 in nanodiscs. Biochim Biophys Acta. 2011;1814:223–9. doi: 10.1016/j.bbapap.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuler MA, Denisov IG, Sligar SG. Nanodiscs as a new tool to examine lipid-protein interactions. Methods Mol Biol. 2013;974:415–33. doi: 10.1007/978-1-62703-275-9_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luthra A, Gregory M, Grinkova YV, Denisov IG, Sligar SG. Nanodiscs in the studies of membrane-bound cytochrome P450 enzymes. In: Phillips IR, Shepard EA, Montellano PRO De, editors. Cytochrome P450 Protocols: Methods in Molecular Biology. Humana Press; New York: 2013. pp. 115–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mulrooney SB, Waskell L. High-level expression in Escherichia coli and purification of the membrane-bound form of cytochrome b(5) Protein Expr Purif. 2000;19:173–8. doi: 10.1006/prep.2000.1228. [DOI] [PubMed] [Google Scholar]
- 39.Grinkova YV, Denisov IG, Sligar SG. Functional reconstitution of monomeric CYP3A4 with multiple cytochrome P450 reductase molecules in Nanodiscs. Biochem Biophys Res Commun. 2010;398:194–8. doi: 10.1016/j.bbrc.2010.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y, Ortiz de Montellano PR. Reaction intermediates and single turnover rate constants for the oxidation of heme by human heme oxygenase-1. J Biol Chem. 2000;275:5297–307. doi: 10.1074/jbc.275.8.5297. [DOI] [PubMed] [Google Scholar]
- 41.Batabyal D, Li H, Poulos TL. Synergistic effects of mutations in cytochrome P450cam designed to mimic CYP101D1. Biochemistry. 2013;52:5396–402. doi: 10.1021/bi400676d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Groves JT. High-valent iron in chemical and biological oxidations. J Inorg Biochem. 2006;100:434–47. doi: 10.1016/j.jinorgbio.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 43.Shimada H, Sligar SG, Yeom H, Ishimura Y. Heme Monooxygenases. In: Funabiki T, editor. Oxygenases and Model Systems. Kluwer Academic Publishers; Dordrecht: 1997. pp. 195–221. [Google Scholar]


