Abstract
Background
Intra-arterial recanalization therapy (IAT) is increasingly utilized for acute stroke. Despite high rates of recanalization the outcome is variable. We attempted to identify predictors of outcome that will enable better patient selection for IAT.
Methods
All patients who underwent IAT at the UT Houston Stroke Center were reviewed. Poor outcome was defined as modified Rankin Scale score 4–6 on hospital discharge. Findings were validated in an independent dataset of 175 patients from UCLA Stroke Center
Results
190 patients were identified. Mean age 62, median baseline NIHSS .18. Recanalization rate 75%, symptomatic hemorrhage rate 6%, and poor outcome rate 66%. Variables associated with poor outcome were: age, baseline NIHSS, admission glucose, diabetes, heart disease, previous stroke and the absence of mismatch on the pre-treatment MRI. Logistic regression identified three variables independently associated with poor outcome: age (p=0.049, OR=1.028), NIHSS (p=0.013, OR=1.084), admission glucose (p=0.031, OR=1.011). Using this data, we devised the Houston IAT (HIAT) score: 1 point for age>75; 1 for NIHSS>18 and 1 point for glucose>150mg/dL (range 0 to 3). The percentage of poor outcome by HIAT score was: score of 0: 44%; 1: 67%; 2: 97%; 3: 100%. Recanalization rates were similar across the scores (p=0.4). Applying HIAT to the external cohort showed comparable trends in outcome and nearly identical rates in the HIAT 3 tier.
Conclusions
The HIAT score estimates the chances of poor outcome after IAT even with recanalization. It may be useful in comparing cohorts of patients and when assessing the results of clinical trials.
Introduction
Intra-arterial therapy (IAT) is increasingly utilized in the treatment of acute stroke either as the primary modality (pIAT) for patients presenting 3–8 hours from symptom onset, or as an adjuvant measure (aIAT) in patients treated with IV tPA who do not improve in a timely fashion. pIAT has been shown to be improve clinical outcome when administered 3 to 6 hours from symptom onset using intra-arterial thrombolytics1 2, and is approved up to 8 hours using mechanical clot retrieval (Merci Retriever) or suction thrombectomy (Penumbra System) 3, 4. aIAT has been studied in phase I and II trials5 and two randomized trials are ongoing6. The primary goal of IAT is recanalization of the occluded artery and reperfusion of the ischemic territory. Recanalization has been shown to correlate with a better outcome in stroke patients7;, however this correlation may be confounded by several factors including the time from symptom onset to recanalization and the degree of collateral circulation to the ischemic region8 and the extent of infarct prior to recanalization9. Therefore, it is not surprising that despite high rates of recanalization with IAT, the rate of functional independence is reported to be only 40–50%4, 10–12. A recent meta-analysis of uncontrolled IAT cohort studies failed to detect benefit compared to a model predicting outcome without IAT,13 suggesting a need for more rigorous evaluation of the efficacy and safety of this approach. IAT is invasive, frequently requiring intubation and admission to an intensive care unit (ICU). IAT is also a very time and resource intensive procedure requiring a trained and dedicated interventional team. It may therefore be important both clinically and economically to improve patient selection for IAT. Important predictors of outcome in IAT have already been identified. In a post-hoc analyses of the PROACT II trial1 the investigators identified age and NIHSS14 and the ASPECT score15 (a semiquantitive measure of early ischemic changes in the MCA territory on admission CT) as independent predictors of outcome. Those predictors were used to evaluate the treatment effect of pro-Urokinase in MCA strokes and may not be applicable to current IAT techniques, thrombolytics, and devices. Older patients have also been previously shown to benefit less from IAT in a single-center series of 114 patients16. Based on these data, we hypothesized that patients with severe strokes, advanced age and co-morbidities are less likely to benefit from IAT. The purpose of this study was therefore to determine, in stroke patients subjected to IAT in routine clinical practice, admission criteria that will identify patients who are not likely to benefit from IAT.
Methods
Study Population
IAT patients were identified using the University of Texas at Houston (UTH) prospective stroke registry from 1998 to 2007. In our institution, the criteria for considering IAT are: ischemic stroke within 6 hours of time first evaluated by the stroke team, disabling symptoms, large vessel occlusion (suspected or documented), and less than a third of the MCA territory showing hypodensity on the admission CT. For patients eligible for IV tPA, the same criteria are applied after tPA is given if the patients do not recanalize (by transcranial Doppler, CT angiography or MR angiography) and show no clinical improvement at the end of the infusion. Patients were excluded from this study if they were treated more than 8 hours from symptom onset or participating in a clinical trial.
Measurements
MRI was obtained before IAT depending on the availability of the MRI machine. At our institution, candidates for IAT are routinely intubated before the procedure. In addition, follow-up imaging with either CT or MRI, and clinical assessment using NIHSS are routinely obtained as close as possible to 24 hours after IV tPA or IAT. We reviewed the records and neuroimaging of all patients taken to IAT from 1998 to 2007. Clinical data points included demographics, medical history, symptom onset time, baseline NIHSS, laboratory values, IV tPA treatment, symptomatic intracerebral hemorrhage (sICH; defined as a parenchymal hematoma grade 217 associated with worsening neurological status thought to be related to the hematoma) and functional outcome on discharge as measured by the modified Rankin Scale (mRS). Poor outcome was defined as mRS 4–6 on hospital discharge. Radiological data points included pre IAT MRI if performed (including DWI lesion volume and presence of mismatch); and post-IAT CTs or MRIs. “Malignant” lesion was defined as DWI infarct volume >100cc on pre-IAT MRI. Mismatch was defined as more than 20% difference between the DWI lesion and perfusion(PWI) deficit (by eyeballing the lesion) on pre- IAT MRI. Angiography data included IAT duration (defined from groin puncture to the time of the last angiogram), responsible vessel, degree of occlusion, thrombolytic used, mechanical device used, and degree of recanalization at the end of the procedure. For recanalization we used TICI score18. Recanalization (partial and complete) was defined as TICI 2b or higher as this was previously shown to better correlate with good outcome19.
The study was approved by the institutional review board.
Validation Cohort
For the validation dataset, we used a cohort of consecutive patients treated with IAT within 8 hours of symptom onset at the University of California Los Angeles (UCLA) stroke center between July 1992 and December 2007.
Statistical analysis
The analysis was carried out using SPSS for Windows version 15 (SPSS Inc. Chicago, IL). We conducted a univariate analysis using Chi-square for catergorical variables and logistic regression for continuous variables, to identify potential predictors of poor outcome. To reduce the likelihood of type I error, we prespecified the following variables to be tested: age, admission NIHSS, admission glucose, presence of mismatch on pre-treatment MRI, presence of a malignant lesion on pre-treatment MRI, co-morbidities (hypertension, coronary artery disease, diabetes, previous stroke) and time from symptom onset to IAT (this was included since in most cases this time can be estimated in the ER). Variables from univariate analysis resulting in p-values of less than 0.2 were entered into the multivariate logistic regression model using the forced entry method. At the multiple regression level, variables with a p-value greater than 0.05 were excluded. The Hosmer-Lemeshow goodness of fit statistics was used to assess the final model. We also tested the association of procedure-related variables (recanalization and IAT duration) to outcome; however, as the primary goal of the study was predicting IAT outcome before the procedure, these were not used in the multivariate model.
The independent predictors identified in the multivariate analysis were used to create a score. Continuous variables were dichotomized at the median or the 75th percentile where appropriate. Spearman’s correlation explored how well the final score correlated with discharge mRS.
Receiver Operator Characteristics (ROC) curves were utilized to explore agreement between the predictive score and outcomes in the original and validation cohorts. Chi square test was used to compare the proportion of poor outcome of each tier of the HIAT score between the UTH and UCLA cohorts.
Results
We identified 190 IAT patients in the UTH dataset. 74 (38.9%) underwent pIAT and 116 (61.1%) aIAT. 41 (21.6%) had pre-IAT MRI. The IAT techniques used were as follows: intra-arterial thrombolytics only 30.5% (58/190); intra-arterial thrombolytics with guide-wire clot-disruption 48.9% (93/190); guide-wire clot-disruption only 2.1% (4/190); Merci clot retriever 18.5% (35/190). The stroke was in the dominant hemisphere in 94 patients (49.5%). Symptomatic ICH occurred in 13 patients (6.8%) and the rate of recanalization was 75.6%. Table 1 shows the main baseline and outcome characteristics.
Table 1.
Baseline characteristics and outcome
| Cohort | UTH (n=190) | UCLA (n=175) | P value (test used) |
|---|---|---|---|
| Age (mean± SD) | 62±14 | 70±17 | <0.001 (TT) |
| Admission NIHSS, median (IQR) | 19 (16.5–22.5) | 17 (12–22) | 0.126 (MW) |
| Admission glucose (mg/dL), mean ± SD | 144±55 | 136±56 | 0.181 (TT) |
| Mortality (%) | 42 (22.1) | 29 (16.6) | 0.182 (CS) |
| Poor outcome (%) | 126 (66) | 84 (48) | <0.001 (CS) |
IQR- interquartile range; TT- t-test; CS Chisquare test; MW- Mann-Whitney test
Pre-IAT predictors of outcome and score development
The results of univariate analysis in the derivation cohort for all variables are listed in Table 2. The following predictors of poor outcome were identified in univariate analysis and entered into the multivariate analysis: Age, NIHSS, admission glucose, history of coronary artery disease, history of diabetes mellitus and previous stroke. Presence of a non-malignant DWI lesion or mismatch on the pre-IAT MRI was associated with a favorable outcome. Following logistic regression, only age (Wald=3.9, p=0.049, OR=1.028), NIHSS (Wald=6.1, p=0.013, OR=1.084) and admission glucose (Wald=4.7, p=0.031, OR=1.011) remained as significant predictors of poor outcome. We then proceeded to dichotomize the predictors according to our primary hypothesis: age and admission glucose at the 75th percentile and admission NIHSS at the median. We scored 1 point for each variable as follows: age>75; NIHSS > 18; admission glucose > 150mg/dL. The sum of points resulted in a score we named the UTH IAT (HIAT) score. HIAT score ranges from 0 to 3.
Table 2.
univariate analysis of outcome predictors
| Variable | Good outcome (mRS 0–3) | Poor outcome (mRS 4–6) | P value (test used) |
|---|---|---|---|
| N=64 | N=126 | ||
| Pre-IAT variables | |||
| Median NIHSS (range) | 16 (5–29) | 20 (3–39) | <0.001 (MW) |
| Mean Age ± SD | 59 ± 12 | 63.6 ± 14.5 | 0.032 (TT) |
| Mean Glucose ± SD | 126 ± 37 | 153 ± 61 | 0.001 (TT) |
| Diabetes (%) | 11 (17) | 31 (26) | 0.16 (CS) |
| PVD (%) | 1 (2) | 4 (4) | 0.42 (CS) |
| CHF (%) | 3 (5) | 10 (10) | 0.27 (CS) |
| Hypertension (%) | 126.4 ± 36.6 | 153 ± 60.6 | 0.37 (CS) |
| CAD (%) | 12 (19) | 36 (30) | 0.092(CS) |
| Previous stroke (%) | 6 (9) | 21 (18) | 0.116 (CS) |
| Malignant pattern on MRI (%) | 1 (2) | 5 (4) | 0.37 (CS) |
| Non-malignant pattern or mismatch pattern on MRI (%) | 16 (25) | 15 (12) | 0.021 (CS) |
| IAT variables | |||
| Mean Onset to IAT time (minutes) ± SD | 266 ± 80 | 282 ± 83 | 0.28 (TT) |
| Mean IAT duration (minutes) ± SD | 82 ± 45 | 104 ± 44 | 0.002 (TT) |
| Mean Onset to recanalization time (minutes) ± SD | 348 ± 91 | 387 ± 94 | 0.029 (TT) |
| Recanalization (%) | 50 (85) | 76 (70) | 0.042 (CS) |
TT- t-test; CS Chi square test; MW- Mann-Whitney test
Procedural outcomes
Table 2 also shows the association between the prespecified procedural outcomes and clinical outcomes in the univariate analysis. Recanalization was associated with favorable outcome and IAT duration with poor outcome. There was no association between the time from symptom onset to IAT and outcome. Also, there was no association between the rates of recanalization and the presence of mismatch on MRI (p=0.15). Table 3 shows the procedural and clinical outcomes according to the occluded artery, IAT technique used and whether or not IV tPA was given before IAT.
Table 3.
Procedural and clinical outcomes by arterial cooclusion, IAT technique and combination therapy
| pICA | tICA | M1 | M2 | BA | P value | IAL | IAL+M | MERCI | MERCI+IAL | P value | pIAT | aIAT | P value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Recanal. (%) | 14 (67) | 14 (74) | 48 (74) | 28 (78) | 21 (84) | 0.7 | 38 (73) | 61 (71) | 6 (100) | 19 (86) | 0.2 | 50 (71) | 76 (78) | 0.36 |
| sICH (%) | 2 (10) | 2 (10) | 4 (6) | 2 (6) | 0 (0) | 0.6 | 3 (5) | 4 (4) | 0 (0) | 5 (17) | 0.08 | 6 (8) | 7 (6) | 0.6 |
| Mortality (%) | 8 (38) | 4 (20) | 12 (17) | 3 (8) | 12 (48) | 0.002 | 12 (21) | 17 (18) | 2 (33) | 10 (35) | 0.3 | 21 (28) | 21 (18) | 0.1 |
pICA- proximal ICA; tICA- terminal ICA; BA- basilar artery; IAL- intra-arterial thrombolithics; M- mechanical clot disruption; MERCI- Merci© retriever; pIAT- primary intraarterial therapy; aIAT- adjuvant intra-arterial therapy; Recanal- recanlization; sICH- symptomatic intracranial hemorrhage
Exploring clinical and procedural outcomes using the HIAT score
We next plotted the percentage of patients with the various study end points by the HIAT score (Table 4). The HIAT score demonstrated an increase in the proportion of poor outcome up to 100% in patients with HIAT of 3. A similar pattern was observed when we looked at mortality and symptomatic ICH. There was no difference in the time from symptom onset to IAT and the rates of recanalization across scores; however, IAT duration was significantly increased in the HIAT 2 and 3 groups. The time from symptom onset to recanalization was likewise increased; however, this was not statistically significant (Table 4)
Table 4.
study end-points by HIAT score
| HIAT 0 (n=57) | HIAT 1 (n=89) | HIAT 2 (n=35) | HIAT 3 (n=9) | P value (test used) | |
|---|---|---|---|---|---|
| Poor outcome (%) | 25 (43.9) | 58 (65.2) | 34 (97.1) | 9 (100) | <0.001 (CS) |
| Mortality (%) | 6 (10.5) | 17 (19.1) | 15 (42.9) | 4 (44.4) | 0.001 (CS) |
| sICH (%) | 3 (5.3) | 4 (4.5) | 5 (14.3) | 1 (11.1) | 0.23 (CS) |
| Median time from stroke onset to IAT (minutes) | 276 | 277 | 283 | 294 | 0.89 (KW) |
| Median IAT duration (minutes) | 86 | 79 | 106 | 133 | 0.003 (KW) |
| Recanalization (%) | 38 (79.2) | 58 (72.5) | 22 (71.0) | 8 (88.9) | 0.6 (CS) |
| Median time from stroke onset to recanalization (minutes) | 361 | 359 | 403 | 401 | 0.29 (KW) |
CS- Chisquare test; MW- Mann-Whitney test
HIAT score validation
The UCLA cohort consisted of 175 patients. ROC curve analysis of the HIAT score showed that the score performed equally well in both cohorts (Figure 1). The rates of poor outcome and mortality were comparable in the HIAT 3 tier; however, in the lower tiers of 0, 1 and 2 there were lower rates of poor outcome and mortality in the UCLA cohort (Figure 2).
Figure 1.
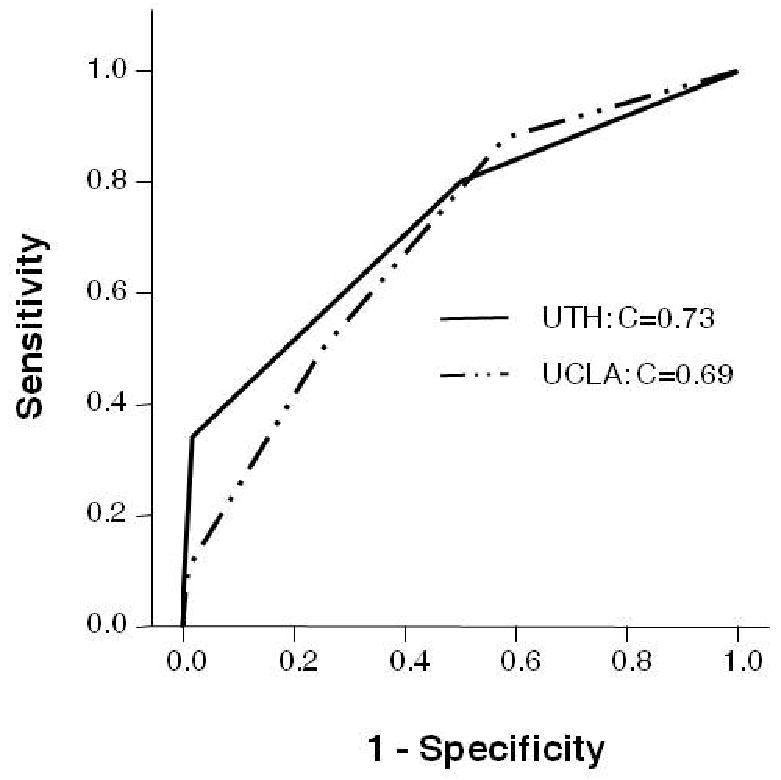
ROC curves comparing the performance of HIAT in the UTH and UCLA cohorts.
Figure 2.
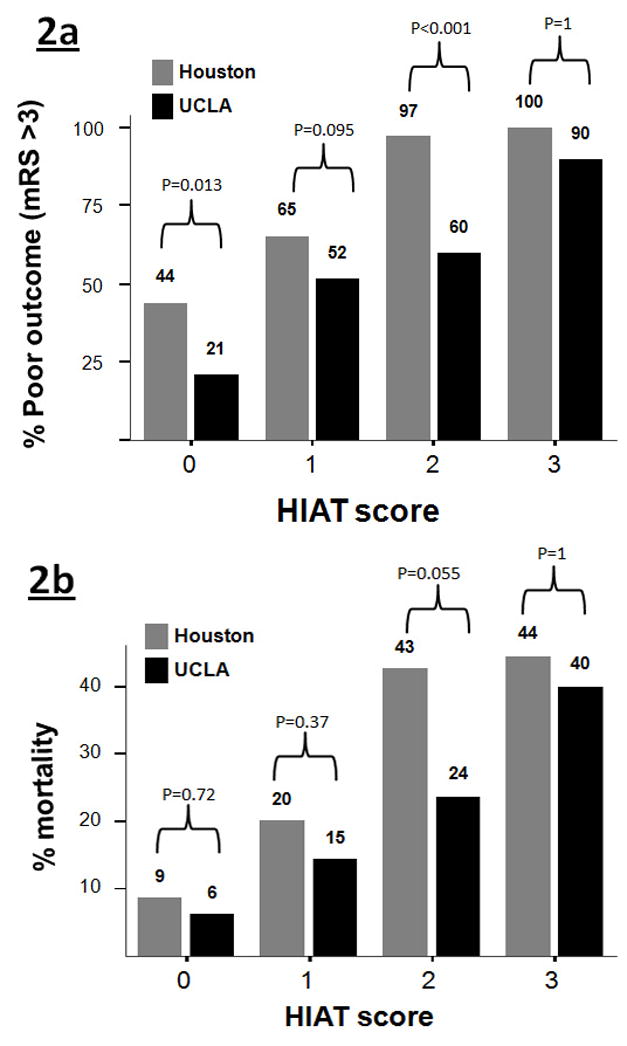
Comparing the proportion of poor outcome (a) and mortality (b) between the UTH and UCLA cohorts across HIAT score tiers.
Discussion
Stroke patients with a large vessel occlusion are often screened for IAT if they present in extended time windows to a comprehensive stroke center. Our work introduces a novel score consisting of three clinical variables: age, admission glucose, and admission NIHSS. These variables were assessed to aid in the process of decision making before IAT. Older patients have been shown to benefit from thrombolytic therapy (both IV and IA)16, 20; however they have worse outcomes and lower rates of recovery. Older people also may have reduced physiological reserves rendering them more susceptible to complications from intubation and sedation that frequently accompany IAT21. NIHSS measures stroke severity and is a powerful predictor of outcome14; and admission hyperglycemia has been shown22 to be associated with poor outcome after ischemic stroke. It is, thus, not surprising that these three variables were identified in our cohort as independent predictors of outcome. The HIAT score is unique in incorporating the three variables into a unifying score and providing an assessment of poor outcome after IAT. In both cohorts studied, we observed an increase in the rate of poor outcome and mortality with increasing HIAT scores. While these rates were variable among centers in the lower tiers (HIAT 0,1,2), the rate of poor outcome and mortality is uniformly high in the HIAT 3 tier. The outcome of any treatment is always a trade-off between the potential benefits versus the risks. In the HIAT 3 tier, the balance appears heavily weighted towards the latter. This result is likely due to the severity of the stroke combined with a poor metabolic state aggravating the ischemic injury and possibly reducing the benefit of recanalization, combined with decreased functional reserve of older patients.
The decision to proceed to IAT is determined individually using clinical judgment and family discussions. Until prospective data from randomized trials are available, the HIAT score could be useful to provide prognostic data and to adjust expectations of outcome. It is also possible that patients with a HIAT of 2 or 3 may benefit from additional studies such as penumbral imaging to select those who may be harmed by recanalization therapies9. Most importantly, HIAT 2 and 3 groups could serve as a means of stratifying patients in future IAT trials. The poor outcome of these groups from two separate centers supports the need to enroll such patients into prospective, randomized trials such as IMS-3 and MR RESCUE6, 23.
Our work is in agreement with previous studies7 that identified recanalization as an important predictor of outcome. However, recanalization rates were evenly distributed among the four HIAT groups implying that the HIAT score predicts outcome in both recanalizers and non-recanalizers. An interesting finding in our work is the increased duration of IAT and the resultant increase in the time from symptom onset to recanalization in the HIAT 2 and 3 tiers. This may be related to a more difficult access and navigation of the intraarterial catheter in older patients with atherosclerotic vessels. It is possible that this delay in recanalization contributed to the poor outcome in these patients.
Imaging is being used to select patients who may benefit from reperfusion therapy in extended time windows. We showed that a non-malignant pattern or mismatch pattern correlated with a favorable outcome. The imaging results did not, however, predict outcome independently; most likely this is due to the small percentage of patients undergoing MRI in our cohort of patients. We are accumulating more patients with pre-IAT imaging data and will re-analyze our prediction score after sufficient numbers of patients are collected.
This work has several limitations. It is retrospective and as such needs to be validated prospectively. We have used both pIAT and aIAT cohorts to create the HIAT score. It is possible that there are subtle differences between groups that may bias the results. Our study spans across 8 years. During that time, there have been advancements in IAT techniques – notably the introduction of the MERCI retriever4. As a result, there is lack of uniformity in our IAT methods. It is possible that newer techniques may alter the pattern of outcome observed in our study. Our patients were routinely intubated per institutional protocol. This may contribute significantly to IAT morbidity (especially in older patients). Although the HIAT score performed equally well in the independent UCLA validation cohort that is derived from another academic stroke center, the reduced rates of poor outcome in this cohort in the HIAT 0,1 and 2 tiers may indicate important differences in either patient population, patient selection or techniques. For instance, in UCLA intubation is not done routinely before IAT. This study was not designed to explore these differences. Whether the HIAT score may apply at non-university based hospitals remains to be proven. Finally, our clinical endpoint only includes hospital discharge but patients continue to recover over time and therefore our results must be interpreted with caution.
In summary, the HIAT score may provide useful information to clinicians who are considering IAT options for acute ischemic stroke patients in extended windows after symptom onset. The score may help in treatment decisions where evidence from randomized trials is still lacking and the pursuit of IAT is made based on clinical judgment alone. At the very least, the concordance of our treated cohort data across two separate stroke centers showing poor outcome in HIAT 3 patients supports the need to enroll patients into prospective, randomized IAT trials such as IMS-36 and MR RESCUE23.
Acknowledgments
The UCLA Intra-Arterial Therapy Study Group: Latisha Ali, MD, Gary Duckwiler, MD, Reza Jahan, MD, Doojin Kim, MD, Bruce Ovbiagele, MD, Sidney Starkman, MD, Satoshi Tateshima, MD, Fernando Vinuela, MD.
Source of funding: 1. H.H, A.D.B. and A.T.A were supported by NIH training grant T32NS04712
2. H.H, A.D.B., M.M.M., A.T.A., J.C.G. and S.I.S were supported by NIH grant P50 NS044227
3. D.S.L., J.L.S. and J.G were supported by NIH grant P50 NS044378
Footnotes
Conflicts of interest: The authors report no conflict of interests for this work.
References
- 1.Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, Pessin M, Ahuja A, Callahan F, Clark WM, Silver F, Rivera F. Intra-arterial prourokinase for acute ischemic stroke. The proact ii study: A randomized controlled trial. Prolyse in acute cerebral thromboembolism. JAMA. 1999;282:2003–2011. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- 2.Ogawa A, Mori E, Minematsu K, Taki W, Takahashi A, Nemoto S, Miyamoto S, Sasaki M, Inoue T. Randomized trial of intraarterial infusion of urokinase within 6 hours of middle cerebral artery stroke: The middle cerebral artery embolism local fibrinolytic intervention trial (melt) japan. Stroke. 2007;38:2633–2639. doi: 10.1161/STROKEAHA.107.488551. [DOI] [PubMed] [Google Scholar]
- 3.Bose A, Henkes H, Alfke K, Reith W, Mayer TE, Berlis A, Branca V, Sit SP. The penumbra system: A mechanical device for the treatment of acute stroke due to thromboembolism. AJNR Am J Neuroradiol. 2008 doi: 10.3174/ajnr.A1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith WS, Sung G, Starkman S, Saver JL, Kidwell CS, Gobin YP, Lutsep HL, Nesbit GM, Grobelny T, Rymer MM, Silverman IE, Higashida RT, Budzik RF, Marks MP. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: Results of the merci trial. Stroke. 2005;36:1432–1438. doi: 10.1161/01.STR.0000171066.25248.1d. [DOI] [PubMed] [Google Scholar]
- 5.The interventional management of stroke (IMS) II study. Stroke. 2007;38:2127–2135. doi: 10.1161/STROKEAHA.107.483131. [DOI] [PubMed] [Google Scholar]
- 6.Broderick JP, Tomsick TA. Interventional management of stroke (IMS) III trial.clinicaltrials.gov; Trial ID NCT00359424
- 7.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: A meta-analysis. Stroke. 2007;38:967–973. doi: 10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]
- 8.Higashida RT, Furlan AJ. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34:e109–137. doi: 10.1161/01.STR.0000082721.62796.09. [DOI] [PubMed] [Google Scholar]
- 9.Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, Bammer R, Kakuda W, Lansberg MG, Shuaib A, Coplin W, Hamilton S, Moseley M, Marks MP. Magnetic resonance imaging profiles predict clinical response to early reperfusion: The diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60:508–517. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 10.Smith WS. Safety of mechanical thrombectomy and intravenous tissue plasminogen activator in acute ischemic stroke. Results of the multi mechanical embolus removal in cerebral ischemia (MERCI) trial, part I. AJNR Am J Neuroradiol. 2006;27:1177–1182. [PMC free article] [PubMed] [Google Scholar]
- 11.Shaltoni HM, Albright KC, Gonzales NR, Weir RU, Khaja AM, Sugg RM, Campbell MS, 3rd, Cacayorin ED, Grotta JC, Noser EA. Is intra-arterial thrombolysis safe after full-dose intravenous recombinant tissue plasminogen activator for acute ischemic stroke? Stroke. 2007;38:80–84. doi: 10.1161/01.STR.0000251720.25337.b0. [DOI] [PubMed] [Google Scholar]
- 12.Lisboa RC, Jovanovic BD, Alberts MJ. Analysis of the safety and efficacy of intra-arterial thrombolytic therapy in ischemic stroke. Stroke. 2002;33:2866–2871. doi: 10.1161/01.str.0000038987.62325.14. [DOI] [PubMed] [Google Scholar]
- 13.Mandava P, Kent TA. Intra-arterial therapies for acute ischemic stroke. Neurology. 2007;68:2132–2139. doi: 10.1212/01.wnl.0000264898.55747.80. [DOI] [PubMed] [Google Scholar]
- 14.Wechsler LR, Roberts R, Furlan AJ, Higashida RT, Dillon W, Roberts H, Rowley HA, Pettigrew LC, Callahan AS, 3rd, Bruno A, Fayad P, Smith WS, Firszt CM, Schulz GA. Factors influencing outcome and treatment effect in PROACT II. Stroke. 2003;34:1224–1229. doi: 10.1161/01.STR.0000068782.15297.28. [DOI] [PubMed] [Google Scholar]
- 15.Hill MD, Rowley HA, Adler F, Eliasziw M, Furlan A, Higashida RT, Wechsler LR, Roberts HC, Dillon WP, Fischbein NJ, Firszt CM, Schulz GA, Buchan AM. Selection of acute ischemic stroke patients for intra-arterial thrombolysis with pro-urokinase by using aspects. Stroke. 2003;34:1925–1931. doi: 10.1161/01.STR.0000082483.37127.D0. [DOI] [PubMed] [Google Scholar]
- 16.Kim D, Ford GA, Kidwell CS, Starkman S, Vinuela F, Duckwiler GR, Jahan R, Saver JL. Intra-arterial thrombolysis for acute stroke in patients 80 and older: A comparison of results in patients younger than 80 years. AJNR Am J Neuroradiol. 2007;28:159–163. [PMC free article] [PubMed] [Google Scholar]
- 17.Fiorelli M, Bastianello S, von Kummer R, del Zoppo GJ, Larrue V, Lesaffre E, Ringleb AP, Lorenzano S, Manelfe C, Bozzao L. Hemorrhagic transformation within 36 hours of a cerebral infarct: Relationships with early clinical deterioration and 3-month outcome in the european cooperative acute stroke study I (ECASS I) cohort. Stroke. 1999;30:2280–2284. doi: 10.1161/01.str.30.11.2280. [DOI] [PubMed] [Google Scholar]
- 18.Noser EA, Shaltoni HM, Hall CE, Alexandrov AV, Garami Z, Cacayorin ED, Song JK, Grotta JC, Campbell MS., 3rd Aggressive mechanical clot disruption: A safe adjunct to thrombolytic therapy in acute stroke? Stroke. 2005;36:292–296. doi: 10.1161/01.STR.0000152331.93770.18. [DOI] [PubMed] [Google Scholar]
- 19.Shaltoni HM, Sugg RM, Gonzales NR, Choi JY, Cacayorin ED, Weir RU, Alexandrov AV, Malkoff MD, Grotta JC. TIMI flow grade 2b or higher predicts better outcome after intraarterial thrombolysis. Presented at the International Stroke Conference; February 2005; New Orleans, LA. 2005. [Google Scholar]
- 20.De Keyser J, Gdovinova Z, Uyttenboogaart M, Vroomen PC, Luijckx GJ. Intravenous alteplase for stroke: Beyond the guidelines and in particular clinical situations. Stroke. 2007;38:2612–2618. doi: 10.1161/STROKEAHA.106.480566. [DOI] [PubMed] [Google Scholar]
- 21.Marik PE. Management of the critically ill geriatric patient. Crit Care Med. 2006;34:S176–182. doi: 10.1097/01.CCM.0000232624.14883.9A. [DOI] [PubMed] [Google Scholar]
- 22.Alvarez-Sabin J, Molina CA, Ribo M, Arenillas JF, Montaner J, Huertas R, Santamarina E, Rubiera M. Impact of admission hyperglycemia on stroke outcome after thrombolysis: Risk stratification in relation to time to reperfusion. Stroke. 2004;35:2493–2498. doi: 10.1161/01.STR.0000143728.45516.c6. [DOI] [PubMed] [Google Scholar]
- 23.Kidwell CS. Mr and recanalization of stroke clots using embolectomy (mr rescue).clinicaltrials.gov NCT00389467


