Abstract
In liver transplantation, the efficacy of mycophenolate mofetil (MMF) has been confirmed in clinical trials and studies. However, therapeutic drug monitoring for mycophenolic acid (MPA) has not been fully accepted in liver transplantation as no long-term prospective study of concentration controlled vs fixed-dose prescribing of MMF has been done. This review addressed MPA measurement, pharmacokinetic variability and reasons of this variation, exposure related to acute rejection and MMF-associated side effects in liver transplant recipients. Limited sampling strategies to predict MPA area under the concentration-time curve have also been described, and the value of clinical use needs to be investigated in future. The published data suggested that a fixed-dosage MMF regimen might not be suitable and monitoring of MPA exposure seems helpful in various clinical settings of liver transplantation.
Keywords: Mycophenolate mofetil, Mycophenolic acid, Pharmacokinetics, Therapeutic drug monitoring, Liver transplantation
Core tip: We discussed the methods of mycophenolic acid (MPA) monitoring, pharmacokinetic characteristics, clinical exposure related to acute rejection and mycophenolate mofetil (MMF) associated side effects in liver transplant recipients. We also introduced the methods of limited sampling strategies to predict the MPA area under the concentration-time curve. It demonstrated that a fixed-dosage MMF regimen might not be suitable. In clinical settings, monitoring of MPA exposure seems reasonable and necessary.
INTRODUCTION
Mycophenolate mofetil (MMF, CellCept, Hoffman-La Roche) has almost full bioavailability by oral intake and is a pro-drug that is hydrolyzed to release mycophenolic acid (MPA)[1]. Subsequently MPA is metabolized to a major phenolic glucuronide, mycophenolic acid glucuronide (MPAG), and a minor acyl glucuronide (AcMPAG)[2-4]. MPA, the active compound of MMF, is a selective, reversible and non-competitive inhibitor of inosine monophosphate dehydrogenase in process of de novo purine synthesis in T and B lymphocytes[5]. As a result nucleic acid synthesis is arrested and immune reaction to allograft is inhibited.
As a major immunosuppressive agent, MPA has been widely used for the prevention of acute rejection in transplant recipients[6]. A dose of 1-1.5 g (fixed-dose) administered orally or intravenously twice a day is recommended for use in renal, cardiac and liver transplant patients in the product leaflet of Hoffman-La Roche Ltd[7]. However, wide inter-patient variability in MPA exposure has been showed in renal, heart and liver transplant patients on a fixed MMF dose[1,8,9]. It is confirmed in renal transplantation that compared with fixed-dose regimen, MPA concentration controlled regimen can reduce the risk of treatment failure and acute rejection in recipients 12 mo post-transplant with no increase in adverse events[10]. Individualizing MMF dose instead of using a fixed dose might be helpful to optimize immunosuppression and minimize potential toxic effects. Carrying out therapeutic drug monitoring (TDM) seems reasonable and necessary and routine monitoring for MPA is increasingly performed. However, the experience with TDM for MPA in liver transplantation is much limited compared to lots of investigations performed in kidney transplant patients. At present, a fixed dose of 1 or 1.5 g twice daily of MMF is the standard protocol in liver transplantation with adjustments only in relation to side effects or to its efficacy[11]. No more MPA monitoring-based guidelines for MMF dosage have been set up[12]. It is necessary to study the MPA pharmacokinetics and to carry out TDM of MMF in liver transplant recipients.
In this review, we will focus on five areas in liver transplant recipients: (1) MPA efficacy and MMF-related side effects; (2) methods for measuring MPA concentration; (3) MPA pharmacokinetics; (4) limited sampling strategy (LSS); and (5) MPA concentration-effect relationship.
MPA EFFICACY AND MMF-RELATED SIDE EFFECTS IN LIVR TRANSPLANATION
MMF has been successfully used with a reduced dosage of calcineurin inhibitor (CNI) and steroids to reduce the rate of acute rejection, lessen side effects of CNI after liver transplantation and improve long-term survival rates of allografts and recipients[13-15]. In a randomized double-blind comparative study of MMF and azathioprine in primary liver transplant recipients, the incidence of acute rejection or graft loss was 47.7% in the azathioprine-treated patients and 38.5% in the MMF-treated patients during the first 6 mo after transplantation[16]. Recently, Goralczyk et al[17] reported the results of a systematic review and meta-analysis of randomized controlled trials of CNI sparing with MMF in liver transplantation. The authors obtained the conclusion that de novo use of MMF in combination with low-dose tacrolimus (TAC) is not associated with an increased risk of acute rejection, graft loss, or death and has an acceptable side effect profile. Ringe et al[18] reported that use of TAC plus MMF immunosuppressive regimen without corticosteroids from the beginning after liver transplantation led to a graft survival rate of 83.9 % at 2 years.
MMF has no nephrotoxity and no effect on the lipid profile or other cardiovascular risk factors such as systemic hypertension or diabetes mellitus[19]. MMF has been widely used to improve the renal function commonly associated with CNI[20,21]. Its nephroprotective effect and promotion of allograft tolerance after liver transplantation were confirmed with replaced CNI or reduced or interrupted CNI therapy in three randomized controlled trials[22-24]. Recently, Kriss et al[25] reported that serum creatinine and calculated glomerular filtration rate (GFR) improved in 23 cases on MMF monotherapy compared with 23 recipients remaining on CNI-based therapy. Improvement was significantly pronounced in patients with milder renal dysfunction with a decrease in serum creatinine (1.63 ± 0.29 mg/dL vs 1.34 ± 0.26 mg/dL, P = 0.02) at last follow-up. In a retrospective analysis of pediatric liver transplantation by Evans et al[26], there was a statistically significant increase to a median calculated GFR of 69 (28-114) mL/min per 1.73 m2 by 1 mo and a further increase to a median calculated GFR of 77 (24-105) mL/min per 1.73 m2 by 2 mo with MMF monotherapy or low-dose cyclosporine A (CsA) or TAC, after which time calculated GFR was maintained. MMF treatment provided safe and effective immunosuppression and allowed CsA or TAC to be discontinued or reduced, leading to improvement of renal function.
CNI increased cardiovascular risk after liver transplantation. Aberg et al[27] analyzed the cardiovascular risk of 77 recipients based on CNI and antibodies at 5 years after liver transplantation. At least one cardiovascular risk factor developed in 92% of patients, and the prevalence of treated hypertension, dyslipidemia, overweight, obesity and diabetes were 71%, 61%, 32%, 13% and 10%, respectively. Antibody therapy was associated with a 1.49-fold increase in the risk of hypertension (95%CI: 1.15-1.94) and a 6.43-fold increase in the risk of diabetes. In a randomized prospective study by Junge et al[28], TAC with MMF compared TAC with corticosteroid significantly decreased glucose levels with lower HbA1c and the need for insulin as well as significantly reduced serum cholesterol and the incidence of osteopenia. It was confirmed in some studies that immunosuppressive protocol based on reduced doses of TAC[22,29] or corticosteroids[30] with MMF could improve blood pressure with reduction of antihypertensive medication.
In summary, the protocol using MMF with reduced TAC improves renal function, decreases the cardiovascular risk and avoids steroid-associated adverse effects.
The principal complications of MMF are gastrointertinal effects (nausea, vomiting, abdominal pain and diarrhea) and myelosuppression (leucopenia, anaemia and thrombocytopenia)[19]. In a study by Hao et al[31], 66.7 % of the patients had at least one episode of MMF-related side effects of hematologic disorder (36.51%), gastrointestinal reaction (25.40%) and infection (20.63%) during the study evaluation up to the third post-transplantation month. For 34 of the patients (53.97%), the symptoms disappeared until MMF was decreased gradually in dosage or stopped. Tredger et al[32] reported that a total of 96 adverse events possibly associated with MMF therapy were well documented in the 147 adult patients, mainly including gastrointestinal dysfunction, leucopenia and infection.
In the study by Wiesner et al[16], diarrhea occurred in 51.3% of liver transplant recipients receiving MMF (1.5 g, twice daily) and corticosteroids. It seems that CNI therapy with MMF is associated with a higher incidence of diarrhea than monotherapy with MMF in liver transplantation. Diarrhea was observed in 31.4% of cases using MMF combined with CNIs[33]. For mono-therapy with MMF, a lower rate of diarrhea (14%-15%) was showed[34-36]. In stable renal transplant recipients, Maes et al[37] reported that gastric emptying of solids was significantly faster in patients treated with TAC compared with those with CsA. Cantarovich et al[13] reported that the incidence of diarrhea was 18% in liver transplantation patients using cyclosporine and MMF regimen, while the incidence of diarrhea was 38.63% in patients using MMF combined with TAC in a study by Xia et al[38].
METHODS FOR MEASURING MPA CONCENTRATION
Methods used for measurement of MPA concentration should be sensitive, accurate, specific, rapid, convenient and economical. Different methods were developed to determine total or unbound MPA (free MPA, fMPA) and MPA metabolites. These methods can be classified as chromatographic methods and immunoassays.
Chromatographic methods
Chromatographic methods have the advantages of good specificity and sensitivity. They are especially useful in monitoring the MPA and its metabolites simultaneously. However, these methods have the common shortcomings including complex sample preparation, which is labor-intensive and time-consuming. Chromatographic methods are suitable for laboratories with large sample load. Based on the variance in the detective method, chromatographic based assays used for MPA monitoring can be classified as high-performance liquid chromatography (HPLC) with ultraviolet (UV) or fluorescence detector and LC-MS/MS assay.
Determination of total MPA
Although LC-MS/MS is the most sensitive assay, HPLC-UV is sufficient in the monitoring of total MPA. Different UV absorption wavelengths were selected for MPA monitoring[39-41]. Most of these assays had the lower limit of quantification (LLOQ) of about 0.2 μg/mL. The sample preparation procedure in previous studies includes solid phase extraction (SPE)[40], liquid-liquid extraction (LLE), and protein precipitation. There is less interference on the chromatographs obtained by SPE or LLE method than by protein precipitation. However, sample preparation by SPE method consists of several steps. It is time-consuming and the SPE columns add the cost of determination. LLE method is also labor-intensive, and large quantity of organic solvents used may be harmful. Although protein precipitation does not provide clean extractions like SPE and LLE, it is simpler, more rapid and more economical compared with SPE and LLE. Shipkova et al[42] used acetontrile, sodium tungstate and perchloric acid to precipitate protein. Khoschsorur et al[43] used 2 folds of acetontrile as the sample precipitation reagent. In the study by Chen et al[41], one fold of methanol containing 5% ZnSO4 was used as the precipitation reagent. The procedure is very simple and rapid, and the result is reliable.
Determination of total MPA and its metabolites
As mentioned in the former part, MPA is metabolized primarily by glucuronidation to form MPAG and AcMPAG. Although MPAG is pharmacologically inactive, it can be hydrolyzed back to MPA and absorbed again during enterohepatic recirculation (EHC). AcMPAG has been observed regularly in the plasma of liver, kidney, and heart transplant recipients undergoing treatment with MMF. Chromatically based methods were established to monitor MPA, MPAG and AcMPAG simultaneously, including HPLC-UV methods[39-41] and LC-MS/MS methods[44,45]. To separate MPA from its metabolites sufficiently, both isocratic[41] and gradient[39,40] mobile phase systems were used. The peak areas of MPA, MPAG and AcMPAG at 304 nm were significantly lower than those at 215 nm (8.3, 21.8 and 9.4-fold lower, respectively) or 254 nm (2.0, 5.0 and 2.7-fold lower, respectively). Higher sensitivity was attained at 215 and 254 nm compared with 304 nm. However, the chromatography at 304 nm provided a cleaner baseline and more reproducible results in our study[41].
Klepacki et al[45] established an UHPLC-MS/MS assay using liquid-handling robotic extraction for the quantification of MPA and its metabolites in human plasma and urine. The LLOQ of MPA and its metabolites was 0.097 μg/mL for MPA and MPAG and 0.156 μg/mL for AcMPAG. The total assay run time was 2.3 min. The assay has proven to be robust and reliable during the measurement of samples from several pharmacokinetics trials.
Determination of total fMPA
The assays for detection fMPA are more complicated due to its very low level in plasma, therefore establishment of more sensitive methods is needed[46-49]. The pivotal sample treatment step is to separate fMPA from protein-bound MPA. Equilibrium dialysis and ultrafiltration can generate comparable results, and most studies selected ultrafiltration due to its practicability, accuracy and reproducibility. In the study by Aresta et al[46], plasma samples were ultrafiltrated in combination with SPE. The detection wavelength was UV 215 nm. The LLOQ was 26 ng/mL. Shen et al[47] used a HPLC-fluorescence method to determine total MPA and fMPA. The LLOQ of fMPA was 5 ng/mL. Chen et al[48] also developed a HPLC-fluorecence method to determine fMPA in plasma previously. The authors found that at a solvent pH of 8.5, the LLOQ of fMPA reached 2.5 ng/mL, which was much lower than that of HPLC-UV and comparable with that of LC-MS/MS. The retention time of MPA was about 3 min when pH of the mobile phase was increased to 8.5. To prevent the endogenous interference, TBA was used as the ion-pair reagent[48].
The lower limit of assay sensitivity of LC-MS/MS made it the best choice in measuring fMPA concentration. Patel et al[49] established an LC-MS/MS assay, and the plasma was subjected to ultrafiltration followed by SPE using C18 cartridges. The assay has a LLOQ of 1 ng/mL and an accuracy > 95%. The method reported has an adequate degree of robustness and dynamic concentration range for the measurement of fMPA for TDM purposes or pharmacokinetics investigations. TDM of MPA in saliva offers a favorable non-invasive approach. Besides, concentration of MPA in saliva can be considered as the fMPA approximately. The LC-MS/MS assays for monitoring MPA in saliva were established for adult and pediatric patients.
Immunoassays
Immunoassays include a series of methods, and the mechanism of these methods is the competent combination of antibody between the MPA in plasma and labeled MPA. The most frequently used assay was commercial enzyme multiplied immunoassay technology (EMIT) assay. The advantage of being less labor intensive of EMIT rendered this assay more suitable for conventional clinical TDM. Although several studies revealed a 9%-15% of systematic positive bias between EMIT and HPLC assay, EMIT has been proven to be an efficient method for monitoring of MPA[50-52]. In the study by Chen et al[48] on liver transplant patients, 470 total MPA concentrations were determined by both HPLC and EMIT methods. The authors found the relationship of the two methods was EMIT = 1.074 × HPLC + 0.582 (r2 = 0.918, n = 470, P < 0.05) for total MPA, and a good correlation between HPLC and EMIT was obtained with a positive bias of EMIT for total MPA (27.0%). The bias of EMIT is suggested to be caused by the cross-reactivity of AcMPAG.
Chen et al[48] established an EMIT method for the determination of fMPA for the first time. The calibration range of fMPA was 0.0050-0.50 μg/mL for EMIT method. Mean recovery of the two methods was 97.1%. The intra-day and inter-day variation coefficients were 4.51%-15.8% and 5.83%-19.5% for EMIT, respectively. The authors determined 297 fMPA concentrations by both HPLC and EMIT methods, and found that the relationship of the two methods was EMIT = 1.068 × HPLC + 0.004 (r2 = 0.945, n = 297, P < 0.05), and a good correlation between HPLC and EMIT was obtained with a positive bias of EMIT for total MPA (23.3%). Although the LLOQ of EMIT is higher than that of HPLC method, more than 95% of fMPA samples determined by EMIT have concentrations higher than LLOQ. EMIT can also be used in monitoring of fMPA.
Other immunoassays include the cloned enzyme donor immunoassay, enzyme inhibition assay[53], and particle enhanced turbidimetric inhibition immunoassay[54]. These methods are either under-development or not widely used.
CHARACERISTICS OF PHARMACOKINETICS OF MPA
At present, a fixed dose of 1 or 1.5 g twice daily of MMF is the standard protocol in liver transplantation with adjustments only in relation to side effects or to its efficacy[11]. However, there are wide variations in MPA pharmacokinetics reported with standard MMF dosing in liver transplant recipients. Shaw et al[8] in his review reported that the range of MPA AUC was 5-160 mg.h/L in 22 liver transplant recipients receiving 1.0 g MPA, twice daily. This kind of variation has been confirmed in some studies in adult (Table 1) or pediatric liver transplantation[55].
Table 1.
Pharmacokinetic data of mycophenolic acid in adult liver transplant recipients
| Ref. | Year | Regimen | Time since LT | n | Method | AUC0-12h (mg.h /L) | Mean tmax (h) | Mean C0h (mg/L) | Mean Cmax (mg/L) |
| Jain et al[65] | 2001 | TAC + MMF | Days 6-30 | 8 | HPLC | 40.0 ± 30.9 (7.3-102.3) | 1.8 ± 1.6 | 10.6 ± 7.5 | |
| Mardigyan et al[92] | 2005 | TAC + MMF | > 12 mo | 14 | EMIT | 45 ± 22 | 0.5 | 2.1 ± 1.5 | 12.2 ± 75 |
| Pisupati et al[60] | 2005 | TAC + MMF | < week 1 | 10 | HPLC | 50.8 ± 42.1 | 1.8 ± 1.2 | 9.1 ± 7.2 | |
| Weeks 1-2 | 60.3 ± 38.5 | 1.8 ± 1.4 | 11.6 ± 6.7 | ||||||
| Weeks 3-6 | 118.0 ± 57.6 | 1.3 ± 0.7 | 36.7 ± 15.6 | ||||||
| Brunet et al[11] | 2006 | TAC + MMF | Day 6 | 13 | HPLC-UC | 17.4 (13.2-39.7) | 2 | 0.4 | 4.6 |
| Day 16 | 13 | 26.3 (13.1-45.8) | 1.2 | 0.6 | 7.7 | ||||
| Month 3 | 14 | 33.6 (15.1-54.6) | 0.7 | 1.3 | 6.6 | ||||
| Chen et al[71] | 2007 | TAC + MMF | Day 7 | 38 | HPLC | 44.6 ± 16.50 (17.99-96.87) | 1.42 ± 0.77 | 8.45 ± 4.77 | |
| Day 14 | 34 | 50.54 ± 18.60 (22.78-98.73) | 1.45 ± 0.81 | 11.29 ± 5.51 | |||||
| Chen et al[76] | 2008 | TAC + MMF | Days 7-14 | 48 | EMIT | 45.77 ± 18.69 (10.66-117.01) | 1.94 ± 1.65 | 2.02 ± 1.57 | 11.76 ± 6.34 |
| Kamar et al[93] | 2009 | TAC + MMF | Day 7 | 15 | HPLC | 36.8 ± 27 | |||
| Day 14 | 15 | 32.6 ± 11 | |||||||
| Day 30 | 15 | 36.7 ± 13 | |||||||
| Beckebaum et al[94] | 2009 | TAC + MMF | Day 60 (14-230 d) | 18 | LC-MS/MS | 55.9 (22.9-144.8) | 0.5 | 3 | 14.2 |
| CsA + MMF | Day 70 (11-87 d) | 12 | 52.2 (31.8-102.1) | 1 | 2.5 | 15.3 | |||
| Benichou et al[61] | 2010 | TAC + MMF | Day 12 (4-20 d) | 26 | EMIT | 26.8 (21.8-39.7) | |||
| Day 36 (24-90 d) | 25 | 45.2 (26.0-57.0) |
TAC: Tacrolimus; MMF: Mycophenolate mofetil; CsA: Cyclosporine A; HPLC: High-performance liquid chromatography; EMIT: Enzyme multiplied immunoassay technology.
The investigations for MPA pharmacokinetics in liver transplantation are focused on the early period after operation. There are several characteristics of MPA pharmacokinetics in early phase (about within 6 mo). First, mean MPA AUC will increase in a time dependent manner, especially in two or three weeks after liver transplantation. Second, a large range of intra-patient and/or within-patient MPA pharmacokinetic variability is observed. Third, the relationship between MMF dosage and MPA pharmacokinetic parameters is variable. Fourth, MPA exposure is different when different immunosuppressive drugs (TAC or CsA) are used.
Reasons of variation of MPA exposure may include type of recipient and donor graft, the process of liver transplantation, dosage of MMF, EHC, bowel, liver, and renal dysfunction and drug interactions.
Type of recipient and donor graft
In a control study by Jain et al[56], the MPA AUC in living donor liver transplant (LDLT) patients were 4-fold higher than in deceased donor liver transplant (DDLT) patients per 1 g MMF intravenously. The mean plasma concentration of MPAG was 1.4-2.0 times higher in deceased donor liver transplant patients compared with live donor liver transplant patients. A reduced size living donor graft may have lower metabolizing capacity and reduced glucuronidation activity during regeneration. Importantly, the authors suggested the need to use a lower dosage (approximately 30%) of MMF in live donor liver transplant patients compared with deceased donor liver transplant patients. Jain et al[57] showed a low bioavailability of oral MMF (mean, 48.5%, within 1 wk). The protocol using intravenous MMF can restore full bioavailability and conserve renal function after liver transplantation[58].
In another control study by Shen et al[59], the comparison of the pharmacokinetics of MPA and its metabolites between LDLT patients and DDLT patients was performed after oral administration of MMF (1 g, bid). Although the AUC0-12h of MPA and MPAG is not significantly different between the two groups, MPA AUC6-12h was significantly higher in the DDLT group than in the LDLT group (P < 0.05). Inversely, higher free MPA AUC0-12h and significantly higher free MPA fraction (P < 0.05) were observed in DDLT patients when compared with the DDLT group. AcMPAG AUC0-12h was also significantly higher in the DDLT group (P < 0.05). The activity of glucuronide-conjugating enzymes was decreased due to reduced liver mass during the hepatic regeneration process. These observations suggested that the ability of clearance of MPA has decreased in LDLT patients during the early period after operation. The authors suggested that DDLT patients had higher EHC contributing to total MPA exposure compared with LDLT patients. As free MPA is the pharmacologically active form, lower oral dose of MMF may be administered for LDLT patients.
Post-transplant duration
MPA exposure significantly increases with post-transplantation time. In the investigation by Brunet et al[11] of 15 liver transplant recipients on a standard 1 g twice-daily dose, mean MPA AUC was 17.4 mg.h/L on day 6, 26.3 mg.h/L on day 10 and 33.6 mg.h/L at month 3. Low MPA AUC in their data was perhaps caused by the external biliary drainage and abnormal values of serum albumin and bilirubin. In another study by Xia et al[38], dose-normalized AUC0-12h of MPA, MPAG and AcMPAG increased significantly in the later stage (> 1 mo) when compared with the data from the early stage (within 2 wk after liver transplantation). Pisupati et al[60] observed that MPA AUC0-12h had doubled with 3-6 wk compared with that at first week after transplantation (50.8 mg.h/L vs 118 mg.h/L). However, the MPA AUC tended to be stable after 3 to 6 mo. Benichou et al[61] showed that there is no change of MPA AUC or free MPA AUC between at mean 36 d (24-90 d) and at mean 867 d (124-6586 d).
The lower MPA AUC0-12h in the immediate postoperative period is due to a higher apparent oral clearance (CL/F), which may result from a reduced absorption (F) or an increased clearance (CL). Benichou et al[61] assumed that the increase in CL/F is related to an increase in MPA free fraction, leading to lower total MPA AUC0-12h value during the immediate postoperative period. Free fraction of MPA related well with MPA CL/F and decreased significantly as serum albumin level returned to normal, which would be consistent with more rapid hepatic and renal extraction, and subsequent biliary and urinary excretion. Pisupati et al[60] showed that total MPA CL/F decreased from 32.9 ± 21.4 L/h during the first week to 9.0 ± 4.4 L/h during 3-6 wk. The same authors also showed that there was no change in the intrinsic CL of MPA among the patients and suggested that the lack of a significant change in the intrinsic clearance indicates that the inherent ability of the liver to metabolize and eliminate MPA did not change significantly over time.
The other causes of low MPA exposure during the early stage may be related to the reduction of EHC and low bioavailability.
Dosage of MMF
The relationship between MMF dosage and MPA exposure is variable, usually weak or absent. In adult liver transplant recipients, Hwang et al[62] showed that there was a crude interindividual correlation between MMF dosage and MPA concentration (r2 = 0.271, P < 0.001). When assorted according to the post-transplant period, r2 was 0.153 during the first three months, 0.228 for months 4-12, 0.508 for years 1-2, 0.293 for years 3-5, and 0.247 after 5 years. With minimal TAC, a similar degree of interindividual variation was observed (r2 = 0.247, P < 0.001). In pediatric liver recipients, Aw et al[63] showed that MPA AUC0-7h correlated significantly with MMF dose (r = 0.552, P = 0.010) and MPA C0h (r = 0.844, P < 0.001). When assorted according to the post-transplant period, r2 was 0.056 during the first three months, 0.162 for months 4-12, 0.085 for years 1-2, 0.071 for years 3-5, and 0.213 after 5 years.
EHC
MPA undergoes extensive EHC after hydrolysis of its biliary MPAG conjugate by intestinal bacteria and re-absorption of MPA. Hesselink et al[64] estimated that the contribution of EHC to the MPA AUC ranges between 10 % and 61 % in human. However, secondary peak is very rare in the initial period after liver transplantation, which occurs in approximately 50 % of patients at 1 mo[65]. In some liver transplant patients, the EHC reestablishes around 4 to 8 h after MMF dosage[66]. Pisupatic et al[60] showed that a secondary peak in MPA was seen between 4 and 6 h after MMF administration in 4 of 10 patients during 3-6 wk and not seen during 1-2 wk. MPA AUC increased approximately 3-fold, which indicated the possible contribution of EHC. In pediatric liver recipients treated with CsA and MMF, Lobritto et al[55] observed that a second smaller peak was exhibited by some patients (probably due to EHC) although CsA was used, which decreased re-circulated MPA concentrations[67].
Impact of liver and renal dysfunction
Impairment of liver function has complex effects on MPA kinetics, although cirrhosis affects neither MPA absorption nor MPA plasma protein binding or pharmacokinetics[68]. It is believed that free MPA levels are affected by hypoalbuminemia, uremia and hyperbilirubinemia[8,69]. Free MPA levels increase markedly in patients with severe renal insufficiency[70].
Chen et al[71] showed that MPA AUC0-12h in patients with abnormal albumin levels were significantly lower than that in patients with normal albumin levels (P = 0.009). MPA AUC0-12h was related significantly with serum albumin levels (r2 = 0.412, P = 0.001). However, other parameters of hepatic function including total serum bilirubin concentration did not influence the change of MPA AUC0-12h. In 8 liver graft recipients, Jain et al[65] reported that MPA AUC correlated with serum bilirubin and MPA C0h with albumin concentration. Higher serum bilirubin levels may impair hepatic MPAG production, transport and biliary excretion during cholestasis[68]. The decreased hepatic glucuronidation and EHC with moderate hepatic impairment may result in increased urinary MPAG concentrations[65]. Tredger et al[32] showed that recipients with low serum albumin levels (< 35 g/L) frequently failed to achieve the therapeutic levels of MPA. In adults and children with lower serum albumin concentrations, median levels of MPA C0h were 42 % and 19 %, respectively, of those in patients with normal serum albumin levels given corresponding doses (P < 0.001). However, Brunet et al[11] showed no relationship between liver function and MPA exposure.
Tredger et al[32] also reported that elevated serum creatinine levels (> 120 mmol/L) were related to higher MPA C0h per unit MMF dose (median increase by 38% early and 50% late after transplantation, P < 0.04) only in adult patients.
Concomitant immunosuppressive drugs
CsA but not TAC decreased MPA AUC and increased MPAG AUC0-24h because CsA inhibits excretion of MPAG into bile[67]. Inhibition of the biliary excretion of MPAG by CsA is mediated by the multidrug resistance-associated protein 2 transporter which leads to the reduction of MPA AUC[72].
In 21 stable pediatric liver transplant recipients, Brown et al[73] observed that MPA C0h was significantly lower during co-therapy with CsA compared with co-therapy with TAC (2.8 mg/L vs 5.6 mg/L, P = 0.006), while MPAG AUC was correspondingly higher (229 mg/L/h vs 94 mg/L/h, P = 0.012). Higher MMF dosage was demanded with CsA to achieve equivalent MPA C0h level than with TAC (362 mg vs 178 mg, P = 0.004). The authors suggested contrasting effects of CsA and TAC on MPA glucuronidation or its excretion and EHC.
Molina Perez et al[74] reported no interaction between total dose or BMI-adjusted dose of VGC and concomitant administration of MMF in liver transplant recipients.
LSS FOR MPA
Till now, there have been some studies establishing model equations for estimation of MPA AUC using LSS in liver transplant recipients.
Multiple regression analysis
The most reliable method for judging the exposure of MPA is to calculate MPA AUC0-12h. But monitoring MPA AUC0-12h requires frequent blood withdrawal. It is impractical to obtain 6-10 plasma samples for measuring full MPA AUC within a 12-h dose interval in clinical settings. Therefore, abbreviated sampling strategies by limited MPA concentrations have been under investigation.
For LSS study, Ting et al[75] have some important suggestions: (1) it is essential to validate the predictive performance of the LSS in other patient populations. The prediction bias and prediction precision of the LSS should be determined; (2) a clinically feasible LSS should use 3 or less blood samples, preferably within a short period of time in order to reduce the inconvenience of TDM; and (3) the application of a specific LSS is ideally limited to the population and drug formulation that is used to develop it.
Some studies tried to test whether MPA AUC can be accurately estimated from plasma concentrations at single time points, especially at MPA C0h. However, it is very regretful that the relationship between MPA C0h and MPA AUC0-12h is not strong enough. In two studies by Chen et al[71] the r2 value of MPA C0h was also lower in monitoring MPA concentrations by HPLC (r2 = 0.300, number of sample = 72) or EMIT (r2 = 0.0677, number of samples = 48)[76] at the early stage after liver transplantation. In the study by Brunet et al[11], an acceptable correlation between MPA C0h and MPA AUC0-12h was found (r = 0.742, number of samples = 63). In pediatric liver transplantation, Brown et al[73] showed a moderate correlation between MPA C0h and MPA AUC0-12h (r2 = 0.65, number of samples = 21). In conclusion, MPA AUC0-12h could not be substituted correctly by MPA C0h as well as other single time-point MPA concentrations.
Stepwise regression analysis was used to establish the abbreviated equations for estimated MPA AUC0-12h. All combined models were obtained by using MPA concentrations at 1 to 4 time points. A number of regression equations that predict MPA AUC0-12h are undertaken and take the form of the following function:
Estimated MPA AUC0-12h = I + β1 × C1h + ··· βn × Cnh
Where I is intercept, β is partial correlation coefficient and C is MPA concentration. The largest r2 value was considered the best regression. Equations with a high coefficient of determination (r2) are then validated using data from another group or bootstrap procedure to evaluate their ability to predict the full MPA AUC. The validation step is critically important to assess reliability of the LSS. There are three main methods to validate an LSS: two-group (model-building group and validating group), bootstrap and jackknife methods.
Chen et al[71] developed an LSS for the prediction of MPA AUC using 72 profiles (40 cases) by HPLC (Table 2). These authors found that the relationship between estimated MPA AUC0-12h and measured MPA AUC0-12h based on three or four MPA pharmacokinetic parameters was related significantly in some abbreviated models. The best model for prediction of MPA AUC0-12h was using MPA concentrations at 1, 2, 6 and 8 h time points (r2 = 0.921, P = 0.0001). Bias and prediction are 1.24 ± 11.19% and 8.24 ± 7.61%, respectively. 63 of 72 (88 %) estimated MPA AUC0-12h values were within 15 % of MPA AUC0-12h. Bland-Altman analysis also revealed the best agreement of this equation compared with the others and a mean error of ± 9.89 mg.h/mL. For validation of the accuracy of these equations, Hao et al[77] used another group of liver transplant recipients (30 cases). It was confirmed that the equation based on C1h, C2h, C6h and C8h had the best ability to predict measured MPA AUC0-12h (r2 = 0.936) with the excellent bias (2.18%), precision (5.11%) and the best prediction variation (2SD = ± 7.88 mg.h/L). However, the equation based on C1h, C2h and C4h was more suitable when considering clinical convenience, which had shorter sampling interval, excellent coefficient of determination (r2 = 0.795), excellent bias (3.48%), acceptable precision (14.37%) and good prediction variation (2SD = ± 13.23 mg.h/L).
Table 2.
Limited sampling strategy for prediction of full mycophenolic acid area under the concentration-time curve in liver transplant recipients
| Ref. | Method | Regimen | Patient population | No. of files (cases) | Sampling times investigated (h) | Suggested times of LSS (h) | Predicted AUC = | r2 | LSS validation | Bias | Precision |
| Attard et al[78] | EMIT or | CsA or TAC | Pediatrics | 41 files | 0, 0.33, 0.67, 1.25, 2, 4, 6, 8 | 0, 0.33, 2 | 9.1 + 5.7*C0h + 1.1*C0.33h + 2.1*C2h | 0.740 | No | N/A | N/A |
| HPLC-UV | + MMF | (41 cases) | 0, 0.67, 6 | 5.2 + 7.1*C0h + 1.1*C0.66h + 5.4*C6h | 0.880 | No | N/A | N/A | |||
| Chen et al[71] | HPLC | TAC + MMF | Adults | 72 files | 0.5, 1, 1.5, 2, 4, 6, 8, 10, 12 | 1, 2, 4 | 10.776 + 0.749*C1h + 1.604*C2h + 4.116*C4h | 0.750 | Validation | Yes | Yes |
| (40 cases) | 1, 2, 6 | 10.229 + 0.925*C1h + 1.750*C2h + 4.586*C6h | 0.855 | Group | Yes | Yes | |||||
| 1, 2, 6, 8 | 5.503 + 0.919*C1h + 1.871*C2h + 3.176*C6h + 3.664*C8h | 0.921 | Yes | Yes | |||||||
| 1, 2, 4, 6 | 6.658 + 0.921*C1h + 1.573*C2h + 2.057*C4h + 3.543*C6h | 0.899 | Yes | Yes | |||||||
| Chen et al[76] | EMIT | TAC + MMF | Adults | 48 files | 0.5, 1, 1.5, 2, 4, 6, 8, 10, 12 | 1.5, 6 | 10.56 + 1.55C1.5h + 6.44C6h | 0.859 | Bootstrap | Yes | Yes |
| (48 cases) | 2, 4, 8 | 9.37 + 2.18C2h + 2.10C4h + 4.71C8h | 0.901 | Yes | Yes | ||||||
| 1, 2, 4, 8 | 4.46 + 0.81C1h + 1.78C2h + 2.51C4h + 4.94C8h | 0.950 | Yes | Yes | |||||||
| 1, 2, 4, 6 | 5.92 + 1.10C1h + 1.01C2h + 1.77C4h + 4.80C6h | 0.927 | Yes | Yes |
LSS: Limited sampling strategy; MPA: Mycophenolic acid; AUC: Area under the concentration-time curve; EMIT: Enzyme multiplied immunoassay technology; HPLC: High-performance liquid chromatography; TAC: Tacrolimus; MMF: Mycophenolate mofetil; CsA: Cyclosporine A.
Although the standard technique for monitoring MPA concentration is HPLC, the EMIT has the advantages of convenience and rapidness in clinical settings for TDM of MMF. Due to the cross-reactivity of the antibody in the EMIT assay with the MPA AcMPAG, the EMIT target concentrations are higher than those for HPLC. The average overestimation by EMIT of MPA levels is approximately 10%-30%. As AcMPAG is pharmacologically active in vitro, it has been speculated that EMIT measurement may better reflect immunosuppression than HPLC techniques that only measure the parent compound. Thus, establishment of the abbreviated model for estimation of full MPA AUC by EMIT method is necessary and valuable. Chen et al[76] established some equations for the prediction of MPA AUC using 48 profiles (40 cases) by EMIT (Table 2). The best equation was based on C1h, C2h, C4h and C8h. Forty of 48 (83.33 %) estimated MPA AUC0-12h values were within 15 % of MPA AUC0-12h. The bias and precision are 0.27% ± 1.79% and 8.83% ± 1.24%, respectively. The best agreement between estimated maximum a posteriori (MAP) AUC0-12h and MPA AUC0-12h was also showed by Bland-Altman analysis, with an average error of 9.02 mg.h/L. The authors conducted the Bootstrap analysis with 200 replicated datasets and confirmed the accuracy and robustness of this equation.
In two above investigations by Chen et al[71,76], MPA C6h and/or C8h were necessary in the best equations from MPA concentrations at 3 or 4 time points. The accurate equation by LSS should include one time-point MPA sample during the interval 6-12 h post-dosage. It is probable that in liver transplant recipients MPA EHC importantly contributed to the full MPA AUC.
In a study by Attard et al[78], a total of 41 MPA AUC0-8h values were determined in 41 pediatric liver transplant recipients (Table 2). The best equation by LSS includes MPA C0h, C0.67h and C6h with excellent coefficient of determination (r = 0.88). For clinical practice, the equation with C0h, C0.33h and C2h is suitable (r = 0.74).
Bayesian analysis
MAP Bayesian assay is based on the concept that prior information or beliefs can be combined with observation data, which is known as Bayes’ theorem[75,79]. Briefly, the priori population PK parameters, in combination with demographic, pathophysiological and limited concentration-time data from the individual, are used to predict the individualized parameters. Besides, the uncertainty of the parameters will also be estimated. As the amount of individual data accumulates, the population data contribute less to the overall prediction, and parameter prediction is individualized eventually. Prediction of parameters is achieved by minimizing the Bayesian Function:
Math 1
Math 1.
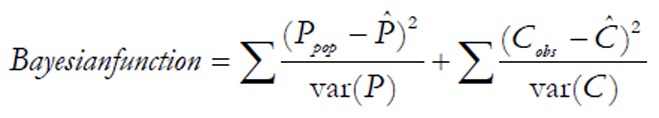
Math(A1).
Where Ppop is the population average of parameter P; P^ is the individual expected average of parameter P; var(P) is the variance of the estimated parameter P; Cobs is the observed concentration value; C^ is the predicted concentration value; and var(C) is the variance of the predicted concentration[80].
Population pharmacokinetic study of MPA
A reliable Bayesian forecasting method is based on the reliability of population pharmacokinetic (PPK) models established. PPK parameters for commonly used drugs are available in popular Bayesian software programs (e.g., NONMEM, ADAPT II, PKS). PPK studies to date have mostly been undertaken in renal transplant recipients, with limited investigation in patients treated with MPA for autoimmune disease or haematopoietic stem cell transplantation. Most of these studies have involved use of the MMF formulation of MPA.
It is a hard work to develop a PPK model of MPA to fully describe the complex physiological processes that occur in relation to the absorption and EHC of this drug. There are more than 20 PPK models that have been developed for MPA, and more complex models for description of MPA pharmacokinetics also include modeling of metabolites and free MPA concentrations. However, most of these studies included less than 100 subjects, which are not sufficient to fully characterize the complex kinetics of this agent in different clinical conditions. Population models applied to MAP Bayesian analysis vary somewhat in structure, and separate covariates have been identified as being significant in different studies.
Sampling time of MPA PPK study varied between various studies, however, most studies using rich-time between two doses of MMF. The data also included various post-transplantation stages, and the longest time of sampling included data at 10 years post-transplantation[81]. The most frequently used structure model is 2-compartmental model. van Hest et al[82] collected data 3-140 d post-transplantation from 140 patients. A total of 6523 samples were obtained, and they tested 1-, 2- and 3-compartment models, and found that the 2-compartment model is most rational and suitable. Similar to other immunosuppressive agents, the absorption of MPA is very complex. Shum et al[83] tested different absorption models including first order absorption, time-dependent model, Emax model, Weibull model and dual sequential first order absorption process. Finally, first order absorption with a lag time improved the model significantly. Le Guellec et al[84] found a 2-compartment model with zero-order absorption, with the absorption duration being estimated from the data, provided the best fitting.
MAP Bayesian estimation of MPA AUC
After the final PPK model of MPA is obtained, the covariate values and selected concentration-time data from individual patients are input in the model to obtain individualized AUC. Most of studies used the trapezoidal method to estimate the full MPA AUC value, which is considered as reference value. Evaluations have been conducted of how closely MAP Bayesian estimation of MPA AUC matches.
External and internal validation methods can be used in the MAP Bayesian estimation of MPA AUC. External validation involves the application of the developed method to a new dataset, which requires the correct covariates and accurate sampling times recorded. It is more stringent in the study design and can provide the strongest evidence for evaluation. Most of studies evaluated using internal validation datasets through data splitting or using a re-sampling technique. In some studies, data were split into a population model-building group and a validation group to evaluate MAP Bayesian forecasting. Other methods of validation include jackknife or Bootstrap method. Optimal sampling theory is based on the notion that there are specific sampling times, or windows of time, containing more information about pharmacokinetic parameters or drug exposure than other sampling times[85]. All these studies tested all combinations of study sampling times in selecting sampling times for Bayesian forecasting. Few studies used D-optimality (within pre-determined time limits). Predictive performance is usually expressed in terms of the r2, mean percentage predicted error (MPPE) and relative root mean-squared error (rRMSE) between reference AUC and estimated AUC.
A study by Barau et al[86] is the only study on the Bayesian estimation of MPA AUC in 28 pediatric patients who received liver transplantation. All patients received MMF therapy combined with TAC or CsA. The PPK model was established by using intensive pharmacokinetic datasets obtained from 16 children. A one-compartment model with first order absorption and first order elimination was selected. CL/F was estimated at 12.7 l h-1. Ka was estimated at 1.7 h-1 at age 8.7 years with IIV of 308%. V/F was 64.7 L, and increased about 2.3 times in children during the immediate post transplantation period. The individual MPA AUC0-12h was estimated by MAP Bayesian method using pharmacokinetic parameters obtained with the final model, including covariates, through Adapt II software. The MPA AUC0-12h estimated from concentrations measured 0, 1 and 4 h after administration of MMF was in good correlation with the data obtained using the trapezoidal method.
MAP Bayesian estimation is more flexible compared with multiple linear regression methods. Drug exposure can be estimated with any number of blood samples taken at any time. Furthermore, with MAP Bayesian forecasting, the information about an individual patient may be helpful in the AUC estimation[87]. However, there are still some problems. First, the PPK model established for MAP Bayesian estimation may be not the best one for the limited cases. Second, the algorithms used to select the optimal sampling time may not be accurate enough. Third, there is still large bias in the prediction in various studies. Finally, the best sampling times by comparison of predictive performance cannot be regarded as truly optimal, because the possible combinations are limited by the study design. These problems should be solved by further studies before the method can be widely used in the individualized therapy with MPA.
CONCENTRATION-EFFECT RELATIONSHIP
It has been clearly shown that MMF is a very powerful immunosuppressive drug in preventing graft rejection. However, there was also plenty of evidence showing that MMF has serious side effects including hematologic and gastrointestinal disorders[4]. The prospective, randomized and double-blind trial performed by van Gelder et al[88] showed that the rate of acute rejection decreased significantly in renal transplantation if MPA AUC was in the target range of 32.2-60.6 mg.h/L. Although the results are conflicting among different transplant settings, MPA concentration monitoring is recommended in kidney transplantation by the therapeutic window of 30 to 60 mg.h/L for MPA AUC and of 1 to 3.5 mg/L for MPA C0h[8]. However, it is still not widely accepted to individualize an oral MPA regimen by routinely monitoring MPA pharmacokinetic parameters in liver transplantation currently.
MPA exposure and acute rejection
In 147 adult liver transplants, Tredger et al[32] observed that nine of the 10 episodes of acute rejection were associated with plasma MPA concentrations less than 1 mg/L, with the exception occurring at 1.8 mg/L in a patient whose serum albumin was 31 g/L and creatinine 236 mmol/L. The relative risk of rejection (95%CI) increased 4.2-, 2.5-, and 1.6-fold, respectively, at plasma MPA concentrations of less than 0.5, 1.0 and 1.5 mg/L (P = 0.003, 0.002 and 0.058, respectively). The authors defined a cutoff of 0.85 mg/L in adult liver recipients by receiver operating characteristic (ROC) curve analysis. Besides, they also observed that MMF doses in the patients with rejection were not different from those in the control cohort. In the study by Hao et al[31], only two cases of acute rejection were proven by hepatic biopsy in 63 patients (3.2 %) within 3 mo after transplantation. Their MPA C0h values were 0.32 and 0.6 mg/L, MPA AUC0-12h values were 15.18 and 32.49 mg.h/L, and TAC C0 values were 7.3 and 2.2 ng/L. Recently, Sarvary et al[89] found the optimal cutoff of MPA C0h for predicting acute rejection (≥ 1.34 mg/L on CsA and ≥ 1.98 mg/L on TAC) in 56 liver transplant recipients during the 6-mo follow-up. In other studies, no relationship between MPA pharmacokinetics and acute rejection was established.
MPA exposure and adverse effects
In 63 liver transplant recipients, Chen et al[31] showed that mean MPA C0h and AUC0-12h in patients with side effects increased significantly compared with those without side effects (C0h: 2.28 mg/L vs 1.31 mg/L, P < 0.05; AUC0-12h: 49.68 mg.h/L vs 37.16 mg.h/L, P < 0.01). In addition, the levels of MPA C0h and MPA Cmax were higher in recipients with leucopenia, diarrhea and infection than in those without these effects, but a significant difference was achieved only during the episode of leucopenia (2.23 vs 1.81, P < 0.01). In 147 adult transplant recipients, Tredger et al[32] also showed that episodes of leukopenia were associated with higher median plasma MPA levels (2.8 mg/L vs 1.4 mg/L, P = 0.004). These authors also observed that MPA levels were higher during episodes of bacterial, fungal and viral infections, although this trend failed to achieve significance (1.8 mg/L vs 1.4 mg/L, P = 0.056) and there were no differences in median MPA levels with regard to gastrointestinal side effects. Brunet et al[11] showed significantly elevated mean MPA concentrations at C0.66h for six of 13 patients with diarrhea compared with symptom free patients (22.9 mg/L vs 7.4 mg/L, P < 0.05) and there was no significant difference significantly in MPA C0h or MPA AUC.
ROC curve analysis is also used to test the ability of MPA pharmacokinetic parameters to discriminate between cases with or without side effects in liver transplantation (Table 3). Hao et al[31] showed that the thresholds of MPA C0h and MPA AUC0-12h for side effects were 2 mg/L (sensitivity, 52.4%; specificity, 90.5%, P = 0.001) and 40 mg.h/L (sensitivity, 71.4%; specificity, 61.9%, P = 0.012), respectively. For individual side effects, only leukopenia was discriminated effectively by ROC analysis using MPA C0h with a threshold of 2 mg/L (sensitivity, 56.5 %; specificity, 75 %, P = 0.026). The relative risks were 1.79 for MPA C0h and 1.65 for MPA AUC to predict the occurrence of MMF-related side effects while 2.11 for MPA C0h and 1.68 for MPA AUC to predict the occurrence of leukopenia. In the study by Tredger et al[32], corresponding more than 3-fold increases in the relative risks for leukopenia, infection and gastrointestinal disturbances were showed when MPA concentration was at 3 to 4 mg/L. The thresholds of MPA C0h were 2.85 mg/L in infectious episodes (ROC area = 0.634, P = 0.056) and 2.25 mg/L in leukopenia (ROC area = 0.780, P = 0.003). Although the relative risk of gastrointestinal disorders increased with the increase in MPA C0h, there was no significant association (P > 0.5). Importantly, the authors observed a significant association between MMF dose and episodes of leukopenia (ROC area = 0.750, P = 0.007). It is suggested that individualizing MMF dose instead of using a fixed dose might be helpful to optimize immunosuppression and minimize potential toxic effects. However, Hao et al[31] showed no significant difference in MPA pharmacokinetic parameters between patients with infection and those without.
Table 3.
Receiver operating characteristic analyses of mycophenolic acid exposure and mycophenolate mofetil-related side effects in liver transplant recipients
| Ref. | Area under ROC curve | 95%CI | Cut-off value | P value | |
| Hao et al[31] | Side effects1 | ||||
| MPA C0h | 0.748 | 0.619-0.877 | 2 mg/L | 0.001 | |
| MPA AUC0-12h | 0.695 | 0.559-0.831 | 40 mg.h/L | 0.012 | |
| Leukopenia | |||||
| MPA C0h | 0.670 | 0.534-0.805 | 2 mg/L | 0.026 | |
| Tredger et al[32] | Leukopenia | ||||
| MPA C0h | 0.780 | 0.642-0.919 | 2.25 mg/L | 0.003 | |
| MMF dose | 0.750 | 0.662-0.837 | 0.007 | ||
| Infection | |||||
| MPA C0h | 0.634 | 0.499-0.770 | 2.85 mg/L | 0.056 |
Side effects include leukopenia, diarrhea and infection. MMF: Mycophenolate mofetil; ROC: Receiver operating characteristic; MPA: Mycophenolic acid.
Among immunosuppressive drugs, MMF is the main cause of diarrhea when compared with other agents. The mechanism responsible for MMF-related diarrhea is not yet elucidated. In liver transplantation[31,32], the levels of MPA C0h or AUC0-12h were not significantly higher in patients with diarrhea than those without diarrhea. However, Xia et al[38] found that MPA C6h, C10h, C12h and MPA AUC6-12h were significantly higher in patients with diarrhea (P < 0.05). These results suggested that higher EHC might contribute to the occurrence of diarrhea.
It was guessed that diarrhea may be related to MPAG or AcMPAG[90]. However, in the study by Xia et al[38], there was no significant difference in MPAG or AcMPAG (P > 0.05) though MPA Cmax and MPA AUC0-12h of MPAG were higher in recipients with diarrhea. Likewise, C0h, Cmax, and AUC0-12h of AcMPAG were also higher in patients with diarrhea, although no significant difference in these parameters was found (P > 0.05). Arns et al[91] suggested that the capacity of enterocytes to participate in MPA metabolism could potentially result in local generation of AcMPAG and MPAG with consequent direct toxic effects on the gastrointestinal tract. Perhaps concentration of AcMPAG in the gastrointestinal tract is more important than plasma concentration of AcMPAG for induction of diarrhea.
Another risk of diarrhea was dependent on dosage of MMF. Diarrhea was controlled by decreasing the dosage or interruption even if these patients had the same starting dosage of MMF as those not suffering from diarrhea[31].
CONCLUSION
Until now, TDM for MPA has not been fully accepted in liver transplantation as no long-term prospective study of concentration controlled vs fixed-dose prescribing of MMF has been done. However, based on published data, it is confirmed that intra- or inter-individual MPA pharmacokinetic variability exists, which is related to greater risk of acute rejection at lower MPA concentrations and MMF-associated side effects at higher MPA concentrations. On the other hand, the standard dose of MMF is rarely necessary in liver transplant recipients who had more MMF-related side effects and less acute rejection. These data suggest that monitoring MPA exposure is helpful in clinical settings.
In liver transplantation, it was showed that MPA C0h has more practical benefits over MPA AUC although the relationship between MPA C0h and MPA AUC is not very strong in some studies. Compared with the therapeutic window in renal transplantation (MPA C0h: 1-3.5 mg/L), acute rejection is more likely at concentrations less than 1 to 2 mg/L (μg/mL) and adverse effects at concentrations 3-4 mg/L or greater in liver transplantation[13]. However, this finding needs more clinical validation in future. Although MPA AUC is much accurate, which reflects the change of MPA pharmacokinetics and is closely related to side effects[31], no recommended therapeutic ranges of MPA AUC could be used in pediatric or adult liver transplant recipients. On the other hand, monitoring of MPA AUC is not practical in clinical settings. It should obtain 6-10 plasma samples for measuring full MPA AUC within a 12-h dose interval. Although abbreviated sampling strategy by limited MPA concentrations is practical in clinical settings, the equations including MPA concentrations within 2 h with good correlation were only seen in pediatric transplant recipients[78]. In adult liver transplantation, good coefficients of determination (r2) were seen in equations including one MPA concentration at least during 6-12 h after oral MMF[71,76]. Monitoring MPA C0h has more practical benefits than MPA AUC in liver transplantation.
Footnotes
P- Reviewer: Antonakopoulos N, Jaeschke H, Kubota K, Sonzogni A S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Zhang DN
References
- 1.Bullingham RE, Nicholls A, Hale M. Pharmacokinetics of mycophenolate mofetil (RS61443): a short review. Transplant Proc. 1996;28:925–929. [PubMed] [Google Scholar]
- 2.Shipkova M, Armstrong VW, Wieland E, Niedmann PD, Schütz E, Brenner-Weiss G, Voihsel M, Braun F, Oellerich M. Identification of glucoside and carboxyl-linked glucuronide conjugates of mycophenolic acid in plasma of transplant recipients treated with mycophenolate mofetil. Br J Pharmacol. 1999;126:1075–1082. doi: 10.1038/sj.bjp.0702399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Budde K, Curtis J, Knoll G, Chan L, Neumayer HH, Seifu Y, Hall M. Enteric-coated mycophenolate sodium can be safely administered in maintenance renal transplant patients: results of a 1-year study. Am J Transplant. 2004;4:237–243. doi: 10.1046/j.1600-6143.2003.00321.x. [DOI] [PubMed] [Google Scholar]
- 4.Shipkova M, Armstrong VW, Oellerich M, Wieland E. Mycophenolate mofetil in organ transplantation: focus on metabolism, safety and tolerability. Expert Opin Drug Metab Toxicol. 2005;1:505–526. doi: 10.1517/17425255.1.3.505. [DOI] [PubMed] [Google Scholar]
- 5.Johnston A, Holt DW. Immunosuppressant drugs--the role of therapeutic drug monitoring. Br J Clin Pharmacol. 2001;52 Suppl 1:61S–73S. doi: 10.1046/j.1365-2125.2001.0520s1061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srinivas TR, Kaplan B, Meier-Kriesche HU. Mycophenolate mofetil in solid-organ transplantation. Expert Opin Pharmacother. 2003;4:2325–2345. doi: 10.1517/14656566.4.12.2325. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman-La Roche Ltd , CellCept Prescribing information. Accessed 23/2/2010. Available from: http://www.gene.com/gene/products/information/cellcept/pdf/pi.pdf.
- 8.Shaw LM, Korecka M, Aradhye S, Grossman R, Bayer L, Innes C, Cucciara A, Barker C, Naji A, Nicholls A, et al. Mycophenolic acid area under the curve values in African American and Caucasian renal transplant patients are comparable. J Clin Pharmacol. 2000;40:624–633. doi: 10.1002/j.1552-4604.2000.tb05988.x. [DOI] [PubMed] [Google Scholar]
- 9.Oellerich M, Shipkova M, Schütz E, Wieland E, Weber L, Tönshoff B, Armstrong VW. Pharmacokinetic and metabolic investigations of mycophenolic acid in pediatric patients after renal transplantation: implications for therapeutic drug monitoring. German Study Group on Mycophenolate Mofetil Therapy in Pediatric Renal Transplant Recipients. Ther Drug Monit. 2000;22:20–26. doi: 10.1097/00007691-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Le Meur Y, Büchler M, Thierry A, Caillard S, Villemain F, Lavaud S, Etienne I, Westeel PF, Hurault de Ligny B, Rostaing L, et al. Individualized mycophenolate mofetil dosing based on drug exposure significantly improves patient outcomes after renal transplantation. Am J Transplant. 2007;7:2496–2503. doi: 10.1111/j.1600-6143.2007.01983.x. [DOI] [PubMed] [Google Scholar]
- 11.Brunet M, Cirera I, Martorell J, Vidal E, Millán O, Jiménez O, Rojo I, Londoño MC, Rimola A. Sequential determination of pharmacokinetics and pharmacodynamics of mycophenolic acid in liver transplant patients treated with mycophenolate mofetil. Transplantation. 2006;81:541–546. doi: 10.1097/01.tp.0000200307.79962.48. [DOI] [PubMed] [Google Scholar]
- 12.Venkataramanan R, Shaw LM. Therapeutic monitoring of mycophenolic acid in liver transplant patients. Liver Transpl. 2004;10:503–505. doi: 10.1002/lt.20125. [DOI] [PubMed] [Google Scholar]
- 13.Cantarovich M, Brown NW, Ensom MH, Jain A, Kuypers DR, Van Gelder T, Tredger JM. Mycophenolate monitoring in liver, thoracic, pancreas, and small bowel transplantation: a consensus report. Transplant Rev (Orlando) 2011;25:65–77. doi: 10.1016/j.trre.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Beckebaum S, Armstrong VW, Cicinnati VR, Streit F, Klein CG, Gerken G, Paul A, Oellerich M. Pharmacokinetics of mycophenolic acid and its glucuronide metabolites in stable adult liver transplant recipients with renal dysfunction on a low-dose calcineurin inhibitor regimen and mycophenolate mofetil. Ther Drug Monit. 2009;31:205–210. doi: 10.1097/FTD.0b013e31819743d9. [DOI] [PubMed] [Google Scholar]
- 15.McDiarmid SV. Mycophenolate mofetil in liver transplantation. Clin Transplant. 1996;10:140–145. [PubMed] [Google Scholar]
- 16.Wiesner R, Rabkin J, Klintmalm G, McDiarmid S, Langnas A, Punch J, McMaster P, Kalayoglu M, Levy G, Freeman R, et al. A randomized double-blind comparative study of mycophenolate mofetil and azathioprine in combination with cyclosporine and corticosteroids in primary liver transplant recipients. Liver Transpl. 2001;7:442–450. doi: 10.1053/jlts.2001.23356. [DOI] [PubMed] [Google Scholar]
- 17.Goralczyk AD, Bari N, Abu-Ajaj W, Lorf T, Ramadori G, Friede T, Obed A. Calcineurin inhibitor sparing with mycophenolate mofetil in liver transplantion: a systematic review of randomized controlled trials. Am J Transplant. 2012;12:2601–2607. doi: 10.1111/j.1600-6143.2012.04157.x. [DOI] [PubMed] [Google Scholar]
- 18.Ringe B, Braun F, Schütz E, Füzesi L, Lorf T, Canelo R, Oellerich M, Ramadori G. A novel management strategy of steroid-free immunosuppression after liver transplantation: efficacy and safety of tacrolimus and mycophenolate mofetil. Transplantation. 2001;71:508–515. doi: 10.1097/00007890-200102270-00005. [DOI] [PubMed] [Google Scholar]
- 19.Mele TS, Halloran PF. The use of mycophenolate mofetil in transplant recipients. Immunopharmacology. 2000;47:215–245. doi: 10.1016/s0162-3109(00)00190-9. [DOI] [PubMed] [Google Scholar]
- 20.Klupp J, Bechstein WO, Platz KP, Keck H, Lemmens HP, Knoop M, Langrehr JM, Neuhaus R, Pratschke J, Neuhaus P. Mycophenolate mofetil added to immunosuppression after liver transplantation--first results. Transpl Int. 1997;10:223–228. doi: 10.1007/s001470050046. [DOI] [PubMed] [Google Scholar]
- 21.Eckhoff DE, McGuire BM, Frenette LR, Contreras JL, Hudson SL, Bynon JS. Tacrolimus (FK506) and mycophenolate mofetil combination therapy versus tacrolimus in adult liver transplantation. Transplantation. 1998;65:180–187. doi: 10.1097/00007890-199801270-00006. [DOI] [PubMed] [Google Scholar]
- 22.Schlitt HJ, Barkmann A, Böker KH, Schmidt HH, Emmanouilidis N, Rosenau J, Bahr MJ, Tusch G, Manns MP, Nashan B, et al. Replacement of calcineurin inhibitors with mycophenolate mofetil in liver-transplant patients with renal dysfunction: a randomised controlled study. Lancet. 2001;357:587–591. doi: 10.1016/s0140-6736(00)04055-1. [DOI] [PubMed] [Google Scholar]
- 23.Pageaux GP, Rostaing L, Calmus Y, Duvoux C, Vanlemmens C, Hardgwissen J, Bernard PH, Barbotte E, Vercambre L, Bismuth M, et al. Mycophenolate mofetil in combination with reduction of calcineurin inhibitors for chronic renal dysfunction after liver transplantation. Liver Transpl. 2006;12:1755–1760. doi: 10.1002/lt.20903. [DOI] [PubMed] [Google Scholar]
- 24.Cicinnati VR, Yu Z, Klein CG, Sotiropoulos GC, Saner F, Malagó M, Frilling A, Gerken G, Broelsch CE, Beckebaum S. Clinical trial: switch to combined mycophenolate mofetil and minimal dose calcineurin inhibitor in stable liver transplant patients--assessment of renal and allograft function, cardiovascular risk factors and immune monitoring. Aliment Pharmacol Ther. 2007;26:1195–1208. doi: 10.1111/j.1365-2036.2007.03466.x. [DOI] [PubMed] [Google Scholar]
- 25.Kriss M, Sotil EU, Abecassis M, Welti M, Levitsky J. Mycophenolate mofetil monotherapy in liver transplant recipients. Clin Transplant. 2011;25:E639–E646. doi: 10.1111/j.1399-0012.2011.01512.x. [DOI] [PubMed] [Google Scholar]
- 26.Evans HM, McKiernan PJ, Kelly DA. Mycophenolate mofetil for renal dysfunction after pediatric liver transplantation. Transplantation. 2005;79:1575–1580. doi: 10.1097/01.tp.0000163504.29054.3f. [DOI] [PubMed] [Google Scholar]
- 27.Aberg F, Jula A, Höckerstedt K, Isoniemi H. Cardiovascular risk profile of patients with acute liver failure after liver transplantation when compared with the general population. Transplantation. 2010;89:61–68. doi: 10.1097/TP.0b013e3181bcd682. [DOI] [PubMed] [Google Scholar]
- 28.Junge G, Neuhaus R, Schewior L, Klupp J, Guckelberger O, Langrehr JM, Tullius S, Neuhaus P. Withdrawal of steroids: a randomized prospective study of prednisone and tacrolimus versus mycophenolate mofetil and tacrolimus in liver transplant recipients with autoimmune hepatitis. Transplant Proc. 2005;37:1695–1696. doi: 10.1016/j.transproceed.2005.03.145. [DOI] [PubMed] [Google Scholar]
- 29.Bilbao I, Castells L, Rojas L, Cancino J, Dopazo C, Castro E, Pou L, Andino R, Margarit C. Immunosuppression based on mycophenolate mofetil in stable liver transplanted patients. Int Immunopharmacol. 2006;6:1977–1983. doi: 10.1016/j.intimp.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 30.Gerhardt T, Terjung B, Knipper P, Palmedo H, Woitas RP, Kalff J, Sauerbruch T, Spengler U. Renal impairment after liver transplantation - a pilot trial of calcineurin inhibitor-free vs. calcineurin inhibitor sparing immunosuppression in patients with mildly impaired renal function after liver transplantation. Eur J Med Res. 2009;14:210–215. doi: 10.1186/2047-783X-14-5-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hao C, Anwei M, Bing C, Baiyong S, Weixia Z, Chuan S, Erzhen C, Xiaxing D, Weihua Q, Weiping Y, et al. Monitoring mycophenolic acid pharmacokinetic parameters in liver transplant recipients: prediction of occurrence of leukopenia. Liver Transpl. 2008;14:1165–1173. doi: 10.1002/lt.21600. [DOI] [PubMed] [Google Scholar]
- 32.Tredger JM, Brown NW, Adams J, Gonde CE, Dhawan A, Rela M, Heaton N. Monitoring mycophenolate in liver transplant recipients: toward a therapeutic range. Liver Transpl. 2004;10:492–502. doi: 10.1002/lt.20124. [DOI] [PubMed] [Google Scholar]
- 33.Pfitzmann R, Klupp J, Langrehr JM, Uhl M, Neuhaus R, Settmacher U, Steinmüller T, Neuhaus P. Mycophenolatemofetil for immunosuppression after liver transplantation: a follow-up study of 191 patients. Transplantation. 2003;76:130–136. doi: 10.1097/01.TP.0000071522.74885.48. [DOI] [PubMed] [Google Scholar]
- 34.Fairbanks KD, Thuluvath PJ. Mycophenolate mofetil monotherapy in liver transplant recipients: a single center experience. Liver Transpl. 2004;10:1189–1194. doi: 10.1002/lt.20210. [DOI] [PubMed] [Google Scholar]
- 35.Moreno JM, Rubio E, Gómez A, Lopez-Monclus J, Herreros A, Revilla J, Navarrete E, Sánchez Turrión V, Jimenez M, Cuervas-Mons V. Effectiveness and safety of mycophenolate mofetil as monotherapy in liver transplantation. Transplant Proc. 2003;35:1874–1876. doi: 10.1016/s0041-1345(03)00643-2. [DOI] [PubMed] [Google Scholar]
- 36.Moreno Planas JM, Cuervas-Mons Martinez V, Rubio Gonzalez E, Gomez Cruz A, Lopez-Monclus J, Sánchez-Turrion V, Lucena Poza JL, Jimenez Garrido M, Millan I. Mycophenolate mofetil can be used as monotherapy late after liver transplantation. Am J Transplant. 2004;4:1650–1655. doi: 10.1111/j.1600-6143.2004.00556.x. [DOI] [PubMed] [Google Scholar]
- 37.Maes BD, Vanwalleghem J, Kuypers D, Ghoos Y, Rutgeerts PJ, Vanrenterghem YF. Differences in gastric motor activity in renal transplant recipients treated with FK-506 versus cyclosporine. Transplantation. 1999;68:1482–1485. doi: 10.1097/00007890-199911270-00009. [DOI] [PubMed] [Google Scholar]
- 38.Xia ZW, Jun CY, Hao C, Bing C, Min SM, Jie XJ. The occurrence of diarrhea not related to the pharmacokinetics of MPA and its metabolites in liver transplant patients. Eur J Clin Pharmacol. 2010;66:671–679. doi: 10.1007/s00228-010-0833-2. [DOI] [PubMed] [Google Scholar]
- 39.Elbarbry FA, Shoker AS. Liquid chromatographic determination of mycophenolic acid and its metabolites in human kidney transplant plasma: pharmacokinetic application. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;859:276–281. doi: 10.1016/j.jchromb.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 40.Patel CG, Akhlaghi F. High-performance liquid chromatography method for the determination of mycophenolic acid and its acyl and phenol glucuronide metabolites in human plasma. Ther Drug Monit. 2006;28:116–122. doi: 10.1097/01.ftd.0000177664.96726.56. [DOI] [PubMed] [Google Scholar]
- 41.Chen B, Zhang WX, Yu ZC, Cai WM. Determination of Mycophenolic Acid (MPA) and Its Acyl and Phenol Glucuronide Metabolits Simultaneously in Human Plasma by a Simplified HPLC Method. Analytical Letter. 2007;40:2465–2475. [Google Scholar]
- 42.Shipkova M, Schütz E, Armstrong VW, Niedmann PD, Oellerich M, Wieland E. Determination of the acyl glucuronide metabolite of mycophenolic acid in human plasma by HPLC and Emit. Clin Chem. 2000;46:365–372. [PubMed] [Google Scholar]
- 43.Khoschsorur G, Erwa W. Liquid chromatographic method for simultaneous determination of mycophenolic acid and its phenol- and acylglucuronide metabolites in plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;799:355–360. doi: 10.1016/j.jchromb.2003.10.074. [DOI] [PubMed] [Google Scholar]
- 44.Brandhorst G, Streit F, Goetze S, Oellerich M, Armstrong VW. Quantification by liquid chromatography tandem mass spectrometry of mycophenolic acid and its phenol and acyl glucuronide metabolites. Clin Chem. 2006;52:1962–1964. doi: 10.1373/clinchem.2006.074336. [DOI] [PubMed] [Google Scholar]
- 45.Klepacki J, Klawitter J, Bendrick-Peart J, Schniedewind B, Heischmann S, Shokati T, Christians U, Klawitter J. A high-throughput U-HPLC-MS/MS assay for the quantification of mycophenolic acid and its major metabolites mycophenolic acid glucuronide and mycophenolic acid acyl-glucuronide in human plasma and urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;883-884:113–119. doi: 10.1016/j.jchromb.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aresta A, Palmisano F, Zambonin CG, Schena P, Grandaliano G. Simultaneous determination of free mycophenolic acid and its glucuronide in serum of patients under mycophenolate mophetil therapy by ion-pair reversed-phase liquid chromatography with diode array UV detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;810:197–202. doi: 10.1016/j.jchromb.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 47.Shen J, Jiao Z, Yu YQ, Zhang M, Zhong MK. Quantification of total and free mycophenolic acid in human plasma by liquid chromatography with fluorescence detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;817:207–213. doi: 10.1016/j.jchromb.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 48.Chen B, Gu Z, Chen H, Zhang W, Fen X, Cai W, Fan Q. Establishment of high-performance liquid chromatography and enzyme multiplied immunoassay technology methods for determination of free mycophenolic acid and its application in Chinese liver transplant recipients. Ther Drug Monit. 2010;32:653–660. doi: 10.1097/FTD.0b013e3181f01397. [DOI] [PubMed] [Google Scholar]
- 49.Patel CG, Mendonza AE, Akhlaghi F, Majid O, Trull AK, Lee T, Holt DW. Determination of total mycophenolic acid and its glucuronide metabolite using liquid chromatography with ultraviolet detection and unbound mycophenolic acid using tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;813:287–294. doi: 10.1016/j.jchromb.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 50.Beal JL, Jones CE, Taylor PJ, Tett SE. Evaluation of an immunoassay (EMIT) for mycophenolic acid in plasma from renal transplant recipients compared with a high-performance liquid chromatography assay. Ther Drug Monit. 1998;20:685–690. doi: 10.1097/00007691-199812000-00019. [DOI] [PubMed] [Google Scholar]
- 51.Shipkova M, Schütz E, Armstrong VW, Niedmann PD, Wieland E, Oellerich M. Overestimation of mycophenolic acid by EMIT correlates with MPA metabolite. Transplant Proc. 1999;31:1135–1137. doi: 10.1016/s0041-1345(98)01936-8. [DOI] [PubMed] [Google Scholar]
- 52.Hosotsubo H, Takahara S, Imamura R, Kyakuno M, Tanaka T, Yazawa K, Hanafusa T, Matsumiya K, Nonomura N, Okuyama A, et al. Analytic validation of the enzyme multiplied immunoassay technique for the determination of mycophenolic acid in plasma from renal transplant recipients compared with a high-performance liquid chromatographic assay. Ther Drug Monit. 2001;23:669–674. doi: 10.1097/00007691-200112000-00013. [DOI] [PubMed] [Google Scholar]
- 53.van Gelder T, Domke I, Engelmayer J, de Fijter H, Kuypers D, Budde K, Koeger R, Luthe H, Oellerich M. Clinical utility of a new enzymatic assay for determination of mycophenolic acid in comparison with an optimized LC-MS/MS method. Ther Drug Monit. 2009;31:218–223. doi: 10.1097/FTD.0b013e31819a05f2. [DOI] [PubMed] [Google Scholar]
- 54.Vergara Chozas JM, Sáez-Benito Godino A, Zopeque García N, García Pinteño S, Joumady I, Carrasco García C, Vara Gil F. Analytical validation of a homogeneous immunoassay for determination of mycophenolic acid in human plasma. Transplant Proc. 2012;44:2669–2672. doi: 10.1016/j.transproceed.2012.09.063. [DOI] [PubMed] [Google Scholar]
- 55.Lobritto SJ, Rosenthal P, Bouw R, Leung M, Snell P, Mamelok RD. Pharmacokinetics of mycophenolate mofetil in stable pediatric liver transplant recipients receiving mycophenolate mofetil and cyclosporine. Liver Transpl. 2007;13:1570–1575. doi: 10.1002/lt.21274. [DOI] [PubMed] [Google Scholar]
- 56.Jain A, Venkataramanan R, Kwong T, Mohanka R, Orloff M, Abt P, Kashyap R, Tsoulfas G, Mack C, Williamson M, et al. Pharmacokinetics of mycophenolic acid in liver transplant patients after intravenous and oral administration of mycophenolate mofetil. Liver Transpl. 2007;13:791–796. doi: 10.1002/lt.21146. [DOI] [PubMed] [Google Scholar]
- 57.Jain A, Mohanka R, Orloff M, Abt P, Kashyap R, Kelley M, Burlee K, Bozorgzadeh A. Intravenous mycophenolate mofetil with low-dose oral tacrolimus and steroid induction for live donor liver transplantation. Exp Clin Transplant. 2005;3:361–365. [PubMed] [Google Scholar]
- 58.Jain A, Sharma R, Ryan C, Tsoulfas G, Orloff M, Abt P, Kashyap R, Batzold P, Sauberman L, Safadjou S, et al. Potential immunological advantage of intravenous mycophenolate mofetil with tacrolimus and steroids in primary deceased donor liver transplantation and live donor liver transplantation without antibody induction. Liver Transpl. 2008;14:202–209. doi: 10.1002/lt.21348. [DOI] [PubMed] [Google Scholar]
- 59.Shen B, Chen B, Zhang W, Mao H, Shen C, Deng X, Zhan X, Chen H. Comparison of pharmacokinetics of mycophenolic acid and its metabolites between living donor liver transplant recipients and deceased donor liver transplant recipients. Liver Transpl. 2009;15:1473–1480. doi: 10.1002/lt.21895. [DOI] [PubMed] [Google Scholar]
- 60.Pisupati J, Jain A, Burckart G, Hamad I, Zuckerman S, Fung J, Venkataramanan R. Intraindividual and interindividual variations in the pharmacokinetics of mycophenolic acid in liver transplant patients. J Clin Pharmacol. 2005;45:34–41. doi: 10.1177/0091270004270145. [DOI] [PubMed] [Google Scholar]
- 61.Benichou AS, Blanchet B, Conti F, Hornecker M, Bernard D, Taieb F, Scatton O, Abbas H, Harcouet L, Dauphin A, et al. Variability in free mycophenolic acid exposure in adult liver transplant recipients during the early posttransplantation period. J Clin Pharmacol. 2010;50:1202–1210. doi: 10.1177/0091270009358084. [DOI] [PubMed] [Google Scholar]
- 62.Hwang S, Lee SG, Ahn CS, Kim KH, Moon DB, Ha TY, Song GW, Jung DH, Choi NK, Kim KW, et al. A clinical assessment of mycophenolate drug monitoring after liver transplantation. Clin Transplant. 2010;24:E35–E42. doi: 10.1111/j.1399-0012.2009.01166.x. [DOI] [PubMed] [Google Scholar]
- 63.Aw MM, Brown NW, Itsuka T, Gonde CE, Adams JE, Heaton ND, Tredger JM, Mieli-Vergani G, Dhawan A. Mycophenolic acid pharmacokinetics in pediatric liver transplant recipients. Liver Transpl. 2003;9:383–388. doi: 10.1053/jlts.2003.50022. [DOI] [PubMed] [Google Scholar]
- 64.Hesselink DA, van Gelder T. Genetic and nongenetic determinants of between-patient variability in the pharmacokinetics of mycophenolic acid. Clin Pharmacol Ther. 2005;78:317–321. doi: 10.1016/j.clpt.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 65.Jain A, Venkataramanan R, Hamad IS, Zuckerman S, Zhang S, Lever J, Warty VS, Fung JJ. Pharmacokinetics of mycophenolic acid after mycophenolate mofetil administration in liver transplant patients treated with tacrolimus. J Clin Pharmacol. 2001;41:268–276. doi: 10.1177/00912700122010087. [DOI] [PubMed] [Google Scholar]
- 66.Fatela-Cantillo D, Hinojosa-Pérez R, Peralvo-Rodríguez MI, Serrano-Díaz Canedo J, Gómez-Bravo MA. Pharmacokinetic evaluation of mycophenolic acid profiles during the period immediately following an orthotopic liver transplant. Transplant Proc. 2006;38:2482–2485. doi: 10.1016/j.transproceed.2006.08.076. [DOI] [PubMed] [Google Scholar]
- 67.van Gelder T, Klupp J, Barten MJ, Christians U, Morris RE. Comparison of the effects of tacrolimus and cyclosporine on the pharmacokinetics of mycophenolic acid. Ther Drug Monit. 2001;23:119–128. doi: 10.1097/00007691-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 68.Parker G, Bullingham R, Kamm B, Hale M. Pharmacokinetics of oral mycophenolate mofetil in volunteer subjects with varying degrees of hepatic oxidative impairment. J Clin Pharmacol. 1996;36:332–344. doi: 10.1002/j.1552-4604.1996.tb04209.x. [DOI] [PubMed] [Google Scholar]
- 69.Mourad M, Malaise J, Chaib Eddour D, De Meyer M, König J, Schepers R, Squifflet JP, Wallemacq P. Correlation of mycophenolic acid pharmacokinetic parameters with side effects in kidney transplant patients treated with mycophenolate mofetil. Clin Chem. 2001;47:88–94. [PubMed] [Google Scholar]
- 70.Kaplan B, Gruber SA, Nallamathou R, Katz SM, Shaw LM. Decreased protein binding of mycophenolic acid associated with leukopenia in a pancreas transplant recipient with renal failure. Transplantation. 1998;65:1127–1129. doi: 10.1097/00007890-199804270-00019. [DOI] [PubMed] [Google Scholar]
- 71.Chen H, Peng C, Yu Z, Shen B, Deng X, Qiu W, Fei Y, Shen C, Zhou G, Yang W, et al. Pharmacokinetics of mycophenolic acid and determination of area under the curve by abbreviated sampling strategy in Chinese liver transplant recipients. Clin Pharmacokinet. 2007;46:175–185. doi: 10.2165/00003088-200746020-00005. [DOI] [PubMed] [Google Scholar]
- 72.Hesselink DA, van Hest RM, Mathot RA, Bonthuis F, Weimar W, de Bruin RW, van Gelder T. Cyclosporine interacts with mycophenolic acid by inhibiting the multidrug resistance-associated protein 2. Am J Transplant. 2005;5:987–994. doi: 10.1046/j.1600-6143.2005.00779.x. [DOI] [PubMed] [Google Scholar]
- 73.Brown NW, Aw MM, Mieli-Vergani G, Dhawan A, Tredger JM. Mycophenolic acid and mycophenolic acid glucuronide pharmacokinetics in pediatric liver transplant recipients: effect of cyclosporine and tacrolimus comedication. Ther Drug Monit. 2002;24:598–606. doi: 10.1097/00007691-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 74.Molina Perez E, Fernández Castroagudín J, Seijo Ríos S, Mera Calviño J, Tomé Martínez de Rituerto S, Otero Antón E, Bustamante Montalvo M, Varo Perez E. Valganciclovir-induced leukopenia in liver transplant recipients: influence of concomitant use of mycophenolate mofetil. Transplant Proc. 2009;41:1047–1049. doi: 10.1016/j.transproceed.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 75.Ting LS, Villeneuve E, Ensom MH. Beyond cyclosporine: a systematic review of limited sampling strategies for other immunosuppressants. Ther Drug Monit. 2006;28:419–430. doi: 10.1097/01.ftd.0000211810.19935.44. [DOI] [PubMed] [Google Scholar]
- 76.Chen H, Gu Z, Chen B, Mao H, Zhang W, Fan Q. Models for the prediction of mycophenolic acid area under the curve using a limited-sampling strategy and an enzyme multiplied immunoassay technique in Chinese patients undergoing liver transplantation. Clin Ther. 2008;30:2387–2401. doi: 10.1016/j.clinthera.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 77.Hao C, Erzheng C, Anwei M, Zhicheng Y, Baiyong S, Xiaxing D, Weixia Z, Chenghong P, Hongwei L. Validation of limited sampling strategy for the estimation of mycophenolic acid exposure in Chinese adult liver transplant recipients. Liver Transpl. 2007;13:1684–1693. doi: 10.1002/lt.21293. [DOI] [PubMed] [Google Scholar]
- 78.Attard TM, Dhawan A, Tredger JM, Conner K, Kling K, Colombani P, Thompson R, Cuffari C. Mycophenolic acid metabolite levels in pediatric liver transplantation: correlation with a limited sampling strategy. J Appl Res. 2008;8:135–142. [Google Scholar]
- 79.Sheiner LB, Rosenberg B, Melmon KL. Modelling of individual pharmacokinetics for computer-aided drug dosage. Comput Biomed Res. 1972;5:411–459. doi: 10.1016/0010-4809(72)90051-1. [DOI] [PubMed] [Google Scholar]
- 80.David OJ, Johnston A. Limited sampling strategies for estimating cyclosporin area under the concentration-time curve: review of current algorithms. Ther Drug Monit. 2001;23:100–114. doi: 10.1097/00007691-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 81.van Hest RM, Mathot RA, Pescovitz MD, Gordon R, Mamelok RD, van Gelder T. Explaining variability in mycophenolic acid exposure to optimize mycophenolate mofetil dosing: a population pharmacokinetic meta-analysis of mycophenolic acid in renal transplant recipients. J Am Soc Nephrol. 2006;17:871–880. doi: 10.1681/ASN.2005101070. [DOI] [PubMed] [Google Scholar]
- 82.van Hest RM, van Gelder T, Vulto AG, Mathot RA. Population pharmacokinetics of mycophenolic acid in renal transplant recipients. Clin Pharmacokinet. 2005;44:1083–1096. doi: 10.2165/00003088-200544100-00006. [DOI] [PubMed] [Google Scholar]
- 83.Shum B, Duffull SB, Taylor PJ, Tett SE. Population pharmacokinetic analysis of mycophenolic acid in renal transplant recipients following oral administration of mycophenolate mofetil. Br J Clin Pharmacol. 2003;56:188–197. doi: 10.1046/j.1365-2125.2003.01863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Le Guellec C, Bourgoin H, Büchler M, Le Meur Y, Lebranchu Y, Marquet P, Paintaud G. Population pharmacokinetics and Bayesian estimation of mycophenolic acid concentrations in stable renal transplant patients. Clin Pharmacokinet. 2004;43:253–266. doi: 10.2165/00003088-200443040-00004. [DOI] [PubMed] [Google Scholar]
- 85.van der Meer AF, Marcus MA, Touw DJ, Proost JH, Neef C. Optimal sampling strategy development methodology using maximum a posteriori Bayesian estimation. Ther Drug Monit. 2011;33:133–146. doi: 10.1097/FTD.0b013e31820f40f8. [DOI] [PubMed] [Google Scholar]
- 86.Barau C, Furlan V, Debray D, Taburet AM, Barrail-Tran A. Population pharmacokinetics of mycophenolic acid and dose optimization with limited sampling strategy in liver transplant children. Br J Clin Pharmacol. 2012;74:515–524. doi: 10.1111/j.1365-2125.2012.04213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Saint-Marcoux F, Royer B, Debord J, Larosa F, Legrand F, Deconinck E, Kantelip JP, Marquet P. Pharmacokinetic modelling and development of Bayesian estimators for therapeutic drug monitoring of mycophenolate mofetil in reduced-intensity haematopoietic stem cell transplantation. Clin Pharmacokinet. 2009;48:667–675. doi: 10.2165/11317140-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van Gelder T, Hilbrands LB, Vanrenterghem Y, Weimar W, de Fijter JW, Squifflet JP, Hené RJ, Verpooten GA, Navarro MT, Hale MD, et al. A randomized double-blind, multicenter plasma concentration controlled study of the safety and efficacy of oral mycophenolate mofetil for the prevention of acute rejection after kidney transplantation. Transplantation. 1999;68:261–266. doi: 10.1097/00007890-199907270-00018. [DOI] [PubMed] [Google Scholar]
- 89.Sarvary E, Nemes B, Varga M, Gaal I, Monostory K, Langer RM, Gorog D, Fazakas J, Kobori L, Fehervari I, et al. Significance of mycophenolate monitoring in liver transplant recipients: toward the cut-off level. Transplant Proc. 2012;44:2157–2161. doi: 10.1016/j.transproceed.2012.07.124. [DOI] [PubMed] [Google Scholar]
- 90.Bailey MJ, Dickinson RG. Acyl glucuronide reactivity in perspective: biological consequences. Chem Biol Interact. 2003;145:117–137. doi: 10.1016/s0009-2797(03)00020-6. [DOI] [PubMed] [Google Scholar]
- 91.Arns W. Noninfectious gastrointestinal (GI) complications of mycophenolic acid therapy: a consequence of local GI toxicity? Transplant Proc. 2007;39:88–93. doi: 10.1016/j.transproceed.2006.10.189. [DOI] [PubMed] [Google Scholar]
- 92.Mardigyan V, Tchervenkov J, Metrakos P, Barkun J, Deschenes M, Cantarovich M. Best single time points as surrogates to the tacrolimus and mycophenolic acid area under the curve in adult liver transplant patients beyond 12 months of transplantation. Clin Ther. 2005;27:463–469. doi: 10.1016/j.clinthera.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 93.Kamar N, Marquet P, Gandia P, Muscari F, Lavayssière L, Esposito L, Guitard J, Canivet C, Peron JM, Alric L, et al. Mycophenolic acid 12-hour area under the curve in de novo liver transplant patients given mycophenolate mofetil at fixed versus concentration-controlled doses. Ther Drug Monit. 2009;31:451–456. doi: 10.1097/FTD.0b013e3181aa776e. [DOI] [PubMed] [Google Scholar]
- 94.Beckebaum S, Cicinnati VR, Klein CG, Brokalaki E, Yu Z, Malago M, Frilling A, Gerken G, Broelsch CE. Impact of combined mycophenolate mofetil and low-dose calcineurin inhibitor therapy on renal function, cardiovascular risk factors, and graft function in liver transplant patients: preliminary results of an open prospective study. Transplant Proc. 2004;36:2671–2674. doi: 10.1016/j.transproceed.2004.10.008. [DOI] [PubMed] [Google Scholar]


