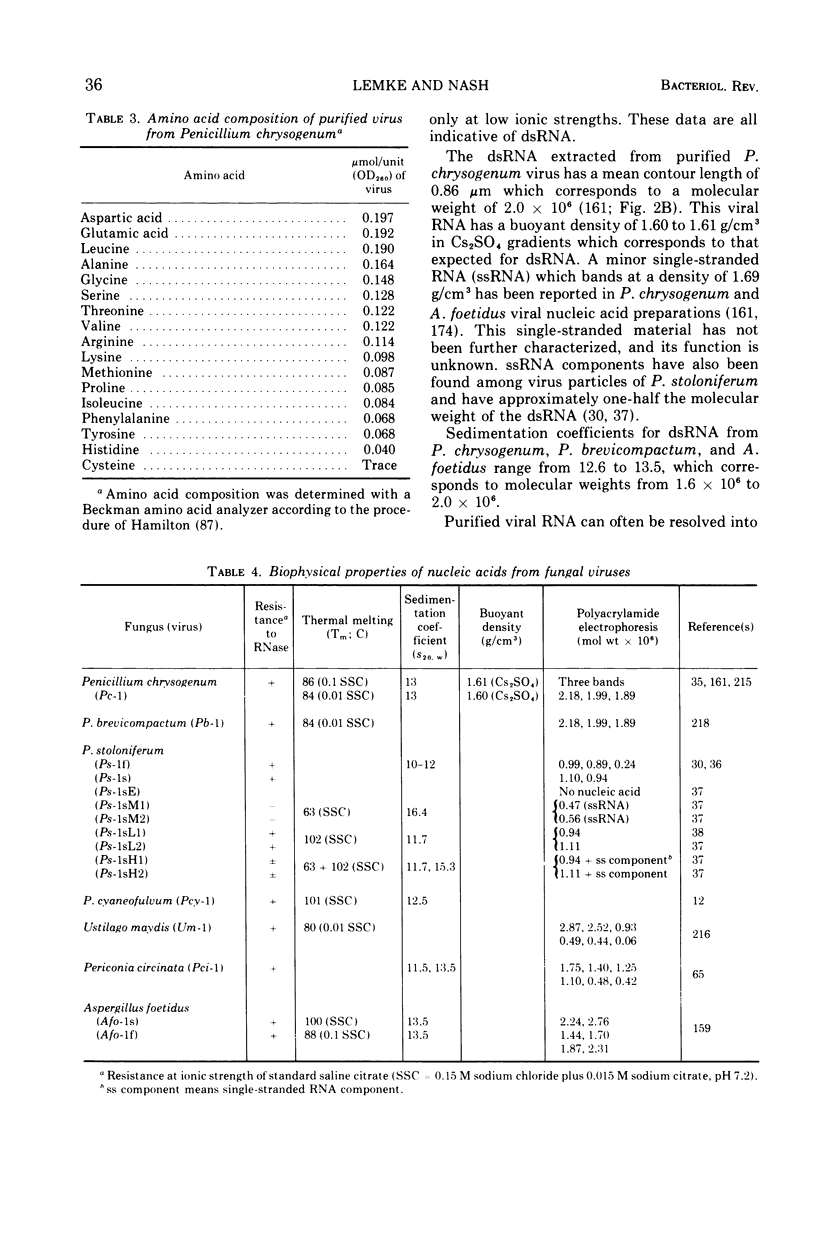
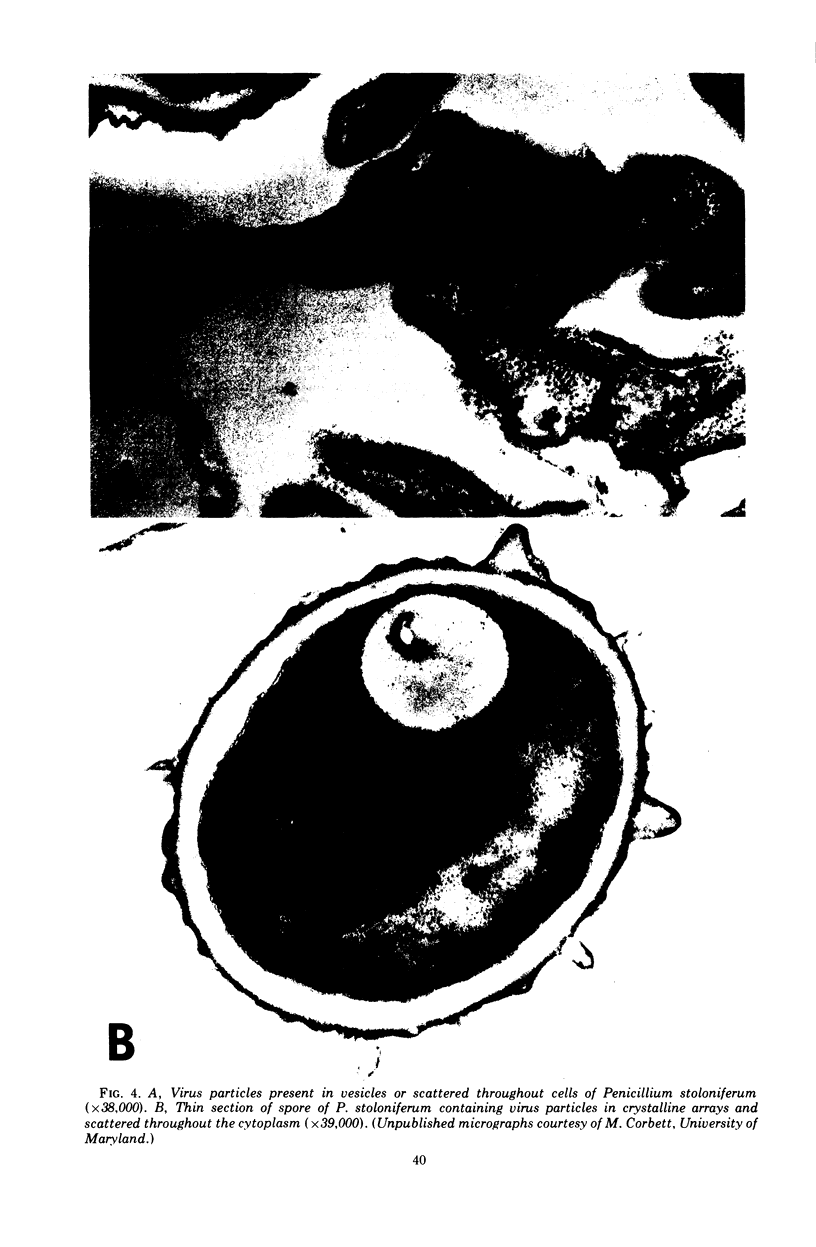
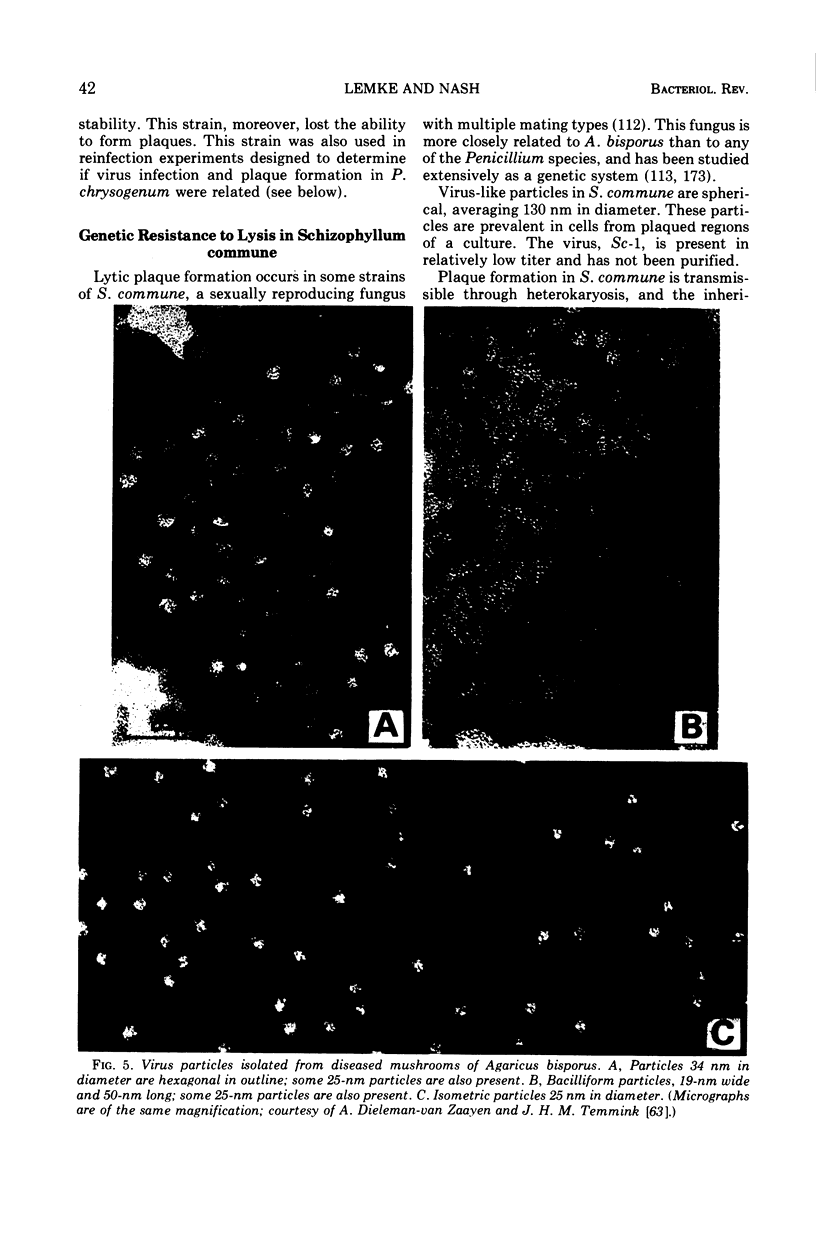
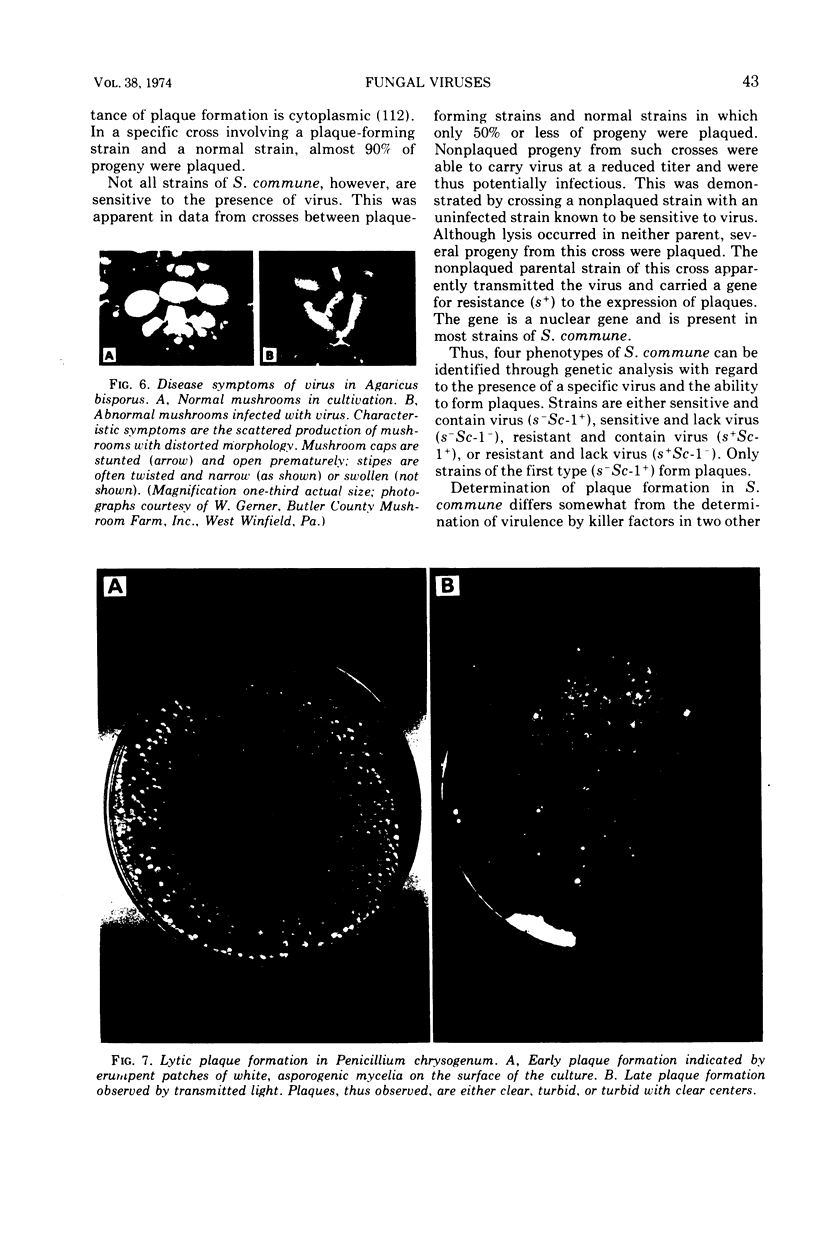
Full text
PDF



















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D. Expression of animal virus genomes. Bacteriol Rev. 1971 Sep;35(3):235–241. doi: 10.1128/br.35.3.235-241.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks G. T., Buck K. W., Chain E. B., Darbyshire J. E., Himmelweit F. Penicillium cyaneo-fulvum virus and interferon stimulation. Nature. 1969 Jul 12;223(5202):155–158. doi: 10.1038/223155a0. [DOI] [PubMed] [Google Scholar]
- Banks G. T., Buck K. W., Chain E. B., Darbyshire J. E., Himmelweit F., Ratti G., Sharpe T. J., Planterose D. N. Antiviral activity of double stranded RNA from a virus isolated from Aspergillus foetidus. Nature. 1970 Aug 1;227(5257):505–507. doi: 10.1038/227505a0. [DOI] [PubMed] [Google Scholar]
- Banks G. T., Buck K. W., Chain E. B., Darbyshire J. E., Himmelweit F. Virus-like particles in penicillin producing strains of Penicillium chrysogenum. Nature. 1969 Apr 5;222(5188):89–90. doi: 10.1038/222089b0. [DOI] [PubMed] [Google Scholar]
- Banks G. T., Buck K. W., Chain E. B., Himmelweit F., Marks J. E., Tyler J. M., Hollings M., Last F. T., Stone O. M. Viruses in fungi and interferon stimulation. Nature. 1968 May 11;218(5141):542–545. doi: 10.1038/218542a0. [DOI] [PubMed] [Google Scholar]
- Berry E. A., Bevan E. A. A new species of double-stranded RNA from yeast. Nature. 1972 Sep 29;239(5370):279–280. doi: 10.1038/239279a0. [DOI] [PubMed] [Google Scholar]
- Bevan E. A., Herring A. J., Mitchell D. J. Preliminary characterization of two species of dsRNA in yeast and their relationship to the "killer" character. Nature. 1973 Sep 14;245(5420):81–86. doi: 10.1038/245081b0. [DOI] [PubMed] [Google Scholar]
- Bevan E. A., Somers J. M. Somatic segregation of the killer (k) and neutral (n) cytoplasmic genetic determinants in yeast. Genet Res. 1969 Aug;14(1):71–77. doi: 10.1017/s0016672300001865. [DOI] [PubMed] [Google Scholar]
- Border D. J., Buck K. W., Chain E. B., Kempson-Jones G. F., Lhoas P., Ratti G. Viruses of Penicillium and Aspergillus species. Biochem J. 1972 Apr;127(2):4P–6P. doi: 10.1042/bj1270004p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Border D. J. Electron microscopy of cells of Saccharomyces cerevisiae infected with double stranded RNA viruses from Aspergillus niger and Penicillium stoloniferum. Nat New Biol. 1972 Mar 22;236(64):87–88. doi: 10.1038/newbio236087a0. [DOI] [PubMed] [Google Scholar]
- Borré E., Morgantini L. E., Ortali V., Tonolo A. Production of lytic plaques of viral origin in Penicillium. Nature. 1971 Feb 19;229(5286):568–569. doi: 10.1038/229568b0. [DOI] [PubMed] [Google Scholar]
- Bozarth R. F., Wood H. A., Mandelbrot A. The Penicillium stoloniferum virus complex: two similar double-stranded RNA virus-like particles in a single cell. Virology. 1971 Aug;45(2):516–523. doi: 10.1016/0042-6822(71)90352-7. [DOI] [PubMed] [Google Scholar]
- Buck K. W., Chain E. B., Darbyshire J. E. High cell wall galactosamine content and virus particles in Penicillium stoloniferum. Nature. 1969 Sep 20;223(5212):1273–1273. doi: 10.1038/2231273a0. [DOI] [PubMed] [Google Scholar]
- Buck K. W., Chain E. B., Himmelweit F. Comparison of interferon induction in mice by purified Penicillium chrysogenum virus and derived double-stranded RNA. J Gen Virol. 1971 Aug;12(2):131–139. doi: 10.1099/0022-1317-12-2-131. [DOI] [PubMed] [Google Scholar]
- Buck K. W., Kempson-Jones G. F. Biophysical properties of Penicillium stoloniferum virus S. J Gen Virol. 1973 Mar;18(3):223–235. doi: 10.1099/0022-1317-18-3-223. [DOI] [PubMed] [Google Scholar]
- Buck K. W., Kempson-Jones G. F. Three types of virus particle in Penicillium stoloniferum. Nature. 1970 Mar 7;225(5236):945–946. doi: 10.1038/225945a0. [DOI] [PubMed] [Google Scholar]
- Bussey H. Effects of yeast killer factor on sensitive cells. Nat New Biol. 1972 Jan 19;235(55):73–75. doi: 10.1038/newbio235073a0. [DOI] [PubMed] [Google Scholar]
- CAMPBELL R. N. Relationship between the lettuce big-vein virus and its vector, Olpidium brassicae. Nature. 1962 Aug 18;195:675–677. doi: 10.1038/195675a0. [DOI] [PubMed] [Google Scholar]
- Carter S. B., Franklin T. J., Jones D. F., Leonard B. J., Mills S. D., Turner R. W., Turner W. B. Mycophenolic acid: an anti-cancer compound with unusual properties. Nature. 1969 Aug 23;223(5208):848–850. doi: 10.1038/223848a0. [DOI] [PubMed] [Google Scholar]
- Caten C. E. Vegetative incompatibility and cytoplasmic infection in fungi. J Gen Microbiol. 1972 Sep;72(2):221–229. doi: 10.1099/00221287-72-2-221. [DOI] [PubMed] [Google Scholar]
- Coutts R. H., Cocking E. C., Kassanis B. Infection of protoplasts from yeast with tobacco mosaic virus. Nature. 1972 Dec 22;240(5382):466–467. doi: 10.1038/240466a0. [DOI] [PubMed] [Google Scholar]
- Cox R. A., Kanagalingam K., Sutherland E. Thermal denaturation in acidic solutions of double-helical ribonucleic acid from virus-like particles found in Penicillium chrysogenum. A spectrophotometric study. Biochem J. 1971 Nov;125(2):655–665. doi: 10.1042/bj1250655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A., Kanagalingam K., Sutherland K. S. Double-helical character of ribonucleic acid from virus-like particles found in Penicillium chrysogenum. Biochem J. 1970 Dec;120(3):549–558. doi: 10.1042/bj1200549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day L. E., Ellis L. F. Virus-like particles in Cephalosporium acremonium. Appl Microbiol. 1971 Nov;22(5):919–920. doi: 10.1128/am.22.5.919-920.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detroy R. W., Freer S. N., Fennell D. I. Relationship between the biosynthesis of virus-like particles and mycophenolic acid in Penicillium stoloniferum and Penicillium brevi-compactum. Can J Microbiol. 1973 Nov;19(11):1459–1462. doi: 10.1139/m73-237. [DOI] [PubMed] [Google Scholar]
- Dias H. F. The relationship between cucumber necrosis virus and its vector, Olpidium cucurbitacearum. Virology. 1970 Sep;42(1):204–211. doi: 10.1016/0042-6822(70)90253-9. [DOI] [PubMed] [Google Scholar]
- Dieleman-Van Zaayen A. Intracellular appearance of mushroom virus in fruiting bodies and basidiospores of Agaricus bisporus. Virology. 1972 Jan;47(1):94–104. doi: 10.1016/0042-6822(72)90242-5. [DOI] [PubMed] [Google Scholar]
- Dieleman-van Zaayen A., Igesz O. Intracellular appearance of mushroom virus. Virology. 1969 Sep;39(1):147–152. doi: 10.1016/0042-6822(69)90359-6. [DOI] [PubMed] [Google Scholar]
- Dieleman-van Zaayen M., Igesz O., Funch J. T. Intracellular appearance and some morphological features of viruslike particles in an ascomycete fungus. Virology. 1970 Oct;42(2):534–537. [PubMed] [Google Scholar]
- Douthart R. J., Burnett J. P., Beasley F. W., Frank B. H. Binding of ethidium bromide to double-stranded ribonucleic acid. Biochemistry. 1973 Jan 16;12(2):214–220. doi: 10.1021/bi00726a006. [DOI] [PubMed] [Google Scholar]
- Ellingboe A. H. ILLEGITIMACY AND SPECIFIC FACTOR TRANSFER IN SCHIZOPHYLLUM COMMUNE. Proc Natl Acad Sci U S A. 1963 Mar;49(3):286–292. doi: 10.1073/pnas.49.3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis L. F., Kleinschmidt W. J. Virus-like particles of a fraction of statolon, a mould product. Nature. 1967 Aug 5;215(5101):649–650. doi: 10.1038/215649a0. [DOI] [PubMed] [Google Scholar]
- Enomoto M., Saito M. Carcinogens produced by fungi. Annu Rev Microbiol. 1972;26:279–312. doi: 10.1146/annurev.mi.26.100172.001431. [DOI] [PubMed] [Google Scholar]
- Esser K. Breeding systems in fungi and their significance for genetic recombination. Mol Gen Genet. 1971;110(1):86–100. doi: 10.1007/BF00276051. [DOI] [PubMed] [Google Scholar]
- Estes A. P., Brakke M. K. Correlation of Polymyxa graminis with transmission of soil-borne wheat mosaic virus. Virology. 1966 Apr;28(4):772–774. doi: 10.1016/0042-6822(66)90266-2. [DOI] [PubMed] [Google Scholar]
- Fink G. R., Styles C. A. Curing of a killer factor in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2846–2849. doi: 10.1073/pnas.69.10.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii-Kawata I., Miura K. I. Segments of genome of viruses containing double-stranded ribonucleic acid. J Mol Biol. 1970 Jul 28;51(2):247–253. doi: 10.1016/0022-2836(70)90140-3. [DOI] [PubMed] [Google Scholar]
- Gauze G. F., Maksimova T. S., Dudnik Iu V., Ol'khovatova O. L., Velikodvorskaia G. A. Novye shtammy virusov, vydelennye iz plesnevykh gribov roda Penicillium. Mikrobiologiia. 1971 May-Jun;40(3):540–543. [PubMed] [Google Scholar]
- HIRANO T., LINDEGREN C. C., BANG Y. N. Electron microscopy of virus-infected yeast cells. J Bacteriol. 1962 Jun;83:1363–1364. doi: 10.1128/jb.83.6.1363-1364.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiruki C. Host specificity in transmission of tobacco stunt virus by Olpidium brassicae. Virology. 1967 Sep;33(1):131–136. doi: 10.1016/0042-6822(67)90101-8. [DOI] [PubMed] [Google Scholar]
- Hollings M., Stone O. M. Viruses in fungi. Sci Prog. 1969 Autumn;57(227):371–391. [PubMed] [Google Scholar]
- KLEINSCHMIDT W. J., CLINE J. C., MURPHY E. B. INTERFERON PRODUCTION INDUCED BY STATOLON. Proc Natl Acad Sci U S A. 1964 Sep;52:741–744. doi: 10.1073/pnas.52.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaempfer R., Kaufman J. Inhibition of cellular protein synthesis by double-stranded RNA: inactivation of an initiation factor. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1222–1226. doi: 10.1073/pnas.70.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazama F. Y., Schornstein K. L. Herpes-type virus particles associated with a fungus. Science. 1972 Aug 25;177(4050):696–697. doi: 10.1126/science.177.4050.696. [DOI] [PubMed] [Google Scholar]
- Kazama F. Y., Schornstein K. L. Ultrastructure of a fungus herpes-type virus. Virology. 1973 Apr;52(2):478–487. doi: 10.1016/0042-6822(73)90343-7. [DOI] [PubMed] [Google Scholar]
- Kleischmidt W. J., Ellis L. F., Van Frank R. M., Murphy E. B. Interferon stimulation by a double stranded RNA of a mycophage in statolon preparations. Nature. 1968 Oct 12;220(5163):167–168. doi: 10.1038/220167a0. [DOI] [PubMed] [Google Scholar]
- Koltin Y., Perick R., Stamberg J., Ben-Shaul Y. Virus-like particles and cytoplasmic inheritance of plaques in a higher fungus. Nat New Biol. 1973 Jan 24;241(108):108–109. doi: 10.1038/newbio241108a0. [DOI] [PubMed] [Google Scholar]
- Koltin Y., Stamberg J., Lemke P. A. Genetic structure and evolution of the incompatibility factors in higher fungi. Bacteriol Rev. 1972 Jun;36(2):156–171. doi: 10.1128/br.36.2.156-171.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács E., Bucz B., Kolompár G. Propagation of mammalian viruses in protista. IV. Experimental infection of C. albicans and S. cerevisiae with polyoma virus. Proc Soc Exp Biol Med. 1969 Dec;132(3):971–977. doi: 10.3181/00379727-132-34348. [DOI] [PubMed] [Google Scholar]
- Kovács E., Bucz B. Propagation of mammalian viruses in Protista II. Isolation of complete virus from yeast and Tetrahymena experimentally infected with picorna viral particles or their infectious RNA. Life Sci. 1967 Feb 15;6(4):347–358. doi: 10.1016/0024-3205(67)90003-3. [DOI] [PubMed] [Google Scholar]
- Kozlova T. M. O virusopodobnykh chastitsakh v drozhzhevykh kletkakh. Mikrobiologiia. 1973 Jul-Aug;42(4):745–747. [PubMed] [Google Scholar]
- Küntzel H., Barath Z., Ali I., Kind J., Althaus H. H. Virus-like particles in an estranuclear mutant of Neurospora crassa. Proc Natl Acad Sci U S A. 1973 May;70(5):1574–1578. doi: 10.1073/pnas.70.5.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDEGREN C. C., BANG Y. N., HIRANO T. Progress report on the zymophage. Trans N Y Acad Sci. 1962 Mar;24:540–566. doi: 10.1111/j.2164-0947.1962.tb01431.x. [DOI] [PubMed] [Google Scholar]
- LINDEGREN C. C., BANG Y. N. The zymophage. Antonie Van Leeuwenhoek. 1961;27:1–18. doi: 10.1007/BF02538417. [DOI] [PubMed] [Google Scholar]
- Lampson G. P., Tytell A. A., Field A. K., Nemes M. M., Hilleman M. R. Inducers of interferon and host resistance. I. Double-stranded RNA from extracts of Penicillium funiculosum. Proc Natl Acad Sci U S A. 1967 Aug;58(2):782–789. doi: 10.1073/pnas.58.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapierre H., Astier-Manifacier S., Cornuet P. Activité RNA polymérase associée aux préparations purifiées de virus du Penicillium stoloniferum. C R Acad Sci Hebd Seances Acad Sci D. 1971 Sep 13;273(11):992–994. [PubMed] [Google Scholar]
- Lapierre H., Lemaire J. M., Jouan B., Molin G. Mise en évidence de particules virales associées à une perte de pathogénicité chez le piétin-échaudage des céréales, Ophiobolus graminis Sacc. C R Acad Sci Hebd Seances Acad Sci D. 1970 Nov 16;271(20):1833–1836. [PubMed] [Google Scholar]
- Lemke P. A., Nash C. H., Pieper S. W. Lytic plaque formation and variation in virus titre among strains of Penicillium chrysogenum. J Gen Microbiol. 1973 Jun;76(2):265–275. doi: 10.1099/00221287-76-2-265. [DOI] [PubMed] [Google Scholar]
- Lemke P. A., Ness T. M. Isolation and characterization of a double-stranded ribonucleic acid from Penicillium chrysogenum. J Virol. 1970 Dec;6(6):813–819. doi: 10.1128/jvi.6.6.813-819.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhoas P. Mating pairs of Saccharomyces cerevisiae infected with double stranded RNA viruses from Aspergillus niger. Nat New Biol. 1972 Mar 22;236(64):86–87. doi: 10.1038/newbio236086a0. [DOI] [PubMed] [Google Scholar]
- Lhoas P. Transmission of double stranded RNA viruses to a strain of Penicillium stoloniferum through heterokaryosis. Nature. 1971 Mar 26;230(5291):248–249. doi: 10.1038/230248a0. [DOI] [PubMed] [Google Scholar]
- Loviny T., Székely M. Fingerprinting double-stranded non-radioactive RNA from a fungal virus. Eur J Biochem. 1973 May;35(1):87–94. doi: 10.1111/j.1432-1033.1973.tb02813.x. [DOI] [PubMed] [Google Scholar]
- Lwoff A., Tournier P. The classification of viruses. Annu Rev Microbiol. 1966;20:45–74. doi: 10.1146/annurev.mi.20.100166.000401. [DOI] [PubMed] [Google Scholar]
- Manier J. F., Vago C. Infection virale chez les Trichomycètes. C R Acad Sci Hebd Seances Acad Sci D. 1971 Oct 4;273(14):1241–1243. [PubMed] [Google Scholar]
- Metitiri P. O., Zachariah K. Viruslike particles and inclusion bodies in penicillus cells of a mutant of Penicillium. J Ultrastruct Res. 1972 Aug;40(3):272–283. doi: 10.1016/s0022-5320(72)90100-1. [DOI] [PubMed] [Google Scholar]
- Mitchell M. B., Mitchell H. K., Tissieres A. Mendelian and Non-Mendelian Factors Affecting the Cytochrome System in Neurospora Crassa. Proc Natl Acad Sci U S A. 1953 Jul;39(7):606–613. doi: 10.1073/pnas.39.7.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt E. M., Lister R. M. Detection of mycoviruses using antiserum specific for dsRNA. Virology. 1973 Mar;52(1):301–304. doi: 10.1016/0042-6822(73)90421-2. [DOI] [PubMed] [Google Scholar]
- Nash C. H., Douthart R. J., Ellis L. F., Van Frank R. M., Burnett J. P., Lemke P. A. On the mycophage of Penicillium chrysogenum. Can J Microbiol. 1973 Jan;19(1):97–103. doi: 10.1139/m73-014. [DOI] [PubMed] [Google Scholar]
- Nienhaus F. Tobacco mosaic virus strains extracted from conidia of powdery mildews. Virology. 1971 Nov;46(2):504–505. doi: 10.1016/0042-6822(71)90054-7. [DOI] [PubMed] [Google Scholar]
- Nii S., Morgan C., Rose H. M. Electron microscopy of herpes simplex virus. II. Sequence of development. J Virol. 1968 May;2(5):517–536. doi: 10.1128/jvi.2.5.517-536.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins F. O. Ultrastructure of vegetative stages in Labyrinthomyxa marina (Dermocystidium marinum), a commercially significant oyster pathogen. J Invertebr Pathol. 1969 Mar;13(2):199–222. doi: 10.1016/0022-2011(69)90211-0. [DOI] [PubMed] [Google Scholar]
- Planterose D. N., Birch P. J., Pilch D. J., Sharpe T. J. Antiviral activity of double stranded RNA and virus-like particles from Penicillium stoloniferum. Nature. 1970 Aug 1;227(5257):504–505. doi: 10.1038/227504a0. [DOI] [PubMed] [Google Scholar]
- Puhalla J. E. Compatibility reactions on solid medium and interstrain inhibition in Ustilago maydis. Genetics. 1968 Nov;60(3):461–474. doi: 10.1093/genetics/60.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rytel M. W., Shope R. E., Kilbourne E. D. An antiviral substance from Penicillium funiculosum. V. Induction of interferon by helenine. J Exp Med. 1966 Apr 1;123(4):577–584. doi: 10.1084/jem.123.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHOPE R. E. An antiviral substance from Penicillium funiculosum. I. Effect upon infection in mice with swine influenza virus and Columbia SK encephalomyelitis virus. J Exp Med. 1953 May;97(5):601–625. doi: 10.1084/jem.97.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansing G. A., Detroy R. W., Freer S. N., Hesseltine C. W. Virus particles from conidia of Penicillium species. Appl Microbiol. 1973 Dec;26(6):914–918. doi: 10.1128/am.26.6.914-918.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnepf E., Soeder C. J., Hegewald E. Polyhedral viruslike particles lysing the aquatic phycomycete Aphelidium sp., a parasite of the green alga Scenedesmus armatus. Virology. 1970 Oct;42(2):482–487. doi: 10.1016/0042-6822(70)90291-6. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J. Viruses with segmented ribonucleic acid genomes: multiplication of influenza versus reovirus. Bacteriol Rev. 1971 Sep;35(3):250–266. doi: 10.1128/br.35.3.250-266.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipkey F. H., Erlandson R. A., Bailey R. B., Babcock V. I., Southam C. M. Virus biographies. II. Growth of herpes simplex virus in tissue culture. Exp Mol Pathol. 1967 Feb;6(1):39–67. doi: 10.1016/0014-4800(67)90005-6. [DOI] [PubMed] [Google Scholar]
- Sirotnak F. M., McCuen R. W. Hyperproduction of dihydrofolate reductase in Diplococcus pneumoniae after mutation in the structural gene. Evidence for an effect at the level of transcription. Genetics. 1973 Aug;74(4):543–556. doi: 10.1093/genetics/74.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers J. M., Bevan E. A. The inheritance of the killer character in yeast. Genet Res. 1969 Feb;13(1):71–83. doi: 10.1017/s0016672300002743. [DOI] [PubMed] [Google Scholar]
- TEAKLE D. S., GOLD A. H. Further studies of Olpidium as a vector of tobacco necrosis virus. Virology. 1963 Mar;19:310–315. doi: 10.1016/0042-6822(63)90069-2. [DOI] [PubMed] [Google Scholar]
- Tuveson R. W., Peterson J. F. Virus-like particles in certain slow-growing strains of Neurospora crassa. Virology. 1972 Feb;47(2):527–531. doi: 10.1016/0042-6822(72)90295-4. [DOI] [PubMed] [Google Scholar]
- Van Frank R. M., Ellis L. F., Kleinschmidt W. J. Purification and physical properties of mycophage PS1. J Gen Virol. 1971 Jul;12(1):33–42. doi: 10.1099/0022-1317-12-1-33. [DOI] [PubMed] [Google Scholar]
- Velikodvorskaia G. A., Bobkova A. F., Maksimova T. S., Klimenko S. M., Tikhonenko T. I. Novye virusy, vydelennye iz kul'tury griba rada Penicillium. Biull Eksp Biol Med. 1972 May;73(5):90–93. [PubMed] [Google Scholar]
- Vodkin M. H., Fink G. R. A nucleic acid associated with a killer strain of yeast. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1069–1072. doi: 10.1073/pnas.70.4.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkoff O., Walters T., Dejardin R. A. An examination of Penicillium notatum for the presence of Penicillium chrysogenum-type virus particles. Can J Microbiol. 1972 Aug;18(8):1352–1353. doi: 10.1139/m72-208. [DOI] [PubMed] [Google Scholar]
- Volkoff O., Walters T. Virus-like particles in abnormal cells of Saccharomyces carlsbergensis. Can J Genet Cytol. 1970 Sep;12(3):621–626. doi: 10.1139/g70-082. [DOI] [PubMed] [Google Scholar]
- Weintraub M., Ragetli H. W. Electron microscopy of the bean and cowpea strains of southern bean mosaic virus within leaf cells. J Ultrastruct Res. 1970 Jul;32(1):167–189. doi: 10.1016/s0022-5320(70)80043-0. [DOI] [PubMed] [Google Scholar]
- Weintraub M., Ragetli H. W., John V. T. Some conditions affecting the intracellular arrangement and concentration of tobacco mosaic virus particles in local lesions. J Cell Biol. 1967 Oct;35(1):183–192. doi: 10.1083/jcb.35.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood H. A., Bozarth R. F., Adler J., Mackenzie D. W. Proteinaceous virus-like particles from an isolate of Aspergillus flavus. J Virol. 1974 Feb;13(2):532–534. doi: 10.1128/jvi.13.2.532-534.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood H. A., Bozarth R. F., Mislivec P. B. Viruslike particles associated with an isolate of penicillium brevi-compactum. Virology. 1971 Jun;44(3):592–598. doi: 10.1016/0042-6822(71)90373-4. [DOI] [PubMed] [Google Scholar]
- Wood H. A., Bozarth R. F. Properties of viruslike particles of Penicillium chrysogenum: one double-stranded RNA molecule per particle. Virology. 1972 Mar;47(3):604–609. doi: 10.1016/0042-6822(72)90549-1. [DOI] [PubMed] [Google Scholar]
- Wood H. A. Viruses with double-stranded RNA genomes. J Gen Virol. 1973 Jun;20(Suppl):61–85. doi: 10.1099/0022-1317-20-Supplement-61. [DOI] [PubMed] [Google Scholar]
- Woods D. R., Bevan E. A. Studies on the nature of the killer factor produced by Saccharomyces cerevisiae. J Gen Microbiol. 1968 Apr;51(1):115–126. doi: 10.1099/00221287-51-1-115. [DOI] [PubMed] [Google Scholar]
- Yamashita S., Doi Y., Yora K. Intracellular appearance of Penicillium chrysogenum virus. Virology. 1973 Oct;55(2):445–452. doi: 10.1016/0042-6822(73)90186-4. [DOI] [PubMed] [Google Scholar]