Abstract
AIM: To investigate whether cold water intake into the stomach affects colonic motility and the involvement of the oxytocin-oxytocin receptor pathway in rats.
METHODS: Female Sprague Dawley rats were used and some of them were ovariectomized. The rats were subjected to gastric instillation with cold (0-4 °C, cold group) or room temperature (20-25 °C, control group) saline for 14 consecutive days. Colon transit was determined with a bead inserted into the colon. Colonic longitudinal muscle strips were prepared to investigate the response to oxytocin in vitro. Plasma concentration of oxytocin was detected by ELISA. Oxytocin receptor expression was investigated by Western blot analysis. Immunohistochemistry was used to locate oxytocin receptors.
RESULTS: Colon transit was slower in the cold group than in the control group (P < 0.05). Colonic smooth muscle contractile response to oxytocin decreased, and the inhibitory effect of oxytocin on muscle contractility was enhanced by cold water intake (0.69 ± 0.08 vs 0.88 ± 0.16, P < 0.05). Atosiban and tetrodotoxin inhibited the effect of oxytocin on colonic motility. Oxytocin receptors were located in the myenteric plexus, and their expression was up-regulated in the cold group (P < 0.05). Cold water intake increased blood concentration of oxytocin, but this effect was attenuated in ovariectomized rats (286.99 ± 83.72 pg/mL vs 100.56 ± 92.71 pg/mL, P < 0.05). However, in ovariectomized rats, estradiol treatment increased blood oxytocin, and the response of colonic muscle strips to oxytocin was attenuated.
CONCLUSION: Cold water intake inhibits colonic motility partially through oxytocin-oxytocin receptor signaling in the myenteric nervous system pathway, which is estrogen dependent.
Keywords: Intragastric cold water stress, Colonic motility, Estradiol, Oxytocin, Oxytocin receptor, Irritable bowel syndrome
Core tip: Colon transit was decreased and oxytocin-induced inhibition of colonic contraction was enhanced in rats with cold water intake. Atosiban and tetrodotoxin inhibited the effect of oxytocin on colonic motility. Cold water intake increased blood concentration of oxytocin and expression of oxytocin receptors in colon. Estradiol regulated blood concentration of oxytocin and oxytocin-induced colonic contraction. The results suggested that cold water intake inhibited colonic motility through oxytocin-oxytocin receptor signaling in the myenteric nervous system pathway, which is estrogen dependent. The estradiol-oxytocin-oxytocin receptor-colonic contractile pathway might be a new therapeutic target for irritable bowel syndrome in females.
INTRODUCTION
Irritable bowel syndrome (IBS) is a functional bowel disorder in which abdominal pain or discomfort is associated with defecation and features of disordered bowel habit[1]. Throughout the world, about 4%-20% of adults and adolescents have symptoms consistent with IBS, and a female predominance has been found by most studies[2-6]. Altered colonic motility, visceral hyperalgesia, disturbance of brain-gut interaction, abnormal central processing, autonomic and hormonal events, genetic and environmental factors, postinfectious sequels, and psychosocial disturbance are variably involved in the development of IBS symptoms[7-9]. Consuming cold meals and cold drinks is common among some people, especially in the industrialized countries. Zuo et al[10,11] found that cold water intake increased plasma 5-HT concentrations and lowered visceral perception thresholds in subjects with IBS. Previous studies also found that cold meal intake affected gastric myoelectrical and contractile activities[12,13]. Healthy women with excessive exposure to cold stress show an abnormal intestinal response to incoming stimuli[14]. Cold water intake might alter colonic motility and be involved in IBS in females.
Oxytocin (OT) is a neuropeptide synthesized by magnocellular neurons in the paraventricular and supraoptic nuclei of the hypothalamus. It is best known as a critical social and reproduction hormone[15-17]. OT effects are more generally associated with trait effects related to social or emotional functions, particularly in females[18]. The oxytocinergic system selectively influences the functional response of females, and as such may be involved in normal and pathologic states that are more common among females. Our previous studies showed that OT decreased the contraction of colon, which is ovarian steroid-dependent[19,20]. In response to a variety of stimuli such as suckling[21], parturition[22], or certain kinds of stress[23-26], the processed OT is released from the posterior pituitary into the systemic circulation. Chronic hyperosmotic stress (drinking water with 2% NaCl solution for 7 d) increased the secretion of OT[27]. Whether intragastric cold stress affects release of OT and then affects colonic contraction in females is still unknown.
OT receptors are expressed in the gastrointestinal tract[28]. OT has been reported to affect colonic smooth muscle contraction via OT receptors[19,20]. OT receptors are found in the gastric smooth muscle[29], the enteric nervous system (ENS) or intestinal epithelium[30-32]. Intragastric cold stress might also affect expression of OT receptors in the colon.
Ovarian hormones are found to regulate the expression of OT receptors in various tissues and the tissue response to OT[33-36]. Estradiol (E2) is known to regulate OT expression in the hypothalamus[37-40].
In the present study, we hypothesized that intragastric cold stress stimulates the secretion of OT and up-regulates the expression of OT receptors to affect colonic motility in female rats and that this effect is exerted via OT receptors in colon, and is estradiol dependent.
MATERIALS AND METHODS
Animals
Female Sprague Dawley rats were housed in a temperature (22 °C)-controlled environment. The use and treatment of animals were approved by the Animal Care and Use Committee of Tongji University (approval no: 2009-0022). All animals were cared for in compliance with the Principles of Laboratory Animal Care and the Guide for the Care and Use of Laboratory Animals, published by the National Science Council, China. The experiments conform to the Convention for the Protection of Vertebrate Animals Used for Experimental and other Scientific Purposes. The experiments were done two days after the last intragastric cold stress procedure. The rats were fasted overnight with water ad libitum before the experiment. Pain or discomfort of the rats was minimized during the experiments.
Intragastric cold stress protocol
The rats were 6-8-week-old and weighed 180 ± 10 g at the beginning of the treatment. Animals were subjected once daily at 8 am to gastric instillation with cold (0-4 °C) physiological saline as the cold group or room temperature (20-25 °C) physiological saline (1 mL) as the control group, for 14 consecutive days. Room temperature regular drinking water and food were offered to rats in both groups all the time.
Ovariectomy
Some of the rats were ovariectomized. Ovariectomy was performed quickly under light ether anesthesia. The ovaries were picked out by forceps through a 1-cm incision made over both flanks. A ligature was placed below the ovary and the ovary was removed, then the incisions of the muscle and skin were sewed up by aseptic suture line, and finally the wounds were disinfected by Iodophors.
Measurement of colonic transit
The rats were lightly anesthetized with sevoflurane. A single 3-3.5 mm glass bead was lubricated with vaseline. The anus was opened with a lubricated glass rod. After the bead was put into the anus, it was inserted into the colon (3 cm proximal to the anus) quickly with the glass rod. The evacuation time was monitored after consciousness was regained.
Preparation of isolated colonic smooth muscle strips
A segment of the colon of approximately 4 cm was collected and put in Krebs solution (composed of NaCl 118.5 mmol/L, KCl 4.8 mmol/L, KH2PO4 1.2 mmol/L, MgSO4 1.2 mmol/L, CaCl2 1.9 mmol/L, NaHCO3 25.0 mmol/L and glucose 10.1 mmol/L). The segment was opened along the mesenteric border and pinned mucosa side up. The mucosa was removed by sharp dissection and the muscle strip (2 mm wide and 8 mm long) was cut along the longitudinal axis. Silk thread was attached to both ends of the muscle strips, and the strips were mounted in 5-mL organ baths. The organ baths contained aerated (5% CO2, 95% O2) Krebs solution which was maintained at 37 °C. Strips were adjusted in length to an initial tension of 1 g, and were allowed to stabilize for 60 min before experimental procedures were initiated. Isometric tension was measured using external force transducers (JH-2B, Beijing, China). Force signals were amplified with a SMUP-PC amplifier (Fudan University, Shanghai, China), and recorded using an MFlab system.
Western blot
Samples of colon stored at -80 °C were homogenized for protein analysis. The homogenates were centrifuged at 2000 rpm for 10 min at 4 °C, and the protein content of the supernatants was evaluated using Protein Quantitative Analysis Kit (k3001-BCA; Shenergy Biocolor, Shanghai, China). Supernatants containing 100 μg protein were diluted in reducing × 2 sample buffer and loaded into 12% SDS-PAGE. After separation by SDS-PAGE, proteins were transferred to nitrocellulose membranes. Membranes were blocked for 3 h at room temperature in blocking buffer (5% nonfat dry milk and TTBS), washed in TTBS (0.1% Tween 20, 50 mmol/L Tris, and 150 mmol/L NaCl) , and incubated overnight with anti-rat oxytocin receptor IgG (1:400, sc 8102; Santa Cruz Biotechnology, CA, United States), followed by peroxidase-conjugated secondary antibodies (1:20000). Finally, immunoreactive proteins were revealed using twin plate Color Scanner (T1200; AGFA, Shenzhen, China).
Immunohistochemistry
Immediately after the animals were anesthetized with sevoflurane, a segment of distal colon was removed and soaked in 4% paraformaldehyde for 12 h. The fixed tissue was rinsed for 100 min and was dehydrated, cleared and mounted in wax. The tissue was cut into 4-μm sections, and stained by a two-step method. Activity of endogenous peroxidase was blocked with 3% hydrogen peroxide. After three rinses in PBS, 10% normal rabbit serum was applied for 15 min, and then the sections were incubated with primary goat anti-oxytocin receptor antibody (Santa Cruz, diluted 1:100 in PBS) overnight in a humid chamber at 4 °C. After the sections were washed, they were incubated with polymer peroxidase-anti-goat serum (ZSGB-BIO, Beijing, China) for 30 min at room temperature. After several rinses, peroxidase was revealed using a 3, 3’-diaminobenzidine tetrahydrochloride substrate kit (ZSGB-BIO, Beijing, China). Negative controls were performed without primary antibody.
ELISA assay
To investigate the involvement of sex hormone in the plasma concentration of OT, female rats were randomly divided into four groups (n = 6 for each group): gastric instillation with room temperature saline (normal group); gastric instillation with cold saline (cold group); stressed rats which had been ovariectomized before gastric instillation with cold saline (OVX + cold group); ovariectomized rats treated with E2 (25 μg/kg, s.c.) once daily for 6 d and then gastric instillation with cold saline (OVX + E2 + cold group). After the animals were anesthetized with sevoflurane, uteri were removed and weighed. The blood from the heart was collected with a syringe into a tube and spun for 10 min at 3000 rpm. Plasma was pipetted out and stored at -80 °C for further OT analysis. OT was determined using an Enzyme Immunoassay Kit (RD, Inc., MI, United States) at a dose of 100 μL plasma per sample per well for the assay, according to the manufacturer’s instructions. Samples were analyzed in duplicate in a single assay.
Statistical analysis
The peak forces of colonic phasic contraction were measured using an MFlab system (Fudan University, Shanghai, China). In each experiment, the peak forces of contractions were evaluated at 0.5 min before and after drug administration. Mean peak force for the 1-min period before drug administration was taken as the baseline. The value of the force after drug treatment was normalized to the baseline value. The ratio of post-treatment force to baseline force was expressed as the ratio R, so that the baseline for each experiment was equal to 1.
Western blots were evaluated by determining the gray scale value of the blot. The value of ratio is the value of the gray scale division between the experimental group and control group.
Data were presented as means ± SD. Statistical analysis was performed by means of Student’s t test for comparisons between two groups or by means of ANOVA analysis for comparisons among groups. A probability level of P < 0.05 was considered statistically significant.
RESULTS
Colonic transit test
Figure 1 presents the colonic transit in rats. The time of the glass bead output was 223.08 ± 90.76 s in the cold group and 102.42 ± 20.21 s in the control group. The colonic transit time in intragastric cold water stressed rats was significantly longer than in control rats (P = 0.029).
Figure 1.
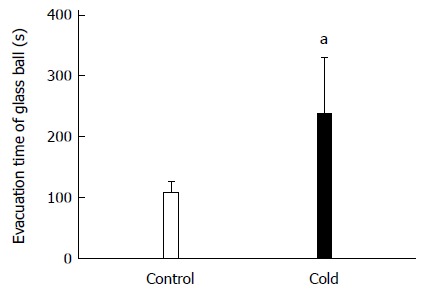
Colonic transit increased in intragastric cold water stressed rats. Control group: the rats treated with intragastric room temperature physiological saline; Cold group: the rats treated with intragastric cold physiological saline. aP < 0.05 vs cold and control rats (n = 10).
Effect of OT on colonic contractility in intragastric cold water stressed rats
As shown in Figure 2, for the control group, low concentrations of OT (0.01-0.3 μmol/L) failed to elicit any effect on the contractions of colonic smooth muscle strips. When the concentration of OT was increased to 1 or 3 μmol/L, the ratio of the contractile force of colonic smooth muscle strips decreased respectively to 0.87 ± 0.15 or 0.88 ± 0.16 (P < 0.05 compared with the data prior to OT administration).
Figure 2.
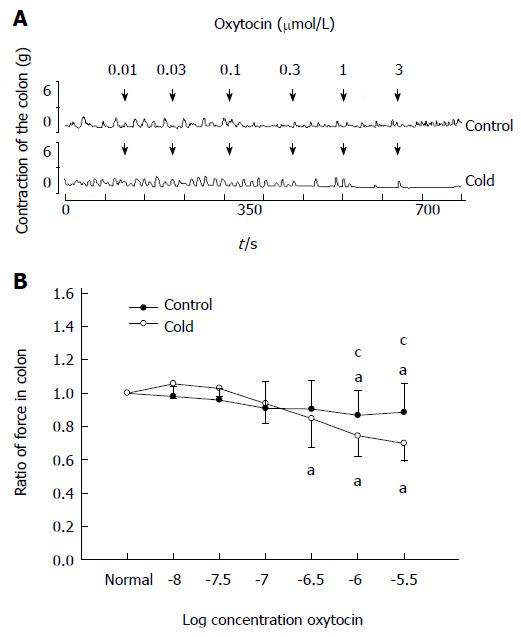
Effects of oxytocin (0.01-0.3 μmol/L) on colon contraction in intragastric cold water stressed rats. A: The dose-dependent contractile response to oxytocin (OT) in colonic smooth muscle strips in cold and control groups. OT was administered at the points marked by the arrows. B: Average response to OT in the colon. aP < 0.05 vs the data prior to OT administration. cP < 0.05 vs cold and control groups (n = 6).
In the cold group, OT (0.01-0.1 μmol/L) failed to elicit any effect on the contractions of colonic smooth muscle strips. When the concentration of OT was increased to 0.3, 1 or 3 μmol/L, the ratio of the contractile force of colonic smooth muscle strips decreased respectively to 0.87 ± 0.15, 0.74 ± 0.12 or 0.69 ± 0.08 (P < 0.05 compared with the data prior to OT administration). Moreover, the ratio of the contractile force of colonic smooth muscle strips induced by OT at 1 or 3 μmol/L was lower than that in control group (0.74 ± 0.12 vs 0.87 ± 0.15, 0.69 ± 0.08 vs 0.88 ± 0.16, respectively, P < 0.05).
Effect of atosiban on OT-induced response of colon in intragastric cold water stressed rats
OT receptor inhibitor atosiban (0.1 mmol/L) showed no effect on the contraction of colonic strips in stressed rats. Thirty minutes after atosiban treatment, OT (0.01-3 μmol/L) was added. The ratio of the contractile force of colonic smooth muscle strips induced by OT at 1 or 3 μmol/L almost returned to normal (0.72 ± 0.10 vs 0.95 ± 0.20, 0.78 ± 0.12 vs 1.0 ± 0.21, respectively, P < 0.05 compared with the data of atosiban treatment, Figure 3).
Figure 3.
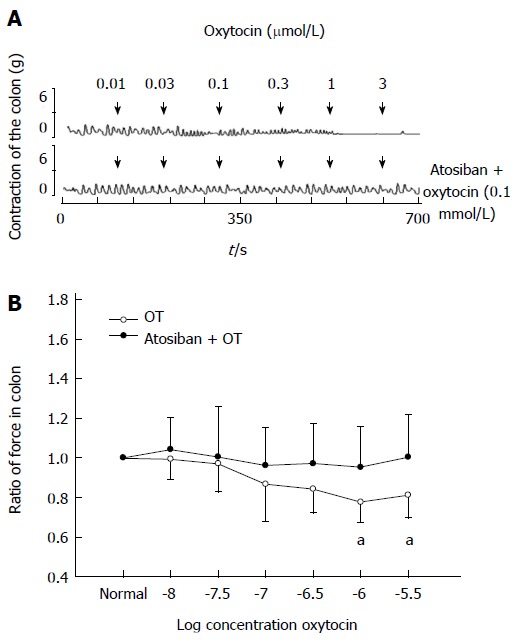
Atosiban (0.1 mmol/L) blocked oxytocin-induced colonic response in intragastric cold water stressed rats. A: Representative traces of the effect of atosiban on oxytocin (OT)-induced colonic response in the cold group. The drug was administered at the points marked by the arrows. B: Results of colonic smooth muscle strips contraction in cold group rats. aP < 0.05 vs the data of atosiban treatment (n = 8).
Effect of tetrodotoxin on OT-induced response of colon in intragastric cold water stressed rats
Addition of tetrodotoxin (TTX) (10 μmol/L) showed no effect on the contraction of colonic strips in stressed rats. Thirty minutes after TTX treatment, OT (0.01-3 μmol/L) was added. The ratio of the contractile force of colonic smooth muscle strips induced by OT (3 μmol/L) recovered from 0.79 ± 0.13 to 0.99 ± 0.08 (P < 0.05 compared with the data of TTX treatment, Figure 4).
Figure 4.
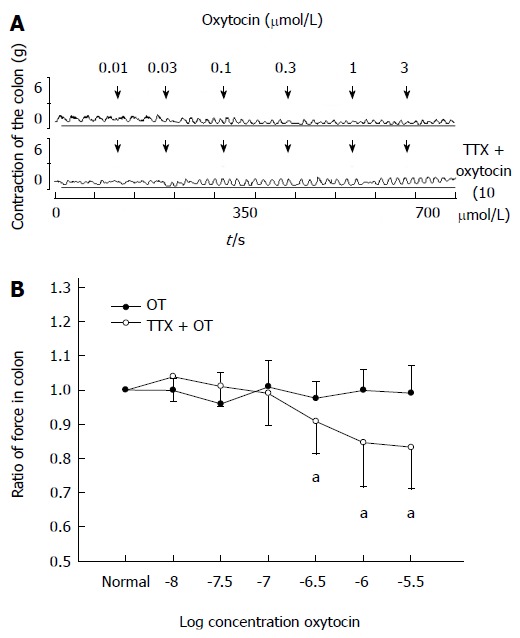
Tetrodotoxin (10 μmol/L) blocked oxytocin-induced colonic response in intragastric cold water stressed rats. A: Representative traces of the effect of tetrodotoxin (TTX) on oxytocin (OT)-induced colonic response in cold group. The drug was administered at the points marked by the arrows. B: Results of colonic smooth muscle strips contraction in cold group rats. aP < 0.05 vs data of TTX treatment (n = 6).
OT receptor expression in the colon of intragastric cold water stressed rats
Compared with the control group, the expression level of OT receptor in the colon of stressed rats was significantly increased (1.82 ± 0.17 vs 1.00 ± 0.31, P < 0.05, Figure 5A, B). The cells with OT receptor immunoreactivity were located in the myenteric plexus of the colon and smooth muscle of the rat uterus (Figure 5C, D).
Figure 5.
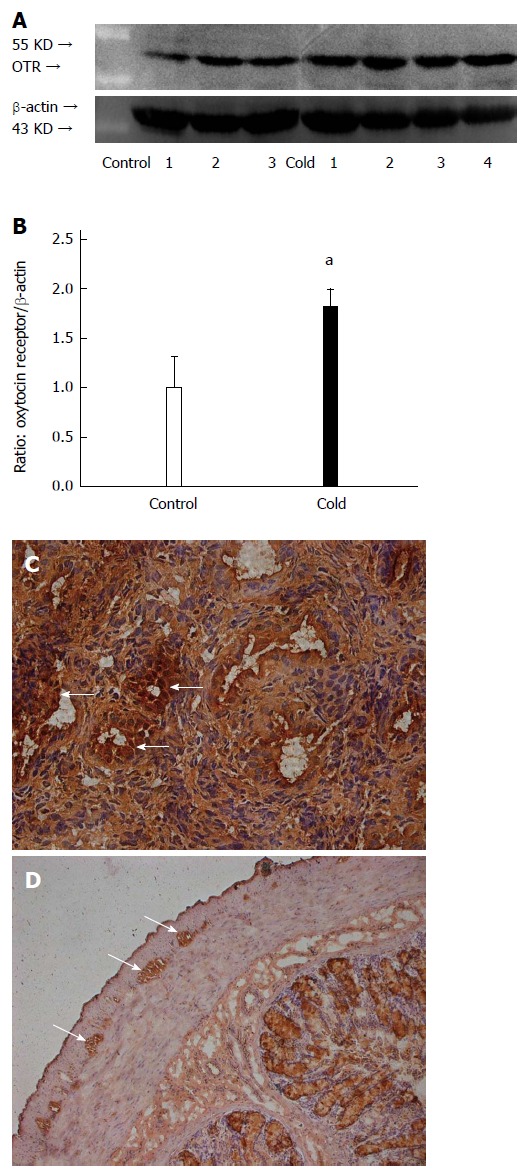
Intragastric cold water stress increased oxytocin receptor expression in colon. A: Representative Western blots. B: Densitometry analysis. C: Positive control of immunoreactivity. Oxytocin (OT) receptor antigen is expressed in the smooth muscle (arrows) of uterus. D: OT receptor immunoreactivity is expressed in myenteric plexus (arrows) of colon. aP < 0.05 vs control group (n = 6). OTR: Oxytocin receptors.
Plasma concentration of OT in rats
As shown in Figure 6A, the weight of the uteri in OVX rats markedly decreased compared with that of control rats (0.14 ± 0.12 g vs 0.46 ± 0.09 g, P < 0.05). After the OVX rats were treated with E2, the weight of the uteri recovered to normal (OVX + E2, 0.51 ± 0.10 g, P < 0.05 vs OVX rats; P > 0.05 vs control rats).
Figure 6.
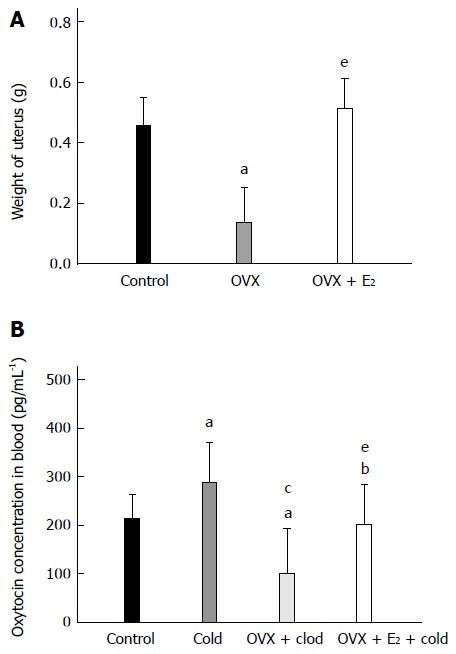
Estradiol regulated the plasma concentration of oxytocin in intragastric cold water stressed rats. A: Weight of uterus. B: The plasma concentration of oxytocin (OT) in the four groups [control, cold, ovariectomy (OVX) + cold and OVX + E2 + cold]. Data are expressed as mean ± SD (n = 6). aP < 0.05 vs control group. cP < 0.05 vs cold group. eP < 0.05 vs OVX + cold group.
The plasma concentration of OT was analyzed in these rats. As shown in Figure 6B, the concentration of OT was significantly increased in the cold group compared with the control group (286.99 ± 83.72 pg/mL vs 212.42 ± 50.62 pg/mL, P = 0.036). After the OVX rats were subjected to gastric instillation with cold saline, the concentration of OT decreased compared with the cold group rats without OVX (100.56 ± 92.71 pg/mL vs 286.99 ± 83.72 pg/mL, P < 0.05 vs the cold group). In OVX + E2 + cold group, the plasma level of OT returned to normal but was still lower than that in the cold group without any treatment (OVX + E2 + cold group, 201.25 ± 80.91 pg/mL; control group, 212.42 ± 50.62 pg/mL; cold group, 286.99 ± 83.72 pg/mL; P > 0.05 vs control group, P < 0.05 vs cold group).
Effect of OVX on OT-induced inhibition of colonic contractility in intragastric cold water stressed rats
After the OVX rats were subjected to gastric instillation with cold saline, OT at 1 or 3 μmol/L still decreased the contractile activity of colon (0.84 ± 0.13, 0.83 ± 0.12, respectively, P < 0.05 vs the data prior to OT administration, Figure 7A, B), but OT-induced inhibition was weaker in the OVX + cold group than that in the cold group (0.84 ± 0.13 vs 0.67 ± 0.11, 0.83 ± 0.12 vs 0.68 ± 0.12, respectively, P < 0.05 vs cold group, Figure 7A, B).
Figure 7.
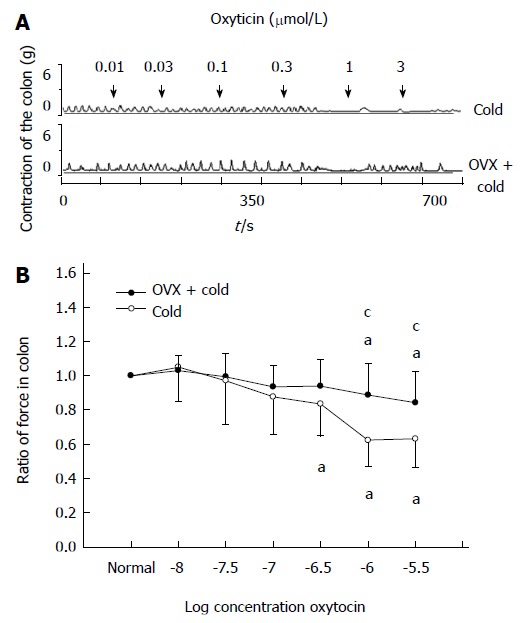
Ovariectomy decreased the oxytocin-induced inhibition of colonic contractility in intragastric cold water stressed rats. A: Representative traces of the effect of ovariectomy (OVX) on oxytocin (OT)-induced colonic response in cold group rats. The drug was administered at the points marked by the arrows. OT-induced inhibition of colonic strips decreased after the rats were ovariectomized. B: The contraction of colonic smooth muscle strips in cold group rats. aP < 0.05 vs the data in normal Krebs solution. cP < 0.05 vs cold group and OVX + cold group (n = 6).
DISCUSSION
The present study showed that the colon transit of cold group rats (stomach irritation with cold physiological saline) was longer than that of control group rats (stomach irritation with room temperature physiological saline). Cold irritation enhanced the inhibitory effect of OT on colonic muscle strips in rats. OT has widespread effects on the motility of the gastrointestinal tract. OT has been shown to inhibit gastric emptying and intestinal transit[41], as well as spontaneous contractions of duodenum in rats[30] and colon in rabbits[19]. In contrast, it excites gastrointestinal motility in rabbits[42], and increases gastric spontaneous contraction[29] and colonic contraction in rats[20]. Our previous study showed that exogenous OT decreased the contractions of proximal colonic smooth muscle strips in control mice, while it increased contractions in antenatal maternal hypoxia mice. OT increased the contractions of distal colonic smooth muscle strips in both antenatal maternal hypoxia and control mice[43]. The effects of OT on gastrointestinal motility might depend on the sex of animal, site of gastrointestinal tract, concentration of OT, level of OT receptors or even the stimulation the animal suffered. Cold water intake might increase the sensitivity of colonic smooth muscle to OT and then affect the colonic motility, which is involved in IBS.
Alterations in bidirectional brain-gut interactions are believed to be involved in the pathogenesis of IBS and related functional gastrointestinal disorders. The central nervous system modulates the gastrointestinal tract via the sympathetic and parasympathetic branches of the autonomic nervous system and the hypothalamic-pituitary axis[44]. OT mRNA expression in the paraventricular nucleus was reported to increase following chronic stress in rats[45]. The present study showed that intragastric cold water stress prolonged colon transit. The inhibitory response of colon to OT was enhanced in intragastric cold water stressed rats. OT is essential to a wide range of stress-related disorders[46,47]. OT might act as a brain-gut signal mediator and be involved in IBS by affecting the colonic motility.
We found that OT receptor antagonist atosiban inhibited the effect of OT on colonic motility in intragastric cold water stressed rats. OT acts on the colon via OT receptors. The voltage-dependent Na+ channel antagonist TTX blocked the inhibitory effect of OT in the colon. Our immunoreactivity study showed that OT receptors were located in the myenteric plexus, rather than in colonic smooth muscle cells. Therefore, OT might affect colonic smooth muscle contraction via OT receptors in the myenteric plexus. Our study further demonstrated that the expression of OT receptors is up-regulated in intragastric cold water stressed rats. Chronic isolation was reported to regulate plasma OT level and gene expression of OT receptors in the heart of prairie voles[48]. Up-regulation of OT receptors may partially account for the enhanced inhibitory effect of OT on colonic contractility in the cold group.
Following chronic homotypic stress, OT-knockout mice fail to restore accelerated colonic transit compared with wild type mice[49]. Proximal colon distension stimulates OT-containing hypothalamic neurons in rats[50]. We also compared the plasma concentration of OT between the cold group and control group, and found that OT level was significantly higher in the cold group than in the control group. Intragastric cold stress might stimulate the synthesis and release of OT, and then increase the concentration of OT in blood. Increased plasma OT might also be partly responsible for the impaired colon transit in the cold group.
OT and OT receptor mRNA levels are regulated by estrogen and are both reduced due to ovariectomy[51,52]. Estradiol was reported to affect OT neurons and modulate the secretion of OT in response to the increase of osmolality induced by refeeding in rats[53]. For the intragastric cold water stressed rats in our study, the plasma levels of OT decreased in the OVX rats; when the OVX rats were treated with estradiol, the plasma levels of OT were almost the same as the control rats, but were still lower than that of the cold group without any treatment. Estradiol also regulates the secretion of OT in response to intragastric cold stress in rats.
Estrogens facilitate social recognition by regulating OT production and OT receptors[54]. Estradiol modulates the cardiovascular responses induced by hemorrhage via enhancement of OT neuron activity[55]. To investigate the involvement of ovarian hormones in OT-induced colonic contraction, the rats were ovariectomized and treated with chronic intragastric cold stress. Our study showed that the response of colonic muscle strips to OT was attenuated in OVX rats. Estradiol facilitates OT-induced colonic contraction in intragastric cold water stressed rats by increasing OT production. OT concentration changes in depressed women, suggesting that OT signaling may provide a mechanism by which to better understand female-biased risk for the development of depressive disorders[56]. OT signaling may also be involved in female-biased risk for IBS.
In conclusion, we found that cold water intake inhibited colonic motility at least partially through oxytocin-oxytocin receptor signaling in the myenteric nervous system pathway, which is estrogen dependent.
COMMENTS
Background
The incidence of irritable bowel syndrome (IBS) is high, predominantly in females, although the cause is largely unknown. Cold meal intake affects gastric myoelectrical and contractile activities. Cold water intake increases plasma 5-HT concentrations in subjects with IBS.
Research frontiers
Oxytocin (OT) is essential to a wide range of stress-related disorders. OT effects are generally related to trait effects related to social or emotional functions, particularly in females. This study suggests that OT might be a potential mechanism involved in IBS.
Innovations and breakthroughs
Cold water intake decreased the colonic transit and OT-induced inhibition of colonic contraction. OT decreased colonic contraction via OT receptors in the myenteric plexus. Cold water intake also increased blood concentration of OT. Estradiol regulated blood concentration of OT and OT-induced colonic contraction.
Applications
By understanding how cold water intake affect colonic motility and the involvement of OT in colonic contraction, this study may represent a future strategy for therapeutic intervention in the treatment of patients with IBS.
Terminology
OT is a neuropeptide synthesized by magnocellular neurons in the paraventricular and supraoptic nuclei of the hypothalamus. OT is released from the pituitary and goes into the blood. OT receptors are expressed in colon. OT acts on the OT receptors of the colon and regulates colonic motility. Hypothalamic OT-colonic OT receptor might be an important brain-gut axis to control colonic motility.
Peer review
The authors examined the effect of cold water intake on colonic motility and on OT-induced colonic contraction. The study revealed that cold water intake inhibited colonic motility at least partly through OT-OT receptor signaling in the myenteric plexus pathway, which is estrogen dependent. The experimental design is reasonable and the findings are interesting.
Footnotes
Supported by National Natural Science Foundation of China, No. 30872475 and No. 31271234
P- Reviewer: Dai GL S- Editor: Ma N L- Editor: Logan S E- Editor: Liu XM
References
- 1.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 2.Saito YA, Schoenfeld P, Locke GR. The epidemiology of irritable bowel syndrome in North America: a systematic review. Am J Gastroenterol. 2002;97:1910–1915. doi: 10.1111/j.1572-0241.2002.05913.x. [DOI] [PubMed] [Google Scholar]
- 3.Gwee KA. Irritable bowel syndrome in developing countries--a disorder of civilization or colonization? Neurogastroenterol Motil. 2005;17:317–324. doi: 10.1111/j.1365-2982.2005.00627.x. [DOI] [PubMed] [Google Scholar]
- 4.Chang L, Toner BB, Fukudo S, Guthrie E, Locke GR, Norton NJ, Sperber AD. Gender, age, society, culture, and the patient’s perspective in the functional gastrointestinal disorders. Gastroenterology. 2006;130:1435–1446. doi: 10.1053/j.gastro.2005.09.071. [DOI] [PubMed] [Google Scholar]
- 5.Zhao Y, Zou D, Wang R, Ma X, Yan X, Man X, Gao L, Fang J, Yan H, Kang X, et al. Dyspepsia and irritable bowel syndrome in China: a population-based endoscopy study of prevalence and impact. Aliment Pharmacol Ther. 2010;32:562–572. doi: 10.1111/j.1365-2036.2010.04376.x. [DOI] [PubMed] [Google Scholar]
- 6.Lovell RM, Ford AC. Effect of gender on prevalence of irritable bowel syndrome in the community: systematic review and meta-analysis. Am J Gastroenterol. 2012;107:991–1000. doi: 10.1038/ajg.2012.131. [DOI] [PubMed] [Google Scholar]
- 7.Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108–2131. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 8.Yu YB, Zuo XL, Zhao QJ, Chen FX, Yang J, Dong YY, Wang P, Li YQ. Brain-derived neurotrophic factor contributes to abdominal pain in irritable bowel syndrome. Gut. 2012;61:685–694. doi: 10.1136/gutjnl-2011-300265. [DOI] [PubMed] [Google Scholar]
- 9.Wong BS, Camilleri M, Busciglio I, Carlson P, Szarka LA, Burton D, Zinsmeister AR. Pharmacogenetic trial of a cannabinoid agonist shows reduced fasting colonic motility in patients with nonconstipated irritable bowel syndrome. Gastroenterology. 2011;141:1638–1647.e1-7. doi: 10.1053/j.gastro.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuo XL, Li YQ, Yang XZ, Guo M, Guo YT, Lu XF, Li JM, Desmond PV. Plasma and gastric mucosal 5-hydroxytryptamine concentrations following cold water intake in patients with diarrhea-predominant irritable bowel syndrome. J Gastroenterol Hepatol. 2007;22:2330–2337. doi: 10.1111/j.1440-1746.2006.04772.x. [DOI] [PubMed] [Google Scholar]
- 11.Zuo XL, Li YQ, Shi L, Lv GP, Kuang RG, Lu XF, Li JM, Desmond PV. Visceral hypersensitivity following cold water intake in subjects with irritable bowel syndrome. J Gastroenterol. 2006;41:311–317. doi: 10.1007/s00535-005-1766-x. [DOI] [PubMed] [Google Scholar]
- 12.Verhagen MA, Luijk HD, Samsom M, Smout AJ. Effect of meal temperature on the frequency of gastric myoelectrical activity. Neurogastroenterol Motil. 1998;10:175–181. doi: 10.1046/j.1365-2982.1998.00089.x. [DOI] [PubMed] [Google Scholar]
- 13.Sun WM, Penagini R, Hebbard G, Malbert C, Jones KL, Emery S, Dent J, Horowitz M. Effect of drink temperature on antropyloroduodenal motility and gastric electrical activity in humans. Gut. 1995;37:329–334. doi: 10.1136/gut.37.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alonso C, Guilarte M, Vicario M, Ramos L, Ramadan Z, Antolín M, Martínez C, Rezzi S, Saperas E, Kochhar S, et al. Maladaptive intestinal epithelial responses to life stress may predispose healthy women to gut mucosal inflammation. Gastroenterology. 2008;135:163–172.e1. doi: 10.1053/j.gastro.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 15.Fuchs AR, Fuchs F, Husslein P, Soloff MS, Fernström MJ. Oxytocin receptors and human parturition: a dual role for oxytocin in the initiation of labor. Science. 1982;215:1396–1398. doi: 10.1126/science.6278592. [DOI] [PubMed] [Google Scholar]
- 16.De Dreu CK, Greer LL, Handgraaf MJ, Shalvi S, Van Kleef GA, Baas M, Ten Velden FS, Van Dijk E, Feith SW. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science. 2010;328:1408–1411. doi: 10.1126/science.1189047. [DOI] [PubMed] [Google Scholar]
- 17.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 18.Love TM, Enoch MA, Hodgkinson CA, Peciña M, Mickey B, Koeppe RA, Stohler CS, Goldman D, Zubieta JK. Oxytocin gene polymorphisms influence human dopaminergic function in a sex-dependent manner. Biol Psychiatry. 2012;72:198–206. doi: 10.1016/j.biopsych.2012.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie D, Chen L, Liu C, Liu K. The inhibitory effects of oxytocin on distal colonic contractile activity in rabbits are enhanced by ovarian steroids. Acta Physiol (Oxf) 2006;186:141–149. doi: 10.1111/j.1365-201X.2005.01506.x. [DOI] [PubMed] [Google Scholar]
- 20.Feng M, Qin J, Wang C, Ye Y, Wang S, Xie D, Wang PS, Liu C. Estradiol upregulates the expression of oxytocin receptor in colon in rats. Am J Physiol Endocrinol Metab. 2009;296:E1059–E1066. doi: 10.1152/ajpendo.90609.2008. [DOI] [PubMed] [Google Scholar]
- 21.Yokoyama Y, Ueda T, Irahara M, Aono T. Releases of oxytocin and prolactin during breast massage and suckling in puerperal women. Eur J Obstet Gynecol Reprod Biol. 1994;53:17–20. doi: 10.1016/0028-2243(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 22.Giraldi A, Enevoldsen AS, Wagner G. Oxytocin and the initiation of parturition. A review. Dan Med Bull. 1990;37:377–383. [PubMed] [Google Scholar]
- 23.Gibbs DM. Vasopressin and oxytocin: hypothalamic modulators of the stress response: a review. Psychoneuroendocrinology. 1986;11:131–139. doi: 10.1016/0306-4530(86)90048-x. [DOI] [PubMed] [Google Scholar]
- 24.Light KC, Grewen KM, Amico JA, Brownley KA, West SG, Hinderliter AL, Girdler SS. Oxytocinergic activity is linked to lower blood pressure and vascular resistance during stress in postmenopausal women on estrogen replacement. Horm Behav. 2005;47:540–548. doi: 10.1016/j.yhbeh.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Seltzer LJ, Ziegler TE, Pollak SD. Social vocalizations can release oxytocin in humans. Proc Biol Sci. 2010;277:2661–2666. doi: 10.1098/rspb.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morhenn V, Beavin LE, Zak PJ. Massage increases oxytocin and reduces adrenocorticotropin hormone in humans. Altern Ther Health Med. 2012;18:11–18. [PubMed] [Google Scholar]
- 27.Kim JS, Kim WB, Kim YB, Lee Y, Kim YS, Shen FY, Lee SW, Park D, Choi HJ, Hur J, et al. Chronic hyperosmotic stress converts GABAergic inhibition into excitation in vasopressin and oxytocin neurons in the rat. J Neurosci. 2011;31:13312–13322. doi: 10.1523/JNEUROSCI.1440-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monstein HJ, Grahn N, Truedsson M, Ohlsson B. Oxytocin and oxytocin-receptor mRNA expression in the human gastrointestinal tract: a polymerase chain reaction study. Regul Pept. 2004;119:39–44. doi: 10.1016/j.regpep.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 29.Qin J, Feng M, Wang C, Ye Y, Wang PS, Liu C. Oxytocin receptor expressed on the smooth muscle mediates the excitatory effect of oxytocin on gastric motility in rats. Neurogastroenterol Motil. 2009;21:430–438. doi: 10.1111/j.1365-2982.2009.01282.x. [DOI] [PubMed] [Google Scholar]
- 30.Lv Y, Feng M, Che T, Sun H, Luo Y, Liu K, Liu C. CCK mediated the inhibitory effect of oxytocin on the contraction of longitudinal muscle strips of duodenum in male rats. Pflugers Arch. 2010;460:1063–1071. doi: 10.1007/s00424-010-0880-7. [DOI] [PubMed] [Google Scholar]
- 31.Welch MG, Tamir H, Gross KJ, Chen J, Anwar M, Gershon MD. Expression and developmental regulation of oxytocin (OT) and oxytocin receptors (OTR) in the enteric nervous system (ENS) and intestinal epithelium. J Comp Neurol. 2009;512:256–270. doi: 10.1002/cne.21872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Che T, Sun H, Li J, Yu X, Zhu D, Xue B, Liu K, Zhang M, Kunze W, Liu C. Oxytocin hyperpolarizes cultured duodenum myenteric intrinsic primary afferent neurons by opening BK(Ca) channels through IP₃ pathway. J Neurochem. 2012;121:516–525. doi: 10.1111/j.1471-4159.2012.07702.x. [DOI] [PubMed] [Google Scholar]
- 33.Soloff MS, Alexandrova M, Fernstrom MJ. Oxytocin receptors: triggers for parturition and lactation? Science. 1979;204:1313–1315. doi: 10.1126/science.221972. [DOI] [PubMed] [Google Scholar]
- 34.Fuchs AR, Periyasamy S, Alexandrova M, Soloff MS. Correlation between oxytocin receptor concentration and responsiveness to oxytocin in pregnant rat myometrium: effects of ovarian steroids. Endocrinology. 1983;113:742–749. doi: 10.1210/endo-113-2-742. [DOI] [PubMed] [Google Scholar]
- 35.Fleming JG, Spencer TE, Safe SH, Bazer FW. Estrogen regulates transcription of the ovine oxytocin receptor gene through GC-rich SP1 promoter elements. Endocrinology. 2006;147:899–911. doi: 10.1210/en.2005-1120. [DOI] [PubMed] [Google Scholar]
- 36.Mamrut S, Harony H, Sood R, Shahar-Gold H, Gainer H, Shi YJ, Barki-Harrington L, Wagner S. DNA methylation of specific CpG sites in the promoter region regulates the transcription of the mouse oxytocin receptor. PLoS One. 2013;8:e56869. doi: 10.1371/journal.pone.0056869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hrabovszky E, Kalló I, Hajszán T, Shughrue PJ, Merchenthaler I, Liposits Z. Expression of estrogen receptor-beta messenger ribonucleic acid in oxytocin and vasopressin neurons of the rat supraoptic and paraventricular nuclei. Endocrinology. 1998;139:2600–2604. doi: 10.1210/endo.139.5.6024. [DOI] [PubMed] [Google Scholar]
- 38.Sakamoto H, Matsuda K, Hosokawa K, Nishi M, Morris JF, Prossnitz ER, Kawata M. Expression of G protein-coupled receptor-30, a G protein-coupled membrane estrogen receptor, in oxytocin neurons of the rat paraventricular and supraoptic nuclei. Endocrinology. 2007;148:5842–5850. doi: 10.1210/en.2007-0436. [DOI] [PubMed] [Google Scholar]
- 39.Sharma D, Handa RJ, Uht RM. The ERβ ligand 5α-androstane, 3β,17β-diol (3β-diol) regulates hypothalamic oxytocin (Oxt) gene expression. Endocrinology. 2012;153:2353–2361. doi: 10.1210/en.2011-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hiroi R, Lacagnina AF, Hinds LR, Carbone DG, Uht RM, Handa RJ. The androgen metabolite, 5α-androstane-3β,17β-diol (3β-diol), activates the oxytocin promoter through an estrogen receptor-β pathway. Endocrinology. 2013;154:1802–1812. doi: 10.1210/en.2012-2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu CL, Doong ML, Wang PS. Involvement of cholecystokinin receptor in the inhibition of gastrointestinal motility by oxytocin in ovariectomized rats. Eur J Pharmacol. 2008;580:407–415. doi: 10.1016/j.ejphar.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 42.Li L, Kong X, Liu H, Liu C. Systemic oxytocin and vasopressin excite gastrointestinal motility through oxytocin receptor in rabbits. Neurogastroenterol Motil. 2007;19:839–844. doi: 10.1111/j.1365-2982.2007.00953.x. [DOI] [PubMed] [Google Scholar]
- 43.Xie DP, Yang X, Cao CY, Wang HH, Li YX, Qin Y, Zhang JP, Chang XW. Exogenous oxytocin reverses the decrease of colonic smooth muscle contraction in antenatal maternal hypoxia mice via ganglia. Regul Pept. 2011;172:30–34. doi: 10.1016/j.regpep.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12:453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshimoto S, Cerjak D, Babygirija R, Bulbul M, Ludwig K, Takahashi T. Hypothalamic circuit regulating colonic transit following chronic stress in rats. Stress. 2012;15:227–236. doi: 10.3109/10253890.2011.614297. [DOI] [PubMed] [Google Scholar]
- 46.Owen SF, Tuncdemir SN, Bader PL, Tirko NN, Fishell G, Tsien RW. Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nature. 2013;500:458–462. doi: 10.1038/nature12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tyzio R, Nardou R, Ferrari DC, Tsintsadze T, Shahrokhi A, Eftekhari S, Khalilov I, Tsintsadze V, Brouchoud C, Chazal G, et al. Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science. 2014;343:675–679. doi: 10.1126/science.1247190. [DOI] [PubMed] [Google Scholar]
- 48.Pournajafi-Nazarloo H, Kenkel W, Mohsenpour SR, Sanzenbacher L, Saadat H, Partoo L, Yee J, Azizi F, Carter CS. Exposure to chronic isolation modulates receptors mRNAs for oxytocin and vasopressin in the hypothalamus and heart. Peptides. 2013;43:20–26. doi: 10.1016/j.peptides.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 49.Babygirija R, Bülbül M, Cerjak D, Ludwig K, Takahashi T. Sustained acceleration of colonic transit following chronic homotypic stress in oxytocin knockout mice. Neurosci Lett. 2011;495:77–81. doi: 10.1016/j.neulet.2011.03.045. [DOI] [PubMed] [Google Scholar]
- 50.Wang L, Martínez V, Larauche M, Taché Y. Proximal colon distension induces Fos expression in oxytocin-, vasopressin-, CRF- and catecholamines-containing neurons in rat brain. Brain Res. 2009;1247:79–91. doi: 10.1016/j.brainres.2008.09.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ho ML, Lee JN. Ovarian and circulating levels of oxytocin and arginine vasopressin during the estrous cycle in the rat. Acta Endocrinol (Copenh) 1992;126:530–534. doi: 10.1530/acta.0.1260530. [DOI] [PubMed] [Google Scholar]
- 52.Larcher A, Neculcea J, Breton C, Arslan A, Rozen F, Russo C, Zingg HH. Oxytocin receptor gene expression in the rat uterus during pregnancy and the estrous cycle and in response to gonadal steroid treatment. Endocrinology. 1995;136:5350–5356. doi: 10.1210/endo.136.12.7588281. [DOI] [PubMed] [Google Scholar]
- 53.Lucio-Oliveira F, Franci CR. Effect of the interaction between food state and the action of estrogen on oxytocinergic system activity. J Endocrinol. 2012;212:129–138. doi: 10.1530/JOE-11-0272. [DOI] [PubMed] [Google Scholar]
- 54.Gabor CS, Phan A, Clipperton-Allen AE, Kavaliers M, Choleris E. Interplay of oxytocin, vasopressin, and sex hormones in the regulation of social recognition. Behav Neurosci. 2012;126:97–109. doi: 10.1037/a0026464. [DOI] [PubMed] [Google Scholar]
- 55.Mecawi AS, Vilhena-Franco T, Araujo IG, Reis LC, Elias LL, Antunes-Rodrigues J. Estradiol potentiates hypothalamic vasopressin and oxytocin neuron activation and hormonal secretion induced by hypovolemic shock. Am J Physiol Regul Integr Comp Physiol. 2011;301:R905–R915. doi: 10.1152/ajpregu.00800.2010. [DOI] [PubMed] [Google Scholar]
- 56.Yuen KW, Garner JP, Carson DS, Keller J, Lembke A, Hyde SA, Kenna HA, Tennakoon L, Schatzberg AF, Parker KJ. Plasma oxytocin concentrations are lower in depressed vs. healthy control women and are independent of cortisol. J Psychiatr Res. 2014;51:30–36. doi: 10.1016/j.jpsychires.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]


