Abstract
Histone acetylation is essential for hippocampal memory formation in young adult rodents. Although dysfunctional histone acetylation has been associated with age-related memory decline in male rodents, little is known about whether histone acetylation is altered by aging in female rodents. In young female mice, the ability of 17β-estradiol (E2) to enhance object recognition memory consolidation requires histone H3 acetylation in the dorsal hippocampus. However, the extent to which histone acetylation is regulated by E2 in middle-aged females is unknown. The mnemonic benefits of E2 in aging females appear to be greatest in middle age, and so pinpointing the molecular mechanisms through which E2 enhances memory at this age could lead to the development of safer and more effective treatments for maintaining memory function without the side effects of current therapies. Here, we show that dorsal hippocampal infusion of E2 rapidly enhanced object recognition and spatial memory, and increased histone H3 acetylation in the dorsal hippocampus, while also significantly reducing levels of histone deacetylase (HDAC2 and HDAC3) proteins. E2 specifically increased histone H3 acetylation at Bdnf promoters pII and pIV in the dorsal hippocampus of both young and middle-aged mice, despite age-related decreases in pI and pIV acetylation. Furthermore, levels of mature BDNF and pro-BDNF proteins in the dorsal hippocampus were increased by E2 in middle-aged females. Together, these data suggest that the middle-aged female dorsal hippocampus remains epigenetically responsive to E2, and that E2 may enhance memory in middle-aged females via epigenetic regulation of Bdnf.
Epigenetic processes such as histone acetylation are essential for memory formation in the hippocampus and other cognitive regions of the brain (Vecsey et al. 2007; Fischer et al. 2010; Sharma 2010; Graff and Tsai 2013a,b; Peixoto and Abel 2013). DNA is supercoiled around four histone proteins (H2A, H2B, H3, and H4), each of which has an amino acid tail that can be acetylated to relax chromatin structure and increase transcription. Acetyl groups are added to lysines by histone acetyltransferases (HATs) and removed by histone deacetylases (HDACs) (Yang 2007). Considerable evidence suggests that histone acetylation promotes memory formation. For example, hippocampal contextual learning increases H3 acetylation (Levenson et al. 2004), whereas expression of certain HDACs, such as HDAC2 and HDAC3, impairs spatial, contextual, and object recognition memories mediated by the hippocampus (Guan et al. 2009; Haettig et al. 2011; Hawk et al. 2011; McQuown et al. 2011). Furthermore, HDAC inhibitors enhance hippocampal memory, synaptic plasticity, and gene expression in young rodents (Stefanko et al. 2009; Zhao et al. 2010), supporting the notion that histone acetylation facilitates the expression of genes necessary for memory formation.
However, much less is known about the contributions of histone acetylation to memory formation in the aging brain. To date, few studies have examined this issue. In middle-aged mice, impaired spatial and contextual memory has been associated with deficits in learning-induced H4 acetylation in the hippocampus (Peleg et al. 2010; Dagnas and Mons 2013). Treatment with HDAC inhibitors reverses these deficits in middle-aged mice (Peleg et al. 2010), which is consistent with other data from middle-aged male rats showing that aging increases HDAC activity in the hippocampus (Dos Santos Sant’ Anna et al. 2013). HDAC inhibition can also reverse memory deficits in mouse models of Alzheimer's disease (Kilgore et al. 2010). Although these studies suggest that dysregulated hippocampal histone acetylation in middle age leads to memory impairment, it is notable that none specifically examined histone acetylation in females. The onset of memory decline occurs earlier in female rats and mice than in males, and is associated with the loss of estrous cycling (Markowska 1999; Frick et al. 2000). In the hippocampus of middle-aged females, levels of the primary estrogen receptors (ERα and ERβ) are reduced (Yamaguchi-Shima and Yuri 2007; Bohacek and Daniel 2009), and prolonged ovarian hormone deprivation reduces the ability of the most biologically active estrogen, 17β-estradiol (E2), to regulate estrogen receptor levels (Daniel et al. 2006; Bohacek et al. 2008; Bohacek and Daniel 2009). Nevertheless, exogenous E2 can still reverse hippocampal memory deficits in middle-aged female rodents (Markham et al. 2002; Fernandez and Frick 2004; Bimonte-Nelson et al. 2006; Daniel et al. 2006), demonstrating that the middle-aged hippocampus remains somewhat responsive to E2. Indeed, our laboratory has shown that a bilateral infusion of E2 into the dorsal hippocampus enhances object recognition memory in middle-aged female mice in a manner dependent on rapid activation of phosphatidylinositol 3-kinase (PI3K) and extracellular signal-regulated kinase (ERK) (Fan et al. 2010). Given our previous reports in young female mice that bilateral infusion of E2 into the dorsal hippocampus increases histone H3 acetylation within 30 min in an ERK-dependent manner (Zhao et al. 2010, 2012), this finding might suggest an important role for E2-induced histone acetylation in memory formation among middle-aged females. The E2-induced increase in H3 acetylation in young females is blocked by HAT inhibition, as is an E2-induced decrease in HDAC2 protein 4 h after infusion (Zhao et al. 2010, 2012). These data suggest that ERK-driven histone acetylation is essential for E2 to regulate memory in young females. However, the extent to which histone acetylation is necessary for E2 to enhance memory in middle age remains unknown, as are the downstream gene targets necessary for E2 to enhance memory at any age.
One potential gene target involved in estrogenic regulation of hippocampal memory consolidation is brain-derived neurotrophic factor (BDNF). BDNF is crucial for synaptic plasticity and hippocampal memory formation (Heldt et al. 2007; Bekinschtein et al. 2014). The Bdnf gene consists of eight untranslated exons and one protein coding exon (Aid et al. 2007). In the hippocampus, BDNF transcripts can be uniquely regulated by L-type calcium channels (Tao et al. 1998) and aging (Chapman et al. 2012; Perovic et al. 2013). Epigenetic regulation of Bdnf in the hippocampus by HDAC inhibition (Koppel and Timmusk 2013) or contextual fear conditioning (Lubin et al. 2008) has also been shown to increase the expression of specific Bdnf exons. E2-induced activation of L-type calcium channels increases CREB phosphorylation to promote gene transcription and exert neurotrophic effects in hippocampal neurons (Boulware et al. 2005; Wu et al. 2005; Zhao et al. 2005), and high levels of E2 during the estrous cycle are associated with increased hippocampal CA1 excitability in a BDNF-dependent manner (Scharfman et al. 2003). Further, aging and ovariectomy significantly decrease the expression of BDNF mRNA in the hippocampus in rodents (Singh et al. 1995; Sohrabji et al. 1995; Chapman et al. 2012; Perovic et al. 2013). Exogenous E2 administered to ovariectomized rodents (Singh et al. 1995; Sohrabji et al. 1995) or elevated E2 in the estrous cycle are associated with increased BDNF mRNA or protein levels (Gibbs 1998; Scharfman et al. 2003). Because E2 modulates many of the same neural mechanisms responsible for epigenetic regulation of Bdnf, and E2 can rapidly activate cell signaling independent of transcriptional genomic mechanisms to enhance hippocampal memory (Packard and Teather 1997b; Fernandez et al. 2008; Zhao et al. 2010), we reasoned that Bdnf may be a key gene that is epigenetically regulated by E2.
Here, we demonstrate that the female middle-aged dorsal hippocampus remains epigenetically responsive to E2. Our findings suggest that E2 may facilitate memory consolidation in the novel object recognition and object placement tasks by increasing histone H3 acetylation at Bdnf promoters in the dorsal hippocampus.
Results
Estradiol enhances novel object recognition and object placement memory consolidation in middle-aged female mice
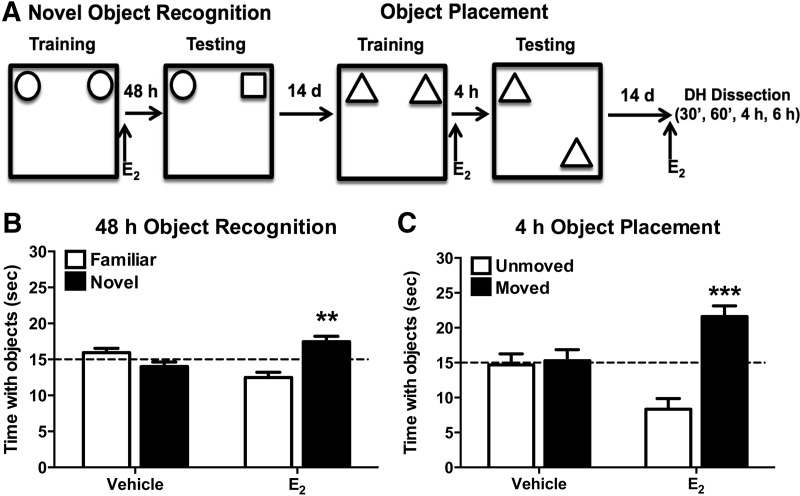
We first sought to replicate our previous findings that a post-training infusion of 5 μg of E2 into the dorsal hippocampus of ovariectomized middle-aged female mice enhances novel object recognition (NOR) memory consolidation (Fan et al. 2010). Mice first explored two identical objects until they had accumulated 30 sec of object exploration (Fig. 1A). Immediately after this training, mice received bilateral dorsal hippocampal infusion of vehicle or 5 μg of E2. Memory for the training objects was tested 48 h later by allowing the mice to explore an object identical to that used in training (i.e., familiar) and a novel object. As in our previous work (Fan et al. 2010), middle-aged mice infused with E2 into the dorsal hippocampus immediately post-training spent significantly more time than chance with the novel object 48 h later (t(16) = 3.41, P = 0.004) (Fig. 1B). In contrast, vehicle-infused mice did not exhibit a significant preference for the novel object (t(15) = −1.56, P = 0.14) (Fig. 1B), suggesting that E2 enhanced NOR memory consolidation in middle-aged females. We next determined whether the beneficial effects of dorsal hippocampal E2 infusion in middle-aged females extend to other forms of memory mediated by the hippocampus. The object placement (OP) task tests hippocampal-dependent spatial memory, and E2 administered to the dorsal hippocampus immediately after training enhances OP memory consolidation in young female mice (Boulware et al. 2013). In the present study, middle-aged females were trained in the OP task 2 wk after NOR testing (Fig. 1A). The training procedure for OP was identical to that for NOR. Immediately after training, the mice were bilaterally infused with vehicle or 5 μg of E2 into the dorsal hippocampus. Twenty-four hours after training, one of the identical objects was moved to a lower corner of the testing arena and mice were again allowed to accumulate 30 sec of exploring the objects. Neither vehicle- nor E2-infused mice showed a significant preference for the moved object in the novel location (data not shown). Because spatial memory decays more rapidly with aging than object memory (Wimmer et al. 2012), we next tested the mice using a shorter 4-h delay. Indeed, 4 h after training, mice infused with E2 spent significantly more time than chance with the moved object (t(7) = 7.14, P < 0.001) (Fig. 1C), demonstrating an intact memory for the unmoved object. In comparison, vehicle-infused mice did not spend more time than chance with the moved object (t(7) = 0.18, P = 0.86) (Fig. 1C). These data show for the first time that acute post-training infusion of E2 into the dorsal hippocampus enhances spatial memory consolidation in middle-aged females, and demonstrate E2 can enhance the consolidation of multiple forms of hippocampal memory in middle age.
Figure 1.
Bilateral dorsal hippocampal (DH) infusion of E2 enhances novel object recognition and object placement memory consolidation. (A) Schematic diagram of the experimental design illustrating the novel object recognition (NOR) and object placement (OP) protocols. In NOR, mice accumulate 30 sec exploring two identical objects and then receive bilateral dorsal hippocampal infusions of 5-µg E2 immediately post-training. Forty-eight hours later, mice accumulate 30 sec exploring a familiar and a novel object. More time than chance spent with the novel object indicates memory for the familiar object. OP is identical to NOR except that testing occurs 4 h after E2 infusion and one of the familiar objects is moved to a new location in the testing arena. More time with the moved object indicates memory for the unmoved object. Fourteen days elapsed between NOR and OP, and between OP and dorsal hippocampal tissue collection. (B) Mice infused with E2 spent significantly more time with the novel object than chance (dashed line at 15 sec) 48 h after training ([**] P < 0.01). (C) Mice infused with E2 also spent significantly more time with the moved object than chance (dashed line at 15 sec) 4 h after training ([***] P < 0.001). In contrast, mice treated with vehicle did not show a preference for either the novel (B) or moved (C) object, indicating that E2 enhanced both NOR and OP memory consolidation. Each bar represents the mean ± SEM.
Estrogenic effects on histone acetylation are restricted to H3
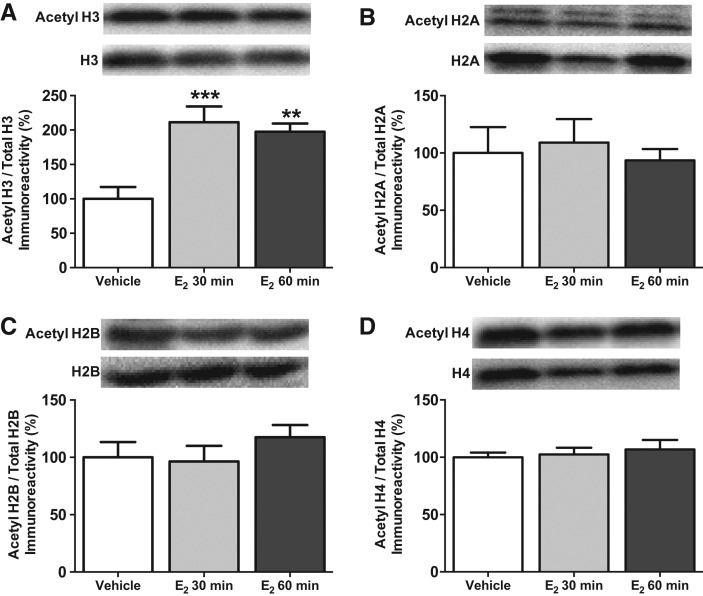
We next determined the extent to which this memory-enhancing dose of E2 triggers epigenetic alterations in the middle-aged dorsal hippocampus. We began with histone acetylation because this process regulates hippocampal memory and the genes necessary for synaptic plasticity (Guan et al. 2009; Graff et al. 2012). Of the four core histones (H2A, H2B, H3, and H4), acetylation of H3 is particularly important in regulating hippocampal learning and memory (Levenson et al. 2004), as well as estrogenic modulation of learning and memory. We previously found in young female mice that dorsal hippocampal infusion of 5-µg E2 significantly increases acetylation of H3 (but not H2B or H4) 30 min later in an ERK-dependent manner, and that histone acetylation is required for E2 to enhance NOR memory consolidation (Zhao et al. 2010, 2012). Because E2 enhances memory consolidation among middle-aged females in a manner similar to young females (Fig. 1; Fan et al. 2010), we hypothesized that E2 would also increase histone acetylation among middle-aged females in an H3-specific manner. Middle-aged mice (n = 27) were bilaterally infused into the dorsal hippocampus with vehicle or 5-µg E2, and the dorsal hippocampus was collected bilaterally 30 or 60 min later for Western blot analysis. The 60-min time point was included in case alterations in histone acetylation were delayed beyond the 30-min time point by aging. E2 significantly increased acetyl H3 levels relative to vehicle (F(2,23) = 10.91, P = 0.0005) (Fig. 2A) both 30 min (P < 0.0001) and 60 min (P < 0.01) after infusion, suggesting that the E2-induced increase in H3 acetylation lasts at least 1 h. In contrast to H3, E2 did not affect acetylation of H2A (F(2,17) = 0.2, P = 0.83) (Fig. 2B), H2B (F(2,24) = 0.8, P = 0.46) (Fig. 2C), or H4 (F(2,21) = 0.29, P = 0.75) (Fig. 2D) at either 30 or 60 min, suggesting that effects of E2 on histone acetylation are specific to H3.
Figure 2.
Dorsal hippocampal histone H3 acetylation is increased by E2. (A) Bilateral dorsal hippocampal infusion of 5-µg E2 significantly increased bulk acetyl H3 levels relative to vehicle 30 min ([***] P < 0.001) and 60 min ([**] P < 0.01) later. In contrast, E2 had no effect on the acetylation of histones H2A (B), H2B (C), or H4 (D). All proteins were normalized to total histone (each bar represents the mean [±SEM] percent change from vehicle). (Insets) Representative Western blots of acetylated and total histone protein.
Estradiol decreases levels of histone deacetylases 2 and 3
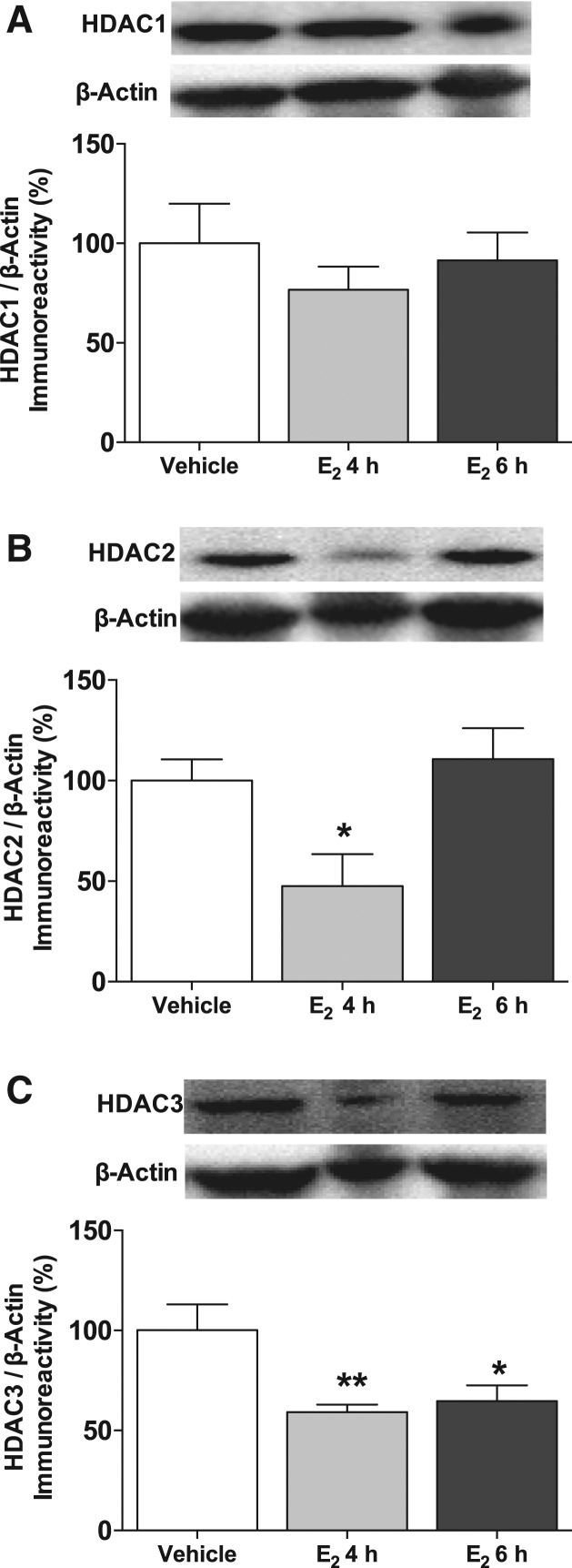
HDACs remove acetyl groups from histone tails, thereby condensing the chromatin and decreasing gene transcription. HDAC2 is a potent negative regulator of both hippocampal memory and synaptic plasticity (Guan et al. 2009). We previously showed that dorsal hippocampal infusion of E2 in young females significantly decreases HDAC2, but not HDAC1, protein in the dorsal hippocampus 4 h after infusion (Zhao et al. 2010, 2012). HDAC3 also negatively regulates object memory consolidation (McQuown et al. 2011), but the effects of E2 on HDAC3 levels in the hippocampus were unknown at any age. Therefore, we next used Western blotting to measure levels of HDAC1, HDAC2, and HDAC3 protein in the middle-aged dorsal hippocampus 4 and 6 h after bilateral dorsal hippocampal infusion of vehicle or 5-µg E2. We hypothesized that E2 would decrease both HDAC2 and HDAC3 levels in middle-aged females because E2-induced memory enhancement is associated with decreased HDAC2 protein expression in young females (Zhao et al. 2010, 2012) and because E2 enhances memory in middle-aged females (Fig. 1). As expected, E2 had no effect on HDAC1 protein levels at either time point (F(2,22) = 0.61, P = 0.6) (Fig. 3A), but significantly decreased HDAC2 (F(2,21) = 5.39, P = 0.01) (Fig. 3B) and HDAC3 (F(2,21) = 6.05, P = 0.008) (Fig. 3C) protein levels in the dorsal hippocampus. For HDAC2, this decrease was evident 4 h after infusion of E2 (P < 0.05), whereas HDAC3 levels were reduced both 4 h (P < 0.01) and 6 h (P < 0.05) after infusion. These data suggest that E2 decreases levels of two key HDACs whose expression is associated with impaired memory.
Figure 3.
E2 decreases levels of histone deacetylases. (A) Bilateral dorsal hippocampal infusion of 5 μg of E2 did not alter HDAC1 protein levels in the dorsal hippocampus at either time point. (B) E2 significantly decreased HDAC2 protein levels 4 h after infusion ([*] P < 0.05 relative to vehicle). (C) E2 significantly decreased HDAC3 protein levels both 4 h ([**] P < 0.01) and 6 h ([*] P < 0.05) later, relative to vehicle. All proteins were normalized to β-Actin (each bar represents the mean [±SEM] percent change from vehicle). (Insets) Representative Western blots of total and phosphorylated protein.
Estradiol increases BDNF protein and regulates acetylation of Bdnf promoters
Traditionally, the estrogenic regulation of Bdnf has been attributed to classical genomic signaling mechanisms due to regulation of the Bdnf gene via an estrogen response element (ERE) (Sohrabji et al. 1995). However, as our lab and others have recently shown, E2 can activate rapid, nonclassical signaling pathways that may ultimately regulate gene transcription independent of the ERE (Bjornstrom and Sjoberg 2005; Spencer et al. 2008b; Fan et al. 2010). The Bdnf gene consists of eight 5′ untranslated exons and one 3′ exon (exon IX) that encodes the BDNF protein (Aid et al. 2007), with the ERE located on Exon V (Sohrabji et al. 1995). Transcription of each exon is driven by its own unique promoter (Aid et al. 2007). Of these promoters, pI, pII, pIV, and pVI are the most common in the brain (Baj et al. 2011). In the hippocampus, memory consolidation is associated with increased histone H3 acetylation at the pIV promoter (Lubin et al. 2008), and fear conditioning increases H3 acetylation at pI and pIV (Fuchikami et al. 2010). In contrast, aging is associated with a decrease in pII and pIV BDNF mRNA in the hippocampus of male rats (Perovic et al. 2013), but changes in acetylation have not been examined. Because E2 increases hippocampal BDNF mRNA (Singh et al. 1995; Sohrabji et al. 1995) and requires ERK-dependent H3 acetylation to enhance memory consolidation (Zhao et al. 2010), we hypothesized that E2 may increase acetylation of H3 at Bdnf promoters pI, pII, and pIV. Further, a role for E2 in regulating H3 acetylation of dorsal hippocampal Bdnf promoters has not been demonstrated at any age, so we used chromatin immunoprecipitation (ChIP) to examine effects in both young and middle-aged female mice.
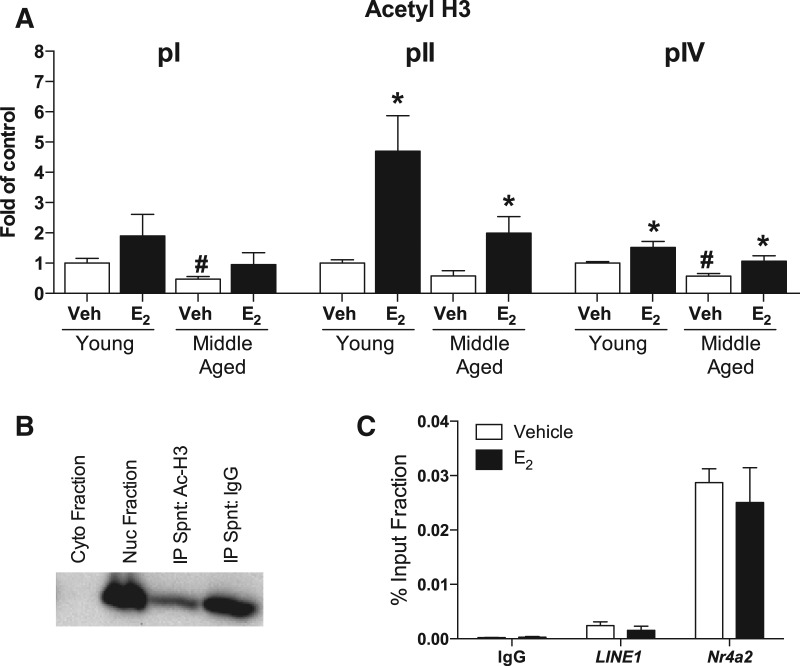
Our control studies demonstrated specificity of the ChIP assay. We first confirmed, using Western blotting, that the pan-acetyl histone H3 was present in the nuclear fraction, but not the cytoplasmic fraction following our shearing protocol (Fig. 4B). Following immunoprecipitation, we observed a reduction in the signal for acetyl H3 in the supernatant samples immunoprecipitated with acetyl H3, suggesting antibody binding to beads coincubated with acetyl H3 rather than control IgG serum (Fig. 4B). Before testing our primers of interest, we used quantitative real-time PCR (qPCR) to ensure that our amplification was specific to the promoters of interest. We found no amplification in an IgG-only sample using primers for our negative control, LINE1 (LINE1). Following immunoprecipitation with acetyl H3, we observed a modest increase in the amplification of LINE1 (LINE1) and a threefold increase in the Nr4a2 positive control (Nr4a2) (Fig. 4C). The vehicle and E2 groups did not differ for any of the three targets.
Figure 4.
E2 increases histone H3 acetylation of Bdnf promoters in the dorsal hippocampus. (A) Chromatin immunoprecipitation analysis of histone H3 acetylation at the Bdnf pI, pII, and pIV promoter regions. Age-related decreases in acetylation were evident among vehicle-infused females at the pI and pIV promoters ([#] P < 0.05 compared to young vehicle-infused mice). E2 increased H3 acetylation at the pII and pIV promoters 30-min post-infusion in both young and middle-aged mice ([*] P < 0.05 vs. age-matched control). Data were normalized to LINE1 for each sample and then normalized to young vehicle-infused mice for each promoter region and represented as fold of control. Each bar represents the mean ± SEM. (B) Western blot demonstrating specificity of sonication parameters. Pan-acetyl histone H3 was not evident in the cytoplasmic fraction (lane 1), but was present in the nuclear fraction (lane 2). The presence of H3 acetylation (Ac-H3) was reduced in the IP supernatant removed from the incubation containing the beads and the acetyl-H3 antibody (lane 3), but remained fully present in the IP supernatant with beads not containing acetyl-H3 antibody (lane 4). (C) qPCR data showing the absence of nonspecific binding in the IgG only control when amplified using primers for LINE1 (LINE1), no difference in the expression of the LINE1 negative control (LINE1), and a threefold increase in expression of the Nr4a2 positive control (Nr4a2) in vehicle- and E2-infused mice.
Next, we compared the effects in young and middle-aged females of dorsal hippocampal vehicle or E2 infusion on H3 acetylation in promoters pI, pII, and pIV. The main effects of age (F(1,12) = 3.18, P = 0.10) and E2 (F(1,12) = 2.68, P = 0.13) were not significant for H3 acetylation at the pI promoter (Fig. 4A). Nevertheless, a t-test comparing young and middle-aged vehicle-infused mice did indicate a significant age-related decrease in H3 acetylation at pI (t(6) = 2.97, P = 0.03). For the pII promoter, main effects of age (F(1,12) = 15.26, P = 0.03) and E2 treatment (F(1,12) = 15.26, P = 0.002) were observed. As suggested by Figure 4A, E2 increased H3 acetylation of pII at both ages, but this effect was more pronounced for young females. E2 increased H3 acetylation at the pII promoter by ∼3.5-fold (P < 0.01) in young females and ∼1.5-fold in middle-aged females (t(6) = 2.49, P = 0.04) (Fig. 4A). There was also a trend for an age-related decrease in H3 acetylation of pII in vehicle-infused females (t(6) = 2.1, P = 0.08) (Fig. 4A). For the pIV promoter, the main effects of age (F(1,19) = 8.36, P = 0.009) and E2 (F(1,19) = 10.84, P = 0.004) were also significant. Aging significantly decreased H3 acetylation at the pIV promoter (t(10) = 4.76, P = 0.0008), and E2 significantly increased H3 acetylation in both young (P < 0.01) and middle-aged females (P = 0.05) by approximately onefold (Fig. 4A). These data demonstrate for the first time that E2 regulates histone acetylation of specific gene promoters in the dorsal hippocampus of adult females at any age, and suggest that Bdnf is a downstream gene target that may be necessary for the mnemonic effects of E2.
Next, to determine if dorsal hippocampal infusion of E2 influences BDNF protein levels, we measured both the uncleaved proform of BDNF, pro-BDNF, and mature BDNF. In middle-aged mice, bilateral dorsal hippocampal infusion of E2 significantly increased levels of pro-BDNF (F(2,24) = 9.26, P = 0.001) (Fig. 5A) in the dorsal hippocampus both 4 and 6 h (P < 0.05) after infusion relative to vehicle. E2 also significantly increased mature BDNF in the dorsal hippocampus of middle-aged mice (F(2,21) = 3.96, P = 0.035) (Fig. 5B). As with pro-BDNF, this effect was evident both 4 h (t(15) = 2.35, P = 0.03) and 6 h (P < 0.05) after infusion relative to vehicle. These data are the first to demonstrate that a direct infusion of E2 in the dorsal hippocampus can regulate pro-BDNF and mature BDNF protein levels in adult females of any age, and the time frame in which this happens suggests that nonclassical cell signaling mechanisms could underlie these changes.
Figure 5.
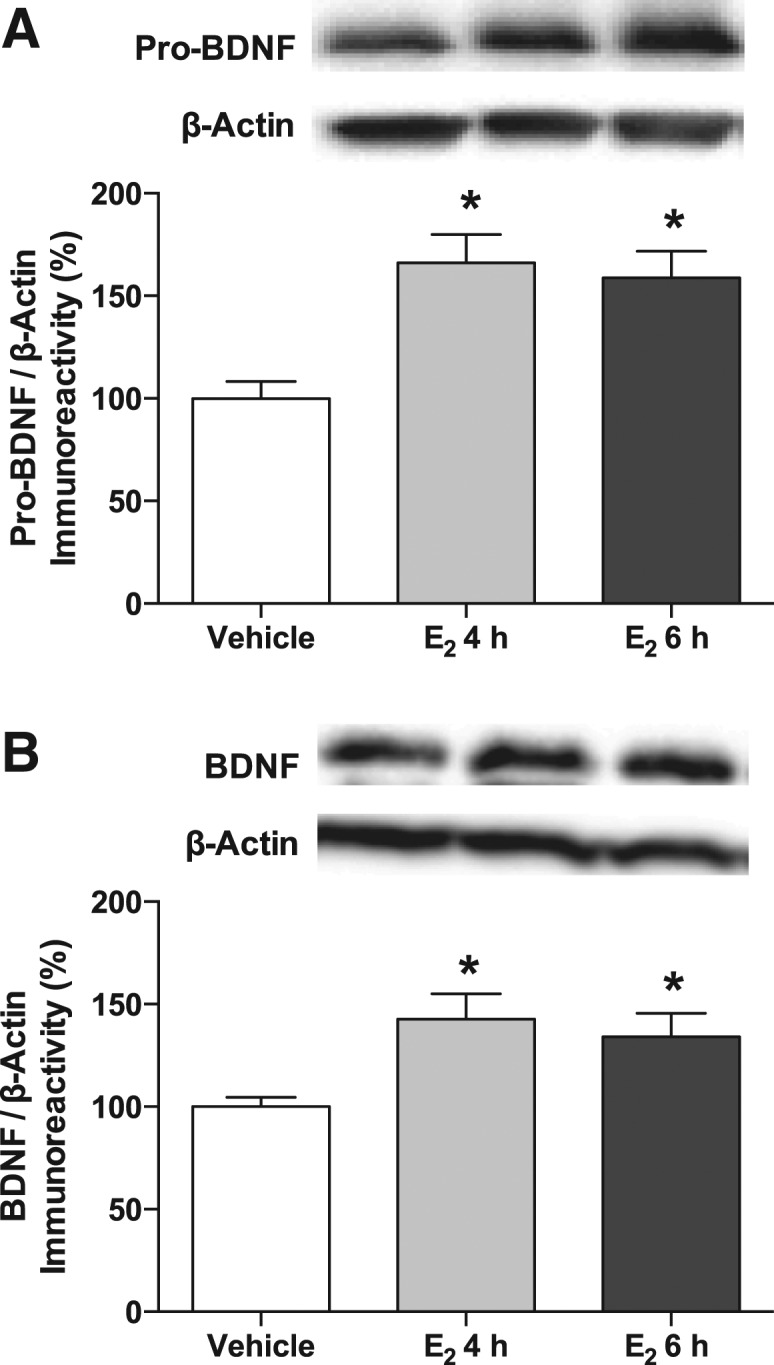
Pro-BDNF and BDNF proteins were increased by E2. (A) Bilateral dorsal hippocampal infusion of 5 μg of E2 significantly increased pro-BDNF protein levels relative to vehicle 4 h ([*] P < 0.05) and 6 h ([*] P < 0.05) after infusion. (B) Similarly, BDNF protein was increased relative to vehicle 4 h ([*] P < 0.05) and 6 h ([*] P < 0.05) after E2 infusion. All proteins were normalized to β-Actin (each bar represents the mean [±SEM] percent change from vehicle). (Insets) Representative Western blots showing total and phosphorylated protein levels.
Discussion
Because E2 is a potent regulator of hippocampal function, the age-related loss of E2 likely contributes to the increased risk of cognitive decline and dementia in postmenopausal women (Zandi et al. 2002; Yaffe et al. 2007). However, our previous and present work suggests that the dorsal hippocampus of the young and middle-aged female hippocampus is similarly responsive to E2. For example, the present data replicate our previous report that post-training dorsal hippocampal infusion of E2 enhances NOR memory consolidation (Fan et al. 2010), and extends this finding to show that dorsal hippocampal E2 infusion also enhances spatial memory consolidation in middle-aged females. Our previous work also demonstrated that rapid ERK phosphorylation in the dorsal hippocampus is necessary for E2 to enhance NOR in both young and middle-aged mice (Fernandez et al. 2008; Fan et al. 2010), which suggests that the middle-aged female hippocampus remains at least partially responsive to E2. Because ERK-induced histone acetylation in the dorsal hippocampus is necessary for E2 to enhance NOR in young females (Zhao et al. 2010, 2012), we hypothesized that E2 would increase histone acetylation in the middle-aged dorsal hippocampus. Indeed, the pattern of E2-induced histone modifications was nearly identical in young and middle-aged females. As in young females (Zhao et al. 2012), E2 selectively increased dorsal hippocampal histone H3 acetylation 30 min after dorsal hippocampal infusion, and decreased dorsal hippocampal levels of HDAC2 (but not HDAC1) 4 h after infusion. This study also extends our previous work to show that E2 decreases levels of HDAC3 in the dorsal hippocampus both 4 and 6 h after infusion. Moreover, we show for the first time that E2 can regulate H3 acetylation of Bdnf promoters and levels of pro-BDNF and BDNF proteins in the dorsal hippocampus of both young and middle-aged females. Together, this work provides the first evidence that the female middle-aged dorsal hippocampus remains epigenetically responsive to E2, and that E2 may enhance memory consolidation in middle-age females by increasing histone H3 acetylation of Bdnf promoters in the dorsal hippocampus.
Our data from middle-aged females showing that the consolidation of NOR and OP was enhanced 48 h and 4 h after dorsal hippocampal E2 infusion are consistent with our previous findings in young female mice (Boulware et al. 2013). These data are also consistent with reports that systemic E2 enhances NOR and OP memory 4 h after treatment in young female rats (Luine et al. 2003; Walf et al. 2006; Frye et al. 2007). However, differences in the timing of spatial memory enhancement between young and middle-aged females should be noted. In young female mice, E2 and estrogen receptor agonists enhance OP 24 h after dorsal hippocampal infusion (Boulware et al. 2013), whereas the present study found that E2 does not enhance OP at this delay in middle-aged females. Rather, effects of E2 on OP were seen here at the shorter 4-h post-infusion delay, suggesting that aging compromises the ability of E2 to enhance spatial memory. However, these data may reflect a detrimental effect of aging on spatial memory rather than a decreased responsiveness to E2, given reports that OP is more susceptible to aging than NOR (Wang et al. 2009; Wimmer et al. 2012). Despite potential age-related decline in spatial memory, memory consolidation in both NOR and OP was facilitated by dorsal hippocampal infusion of E2, suggesting that the molecular mechanisms within the dorsal hippocampus that support object recognition and spatial memory consolidation remain responsive to E2 in middle-aged females.
These mechanisms may include chromatin modifications, as suggested by the present data. As in young females (Zhao et al. 2010), E2 increased histone H3 acetylation in the dorsal hippocampus 30 min after infusion and decreased HDAC2 levels in the dorsal hippocampus 4 h after infusion. Not only were these effects present in middle-aged females, but they were also persistent, lasting at least 60 min for H3 acetylation and 6 h for HDAC3 levels. Such persistent changes may be evident in young females as well, but have not yet been examined. With respect to histone acetylation, the specificity of E2-induced changes to H3 is consistent with our previous work in young females (Zhao et al. 2010, 2012) and with contextual fear-induced changes in the hippocampus of male rats (Levenson et al. 2004; Chwang et al. 2006). With respect to HDAC levels, the failure of E2 to regulate HDAC1 levels is consistent with our previous data from young female mice (Zhao et al. 2010, 2012). These data also support evidence that hippocampal HDAC1 is necessary for the extinction of fear memory, but not for NOR memory (Bahari-Javan et al. 2012). In contrast, the E2-induced reduction of dorsal hippocampal HDAC2 and HDAC3 levels corresponds with other studies showing that these HDACs negatively regulate hippocampal memory (Guan et al. 2009; McQuown et al. 2011). Notably, Hdac2 knockout mice display enhanced acetylation of genes that regulate synaptic plasticity and memory (Guan et al. 2009) and shRNA knockdown of Hdac2 restores memory deficits in a mouse model of Alzheimer's disease (Graff et al. 2012). Similarly, specific deletion of Hdac3 in the dorsal hippocampus enhances long-term NOR memory (McQuown et al. 2011). Here, we provide the first evidence that E2 reduces HDAC3 protein in the dorsal hippocampus, thus supporting HDAC2 and HDAC3 as negative regulators of memory.
Findings showing that E2 administered 2 or 3 h after training does not enhance spatial memory or object recognition (Packard and Teather 1997a,b; Fernandez et al. 2008) support the temporal specificity of E2’s effects on the memory consolidation phase of memory formation. Therefore, E2-induced changes in cell signaling and epigenetic modifications likely follow a specific time course in order to facilitate memory consolidation. Acute systemic injections of E2 in young female rats can increase spine density in the hippocampus within 30 min (Inagaki et al. 2012), an effect that is ERK-dependent in vitro (Srivastava et al. 2008). Our laboratory has shown in young female mice that E2 increases the phosphorylation of p42 ERK and activates ERK-driven mammalian target of rapamycin (mTOR) protein synthesis pathway 5 min after dorsal hippocampal infusion (Fernandez et al. 2008; Boulware et al. 2013; Fortress et al. 2013b), which suggests a possible increase in synaptogenesis as early as 5 min after infusion. We also found in young females that E2 increases dorsal hippocampal HAT activity 30 min after infusion, and that ERK activation is necessary for the E2-induced acetylation of H3 30 min after infusion (Zhao et al. 2010). In young ovariectomized female mice, histone acetylation must be increased within 3 h of NOR training to facilitate memory consolidation (Zhao et al. 2010), and is essential for the reduction in E2-induced HDAC2 observed 4 h after infusion (Zhao et al. 2010, 2012). Therefore, the data suggest that E2-induced alterations in histone acetylation occur within the time frame of memory consolidation, and lead to subsequent alterations in levels of HDAC2 protein. This evidence leads us to hypothesize that E2-induced changes in H3 acetylation are responsible for the initial consolidation of the memory, and that a subsequent decrease in HDAC2 expression may be involved in the maintenance of the memory. Support for this notion comes from evidence that HDAC inhibitors increase the persistence of object recognition memories for up to a week longer than normally observed (Stefanko et al. 2009; Zhao et al. 2010).
Despite our previous findings showing that E2 regulates histone acetylation in the dorsal hippocampus of young females, the identity of the epigenetically regulated genes has remained unknown. Although there are likely numerous gene targets, Bdnf is a logical candidate given the well-documented relationship between E2 and BDNF (for recent reviews, see Harte-Hargrove et al. 2013; Luine and Frankfurt 2013; Pluchino et al. 2013). E2 increases BDNF mRNA and protein, BDNF release, and activation of TrkB in the hippocampus (Sohrabji et al. 1995; Zhou et al. 1996; Scharfman et al. 2003; Sato et al. 2007; Aguirre and Baudry 2009). This regulation of BDNF by E2 is significant because cellular processes necessary for hippocampal function, like dendritic spinogenesis and long-term potentiation (LTP), are dependent on BDNF and its high affinity receptor, TrkB (Luine and Frankfurt 2013). Collectively, this literature led us to hypothesize that E2 would increase histone H3 acetylation at Bdnf promoters. We focused on the Bdnf pI, pII, and pIV promoters because H3 acetylation of these promoters is closely associated with hippocampal learning and aging (Lubin et al. 2008; Fuchikami et al. 2010; Perovic et al. 2013). We found that aging significantly decreased H3 acetylation of pI and pIV, and tended to decrease acetylation of pII. These findings are generally consistent with data from male rats showing significant age-related reductions in Bdnf pII and pIV (Perovic et al. 2013). Despite these reductions, we found that E2 significantly increased H3 acetylation of pII and pIV, but had no effect on pI. The specific roles of each Bdnf mRNA transcript in hippocampal function are still unclear. Bdnf pI is located largely in the soma of CA1 pyramidal neurons, whereas pII and pIV are located primarily in proximal and distal dendrites, respectively (Baj et al. 2011). Interestingly, pIV is regulated by calcium through L-type calcium channels (Zheng et al. 2011). E2 activates the L-type calcium channels necessary for ERK signaling (Wu et al. 2005; Sarkar et al. 2008), and we have shown that ERK signaling is required for H3 acetylation (Zhao et al. 2010). Activation of L-type calcium channels by ERK promotes CREB phosphorylation (Wu et al. 2005), which can facilitate epigenetic changes by increasing CBP occupancy at gene promoters important for synaptic plasticity (Bousiges et al. 2010). In fact, a synthetic CBP analog has recently been shown to increase acetylation of Bdnf pI in addition to increasing BDNF mRNA and protein levels (Chatterjee et al. 2013). As such, E2 may increase H3 acetylation at pIV by activating L-type calcium channels, thereby phosphorylating ERK, and increasing the expression of Bdnf mRNA at distal dendrites. These changes could increase the pool of resident mRNA for local BDNF synthesis to facilitate synaptic plasticity, but this speculation has yet to be tested. It will also be of interest in future studies to determine how E2 regulates expression of other Bdnf transcripts and the extent to which E2 increases H3 acetylation of promoters for other synaptic plasticity genes like Egr1 and c-fos, the latter of which is transcribed within 15 min after dorsal hippocampal E2 infusion in young female mice (Zhao et al. 2010).
E2 also increased pro-BDNF and BDNF protein levels in middle-aged females 4 and 6 h after dorsal hippocampal infusion. Combined with our ChIP data, these results suggest that E2 induces a transcriptionally permissive state at the Bdnf promoter within 30 min, which may increase levels of Bdnf transcripts and BDNF protein within 4 h. Evidence that BDNF is transcribed within 30–60 min and translated within 4–6 h is supported by other studies demonstrating that visual experience affects BDNF synthesis in visual cortex (Schwartz et al. 2011) and that vibrissae stimulation affects BDNF synthesis in somatosensory cortex and hippocampus (Nanda and Mack 2000). Although protein changes in the order of hours could suggest regulation of the BDNF gene by classic genomic signaling through nuclear estrogen receptors at the BDNF ERE (Sohrabji et al. 1995), the present data instead implicate rapid nonclassical epigenetic regulation of BDNF. Other rapid regulation of BDNF by E2 has been observed; for example, E2 facilitates hippocampal synaptogenesis by increasing BDNF release through a rapid PKA-dependent mechanism (Sato et al. 2007). Interestingly, exogenous BDNF increases expression of Bdnf pIV mRNA within 1 h of application (Zheng et al. 2012). These data suggest a feedback mechanism in which BDNF levels regulate BDNF signaling. Such a mechanism is supported by in vitro evidence that BDNF increases BDNF protein by nitrosylating cysteine residues on HDAC2 (Nott et al. 2008; Graff and Tsai 2013a). Thus, one way in which E2 may facilitate memory consolidation in middle-aged females is by priming this BDNF positive feedback loop. Indeed, E2 levels are positively correlated with the expression of activated TrkB receptors (Spencer et al. 2008a). However, the potential interactions between E2 and the BDNF feedback loop will need to be examined in future studies. It should be noted, however, that our studies cannot definitively exclude other mechanisms regulating pro-BDNF and BDNF protein levels. It is unclear whether ERα and ERβ are involved in estrogenic regulation of histone acetylation, but both receptors are present in neurons within the hippocampus. Both ERα and ERβ have been localized to nuclei, axons, and dendritic spine synapses of CA1 pyramidal neurons in the hippocampus (Milner et al. 2001, 2005), and ERα is present in GABAergic interneurons (Murphy et al. 1998b) and cholinergic axons and terminals (Towart et al. 2003) within the hippocampus. In 2- to 3-wk-old hippocampal cell cultures, E2 down-regulates BDNF immunoreactivity in GABAergic interneurons, which reduces inhibition and increases excitatory tone and dendritic spine density in pyramidal neurons (Murphy et al. 1998a). Similarly, other data suggest that estrogenic stimulation of the basal forebrain cholinergic system disinhibits hippocampal pyramidal neuron by reducing GABAergic neurotransmission (Rudick et al. 2003). These data suggest complex interactions between E2 and BDNF involving multiple neuron types within the hippocampus. As such, future studies should specifically examine epigenetic regulation of BDNF in glutamatergic, GABAergic, and cholinergic cell types to gain a better understanding of how E2 regulates BDNF and pro-BDNF proteins.
In conclusion, our findings provide the first evidence that the middle-aged female dorsal hippocampus remains epigenetically responsive to E2, and suggest that E2 may enhance memory in middle-aged females via epigenetic regulation of Bdnf. Because women are at greater risk than men for developing Alzheimer's disease (Yaffe et al. 2007) and mental illnesses such as depression, anxiety, and mood disorders (Kessler et al. 2005), these findings may provide key insight into the hormonal regulation of cognition in women. Understanding how E2 epigenetically regulates Bdnf in females throughout the lifespan will provide essential new information for the development of more effective treatments for women with neurodegenerative and neuropsychiatric illnesses.
Materials and Methods
Subjects
Middle-aged (16-mo-old) female C57BL/6 mice were obtained from the National Institutes on Aging Aged Rodent Colony at Charles River Laboratories. For chromatin immunoprecipitation studies, young (3-mo-old) female C57BL/6 mice were obtained from Taconic. All mice were singly housed and maintained on a 12-h light–dark cycle (lights on at 07:00) with ad libitum access to food and water. All procedures followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Wisconsin-Milwaukee Institutional Animal Care and Use Committee.
Surgery
All mice were bilaterally ovariectomized and implanted with guide cannulae within the same surgical session as described previously (Fortress et al. 2013b). Using a stereotaxic apparatus, bilateral stainless-steel guide cannulae (22-gauge, C232G, Plastics One) were implanted into the dorsal hippocampus (−1.7 mm AP, ±1.5 mm ML, −2.3 mm DV [injection site]) and were fitted with dummy cannulae (C232DC) to preserve the integrity of the guide cannulae. Dental cement (Darby Dental) was used to affix the cannulae to the skull surface and also served to close the wound. Mice were allowed to recover for 7 d before the start of behavioral training.
Drugs and infusions
All mice were infused with vehicle or E2. Cyclodextrin-encapsulated E2 (Sigma-Aldrich) was dissolved to a concentration of 5 μg/0.5 μL in physiological saline as described previously (Fortress et al. 2013b). The vehicle, 2-hydroxypropyl-β-cyclodextrin (HBC, Sigma-Aldrich), was dissolved in saline to the same concentration of cyclodextrin used in the cyclodextrin–E2 solution. During infusions, mice were gently restrained and dummy cannulae were replaced with infusion cannulae (C232I, 26 gauge extending 0.8 mm beyond the 1.5-mm guide cannulae), which were attached to PE50 tubing connected to a 5-μL Hamilton syringe. Using a microinfusion pump (KDS 100, KD Scientific), mice were bilaterally infused into the dorsal hippocampus at a rate of 0.5 μL/min for 1 min, resulting in an E2 dose of 5 μg/hemisphere. Infusion cannulae remained in place for 1 min to prevent diffusion back up the cannula track. Infusions occurred immediately after behavioral training (i.e., post-training). Infusions were spaced out by 2 wk to allow the acute effects of E2 to dissipate, and all mice received the same number of infusions.
Behavioral testing
To examine the effects of E2 on object recognition and spatial memory, mice (n = 79) were tested in both novel object recognition (NOR) and object placement (OP) (Fig. 1A) tasks. NOR was first used to assess nonspatial hippocampal-dependent memory as described previously (Fortress et al. 2013b). Mice were handled for 30 sec/d for 3 d prior to habituation. A small Lego was placed in the home cage from the start of handling until training to habituate the mice to the objects. Mice were habituated to the testing arena for 2 d, during which time they were allowed to freely explore the empty, white testing arena (60-cm W × 60-cm L × 47-cm H) for 5 min. Twenty-four hours after the second habituation session, mice were rehabituated to the testing arena for 2 min, and then placed in a holding cage while two identical objects were placed in the Northeast and Northwest corners of the box (∼5 cm from the walls). Mice were then immediately returned to the testing arena and allowed to freely explore both objects until they accumulated a total of 30 sec of exploration time, with a maximum of 20 min allowed for completion of training. Exploration was recorded when the front paws or nose contacted either object. Immediately after training, mice were removed from the box, gently restrained, and were bilaterally infused with vehicle or E2 into the dorsal hippocampus. Forty-eight hours after infusion, mice were returned to the testing arena and allowed to accumulate 30 sec exploring an object identical to that explored during training (familiar) and a new (novel) object. Novel object location was counterbalanced across mice. Time spent exploring each object was recorded using ANYmaze software (Stoelting). More time than chance (15 sec) spent exploring the novel object indicated memory for the familiar object.
Two weeks after the completion of NOR, spatial memory was tested using an OP protocol as described previously (Boulware et al. 2013). Mice were rehabituated to the testing arena for 2 min and were then allowed to explore two identical objects placed in the Northeast and Northwest corners as in NOR. Immediately after training, mice were infused with vehicle or E2 as above. Either 4 or 24 h after training, mice were returned to the testing arena in which one of the identical objects had been moved to the Southeast or Southwest corner. The location of the moved object was counterbalanced across mice. Mice were again allowed to accumulate 30 sec with the objects. More time than chance (15 sec) spent exploring the moved object indicated memory for the unmoved object.
Western blotting
Two weeks after the completion of OP, mice were again infused with vehicle (n = 16) or E2 (n = 36) into the dorsal hippocampus, and dorsal hippocampal tissues were collected bilaterally on ice 30 min, 60 min, 4 h, or 6 h later for measurement of histone acetylation (H2A, H2B, H3, and H4), and levels of HDAC1, HDAC2, and HDAC3 protein. Our previous studies in young females showed that dorsal hippocampal infusion of E2 increased H3 acetylation 30 min later and decreased HDAC2 protein 4 h later (Zhao et al. 2010, 2012). However, because we also found that activation of ERK is delayed in middle-aged females relative to young females (Fan et al. 2010), and that ERK activation is necessary for E2 to increase H3 acetylation (Zhao et al. 2010), we included the 60-min and 6-h time points here to account for potential age-related delays in histone modifications. Dorsal hippocampal tissues were maintained at −80°C until homogenization.
Tissues were prepared as described previously (Fortress et al. 2013a), with the exception of those used for histone extraction. For tissue collected at 30 and 60 min to measure histone acetylation, tissues were dounce homogenized with a Teflon homogenizer after resuspension in 1:50 w/v of 1× Laemelli buffer diluted from 5× Laemelli buffer (50% glycerol in water containing 587 mM Tris HCl, 38 mM Tris Base, 173 mM SDS) containing 100 mM PMSF, and 1× Protease Inhibitor Cocktail. Homogenates were then incubated in a 37°C water bath for 10 min and centrifuged at 12,000 rpm for 5 min at 4°C. For all other proteins to be assayed at the 4- and 6-h time points, tissues were homogenized in 1:25 w/v dilution in lysis buffer and homogenized with a probe sonicator (Branson Sonifier 250). After total protein content was measured for all samples (Fernandez et al. 2008), 20 μg of sample was electrophoresed on various gradients of Tris-HCl gels and transferred to PVDF membranes using the TransBlot Turbo system (Bio-Rad). Membranes were blocked and incubated with the following anti-rabbit primary antibodies overnight at 4°C: acetyl-H2A (Lys 5), acetyl-H2B (Lys 12) (1:1000, Cell Signaling Technology), acetyl-H3 (pan), acetyl-H4 (Lys 12), HDAC1, HDAC2, or HDAC3 (1:1000, Cell Signaling Technology). The next day, membranes were washed and incubated in secondary antibody (anti-rabbit HRP, 1:5000, Cell Signaling Technology), and then developed in West Dura Chemiluminescent substrate (Pierce). For normalization of histone proteins, blots were stripped and reprobed with anti-rabbit antibodies for corresponding total histone proteins H2A, H2B (1:1000, Cell Signaling Technology), H3, or H4 (1:2000, Millipore). Anti-β-Actin (1:5000, Cell Signaling Technology) was used to normalize all other proteins. Images were captured and densitometry was performed using the Carestream Gel Logic 6000 Pro. All normalized proteins were expressed as a percentage relative to vehicle controls.
Chromatin immunoprecipitation (ChIP)
ChIP was performed using a previously published protocol (Kenney et al. 2012). Thirty minutes after infusion with vehicle or E2, young (n = 15) and middle-aged mice (n = 13) were cervically dislocated, and the dorsal hippocampus was rapidly dissected, flash frozen, and stored at −80°C until use. Tissues were minced and proteins cross-linked by placing them for 10 min in 1% formaldehyde on a rotator at room temperature. Glycine (200 mM) was then added for 5 min to stop the cross-linking reaction, and the tissue was then centrifuged at 2500 rpm for 2 min at 4°C. The supernatant was removed and the samples were washed three times with ice-cold phosphate buffered saline (PBS) containing protease and phosphatase inhibitors (PPI) (Pierce), with centrifugation at 2500 rpm for 2 min at 4°C between each wash. To lyse the cells, tissue was homogenized on ice in 500 μL of lysis buffer (1× PPI, 10 mM Tris-HCl [pH 8.1], 10 mM NaCl, 1.5 mM MgCl, 0.5% Igepal-CA630) using a sterile pestle homogenizer, then centrifuged at 5500 rpm for 5 min at 4°C. The supernatant was then removed and 150 μL of nuclear lysis buffer (1× PPI, 50 mM Tris-HCl [pH 8.1], 5 mM EDTA, 1% SDS) was added. For chromatin shearing, samples were incubated on ice for 10 min and then sonicated on ice using a probe sonicator (10 cycles of 15-sec on, 120-sec off at 25% power) (Branson Sonifier 250). These conditions yielded chromatin fragments in the 100–800 base pair range. Following sonication, 5 μL of sample was eluted in ChIP elution buffer (1% SDS, 0.1 M NaHCO3) and 0.1 µg/µL proteinase K (Invitrogen), and then incubated for 2 h at 62°C on a rotator. Cross-links were then reversed by incubation at 95°C for 10 min. Resulting DNA was purified using QiaQuick spin columns (Qiagen). The following day, purified DNA fragments were used to check DNA concentration and efficiency of chromatin shearing. To detect the size of base pair fragments, samples were electrophoresed in 1.5% agarose in TAE buffer containing Sybr Safe and visualized using UV luminescence on the ChemiDoc MP Gel Imaging System (Bio-Rad). All fragments of DNA were determined to be in the 100–800 base pair range. For the immunoprecipitation (IP) process, 2 μg of DNA was mixed with IP buffer (16.7 mM Tris-HCl [pH 8.1], 1.1% Triton X-100, 0.01% SDS, 167 mM NaCl) with 1× PPI to a volume of 500 μL. Five microliters of the IP mix was removed as 1% input prior to the addition of beads and antibody. This process resulted in three tubes for each sample: input, acetyl-H3, or IgG control. For tubes receiving acetyl-H3, 5 μL of pan-acetyl H3 antibody (Millipore #06-599) was added. For IgG control tubes, 1 μL of rabbit IgG (Cell Signaling Technology) was added. Twenty microliters of protein A magnetic beads (Millipore) were then added to the acetyl-H3 and IgG tubes, and incubated overnight on a rotator at 4°C. Following incubation, the acetyl-H3 and IgG tubes were washed once each with low salt (20 mM Tris HCl, 150 mM NaCl, 2 mM EDTA, 1% Triton-X, 0.1% SDS), high salt (20 mM Tris HCl, 500 mM NaCl, 2 mM EDTA, 1% Triton-X, 0.1% SDS), LiCl (10 mM Tris HCl, 250 mM LiCl, 1 mM EDTA, 1% deoxycholate, 1% Igepal-CA630), and TE (10 mM Tris HCl, 1 mM EDTA) wash buffers. All tubes were then incubated in elution buffer (0.1 M NaHCO3, 1% SDS) and 0.1 µg/µL proteinase K (Invitrogen) for 2 h at 62°C. Cross-links were reversed via incubation at 95°C for 10 min. DNA was isolated and purified using QiaQuick spin columns (Qiagen) and eluted twice in nuclease-free water to a total volume of 200 µL.
Quantitative real-time PCR (qPCR)
For each qPCR reaction, 9 μL of DNA was combined with 250 nM primer solution and 10 μL of EVAGreen SYBR Green Master Mix (Midwest Scientific) for a final volume of 20 μL. Each sample (input, acetyl-H3, and IgG) was loaded in triplicate using the Eppendorf Realplex 2 PCR System (Eppendorf). Cycle conditions were determined by EVAGreen guidelines: 95°C for 10 min, 40 cycles of 95°C for 20 sec, 40 cycles of 95°C for 15 sec, and 60°C for 60 sec. At the completion of the qPCR reactions, melt curves and product size analyses were performed for each primer. Additionally, using mouse genomic DNA, the efficiency of each of the primers was calculated and determined to be at 100 ± 10% before analyzing the results. The following primer sequences were used:
Bdnf pI forward 5′-TGATCATCACTCACGACCACG-3′, pI reverse 5′-CAGCCTCTCTGAGCCAGTTACG-3′ (Graff et al. 2012);
Bdnf pII forward 5′-CCGTCTTGTATTCCATCCTTTG-3′, pII reverse 5′-CCCAACTCCACCACTATCCTC-3′ (Graff et al. 2012);
Bdnf pIV forward 5′-GCGCGGAATTCTGATTCTGGTAAT-3′, reverse 5′-GAGAGGGCTCCACGCTGCCTTGACG-3′ (Zeng et al. 2011);
LINE1 forward 5′-AAACGAGGAGTTGGTTCTTTGAG-3′, reverse 5′-TTTGTCCCTGTGCCCTTTAGTGA-3′ (Kenney et al. 2012);
nr4a2 forward 5′-GTGTGAGGACGCAAGGTCTG-3′, reverse 5′-CACGACTGGGGCTGATTT-3′ (Kenney et al. 2012).
Statistical analyses
For NOR and OP, independent sample t-tests were used to determine if the time each group spent with the novel or moved objects differed significantly from chance (15 sec) (Boulware et al. 2013; Fortress et al. 2013a). This test was used because time spent with the objects is not independent; time spent with one necessarily reduces time spent with the other (Frick and Gresack 2003). Western blot data were analyzed using a one-way analysis of variance with Dunnett's planned comparisons for post hoc analyses (Fortress et al. 2013b). For qPCR analyses, median Ct values were used with the ΔΔCt method then normalized to young, vehicle mice (Zhao et al. 2010). ChIP data are presented as fold change over young vehicle infused mice. A 2 × 2 analysis of variance, with age and treatment as independent variables and selected planned t-tests were used to determine statistical differences for each of the promoter regions. All data were analyzed using GraphPad Prism 6 (GraphPad Software, Inc.) and significance for all analyses was determined as P ≤ 0.05.
Acknowledgments
This project was supported by the University of Wisconsin-Milwaukee, a Research Growth Initiative grant from the UWM Research Foundation to K.M.F; an Ellison Medical Foundation/American Federation for Aging Research Postdoctoral Fellowship in Aging to A.M.F.; and a National Institute on Drug Abuse (NIDA) grant, DA017949, to T.J.G. We thank Dr. Ava Udvadia for helpful advice on the ChIP analyses and the Great Lakes Genomic Center for the use of its NanoDrop spectrophotometer.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.034033.113.
References
- Aguirre CC, Baudry M 2009. Progesterone reverses 17β-estradiol-mediated neuroprotection and BDNF induction in cultured hippocampal slices. Eur J Neurosci 29: 447–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T 2007. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res 85: 525–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahari-Javan S, Maddalena A, Kerimoglu C, Wittnam J, Held T, Bahr M, Burkhardt S, Delalle I, Kugler S, Fischer A, et al. 2012. HDAC1 regulates fear extinction in mice. J Neurosci 32: 5062–5073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baj G, Leone E, Chao MV, Tongiorgi E 2011. Spatial segregation of BDNF transcripts enables BDNF to differentially shape distinct dendritic compartments. Proc Natl Acad Sci 108: 16813–16818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Medina JH 2014. BDNF and memory processing. Neuropharmacology 76Pt C:677–683 [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Francis KR, Umphlet CD, Granholm A-C 2006. Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. Eur J Neurosci 24: 229–242 [DOI] [PubMed] [Google Scholar]
- Bjornstrom L, Sjoberg M 2005. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol 19: 833–842 [DOI] [PubMed] [Google Scholar]
- Bohacek J, Daniel JM 2009. The ability of oestradiol administration to regulate protein levels of oestrogen receptor α in the hippocampus and prefrontal cortex of middle-aged rats is altered following long-term ovarian hormone deprivation. J Neuroendocrinol 21: 640–647 [DOI] [PubMed] [Google Scholar]
- Bohacek J, Bearl AM, Daniel JM 2008. Long-term ovarian hormone deprivation alters the ability of subsequent oestradiol replacement to regulate choline acetyltransferase protein levels in hippocampus and prefrontal cortex of middle-aged rats. J Neuroendocrinol 20: 1023–1027 [DOI] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG 2005. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci 25: 5066–5078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Heisler JD, Frick KM 2013. The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. J Neurosci 33: 15184–15194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousiges O, Vasconcelos AP, Neidl R, Cosquer B, Herbeaux K, Panteleeva I, Loeffler JP, Cassel JC, Boutillier AL 2010. Spatial memory consolidation is associated with induction of several lysine-acetyltransferase (histone acetyltransferase) expression levels and H2B/H4 acetylation-dependent transcriptional events in the rat hippocampus. Neuropsychopharmacology 35: 2521–2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman TR, Barrientos RM, Ahrendsen JT, Hoover JM, Maier SF, Patterson SL 2012. Aging and infection reduce expression of specific brain-derived neurotrophic factor mRNAs in hippocampus. Neurobiol Aging 33: 832.e1–832.e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Mizar P, Cassel R, Neidl R, Selvi BR, Mohankrishna DV, Vedamurthy BM, Schneider A, Bousiges O, Mathis C, et al. 2013. A novel activator of CBP/p300 acetyltransferases promotes neurogenesis and extends memory duration in adult mice. J Neurosci 33: 10698–10712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chwang WB, O'Riordan KJ, Levenson JM, Sweatt JD 2006. ERK/MAPK regulates hippocampal histone phosphorylation following contextual fear conditioning. Learn Mem 13: 322–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagnas M, Mons N 2013. Region- and age-specific patterns of histone acetylation related to spatial and cued learning in the water maze. Hippocampus 23: 581–591 [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL 2006. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology 147: 607–614 [DOI] [PubMed] [Google Scholar]
- Dos Santos Sant’ Anna G, Rostirola Elsner V, Moyses F, Reck Cechinel L, Agustini Lovatel G, Rodrigues Siqueira I 2013. Histone deacetylase activity is altered in brain areas from aged rats. Neurosci Lett 556: 152–154 [DOI] [PubMed] [Google Scholar]
- Fan L, Zhao Z, Orr PT, Chambers CH, Lewis MC, Frick KM 2010. Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signal-regulated kinase and phosphatidylinositol 3-kinase activation. J Neurosci 30: 4390–4400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez SM, Frick KM 2004. Chronic oral estrogen affects memory and neurochemistry in middle-aged female mice. Behav Neurosci 118: 1340–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM 2008. Estradiol-induced enhancement of object memory consolidation involves hippocampal ERK activation and membrane-bound estrogen receptors. J Neurosci 28: 8660–8667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Mungenast A, Tsai LH 2010. Targeting the correct HDAC(s) to treat cognitive disorders. Trends Pharmacol Sci 31: 605–617 [DOI] [PubMed] [Google Scholar]
- Fortress AM, Schram SL, Tuscher JJ, Frick KM 2013a. Canonical Wnt signaling is necessary for object recognition memory consolidation. J Neurosci 33: 12619–12626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortress AM, Fan L, Orr PT, Zhao Z, Frick KM 2013b. Estradiol-induced object recognition memory consolidation is dependent on activation of mTOR signaling in dorsal hippocampus. Learn Mem 20: 147–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Gresack JE 2003. Sex differences in the behavioral response to spatial and object novelty in adult C57BL/6 mice. Behav Neurosci 117: 1283–1291 [DOI] [PubMed] [Google Scholar]
- Frick KM, Burlingame LA, Arters JA, Berger-Sweeney J 2000. Reference memory, anxiety, and estrous cyclicity in C57BL/6NIA mice are affected by age and sex. Neuroscience 95: 293–307 [DOI] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, Walf AA 2007. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem 88: 208–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchikami M, Yamamoto S, Morinobu S, Takei S, Yamawaki S 2010. Epigenetic regulation of BDNF gene in response to stress. Psychiatry Investig 7: 251–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB 1998. Levels of trkA and BDNF mRNA, but not NGF mRNA, fluctuate across the estrous cycle and increase in response to acute hormone replacement. Brain Res 787: 259–268 [DOI] [PubMed] [Google Scholar]
- Graff J, Tsai LH 2013a. Histone acetylation: molecular mnemonics on the chromatin. Nat Rev Neurosci 14: 97–111 [DOI] [PubMed] [Google Scholar]
- Graff J, Tsai LH 2013b. The potential of HDAC inhibitors as cognitive enhancers. Annu Rev Pharmacol Toxicol 53: 311–330 [DOI] [PubMed] [Google Scholar]
- Graff J, Rei D, Guan JS, Wang WY, Seo J, Hennig KM, Nieland TJ, Fass DM, Kao PF, Kahn M, et al. 2012. An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature 483: 222–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, Nieland TJ, Zhou Y, Wang X, Mazitschek R, et al. 2009. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature 459: 55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haettig J, Stefanko DP, Multani ML, Figueroa DX, McQuown SC, Wood MA 2011. HDAC inhibition modulates hippocampus-dependent long-term memory for object location in a CBP-dependent manner. Learn Mem 18: 71–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte-Hargrove LC, Maclusky NJ, Scharfman HE 2013. Brain-derived neurotrophic factor-estrogen interactions in the hippocampal mossy fiber pathway: Implications for normal brain function and disease. Neuroscience 239: 46–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawk JD, Florian C, Abel T 2011. Post-training intrahippocampal inhibition of class I histone deacetylases enhances long-term object-location memory. Learn Mem 18: 367–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt SA, Stanek L, Chhatwal JP, Ressler KJ 2007. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry 12: 656–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Frankfurt M, Luine V 2012. Estrogen-induced memory enhancements are blocked by acute bisphenol A in adult female rats: role of dendritic spines. Endocrinology 153: 3357–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JW, Poole RL, Adoff MD, Logue SF, Gould TJ 2012. Learning and nicotine interact to increase CREB phosphorylation at the jnk1 promoter in the hippocampus. PLoS One 7: e39939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE 2005. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62: 617–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgore M, Miller CA, Fass DM, Hennig KM, Haggarty SJ, Sweatt JD, Rumbaugh G 2010. Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer's disease. Neuropsychopharmacology 35: 870–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel I, Timmusk T 2013. Differential regulation of Bdnf expression in cortical neurons by class-selective histone deacetylase inhibitors. Neuropharmacology 75: 106–115 [DOI] [PubMed] [Google Scholar]
- Levenson JM, O'Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD 2004. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem 279: 40545–40559 [DOI] [PubMed] [Google Scholar]
- Lubin FD, Roth TL, Sweatt JD 2008. Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J Neurosci 28: 10576–10586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V, Frankfurt M 2013. Interactions between estradiol, BDNF and dendritic spines in promoting memory. Neuroscience 239: 34–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ 2003. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology 144: 2836–2844 [DOI] [PubMed] [Google Scholar]
- Markham JA, Pych JC, Juraska JM 2002. Ovarian hormone replacement to aged ovariectomized female rats benefits acquisition of the Morris water maze. Horm Behav 42: 284–293 [DOI] [PubMed] [Google Scholar]
- Markowska AL 1999. Sex dimorphisms in the rate of age-related decline in spatial memory: relevance to alterations in the estrous cycle. J Neurosci 19: 8122–8133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuown SC, Barrett RM, Matheos DP, Post RJ, Rogge GA, Alenghat T, Mullican SE, Jones S, Rusche JR, Lazar MA, et al. 2011. HDAC3 is a critical negative regulator of long-term memory formation. J Neurosci 31: 764–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE 2001. Ultrastructural evidence that hippocampal α estrogen receptors are located at extranuclear sites. J Comp Neurol 429: 355–371 [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE 2005. Ultrastructural localization of estrogen receptor β immunoreactivity in the rat hippocampal formation. J Comp Neurol 491: 81–95 [DOI] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Segal M 1998a. Brain-derived neurotrophic factor mediates estradiol-induced dendritic spine formation in hippocampal neurons. Proc Natl Acad Sci 95: 11412–11417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Greenberger V, Segal M 1998b. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci 18: 2550–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda SA, Mack KJ 2000. Seizures and sensory stimulation result in different patterns of brain derived neurotrophic factor protein expression in the barrel cortex and hippocampus. Brain Res Mol Brain Res 78: 1–14 [DOI] [PubMed] [Google Scholar]
- Nott A, Watson PM, Robinson JD, Crepaldi L, Riccio A 2008. S-Nitrosylation of histone deacetylase 2 induces chromatin remodelling in neurons. Nature 455: 411–415 [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA 1997a. Intra-hippocampal estradiol infusion enhances memory in ovariectomized rats. Neuroreport 8: 3009–3013 [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA 1997b. Posttraining estradiol injections enhance memory in ovariectomized rats: cholinergic blockade and synergism. Neurobiol Learn Mem 68: 172–188 [DOI] [PubMed] [Google Scholar]
- Peixoto L, Abel T 2013. The role of histone acetylation in memory formation and cognitive impairments. Neuropsychopharmacology 38: 62–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Javan S, Agis-Balboa RC, Cota P, Wittnam JL, Gogol-Doering A, Opitz L, et al. 2010. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science 328: 753–756 [DOI] [PubMed] [Google Scholar]
- Perovic M, Tesic V, Mladenovic Djordjevic A, Smiljanic K, Loncarevic-Vasiljkovic N, Ruzdijic S, Kanazir S 2013. BDNF transcripts, proBDNF and proNGF, in the cortex and hippocampus throughout the life span of the rat. Age (Dordr) 35: 2057–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino N, Russo M, Santoro AN, Litta P, Cela V, Genazzani AR 2013. Steroid hormones and BDNF. Neuroscience 239: 271–279 [DOI] [PubMed] [Google Scholar]
- Rudick CN, Gibbs RB, Woolley CS 2003. A role for the basal forebrain cholinergic system in estrogen-induced disinhibition of hippocampal pyramidal cells. J Neurosci 23: 4479–4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar SN, Huang RQ, Logan SM, Yi KD, Dillon GH, Simpkins JW 2008. Estrogens directly potentiate neuronal L-type Ca2+ channels. Proc Natl Acad Sci 105: 15148–15153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Akaishi T, Matsuki N, Ohno Y, Nakazawa K 2007. β-Estradiol induces synaptogenesis in the hippocampus by enhancing brain-derived neurotrophic factor release from dentate gyrus granule cells. Brain Res 1150: 108–120 [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ 2003. Hippocampal excitability increases during the estrous cycle in the rat: a potential role for brain-derived neurotrophic factor. J Neurosci 23: 11641–11652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz N, Schohl A, Ruthazer ES 2011. Activity-dependent transcription of BDNF enhances visual acuity during development. Neuron 70: 455–467 [DOI] [PubMed] [Google Scholar]
- Sharma SK 2010. Protein acetylation in synaptic plasticity and memory. Neurosci Biobehav Rev 34: 1234–1240 [DOI] [PubMed] [Google Scholar]
- Singh M, Meyer EM, Simpkins JW 1995. The effect of ovariectomy and estradiol replacement on brain-derived neurotrophic factor messenger ribonucleic acid expression in cortical and hippocampal brain regions of female Sprague-Dawley rats. Endocrinology 136: 2320–2324 [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RC, Toran-Allerand CD 1995. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc Natl Acad Sci 92: 11110–11114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Milner TA, McEwen BS 2008a. Estrous cycle regulates activation of hippocampal Akt, LIM kinase, and neurotrophin receptors in C57BL/6 mice. Neuroscience 155: 1106–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS 2008b. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol 29: 219–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava DP, Woolfrey KM, Jones KA, Shum CY, Lash LL, Swanson GT, Penzes P 2008. Rapid enhancement of two-step wiring plasticity by estrogen and NMDA receptor activity. Proc Natl Acad Sci 105: 14650–14655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanko DP, Barrett RM, Ly AR, Reolon GK, Wood MA 2009. Modulation of long-term memory for object recognition via HDAC inhibition. Proc Natl Acad Sci 106: 9447–9452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME 1998. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron 20: 709–726 [DOI] [PubMed] [Google Scholar]
- Towart LA, Alves SE, Znamensky V, Hayashi S, McEwen BS, Milner TA 2003. Subcellular relationships between cholinergic terminals and estrogen receptor-α in the dorsal hippocampus. J Comp Neurol 463: 390–401 [DOI] [PubMed] [Google Scholar]
- Vecsey CG, Hawk JD, Lattal KM, Stein JM, Fabian SA, Attner MA, Cabrera SM, McDonough CB, Brindle PK, Abel T, et al. 2007. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J Neurosci 27: 6128–6140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA 2006. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem 86: 35–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Li S, Dong HP, Lv S, Tang YY 2009. Differential impairment of spatial and nonspatial cognition in a mouse model of brain aging. Life Sci 85: 127–135 [DOI] [PubMed] [Google Scholar]
- Wimmer ME, Hernandez PJ, Blackwell J, Abel T 2012. Aging impairs hippocampus-dependent long-term memory for object location in mice. Neurobiol Aging 33: 2220–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TW, Wang JM, Chen S, Brinton RD 2005. 17β-Estradiol induced Ca2+ influx via L-type calcium channels activates the Src/ERK/cyclic-AMP response element binding protein signal pathway and BCL-2 expression in rat hippocampal neurons: a potential initiation mechanism for estrogen-induced neuroprotection. Neuroscience 135: 59–72 [DOI] [PubMed] [Google Scholar]
- Yaffe K, Barnes D, Lindquist K, Cauley J, Simonsick EM, Penninx B, Satterfield S, Harris T, Cummings SR 2007. Endogenous sex hormone levels and risk of cognitive decline in an older biracial cohort. Neurobiol Aging 28: 171–178 [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Shima N, Yuri K 2007. Age-related changes in the expression of ER-β mRNA in the female rat brain. Brain Res 1155: 34–41 [DOI] [PubMed] [Google Scholar]
- Yang X 2007. HATs and HDACs: from structure, function and regulation to novel strategies for therapy and prevention. Oncogene 26: 5310–5318 [DOI] [PubMed] [Google Scholar]
- Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Breitner JCS 2002. Hormone replacement therapy and incidence of Alzheimer disease in older women. JAMA 288: 2123–2129 [DOI] [PubMed] [Google Scholar]
- Zeng Y, Tan M, Kohyama J, Sneddon M, Watson JB, Sun YE, Xie CW 2011. Epigenetic enhancement of BDNF signaling rescues synaptic plasticity in aging. J Neurosci 31: 17800–17810 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhao L, Chen S, Ming Wang J, Brinton RD 2005. 17β-Estradiol induces Ca2+ influx, dendritic and nuclear Ca2+ rise and subsequent cyclic AMP response element-binding protein activation in hippocampal neurons: a potential initiation mechanism for estrogen neurotrophism. Neuroscience 132: 299–311 [DOI] [PubMed] [Google Scholar]
- Zhao Z, Fan L, Frick KM 2010. Epigenetic alterations regulate the estradiol-induced enhancement of memory consolidation. Proc Natl Acad Sci 107: 5605–5610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Fan L, Fortress AM, Boulware MI, Frick KM 2012. Hippocampal histone acetylation regulates object recognition and the estradiol-induced enhancement of object recognition. J Neurosci 32: 2344–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Zhou X, Luo Y, Xiao H, Wayman G, Wang H 2011. Regulation of brain-derived neurotrophic factor exon IV transcription through calcium responsive elements in cortical neurons. PLoS One 6: e28441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Zhou X, Moon C, Wang H 2012. Regulation of brain-derived neurotrophic factor expression in neurons. Int J Physiol Pathophysiol Pharmacol 4: 188–200 [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Watters JJ, Dorsa DM 1996. Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinology 137: 2163–2166 [DOI] [PubMed] [Google Scholar]