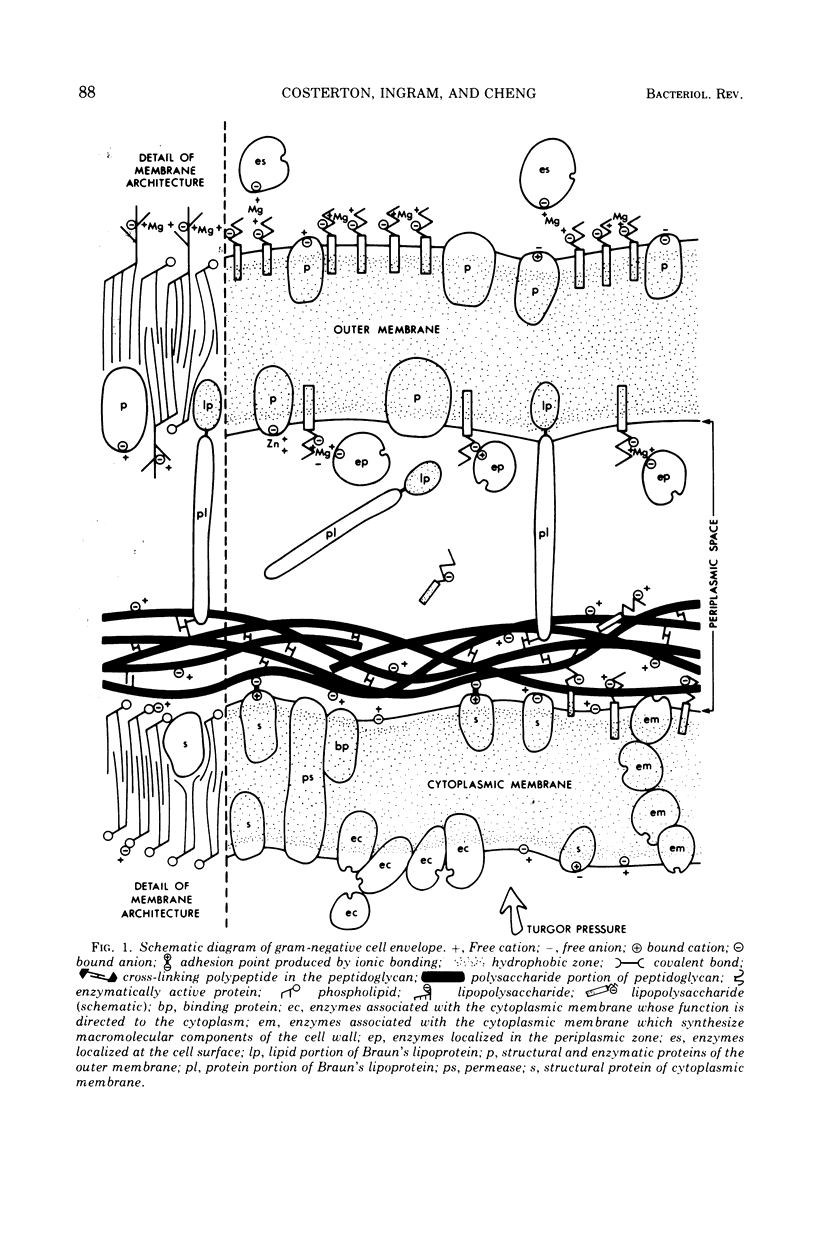
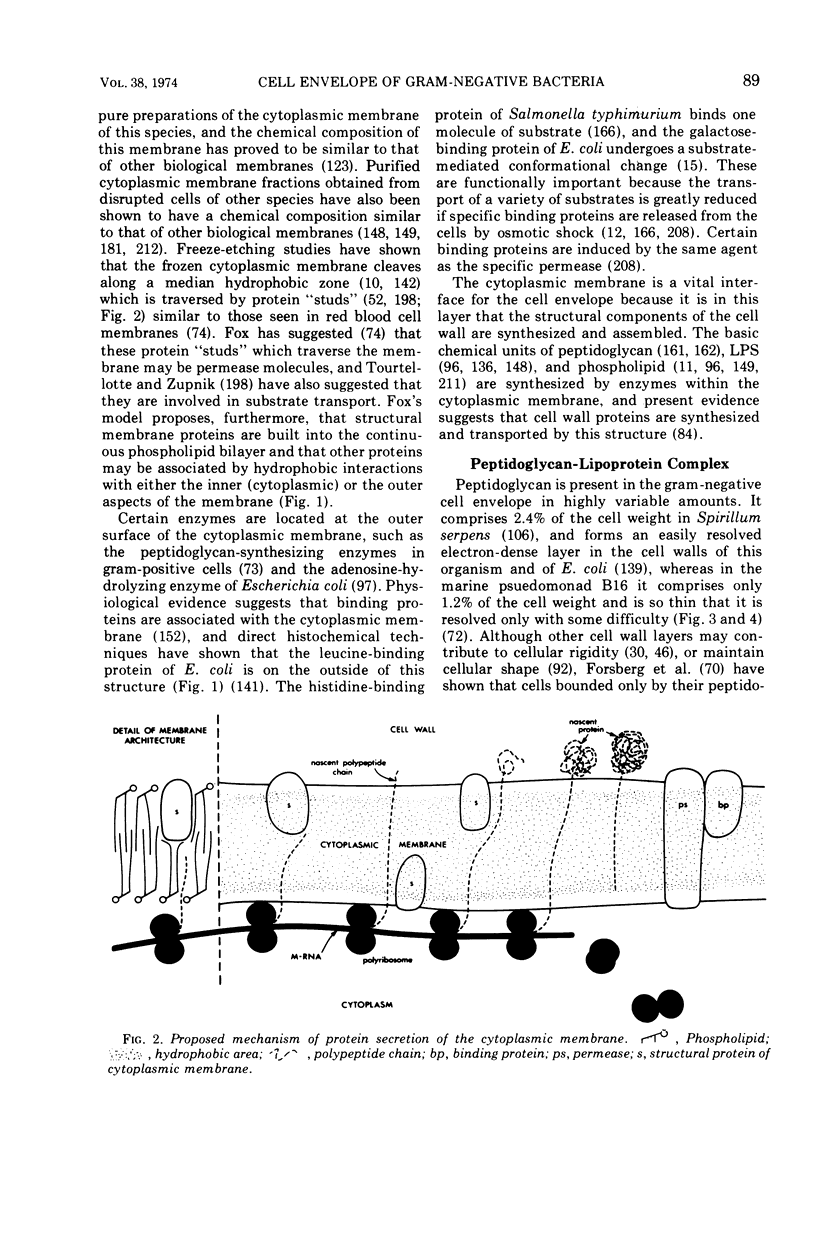
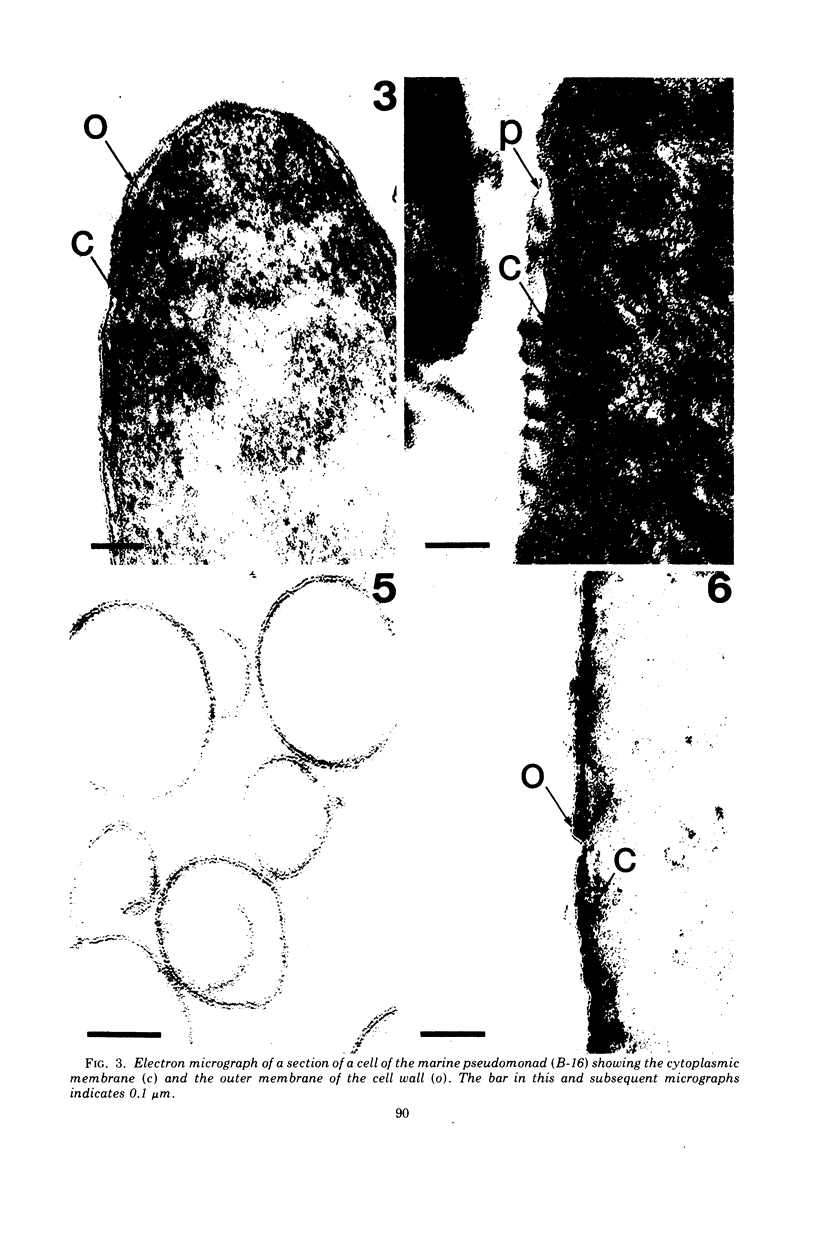
Full text
PDF



















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman M. R., Sabatini D. D., Blobel G. Ribosome-membrane interaction. Nondestructive disassembly of rat liver rough microsomes into ribosomal and membranous components. J Cell Biol. 1973 Jan;56(1):206–229. doi: 10.1083/jcb.56.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agate A. D., Korczynski M. S., Lundgren D. G. Extracellular complex from the culture filtrate of Ferrobacillus ferrooxidans. Can J Microbiol. 1969 Mar;15(3):259–264. doi: 10.1139/m69-048. [DOI] [PubMed] [Google Scholar]
- Agate A. D., Vishniac W. Changes in phospholipid composition of Thiobacillus neapolitanus during growth. Arch Mikrobiol. 1973;89(3):247–255. doi: 10.1007/BF00422205. [DOI] [PubMed] [Google Scholar]
- Anderson R. G., Hussey H., Baddiley J. The mechanism of wall synthesis in bacteria. The organization of enzymes and isoprenoid phosphates in the membrane. Biochem J. 1972 Mar;127(1):11–25. doi: 10.1042/bj1270011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anraku Y. Transport of sugars and amino acids in bacteria. I. Purification and specificity of the galactose- and leucine-binding proteins. J Biol Chem. 1968 Jun 10;243(11):3116–3122. [PubMed] [Google Scholar]
- Aprile M. A., Wardlaw A. C. Availability and specificity of lipopolysaccharide on the surface of 56 degrees C heated Bordetella pertussis. Can J Microbiol. 1973 Apr;19(4):536–538. doi: 10.1139/m73-087. [DOI] [PubMed] [Google Scholar]
- Bae H. C., Cota-Robles E. H., Casida L. E. Microflora of soil as viewed by transmission electron microscopy. Appl Microbiol. 1972 Mar;23(3):637–648. doi: 10.1128/am.23.3.637-648.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E. Adsorption of bacteriophages to adhesions between wall and membrane of Escherichia coli. J Virol. 1968 Apr;2(4):346–356. doi: 10.1128/jvi.2.4.346-356.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E. Areas of adhesion between wall and membrane of Escherichia coli. J Gen Microbiol. 1968 Oct;53(3):395–404. doi: 10.1099/00221287-53-3-395. [DOI] [PubMed] [Google Scholar]
- Bayer M. E., Remsen C. C. Structure of Escherichia coli after freeze-etching. J Bacteriol. 1970 Jan;101(1):304–313. doi: 10.1128/jb.101.1.304-313.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. M., Mavis R. D., Osborn M. J., Vagelos P. R. Enzymes of phospholipid metabolism: localization in the cytoplasmic and outer membrane of the cell envelope of Escherichia coli and Salmonella typhimurium. Biochim Biophys Acta. 1971 Dec 3;249(2):628–635. doi: 10.1016/0005-2736(71)90144-1. [DOI] [PubMed] [Google Scholar]
- Berger E. A., Heppel L. A. A binding protein involved in the transport of cystine and diaminopimelic acid in Escherichia coli. J Biol Chem. 1972 Dec 10;247(23):7684–7694. [PubMed] [Google Scholar]
- Birdsell D. C., Cota-Robles E. H. Production and ultrastructure of lysozyme and ethylenediaminetetraacetate-lysozyme spheroplasts of Escherichia coli. J Bacteriol. 1967 Jan;93(1):427–437. doi: 10.1128/jb.93.1.427-437.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boos W., Gordon A. S., Hall R. E., Price H. D. Transport properties of the galactose-binding protein of Escherichia coli. Substrate-induced conformational change. J Biol Chem. 1972 Feb 10;247(3):917–924. [PubMed] [Google Scholar]
- Boothby D., Daneo-Moore L., Higgins M. L., Coyette J., Shockman G. D. Turnover of bacterial cell wall peptidoglycans. J Biol Chem. 1973 Mar 25;248(6):2161–2169. [PubMed] [Google Scholar]
- Bradley T. J., Parker M. S. Binding of aluminium ions by Staphylococcus aurens 893. Experientia. 1968 Nov 15;24(11):1175–1176. doi: 10.1007/BF02147837. [DOI] [PubMed] [Google Scholar]
- Braun V., Bosch V. Repetitive sequences in the murein-lipoprotein of the cell wall of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Apr;69(4):970–974. doi: 10.1073/pnas.69.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Rehn K., Wolff H. Supramolecular structure of the rigid layer of the cell wall of Salmonella, Serratia, Proteus, and Pseudomonas fluorescens. Number of lipoprotein molecules in a membrane layer. Biochemistry. 1970 Dec 22;9(26):5041–5049. doi: 10.1021/bi00828a001. [DOI] [PubMed] [Google Scholar]
- Braun V., Sieglin U. The covalent murein-lipoprotein structure of the Escherichia coli cell wall. The attachment site of the lipoprotein on the murein. Eur J Biochem. 1970 Apr;13(2):336–346. doi: 10.1111/j.1432-1033.1970.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Braun V., Wolff H. The murein-lipoprotein linkage in the cell wall of Escherichia coli. Eur J Biochem. 1970 Jun;14(2):387–391. doi: 10.1111/j.1432-1033.1970.tb00301.x. [DOI] [PubMed] [Google Scholar]
- Brockman R. W., Heppel L. A. On the localization of alkaline phosphatase and cyclic phosphodiesterase in Escherichia coli. Biochemistry. 1968 Jul;7(7):2554–2562. doi: 10.1021/bi00847a016. [DOI] [PubMed] [Google Scholar]
- Buckmire F. L., Murray R. G. Studies on the cell wall of Spirillum serpens. 1. Isolation and partial purification of the outermost cell wall layer. Can J Microbiol. 1970 Oct;16(10):1011–1022. doi: 10.1139/m70-171. [DOI] [PubMed] [Google Scholar]
- Buckmire F. L., Murray R. G. Studies on the cell wall of Spirillum serpens. II. Chemical characterization of the outer structured layer. Can J Microbiol. 1973 Jan;19(1):59–66. doi: 10.1139/m73-009. [DOI] [PubMed] [Google Scholar]
- Burge R. E., Draper J. C. The structure of the cell wall of the Gram-negative bacterium Proteus vulgaris. 3. A lipopolysaccharide "unit membrane". J Mol Biol. 1967 Sep 14;28(2):205–210. doi: 10.1016/s0022-2836(67)80003-2. [DOI] [PubMed] [Google Scholar]
- Burman L. G., Nordström K., Bloom G. D. Murein and the outer penetration barrier of Escherichia coli K-12, Proteus mirabilis, and Pseudomonas aeruginosa. J Bacteriol. 1972 Dec;112(3):1364–1374. doi: 10.1128/jb.112.3.1364-1374.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPMAN J. A., MURRAY R. G., SALTON M. R. THE SURFACE ANATOMY OF LAMPROPEDIA HYALINA. Proc R Soc Lond B Biol Sci. 1963 Nov 19;158:498–513. doi: 10.1098/rspb.1963.0060. [DOI] [PubMed] [Google Scholar]
- Carson K. J., Eagon R. G. Lysozyme sensitivity of the cell wall of Pseudomonas aeruginosa. Further evidence for the role of the non-peptidoglycan components in cell wall rigidity. Can J Microbiol. 1966 Feb;12(1):105–108. doi: 10.1139/m66-015. [DOI] [PubMed] [Google Scholar]
- Cedar H., Schwartz J. H. Localization of the two-L-asparaginases in anaerobically grown Escherichia coli. J Biol Chem. 1967 Aug 25;242(16):3753–3755. [PubMed] [Google Scholar]
- Cerny G., Teuber M. Differential release of periplasmic versus cytoplasmic enzymes from Escherichia coli B by polymixin B. Arch Mikrobiol. 1971;78(2):166–179. doi: 10.1007/BF00424873. [DOI] [PubMed] [Google Scholar]
- Cheng J. K., Costerton J. W., Singh A. P., Ingram J. M. Susceptibility of whole cells and spheroplasts of Pseudomonas aeruginosa to actinomycin D. Antimicrob Agents Chemother. 1973 Mar;3(3):399–406. doi: 10.1128/aac.3.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J., Costerton J. W. Localization of alkaline phosphatase in three gram-negative rumen bacteria. J Bacteriol. 1973 Oct;116(1):424–440. doi: 10.1128/jb.116.1.424-440.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J., Day D. F., Costerton J. W., Ingram J. M. Alkaline phosphatase subunits in the culture filtrate of Pseudomonas aeruginosa. Can J Biochem. 1972 Mar;50(3):268–276. doi: 10.1139/o72-038. [DOI] [PubMed] [Google Scholar]
- Cheng K. J., Ingram J. M., Costerton J. W. Alkaline phosphatase localization and spheroplast formation of Pseudomonas aeruginosa. Can J Microbiol. 1970 Dec;16(12):1319–1324. doi: 10.1139/m70-218. [DOI] [PubMed] [Google Scholar]
- Cheng K. J., Ingram J. M., Costerton J. W. Interactions of alkaline phosphatase and the cell wall of Pseudomonas aeruginosa. J Bacteriol. 1971 Jul;107(1):325–336. doi: 10.1128/jb.107.1.325-336.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J., Ingram J. M., Costerton J. W. Release of alkaline phosphatase from cells of Pseudomonas aeruginosa by manipulation of cation concentration and of pH. J Bacteriol. 1970 Nov;104(2):748–753. doi: 10.1128/jb.104.2.748-753.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesbro W. R., Lampen J. O. Characteristics of secretion of penicillinase, alkaline phosphatase, and nuclease by Bacillus species. J Bacteriol. 1968 Aug;96(2):428–437. doi: 10.1128/jb.96.2.428-437.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Forsberg C., Matula T. I., Buckmire F. L., MacLeod R. A. Nutrition and metabolism of marine bacteria. XVI. Formation of protoplasts, spheroplasts, and related forms from a gram-negative marine bacterium. J Bacteriol. 1967 Nov;94(5):1764–1777. doi: 10.1128/jb.94.5.1764-1777.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W. Relationship of a wall-associated enzyme with specific layers of the cell wall of a gram-negative bacterium. J Bacteriol. 1973 Jun;114(3):1281–1293. doi: 10.1128/jb.114.3.1281-1293.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W. The structure and function of the cell envelope of gram-negative bacteria. Rev Can Biol. 1970 Sep;29(3):299–316. [PubMed] [Google Scholar]
- Costerton J. W., Thompson J. Induced morphological changes in the stainable layers of the cell envelope of a gram-negative bacterium. Can J Microbiol. 1972 Jun;18(6):937–940. doi: 10.1139/m72-144. [DOI] [PubMed] [Google Scholar]
- Cox S. T., Jr, Eagon R. G. Action of ethylenediaminetetraacetic acid, tris(hydroxymethyl)-aminomethane, and lysozyme on cell walls of Pseudomonas aeruginosa. Can J Microbiol. 1968 Aug;14(8):913–922. doi: 10.1139/m68-153. [DOI] [PubMed] [Google Scholar]
- Crowfoot P. D., Esfahani M., Wakil S. J. Relation between protein synthesis and phospholipid synthesis and turnover in Escherichia coli. J Bacteriol. 1972 Dec;112(3):1408–1415. doi: 10.1128/jb.112.3.1408-1415.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundliffe E. Intracellular distribution of ribosomes and polyribosomes in Bacillus megaterium. J Mol Biol. 1970 Sep 28;52(3):467–481. doi: 10.1016/0022-2836(70)90413-4. [DOI] [PubMed] [Google Scholar]
- DUGAN P. R., LUNDGREN D. G. ENERGY SUPPLY FOR THE CHEMOAUTOTROPH FERROBACILLUS FERROOXIDANS. J Bacteriol. 1965 Mar;89:825–834. doi: 10.1128/jb.89.3.825-834.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Duve C., Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- De Petris S. Ultrastructure of the cell wall of Escherichia coli and chemical nature of its constituent layers. J Ultrastruct Res. 1967 Jul;19(1):45–83. doi: 10.1016/s0022-5320(67)80059-5. [DOI] [PubMed] [Google Scholar]
- De Voe I. W., Thompson J., Costerton J. W., MacLeod R. A. Stability and comparative transport capacity of cells, mureinoplasts, and true protoplasts of a gram-negative bacterium. J Bacteriol. 1970 Mar;101(3):1014–1026. doi: 10.1128/jb.101.3.1014-1026.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L., Adler J. Attachment of flagellar basal bodies to the cell envelope: specific attachment to the outer, lipopolysaccharide membrane and the cyoplasmic membrane. J Bacteriol. 1971 Jan;105(1):396–407. doi: 10.1128/jb.105.1.396-407.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVoe I. W., Costerton J. W., MacLeod R. A. Demonstration by freeze-etching of a single cleavage plane in the cell wall of a gram-negative bacterium. J Bacteriol. 1971 May;106(2):659–671. doi: 10.1128/jb.106.2.659-671.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz G. W., Heppel L. A. Studies on the uptake of hexose phosphates. 3. Mechanism of uptake of glucose 1-phosphate in Escherichia coli. J Biol Chem. 1971 May 10;246(9):2891–2897. [PubMed] [Google Scholar]
- Dreher K. D., Schulman J. H., Anderson O. R., Roels O. A. The stability and structure of mixed lipid monolayers and bilayers. I. Properties of lipid and lipoprotein monolayers on OsO4 solutions and the role of cholesterol, retinol, and tocopherol in stabilizing lecithin monolayers. J Ultrastruct Res. 1967 Aug 30;19(5):586–599. doi: 10.1016/s0022-5320(67)80084-4. [DOI] [PubMed] [Google Scholar]
- Dvorak H. F., Wetzel B. K., Heppel L. A. Biochemical and cytochemical evidence for the polar concentration of periplasmic enzymes in a "minicell" strain of Escherichia coli. J Bacteriol. 1970 Oct;104(1):543–548. doi: 10.1128/jb.104.1.543-548.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIOTT A. M., BAK I. J. THE FATE OF MITOCHONDRIA DURING AGING IN TETRAHYMENA PYRIFORMIS. J Cell Biol. 1964 Jan;20:113–129. doi: 10.1083/jcb.20.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagon R. G., Simmons G. P., Carson K. J. Evidence for the presence of ash and fivalent metals in the cell wall of Pseudomonas aeruginosa. Can J Microbiol. 1965 Dec;11(6):1041–1042. doi: 10.1139/m65-144. [DOI] [PubMed] [Google Scholar]
- Fan D. P., Beckman M. M., Cunningham W. P. Ultrastructural studies on a mutant of Bacillus subtilis whose growth is inhibited due to insufficient autolysin production. J Bacteriol. 1972 Mar;109(3):1247–1257. doi: 10.1128/jb.109.3.1247-1257.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold D. S., Goldman J. N., Kuritz H. M. Locus of the action of serum and the role of lysozyme in the serum bactericidal reaction. J Bacteriol. 1968 Dec;96(6):2118–2126. doi: 10.1128/jb.96.6.2118-2126.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold D. S., Goldman J. N., Kuritz H. M. Locus of the lethal event in the serum bactericidal reaction. J Bacteriol. 1968 Dec;96(6):2127–2131. doi: 10.1128/jb.96.6.2127-2131.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiil A., Branton D. Changes in the plasma membrane of Escherichia coli during magnesium starvation. J Bacteriol. 1969 Jun;98(3):1320–1327. doi: 10.1128/jb.98.3.1320-1327.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finean J. B. Biophysical contributions to membrane structure. Q Rev Biophys. 1969 May;2(1):1–23. doi: 10.1017/s0033583500000779. [DOI] [PubMed] [Google Scholar]
- Forge A., Costerton J. W., Kerr K. A. Biophysical examination of the cell wall of a gram-negative marine pseudomanad. The effects of various treatments on the isolated double-track layer. Can J Microbiol. 1973 Apr;19(4):451–459. doi: 10.1139/m73-073. [DOI] [PubMed] [Google Scholar]
- Forge A., Costerton J. W., Kerr K. A. Freeze-etching and x-ray diffraction of the isolated double-track layer from the cell wall of a gram-negative marine pseudomonad. J Bacteriol. 1973 Jan;113(1):445–451. doi: 10.1128/jb.113.1.445-451.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forge A., Costerton J. W. The effects of phospholipid depletion on the cleavage pattern of the cell walls of frozen Gram-negative bacteria. Can J Microbiol. 1973 Aug;19(8):1056–1057. doi: 10.1139/m73-168. [DOI] [PubMed] [Google Scholar]
- Forsberg C. W., Costerton J. W., Macleod R. A. Quantitation, chemical characteristics, and ultrastructure of the three outer cell wall layers of a gram-negative bacterium. J Bacteriol. 1970 Dec;104(3):1354–1368. doi: 10.1128/jb.104.3.1354-1368.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W., Costerton J. W., Macleod R. A. Separation and localization of cell wall layers of a gram-negative bacterium. J Bacteriol. 1970 Dec;104(3):1338–1353. doi: 10.1128/jb.104.3.1338-1353.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W., Rayman M. K., Costerton J. W., MacLeod R. A. Isolation, characterization, and ultrastructure of the peptidoglycan layer of a marine pseudomonad. J Bacteriol. 1972 Feb;109(2):895–905. doi: 10.1128/jb.109.2.895-905.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W., Ward J. B. N-acetylmuramyl-L-alanine amidase of Bacillus licheniformis and its L-form. J Bacteriol. 1972 Jun;110(3):878–888. doi: 10.1128/jb.110.3.878-888.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg I. Localization of phosphoglucose isomerase in Escherichia coli and its relation to the induction of the hexose phosphate transport system. J Bacteriol. 1972 Dec;112(3):1201–1205. doi: 10.1128/jb.112.3.1201-1205.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganoza M. C., Williams C. A. In vitro synthesis of different categories of specific protein by membrane-bound and free ribosomes. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1370–1376. doi: 10.1073/pnas.63.4.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrard W. T. Selective release of proteins from Spirillum itersonii by tris (hydroxymethyl) aminomethane and ethylenediaminetetraacetate. J Bacteriol. 1971 Jan;105(1):93–100. doi: 10.1128/jb.105.1.93-100.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrard W. T. Synthesis, assembly, and localization of periplasmic cytochrome c. J Biol Chem. 1972 Sep 25;247(18):5935–5943. [PubMed] [Google Scholar]
- Ghosh B. K., Sargent M. G., Lampen J. O. Morphological phenomena associated with penicillinase induction and secretion in Bacillus licheniformis. J Bacteriol. 1968 Oct;96(4):1314–1328. doi: 10.1128/jb.96.4.1314-1328.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh B. K., Wouters J. T., Lampen J. O. Distribution of the sites of alkaline phosphatase(s) activity in vegetative cells of Bacillus subtilis. J Bacteriol. 1971 Nov;108(2):928–937. doi: 10.1128/jb.108.2.928-937.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Stinnett J. D., Roth I. L., Eagon R. G. Freeze-etch study of Pseudomonas aeruginosa: localization within the cell wall of an ethylenediaminetetraacetate-extractable. J Bacteriol. 1973 Jan;113(1):417–432. doi: 10.1128/jb.113.1.417-432.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser M., Bayer W. H., Bell R. M., Vagelos P. R. Regulation of macromolecular biosynthesis in a mutant of Escherichia coli defective in membrane phospholipid biosynthesis. Proc Natl Acad Sci U S A. 1973 Feb;70(2):385–389. doi: 10.1073/pnas.70.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauert A. M., Thornley M. J. The topography of the bacterial cell wall. Annu Rev Microbiol. 1969;23:159–198. doi: 10.1146/annurev.mi.23.100169.001111. [DOI] [PubMed] [Google Scholar]
- Glew R. H., Heath E. C. Studies on the extracellular alkaline phosphatase of Micrococcus sodonensis. II. Factors affecting secretion. J Biol Chem. 1971 Mar 25;246(6):1566–1574. [PubMed] [Google Scholar]
- Glick M. C., Warren L. Membranes of animal cells. 3. Amino acid incorporation by isolated surface membranes. Proc Natl Acad Sci U S A. 1969 Jun;63(2):563–570. doi: 10.1073/pnas.63.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L. Degradation of abnormal proteins in Escherichia coli (protein breakdown-protein structure-mistranslation-amino acid analogs-puromycin). Proc Natl Acad Sci U S A. 1972 Feb;69(2):422–426. doi: 10.1073/pnas.69.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant W. D., Sutherland I. W., Wilkinson J. F. Exopolysaccharide colanic acid and its occurrence in the Enterobacteriaceae. J Bacteriol. 1969 Dec;100(3):1187–1193. doi: 10.1128/jb.100.3.1187-1193.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton-Miller J. M. Damaging effects of ethylenediaminetetra-acetate and penicillins on permeability barriers in Gram-negative bacteria. Biochem J. 1966 Sep;100(3):675–682. doi: 10.1042/bj1000675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton-Miller J. M. Effect of EDTA upon bacterial permeability to benzylpenicillin. Biochem Biophys Res Commun. 1965 Sep 22;20(6):688–691. doi: 10.1016/0006-291x(65)90070-7. [DOI] [PubMed] [Google Scholar]
- Hartmann R., Höltje J. V., Schwarz U. Targets of penicillin action in Escherichia coli. Nature. 1972 Feb 25;235(5339):426–429. doi: 10.1038/235426a0. [DOI] [PubMed] [Google Scholar]
- Henning U., Rehn K., Hoehn B. Cell envelope and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2033–2036. doi: 10.1073/pnas.70.7.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Procaryotic cell division with respect to wall and membranes. CRC Crit Rev Microbiol. 1971 May;1(1):29–72. doi: 10.3109/10408417109104477. [DOI] [PubMed] [Google Scholar]
- Hinckley, Müller E., Rothfield L. Reassembly of a membrane-bound multienzyme system. I. Formation of a particle containing phosphatidylethanolamine, lipopolysaccharide, and two glycosyltransferase enzymes. J Biol Chem. 1972 Apr 25;247(8):2623–2628. [PubMed] [Google Scholar]
- Hochstadt-Ozer J. The regulation of purine utilization in bacteria. IV. Roles of membrane-localized and pericytoplasmic enzymes in the mechanism of purine nucleoside transport across isolated Escherichia coli membranes. J Biol Chem. 1972 Apr 25;247(8):2419–2426. [PubMed] [Google Scholar]
- Hofschneider P. H., Martin H. H. Diversity of surface layers in L-forms of Proteus mirabilis. J Gen Microbiol. 1968 Apr;51(1):23–33. doi: 10.1099/00221287-51-1-23. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Leadbetter E. R. Comparative ultrastructure of selected aerobic spore-forming bacteria: a freeze-etching study. Bacteriol Rev. 1969 Jun;33(2):346–378. doi: 10.1128/br.33.2.346-378.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram J. M., Cheng K. J., Costerton J. W. Alkaline phosphatase of Pseudomonas aeruginosa: the mechanism of secretion and release of the enzyme from whole cells. Can J Microbiol. 1973 Nov;19(11):1407–1415. doi: 10.1139/m73-227. [DOI] [PubMed] [Google Scholar]
- Inouye M., Shaw J., Shen C. The assembly of a structural lipoprotein in the envelope of Escherichia coli. J Biol Chem. 1972 Dec 25;247(24):8154–8159. [PubMed] [Google Scholar]
- Inouye M., Yee M. L. Specific removal of proteins from the envelope of Escherichia coli by protease treatments. J Bacteriol. 1972 Oct;112(1):585–592. doi: 10.1128/jb.112.1.585-592.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk R. G., Ginzburg M. Ultrastructure of two species of halobacterium. J Ultrastruct Res. 1972 Oct;41(1):80–94. doi: 10.1016/s0022-5320(72)90040-8. [DOI] [PubMed] [Google Scholar]
- Knox K. W., Vesk M., Work E. Relation between excreted lipopolysaccharide complexes and surface structures of a lysine-limited culture of Escherichia coli. J Bacteriol. 1966 Oct;92(4):1206–1217. doi: 10.1128/jb.92.4.1206-1217.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Ensign J. C. Isolation and chemical structure of the peptidoglycan of Spirillum serpens cell walls. J Bacteriol. 1968 Jan;95(1):201–210. doi: 10.1128/jb.95.1.201-210.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnarev V. M., Smirnova T. A. Electron microscopy of alkaline phosphatase of Escherichia coli. Can J Microbiol. 1966 Aug;12(4):605–607. doi: 10.1139/m66-086. [DOI] [PubMed] [Google Scholar]
- LEIVE L. A NONSPECIFIC INCREASE IN PERMEABILITY IN ESCHERICHIA COLI PRODUCED BY EDTA. Proc Natl Acad Sci U S A. 1965 Apr;53:745–750. doi: 10.1073/pnas.53.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampen J. O. Cell-bound penicillinase of Bacillus licheniformis; properties and purification. J Gen Microbiol. 1967 Aug;48(2):249–259. doi: 10.1099/00221287-48-2-249. [DOI] [PubMed] [Google Scholar]
- Lampen J. O. External enzymes of yeast: their nature and formation. Antonie Van Leeuwenhoek. 1968;34(1):1–18. doi: 10.1007/BF02046409. [DOI] [PubMed] [Google Scholar]
- Lazdunski C., Shapiro B. M. Isolation and some properties of cell envelope altered mutants of Escherichia coli. J Bacteriol. 1972 Aug;111(2):495–498. doi: 10.1128/jb.111.2.495-498.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L. Studies on the permeability change produced in coliform bacteria by ethylenediaminetetraacetate. J Biol Chem. 1968 May 10;243(9):2373–2380. [PubMed] [Google Scholar]
- Lickfeld K. G., Achterrath M., Hentrich F., Kolehmainen-Seveus L., Persson A. Die Feinstrukturen von Pseudomonas aeruginosa in ihrer Deutung durch die Gefrierätztechnik, Ultramikrotomie und Kryo-Ultramikrotomie. J Ultrastruct Res. 1972 Jan;38(1):27–45. doi: 10.1016/s0022-5320(72)90082-2. [DOI] [PubMed] [Google Scholar]
- Lindberg A. A., Hellerqvist C. G. Bacteriophage attachment sites, serological specificity, and chemical composition of the lipopolysaccharides of semirough and rough mutants of Salmonella typhimurium. J Bacteriol. 1971 Jan;105(1):57–64. doi: 10.1128/jb.105.1.57-64.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay S. S., Wheeler B., Sanderson K. E., Costerton J. W., Cheng K. J. The release of alkaline phosphatase and of lipopolysaccharide during the growth of rough and smooth strains of Salmonella typhimurium. Can J Microbiol. 1973 Mar;19(3):335–343. doi: 10.1139/m73-056. [DOI] [PubMed] [Google Scholar]
- Lopes J., Gottfried S., Rothfield L. Leakage of periplasmic enzymes by mutants of Escherichia coli and Salmonella typhimurium: isolation of "periplasmic leaky" mutants. J Bacteriol. 1972 Feb;109(2):520–525. doi: 10.1128/jb.109.2.520-525.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURRAY R. G., STEED P., ELSON H. E. THE LOCATION OF THE MUCOPEPTIDE IN SECTIONS OF THE CELL WALL OF ESCHERICHIA COLI AND OTHER GRAM-NEGATIVE BACTERIA. Can J Microbiol. 1965 Jun;11:547–560. doi: 10.1139/m65-072. [DOI] [PubMed] [Google Scholar]
- MURRAY R. G., WATSON S. W. STRUCTURE OF NITROSOCYSTIS OCEANUS AND COMPARISON WITH NITROSOMONAS AND NITROBACTER. J Bacteriol. 1965 Jun;89:1594–1609. doi: 10.1128/jb.89.6.1594-1609.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAlister T. J., Costerton J. W., Cheng K. J. Effect of the removal of outer cell wall layers on the actinomycin susceptibility of a gram-negative bacterium. Antimicrob Agents Chemother. 1972 May;1(5):447–449. doi: 10.1128/aac.1.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAlister T. J., Costerton J. W., Thompson L., Thompson J., Ingram J. M. Distribution of alkaline phosphatase within the periplasmic space of gram-negative bacteria. J Bacteriol. 1972 Sep;111(3):827–832. doi: 10.1128/jb.111.3.827-832.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier S., Murray R. G. The fine structure of Thioploca ingrica and a comparison with Beggiatoa. Can J Microbiol. 1965 Aug;11(4):645–655. doi: 10.1139/m65-087. [DOI] [PubMed] [Google Scholar]
- Mangiarotti G., Apirion D., Schlessinger D. Selection of sucrose-dependent Escherichia coli to obtain envelope mutants and fragile cultures. Science. 1966 Aug 19;153(3738):892–894. doi: 10.1126/science.153.3738.892. [DOI] [PubMed] [Google Scholar]
- Martin E. L., MacLeod R. A. Isolation and chemical composition of the cytoplasmic membrane of a gram-negative bacterium. J Bacteriol. 1971 Mar;105(3):1160–1167. doi: 10.1128/jb.105.3.1160-1167.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu L. G., Legault-Hetu D. Decreased sensitivity to polymyxin B in colicin K tolerant cells of Escherichia coli K-12 in the presence of colicin K. Can J Microbiol. 1973 Mar;19(3):345–351. doi: 10.1139/m73-057. [DOI] [PubMed] [Google Scholar]
- Matzura H., Broda P. Sensitization of Escherichia coli to actinomycin D by the arrest of protein synthesis. J Bacteriol. 1968 Nov;96(5):1877–1879. doi: 10.1128/jb.96.5.1877-1879.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May B. K., Elliott W. H. Characteristics of extracellular protease formation by Bacillus subtilis and its control by amino acid repression. Biochim Biophys Acta. 1968 May 21;157(3):607–615. doi: 10.1016/0005-2787(68)90158-5. [DOI] [PubMed] [Google Scholar]
- McGroarty E. J., Koffler H., Smith R. W. Regulation of flagellar morphogenesis by temperature: involvement of the bacterial cell surface in the synthesis of flagellin and the flagellum. J Bacteriol. 1973 Jan;113(1):295–303. doi: 10.1128/jb.113.1.295-303.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcelhaney R. N., de Gier J., van der Neut-Kok E. C. The effect of alterations in fatty acid composition and cholesterol content on the nonelectrolyte permeability of Acholeplasma laidlawii B cells and derived liposomes. Biochim Biophys Acta. 1973 Mar 16;298(2):500–512. doi: 10.1016/0005-2736(73)90376-3. [DOI] [PubMed] [Google Scholar]
- Mergenhagen S. E., Bladen H. A., Hsu K. C. Electron microscopic localization of endotoxic lipopolysaccharide in gram-negative organisms. Ann N Y Acad Sci. 1966 Jun 30;133(2):279–291. doi: 10.1111/j.1749-6632.1966.tb52371.x. [DOI] [PubMed] [Google Scholar]
- Merton P. A. How we control the contraction of our muscles. Sci Am. 1972 May;226(5):30–37. doi: 10.1038/scientificamerican0572-30. [DOI] [PubMed] [Google Scholar]
- Miller O. L., Jr, Hamkalo B. A., Thomas C. A., Jr Visualization of bacterial genes in action. Science. 1970 Jul 24;169(3943):392–395. doi: 10.1126/science.169.3943.392. [DOI] [PubMed] [Google Scholar]
- Miller O. L., Jr, Hamkalo B. A. Visualization of RNA synthesis on chromosomes. Int Rev Cytol. 1972;33:1–25. doi: 10.1016/s0074-7696(08)61446-1. [DOI] [PubMed] [Google Scholar]
- Morris H., Schlesinger M. J. Effects of proline analogues on the formation of alkaline phosphatase in Escherichia coli. J Bacteriol. 1972 Jul;111(1):203–210. doi: 10.1128/jb.111.1.203-210.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller E., Hinckley A., Rothfield L. Studies of phospholipid-requiring bacterial enzymes. 3. Purification and properties of uridine diphosphate glucose:lipopolysaccharide glucosyltransferase I. J Biol Chem. 1972 Apr 25;247(8):2614–2622. [PubMed] [Google Scholar]
- Nakane P. K., Nichoalds G. E., Oxender D. L. Cellular localization of leucine-binding protein from Escherichia coli. Science. 1968 Jul 12;161(3837):182–183. doi: 10.1126/science.161.3837.182. [DOI] [PubMed] [Google Scholar]
- Nanninga N. Ultrastructure of the cell envelope of Escherichia coli B after freeze-etching. J Bacteriol. 1970 Jan;101(1):297–303. doi: 10.1128/jb.101.1.297-303.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Chou J. Release of surface enzymes in Enterobacteriaceae by osmotic shock. J Bacteriol. 1967 Dec;94(6):1934–1945. doi: 10.1128/jb.94.6.1934-1945.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisonson I., Tannenbaum M., Neu H. C. Surface localization of Escherichia coli 5'-nucleotidase by electron microscopy. J Bacteriol. 1969 Nov;100(2):1083–1090. doi: 10.1128/jb.100.2.1083-1090.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary G. P., Nelson J. D., Jr, MacLeod R. A. Requirement for salts for the isolation of lipopolysaccharide from a marine pseudomonad. Can J Microbiol. 1972 May;18(5):601–606. doi: 10.1139/m72-095. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Site of synthesis of lipopolysaccharide. J Biol Chem. 1972 Jun 25;247(12):3973–3986. [PubMed] [Google Scholar]
- Osborn M. J. Structure and biosynthesis of the bacterial cell wall. Annu Rev Biochem. 1969;38:501–538. doi: 10.1146/annurev.bi.38.070169.002441. [DOI] [PubMed] [Google Scholar]
- Page W. J., Stock J. J. Isolation and characterization of Microsporum gypseum lysosomes: role of lysosomes in macroconidia germination. J Bacteriol. 1972 Apr;110(1):354–362. doi: 10.1128/jb.110.1.354-362.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangborn J., Starr M. P. Ultrastructure of Lampropedia hyalina. J Bacteriol. 1966 May;91(5):2025–2030. doi: 10.1128/jb.91.5.2025-2030.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B., Watanabe K. Location of sulfate-binding protein in Salmonella typhimurium. J Bacteriol. 1968 Oct;96(4):1049–1054. doi: 10.1128/jb.96.4.1049-1054.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate J. L., Ordal E. J. The fine structure of Chondrococcus columnaris. 3. The surface layers of Chondrococcus columnaris. J Cell Biol. 1967 Oct;35(1):37–51. doi: 10.1083/jcb.35.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne J. W., Gilvarg C. Size restriction on peptide utilization in Escherichia coli. J Biol Chem. 1968 Dec 10;243(23):6291–6299. [PubMed] [Google Scholar]
- Pruul H., Reynolds B. L. Interaction of complement and polymyxin with gram-negative bacteria. Infect Immun. 1972 Nov;6(5):709–717. doi: 10.1128/iai.6.5.709-717.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman C. M., Cherian M. G. The secretory pathways of rat serum glycoproteins and albumin. Localization of newly formed proteins within the endoplasmic reticulum. J Cell Biol. 1972 Feb;52(2):231–245. doi: 10.1083/jcb.52.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman C. M., Siekevitz P., Palade G. E. Synthesis and transfer of amylase in pigeon pancreatic micromosomes. J Biol Chem. 1966 Mar 10;241(5):1150–1158. [PubMed] [Google Scholar]
- Redman C. M. Studies on the transfer of incomplete polypeptide chains across rat liver microsomal membranes in vitro. J Biol Chem. 1967 Feb 25;242(4):761–768. [PubMed] [Google Scholar]
- Reynolds B. L., Pruul H. Protective role of smooth lipopolysaccharide in the serum bactericidal reaction. Infect Immun. 1971 Dec;4(6):764–771. doi: 10.1128/iai.4.6.764-771.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J. Bacterial growth and the cell envelope. Bacteriol Rev. 1970 Jun;34(2):194–214. doi: 10.1128/br.34.2.194-214.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo D., Girard A., Rothfield L. Reconstitution of a functional membrane enzyme system in a monomolecular film. I. Formation of a mixed monolayer of lipopolysaccharide and phospholipid. J Mol Biol. 1970 Nov 14;53(3):475–490. doi: 10.1016/0022-2836(70)90078-1. [DOI] [PubMed] [Google Scholar]
- Romeo D., Hinckley A., Rothfield L. Reconstitution of a functional membrane enzyme system in a monomolecular film. II. Formation of a functional ternary film of lipopolysaccharide, phospholipid and transferase enzyme. J Mol Biol. 1970 Nov 14;53(3):491–501. doi: 10.1016/0022-2836(70)90079-3. [DOI] [PubMed] [Google Scholar]
- Rosen B. P., Hackette S. L. Effects of fatty acid substitution on the release of enzymes by osmotic shock. J Bacteriol. 1972 Jun;110(3):1181–1189. doi: 10.1128/jb.110.3.1181-1189.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen B. P., Vasington F. D. Purification and characterization of a histidine-binding protein from Salmonella typhimurium LT-2 and its relationship to the histidine permease system. J Biol Chem. 1971 Sep 10;246(17):5351–5360. [PubMed] [Google Scholar]
- Rothfield L., Finkelstein A. Membrane biochemistry. Annu Rev Biochem. 1968;37:463–496. doi: 10.1146/annurev.bi.37.070168.002335. [DOI] [PubMed] [Google Scholar]
- Rothfield L., Takeshita M., Pearlman M., Horne R. W. Role of phospholipids in the enzymatic synthesis of the bacterial cell envelope. Fed Proc. 1966 Sep-Oct;25(5):1495–1502. [PubMed] [Google Scholar]
- SCHLESSINGER D. PROTEIN SYNTHESIS BY POLYRIBOSOMES ON PROTOPLAST MEMBRANES OF B. MEGATERIUM. J Mol Biol. 1963 Nov;7:569–582. doi: 10.1016/s0022-2836(63)80103-5. [DOI] [PubMed] [Google Scholar]
- Sabet S. F., Schnaitman C. A. Purification and properties of the colicin E3 receptor of Escherichia coli. J Biol Chem. 1973 Mar 10;248(5):1797–1806. [PubMed] [Google Scholar]
- Sargent M. G., Lampen J. O. A mechanism for penicillinasesecretion in Bacillus licheniformis. Proc Natl Acad Sci U S A. 1970 Apr;65(4):962–969. doi: 10.1073/pnas.65.4.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T. Further studies on the binding of vitamin B 12 to the cell wall of a B 12 -requiring Lactobacillus. J Bacteriol. 1972 Jan;109(1):169–178. doi: 10.1128/jb.109.1.169-178.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlecht S., Westphal O. Untersuchungen zur Typisierung von Salmonella-R-Formen. 4. Typisierung von S. minnesota-R-Mutanten mittels Antibiotica. Zentralbl Bakteriol Orig. 1970 Apr;213(3):356–380. [PubMed] [Google Scholar]
- Schlesinger M. J., Olsen R. Expression and localization of Escherichia coli alkaline phosphatase synthesized in Salmonella typhimurium cytoplasm. J Bacteriol. 1968 Nov;96(5):1601–1605. doi: 10.1128/jb.96.5.1601-1605.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. J. Secretion of alkaline phosphatase subunits by spheroplasts of Escherichia coli. J Bacteriol. 1968 Sep;96(3):727–733. doi: 10.1128/jb.96.3.727-733.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Effect of ethylenediaminetetraacetic acid, Triton X-100, and lysozyme on the morphology and chemical composition of isolate cell walls of Escherichia coli. J Bacteriol. 1971 Oct;108(1):553–563. doi: 10.1128/jb.108.1.553-563.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Examination of the protein composition of the cell envelope of Escherichia coli by polyacrylamide gel electrophoresis. J Bacteriol. 1970 Nov;104(2):882–889. doi: 10.1128/jb.104.2.882-889.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J. R., Landau J. V. Hydrostatic pressure effects on Escherichia coli: site of inhibition of protein synthesis. J Bacteriol. 1972 Feb;109(2):945–948. doi: 10.1128/jb.109.2.945-948.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi M., Iida S. Mutants of Escherichia coli permeable to actinomycin. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2315–2320. doi: 10.1073/pnas.58.6.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellin H. G., Srinivasan P. R., Borek E. Studies of a phage-induced DNA methylase. J Mol Biol. 1966 Aug;19(1):219–222. doi: 10.1016/s0022-2836(66)80065-7. [DOI] [PubMed] [Google Scholar]
- Shands J. W. Localization of somatic antigen on gram-negative bacteria using ferritin antibody conjugates. Ann N Y Acad Sci. 1966 Jun 30;133(2):292–298. doi: 10.1111/j.1749-6632.1966.tb52372.x. [DOI] [PubMed] [Google Scholar]
- Shilo M. Lysis of blue-green algae by myxobacter. J Bacteriol. 1970 Oct;104(1):453–461. doi: 10.1128/jb.104.1.453-461.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shires T. K., Ekren T., Narurkar L. M., Pitot H. C. Protein synthesis on rat liver polysome-membrane complexes formed in vitro and disposition of the discharges chains. Nat New Biol. 1973 Apr 18;242(120):198–201. doi: 10.1038/newbio242198a0. [DOI] [PubMed] [Google Scholar]
- Singh A. P., Cheng K. J., Costerton J. W., Idziak E. S., Ingram J. M. Sensitivity of normal and mutant strains of Escherichia coli to actinomycin-D. Can J Microbiol. 1972 Jun;18(6):909–915. doi: 10.1139/m72-139. [DOI] [PubMed] [Google Scholar]
- Starr M. P., Huang J. C. Physiology of the bdellovibrios. Adv Microb Physiol. 1972;8:215–261. doi: 10.1016/s0065-2911(08)60191-5. [DOI] [PubMed] [Google Scholar]
- Stinnett J. D., Gilleland H. E., Jr, Eagon R. G. Proteins released from cell envelopes of Pseudomonas aeruginosa on exposure to ethylenediaminetetraacetate: comparison with dimethylformamide-extractable proteins. J Bacteriol. 1973 Apr;114(1):399–407. doi: 10.1128/jb.114.1.399-407.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagaki Y., Kunugita K., Matsuhashi M. Evidence for the direct action of colicin K on aerobic 32 P i uptake in Escherichia coli in vivo and in vitro. J Bacteriol. 1973 Jan;113(1):42–50. doi: 10.1128/jb.113.1.42-50.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki S., Matsuhashi M. Increase in sensitivity to antibiotics and lysozyme on deletion of lipopolysaccharides in Escherichia coli strains. J Bacteriol. 1973 Apr;114(1):453–454. doi: 10.1128/jb.114.1.453-454.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornley M. J., Glauert A. M. Fine structure and radiation resistance in Acinetobacter: studies on a resistant strain. J Cell Sci. 1968 Jun;3(2):273–294. doi: 10.1242/jcs.3.2.273. [DOI] [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. XII. Inhibition of cross-linking by penicillins and cephalosporins: studies in Staphylococcus aureus in vivo. J Biol Chem. 1968 Jun 10;243(11):3169–3179. [PubMed] [Google Scholar]
- Torriani A. Alkaline phosphatase subunits and their dimerization in vivo. J Bacteriol. 1968 Oct;96(4):1200–1207. doi: 10.1128/jb.96.4.1200-1207.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourtellotte M. E., Zupnik J. S. Freeze-fractured Acholeplasma laidlawii membranes: nature of particles observed. Science. 1973 Jan 5;179(4068):84–86. doi: 10.1126/science.179.4068.84. [DOI] [PubMed] [Google Scholar]
- Vaituzis Z., Doetsch R. N. Relationship between cell wall, cytoplasmic membrane, and bacterial motility. J Bacteriol. 1969 Oct;100(1):512–521. doi: 10.1128/jb.100.1.512-521.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varricchio F. "Compartmentalization" of Escherichia coli ribosomes and ribonucleic acid. J Bacteriol. 1972 Mar;109(3):1284–1294. doi: 10.1128/jb.109.3.1284-1294.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W. Bacterial viruses; with particular reference to adsorption/penetration. Annu Rev Microbiol. 1958;12:27–48. doi: 10.1146/annurev.mi.12.100158.000331. [DOI] [PubMed] [Google Scholar]
- WEIDEL W., FRANK H., LEUTGEB W. Autolytic enzymes as a source of error in the preparation and study of gram-negative cell walls. J Gen Microbiol. 1963 Jan;30:127–130. doi: 10.1099/00221287-30-1-127. [DOI] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]
- Ward J. B., Glaser L. An E. coli mutant with cryptic UDP-sugar hydrolase and altered metabolite regulation. Biochem Biophys Res Commun. 1968 Jun 10;31(5):671–677. doi: 10.1016/0006-291x(68)90614-1. [DOI] [PubMed] [Google Scholar]
- Watson S. W., Remsen C. C. Macromolecular subunits in the walls of marine nitrifying bacteria. Science. 1969 Feb 14;163(3868):685–686. doi: 10.1126/science.163.3868.685. [DOI] [PubMed] [Google Scholar]
- Wetzel B. K., Spicer S. S., Dvorak H. F., Heppel L. A. Cytochemical localization of certain phosphatases in Escherichia coli. J Bacteriol. 1970 Oct;104(1):529–542. doi: 10.1128/jb.104.1.529-542.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. A., Albright F. R., Lennarz W. J., Schnaitman C. A. Distribution of phospholipid-synthesizing enzymes in the wall and membrane subfractions of the envelope of Escherichia coli. Biochim Biophys Acta. 1971 Dec 3;249(2):636–642. doi: 10.1016/0005-2736(71)90145-3. [DOI] [PubMed] [Google Scholar]
- White D. A., Lennarz W. J., Schnaitman C. A. Distribution of lipids in the wall and cytoplasmic membrane subfractions of the cell envelope of Escherichia coli. J Bacteriol. 1972 Feb;109(2):686–690. doi: 10.1128/jb.109.2.686-690.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D., Dworkin M., Tipper D. J. Peptidoglycan of Myxococcus xanthus: structure and relation to morphogenesis. J Bacteriol. 1968 Jun;95(6):2186–2197. doi: 10.1128/jb.95.6.2186-2197.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins A. S. Physiological factors in the regulation of alkaline phosphatase synthesis in Escherichia coli. J Bacteriol. 1972 May;110(2):616–623. doi: 10.1128/jb.110.2.616-623.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S. G. The sensitivity of pseudomonads to ethylenediaminetetra-acetic acid. J Gen Microbiol. 1967 Apr;47(1):67–76. doi: 10.1099/00221287-47-1-67. [DOI] [PubMed] [Google Scholar]
- Willsky G. R., Bennett R. L., Malamy M. H. Inorganic phosphate transport in Escherichia coli: involvement of two genes which play a role in alkaline phosphatase regulation. J Bacteriol. 1973 Feb;113(2):529–539. doi: 10.1128/jb.113.2.529-539.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson L. A., Spitznagel J. K. Molecular and structural damage to Escherichia coli produced by antibody, complement, and lysozyme systems. J Bacteriol. 1968 Oct;96(4):1339–1348. doi: 10.1128/jb.96.4.1339-1348.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurewicz E. C., Ghalambor M. A., Duckworth D. H., Heath E. C. Catalytic and molecular properties of a phage-induced capsular polysaccharide depolymerase. J Biol Chem. 1971 Sep 25;246(18):5607–5616. [PubMed] [Google Scholar]
- Yurewicz E. C., Ghalambor M. A., Heath E. C. The structure of Aerobacter aerogenes capsular polysaccharide. J Biol Chem. 1971 Sep 25;246(18):5596–5606. [PubMed] [Google Scholar]
- Zagury D., Uhr J. W., Jamieson J. D., Palade G. E. Immunoglobulin synthesis and secretion. II. Radioautographic studies of sites of addition of carbohydrate moieties and intracellular transport. J Cell Biol. 1970 Jul;46(1):52–63. doi: 10.1083/jcb.46.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf F. K., van Vught A. M., Stouthamer A. H. Cell-envelope changes in mutants of Citrobacter freundii with altered response to colicin A. Antonie Van Leeuwenhoek. 1973;39(1):51–63. doi: 10.1007/BF02578841. [DOI] [PubMed] [Google Scholar]
- van Gool A. P., Lambert R., Laudelout H. The fine structure of frozen etched nitrobacter cells. Arch Mikrobiol. 1969;69(4):281–293. doi: 10.1007/BF00408570. [DOI] [PubMed] [Google Scholar]