Abstract
We describe Resonance Raman based skin carotenoid measurements in newborns and infants. Skin- and serum carotenoid levels correlate with high statistical significance in healthy newborns and infants, and with reduced accuracy also in prematurely born infants, who in general feature very low carotenoid levels and thin transparent skin giving rise to large background absorption effects. Skin carotenoid levels can be easily compared among subjects and/or tracked in longitudinal studies with the highly molecule-specific Raman method. It therefore holds promise as a rapid, non-invasive, carotenoid antioxidant assessment method for newborns and infants in the field of pediatrics.
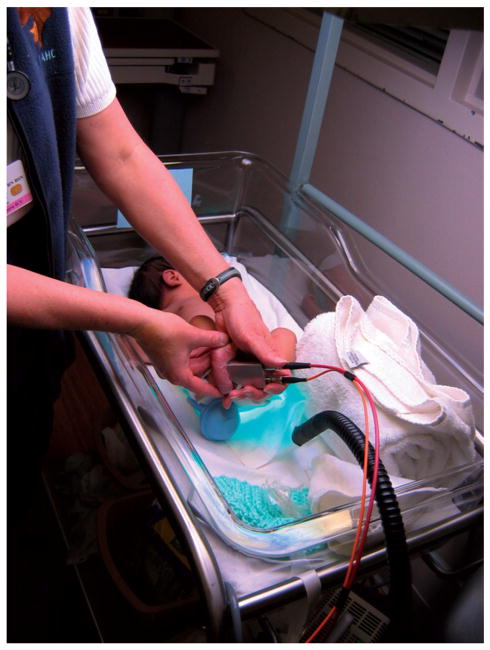
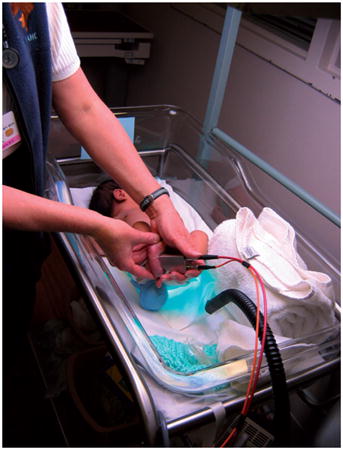
Photograph of an infant’s skin carotenoid measurement via Resonance Raman spectroscopy. The instrument’s fiber-coupled light delivery and collection module is held against the foot, exposing the heel skin to weak 488 nm laser light for 20 seconds. From spectral analysis of the Raman scattered light intensities, which occur in the green wavelength region, the carotenoid levels in the heel skin are obtained in a rapid, non-invasive, and painless fashion.
Keywords: newborns, carotenoids, antioxidants, Raman spectroscopy
1. Introduction
Carotenoids are fat-soluble, extensively conjugated polyene pigments synthesized exclusively by plants and micro-organisms, with widespread distributions in fruits and vegetables [1]. Adults and children obtain carotenoids from the diet, with fruits and vegetables as the major source of carotenoids. The fetus, on the other hand, obtains carotenoids only from the mother’s diet prenatally. In the first months after birth the infant receives carotenoids from breast milk and/or commercial formula preparations that have been enriched with carotenoids. There are over 600 known carotenoids in plants, but only about 15 are routinely detected in human tissues, serum, and milk. The major carotenoids in human serum include β-carotene, lycopene, lutein, α-carotene, β-crypto-xanthin, and zeaxanthin. The various carotenoids are deposited with widely varying concentrations and spatial distributions in many tissues of the body, including retina, skin, and fat. The presence of carotenoids in adult human skin can serve as a biomarker for fruit and vegetable consumption [2].
As abundantly available phyto-nutrients, carotenoids are thought to be beneficial for human development and health via their function as antioxidants. In adults, they appear to protect against various cancers [3–6], cardiovascular disease [7], macular degeneration [8], and all-cause mortality [9]. In infants, carotenoids may function as antioxidants and anti-inflammatory mediators, although relatively little is known about carotenoids in infants. Preterm infants are often exposed to high oxygen levels due to immature lung development and may be at heightened risk for oxidative stress because of their deficient antioxidant system and low carotenoid stores. Almost all preterm infants born have low stores of carotenoids since the in utero transfer of carotenoids occurs mainly during the last trimester. Postnatally, the source of carotenoids is also very limited for preterm infants and this poses a risk for oxidative stress, which may play a causative role in certain diseases of the preterm infant such as chronic lung disorder, necrotizing enterocolitis, sepsis, intraventricular hemorrhage, and retinopathy of prematurity [10, 11].
In the infant’s eye development, the carotenoid species lutein and zeaxanthin are increasingly concentrated in the macula postnatally. Only very small concentrations accumulate in the fetus at earlier stages. In this tissue, carotenoids are thought to be protective also through their optical filtering function, since due to their strong absorption in the blue wavelength region, they block phototoxic short-wavelength light from reaching the cone and rod cells in the macula, and therefore may be important in maturation and protection of the visual system.
The assessment of carotenoid status is traditionally based on the collection of plasma or serum samples for high performance liquid chromatography analysis, HPLC [12, 13]. While considered the current standard, this approach has several important limitations, including high cost and the necessity to extract the carotenoids from very limited quantities of infant blood [14]. Also, not all of the half dozen carotenoid species circulating in blood are equally taken up by the various body tissues. For example, due to protein binding effects, the human macula takes up only lutein and zeaxanthin from the blood stream. As a consequence there is at best only a modest correlation between retinal tissue and plasma levels, and it is compelling to measure carotenoid levels directly in the tissue of interest.
For newborns, only few carotenoid studies exist, mostly carried out via HPLC analysis, and only for short time periods. It is known that the plasma concentration of β-carotene on the day of birth is about one order of magnitude lower compared to the mother, and that plasma β-carotene increases in breast-fed infants [15]. Furthermore, carotenoid concentrations in plasma can differ based on feeding procedures. For example, formula preparations can have diminished carotenoid profiles compared to breast milk, and important carotenoid species such as lycopene, which is a highly effective singlet-oxygen scavenger, may be absent from the plasma [16]. This could negatively affect the antioxidant capacity of these infants and hence their healthy development.
A non-invasive optical method well suited for the detection of carotenoids in living human tissue is based on Resonance Raman spectroscopy, RRS. It can be readily used as an objective indicator of carotenoid status in the skin of adults [17–20]. The RRS method is highly molecule-specific since it is based on the Raman response originating from the conjugated carbon backbone that is common to all carotenoids. The backbone’s carbon-carbon single-bonds (C–C) and double-bonds (C=C) each generate a spectrally sharp, resonantly enhanced, Raman scattered intensity (“RRS line”) when excited in any of the molecules’ vibronic absorption transitions in the visible wavelength region. The RRS lines are shifted from the light excitation frequency, which is preferentially a laser line, by exactly the amount of the respective carbon bond stretch frequency, i.e. by 1159 cm−1 for C–C bonds, and 1525 cm−1 for C=C bonds. Since these frequencies are relatively large, they translate into large wavelength shifts of about 30 nm away from the laser excitation wavelength when excited in the visible. Typically superimposed on a fluorescence background, the Raman lines are readily isolated from the excitation light with a medium-resolution (~1 nm) spectrograph, and their strengths can be easily quantified with a linear detector array provided it has a high dynamic detection range at the spectral location of the Raman scattered light [18]. The intensity of the Raman scattered light, IRRS, is given by
| (1) |
where σ is the RRS scattering cross section of the carotenoids, N is their population density, l the sample path length monitored by the detection system, and IL the laser excitation intensity. Keeping the laser intensity and scattering geometry fixed among subjects, the skin carotenoid RRS intensity scales linearly with the tissue carotenoid concentration, and therefore allows one to compare levels among subjects or level changes in longitudinal studies.
Choosing an excitation wavelength in the spectral vicinity of 480 nm, RRS measures the combined concentrations of all resonantly excited carotenoids in skin, including beta-carotene, lycopene, beta-cryp-toxanthin, lutein, zeaxanthin, as well as their isomers. Phytoene and phytofluene, two carotenoids found in skin that have shorter conjugation lengths and corresponding absorptions in the UV, are not detected [20]. In the adult human retina, RRS can be used to measure the combined concentration of lutein and zeaxanthin in the ~1 mm diameter macular region with spatially integrating detection [21, 22] or with spatially resolved imaging configurations [23].
One of the preferred body sites for RRS measurements is the palm of the hand or heel of the foot, because the melanin pigment is lighter and less variable among individuals of different racial and ethnic backgrounds in these tissue sites [18]. Additionally, the stratum corneum, the outer skin tissue layer, is relatively thick in the palm and heel (at least ~400 μm). For term neonates, an average skin thickness of ~300 μm has been reported, whereas the skin of a premature neonate is 40–60% thinner than the skin of an adult. However, by 2 to 3 weeks of age, the premature infant will have skin maturation similar to term infants [24]. Estimating a shallow light penetration depth of ~200 μm into the skin due to the strong scattering of the stratum corneum [25], this insures that in adults and healthy neonates the excitation light does not penetrate beyond this strongly scattering layer into the deeper tissue layers. In premature neonates, however, the Raman excitation light can be expected to penetrate into the dermis, with potentially confounding effects on the RRS skin carotenoid scores.
In field applications with portable instrument configurations, we could demonstrate the usefulness of the RRS methodology for the rapid measurement of large subject populations. Measurements of the palms of subjects showed a bell-shaped distribution with significant width (~50% of the central value), proving that important characteristics of an objective biomarker of carotenoid status, such as inter-subject variability, could be easily reproduced in a non-invasive fashion [18]. Carotenoid levels measured with RRS in the inner palm of the hand could be shown to correlate strongly with HPLC-derived carotenoid levels of fasting serum, with R = 0.78 (p < 0.001) for a study involving 104 mostly Caucasian subjects [26], and R = 0.62 (p = 0.006) for a more ethnically diverse population of 28 subjects [2], thus validating the method in an indirect way. Recently, direct validation experiments were completed that involved skin carotenoid RRS measurements followed by biopsy of the measured tissue volume, and subsequent HPLC analysis [2, 27]. Again, a high correlation was found between both methods, with a correlation coefficient of R = 0.9 for excised heel skin [27], and R = 0.7 for excised abdominal tissue [2].
2. RRS-based skin carotenoid measurements in infants
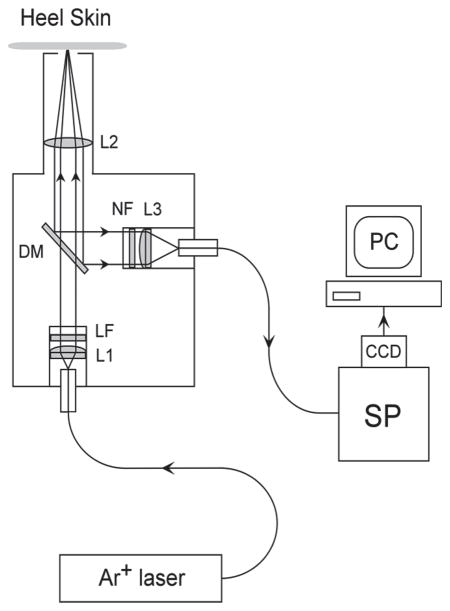
RRS-based carotenoid measurements of infants were carried out with a portable instrument developed specifically for clinical use in the newborn intensive care facility. It uses a fiber-coupled, hand-held, light delivery and detection module with a window port that is brought into direct contact with the skin tissue site of the sole of the infant who is lying in a crib, as illustrated in Figure 1. Further, the instrument includes a compact air-cooled 488 nm argon laser excitation source (National Laser, Inc., Model H210), a small spectrograph (Jarrel Ash, Inc., Model MonoSpec 18) with coupled CCD camera (Santa Barbara Instrument Group, Inc., Model ST6v), and a laptop computer operating custom-tailored software for instrumentation control and data acquisition. The layout of the instrument is schematically shown in Figure 2. The argon laser is routed through a 200 μm diameter multi-mode light delivery fiber to the optics module, expanded with a 25 mm focal length lens, sent through a 488 nm band-pass laser line filter and dichroic beam splitter, and focused with a 50 mm focal length lens onto the external surface of the window port, where it forms a ~2 mm diameter light excitation disk. The band-pass filter removes any light components other than the laser excitation light. The Raman-scattered, spectrally shifted, light is collected in a 180 degree backscattering geometry with the same focusing lens, expanded, and reflected by the dichroic beam splitter into a separate light detection path, which contains a holographic Rayleigh light rejection filter, and is focused via 25 mm focal length fiber coupling lens into a fiber bundle that routes the collected light to the spectrograph. The light collection fiber bundle consists of 61 multi-mode fibers each having 70 μm core diameter multi-mode fibers. To optimize light throughput of the whole system, the bundle is shaped in form of a disk at the optics module, providing a match to the circular focal area of the associated fiber coupling lens, and it is shaped as a rectangle at the input of the spectrograph, where it serves as the input slit. The f/3.8 spectrograph employs a 1200 grooves/mm grating blazed for 500 nm. The CCD camera contains a 750 × 240 pixel array, with each pixel of 11.5 × 24 μm size, and is operated at −20 °C. The instrument is designed for high light throughput at a moderate spectral resolution of ~35 cm−1 that still allows for easy separation of the spectrally narrow Raman Stokes lines from spectrally broad fluorescence backgrounds.
Figure 1.
Photograph of an infant’s skin carotenoid measurement via Resonance Raman spectroscopy, RRS. The instrument’s fiber-coupled light delivery and collection module is held against the foot, exposing the heel skin to weak 488 nm laser light for 20 seconds. From spectral analysis of the Raman scattered light intensities, which occur in the green wavelength region, the carotenoid levels in the heel skin are obtained in a rapid, non-invasive, and painless fashion.
Figure 2.
Schematic diagram of RRS instrument and fiber based light delivery/detection module used in this study (not to scale). L1, L3: 25 mm focal length fiber coupling lenses; L2: 50 mm focal length lens; DM: dichroic mirror; LF: laser line filter; NF: Notch filter.
The measured raw skin spectra as well as the specific skin carotenoid RRS intensity levels derived from the spectra are displayed in near real-time on the computer monitor. The laser generates 10 mW output at 488 nm. For measurements of infants, their heel was chosen as a conveniently accessible skin tissue site, and the contact module was held against the sole of the foot for approximately 30 seconds. At a power of 0.2 W/cm2 it is safe to expose skin to the laser light for 30 hours. The light exposure is approximately 1,000 times less than the exposure limit set by the ANSIz136.1-2000 standard, which assures the instrument was safe for use in human skin. Three measurements were taken for each tissue site, and the computer-derived corresponding skin carotenoid RRS levels were averaged for the determination of a subject’s carotenoid level. To prevent eye injuries of bystanders and the measured subject, the optics module was placed in a black enclosure prior to skin measurements, carefully pointed to the bedding of the crib supporting the infant, and guided to the heel skin tissue site where at contact any light was essentially blocked. Also, protective goggles (Kentek Corporation, Pittsfield, New Hampshire) were worn by the research assistant. Since the optics module window is shielded against ambient light upon contact with the measured skin tissue, the measurements could be performed in a normally lighted environment.
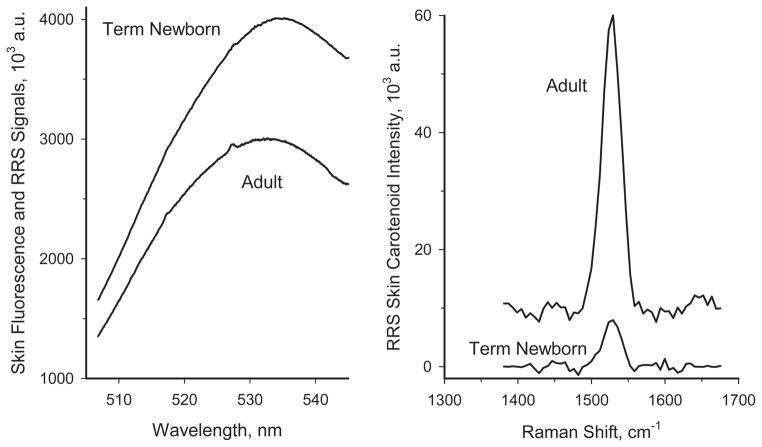
Typical heel skin spectra are shown in Figure 3 for a healthy newborn and for an adult with medium-level skin carotenoid score. In both subjects, a spectrally broad background fluorescence (“autofluorescence”) dominates the skin response, with the carotenoid C=C RRS peaks appearing as small spectral features superimposed on the spectrally broad background fluorescence response of the skin. For the adult subject, featuring a relatively high carotenoid level, the C=C RRS peak is discernible as a small blip near 527 nm, while in the infant case the RRS peak is too small to be seen on the fluorescence intensity scale. The strengths of the carotenoid C=C RRS intensities are calculated in each case via fitting of the fluorescence background with a third-order polynomial and subsequent subtraction from the skin spectra. This is achieved automatically by the computer’s data processing software, and results in background-subtracted RRS spectra displayed in the right panel of Figure 3. The autofluorescence originates from non-carotenoid chromophores such as elastin, collagen and porphyrin, which all are excited by the instrument’s argon laser simultaneously with the carotenoids of interest. Typically, the autofluorescence intensities are about two orders of magnitude stronger than the carotenoid RRS signals.
Figure 3.
Comparison of RRS spectra for the skin of an adult subject and a newborn term infant. Left panel: raw spectra consisting of large fluorescence background and superimposed, very weak, spectrally narrow, carotenoid C=C RRS lines near 527 nm. Right panel: Carotenoid C=C RRS lines obtained for both subjects after signal processing (subtraction of fluorescence background). Note the approximately 7-fold lower RRS carotenoid level in the skin of the infant skin relative to the adult subject.
The results in Figure 3 show that while the RRS signals for the adult subject are well above the noise floor, the infant RRS signals are significantly weaker (by a factor of ~7), with a resulting relatively low signal-to-noise level. This finding demonstrates significantly reduced skin carotenoid levels in infants, which is in agreement with level differences in plasma [15], and it raises the possibility that the autofluorescence sources in infant skin could play an increasingly confounding role in the RRS based carotenoid measurements at low carotenoid levels.
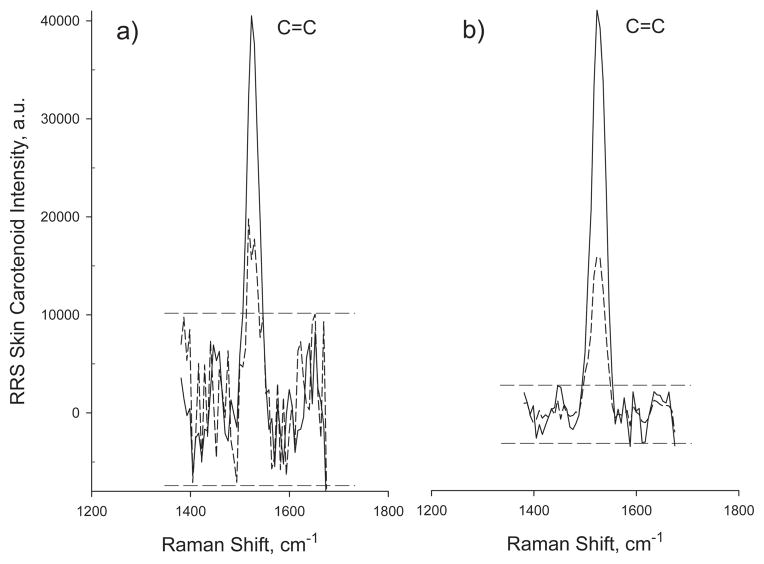
In order to address the generally lower carotenoid levels encountered in infant skin, we increased the sensitivity of our standard RRS instrument by binning 3 horizontal and 8 vertical pixels of the CCD detector array into effective individual active areas of 34.5 μm by 216 μm. This resulted in significantly reduced readout noise, and also improved the dynamic range of the binned array (by a factor of ~12). Figure 4 shows skin carotenoid RRS results obtained with the RRS instrument before and after sensitivity enhancement for an adult and for an infant, demonstrating significantly improved signal-to-noise ratios after pixel binning. For the infant, for example, having a relatively low skin carotenoid RRS intensity level of 12,000, the level is still about 10 times above the noise floor after sensitivity enhancement. Setting an acceptable limit of at least 3 : 1 for the signal-to-noise ratios for meaningful infant RRS measurements, this allowed for the detection of infant skin RRS levels down to about 500–1000 Raman intensity units in the sensitivity enhanced instrument, depending on the magnitude of the autofluorescence background in the particular subject. RRS levels below the 3 : 1 limit were counted as zero. Consecutive measurements of the same skin spot revealed a low standard error of ~8.2% with the binned detector array.
Figure 4.
RRS carotenoid spectra measured for a healthy infant (dashed curves) and adult subject (solid curves) before and after sensitivity enhancement of the RRS instrument.
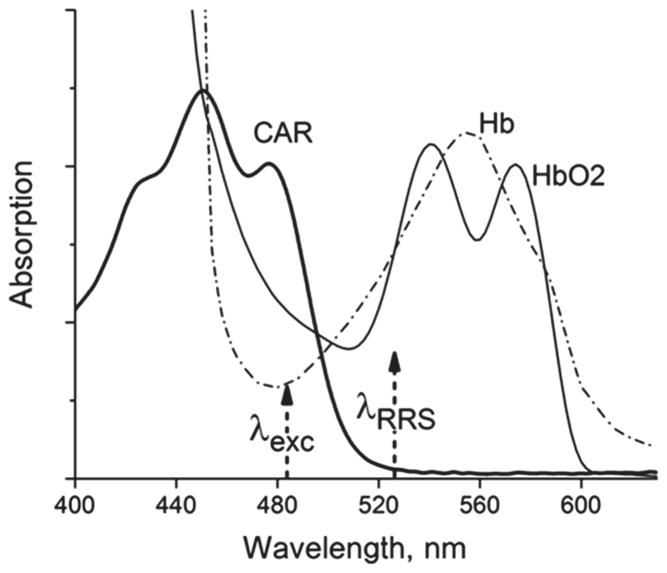
Next we re-examined the light-tissue interaction scenario and main assumptions underlying the skin carotenoid RRS methodology. The influence of tissue chromophores can be judged from Figure 5, which shows schematically the three major chromophores encountered by both the laser excitation and the RRS scattered light in melanin-free skin. These are carotenoids, oxy-hemoglobin (HbO2), and de-oxy-hemoglobin (Hb). Also present, but omitted in Figure 5, are all absorption sources for the autofluorescence response overlapping the carotenoid absorption, and a superimposed strong scattering background that rises monotonically and strongly with decreasing wavelengths but that is otherwise featureless. The 488 nm laser excites the carotenoids approximately in the absorption peak of their longest-wavelength vibronic transition. At this spectral position, there is significant absorption overlap with a higher-energy transition of oxy-hemoglobin (~50% overlap), and, to a lesser extent, also with de-oxy-hemoglobin (~30% overlap). Furthermore, at the carotenoid C=C RRS line position at ~527 nm – the RRS carotenoid detection wavelength of interest-both hemoglobin and de-oxy-hemoglobin chromophores present significant additional losses via their fundamental absorption transitions (~60% of peak absorption for either one). In adult skin, the confounding influence of all blood chromophores and melanin can be largely avoided by limiting the laser excitation light to the bloodless outer skin layer, the stratum corneum, which also features very low melanin concentrations, independent of ethnicity. All tissue chromophores causing autofluorescence, like collagen, etc., can be considered unchanged between adult subjects. In infant skin, however, particularly in newborns and prematurely born infants, much thinner and softer skin is present. This allows the excitation light to penetrate down to deeper skin layers. As a consequence, increased absorption due to blood chromophores can be expected at the laser excitation wavelength, and also at the carotenoid RRS wavelength.
Figure 5.
Model absorption spectra of carotenoid and blood chromophores encountered in human skin. The dashed arrows indicate, respectively, the wavelength positions of the argon laser used for excitation, and the C=C skin carotenoid RRS signal.
To check the importance of these factors in infant skin, we used pressure-mediated reflection spectroscopy [28] to directly measure the skin absorption behavior in-vivo, and to compare it with the optical properties of adult skin. In the reflection method, a broad-band white light source is used to illuminate the skin over a wide wavelength region from the near UV to near IR; the reflected light is measured with a spectrograph/CCD detector array combination which is interfaced to a computer, and the apparent absorption spectrum is calculated and displayed in near-real time on a monitor.
The instrument software calculates the reflectivity spectrum R(λ) according to the expression
| (2) |
where T(λ) and S(λ) are the intensities measured at wavelength λ from the skin tissue and a white reflectivity standard, respectively, and D(λ) is the signal dark spectrum intensity. The apparent optical density spectrum A(λ) is derived by taking the common (base 10) logarithm for each spectral data point of the reflectivity spectrum, using the relation
| (3) |
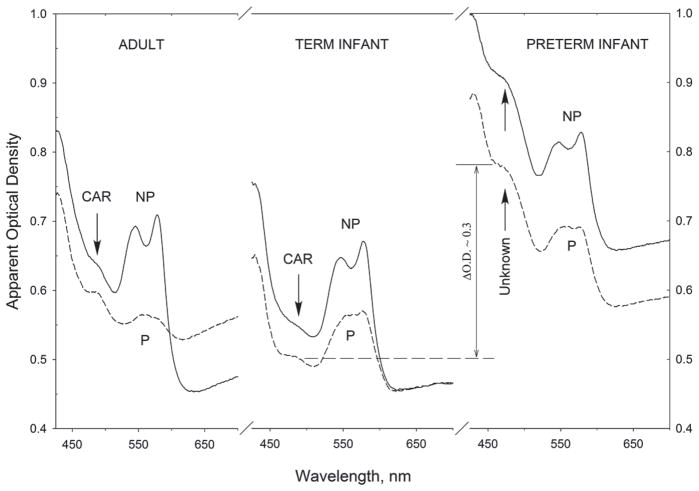
Representative results are summarized in Figure 6, which shows reflection-derived representative skin absorption spectra for adult skin, skin of a term infant, and skin of a preterm infant. For each subject, two spectra are shown: one spectrum measured without application of any topical pressure (labeled NP), and one measured after applying modest pressure of ~3 × 105 Pa to the measured tissue site for 10–15 seconds (labeled P). The topical pressure removes blood temporarily from the measured tissue volume. In the adult skin, the background absorption in the carotenoid wavelength range of interest is about 0.65 optical density units before applying pressure to the tissue volume, and 0.60 density units afterward. The blood chromophore absorption is significantly reduced in the process, as evidenced by the strong absorption decrease of the characteristic oxy-hemoglobin bands in the 530–580 nm wavelength range. Simultaneously, a related absorption drop of oxy-hemoglobin occurs at shorter wavelengths, in the absorption range of carotenoids near 480 nm, and this effect renders the carotenoid absorption band more clearly resolved.
Figure 6.
Representative reflection-derived heel skin absorption spectra for an adult, term infant, and prematurely born infant. Compared to infants and adults, significantly higher chromophore absorptions exist in the visible wavelength range for the skin of prematurely born infants. This effect can be expected to reduce the accuracy of RRS measurements in prematurely born infants.
For the infant skin, the absorption changes are very similar, including the appearance of a weaker but still resolvable carotenoid absorption band in the 480 nm range after application of topical pressure. In the preterm baby, however, the optical behavior is very different. First, the absorption is stronger throughout the whole UV-NIR spectral region, with ~0.3 density units increased absorption in the 480 nm carotenoid region, and even after temporary blood removal, the absorption spectrum in the 480 nm region does not reduce to any absorption feature indicative of carotenoids. This behavior therefore constitutes a significant confounding factor for RRS probing of carotenoids in these cases, since excitation and RRS scattered light both encounter strongly increased background absorption due to unknown chromophore absorptions (increased combined absorption losses of ~ 0.6 optical density units per Figure 5). The finding of increased background absorption in prematurely born infants is consistent with findings by other researchers [29], who measured red, green, and blue light reflectance at the sternum of 99 Caucasian infants ranging in gestational age from 26 to 44 weeks, and found increased reflectance at blue and green wavelengths in correlation with low gestational age.
3. RRS-validation in infants via HPLC-based serum analysis
In order to systematically validate the RRS method for infants, we first measured heel skin carotenoid levels in 32 healthy infants aged 1 day to 6 years, and compared the obtained RRS results with HPLC-derived data for blood samples obtained from venipuncture. Optical measurements were carried out at the same time period as the blood draws. All study procedures were approved by the University of Utah Institutional Review Board, IRB, and informed consent was obtained from the parents.
Plasma samples (100 μl) were treated with ethanol and hexane containing 0.1% (w/v) BHT, and centrifuged to remove the proteins. The proteins were re-extracted with hexane (3 × 300 μl), and the combined extract was evaporated to dryness under reduced pressure at below 40 °C. After evaporation of the solvent, the residue was reconstituted in the appropriate HPLC solvents and centrifuged at 2000 × g prior to analysis. The chromatographic conditions for carotenoid separation and quantitation were similar to those reported earlier [30].
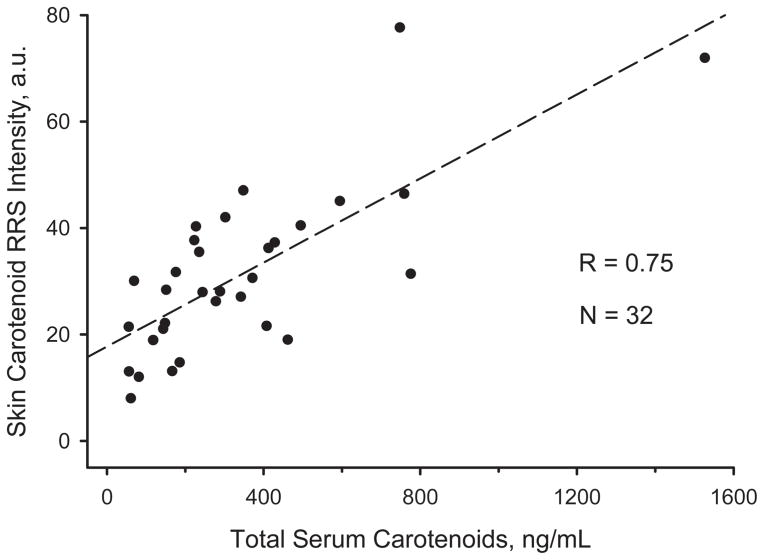
The HPLC and RRS data are compared against each other in Figure 7. Importantly, total serum and corresponding skin carotenoid RRS levels vary drastically between the infants, from near zero total serum level in the lowest cases, to 1500 ng/ml in the highest one, with corresponding RRS intensity variations between zero and 80,000. Interviews with parents about the type of food fed to the infants confirmed the generation of high serum and skin carotenoid levels whenever the individuals were fed a diet with carotenoid-enriched baby food products (purees, juices, etc.). Using a linear fit to the data, a very satisfactory high correlation with coefficient R = 0.75 is obtained between optical and biochemical method for all these subjects. This result is therefore very similar to the previously observed high correlation for adults. Indirectly, this validation project shows that the light-tissue interaction effects involved in RRS measurements of infant skin are similar to those of adults.
Figure 7.
Correlation between HPLC-derived carotenoid serum data and RRS-based heel skin carotenoid data for infants aged 1 day to 6 years.
4. RRS-based monitoring of skin carotenoid levels in infants
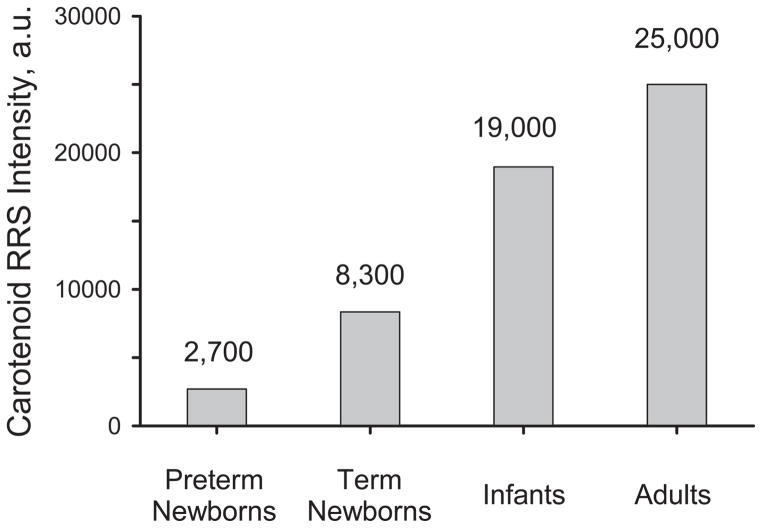
Non-invasive RRS measurements permit the assessment of skin carotenoid levels in a rapid fashion, and therefore allow one to generate statistical results for larger subject populations. In Figure 8 we plot average skin carotenoid levels obtained for the above preterm and term infants, and compare them with results obtained for adults [18] in separate studies. The average skin carotenoid levels are seen to increase with increasing maturity. In pre-school children, RRS-based skin carotenoid measurements have already proven useful to investigate the effects of fruit/vegetable effects and demographic factors [31]. In infants, RRS based skin carotenoid measurements should be useful as well to monitor effects of oxidative stress and feeding correlated changes. As shown above, drastically increased background absorption levels can exist in the skin of prematurely born infants. These will have a confounding effect on the RRS carotenoid measurements, particularly if the individual’s carotenoid levels are very low. In spite of these difficulties we could measure RRS based heel skin carotenoid levels, exceeding the 3 : 1 signal-to-noise limit, in a relatively large number of prematurely born infants. Skin carotenoid RRS measurements and total serum HPLC carotenoid levels still showed a statistically significant correlation, with R = 0.44 (p = 0.01), which is reduced by roughly a factor of 2 relative to healthy infants and adults.
Figure 8.
Bar graph for average RRS skin carotenoid levels in preterm infants, term infants, infants aged 1 day to 12 months, and adults.
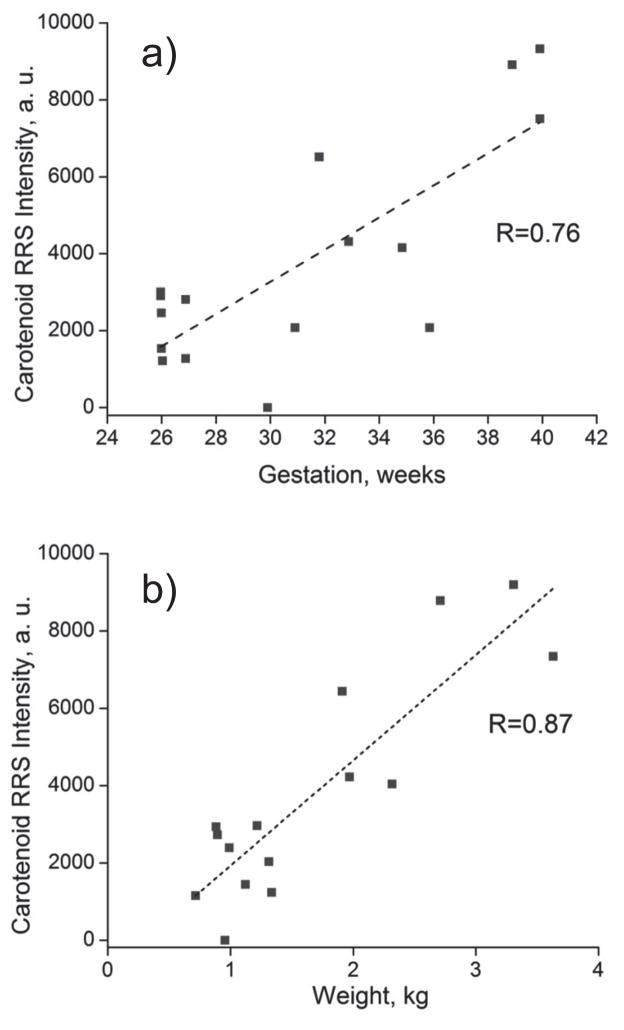
Examples for non-invasive RRS tracking of infant skin carotenoids are shown in Figure 9, where linear increases are seen to occur with increasing body weight at birth and gestation, respectively. While in general the levels are relatively low, sizable carotenoid levels can already exist in some cases. This is probably correlated with the carotenoid status of the infant’s mother and the placental transfer of carotenoids during the last trimester of gestation. In a further study, we enrolled forty preterm infants in an IRB approved study to explore the tracking of skin carotenoid level changes upon feeding with either mother’s milk or preterm formula. Infants fed human milk had higher skin carotenoid RRS levels and corresponding HPLC serum total carotenoid concentrations than formula fed infants [32].
Figure 9.
Heel skin RRS carotenoid levels as function of gestation time (a) and weight at birth (b), showing nearly linear increase of carotenoid levels in both cases.
5. Discussion
Compared to adults, average skin carotenoid levels are significantly lower in infants and even more so in prematurely born babies. Using RRS instrumentation with enhanced sensitivity, however, skin carotenoid levels in term infants can be measured with the same high accuracy as in adults. RRS-based skin carotenoid measurements are therefore well suited for applications in pediatric research. Skin carotenoid status can be optically assessed in a rapid non-invasive fashion, levels can be compared between subjects, and they can be tracked in response to feeding interventions. In prematurely born infants, a reduced accuracy of the Raman measurements can be caused by high background absorption effects due to the deeper penetration of the laser light into the relatively thin transparent skin, and by the generally very low carotenoid levels in these cases. Reflection spectroscopy is demonstrated as a simple non-invasive method useful to quantify the strength of this background absorption in the carotenoid range, which can be expected to become more problematic with shorter gestation periods, and in extreme cases even prevent acceptable skin carotenoid RRS results in the first few weeks after birth prior to skin maturation. A larger number of prematurely born infants will have to be measured with both methods and correlated with HPLC derived serum levels to establish this limit. In any case, if RRS based skin carotenoid levels can be detected above the noise floor in a particular prematurely born infant, the method still holds promise as a convenient non-invasive diagnostic tool in longitudinal studies, allowing one to study correlations between tissue carotenoid content and health outcomes such as sepsis, broncho-pulmonary dysplasia, retinopathy of prematurity, etc.
Acknowledgments
We would like to acknowledge Mohsen Sharifzadeh for help with preparation of the Figures, and loan of the reflection instrument by Image Technologies, Inc. Grant support was provided by the National Institutes of Health grant EY-11600, Research to Prevent Blindness, and Abbott Nutrition.
Footnotes
Author biographies Please see Supporting Information online.
References
- 1.Krinsky N, Mayne ST, Sies H. Carotenoids in Health and Disease. Marcel Dekker, Inc; NY, NY: 2004. [Google Scholar]
- 2.Mayne ST, Cartmel B, Scarmo S, Lin H, Leffell DJ, Welch E, Ermakov IV, Bhosale P, Bernstein P, Gellermann W. Am J Clin Nutr. 2010;92:794–800. doi: 10.3945/ajcn.2010.29707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michaud DS, Feskanich D, Rimm EB, Colditz GA, Speizer FE, Willett WC, Giovannucci E. Am J Clin Nutr. 2000;72:990–997. doi: 10.1093/ajcn/72.4.990. [DOI] [PubMed] [Google Scholar]
- 4.Zhang M, Holman CD, Binns CW. Br J Nutr. 2007;98:187–193. doi: 10.1017/S0007114507690011. [DOI] [PubMed] [Google Scholar]
- 5.Giovannucci E, Ascherio A, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. J Natl Cancer Inst. 1995;87:1767–1776. doi: 10.1093/jnci/87.23.1767. [DOI] [PubMed] [Google Scholar]
- 6.Le Marchand L, Hankin JH, Kolonel LN, Beecher GR, Wilkens LR, Zhao LP. Cancer Epidemiol Biomarkers Prev. 1993;2:183–187. [PubMed] [Google Scholar]
- 7.Wang L, Gaziano JM, Norkus EP, Buring JE, Sesso HD. Am J Clin Nutr. 2008;88:747–754. doi: 10.1093/ajcn/88.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Age-Related Eye Disease Study Group. ARES Rep. No. 22. Arch Ophthalmol (Chicago) 2007;125:1225–1232. [Google Scholar]
- 9.Ray AL, Semba RD, Walston J, Ferrucci L, Cappola AR, Ricks MO, Xue QL, Fried LP. J Nutr. 2006;136:172–176. doi: 10.1093/jn/136.1.172. [DOI] [PubMed] [Google Scholar]
- 10.Thibeault DW. Am J Perinatol. 2000;17:167–181. doi: 10.1055/s-2000-9422. [DOI] [PubMed] [Google Scholar]
- 11.Jewell VC, Northrop-Clewes CA, Tubman R, Thurnham DI. Proc Nutr Soc. 2001;60:171–178. doi: 10.1079/pns200089. [DOI] [PubMed] [Google Scholar]
- 12.Parker RS. Methods Enzymol. 1993;214:86–93. doi: 10.1016/0076-6879(93)14056-o. [DOI] [PubMed] [Google Scholar]
- 13.Karppi J, Nurmi T, Olmedilla-Alonso B, Granado-Lorencio F, Nyyssonen K, Chromatogr J, Analyt B. Technol Biomed Life Sci. 2008;867:226–232. doi: 10.1016/j.jchromb.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Rock CL, Swendseid ME, Jacob RA, McKee RW. J Nutr. 1992;122:96100. doi: 10.1093/jn/122.1.96. [DOI] [PubMed] [Google Scholar]
- 15.Ostrea EM, Jr, Balun JE, Winkler R, Porter T. Am J Obstet Gynecol. 1986;154:1014–1017. doi: 10.1016/0002-9378(86)90740-4. [DOI] [PubMed] [Google Scholar]
- 16.Sommerburg O, Meissner K, Nelle M, Lenhartz H, Leichsenring M. Eur J Pediatr. 2000;159:86–90. doi: 10.1007/pl00013811. [DOI] [PubMed] [Google Scholar]
- 17.Gellermann W, McClane RW, Katz NB, Bernstein PS. 6,205,354. US Patent. 2001 Mar;
- 18.Ermakov IV, Ermakova MR, McClane RW, Gellermann W. Opt Lett. 2001;26:1179–1181. doi: 10.1364/ol.26.001179. [DOI] [PubMed] [Google Scholar]
- 19.Hata TR, Scholz TA, Ermakov IV, McClane RW, Khachik F, Gellermann W, Pershing LK. J Invest Dermatol. 2000;115:441–448. doi: 10.1046/j.1523-1747.2000.00060.x. [DOI] [PubMed] [Google Scholar]
- 20.Ermakov IV, Sharifzadeh M, Bernstein PS, Gellermann W. In: Carotenoids-Physical, Chemical and Biological Functions and Properties. Landrum JT, editor. CRC Press; Atlanta, GA: 2009. [Google Scholar]
- 21.Gellermann W, Ermakov IV, Ermakova MR, McClane RW, Zhao DY, Bernstein PS. JOSA A. 2002;19:1172–1186. doi: 10.1364/josaa.19.001172. [DOI] [PubMed] [Google Scholar]
- 22.Ermakov IV, Ermakova MR, Gellermann W. Appl Spect. 2005;59:861–867. doi: 10.1366/0003702054411616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharifzadeh M, Zhao DY, Bernstein PS, Gellermann W. J Opt Soc A. 2008;25:947–957. doi: 10.1364/josaa.25.000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiou YB, Blume-Peytavi U. Skin Pharmacol Physiol. 2004;17:57–66. doi: 10.1159/000076015. [DOI] [PubMed] [Google Scholar]
- 25.Bahkatov AN, Genina EA, Kochubey VI, Tuchin VV. J Phys D: Appl Phys. 2005;38:2543–2555. [Google Scholar]
- 26.Gellermann W, Zidichouski JA, Smidt CR, Bernstein PS. In: Carotenoids and Retinoids: Molecular Aspects and Health Issues. Packer L, Kraemer K, Obermueller-Jervic U, Sies H, editors. chap 6. AOCS Press; Champain, Illinois: 2005. pp. 86–114. [Google Scholar]
- 27.Ermakov IV, Gellermann W. Arch Biochem Biophys. 2010;504:40–49. doi: 10.1016/j.abb.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 28.Ermakov IV, Gellermann W. J Biophotonics. 2012;5:559–570. doi: 10.1002/jbio.201100122. [DOI] [PubMed] [Google Scholar]
- 29.Krauss AN, Post PW, Waldman S, Auld PAM. Pediat Res. 1976;10:776–778. doi: 10.1203/00006450-197609000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Weissenberg M, Schaeffler I, Menagem E, Barzilai M, Levy A. J Chromatogr A. 1997;757:89–98. doi: 10.1016/s0021-9673(96)00665-6. [DOI] [PubMed] [Google Scholar]
- 31.Scarmo S, Henebery K, Peracchio H, Cartmel B, Lin H, Ermakov IV, Gellermann W, Bernstein PS, Duffy VB, Mayne ST. European J Clin Nutr. 2012;66:555–560. doi: 10.1038/ejcn.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan GM, Chan M, Gellermann W, Ermakov IV, Ermakova M, Bhosale P, Bernstein PS, Rau C. J Pediatric Gastroenterology and Nutrition. doi: 10.1097/MPG.0b013e318282a8fe. accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]