Abstract
End stage liver disease from hepatitis C is the most common indication for liver transplantation in many parts of the world accounting for up to 40% of liver transplants. Antiviral therapy either before or after liver transplantation is challenging due to side effects and lower efficacy in patients with cirrhosis and liver transplant recipients, as well as from drug interactions with immunosuppressants. Factors that may affect recurrent hepatitis C include donor age, immunosuppression, IL28B genotype, cytomegalovirus infection, and metabolic syndrome. Older donor age has persistently been shown to have the greatest impact on recurrent hepatitis C. After liver transplantation, distinguishing recurrent hepatitis C from acute cellular rejection may be difficult, although the development of molecular markers may help in making the correct diagnosis. The advent of interferon free regimens with direct acting antiviral agents that include NS3/4A protease inhibitors, NS5B polymerase inhibitors and NS5A inhibitors holds great promise in improving outcomes for liver transplant candidates and recipients.
Keywords: Hepatitis C, Liver transplant, Donor risk factors, Immunosuppression, Protease inhibitors, Fibrosing cholestatic hepatitis C, Acute cellular rejection, Cytomegalovirus
Core tip: Recurrent hepatitis C impacts graft and patient survival following liver transplant. Preventing aggressive hepatitis C virus (HCV) recurrence by selecting appropriate donor allografts for HCV patients and careful management of immunosuppression in the post-transplant setting remain crucial. Direct acting antiviral therapy in patients awaiting transplant may prevent HCV re-infection post-transplant and has the potential to fundamentally change the natural history of hepatitis C in liver transplant recipients.
BACKGROUND
End stage liver disease due to hepatitis C virus (HCV) infection remains a leading indication for liver transplantation (LT) worldwide. While eradication of virus prior to LT is ideal, currently available antiviral therapy for those awaiting transplant is limited by toxicities and low response rates. Viral recurrence following LT is immediate and universal. There are donor, host, and transplant related factors which increase the likelihood and severity of recurrence. Some of these factors are modifiable.
Recurrent HCV infection is associated with more rapid fibrosis progression leading to higher rates of graft loss and patient mortality compared to patients transplanted for non-HCV etiologies. Post-transplant HCV antiviral treatment is also challenging due to poor tolerability, drug-drug interactions with immunosuppressant agents, and low response rates. Re-transplantation for allograft cirrhosis due to recurrent HCV remains controversial. This review will address pre- and post-transplant hepatitis C infection, antiviral treatment strategies, and the future role of direct acting antiviral agents.
TREATMENT OF HEPATITIS C IN CIRRHOTIC PATIENTS AWAITING LIVER TRANSPLANTATION
The most effective way to avoid post-LT HCV recurrence is to eradicate virus prior to transplant. Treating patients who are awaiting transplant, however, has been difficult with currently available regimens due to side effects and limited efficacy. The best-suited treatment candidates are those with compensated cirrhosis [Child-Pugh class A, model for end-stage liver disease (MELD) < 13] or those with hepatocellular carcinoma (HCC) as the primary indication for transplant. Unfortunately, this scenario is not the norm for most liver transplant candidates who have advanced liver failure.
Several published studies have examined the role of standard or pegylated interferon (PEG-IFN) with or without ribavirin (RBV) before liver transplantation in cirrhotic patients (Table 1). One large single-center study treated 124 patients (mean MELD score 11.0 ± 3.7) with a low accelerating dosage regimen (LADR) of antiviral therapy[1]. Most patients were treated with interferon alfa-2b and RBV; PEG-IFN and RBV was reserved for use in the retreatment of 15 patients. Treatment was initiated with half doses of interferon and RBV. Dose adjustments were made every 2 wk to reach maximally tolerated or target standard doses. Sustained virologic response (SVR) was achieved in 13% of patients with genotype 1 HCV and 50% of patients with non-1 genotypes. Twelve of 15 patients who were HCV-RNA negative prior to LT remained virus negative 6 mo or more after transplant. Fifteen patients experienced 22 serious adverse events (SAEs) including infection, diabetes mellitus, severe thrombocytopenia, and venous thromboembolism[1]. This study showed an acceptable SVR for non-genotype 1 patients with advanced disease, but highlighted the importance of following these patients closely while on treatment, preferably with the safety net of liver transplantation in place.
Table 1.
Treatment of hepatitis C virus cirrhosis with interferon and ribavirin
| Ref | n | Child score | Treatment | Mean treatment duration (mo) | End of treatment response/post-LT SVR |
| Everson et al[1] | 124 | 7 | IFN and RBV | 6-12 | 46%/30% |
| Carrion et al[2] | 51 | 88% A or B | PEG-IFN and RBV | 3 | 29%/20% |
| Everson et al[3] | 63 | 7 | PEG-IFN and RBV | 4 | 59%/25% |
LT: Liver transplantation; PEG-IFN: Pegylated-interferon; RBV: Ribavirin; IFN: Interferon; SVR: Sustained viral response rate.
Carrión et al[2] published the first study using PEG-IFN and RBV in hepatitis C patients awaiting LT. Fifty-one patients with HCV cirrhosis (mean MELD 12) were matched to 51 untreated controls. While the on-treatment virologic response was 47%, 29% were HCV RNA negative at the time of LT, and only 20% achieved a SVR after LT. Early virologic response and non-1 genotype were the strongest predictors of viral clearance. Child-Pugh B/C patients had a particularly high incidence of bacterial infection with bacteremia and spontaneous bacterial peritonitis. Three control and 12 treated patients developed 3 and 19 bacterial infections, respectively. The authors recommended using caution when treating those with decompensated disease who are not on prophylactic antibiotics[2].
A randomized controlled trial (LADR-A2ALL) evaluated PEG-IFN and RBV in a cohort of 79 patients with advanced HCV who were candidates for adult living donor LT[3]. Patients with genotypes 1/4/6 (n = 44/2/1) were randomized 2:1 to treatment or untreated control; HCV genotypes 2/3 (n = 32) were assigned to treatment. Two groups of adult patients were included: those who had a potential living donor and those with HCC eligible for a MELD upgrade; the average native MELD score was 12 in both the treated and control groups. Pre-transplant treatment achieved post-transplant viral clearance (pTVR = negative viral load 12 wk after transplant) in 25% of patients. The only factor predictive of pTVR was longer duration of pre-transplant treatment. More specifically, pTVR was 0%, 18%, and 50% in patients treated for < 8, 8-16, and > 16 wk, respectively. SAEs occurred during the course of treatment and the number of SAEs per patient was higher in the treated group (2.7 vs 1.3, P = 0.003). In fact, after several serious infections, the authors broadened the use of antibiotic prophylaxis to include patients with a current or past history of ascites.
Triple therapy using first generation HCV NS3/4A protease inhibitors for genotype 1 HCV led to improved SVR rates overall. However, it’s important to note that relatively few patients with advanced fibrosis were included in the phase 3 registration trials of telaprevir and boceprevir[4,5]. Patients with advanced fibrosis and prior relapse to interferon therapy actually did better than treatment naïve patients with advanced fibrosis (Table 2)[6,7]. Unfortunately, the initial enthusiasm to treat those chronic hepatitis C patients with cirrhosis with first generation triple therapy was tempered by an unfavorable side effect profile. The risks associated with triple therapy in cirrhotics were perhaps best outlined by the prospective observational multi-center French study (ANRS-CUPIC) study which compared on-treatment response with TVR (n = 285) and BOC-based (n = 204) triple therapy in compensated genotype 1 cirrhotics who were partial responders or relapsers to prior dual therapy[8]. The week 16 on-treatment virologic response data showed that 67% of the TVR group and 58% of the BOC group had undetectable HCV viral loads. The high rate of SAEs in both groups (33%-45%) contrasted starkly to the 9%-14% SAE rate seen in the phase 3 trials that led to licensing of the protease inhibitors. Anemia was especially problematic as 46%-54% of patients required erythropoietin and 6%-16% required blood transfusions[8]. The availability of better and safer next generation direct acting antiviral therapy, such as sofosbuvir, has quickly caused providers to abandon first generation triple therapy with TVR and BOC as a treatment strategy.
Table 2.
Treatment of hepatitis C cirrhosis with triple therapy
| Fibrosis stage | Drug | Prior PEG-IFN responsiveness (n) | Treatment response | Serious adverse events1 | Ref. |
| Cirrhosis | Telaprevir | 21 | 62% SVR | 9% | [4] |
| Bridging fibrosis and cirrhosis | Boceprevir | 76 | 47% SVR | 12% | [5] |
| Relapse (n = 119) | 85% SVR | ||||
| Partial response (n = 50) | 42% SVR | ||||
| Null response (n = 88) | 24% SVR | ||||
| Bridging fibrosis and cirrhosis | Boceprevir | Relapse and partial response (n = 63) | 56% SVR | 12% | [7] |
| Child A | Telaprevir | 285 | 67% (16 wk) | 45% | [8] |
| Boceprevir | 204 | 58% (16 wk) | 33% |
Serious adverse event rate for entire study population, % of patients with undetectable hepatitis C virus RNA at 16 wk following drug initiation, an interim analysis. PEG-IFN: Pegylated-interferon; SVR: Sustained viral response rate.
NATURAL HISTORY OF HEPATITIS C AFTER LIVER TRANSPLANT
HCV infection recurs universally in LT recipients who are viremic at transplantation. Viral kinetic studies have shown that replication begins as early as the first post-operative week and typically peaks by the fourth post-operative month. Virus levels at one year post-LT are 10-20 fold greater than pre-transplant[9]. Histologic studies have shown accelerated fibrosis progression compared to immunocompetent patients infected with HCV. A retrospective cohort study of 183 liver transplant recipients with HCV looked at fibrosis progression based on protocol liver biopsies done over a 10 year period. Fibrosis progression was non-linear, increasing exponentially during the first three years post-LT. Having advanced fibrosis (> stage 2) 1 year post-LT led to a 15-fold increase in HCV-related graft loss[10]. Cirrhosis occurs in up to 20% of patients within 5 years of LT. The cumulative probability of decompensation 1 year after developing cirrhosis is 30%. Once decompensated cirrhosis occurs, the 1 year-survival rate is poor at 46%[11].
CLASSIFICATION OF RECURRENT HEPATITIS C
Standardized definitions of recurrent hepatitis C and its various forms were proposed by an international consensus conference and published in 2003[12]. Recurrent HCV infection is defined by the presence of HCV RNA in serum and/or liver. Acute recurrent HCV is often associated with elevated aminotransferases and typically occurs within 6 mo of LT, though it can occur any time post LT. Histologically, reinfection of the graft with HCV is characterized by lobular hepatitis, focal hepatocyte necrosis, acidophil bodies, and macrovesicular steatosis. Chronic recurrent HCV disease develops as a result of acute recurrent HCV. Liver biopsy findings include chronic hepatitis with mixed portal, periportal, and lobular inflammation with variable degrees of portal and periportal fibrosis[12].
A more detailed set of criteria was proposed for fibrosing cholestatic hepatitis C (FCH). This includes (1) onset greater than 1 mo and usually < 6 mo after LT; (2) serum bilirubin greater than 6 mg/dL; (3) serum alkaline phosphatase and gamma-glutamyltransferase levels greater than 5 times the upper limit of normal; (4) characteristic histology with hepatocyte ballooning, a paucity of inflammation, and cholestasis; (5) very high HCV RNA levels; and (6) absence of biliary or vascular complications[12].
IMPACT OF DONOR AND RECIPIENT RISK FACTORS ON HCV RECURRENCE AFTER TRANSPLANT
Risks due to donor allograft
The challenge to mitigate the risk of HCV recurrence begins prior to transplant with the selection of the appropriate donor allograft when possible. Donor age influences the risk of recurrent HCV and graft survival. For HCV positive recipients, donor age over 40[13] (or 50[14]) years old was found to be an independent predictor of graft loss and patient death in two large retrospective reports of liver transplant recipients from the Scientific Registry of Transplant Recipients (SRTR) and United Network of Organ Sharing (UNOS) databases. Recent data also points to an association between the incidence of FCH and the use of allografts from older donors. Verna et al[15] reviewed 179 post-LT biopsies that had been initially categorized as demonstrating cholestatic hepatitis C and refined the classification of FCH to include only those patients meeting at least 3 of the following 4 pathologic criteria: (1) ductular reaction; (2) cholestasis; (3) hepatocyte ballooning with lobular disarray; and (4) periportal sinusoidal/pericellular fibrosis (Figure 1). With these more stringent standards, donor age (OR = 1.37, 95%CI: 1.02-1.84, P = 0.04) and prior history of acute cellular rejection (OR = 4.19, 95%CI: 1.69-10.4, P = 0.002) were the strongest predictors of developing FCH on multivariate analysis. Thus, transplanting older allografts into HCV recipients leads to worse outcomes due to recurrent HCV both in the short and long-term.
Figure 1.
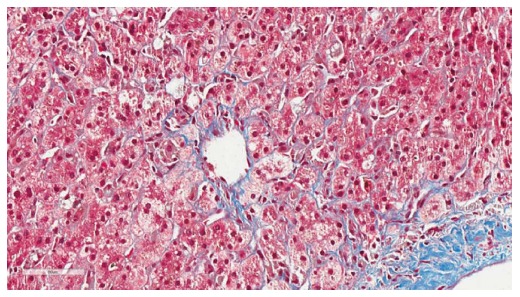
Histopathology of fibrosing cholestatic hepatitis C. Histopathology of fibrosing cholestatic hepatitis C demonstrating periportal sinusoidal and pericellular “chicken wire” fibrosis (trichrome, image magnification × 40) (Courtesy of Carl Jacobs, MD, Department of Pathology, Carolinas Medical Center, Charlotte, NC, United States).
Weighing the risk-benefit ratio of selecting an allograft from a donor of advanced age reflects the imbalance between supply and demand in liver transplantation today. Another consequence of this disparity is the increased reliance on potential donors with hepatic steatosis. Overall, recipients of allografts with over 30% macrovesicular steatosis are at increased risk of delayed graft function and primary graft non-function. However, the natural history of hepatitis C recurrence in recipients of fatty livers is less clear. Interestingly, macrovesicular steatosis at the time of transplant has been shown to be a transient and reversible phenomenon[16,17]. As such, the mechanistic link between allograft steatosis and HCV reinfection is ambiguous. In a prospective study comparing 56 HCV+ and 60 HCV- LT recipients[18], Burra and colleagues did not find a statistically significant difference between donor biopsy steatosis and patient or graft survival at 3 years. However, only 9.5% (11/116) of the donor biopsies in their study population had > 33% steatosis, limiting their ability to detect a statistical difference. In contrast, Briceño et al[19] found an inverse relationship between graft survival and donor liver steatosis in 120 HCV+ LT recipients, of whom 48/120 (40%) had > 30% donor steatosis. One year following LT, 40% of patients who received a donor allograft with > 30% steatosis had histologic evidence of HCV recurrence with ≥ stage 2 fibrosis in comparison to only 17% in patients who received a graft with < 30% steatosis. Their conclusion that HCV recurrence is more aggressive in patients receiving allografts with moderate/severe steatosis is subject to selection bias, though, since their enrollment included only patients biopsied for elevated aminotransferases[20]. In summary, the presence of allograft steatosis > 30% can be problematic for any LT recipient, but particularly in the context of other extended donor criteria such as advanced donor age or cold ischemic time > 10 h. Whether steatosis in and of itself modifies the risk of HCV recurrence remains subject to interpretation and undoubtedly the topic of future investigation.
The influence of HCV recurrence has been studied in regard to split liver and living donor liver transplants as well. Two studies comparing recipients of either a deceased or living donor liver transplant did not find any difference in HCV recurrence or graft survival at 2[21] and 3[22] years. A study by Selzner et al[23] found that HCV+ recipients of a living donor liver transplantation (LDLT) had less fibrosis progression at 24 mo than deceased donor HCV+ transplant recipients. Not surprisingly, the average donor age of the LDLT recipients was younger than that deceased donor liver transplantations (DDLTs). Age > 45 years old was the only variable independently associated with fibrosis progression from their cohort of 46 LDLTs and 155 DDLTs. Since split liver transplant recipients receive younger organs on average, it is not surprising that no differences have been shown in the small studies[24,25] with these grafts, either. Interestingly, Yoshizawa et al[26] reported two cases in which identical twins underwent LDLT from their respective siblings. In both cases, HCV recurrence occurred shortly after transplant even in the absence of immunosuppression. Fortunately, both patients responded well to antiviral therapy.
Peri-operative factors to take into consideration include ischemia-reperfusion injury (IRI) and ischemia time. Several factors contribute to IRI, most notably donor status (cardiac vs brain death); and warm and cold ischemic time. A small, but well-controlled study by Watt et al[27] demonstrated increased mortality at 3 years (59% vs 82%, 59% vs 88%, P = 0.0055) in HCV+ recipients with preservation injury (PI) compared to non-HCV recipients with PI and HCV+ recipients without PI, who were matched for gender, age, and immunosuppression. In contrast, time to histologic HCV recurrence did not correlate with the severity of IRI as defined biochemically by peak alanine aminotransfersase levels and on liver biopsy in a large retrospective cohort of HCV+ transplant recipients[28]. The impact of IRI on the risk of HCV recurrence remains unclear due to potential confounding from perioperative and donor factors. Taner et al[29] attempted to address confounding by exclusively comparing HCV+ recipients of a donation after cardiac death (DCD) allograft to HCV- recipients of a DCD organ and HCV+ recipients of a brain dead donor graft. In this large, single center experience no difference in patient or graft survival was found up to five years after transplant.
Lastly, HCV+ donors constitute a subset of extended criteria donor allografts that may be considered given the organ shortage in liver transplantation. Marroquin et al[30] studied UNOS data from 1994-1997 and identified 96 HCV+ allografts transplanted into HCV+ recipients, who were more likely to have been transplanted due to underlying hepatocellular carcinoma (8.3%) than those patients receiving an HCV- allograft (3.1%). Patients who received the HCV+ allografts had improved survival at 24 mo compared HCV- allograft recipients (90% vs 77%, P = 0.01). A retrospective case control study of 39 HCV+ allografts transplanted over a 16-year period also showed no difference in overall survival, though fibrosis progression was more advanced in the HCV+ allograft recipients, and particularly those patients who received an HCV+ organ from a donor over age 50[31]. The synergism in risk encountered by transplanting an HCV+ organ from a donor > 45 years old was recently reaffirmed by the CRUSH-C investigators[32]. Nonetheless, survival data up to 5 years following OLT with HCV+ donors[33] confirms the utility of these organs for genotype 1-positive recipients who have not achieved a sustained virological response to anti-HCV therapy before transplantation. With the promise of direct acting antivirals for HCV now in view, transplant centers may continue to make use of these organs for appropriate patients.
Viral factors
HCV genotype affects recurrent hepatitis C with fibrosis progression from stage 1 to 2 more common in HCV genotype 1 transplant recipients compared to non-1 genotypes (HR = 2.739, 95%CI: 1.047-7.143, P = 0.04)[29]. Pre and post-transplant viral loads appear to influence the risk of HCV recurrence as well. A pre-transplant viral load > 1 × 106 vEq/mL was associated with increased mortality at 5-years post LT from an early study[34]. However, when pre-transplant HCV RNA level was incorporated into a model by the same investigators to predict outcomes in HCV+ transplant recipients, no difference in survival at 10 years was seen when compared to uninfected transplant recipients[35]. Post-transplant HCV RNA ≥ 1 × 109 copies/mL at 4 mo following LT was associated with worse necroinflammatory activity as assessed by hepatitis activity index on protocol biopsies at 1 and 3 years following LT[36]. Hanouneh et al[37] also reported an independent association (HR = 1.1, P = 0.004) between fibrosis progression and HCV RNA level at 4 mo following transplant.
Cytomegalovirus (CMV) co-infection has consistently been shown to impact the risk of HCV recurrence. CMV infection, defined as viremia requiring antiviral therapy, resulted in graft failure in 52% vs 19.1% (P = 0.002) of 93 consecutive HCV+ transplant recipients[38]. Fibrosis (stage ≥ 2) on protocol liver biopsies at month 4 was significantly higher (45% vs 16.4%, P = 0.01) in the co-infected patients compared to the CMV-group. CMV co-infection was associated with fibrosis progression by univariate analysis in a study of HCV+ transplant recipients with and without metabolic syndrome[37]. In the aforementioned study on the risk of DCD donation in HCV+ patients, CMV infection post-transplant was a significant factor for graft loss (HR = 3.367, 95%CI: 1.493-7.593, P = 0.003)[29]. CMV viremia may alter the host immunological profile independent of its effect on HCV replication[39].
Recent prospective data from a US consortium of human immunodeficiency virus (HIV)/HCV investigators establishes HIV co-infection as a significant risk for graft failure[40]. Patient survival at 3 years post-LT was 60% in 89 HCV/HIV-co-infected patients compared to 75% in 235 HCV mono-infected patients (P < 0.001). However, after excluding 25 (28%) co-infected patients who met at least one of the following criteria: (1) a body mass index < 21 kg/m2; (2) a HCV+ donor allograft; or (3) a combined liver kidney transplant; patient survival was not statistically different in comparison to all the United States transplant recipients ≥ 65 years old or liver transplant recipients transplanted for other indications. Acute rejection occurred more frequently in co-infected patients (39% vs 24%, HR = 2.1, P = 0.01), but HCV disease severity assessed on biopsy was not statistically different between the two groups. This finding is in contrast to the study by Duclos-Vallée et al[41] where time to fibrosis progression (stage ≥ 2) was significantly shorter in the co-infected population. The failure to detect a difference in fibrosis progression in the United States cohort is almost certainly a limitation in power (only 62% of HIV/HCV patients had liver biopsies and multiple deaths occurred early from sepsis and multi-organ failure), though it may also reflect different centers’ thresholds to begin antiviral therapy. In summary, HIV infection likely impacts HCV fibrosis progression in co-infected transplant recipients. Careful patient selection is essential in order to achieve good outcomes.
Host factors
A number of studies identify sex and race as modifying the risk of HCV recurrence following transplant. The CRUSH-C investigators found that HCV+ women were at increased risk for bridging fibrosis or cirrhosis after transplant in comparison to their HCV+ male counterparts[42]. After multivariate analysis, female sex was found to be an independent predictor of advanced fibrosis (HR = 1.31, 95%CI: 1.02-1.70, P = 0.04) and mortality (HR = 1.30, 95%CI: 1.01-1.67, P = 0.04). African American patients transplanted for HCV who receive an allograft from a racially matched donor have been shown to have excellent outcomes following transplant. Conversely, African American HCV+ recipients of a racially mismatched allograft are at increased risk of graft failure and death. Pang et al[43] reviewed UNOS data from 1998 through 2007 and found a 5-year survival rate of 45% for racially mismatched pairs compared to 59% for a racially matched pairs. The survival rates for African American matched donor and recipient pairs was on par with HCV+ Caucasian transplant recipients. The risk associated with a mismatched donor was restricted to HCV+ African American recipients and not HCV negative African American transplant recipients. Two studies recently published by Saxena et al[44] and Layden et al[45] reinforced the survival data by finding that racial mismatch is a significant independent predictor of advanced fibrosis. While the exact interaction between HCV, the host immune system, and the donor allograft genetic profile isn’t clear, the overwhelming data supports that racial mismatch is associated with poor outcomes in HCV+ African American transplant recipients due to recurrent HCV disease.
Metabolic syndrome (MS) following LT is associated with worse outcomes after LT and may be an important modifiable risk factor. Hanouneh et al[37] reported an independent association between metabolic syndrome and fibrosis progression in HCV+ transplant recipients 1-year following OLT. Similarly, Veldt et al[46] calculated the homeostasis model assessment of insulin resistance (HOMA-IR) in 160 HCV+ patients 4 mo following transplant and found that insulin resistance as defined by a HOMA-IR > 2.5 was independently associated with advanced fibrosis (HR = 2.07, 95%CI: 1.10-3.91, P = 0.024). The above two studies suggest a link between MS and fibrosis progression in HCV+ transplant recipients. Whether the influence of MS on fibrosis progression is specific to HCV+ recipients or all transplants remains a question and further studies are needed. Lastly, HCV+ transplant recipients with hepatic steatosis (≥ 5%) on an index liver allograft biopsy 1 year post-LT may be at increased risk of fibrosis progression[47]. The cumulative rate of significant fibrosis (Ludwig-Batts F2-F4) after a median follow-up of 2 years after an index biopsy was 49% compared to 24% for HCV+ recipients without steatosis on their index biopsy. Overall, MS is an important consideration following liver transplant for any cause, particularly in view of the side effects of lifelong immunosuppression. A comprehensive assessment for MS should be integrated into post-transplant care, especially in the HCV+ and nonalcoholic fatty liver disease population (Table 3).
Table 3.
Risk factors studied for association with more severe hepatitis C virus recurrence
| Factor | Evidence |
| Donor | |
| Age > 40 yr | ↑↑↑ |
| Living donor | ↔ |
| Split liver | ↔ |
| DCD | ↔ |
| HCV+ | ↔ |
| Macrovesicular steatosis > 30% | ↑↓ |
| IRI | ↑↓ |
| IL28B “CC” genotype | ↑↓ |
| Virus | |
| HCV genotype 1 | ↑ |
| High pre-transplant HCV RNA | ↑ |
| HCV RNA 4 mo post LT ≥ 1 × 109 mEq/mL | ↑↑ |
| CMV viremia | ↑↑↑ |
| HIV coinfection | ↑↑ |
| Recipient | |
| Female sex | ↑↑ |
| African American D/R mismatch | ↑↑ |
| African American D/R match | ↔ |
| Metabolic syndrome1 | ↑ |
| IL28B non-“CC” genotype | ↑ |
| Immunosuppression | |
| Pulsed corticosteroids for American College of Rheumatology | ↑↑↑ |
| Tacrolimus (vs CsA) | ↑↓ |
| Sirolimus | ↑ |
| Thymoglobulin | ↔ |
| Basiliximab | ↔ |
| OKT3 | ↑ |
Metabolic syndrome defined by ATP-III criteria 1 yr post liver transplantation (LT)[37], homeostasis model assessment-estimated insulin resistance > 2.5 4 mo post LT[46], or hepatic steatosis ≥ 5% on an index liver allograft biopsy 1 yr post-LT[47]. ↑: Evidence of increased risk; ↔: Evidence of no increased risk; ↑↓: Indeterminate risk; DCD: Donation after cardiac death; HCV: Hepatitis C virus; CMV: Cytomegalovirus; HIV: Human immunodeficiency virus; CsA: Cyclosporin A; OKT3: Muromonab-CD3; IRI: Ischemia reperfusion injury.
IL28B genotype
Genetic variation upstream of the IL28B gene was originally found in GWAS to be associated with response to interferon therapy as well as spontaneous clearance after acute HCV infection, with the presence of the “CC” genotype for rs12979860 predicting both of the above favorable outcomes in the non-transplant setting. The impact of donor and recipient IL28B genotype (for rs12979860) on HCV recurrence and outcomes following liver transplant has recently been the topic of a number of studies[48,49]. Duarte-Rojo et al[50] found that donor-CC genotype allograft recipients had a significantly higher average ALT, viral load, and rate of fibrosis ≥ stage 2 at 1 year compared to non-CC donor graft recipients. On the other hand, recipient “CC” genotype was associated with the exact opposite result with rates of fibrosis ≥ stage 2 at 1 year of only 19% compared to 38% for non-CC recipient genotype (P = 0.012). The combination of both donor and recipient “CC” genotype was associated with a 90% sustained virological response to antiviral therapy. Interestingly, Duarte-Rojo found that donor-CC genotype was independently associated with adverse outcomes - defined as cirrhosis, liver-related death, or retransplantation. This finding was not a result of increased rates of acute cellular rejection, however. In contrast, a survival analysis performed recently by Allam et al[51] did not demonstrate the same impact on donor IL28B genotype, perhaps due to grouping of genotypes “CC” and “CT” together rather than comparing “CC” with non-CC genotypes. While it is safe to conclude that the unfavorable “T” allele in recipients is associated with a worse response to antiviral therapy as in the pre-transplant setting, a consensus on the influence of donor and recipient IL28B on outcomes following LT in HCV+ patients has yet to emerge. A comprehensive review of IL28B genotype in transplantation for HCV is beyond the scope of this article, but the topic of an excellent recent review[52]. Hopefully, the promise of direct acting antiviral therapy will reduce the impact of IL28B genotype.
Immunosuppression: Corticosteroids
Immunosuppression represents arguably the most critical factor to address with respect to HCV recurrence following transplantation. A balance exists between maintaining appropriate immunosuppression and preventing aggressive HCV recurrence. HCV+ recipients who receive high dose steroid treatment for acute cellular rejection are at risk for developing FCH. Donor age (OR = 1.37, P = 0.04) and previous rejection defined as Banff grade ≥ 5 (OR = 4.19, P = 0.002) were found to be the two most important predictors of developing FCH[15]. The association between steroid bolus therapy and early graft loss as well as death has been reported by others[34,53]. Data on the impact of maintenance steroids following liver transplantation for HCV is less clear. The decision and timing to stop steroids varies across transplant centers[54]. Nonetheless, a meta-analysis by Segev et al[55] reported a reduced risk of HCV recurrence for steroid free regimens (RR = 0.90, P = 0.03), even though no individual trial met statistical significance. A recent study by Takada et al[56] evaluated the impact of steroid avoidance on HCV recurrence following LDLT. Seventy-five patients were randomized to immunosuppression with either tacrolimus (TAC) plus a corticosteroid or TAC with mycophenolate mofetil (MMF). HCV recurrence rates defined by a METAVIR fibrosis score ≥ 1 were not statistically different at either 1 or 3 years following transplant.
Calcineurin inhibitors
Cyclosporine (CsA) has been shown to inhibit hepatitis C viral replication in vitro, however, evidence supporting a benefit over tacrolimus with regard to HCV recurrence and fibrosis progression following transplant is inconsistent. The LIS2T trial was a prospective, open-label, randomized trial comparing CsA to tacrolimus[57]. In the HCV+ transplant recipients, death and graft loss were higher with tacrolimus compared to CsA (16% vs 6%, P ≤ 0.03) at 12 mo. However, it’s not clear that any of those deaths occurred as a result of recurrent or fibrosing cholestatic hepatitis C. Moreover, there was no difference in fibrosis stage between the CsA and tacrolimus groups. A meta-analysis by Berenguer et al[58] that included 5 trials, totaling 366 patients failed to detect a difference between CsA vs tacrolimus-based regimens. A subsequent prospective study comparing CsA and tacrolimus by Berenguer et al[59] involved 253 patients transplanted for HCV between 2001 and 2007. Severe recurrent disease defined as bridging fibrosis, cirrhosis, FCH, graft failure, and death occurred with the same frequency in both groups (CsA: 27% vs Tacrolimus: 26%, P = 0.68)
The decision to incorporate a CsA-based immunosuppression strategy in HCV+ patients after transplant typically revolves around beginning antiviral therapy for recurrent disease (see discussion below). Inhibiting HCV replication with CsA in vivo might conceivably augment the response to PEG-IFN and RBV. A study by Firpi et al[60] supported this claim with improved SVR rates with CsA vs tacrolimus (46% vs 27%, P = 0.03). However, a smaller controlled trial of mostly genotype 1 patients randomized to either CsA or tacrolimus did not find a difference in SVR rates (39% vs 35%, P = 0.8)[61].
mTOR inhibitors
Sirolimus may be prescribed after liver transplantation in patients intolerant to calcineurin inhibitors or for hepatocellular carcinoma or as primary immunosuppression. SRTR data from 26414 liver transplants (12589 for HCV) was analyzed to address risk factors for patient and graft survival[62]. 6.5% (795/12269) of HCV+ transplant recipients were prescribed sirolimus at the time of discharge from LT, and 3.5% of these patients remained on the drug 1 year after transplant. Sirolimus was found on multivariate analysis to be associated with increased mortality within three years of liver transplant in HCV+ recipients (HR = 1.26, 95%CI: 1.08-1.48, P = 0.0044), but not in non-HCV patients. On the other hand, all patients (HCV+ or HCV-) on tacrolimus-based regimens had improved overall survival. The authors performed a propensity analysis to account for the fact that patients who received sirolimus were more likely to have had HCC and also had significantly higher creatinine and MELD score prior to transplant. Sirolimus at baseline was still an independent risk factor for increased mortality at 3 years (HR = 1.29, 95%CI: 1.08-1.55, P = 0.0053). While the SRTR database does not capture biopsy data to determine whether the increased mortality in the sirolimus group was a result of HCV recurrence, the mortality data certainly warrants pause and further investigation into the mechanism which may underlie the association. The larger studies with everolimus in LT from Toronto[63] and Italy[64] have been powered to determine efficacy, safety, and renal protective benefits and not the impact on HCV recurrence.
MMF, T-cell depleting therapies, and IL-2 receptor inhibition
The addition of MMF to immunosuppression regimens in the mid to late 1990s has had a positive impact on long-term outcomes following LT for all causes including hepatitis C[65]. The data for thymoglobulin induction suggests that it is safe to use in HCV+ patients and that it may provide a benefit in terms of slowing fibrosis progression[66]. This benefit may derive from lower rates of acute cellular rejection. Interestingly, a study comparing outcomes with routine induction with either thymoglobulin or basiliximab after living donor liver transplant demonstrated more frequent HCV recurrence requiring antiviral therapy in patients who received rabbit thymoglobulin[67]. Data demonstrate the safety profile of basiliximab induction therapy in HCV+ transplant recipients. A randomized trial comparing basiliximab with or without steroids indeed found that the steroid free group had less fibrosis at 6 mo, 1 year, and 2 years following transplant[68]. With the exception of OKT3, which has historically been associated with poor outcomes when used in HCV+ transplant recipients[69], the overall evidence suggests an acceptable safety profile with the use of thymoglobulin or basiliximab.
ROLE OF PROTOCOL LIVER BIOPSIES
Transplant centers may perform annual protocol liver biopsies on HCV transplant recipients to assess disease progression. However, there is a lack of uniformity regarding their use. A study from Spain evaluated protocol liver biopsies from 245 patients between 1991 and 1997. HCV infection +/- alcohol was the cause of cirrhosis in 125 patients. Histologic evidence of recurrent hepatitis C was present in 66% of patients at 1 year and 80% at 3 years post-LT. The cumulative probability of developing stage 3 or 4 fibrosis was 41% at 5 years post-LT[70]. Based on these results, the authors concluded that the high prevalence of abnormal histology and the rate of fibrosis progression justify the use of protocol biopsies.
A cohort of 264 HCV-infected liver transplant recipients who underwent protocol liver biopsies showed that the 12-mo biopsy had the best ability to stratify fibrosis progression. Twenty one percent of patients with stage 2-3/6 fibrosis at month 12 progressed to cirrhosis (stage 5-6) within 5 years. The degree of inflammation also correlated with fibrosis progression[71]. These studies support the role of protocol liver biopsies and suggest that rapid fibrosis progressors can be identified within a year of transplant.
LIVER BIOPSY INTERPRETATION AFTER LIVER TRANSPLANTATION
One of the most challenging diagnostic dilemmas for the hematopathologist and transplant team is distinguishing recurrent hepatitis C from acute cellular rejection on liver biopsy. The treatments are diametrically opposed; treatment of rejection requires more intensive immunosuppression which may exacerbate recurrent hepatitis C and treatment of hepatitis C with interferon based therapy may provoke an immune mediated injury or plasma cell hepatitis.
An understanding of the timing of reinfection and features on liver biopsy can help distinguish recurrent hepatitis C from rejection. Immediately after liver transplant hepatitis C virus infects the liver allograft. Within the first several weeks after liver transplantation serum HCV RNA levels are approximately 1-log higher compared to non-transplant HCV patients. Elevations in aminotransferases and an acute hepatitis with a lobular lymphocytic infiltrate may be seen two to six months after transplant. Thus, monitoring HCV viral load within the first 3 mo after transplant may be helpful because high viral load may support recurrent hepatitis C as a cause for elevated liver tests.
Although recurrent hepatitis C and acute cellular rejection may have similar pathologic findings on liver biopsy several features on biopsy may be helpful in distinguishing the two diagnoses (Table 4). The histopathologic variants of hepatitis C that have been described include usual or conventional hepatitis C, fibrosing cholestatic hepatitis C, plasma-cell rich hepatitis C, and HCV overlapping with acute and chronic rejection. The plasma cell rich hepatitis is seen in patients on interferon based antiviral therapy who typically are on low levels of immunosuppression and have low or undetectable serum HCV RNA. Plasma cells are not a feature of acute cellular rejection, although they may be seen in antibody mediated rejection.
Table 4.
Liver biopsy findings in recurrent hepatitis C and acute cellular rejection
| Favors hepatitis C | Favors rejection |
| Apoptotic (Councilman) bodies | Central venulitis |
| Lymphoid aggregates | Perivenular necrosis |
| Kupffer cell hypertrophy | Inflammatory bile duct damage |
| Mononuclear portal inflammation | Biliary epithelial senescence changes |
| Ballooning degeneration |
HCV evolution in liver allografts in the acute phase of reinfection during the first 1-3 mo after liver transplant shows lobular disarray, Kupffer cell hypertrophy, hepatocyte apoptosis, macrovesicular steatosis, mild sinusoid lymphocytosis and portal inflammation[72]. Damage of bile ducts by infiltrating lymphocytes is usual mild, in contrast to acute cellular rejection where biliary epithelium damage can be severe. This constellation of findings can be useful in distinguishing recurrent hepatitis C from acute cellular rejection (Table 4).
Molecular and immunologic diagnostics have been used to distinguish hepatitis C from rejection or to identify recipients with aggressive hepatitis C[73-76]. A cirrhosis risk score was developed from a 7-gene signature that accurately identified liver transplant recipients who developed advanced fibrosis on liver biopsy after liver transplantation[73]. A prospective study of an immune functional assay found that the immune response was significantly higher in recipients with features of acute cellular rejection on liver biopsy compared to recipients with features of recurrent hepatitis C (P < 0.001)[74]. Hepatitis C recurrence has been associated with genes associated with cytotoxic T cells profile and acute cellular rejection was associated with an inflammatory response gene profile[75]. Increased expression of miRNA-146a, miRNA-19a, miRNA-20a, and miRNA-let7e was seen in hepatitis C recipients with slow fibrosis progression[76]. Molecular profiling is not widespread and needs further validation for distinguishing rejection from hepatitis C recurrence or for identifying hepatitis C recipients at greatest risk for progressing to cirrhosis.
TREATMENT OF RECURRENT HCV AFTER TRANSPLANT
Cirrhosis develops in approximately 20% of HCV+ transplant recipients within 5 years of LT[77,78]. The 5-year survival following a liver transplant for HCV is significantly worse than for non-HCV related disease (69.9% vs 76.6%, P < 0.0001)[79]. Furthermore, only 25% of patients treated with standard dual therapy following transplant (PEG-IFN and RBV) achieve an SVR. Thus, the impact of direct acting antiviral therapy (DAA) for HCV cirrhotic patients awaiting transplant and for patients with recurrent disease requiring treatment, is potentially life prolonging - if not life altering.
Preventing allograft reinfection by treating HCV+ patients awaiting transplant will continue to be a key objective. As discussed above, clinical trials with dual therapy (PEG-IFN and RBV) in HCV+ patients awaiting transplant have had disappointing rates of SVR following LT (Table 1). Triple therapy (PEG-IFN, RBV, and either TVR or BOC), even in compensated cirrhotics, is poorly tolerated and risks serious adverse outcomes in Child’s A patients[8]. Interferon free trials with DAAs in patients awaiting transplant is an exciting area of research. One such trial recently presented in abstract form is (NCT01559844), an open-label study with the NS5B polymerase inhibitor, sofosbuvir, and RBV, which was designed specifically to determine the SVR rate 12 wk following LT in patients with HCV and HCC awaiting transplant[80]. Of the 26 patients who received sofosbuvir and RBV prior to transplant and who had HCV RNA levels < 25 IU/mL just prior to LT, 18 (69%) attained an SVR 12-wk following LT. Based upon this compelling data, sofosbuvir gained FDA approval with an additional indication for treatment in the pre-transplant setting for up to 48 wk in combination with RBV in chronic hepatitis C patients with HCC awaiting LT. This strategy promises to have an immediate and profound impact on HCV+ transplant recipients. Finally, antibody therapy directed against the HCV envelope protein is another tactic under investigation to prevent allograft reinfection which may yet prove efficacious particularly in combination with DAAs[81].
No data supports pre-emptive antiviral therapy in the first six months after LT for HCV[82]. Nevertheless, this approach will likely be reevaluated when safer oral therapies are available. Early post-transplant therapy is reserved for patients with FCH, who are at risk for rapid graft failure without treatment. Unfortunately, PEG-IFN and RBV are rarely successful for FCH, and the addition of boceprevir or telaprevir has not afforded dramatically improved response rates, either. Moreover, triple therapy is even harder for these typically decompensated patients to tolerate. The pharmaceutical industry, recognizing the high mortality associated with a diagnosis of FCH has made some of their DAAs available to physicians on a compassionate use basis with some excellent reported outcomes[83,84].
Apart from FCH, antiviral therapy for recurrent HCV after liver transplant is typically reserved for those patients with at least stage 2 fibrosis and/or moderate to severe necroinflammatory activity on liver biopsy. A meta-analysis by Wang et al[85] evaluated the efficacy of PEG-IFN and RBV after transplant and found a pooled SVR rate of 27% and a pooled discontinuation rate of 26%. The decision to begin dual therapy must also weigh the risk of immune-mediated graft dysfunction developing from exposure to PEG-IFN[86]. Historically, maintenance therapy with long-term, low-dose PEG-IFN, with or without RBV, has been used with a hope of delaying progression of fibrosis in recurrent HCV patients who do not attain an SVR with treatment after transplant, but do achieve a reduction in viral load and improvement in LFTs with treatment. However, no clinical trial has demonstrated a benefit of this approach.
Given the unsatisfactory SVR rates for standard therapy, it is not surprising that a flurry of off-label use of TVR and BOC began when the first generation protease inhibitors became available for genotype 1 patients. Triple therapy after transplant is particularly challenging since TVR and BOC inhibit cytochrome P450 3A4, which is responsible for the metabolism of CsA and tacrolimus. CsA levels increased approximately 4.6-fold following co-administration with TVR in healthy controls subjects while the corresponding number with tacrolimus was 70-fold[87]. An analysis of pharmacokinetic data with TVR 1125 mg BID combined with PEG-IFN/RBV in liver transplant recipients requiring HCV therapy was recently presented[88]. Among the 19 subjects, 16 were maintained on tacrolimus. With co-administration of TVR, the average dose of tacrolimus was 0.5 mg given at an average interval of every 168 h. (range = 96-607 h). Transplant centers are carefully utilizing triple therapy, often, but not exclusively, after converting patients from tacrolimus to CsA. Pungpapong et al[89] recently reported the Mayo experience of 66 patients who received triple therapy. TVR was given for 12 wk in combination with PEG-IFN (starting dose 135 μg weekly) and RBV, followed by PEG-IFN and RBV for 36 wk. The CsA dose was reduced by 75%-100%. Sixty-seven percent (14/21) of TVR-treated patients had an undetectable HCV RNA at week 24 in this preliminary analysis. Forty-five percent (10/22) of the BOC-treated patients had undetectable HCV RNA at week 24. Dose reductions of PEG-IFN were required for leukopenia in 75% of patients and RBV dose reductions occurred in all but 4 of the 66 study patients. Recent results from the CRUSH-C consortium confirm similar efficacy with triple therapy[90] (Figure 2). Ninety-six percent of the 112 patients studied had a lead-in period of dual therapy prior to beginning TVR (88%) or BOC (12%). The more frequent calcineurin inhibitor used was CsA (61%) with an average dose reduction of 75%. The median time from liver transplant for the population was 3.7 years and 84% had ≥ stage 2 fibrosis. Forty-three patients had follow-up of sufficient duration to measure an HCV viral load 4 wk (SVR4) after completing 48 wk. The SVR4 in this group was 65%. Ideally, the natural history of hepatitis C infection after transplant will change significantly with the availability of DAA, but these rates are encouraging for those patients who have not had the luxury of waiting for the newer agents.
Figure 2.
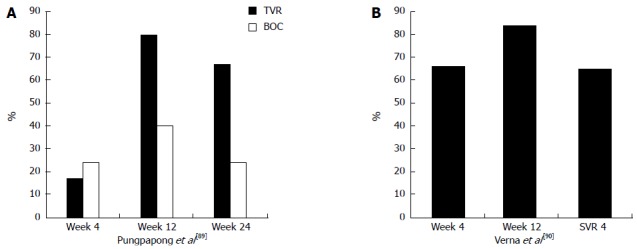
Percent of patients with hepatitis C virus RNA. A: Percent of patients with undetectable hepatitis C virus (HCV) RNA treated with transplant viral clearance (TVR) or boceprevir (BOC) plus pegylated-interferon/ribavirin post-liver transplantation[35]; B: Percent of patients on triple therapy (88%, TVR and 12%, BOC) with HCV RNA below level of detection. Sustained viral response rate (SVR4) refers to percent of patients with undetectable HCV RNA 4 wk following completion of 48 wk of treatment[36].
RE-TRANSPLANT FOR HEPATITIS C
While re-transplantation for primary non-function or hepatic artery thrombosis is generally accepted, re-transplant for allograft failure due to recurrent HCV is a more contentious issue. A United States study group comprising 11 transplant centers compared survival after re-transplantation in patients with recurrent HCV and those re-transplanted for other indications. The overall 1-year and 3-year survival rates were lower, but not significantly different, for patients re-transplanted for recurrent HCV (1 year, 69%, 3 year, 49%) compared to those re-transplanted for other causes (1 year, 73%, 3 year, 55%, P = 0.74). None of the 4 patients re-transplanted FCH were survived 1 year after re-transplant. Most notably, there seemed to be little enthusiasm for evaluating patients with HCV for re-transplant. Thirty percent of patients with allograft failure from recurrent HCV were not considered for re-transplant and only half of those evaluated for re-transplant were listed for transplant. The most common problems precluding re-transplant were recurrent HCV within 6 mo (22%), FCH (19%), and renal insufficiency (9%)[91].
There have been multiple studies evaluating risk factors of mortality following re-transplantation for HCV. Ghabril et al[92] evaluated 1034 HCV-infected patients and 1249 non-HCV-infected patients who underwent re-transplantation. Based on multivariate analysis, the independent predictors of mortality were recipient age, MELD score > 25, re-transplant during the first year after LT, donor age > 60, and a warm ischemia time of ≥ 75 min. Predictive models have been evaluated to select re-transplant candidates with the best potential outcomes. One such score was devised by focusing on HCV-infected patients from a large registry population. Variables included donor age, recipient age, creatinine, albumin, INR at the second transplant, and the interval between transplants. However, the receiver operating characteristic area under curve was a disappointing 0.643 at 3 years[93]. Though some of the above-mentioned risk factors are modifiable, performing re-transplants in patients with lower MELD scores using high quality donors may not be feasible given the donor shortage.
CONCLUSION
For more than a decade clinicians managing patients with hepatitis C awaiting liver transplant or who have had a liver transplant have been challenged in treating these patients. Antiviral therapy has been associated with substantial side effects with only modest efficacy. In addition, some liver transplant recipients develop rapid fibrosis progression while others coexist with the virus for years seemingly without any significant problems. Few modifiable factors have been identified to distinguish the two groups, although molecular markers hold promise as a predictive tool for fibrosis progression. The development of potent direct acting antiviral agents will hopefully obviate the need for interferon, and in the long-term provide a panacea that fundamentally changes the outcome for patients infected with this virus.
Footnotes
P- Reviewer: Kornberg A, Mizuno S, Ohkohchi N, Sugawara Y, Yin DP, Zezos P S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
References
- 1.Everson GT, Trotter J, Forman L, Kugelmas M, Halprin A, Fey B, Ray C. Treatment of advanced hepatitis C with a low accelerating dosage regimen of antiviral therapy. Hepatology. 2005;42:255–262. doi: 10.1002/hep.20793. [DOI] [PubMed] [Google Scholar]
- 2.Carrión JA, Martínez-Bauer E, Crespo G, Ramírez S, Pérez-del-Pulgar S, García-Valdecasas JC, Navasa M, Forns X. Antiviral therapy increases the risk of bacterial infections in HCV-infected cirrhotic patients awaiting liver transplantation: A retrospective study. J Hepatol. 2009;50:719–728. doi: 10.1016/j.jhep.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Everson GT, Terrault NA, Lok AS, Rodrigo del R, Brown RS, Saab S, Shiffman ML, Al-Osaimi AM, Kulik LM, Gillespie BW, et al. A randomized controlled trial of pretransplant antiviral therapy to prevent recurrence of hepatitis C after liver transplantation. Hepatology. 2013;57:1752–1762. doi: 10.1002/hep.25976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–2416. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 5.Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195–1206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, Focaccia R, Younossi Z, Foster GR, Horban A, et al. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417–2428. doi: 10.1056/NEJMoa1013086. [DOI] [PubMed] [Google Scholar]
- 7.Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207–1217. doi: 10.1056/NEJMoa1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hézode C, Fontaine H, Dorival C, Larrey D, Zoulim F, Canva V, de Ledinghen V, Poynard T, Samuel D, Bourlière M, et al. Triple therapy in treatment-experienced patients with HCV-cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS CO20-CUPIC) - NCT01514890. J Hepatol. 2013;59:434–441. doi: 10.1016/j.jhep.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 9.Charlton M. Liver biopsy, viral kinetics, and the impact of viremia on severity of hepatitis C virus recurrence. Liver Transpl. 2003;9:S58–S62. doi: 10.1053/jlts.2003.50245. [DOI] [PubMed] [Google Scholar]
- 10.Neumann UP, Berg T, Bahra M, Seehofer D, Langrehr JM, Neuhaus R, Radke C, Neuhaus P. Fibrosis progression after liver transplantation in patients with recurrent hepatitis C. J Hepatol. 2004;41:830–836. doi: 10.1016/j.jhep.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 11.Firpi RJ, Clark V, Soldevila-Pico C, Morelli G, Cabrera R, Levy C, Machicao VI, Chaoru C, Nelson DR. The natural history of hepatitis C cirrhosis after liver transplantation. Liver Transpl. 2009;15:1063–1071. doi: 10.1002/lt.21784. [DOI] [PubMed] [Google Scholar]
- 12.Wiesner RH, Sorrell M, Villamil F. Report of the first International Liver Transplantation Society expert panel consensus conference on liver transplantation and hepatitis C. Liver Transpl. 2003;9:S1–S9. doi: 10.1053/jlts.2003.50268. [DOI] [PubMed] [Google Scholar]
- 13.Lake JR, Shorr JS, Steffen BJ, Chu AH, Gordon RD, Wiesner RH. Differential effects of donor age in liver transplant recipients infected with hepatitis B, hepatitis C and without viral hepatitis. Am J Transplant. 2005;5:549–557. doi: 10.1111/j.1600-6143.2005.00741.x. [DOI] [PubMed] [Google Scholar]
- 14.Condron SL, Heneghan MA, Patel K, Dev A, McHutchison JG, Muir AJ. Effect of donor age on survival of liver transplant recipients with hepatitis C virus infection. Transplantation. 2005;80:145–148. doi: 10.1097/01.tp.0000164291.35925.7a. [DOI] [PubMed] [Google Scholar]
- 15.Verna EC, Abdelmessih R, Salomao MA, Lefkowitch J, Moreira RK, Brown RS. Cholestatic hepatitis C following liver transplantation: an outcome-based histological definition, clinical predictors, and prognosis. Liver Transpl. 2013;19:78–88. doi: 10.1002/lt.23559. [DOI] [PubMed] [Google Scholar]
- 16.Selzner M, Clavien PA. Fatty liver in liver transplantation and surgery. Semin Liver Dis. 2001;21:105–113. doi: 10.1055/s-2001-12933. [DOI] [PubMed] [Google Scholar]
- 17.Imber CJ, St Peter SD, Handa A, Friend PJ. Hepatic steatosis and its relationship to transplantation. Liver Transpl. 2002;8:415–423. doi: 10.1053/jlts.2002.32275. [DOI] [PubMed] [Google Scholar]
- 18.Burra P, Loreno M, Russo FP, Germani G, Galligioni A, Senzolo M, Cillo U, Zanus G, Fagiuoli S, Rugge M. Donor livers with steatosis are safe to use in hepatitis C virus-positive recipients. Liver Transpl. 2009;15:619–628. doi: 10.1002/lt.21761. [DOI] [PubMed] [Google Scholar]
- 19.Briceño J, Ciria R, Pleguezuelo M, de la Mata M, Muntané J, Naranjo A, Sánchez-Hidalgo J, Marchal T, Rufián S, López-Cillero P. Impact of donor graft steatosis on overall outcome and viral recurrence after liver transplantation for hepatitis C virus cirrhosis. Liver Transpl. 2009;15:37–48. doi: 10.1002/lt.21566. [DOI] [PubMed] [Google Scholar]
- 20.Yilmaz N, Shiffman ML. Impact of the donor liver with steatosis in patients with hepatitis C virus: not so FAst. Liver Transpl. 2009;15:4–6. doi: 10.1002/lt.21661. [DOI] [PubMed] [Google Scholar]
- 21.Guo L, Orrego M, Rodriguez-Luna H, Balan V, Byrne T, Chopra K, Douglas DD, Harrison E, Moss A, Reddy KS, et al. Living donor liver transplantation for hepatitis C-related cirrhosis: no difference in histological recurrence when compared to deceased donor liver transplantation recipients. Liver Transpl. 2006;12:560–565. doi: 10.1002/lt.20660. [DOI] [PubMed] [Google Scholar]
- 22.Shiffman ML, Stravitz RT, Contos MJ, Mills AS, Sterling RK, Luketic VA, Sanyal AJ, Cotterell A, Maluf D, Posner MP, et al. Histologic recurrence of chronic hepatitis C virus in patients after living donor and deceased donor liver transplantation. Liver Transpl. 2004;10:1248–1255. doi: 10.1002/lt.20232. [DOI] [PubMed] [Google Scholar]
- 23.Selzner N, Girgrah N, Lilly L, Guindi M, Selzner M, Therapondos G, Adeyi O, McGilvray I, Cattral M, Greig PD, et al. The difference in the fibrosis progression of recurrent hepatitis C after live donor liver transplantation versus deceased donor liver transplantation is attributable to the difference in donor age. Liver Transpl. 2008;14:1778–1786. doi: 10.1002/lt.21598. [DOI] [PubMed] [Google Scholar]
- 24.Humar A, Horn K, Kalis A, Glessing B, Payne WD, Lake J. Living donor and split-liver transplants in hepatitis C recipients: does liver regeneration increase the risk for recurrence? Am J Transplant. 2005;5:399–405. doi: 10.1111/j.1600-6143.2004.00704.x. [DOI] [PubMed] [Google Scholar]
- 25.Lawal A, Ghobrial R, Te H, Artinian L, Eastwood D, Schiano TD. Comparison of hepatitis C histological recurrence rates and patient survival between split and deceased donor liver transplantation. Transplant Proc. 2007;39:3261–3265. doi: 10.1016/j.transproceed.2007.08.106. [DOI] [PubMed] [Google Scholar]
- 26.Yoshizawa A, Takada Y, Fujimoto Y, Koshiba T, Haga H, Nabeshima S, Uemoto S. Liver transplantation from an identical twin without immunosuppression, with early recurrence of hepatitis C. Am J Transplant. 2006;6:2812–2816. doi: 10.1111/j.1600-6143.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- 27.Watt KD, Lyden ER, Gulizia JM, McCashland TM. Recurrent hepatitis C posttransplant: early preservation injury may predict poor outcome. Liver Transpl. 2006;12:134–139. doi: 10.1002/lt.20583. [DOI] [PubMed] [Google Scholar]
- 28.Killackey MT, Gondolesi GE, Liu LU, Paramesh AS, Thung SN, Suriawinata A, Nguyen E, Roayaie S, Schwartz ME, Emre S, et al. Effect of ischemia-reperfusion on the incidence of acute cellular rejection and timing of histologic hepatitis C virus recurrence after liver transplantation. Transplant Proc. 2008;40:1504–1510. doi: 10.1016/j.transproceed.2008.03.101. [DOI] [PubMed] [Google Scholar]
- 29.Taner CB, Bulatao IG, Keaveny AP, Willingham DL, Pungpapong S, Perry DK, Rosser BG, Harnois DM, Aranda-Michel J, Nguyen JH. Use of liver grafts from donation after cardiac death donors for recipients with hepatitis C virus. Liver Transpl. 2011;17:641–649. doi: 10.1002/lt.22258. [DOI] [PubMed] [Google Scholar]
- 30.Marroquin CE, Marino G, Kuo PC, Plotkin JS, Rustgi VK, Lu AD, Edwards E, Taranto S, Johnson LB. Transplantation of hepatitis C-positive livers in hepatitis C-positive patients is equivalent to transplanting hepatitis C-negative livers. Liver Transpl. 2001;7:762–768. doi: 10.1053/jlts.2001.27088. [DOI] [PubMed] [Google Scholar]
- 31.Khapra AP, Agarwal K, Fiel MI, Kontorinis N, Hossain S, Emre S, Schiano TD. Impact of donor age on survival and fibrosis progression in patients with hepatitis C undergoing liver transplantation using HCV+ allografts. Liver Transpl. 2006;12:1496–1503. doi: 10.1002/lt.20849. [DOI] [PubMed] [Google Scholar]
- 32.Lai JC, O’Leary JG, Trotter JF, Verna EC, Brown RS, Stravitz RT, Duman JD, Forman LM, Terrault NA. Risk of advanced fibrosis with grafts from hepatitis C antibody-positive donors: a multicenter cohort study. Liver Transpl. 2012;18:532–538. doi: 10.1002/lt.23396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Northup PG, Argo CK, Nguyen DT, McBride MA, Kumer SC, Schmitt TM, Pruett TL. Liver allografts from hepatitis C positive donors can offer good outcomes in hepatitis C positive recipients: a US National Transplant Registry analysis. Transpl Int. 2010;23:1038–1044. doi: 10.1111/j.1432-2277.2010.01092.x. [DOI] [PubMed] [Google Scholar]
- 34.Charlton M, Seaberg E, Wiesner R, Everhart J, Zetterman R, Lake J, Detre K, Hoofnagle J. Predictors of patient and graft survival following liver transplantation for hepatitis C. Hepatology. 1998;28:823–830. doi: 10.1002/hep.510280333. [DOI] [PubMed] [Google Scholar]
- 35.Charlton M, Ruppert K, Belle SH, Bass N, Schafer D, Wiesner RH, Detre K, Wei Y, Everhart J. Long-term results and modeling to predict outcomes in recipients with HCV infection: results of the NIDDK liver transplantation database. Liver Transpl. 2004;10:1120–1130. doi: 10.1002/lt.20211. [DOI] [PubMed] [Google Scholar]
- 36.Sreekumar R, Gonzalez-Koch A, Maor-Kendler Y, Batts K, Moreno-Luna L, Poterucha J, Burgart L, Wiesner R, Kremers W, Rosen C, et al. Early identification of recipients with progressive histologic recurrence of hepatitis C after liver transplantation. Hepatology. 2000;32:1125–1130. doi: 10.1053/jhep.2000.19340. [DOI] [PubMed] [Google Scholar]
- 37.Hanouneh IA, Feldstein AE, McCullough AJ, Miller C, Aucejo F, Yerian L, Lopez R, Zein NN. The significance of metabolic syndrome in the setting of recurrent hepatitis C after liver transplantation. Liver Transpl. 2008;14:1287–1293. doi: 10.1002/lt.21524. [DOI] [PubMed] [Google Scholar]
- 38.Burak KW, Kremers WK, Batts KP, Wiesner RH, Rosen CB, Razonable RR, Paya CV, Charlton MR. Impact of cytomegalovirus infection, year of transplantation, and donor age on outcomes after liver transplantation for hepatitis C. Liver Transpl. 2002;8:362–369. doi: 10.1053/jlts.2002.32282. [DOI] [PubMed] [Google Scholar]
- 39.Nebbia G, Mattes FM, Cholongitas E, Garcia-Diaz A, Samonakis DN, Burroughs AK, Emery VC. Exploring the bidirectional interactions between human cytomegalovirus and hepatitis C virus replication after liver transplantation. Liver Transpl. 2007;13:130–135. doi: 10.1002/lt.21037. [DOI] [PubMed] [Google Scholar]
- 40.Terrault NA, Roland ME, Schiano T, Dove L, Wong MT, Poordad F, Ragni MV, Barin B, Simon D, Olthoff KM, et al. Outcomes of liver transplant recipients with hepatitis C and human immunodeficiency virus coinfection. Liver Transpl. 2012;18:716–726. doi: 10.1002/lt.23411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duclos-Vallée JC, Féray C, Sebagh M, Teicher E, Roque-Afonso AM, Roche B, Azoulay D, Adam R, Bismuth H, Castaing D, et al. Survival and recurrence of hepatitis C after liver transplantation in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology. 2008;47:407–417. doi: 10.1002/hep.21990. [DOI] [PubMed] [Google Scholar]
- 42.Lai JC, Verna EC, Brown RS, O’Leary JG, Trotter JF, Forman LM, Duman JD, Foster RG, Stravitz RT, Terrault NA. Hepatitis C virus-infected women have a higher risk of advanced fibrosis and graft loss after liver transplantation than men. Hepatology. 2011;54:418–424. doi: 10.1002/hep.24390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pang PS, Kamal A, Glenn JS. The effect of donor race on the survival of Black Americans undergoing liver transplantation for chronic hepatitis C. Liver Transpl. 2009;15:1126–1132. doi: 10.1002/lt.21835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saxena V, Lai JC, O’Leary JG, Verna EC, Brown RS, Stravitz RT, Trotter JF, Krishnan K, Terrault NA. Recipient-donor race mismatch for African American liver transplant patients with chronic hepatitis C. Liver Transpl. 2012;18:524–531. doi: 10.1002/lt.22461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Layden JE, Cotler SJ, Grim SA, Fischer MJ, Lucey MR, Clark NM. Impact of donor and recipient race on survival after hepatitis C-related liver transplantation. Transplantation. 2012;93:444–449. doi: 10.1097/TP.0b013e3182406a94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veldt BJ, Poterucha JJ, Watt KD, Wiesner RH, Hay JE, Rosen CB, Heimbach JK, Janssen HL, Charlton MR. Insulin resistance, serum adipokines and risk of fibrosis progression in patients transplanted for hepatitis C. Am J Transplant. 2009;9:1406–1413. doi: 10.1111/j.1600-6143.2009.02642.x. [DOI] [PubMed] [Google Scholar]
- 47.Brandman D, Pingitore A, Lai JC, Roberts JP, Ferrell L, Bass NM, Terrault NA. Hepatic steatosis at 1 year is an additional predictor of subsequent fibrosis severity in liver transplant recipients with recurrent hepatitis C virus. Liver Transpl. 2011;17:1380–1386. doi: 10.1002/lt.22389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coto-Llerena M, Pérez-Del-Pulgar S, Crespo G, Carrión JA, Martínez SM, Sánchez-Tapias JM, Martorell J, Navasa M, Forns X. Donor and recipient IL28B polymorphisms in HCV-infected patients undergoing antiviral therapy before and after liver transplantation. Am J Transplant. 2011;11:1051–1057. doi: 10.1111/j.1600-6143.2011.03491.x. [DOI] [PubMed] [Google Scholar]
- 49.Eurich D, Boas-Knoop S, Bahra M, Neuhaus R, Somasundaram R, Neuhaus P, Neumann U, Seehofer D. Role of IL28B polymorphism in the development of hepatitis C virus-induced hepatocellular carcinoma, graft fibrosis, and posttransplant antiviral therapy. Transplantation. 2012;93:644–649. doi: 10.1097/TP.0b013e318244f774. [DOI] [PubMed] [Google Scholar]
- 50.Duarte-Rojo A, Veldt BJ, Goldstein DD, Tillman HL, Watt KD, Heimbach JK, McHutchison JG, Poterucha JJ, Vargas-Vorackova F, Charlton MR. The course of posttransplant hepatitis C infection: comparative impact of donor and recipient source of the favorable IL28B genotype and other variables. Transplantation. 2012;94:197–203. doi: 10.1097/TP.0b013e3182547551. [DOI] [PubMed] [Google Scholar]
- 51.Allam SR, Krüger B, Mehrotra A, Schiano T, Schröppel B, Murphy B. The association of IL28B polymorphism and graft survival in patients with hepatitis C undergoing liver transplantation. PLoS One. 2013;8:e54854. doi: 10.1371/journal.pone.0054854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duarte-Rojo A, Deneke MG, Charlton MR. Interleukin-28B polymorphism in hepatitis C and liver transplantation. Liver Transpl. 2013;19:49–58. doi: 10.1002/lt.23554. [DOI] [PubMed] [Google Scholar]
- 53.Sheiner PA, Schwartz ME, Mor E, Schluger LK, Theise N, Kishikawa K, Kolesnikov V, Bodenheimer H, Emre S, Miller CM. Severe or multiple rejection episodes are associated with early recurrence of hepatitis C after orthotopic liver transplantation. Hepatology. 1995;21:30–34. [PubMed] [Google Scholar]
- 54.Gedaly R, Clifford TM, McHugh PP, Jeon H, Johnston TD, Ranjan D. Prevalent immunosuppressive strategies in liver transplantation for hepatitis C: results of a multi-center international survey. Transpl Int. 2008;21:867–872. doi: 10.1111/j.1432-2277.2008.00699.x. [DOI] [PubMed] [Google Scholar]
- 55.Segev DL, Sozio SM, Shin EJ, Nazarian SM, Nathan H, Thuluvath PJ, Montgomery RA, Cameron AM, Maley WR. Steroid avoidance in liver transplantation: meta-analysis and meta-regression of randomized trials. Liver Transpl. 2008;14:512–525. doi: 10.1002/lt.21396. [DOI] [PubMed] [Google Scholar]
- 56.Takada Y, Kaido T, Asonuma K, Sakurai H, Kubo S, Kiuchi T, Inomata Y, Isaji S, Tsumura H, Teramukai S, et al. Randomized, multicenter trial comparing tacrolimus plus mycophenolate mofetil to tacrolimus plus steroids in hepatitis C virus-positive recipients of living donor liver transplantation. Liver Transpl. 2013;19:896–906. doi: 10.1002/lt.23679. [DOI] [PubMed] [Google Scholar]
- 57.Levy G, Villamil F, Samuel D, Sanjuan F, Grazi GL, Wu Y, Marotta P, Boillot O, Muehlbacher F, Klintmalm G. Results of lis2t, a multicenter, randomized study comparing cyclosporine microemulsion with C2 monitoring and tacrolimus with C0 monitoring in de novo liver transplantation. Transplantation. 2004;77:1632–1638. doi: 10.1097/01.tp.0000129095.51031.42. [DOI] [PubMed] [Google Scholar]
- 58.Berenguer M, Royuela A, Zamora J. Immunosuppression with calcineurin inhibitors with respect to the outcome of HCV recurrence after liver transplantation: results of a meta-analysis. Liver Transpl. 2007;13:21–29. doi: 10.1002/lt.21035. [DOI] [PubMed] [Google Scholar]
- 59.Berenguer M, Aguilera V, San Juan F, Benlloch S, Rubin A, López-Andujar R, Moya A, Pareja E, Montalva E, Yago M, et al. Effect of calcineurin inhibitors in the outcome of liver transplantation in hepatitis C virus-positive recipients. Transplantation. 2010;90:1204–1209. doi: 10.1097/TP.0b013e3181fa93fa. [DOI] [PubMed] [Google Scholar]
- 60.Firpi RJ, Zhu H, Morelli G, Abdelmalek MF, Soldevila-Pico C, Machicao VI, Cabrera R, Reed AI, Liu C, Nelson DR. Cyclosporine suppresses hepatitis C virus in vitro and increases the chance of a sustained virological response after liver transplantation. Liver Transpl. 2006;12:51–57. doi: 10.1002/lt.20532. [DOI] [PubMed] [Google Scholar]
- 61.Firpi RJ, Soldevila-Pico C, Morelli GG, Cabrera R, Levy C, Clark VC, Suman A, Michaels A, Chen C, Nelson DR. The use of cyclosporine for recurrent hepatitis C after liver transplant: a randomized pilot study. Dig Dis Sci. 2010;55:196–203. doi: 10.1007/s10620-009-0981-3. [DOI] [PubMed] [Google Scholar]
- 62.Watt KD, Dierkhising R, Heimbach JK, Charlton MR. Impact of sirolimus and tacrolimus on mortality and graft loss in liver transplant recipients with or without hepatitis C virus: an analysis of the Scientific Registry of Transplant Recipients Database. Liver Transpl. 2012;18:1029–1036. doi: 10.1002/lt.23479. [DOI] [PubMed] [Google Scholar]
- 63.Levy G, Schmidli H, Punch J, Tuttle-Newhall E, Mayer D, Neuhaus P, Samuel D, Nashan B, Klempnauer J, Langnas A, et al. Safety, tolerability, and efficacy of everolimus in de novo liver transplant recipients: 12- and 36-month results. Liver Transpl. 2006;12:1640–1648. doi: 10.1002/lt.20707. [DOI] [PubMed] [Google Scholar]
- 64.De Simone P, Nevens F, De Carlis L, Metselaar HJ, Beckebaum S, Saliba F, Jonas S, Sudan D, Fung J, Fischer L, et al. Everolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: a randomized controlled trial. Am J Transplant. 2012;12:3008–3020. doi: 10.1111/j.1600-6143.2012.04212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wiesner RH, Shorr JS, Steffen BJ, Chu AH, Gordon RD, Lake JR. Mycophenolate mofetil combination therapy improves long-term outcomes after liver transplantation in patients with and without hepatitis C. Liver Transpl. 2005;11:750–759. doi: 10.1002/lt.20453. [DOI] [PubMed] [Google Scholar]
- 66.Belli LS, Burroughs AK, Burra P, Alberti AB, Samonakis D, Cammà C, De Carlis L, Minola E, Quaglia A, Zavaglia C, et al. Liver transplantation for HCV cirrhosis: improved survival in recent years and increased severity of recurrent disease in female recipients: results of a long term retrospective study. Liver Transpl. 2007;13:733–740. doi: 10.1002/lt.21093. [DOI] [PubMed] [Google Scholar]
- 67.Ghanekar A, Kashfi A, Cattral M, Selzner N, McGilvray I, Selzner M, Renner E, Lilly L, Levy G, Grant D, et al. Routine induction therapy in living donor liver transplantation prevents rejection but may promote recurrence of hepatitis C. Transplant Proc. 2012;44:1351–1356. doi: 10.1016/j.transproceed.2012.01.117. [DOI] [PubMed] [Google Scholar]
- 68.Lladó L, Fabregat J, Castellote J, Ramos E, Xiol X, Torras J, Serrano T, Baliellas C, Figueras J, Garcia-Gil A, et al. Impact of immunosuppression without steroids on rejection and hepatitis C virus evolution after liver transplantation: results of a prospective randomized study. Liver Transpl. 2008;14:1752–1760. doi: 10.1002/lt.21629. [DOI] [PubMed] [Google Scholar]
- 69.Rosen HR, Shackleton CR, Higa L, Gralnek IM, Farmer DA, McDiarmid SV, Holt C, Lewin KJ, Busuttil RW, Martin P. Use of OKT3 is associated with early and severe recurrence of hepatitis C after liver transplantation. Am J Gastroenterol. 1997;92:1453–1457. [PubMed] [Google Scholar]
- 70.Berenguer M, Rayón JM, Prieto M, Aguilera V, Nicolás D, Ortiz V, Carrasco D, López-Andujar R, Mir J, Berenguer J. Are posttransplantation protocol liver biopsies useful in the long term? Liver Transpl. 2001;7:790–796. doi: 10.1053/jlts.2001.23794. [DOI] [PubMed] [Google Scholar]
- 71.Firpi RJ, Abdelmalek MF, Soldevila-Pico C, Cabrera R, Shuster JJ, Theriaque D, Reed AI, Hemming AW, Liu C, Crawford JM, et al. One-year protocol liver biopsy can stratify fibrosis progression in liver transplant recipients with recurrent hepatitis C infection. Liver Transpl. 2004;10:1240–1247. doi: 10.1002/lt.20238. [DOI] [PubMed] [Google Scholar]
- 72.Demetris AJ. Evolution of hepatitis C virus in liver allografts. Liver Transpl. 2009;15 Suppl 2:S35–S41. doi: 10.1002/lt.21890. [DOI] [PubMed] [Google Scholar]
- 73.do O NT, Eurich D, Schmitz P, Schmeding M, Heidenhain C, Bahra M, Trautwein C, Neuhaus P, Neumann UP, Wasmuth HE. A 7-gene signature of the recipient predicts the progression of fibrosis after liver transplantation for hepatitis C virus infection. Liver Transpl. 2012;18:298–304. doi: 10.1002/lt.22475. [DOI] [PubMed] [Google Scholar]
- 74.Cabrera R, Ararat M, Soldevila-Pico C, Dixon L, Pan JJ, Firpi R, Machicao V, Levy C, Nelson D, Morelli G. Using an immune functional assay to differentiate acute cellular rejection from recurrent hepatitis C in liver transplant patients. Liver Transpl. 2009;15:216–222. doi: 10.1002/lt.21666. [DOI] [PubMed] [Google Scholar]
- 75.Gehrau R, Maluf D, Archer K, Stravitz R, Suh J, Le N, Mas V. Molecular pathways differentiate hepatitis C virus (HCV) recurrence from acute cellular rejection in HCV liver recipients. Mol Med. 2011;17:824–833. doi: 10.2119/molmed.2011.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Joshi D, Salehi S, Brereton H, Arno M, Quaglia A, Heaton N, O’Grady J, Agarwal K, Aluvihare V. Distinct microRNA profiles are associated with the severity of hepatitis C virus recurrence and acute cellular rejection after liver transplantation. Liver Transpl. 2013;19:383–394. doi: 10.1002/lt.23613. [DOI] [PubMed] [Google Scholar]
- 77.Pelletier SJ, Iezzoni JC, Crabtree TD, Hahn YS, Sawyer RG, Pruett TL. Prediction of liver allograft fibrosis after transplantation for hepatitis C virus: persistent elevation of serum transaminase levels versus necroinflammatory activity. Liver Transpl. 2000;6:44–53. doi: 10.1002/lt.500060111. [DOI] [PubMed] [Google Scholar]
- 78.Berenguer M, Ferrell L, Watson J, Prieto M, Kim M, Rayón M, Córdoba J, Herola A, Ascher N, Mir J, et al. HCV-related fibrosis progression following liver transplantation: increase in recent years. J Hepatol. 2000;32:673–684. doi: 10.1016/s0168-8278(00)80231-7. [DOI] [PubMed] [Google Scholar]
- 79.Forman LM, Lewis JD, Berlin JA, Feldman HI, Lucey MR. The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology. 2002;122:889–896. doi: 10.1053/gast.2002.32418. [DOI] [PubMed] [Google Scholar]
- 80.Curry MP, Forns X, Chung RT, Terrault NA, Brown RS, Fenkel JM, Gordon FD, O’Leary JG, Kuo A, Schiano T, et al. Pretransplant Sofosbuvir and Ribavirin to Prevent Recurrence of HCV Infection after Liver Transplantation [oral presentation] Hepatology. 2013;58(S1):36A–91A. doi: 10.1053/j.gastro.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 81.Chung RT, Gordon FD, Curry MP, Schiano TD, Emre S, Corey K, Markmann JF, Hertl M, Pomposelli JJ, Pomfret EA, et al. Human monoclonal antibody MBL-HCV1 delays HCV viral rebound following liver transplantation: a randomized controlled study. Am J Transplant. 2013;13:1047–1054. doi: 10.1111/ajt.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bzowej N, Nelson DR, Terrault NA, Everson GT, Teng LL, Prabhakar A, Charlton MR. PHOENIX: A randomized controlled trial of peginterferon alfa-2a plus ribavirin as a prophylactic treatment after liver transplantation for hepatitis C virus. Liver Transpl. 2011;17:528–538. doi: 10.1002/lt.22271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fontana RJ, Hughes EA, Bifano M, Appelman H, Dimitrova D, Hindes R, Symonds WT. Sofosbuvir and daclatasvir combination therapy in a liver transplant recipient with severe recurrent cholestatic hepatitis C. Am J Transplant. 2013;13:1601–1605. doi: 10.1111/ajt.12209. [DOI] [PubMed] [Google Scholar]
- 84.Fontana RJ, Hughes EA, Appelman H, Hindes R, Dimitrova D, Bifano M. Case report of successful peginterferon, ribavirin, and daclatasvir therapy for recurrent cholestatic hepatitis C after liver retransplantation. Liver Transpl. 2012;18:1053–1059. doi: 10.1002/lt.23482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang CS, Ko HH, Yoshida EM, Marra CA, Richardson K. Interferon-based combination anti-viral therapy for hepatitis C virus after liver transplantation: a review and quantitative analysis. Am J Transplant. 2006;6:1586–1599. doi: 10.1111/j.1600-6143.2006.01362.x. [DOI] [PubMed] [Google Scholar]
- 86.Levitsky J, Fiel MI, Norvell JP, Wang E, Watt KD, Curry MP, Tewani S, McCashland TM, Hoteit MA, Shaked A, et al. Risk for immune-mediated graft dysfunction in liver transplant recipients with recurrent HCV infection treated with pegylated interferon. Gastroenterology. 2012;142:1132–1139.e1. doi: 10.1053/j.gastro.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 87.Garg V, van Heeswijk R, Lee JE, Alves K, Nadkarni P, Luo X. Effect of telaprevir on the pharmacokinetics of cyclosporine and tacrolimus. Hepatology. 2011;54:20–27. doi: 10.1002/hep.24443. [DOI] [PubMed] [Google Scholar]
- 88.Vargas HE, Dai Y, Brown KA, Russo MW, Yoshida E, Fontana RJ, Levitsky J, Rubin R, Garg V, Brown RS. Twice Daily Telaprevir in Combination With Peginterferon Alfa-2a/Ribavirin in HCV Genotype 1 Liver Transplant Recipients: Interim Pharmacokinetics of the REFRESH Study [abstract] Am J Transplant. 2013;13(S5):B1062. [Google Scholar]
- 89.Pungpapong S, Aqel BA, Koning L, Murphy JL, Henry TM, Ryland KL, Yataco ML, Satyanarayana R, Rosser BG, Vargas HE, et al. Multicenter experience using telaprevir or boceprevir with peginterferon and ribavirin to treat hepatitis C genotype 1 after liver transplantation. Liver Transpl. 2013;19:690–700. doi: 10.1002/lt.23669. [DOI] [PubMed] [Google Scholar]
- 90.Verna EC, Burton JR, Jr O’Leary JG, Lai JC, Saxena V, Dodge JL, Everson GT, Trotter JF, Stravitz RT, Brown RS. A multicenter study of protease inhibitor-triple therapy in HCV-infected liver transplant recipients: report from the CRUSH-C group [abstract] J Hepatol. 2013;58:S10–S11. doi: 10.1016/j.jhep.2014.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McCashland T, Watt K, Lyden E, Adams L, Charlton M, Smith AD, McGuire BM, Biggins SW, Neff G, Burton JR, et al. Retransplantation for hepatitis C: results of a U.S. multicenter retransplant study. Liver Transpl. 2007;13:1246–1253. doi: 10.1002/lt.21322. [DOI] [PubMed] [Google Scholar]
- 92.Ghabril M, Dickson R, Wiesner R. Improving outcomes of liver retransplantation: an analysis of trends and the impact of Hepatitis C infection. Am J Transplant. 2008;8:404–411. doi: 10.1111/j.1600-6143.2007.02082.x. [DOI] [PubMed] [Google Scholar]
- 93.Andres A, Gerstel E, Combescure C, Asthana S, Merani S, Majno P, Berney T, Morel P, Kneteman N, Mentha G, et al. A score predicting survival after liver retransplantation for hepatitis C virus cirrhosis. Transplantation. 2012;93:717–722. doi: 10.1097/TP.0b013e318246f8b3. [DOI] [PubMed] [Google Scholar]


