Abstract
The relationship between superior longitudinal fasciculus microstructural integrity and neuropsychological functions were examined in 49 healthy children (range: 5–17 years) using diffusion tensor imaging. Seven major cognitive domains (intellegience, fine-motor, attention, language, visual-spatial, memory, executive function) were assessed. Data analyses utilized correlational methods. After adjusting for age and gender, fractional anisotropy and axial diffusivity values in the superior longitudinal fasciculus were positively correlated with executive functions of set-shifting; while left superior longitudinal fasciculus fractional anisotropy values correlated with attention and language. Apparent diffusion coefficient values in the left superior longitudinal fasciculus negatively correlated with inhibitory control. In the left arcuate fasciculus, fractional anisotropy correlated with IQ and attention; while radial diffusivity values negatively correlated with IQ, fine-motor skills, and expressive language. Findings from this study provide an examination of the relationship between superior longitudinal fasciculus integrity and children’s neuropsychological abilities that can be useful in monitoring pediatric neurological diseases.
Keywords: superior longitudinal fasciculus, diffusion tensor imaging, typically developing youth, cognitive function, diffusion tensor imaging
Introduction
The superior longitudinal fasciculus is a long myelinated bundle of neurons that extends from the anterior region of the cortex to the posterior region, running through the major lobules in each hemisphere. The superior longitudinal fasciculus is bi-directional in its neural transmission1. The arcuate fasciculus is a subdivision of the superior longitudinal fasciculus whose primary axons are thought to be involved in language processing2. Based on various magnetic resonance imaging-based studies, the superior longitudinal fasciculus is associated with verbal working memory 3 in the left hemisphere, and nonverbal-auditory information (e.g., pitch)4,5, attention 6 and visual-spatial functions 7 in the right hemisphere. In the left hemisphere, the microstructural integrity of the superior longitudinal fasciculus and arcuate fasciculus have been associated with various language functions (e.g., expressive speech, naming) in children, adolescents, and adults8–10.
The integrity of children’s superior longitudinal fasciculus and arcuate fasciculus can be safely studied using diffusion tensor imaging, an advanced neuroimaging technique that provides microstructural information about the orientation and diffusion characteristics of the brain's myelin tracts. This process utilizes the natural transport process of diffusion to measure how water moves through the different brain structures as water is more likely to diffuse along myelinated fiber tracts, rather than across them. This process produces a 3 dimensional brain image represented by three eigenvectors (lambda1, lambda 2 and lambda 3) which define maximum water diffusivity and reveal the direction of myelin tracts. Lambda 1 represents the axial diffusivity parallel to the axon fibers. Higher axial diffusivity reflects axon maturation. Radial diffusivity is the mean of lambda 2 and 3 and represents the diffusivity perpendicular to axonal fibers. Greater radial diffusivity reflects decreased or incomplete myelination. The deviation from isotropy measure (i.e., random water diffusion) is called fractional anisotropy. Fractional anisotropy is a scalar value between 0 and 1 that measures the directionality of water diffusion. Fractional anisotropy is greatest perpendicular to myelin tracts, represents restriction barriers to diffusion, and results in a signal intensity increase on an image. Fractional anisotropy serves as an important index of structural connectivity and myelin fiber integrity and coherence. This impedance of diffusion of water molecules is also quantitatively assessed using the apparent diffusion coefficient, a measure of flow. The apparent diffusion coefficient is greatest along fiber tracts, whereas fractional anisotropy is greatest in a direction perpendicular to myelin tracts. Apparent diffusion coefficient and fractional anisotropy measures reflect axonal maturation (for review of diffusion tensor imaging methods see11).
Thus, diffusion tensor imaging is a well-accepted method for assessing the degree of microstructural maturation of white matter tracts in children, with fractional anisotropy values increasing and the apparent diffusion coefficient and radial diffusivity values decreasing with advancing age12. Using diffusion tensor imaging, several initial studies have suggested a relationship of the superior longitudinal fasciculus with language13, spatial working memory14,15, verbal working memory16, attention 17 and reading problems 18 in children. To our knowledge, however, no studies have examined the relationship of the microstructure of superior longitudinal fasciculus and arcuate fasciculus with a broader array of neuropsychological functions in a typically-developing pediatric sample.
The primary purpose of this study was to compare the degree of microstructural maturation of the superior longitudinal fasciculus and arcuate fasciculus (as assessed by diffusion tensor imaging) with neuropsychological functions in a sample of typically -developing children and adolescents. Given that the superior longitudinal fasciculus has bidirectional connections from the anterior region to the posterior region of the cortex which involve connections to all major cortical lobules in both the right and left hemispheres, we hypothesized that all major neuropsychological domains would be related to diffusion tensor imaging values. We hypothesized that higher fractional anisotropy values, higher axial diffusivity (lambda 1) values, lower apparent diffusion coefficient and radial diffusivity values, which all likely represent a higher degree of myelination or maturation in the superior longitudinal fasciculus, would be significantly associated with major neurocognitive domains. We were also particularly interested in two hypotheses. First, we specifically hypothesized that diffusion tensor imaging measures of the left superior longitudinal fasciculus and arcuate fasciculus, would significantly correlate with higher scores on measures of receptive and expressive language, and verbal working memory. Second, we hypothesized that higher fractional anisotropy values, higher lambda 1 values, lower apparent diffusion coefficient values, and lower radial diffusivity values in the right superior longitudinal fasciculus and arcuate fasciculus would significantly correlate with higher scores on visual working memory and visual-spatial measures, as these are cognitive skills required for reading and language function. In this study, we also examined the relationship between the right and left superior longitudinal fasciculus and arcuate fasciculus and seven major neuropsychological domains (intellegience, fine-motor, attention, language, visual-spatial, memory, executive function) while controlling for the variables of age and gender that have known relationships with these major neuropsychological domains in the developing child.
Methods
Participants
The clinical assessment portion of the study was undertaken at the Healthy Childhood Brain Development Developmental Traumatology Research Program and included interviews of both adolescents and their legal guardians using the Schedule for Affective Disorders and Schizophrenia for School-Aged Children Present and Lifetime Version, which includes a comprehensive post-traumatic stress disorder interview19. Subjects underwent extensive neuropsychological testing described below to verify that they were age-typical. The sample of this cross-sectional study included 49 typically developing children and adolescents ranging from 5 to 17 years of age (Mean = 11.08 years, SD = 3.49). The participants were recruited from the community, with overall socioeconomic status falling within the middle stratum. As above, all participants received a comprehensive neuropsychological, psychiatric, and developmental history to rule out the presence of any medical or mental disorders. Additionally, prenatal and birth records of the subject were gathered and reviewed to insure no prenatal confounds such as illicit drug exposure. Exclusion criteria included left hand dominance, Full Scale IQ < 70, a disability that made a comprehensive interview of the child difficult, a significant medical illness, lifetime history of Axis I mental disorders or learning disability, documented brain injury or neurological disorder, Autism Spectrum Disorder, birth weight under 5 pounds, or severe prenatal compromise or neonatal intensive care unit stay, documented history of involvement with child protective services for abuse and/or neglect, and any contraindications to safe magnetic resonance imaging. The local university hospital Institutional Review Board committee approved the study. All legal guardians gave informed consent and children assented prior to participation in accordance with the guidelines of the Helsinki Declaration. Participant demographics are shown in Table 1.
Table 1.
Demographic Characteristics of Samples
| Superior Longitudinal Fasciculus Sample |
Arcuate Fasciculus Sample |
|
|---|---|---|
| N Mean Age in years* |
49 11.08 (3.45) |
29 11.55 (3.90) |
| IQ | 113.45 (16.47) | 114.62 (13.77) |
| Mean Hollingshead Socioeconomic status Index* |
48.80 (10.56) | 49.59 (9.58) |
| Sex | ||
| Number (Male/ Female) | 24/25 | 15/14 |
| Race | ||
| Number (White/ Other) | 29/20 | 18/11 |
Data given as means (standard deviations).
Measures
All participants in this study received a comprehensive neuropsychological evaluation at study entry; which included examination of seven of the major cognitive domains (Intelligence, fine-motor, attention, language, visual-spatial, memory, executive function), and a magnetic resonance imaging scan that included diffusion tensor imaging within two weeks of the neuropsychological evaluation.
Neuropsychological Measures
Neuropsychological measures included a comprehensive battery of tasks that were conceptually grouped into neuropsychological domains in order to facilitate data reduction. The neuropsychological domains and their associated measures included seven of the major neuropsychological domains (Intelligence, fine-motor, attention, language, visual-spatial, memory, executive function), Intelligence was assessed via a composite IQ from two-subtest short-forms of the Wechsler Preschool and Primary Scale of Intelligence-III, the Wechsler Intelligence Scale for Children-III, or the Wechsler Abbreviated Scale of Intelligence, depending on the age of the child. Fine motor speed and control was assessed with Trail-Making Test Part A, Finger Tapping, Grooved Pegboard). Attention was assessed with the Conners Continuous Performance Test Variability T-Score. Language was assessed as receptive language (Peabody Picture Vocabulary Test, Clinical Evaluation of Language Fundamentals Concepts and Directions Subtest) and expressive language (NEPSY Verbal Fluency Subtest, Wechsler Preschool and Primary Scale of Intelligence-III/ Wechsler Intelligence Scale for Children-III/ Wechsler Abbreviated Scale of Intelligence Vocabulary Subtest). Visual-spatial skills were assessed with the Judgment of Line Orientation Test, Rey-Osterrieth Complex Figure Copy Condition, NEPSY Block Construction, NEPSY Route Finding. Memory was assessed as short-term verbal memory (age-appropriate version of the California Verbal Learning Test Total Recall, Test of Memory and Learning Object Memory Recall), verbal memory-delayed (age-appropriate version of the California Verbal Learning Test Long Delay Free Recall), and visual memory- delayed (Rey-Osterrieth Complex Figure Delayed Recall). Executive functions were assessed as planning and problem solving (Wisconsin Card Sorting Test Categories Completed); working memory (Woodcock-Johnson-III Test of Cognitive Abilities Numbers Reversed Subtest); set-shifting (Trail-Making Test Part B, Stroop Color and Word Test Interference, Wisconsin Card Sorting Test Perseverative Responses); cognitive efficiency (NEPSY Verbal Fluency Subtest, NEPSY Design Fluency Subtest,
WISC-III Coding); and inhibitory control (Conners Continuous Performance Test Errors of Commission). Age-based standard scores were calculated for all of the tests for data analyses. Reliability and validity for all of the measures were deemed satisfactory. Note that for all z-scores, a higher score indicated a better performance with the exception of the attention z-score, where a lower score indicated a better performance. The cognitive domains were created by converting the previously mentioned neuropsychological measures within each domain to z-scores and then averaging the available variables within each domain except for the measure of attention which used only one measure, the Conners Continuous Performance Test Variability T-Score.
Magnetic resonance imaging technique
Magnetic resonance imaging was performed using a Siemens Trio 3.0 Tesla magnetic resonance imaging system (Trio, Siemens Medical Systems) running version VA 24 software. Diffusion weighted images were acquired using a single-shot echo-planar imaging pulse sequence. Imaging parameters were TE = 90 msec, TR = 7200 msec, bandwidth of 1346 Hz/pixel, acquisition matrix of 128 × 64, FOV of 220 mm, contiguous 3-mm slice thickness. All axial slices were acquired parallel to the anterior commissure-posterior commissure line. Images were acquired with diffusion weighting in each of 6 different directions, all with a b-value (diffusion weighting factor) of 1000. An image with no diffusion weighting (b-value of 0) was acquired as reference. The set of seven diffusion weighted images were acquired a total of 4 times for retrospective averaging to improve image quality and then averaged together after the magnitude-image reconstruction. The diffusion tensor eigenvalues were calculated in each voxel allowing the calculation of the apparent diffusion coefficient and the fractional anisotropy value in each voxel using established methods20. Our study had very strict inclusion criteria. For example, careful examination for both a lifetime history of mental illness and learning disorders, as well as examination of prenatal and birth records to insure no prenatal confounds as described above. Because of these criteria, data collection began in 2003 and ended in 2011. Since our initial data were obtained by using 6 diffusion-encoding directions, we continued this practice to insure image comparability across subjects. A neuroradiologist reviewed scans and excluded scans with any clinically significant abnormalities. Subjects tolerated the procedure well. No sedation was used.
Data-processing
A radiologist with a four-year experience in Neuroradiology and one year experience in diffusion tensor imaging analyses analyzed the diffusion tensor imaging data. Diffusion tensor matrices from sets of the seven diffusion-weighted images were generated, and the three eigenvalues and eigenvectors were calculated via matrix diagonalization in Diffusion Tensor Imaging Studio (H. Jiang and S. Mori, Department of Radiology, Johns Hopkins University, Baltimore). Maps were generated according to the following standard algorithms:
Where sb=1000 was obtained by averaging the six diffusion-weighted images;
Where λn is the eigenvalue describing the diffusion tensor and <λ> = (λ1+λ2+ λ3)/3. Radial diffusivity was calculated as (λ2 + λ3)/2.
Simple head motion of diffusion weighted images was corrected by affine co-registration of all diffusion weighted images to b=0 image. Fiber assignment by means of continuous tracking (FACT) was used with a fractional anisotropy threshold value of 0.2 and angle transition threshold of 80° during tracking.
Tractography Method for the superior longitudinal fasciculus
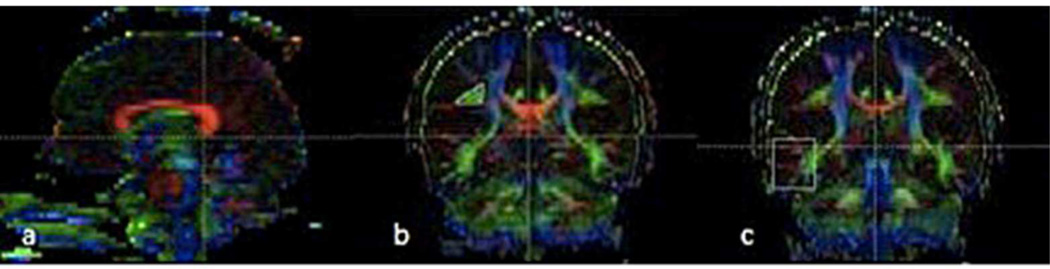
At the level of the rostral aspect of the splenium of corpus callosum, lateral to the superior-to-inferior corticospinal blue fibers, bilateral green triangular-shaped regions representing the superior longitudinal fasciculus fibers were encircled using a manual tracing for the region of interest on coronal color map images (Figure-1). The entire superior longitudinal fasciculus, including the arcuate fasciculus, were obtained (Figure-2) and depicted on the color map and then automatically transferred to the fractional anisotropy, apparent diffusion coefficient, lambda 1(λ1), lambda 2 (λ2), and lambda 3 (λ3) maps to generate the values for each of these parameters.
Figure 1.
Region of interest placement for the diffusion tensor imaging superior longitudinal fasciculus and arcuate fasciculus measures on coronal color map images. Figure-1a: To obtain superior longitudinal fasciculus, the first region of interest was placed at the level of the rostral aspect of the splenium of the corpus callosum, depicted on sagittal color map image with slice marker. Figure-1b: Bilateral green triangular shaped regions lateral to the corticospinal blue fibers on coronal color map image, representing superior longitudinal fasciculus, are encircled using a manual tracing for this region of interest. Figure-1c: For further arcuate fasciculus segmentation, a second, rectangular-shaped 30×10 pixel region of interest was placed next to the superior posterior temporal lobe on coronal color map image.
Figure 2.
a: Sagittal view of superior longitudinal fasciculus with all segments (in red). Figure-2b: Sagittal view of the arcuate fasciculus. Figure-2c: Axial views depicting arcuate fasciculus.
Tractography Method for arcuate fasciculus
After obtaining the whole superior longitudinal fasciculus, we put a second region of interest (30×10 pixel regions) to the superior posterior temporal lobe on coronal color map images (Figure-1). The fibers between the first and second regions of interest represented the arcuate fasciculus (Figure-2). Maps for fractional anisotropy, apparent diffusion coefficient and λ1, λ2, and λ3 were generated to obtain the values for each of these parameters.
Laterality Indices
We calculated laterality indices to compare the left and right superior longitudinal fasciculus, fractional anisotropy, and apparent diffusion coefficient values. The mean laterality index was calculated by (left – right) / (left + right). For this formula, a positive mean indicates that the left is larger than the right, and a negative mean indicates that the right is larger than the left. A mean laterality index close to zero indicates more symmetry in the integrity of the superior longitudinal fasciculus in both hemispheres. Because of diffusion tensor imaging acquisition parameters or motion artifacts, which may have had a negative effect on detecting thin arcuate fasciculus, we were only able to validly measure both left and right observations in 29 of the 49 subjects for the arcuate fasciculus measures that were included in this aspect of the study.
Intra-Observer Variability
A single rater collected tractography measurements of the superior longitudinal fasciculus twice, two months apart. Because the measurements are continuous variables, Cronbach’s alpha was used as a measure of internal consistency21. Cronbach’s alpha coefficient ranges from 0 to 1, and higher scores (above .70) suggest that the two measurements were identifying the same item22. With regard to fractional anisotropy values in the superior longitudinal fasciculus, the repeated tractography measurements of the right side showed a Cronbach’s alpha of .79, while the left side showed an alpha of .97. These findings indicated very good agreement on each side. Aside from the right fractional anisotropy measurements, no measurements had a Cronbach’s alpha coefficient lower than .92, illustrating extremely high intra-rater reliability.
Data Analyses
To address the research questions, Pairwise Partial Pearson correlations controlling for age and gender were conducted between the 15 neuropsychological measures and the left and right elements of the superior longitudinal fasciculus and arcuate fasciculus. As described in other studies from our group23, each cognitive domain was adjusted with a Bonferroni correction for the number of neuropsychological measures in each domain. For example, for the domain of Intelligence, the p-value was set at p ≤.05; while for executive function domain, the p-value was set at p ≤ 0.01.
Results
Correlations of superior longitudinal fasciculus and cognitive functions
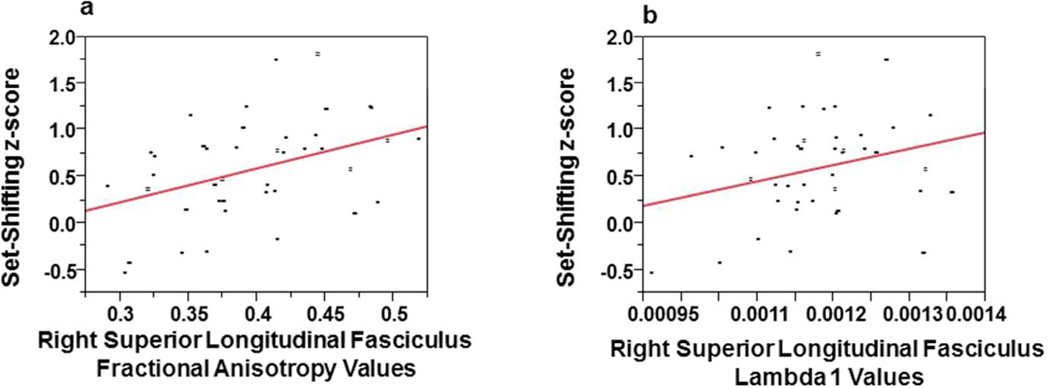
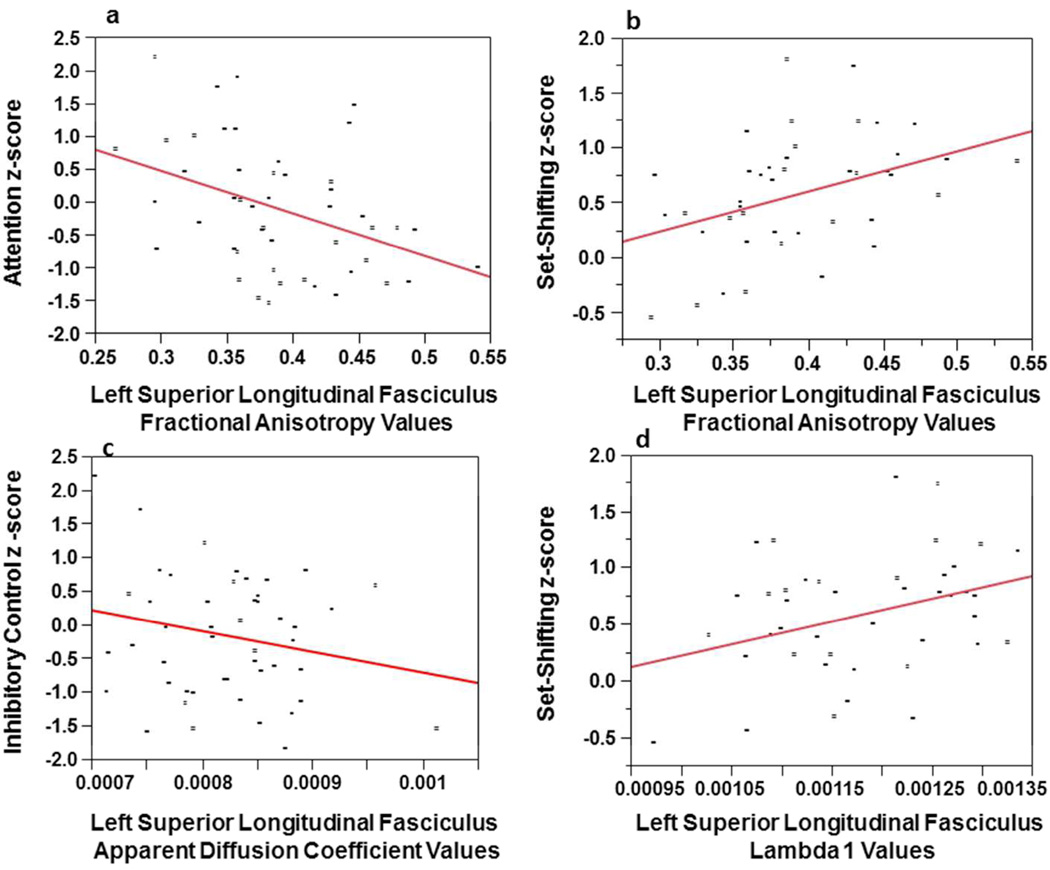
Table 2 provides partial correlations between diffusion tensor imaging indices from the right and left superior longitudinal fasciculus with the seven cognitive domains (intellegience, fine-motor, attention, language, visual-spatial, memory, executive function) controlling for age and gender. Table 2 shows that the executive function and attention domains were significantly associated with both right and left diffusion tensor imaging values. Specifically, higher values of fractional anisotropy and λ1 in the right and left superior longitudinal fasciculus correlated with better set-shifting abilities. Figures-3a and 3b show that right superior longitudinal fasciculus fractional anisotropy and λ1 account for about 13% (Adjusted R2 = .125) and 5% (Adjusted R2 = .052) of the variance, respectively, in set-shifting. Higher values of both right and left fractional anisotropy were significantly associated with better performance on an attention task (note higher scores on the attention domain indicated worse performance). Figure 4a and 4b show that the left superior longitudinal fasciculus, fractional anisotropy values significantly correlated with attention and set-shifting and in the expected direction. These relationships account for approximately 15% (Adjusted R2 = .145) and 13% (Adjusted R2 = .127) of the variance, respectively. Apparent diffusion coefficient values in the left superior longitudinal fasciculus inversely correlated with inhibitory control, and in the expected direction, so poorer inhibitory control was associated with higher apparent diffusion coefficient values. Figure-4c shows that as apparent diffusion coefficient values decreased (a mature of myelin maturation), inhibitory control improved. This relationship accounted for approximately 2% of the variance (Adjusted R2 = .024). Values of λ1 in the left superior longitudinal fasciculus were found to be positively correlated with set-shifting. Figure-4d shows that left superior longitudinal fasciculus λ1 accounts for about 9% of the variance in set-shifting (Adjusted R2 = .089).
Table 2.
Pairwise Partial Pearson Correlation Coefficients of Superior Longitudinal Fasciculus Diffusion Tensor Imaging Measures with Seven Neuropsychological Domains after controlling for chronological age and gender (N=49).
| Right Superior Longitudinal Fasciculus | Left Superior Longitudinal Fasciculus | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Domain | Apparent Diffusion Coefficient |
Fractional Anisotropy |
Axial Diffusivity (λ1) |
Radial Diffusivity |
Apparent Diffusion Coefficient |
Fractional Anisotropy |
Axial Diffusivity (λ1) |
Radial Diffusivity |
|
| Intelligence | |||||||||
| Child IQ | −.06 (.68) |
.23 (.11) |
.22 (.13) |
−.15 (.30) |
−.09 (.54) |
.22 (.13) |
.13 (.37) |
−.22 (.14) |
|
| Fine-Motor Speed and Control | |||||||||
| Fine-Motor Dominant |
−.11 (.46) |
−.16 (.30) |
−.15 (.32) |
.06 (.70) |
−.12 (.41) |
−.15 (.32) |
−.24 (.11) |
−.02 (.91) |
|
| Fine-Motor Nondominant |
−.02 (.92) |
.14 (.45) |
.07 (.71) |
−.09 (.64) |
−.12 (.52) |
.23 (.21) |
−.02 (.89) |
−.17 (.35) |
|
| Attention | |||||||||
| Conners Continuous Performance Variability T-Score |
.07 (.62) |
−.30 (.04) |
−.14 (.34) |
.17 (.26) |
.04 (.80) |
−.39 (.006) |
−.25 (.08) |
.22 (.13) |
|
| Language | |||||||||
| Receptive Language |
−.10 (.48) |
.25 (.08) |
.18 (.23) |
−.18 (.23) |
−.02 (.87) |
.31 (.03) |
.16 (.27) |
−.15 (.32) |
|
| Expressive Language |
−.22 (.14) |
.25 (.10) |
.05 (.75) |
−.25 (.09) |
−.19 (.20) |
.34 (.02) |
.05 (.75) |
−.31 (.04) |
|
| Visual-Spatial | |||||||||
| Visual-Spatial | .17 (.26) |
−.03 (.84) |
.25 (.10) |
.07 (.62) |
.18 (.23) |
−.01 (.96) |
.21 (.17) |
.10 (.50) |
|
| Memory | |||||||||
| Verbal Memory |
.11 (.49) |
−.26 (.09) |
.01 (.93) |
.18 (.25) |
.25 (.11) |
−.24 (.13) |
.05 (.75) |
.32 (.04) |
|
| Verbal Memory Delayed |
−.12 (.45) |
.06 (.72) |
−.01 (.95) |
−.14 (.36) |
−.07 (.66) |
.07 (.64) |
.03 (.85) |
−.13 (.40) |
|
| Visual Memory Delayed |
.09 (.63) |
−.12 (.49) |
.03 (.88) |
.10 (.59) |
.12 (.49) |
−.04 (.81) |
.06 (.72) |
.13 (.48) |
|
| Executive Function | |||||||||
| Problem Solving |
−.03 (.87) |
.27 (.09) |
.23 (.15) |
−.18 (.25) |
.08 (.64) |
.23 (.15) |
.26 (.10) |
−.10 (.54) |
|
| Working Memory |
−.19 (.22) |
.15 (.35) |
−.03 (.83) |
−.20 (.20) |
−.17 (.30) |
.20 (.21) |
.06 (.72) |
−.28 (.08) |
|
| Set-Shifting | .05 (.73) |
.38 (.01) |
.42 (.006) |
−.21 (.19) |
.17 (.29) |
.40 (.01) |
.51 (.001) |
−.16 (.32) |
|
| Cognitive Efficiency |
.11 (.51) |
−.10 (.53) |
.07 (.67) |
.12 (.45) |
.13 (.41) |
−.21 (.19) |
.08 (.63) |
.14 (.40) |
|
| Inhibitory Control |
−.28 (.05) |
.21 (.15) |
−.20 (.17) |
−.32 (.02) |
−.39 (.006) |
.06 (.70) |
−.33 (.02) |
−.32 (.03) |
|
Statistically significant correlations are bolded. Each cognitive domain was adjusted with a Bonferroni correction for the number of psychological measures in each domain.
Figure 3.
a: Linear regression plots of set-shifting z-score and right superior longitudinal fasciculus fractional anisotropy values; and Figure-3b: set-shifting z-score and right superior longitudinal fasciculus lambda 1(λ1) values, both correlations adjusted for chronological age and gender.
Figure 4.
a: Linear regression plots of attention z-score and left superior longitudinal fasciculus fractional anisotropy values. Note the higher the attention z-score, the poorer the performance on the attention task so higher attention scores were associated with less mature measures of myelination. Figure-4b: Linear regression plots of the set-shifting z-score and left superior longitudinal fasciculus fractional anisotropy values. Figure-4c: Linear regression plots of the inhibitory control z-score and left superior longitudinal fasciculus apparent diffusion coefficient values. Figure-4d: Linear regression plots of the set-shifting z-score and left superior longitudinal fasciculus lambda 1(λ1) values. All correlations were adjusted for chronological age and gender.
As hypothesized, expressive language significantly correlated with left superior longitudinal fasciculus fractional anisotropy values; while there was a trend for a correlation with left longitudinal fasciculus fractional anisotropy values and receptive language.
No relationships were seen between the domains of intelligence, fine-motor, memory, and visual-spatial skills, and diffusion tensor imaging measures of the longitudinal fasciculus.
Correlations of arcuate fasciculus and Neuropsychological Functions
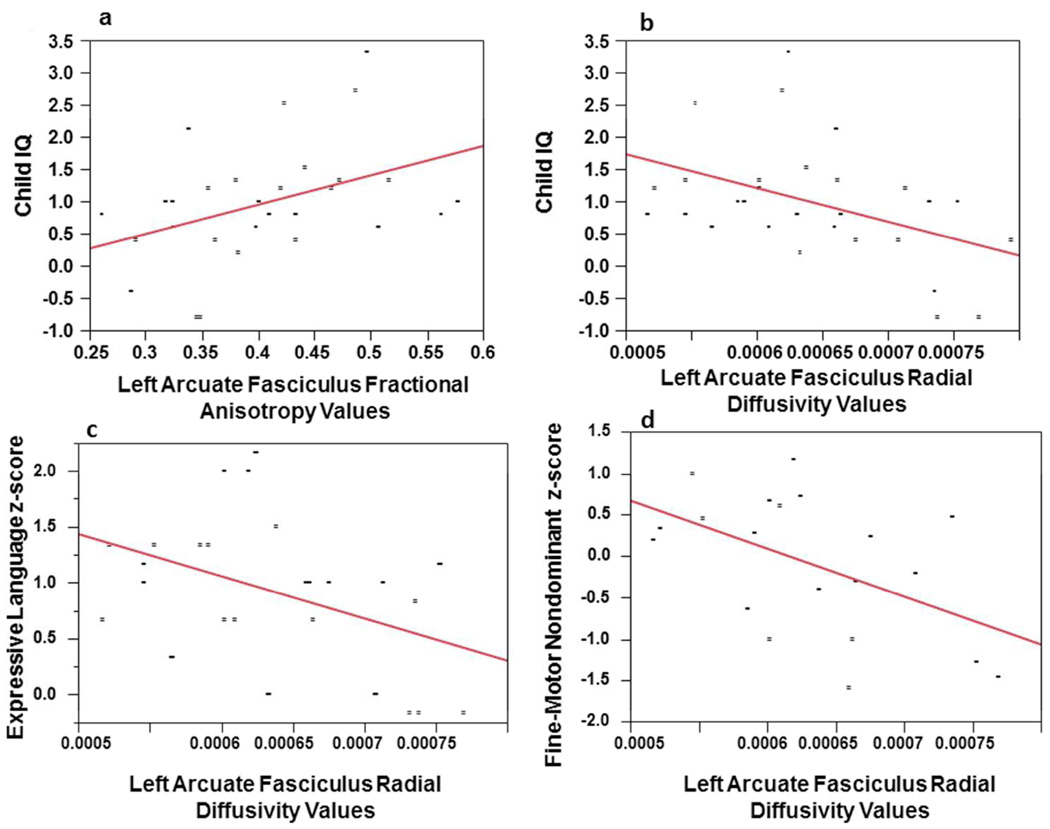
Table 3 provides the partial correlations between the arcuate fasciculus diffusion tensor imaging measures with the seven cognitive domains (intellegience, fine-motor, attention, language, visual-spatial, memory, executive function) controlling for age and gender. As can be seen in Table 3, no significant correlations were seen between the right arcuate fasciculus diffusion tensor imaging measures and any of the neuropsychological measures. However, when the left arcuate fasciculus is examined, fractional anisotropy and radial diffusivity were found to significantly correlate with IQ in the expected direction. Figures-5a depicts linear regressions of the left fractional anisotropy value with IQ. This relationship accounted for approximately 13% of the variance (Adjusted R2 = .128). The left-sided radial diffusivity also significantly correlated with non-dominant (left) fine-motor speed, and expressive language. These correlations were all in inverse direction as expected. The linear regressions for these relationships can be seen in Figures-5b, 5c and 5d, and accounted for approximately 15% to 22% of the variance. The respective Adjusted R2 statistics for these relationships were .158, .149, and .216, respectively. The attention domain significantly correlated with left-sided fractional anisotropy and radial diffusivity values in the expected direction.
Table 3.
Pairwise Partial Pearson Correlation Coefficients of Arcuate Fasciculus Diffusion Tensor Imaging Measures with Neuropsychological Domains after controlling for chronological age and gender (N=29).
| Right Arcuate Fasciculus | Left Arcuate Fasciculus | |||||||
|---|---|---|---|---|---|---|---|---|
| Domain | Apparent Diffusion Coefficient |
Fractional Anisotropy |
Axial Diffusivity (λ1) |
Radial Diffusivity |
Apparent Diffusion Coefficient |
Fractional Anisotropy |
Axial Diffusivity (λ1) |
Radial Diffusivity |
| Intelligence | ||||||||
| Child IQ | −.18 (.37) |
.32 (.11) |
.23 (.23) |
−.18 (.37) |
−.23 (.25) |
.46 (.01) |
.30 (.13) |
−.48 (.01) |
| Fine-Motor Speed and Control | ||||||||
| Fine-Motor Dominant |
.01 (.96) |
−.21 (.29) |
−.20 (.31) |
.25 (.20) |
−.33 (.09) |
.15 (.44) |
−.17 (.40) |
−.23 (.24) |
| Fine-Motor Nondominant |
.37 (.13) |
.06 (.82) |
.24 (.33) |
.21 (.41) |
−.36 (.14) |
.30 (.22) |
.19 (.44) |
−.57 (.01) |
| Attention | ||||||||
| Conners Continuous Performance Variability T-Score |
.07 (.72) |
-.10 (.60) |
-.25 (.21) |
.16 (.43) |
.20 (.31) |
-.37 (.05) |
-.21 (.29) |
.37 (.05) |
| Language | ||||||||
| Receptive Language |
.02 (.92) |
.05 (.80) |
.34 (.08) |
−.01 (.94) |
.02 (.91) |
.27 (.17) |
.25 (.21) |
−.18 (.38) |
| Expressive Language |
−.34 (.09) |
.22 (.28) |
.17 (.41) |
−.28 (.18) |
−.36 (.07) |
.41 (.04) |
.070 (.74) |
−.47 (.01) |
| Visual-Spatial | ||||||||
| Visual-Spatial | −.01 (.95) |
−.16 (.43) |
.01 (.95) |
.01 (.96) |
−.14 (.49) |
.17 (.39) |
.13 (.52) |
−.26 (.20) |
| Memory | ||||||||
| Verbal Memory |
.03 (.88) |
−.14 (.51) |
.12 (.58) |
.02 (.93) |
.27 (.20) |
−.25 (.24) |
−.10 (.65) |
.41 (.05) |
| Verbal Memory Delayed |
.11 (.62) |
−.01 (.98) |
.13 (.55) |
.05 (.81) |
.126 (.56) |
−.147 (.49) |
.020 (.93) |
.125 (.56) |
| Visual Memory Delayed |
−.03 (.91) |
.06 (.81) |
.14 (.55) |
−.09 (.71) |
−.25 (.30) |
.25 (.30) |
.04 (.86) |
−.32 (.19) |
| Executive Function | ||||||||
| Problem Solving |
−.09 (.69) |
.10 (.65) |
.27 (.21) |
−.25 (.25) |
.17 (.44) |
.22 (.31) |
.34 (.11) |
−.10 (.64) |
| Working Memory |
−.16 (.44) |
.13 (.54) |
.12 (.56) |
−.17 (.42) |
−.11 (.59) |
.11 (.60) |
.05 (.80) |
−.22 (.29) |
| Set-Shifting | .32 (.13) |
−.33 (.11) |
.19 (.37) |
.28 (.18) |
.30 (.15) |
.09 (.67) |
.27 (.20) |
.10 (.62) |
| Cognitive Efficiency |
.18 (.40) |
−.20 (.34) |
−.23 (.28) |
.39 (.06) |
.13 (.54) |
−.13 (.53) |
.19 (.36) |
−.05 (.82) |
| Inhibitory Control |
−.14 (.47) |
.06 (.77) |
−.12 (.56) |
−.19 (.35) |
−.34 (.08) |
−.06 (.77) |
−.16 (.42) |
−.24 (.23) |
Statistically significant correlations are bolded. Each cognitive domain was adjusted with a Bonferroni correction for the number of psychological measures in each domain.
Figure 5.
Figure-5a: Linear regression plots of child IQ z-score and left arcuate fasciculus fractional anisotropy values. Figure-5b: Linear regression plots of child IQ z-score and left arcuate fasciculus radial diffusivity values. Figure-5c: Linear regression plots of expressive language z-score and left arcuate fasciculus radial diffusivity values. Figure-5d: Linear regression plots of fine-motor speed non-dominant hand z-score and left arcuate fasciculus radial diffusivity values. All correlations were adjusted for chronological age and gender.
Laterality Indices
As can be seen in Table 4, when laterality indices are calculated for the superior longitudinal fasciculus fractional anisotropy, the superior longitudinal fasciculus apparent diffusion coefficient, the arcuate fasciculus fractional anisotropy, and the arcuate fasciculus apparent diffusion coefficient, there is very little difference between these diffusion tensor imaging hemispheric indices. This suggested symmetrical integrity of the superior longitudinal fasciculus and arcuate fasciculus in healthy youth.
Table 4.
Laterality Indices for superior longitudinal fasciculus and arcuate fasciculus
| N | Minimum | Maximum | Mean | Deviation | |
|---|---|---|---|---|---|
| Superior longitudinal fasciculus fractional anisotropy laterality index |
49 | −.06 | .04 | −.0069 | .02062 |
| Superior longitudinal fasciculus apparent diffusion coefficient laterality index |
49 | −.05 | .02 | .0033 | .01140 |
| Arcuate fasciculus fractional anisotropy laterality index |
29 | −.09 | .09 | −.0056 | .04862 |
| Arcuate fasciculus Apparent apparent diffusion coefficient laterality index |
29 | −.05 | .03 | .0018 | .01699 |
Discussion
In this study, we compared the degree of maturation of the superior longitudinal fasciculus and arcuate fasciculus with seven cognitive domains in a sample of typically-developing children and adolescents. We found a number of correlations suggesting that a greater degree of microstructural maturation of the superior longitudinal fasciculus (and, in particular, the left superior longitudinal fasciculus) was associated with higher performance on neuropsychological tests. In particular, we found that higher fractional anisotropy and axial diffusivity values in the left superior longitudinal fasciculus and right superior longitudinal fasciculus were significantly correlated with set-shifting. There was a positive relationship of fractional anisotropy in the right and left superior longitudinal fasciculus with better performance on the attention task. Furthermore, a significant correlation was found between lower apparent diffusion coefficient values in the left superior longitudinal fasciculus and inhibitory control. Finally, examination of the arcuate fasciculus, a component of the superior longitudinal fasciculus, showed higher fractional anisotropy values significantly correlated with IQ, and lower radial diffusivity values in the left arcuate fasciculus significantly correlated with IQ, fine-motor speed and expressive language; while the attention domain significantly correlated with left-sided fractional anisotropy and radial diffusivity values in the expected direction.
Our correlations of diffusion tensor imaging parameters with language function are supported by a number of findings in previous studies by other investigators8–10,24,25. Previous studies have found correlations between language processing abilities and diffusion tensor imaging metrics of the superior longitudinal fasciculus in healthy individuals3,8,26. For example, one study found that speed of visual word recognition, a factor in linguistic competence, correlated with regional fractional anisotropy values in tracts aligned in the anterior-posterior direction at or near the left superior longitudinal fasciculus27. Furthermore, diffusion tensor imaging evidence of damage to these regions has been associated with language dysfunction. In patients with primary progressive aphasia, microstructural damage to the left superior longitudinal fasciculus, as evidenced by reduced fractional anisotropy, strongly correlated with both deficits of syntactic comprehension and production28.
The association of left superior longitudinal fasciculus integrity (as measured using diffusion tensor imaging) with language also extends to a number of disease states. For instance, schizophrenia is an illness characterized by (among other signs) disordered speech and auditory hallucinations. Diffusion tensor imaging studies of childhood-onset schizophrenia have shown increased axial diffusivity and radial diffusivity in a subgroup of individuals with linguistic impairment compared to children with schizophrenia without linguistic impairment. Abnormalities were particularly noted in the left superior longitudinal fasciculus and the left inferior longitudinal fasciculus29.
As expected, the left arcuate fasciculus radial diffusivity values were found to be significantly correlated with expressive language. The left superior longitudinal fasciculus was correlated with receptive and expressive language functions in our study in a small to moderate effect (p < 0.05); however, it was not statistically significant given our more conservative criterion for significance (i.e., p < 0.025) nor did it account for a large proportion of the variance. Two major pathways in the language network exist in the left hemisphere: a ventral pathway via the extreme capsule fiber system and a dorsal pathway via the superior longitudinal fasciculus/ arcuate fasciculus30,31. It has been shown that the dorsal pathway does not solely support language comprehension in children. Instead, supplementary involvement of the ventral pathway exists to facilitate this language function13. We did not analyze the maturity of the ventral pathway, but the neurological immaturity of this dorsal connection might explain the lack of correlation of language function with the superior longitudinal fasciculus in our study of typically developing children and adolescents.
The correlation of the superior longitudinal fasciculus with attention 17 and the arcuate fasciculus with intelligence 32 has been reported previously. Correlation of set shifting with the superior longitudinal fasciculus 33 and correlation of fine-motor speed with arcuate fasciculus 34 has been reported in adults but not examined in youth; however, examination of the literature did not reveal any diffusion tensor Imaging studies showing the correlation of the superior longitudinal fasciculus with inhibitory control and set shifting, or any findings showing a correlation of arcuate fasciculus with fine motor speed and control in children, which we believe are a new finding presented in our study.
We did not find any correlations between diffusion tensor imaging indices in the right superior longitudinal fasciculus and the fine motor, memory, and visual-spatial domains. More generally, our relative lack of association between right-sided superior longitudinal fasciculus and neuropsychological functioning was somewhat unexpected, but was consistent with findings in the adult literature where even visual memory tasks have been more strongly associated with the left rather than the right superior longitudinal fasciculus35. With regard to this relationship, a previous study found significantly reduced intervoxel coherence values (indicative of diminished structural connectivity) in the left superior longitudinal fasciculus, but not the right, in low performers on a visual memory task compared to high performers35. Several other studies also have reported that spatial working memory was correlated with the left superior longitudinal fasciculus, but not the right14,15. There was also a lack of correlation between left superior longitudinal fasciculus and verbal working memory, again contrary to our initial hypotheses. This may be related to ongoing brain maturation in children, as it has previously been demonstrated that adults use a more confined language network compared with children13, or perhaps is a result of measurement limitations of our study, which are discussed below.
With respect to the lateralization indices for the superior longitudinal fasciculus and arcuate fasciculus, there are a few studies that have examined language lateralization and associated fractional anisotropy values in children. Tiwari et al. 36measured fractional anisotropy of the arcuate fasciculus after tractography to determine language laterality in children and found no significant asymmetry. This was consistent with our findings. This relatively even neurodevelopment of the arcuate fasciculus component of the microstructure of the superior longitudinal fasciculus, which reflects both symmetrical maturation of intracellular machinery and the microstructure surrounding the axon in both hemispheres, may be related to ongoing myelination and maturation of the pediatric brain.
Recent articles have concluded that more accurate results of scalar diffusion parameters are obtained with increasing numbers of diffusion-encoding gradient directions37. Nevertheless, diffusion-encoding directions less than 12 are still in use, give accurate results, and are published in esteemed articles in literature26,38. Our study had very strict inclusion criteria. For example, we excluded participants with lifetime mental disorders or learning disabilities and we examined prenatal and birth records to insure no prenatal confounds. Data collection began in 2003 and ended in 2011. Because our initial data were obtained by using 6 diffusion-encoding directions, we continued this practice to insure image comparability across subjects. The images were acquired a total of 4 times to improve image quality and then averaged together. Simple head motions were corrected by affine co-registration of all diffusion weighted images to b=0 image. Because all children were scanned in the same scanner, under the same imaging protocol, and with the same number of diffusion-encoding gradient directions, the data obtained was expected to have similar degree of signal-to-noise ratio, which allows for the accurate comparison in our data.
Our study has several limitations. First, although we believe that our correlation coefficients were relatively stable, our small sample size clearly limited our power to detect additional relationships between the diffusion tensor imaging measures of the superior longitudinal fasciculus and various neuropsychologicalmeasures. We also attempted to control for spurious correlations by using a Bonferroni correction adjusted for the number of neuropsychological measures in each domain to determine significance as we have done in previously published preliminary studies23. Second, we are aware that different neuropsychological measures may have produced different cognitive associations with the diffusion tensor imaging indices. Although all of our neuropsychological measures had good reliability and validity, a different set of cognitive measures with different psychometric properties could have produced different results. Third, although conceptually based, the cognitive domains might have produced a different pattern and/or strength of correlations if the factors had been derived using empirical methods (e.g., factor analysis); however, our sample size limited the formation of empirical factors. Finally, although we adjusted for both age and gender in the derivation of our correlations, the study remains cross-sectional in nature, and it will be important to determine if these relationships persist in longitudinal designs and with different sample of children and adolescents with diseases or disorders.
The novel findings reported here showing significant correlations of the superior longitudinal fasciculus with inhibitory control and set shifting in a typically developing sample of children and adolescents have the potential to contribute to our understanding of both normal and atypical brain development. This study is the first to describe the correlation of the superior longitudinal fasciculus with inhibitory control and set shifting in children and adolescents, and to show the correlation of arcuate fasciculus with fine-motor speed in a typically-developing sample of youth. Diffusion tensor imaging measures of the superior longitudinal fasciculus and arcuate fasciculus were found to be correlated with expressive language functions, particularly with the generation of verbal output. The structural integrity of white matter tracts increases in childhood and adolescence and decreases in elderly people accompanying cognitive and motor decline. The integrity of the tract may also decrease in certain pediatric or neurological disorders, which might affect the function related to that tract. This study provides important preliminary data that increase our understanding of targeted brain-behavior relationships in typically developing children and adolescents, and also lays the foundation for examining neuropsychological functions in pediatric populations with diseases and disorders. Being aware of these significant associations between diffusion tensor imaging measures and cognitive function may help clinicians better diagnosis and clinically monitor their pediatric patients. This data may also be of value to neurosurgeons as they may be able to make a more preventive surgical plan to either save or monitor important neuropsychological functions whose relationship to the superior longitudinal fasciculus was previously unknown (i.e., executive functions of inhibitory control and set shifting) in brain tumor treatment or in the prediction of outcome after neurosurgery or radiation procedures. When interpreting the brain imaging examination in many diseases (including stroke, trauma, masses, demyelinating diseases or metabolic disorders), knowledge of these cognitive correlations with the right and left superior longitudinal fasciculus and arcuate fasciculus, can be complementary to a patient’s clinical status. We hope that accumulation of the knowledge about diffusion tensor imaging correlations with neuropsychological measures will help pediatric patients during their treatment; which is the main goal of these types of investigations.
Acknowledgements
The authors thank the staff of the Duke Healthy Childhood Brain Development Developmental Traumatology Research Program and the participants and their families for making this work possible.
Funding
This work was supported by funding from National Institutes of Health [K24 MH071434, K24-DA028773 and R01- MH61744, R01-AA12479, R01-MH63407 to De Bellis].
Footnotes
Author Contributions
SRH and SEU performed the literature search. SEU, SC, and SRH were included in acquisition of the data. SEU, SC, DW, MDDB, JP and SRH worked on analysis and interpretation of data.. SRH worked on the conception and design. SRH drafted the article. SEU prepared the manuscript. SRH, JP and MDDB revised and approved the final version for publication.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval
The local university hospital Institutional Review Board committee approved the study. All legal guardians gave informed consent and children assented prior to participation in accordance with the guidelines of the Helsinki Declaration.
Contributor Information
Sacide E. Urger, Istanbul Cerrahi Hastanesi, Tesvikiye mahallesi, 34365 Sisli, Istanbul, Turkey
Michael D. De Bellis, Department of Psychiatry and Behavioral Sciences, Duke University Medical School, Durham, NC 27710.
Stephen R. Hooper, Department of Psychiatry and The Carolina Institute for Developmental Disabilities, University of North Carolina School of Medicine, Chapel Hill, North Carolina 27514.
Donald P. Woolley, Department of Psychiatry and Behavioral Sciences, Duke University Medical School, Durham, NC 27710
Steven D. Chen, Department of Radiology, Duke University Medical School, Durham, NC 27710
James Provenzale, Department of Radiology, Duke University Medical School Durham, NC 27710 and Departments of Radiology and Imaging Sciences, Oncology and Biomedical Engineering, Emory University School of Medicine, Atlanta, GA 30322.
References
- 1.Makris N, Kennedy DN, McInerney S, et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cerebral Cortex. 2005;15(6):854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- 2.Geschwind N. The organization of language and the brain. Science. 1970;170:940–944. doi: 10.1126/science.170.3961.940. [DOI] [PubMed] [Google Scholar]
- 3.Peters BD, Szeszko PR, Radua J, et al. White Matter Development in Adolescence: Diffusion Tensor Imaging and Meta-Analytic Results. Schizophrenia Bulletin. 2012;38(6):1308–1317. doi: 10.1093/schbul/sbs054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loui P, Kroog K, Zuk J, Winner E, Schlaug G. Relating pitch awareness to phonemic awareness in children: implications for tone-deafness and dyslexia. Frontiers in Psychology. 2011;2:111. doi: 10.3389/fpsyg.2011.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loui P, Wu EH, Wessel DL, Knight RT. A generalized mechanism for perception of pitch patterns. The Journal of Neuroscience. 2009;29(2):454–459. doi: 10.1523/JNEUROSCI.4503-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frye RE, Hasan K, Malmberg B, et al. Superior longitudinal fasciculus and cognitive dysfunction in adolescents born preterm and at term. Developmental Medicine & Child Neurology. 2010;52(8):760–766. doi: 10.1111/j.1469-8749.2010.03633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoeft F, Barnea-Goraly N, Haas BW, et al. More is not always better: increased fractional anisotropy of superior longitudinal fasciculus associated with poor visuospatial abilities in Williams syndrome. The Journal of Neuroscience. 2007;27(44):11960–11965. doi: 10.1523/JNEUROSCI.3591-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lebel C, Beaulieu C. Lateralization of the arcuate fasciculus from childhood to adulthood and its relation to cognitive abilities in children. Human Brain Mapping. 2009;30(11):3563–3573. doi: 10.1002/hbm.20779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeatman JD, Feldman HM. Neural plasticity after pre-linguistic injury to the arcuate and superior longitudinal fasciculi. Cortex. 2011;49(1):301–311. doi: 10.1016/j.cortex.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Wang C, Zhao X, Chen H, Han Z, Wang Y. Diffusion tensor imaging depicting damage to the arcuate fasciculus in patients with conduction aphasia: a study of the Wernicke-Geschwind model. Neurological Research. 2010;32(7):775–778. doi: 10.1179/016164109X12478302362653. [DOI] [PubMed] [Google Scholar]
- 11.Le Bihan D, Mangin J-F, Poupon C, et al. Diffusion tenson imaging: concepts and applications. Journal of Magnetic Resonnanc e Imaging. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- 12.Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40(3):1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- 13.Brauer J, Anwander A, Friederici AD. Neuroanatomical prerequisites for language functions in the maturing brain. Cerebral Cortex. 2011;21(2):459–466. doi: 10.1093/cercor/bhq108. [DOI] [PubMed] [Google Scholar]
- 14.Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. J Cogn Neurosci. 2004;16(7):1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- 15.Vestergaard M, Madsen KS, Baaré WF, et al. White matter microstructure in superior longitudinal fasciculus associated with spatial working memory performance in children. Journal of Cognitive Neuroscience. 2011;23(9):2135–2146. doi: 10.1162/jocn.2010.21592. [DOI] [PubMed] [Google Scholar]
- 16.Østby Y, Tamnes CK, Fjell AM, Walhovd KB. Morphometry and connectivity of the fronto-parietal verbal working memory network in development. Neuropsychologi a. 2011;49(14):3854–3862. doi: 10.1016/j.neuropsychologia.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton LS, Levitt JG, O'Neill J, et al. Reduced white matter integrity in attention-deficit hyperactivity disorder. Neuroreport. 2008;19(17):1705–1708. doi: 10.1097/WNR.0b013e3283174415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niogi SN, McCandliss BD. Left lateralized white matter microstructure accounts for individual differences in reading ability and disability. Neuropsychologia. 2006;44(11):2178–2188. doi: 10.1016/j.neuropsychologia.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman J, Birmaher B, Brent D, et al. Schedule for affective Disorders and Schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 20.Taylor WD, Hsu E, Krishnan KR, MacFall JR. Diffusion tensor imaging: background, potential, and utility in psychiatric research. Biological Psychiatry. 2004;55(3):201–207. doi: 10.1016/j.biopsych.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Crocker L, Algina J. Procedures for Estimating Reliability. In: Crocker L, Algina J, editors. Introduction to Classical and Modern Test Theory. Orlando FL: Holt Rinehart and Winston; 1986. pp. 131–156. [Google Scholar]
- 22.Nunnally JC. The Assessment of Reliability. In: Nunnally JC, editor. Psychometric Theory 3rd edition. New York NY: McGraw-Hill; 1994. pp. 248–292. [Google Scholar]
- 23.Beers SR, De Bellis MD. Neuropsychological function in children with maltreatment-related posttraumatic stress disorder. American Journal of Psychiatry. 2002;159:483–486. doi: 10.1176/appi.ajp.159.3.483. [DOI] [PubMed] [Google Scholar]
- 24.Marchina S, Zhu LL, Norton A, Zipse L, Wan CY, Schlaug G. Impairment of speech production predicted by lesion load of the left arcuate fasciculus. Stroke. 2011;42(8):2251–2256. doi: 10.1161/STROKEAHA.110.606103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papoutsi M, Stamatakis EA, Griffiths J, Marslen-Wilson WD, Tyler LK. Is left fronto-temporal connectivity essential for syntax? Effective connectivity, tractography and performance in left-hemisphere damaged patients. Neuroimage. 2011;58(2):656–664. doi: 10.1016/j.neuroimage.2011.06.036. [DOI] [PubMed] [Google Scholar]
- 26.Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage. 2012;60:340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- 27.Gold BT, Powell DK, Xuan L, Jiang Y, Hardy PA. Speed of lexical decision correlates with diffusion anisotropy in left parietal and frontal white matter: evidence from diffusion tensor imaging. Neuropsychologia. 2007;45(11):2439–2446. doi: 10.1016/j.neuropsychologia.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson SM, Galantucci S, Tartaglia MC, et al. Syntactic processing depends on dorsal language tracts. Neuron. 2011;72(2):397–403. doi: 10.1016/j.neuron.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark K, Narr KL, O'Neill J, et al. White matter integrity, language, and childhood onset schizophrenia. Schizophrenia Bulletin. 2012;138:150–156. doi: 10.1016/j.schres.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frey S, Campbell JS, Pike GB, Petrides M. Dissociating the human language pathways with high angular resolution diffusion fiber tractography. The Journal of Neuroscience. 2008;28(45):11435–11444. doi: 10.1523/JNEUROSCI.2388-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saur D, Kreher BW, Schnell S, et al. Ventral and dorsal pathways for language. Proc Natl Acad Sci USA. 2008;105(46):18035–18040. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Cognitive functions correlate with white matter architecture in a normal pediatric population: a diffusion tensor MRI study. Human Brain Mapping. 2005;26(2):139–147. doi: 10.1002/hbm.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perry ME, McDonald CR, HaglerJr DJ, et al. White matter tracts associated with set-shifting in healthy aging. Neuropsychologia. 2009;47(13):2835–2842. doi: 10.1016/j.neuropsychologia.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlaug G, Marchina S, Norton A. Evidence for plasticity in white-matter tracts of patients with chronic Broca’s aphasia undergoing intense intonation-based speech therapy. Ann NY Acad Sci. 2009;1169:385–394. doi: 10.1111/j.1749-6632.2009.04587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Begré S, Frommer A, von Känel R, Kiefer C, Federspiel A. Relation of white matter anisotropy to visual memory in 17 healthy subjects. Brain Research. 2007;7(1168):60–66. doi: 10.1016/j.brainres.2007.06.096. [DOI] [PubMed] [Google Scholar]
- 36.Tiwari VN, Jeong JW, Asano E, Rothermel R, Juhasz C, Chugani HT. A sensitive diffusion tensor imaging quantification method to detect language laterality in children: correlation with the Wada test. J Child Neurol. 2011;26(12):1516–1521. doi: 10.1177/0883073811409225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Correia MM, Carpenter TA, Williams GB. Looking for the optimal Diffusion Tensor Imaging acquisition scheme given a maximum scan time: are more b-values a waste of time? Magn Reson Imaging. 2009;27(2):163–175. doi: 10.1016/j.mri.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 38.Ojemann JG, Partridge SC, Poliakov AV, et al. Diffusion tensor imaging of the superior cerebellar peduncle identifies patients with posterior fossa syndrome. Childs Nerv Syst. 2013 Jul 2; doi: 10.1007/s00381-013-2205-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]