Abstract
AIM: To establish a model to predict long-term survival of hepatocellular carcinoma (HCC) patients after liver transplantation (MHCAT).
METHODS: Two hundred and twenty-three patients with HCC were followed for at least six years to identify independent risk factors for long-term survival after liver transplantation (LT). The criteria for HCC liver transplantation included the Milan, University of California San Francisco, Hangzhou and Shanghai Fudan criteria. The Cox regression model was used to build MHCAT specifying these criteria. A survival analysis was carried out for patients with high or low risk.
RESULTS: The one-, three- and five-year cumulative survival of HCC patients after LT was 78.9%, 53.2% and 46.4%, respectively. Of the HCC patients, the proportion meeting the Hangzhou and Fudan criteria was significantly higher than the proportion meeting the Milan criteria (64.6% vs 39.5%, 52.0% vs 39.5%, P < 0.05). Moreover, the proportion meeting the Hangzhou criteria was also significantly higher than the proportion meeting other criteria (P < 0.01). Pre-operative alfa-fetoprotein level, intraoperative blood loss and retransplantation were common significant predictors of long-term survival in HCC patients with reference to the Milan, University of California San Francisco and Fudan criteria, whereas in MHCAT based on the Hangzhou criteria, total bilirubin, intraoperative blood loss and retransplantation were independent predictors. The c-statistic for MHCAT was 0.773-0.824, with no statistical difference among these four criteria. According to the MHCAT scoring system, patients with low risk showed a higher five-year survival than those with high risk (P < 0.001).
CONCLUSION: MHCAT can effectively predict long-term survival for HCC patients, but needs to be verified by multi-center retrospective or randomized controlled trials.
Keywords: Criteria, Hepatocellular carcinoma, Liver transplantation, MHCAT, Survival model
Core tip: This study was conducted to establish a model to predict long-term survival of hepatocellular carcinoma (HCC) patients after liver transplantation (MHCAT) with reference to different criteria and peri-transplant risk factors. We found that MHCAT can effectively predict long-term survival for HCC patients, but needs to be verified by multi-center retrospective or randomized controlled trials.
INTRODUCTION
Liver transplantation (LT) is now a widely accepted treatment for patients with hepatocellular carcinoma (HCC), curing both the underlying disease and the cancer. HCC is currently an indication for LT, which accounts for 16% and 20.9% of all cases in Europe and the United States, respectively[1,2]. In China, the rate is as high as 40%[3]. The progression of HCC and its prognosis after LT are quite different from benign end-stage liver diseases and the success of LT for HCC depends largely on the tumor load, such as size, number of lesions and biological activities. Therefore, the Milan criteria (a solitary HCC nodule 5.0 cm or less in diameter, or no more than three tumor nodules with the largest lesion 3.0 cm or less in diameter, without tumor invasion of blood vessels or lymph nodes) were introduced to achieve a compatible post-transplant survival for HCC and other indications for LT[4,5]. This has also led to controversy regarding the extension of criteria boundaries, such as the University of California San Francisco (UCSF) criteria (similar to the Milan criteria, extending the diameter to 6.5 cm for a solitary nodule and 4.5 cm for the largest, and 8.0 cm as the total when multiple nodules are present)[6,7], Shanghai Fudan criteria (similar to the UCSF criteria, extending the diameter to 9.0 cm for a solitary nodule and 5.0 cm for the largest, and 9.0 cm as the total when multiple nodules are present) and Hangzhou criteria (a total tumor diameter 8 cm or less or total tumor diameter more than 8 cm, with Edmondson grade I or II and pre-operative alfa-fetoprotein (AFP) level 400 ng/mL or less, simultaneously)[8,9]. In view of the global shortage of organ donation, it is critical to achieve a balance between not only a waiting list and post-transplant survival, but also benefit in HCC patients and other recipients. This study was conducted to build a model to predict long-term survival of HCC patients after LT with reference to different criteria and peri-transplant risk factors.
MATERIALS AND METHODS
Subjects and data collection
We followed 223 HCC patients who received deceased donor LT from January 2001 to December 2006 at Changzheng Hospital (Shanghai, China), including four cases of retransplantation due to HCC recurrence. Patients with cholangiocellular carcinoma, mixed liver cancer and malignancies discovered incidentally during transplant were excluded from the study. The follow-up of all 223 cases started on the day of LT until death, retransplantation or the end of the study (December 31, 2012).
Follow-up ended at the second transplantation in some recipients. This was to avoid the halo effect of retransplantation on modeling survival after the first LT. Pre- and intra-operative potential risk factors and criteria for HCC are listed in Table 1. All data included in the final analysis were extracted from the records of our center in the China Liver Transplant Registry. Ethical approval for the use of human subjects was obtained from the Research Ethics Committee of Changzheng Hospital, consistent with the ethical guidelines of the 1975 Declaration of Helsinki. Informed consent was obtained from each patient.
Table 1.
Characteristics of hepatocellular carcinoma patients and univariate assessment of long-term survival after liver transplantation n (%)
| Variables | Value | P value |
| Age (yr) | 47.99 ± 9.09 | < 0.001 |
| Gender | 0.048 | |
| Male | 204 (91.5) | |
| Female | 19 (8.5) | |
| Blood type | 0.951 | |
| A | 66 (29.6) | |
| B | 62 (27.8) | |
| O | 75 (33.6) | |
| AB | 20 (9.0) | |
| Identical | 195 (87.4) | 0.492 |
| History | ||
| Cardiovascular disease | 17 (7.6) | 0.358 |
| Respiratory disease | 3 (1.3) | 0.296 |
| Diabetes mellitus | 17 (7.6) | 0.736 |
| Hepatitis B virus infection | 223 (100.0) | 0.252 |
| Ascites | 144 (64.6) | 0.426 |
| Variceal bleeding | 25 (11.2) | 0.023 |
| Encephalopathy | 4 (1.8) | 0.799 |
| Treatment related to hepatocellular carcinoma | ||
| Hepatectomy | 21 (9.4) | 0.693 |
| Transplantation | 4 (1.8) | < 0.001 |
| TACE | 44 (19.7) | 0.756 |
| Criteria | ||
| Milan | < 0.001 | |
| Met | 88 (39.5) | |
| Exceeded | 135 (60.5) | |
| UCSF | < 0.001 | |
| Met | 97 (43.5) | |
| Exceeded | 126 (56.5) | |
| Shanghai Fudan | < 0.001 | |
| Met | 116 (52.0) | |
| Exceeded | 107 (48.0) | |
| Hangzhou | < 0.001 | |
| Met | 144 (64.6) | |
| Exceeded | 79 (35.4) | |
| MELD score | 14.12 ± 7.02 | 0.086 |
| SCr (μmol/L) | 71.41 ± 69.10 | 0.173 |
| TB (μmol/L) | 89.45 ± 174.19 | < 0.001 |
| INR | 1.42 ± 0.45 | 0.010 |
| AFP (ng/mL) | 5694.77 ± 12584.00 | < 0.001 |
| Cold ischemia time (h) | 9.21 ± 2.07 | 0.129 |
| Intraoperative blood loss (IU) | 9.24 ± 8.50 | 0.002 |
| Operation duration (h) | 8.29 ± 1.58 | 0.200 |
| Liver transplantation technique | 0.001 | |
| Classic | 212 (95.1) | |
| Piggyback | 11 (4.9) | |
| Biliary reconstruction | < 0.001 | |
| Duct-to-duct | 221 (99.1) | |
| Roux-en-Y | 2 (0.9) | |
| Edmondson grading | 0.790 | |
| I | 8 (3.6) | |
| II | 200 (89.7) | |
| III | 13 (5.8) | |
| IV | 2 (0.9) | |
MELD: Model for end-stage liver disease; SCr: Serum creatinine; TB: Total bilirubin; INR: International normalized ratio; AFP: Alfa-fetoprotein; UCSF: University of California San Francisco; TACE: Transcatheter arterial chemoembolization.
Immunosuppressive protocol and follow-up
The post-LT immunosuppressive protocols were tacrolimus or cyclosporine, mycophenolate mofetil and steroids. Steroids were usually tapered and withdrawn within the first month after LT. Follow-up was routinely conducted in the outpatient clinics. Patients were followed up every 2 mo during the first postoperative year and at least every 3 to 4 mo thereafter. All patients were monitored prospectively by serum AFP, abdominal ultrasonography, and chest X-ray every 1 to 6 mo, according to the postoperative time. For patients with test results suggestive of recurrence, computed tomography (CT) and/or magnetic resonance imaging (MRI) were used to verify whether intrahepatic recurrence and/or distal metastasis had occurred. A diagnosis of recurrence was based on typical imaging appearance on CT and/or MRI scan and an elevated AFP level.
Statistical analysis
Data were analyzed using statistical software (PASW Statistics@ 18; Chicago, IL, United States). Continuous variables were reported as mean ± SD or as median (range, minimum to maximum) if the variable was not normally distributed. Categorical variables were given as frequencies (%). The primary outcome was death or retransplantation of patients. The patients remained at risk as long as they were free from recurrence and alive during the follow-up. Kaplan-Meier analysis with the log-rank test and Cox proportional hazards model were used for time-to-event analysis. Covariate selection was a non-automated form of backward elimination. An adjusted hazard ratio, together with 95%CI, was used as the risk measurement for mortality. The receiver operating characteristic (ROC) curve was used to determine the efficacy of the survival models, and the area under the ROC curve (c-statistic) was compared using Kruskal and Wallis analysis. P values and 95%CIs were estimated in a two-tailed manner. Differences were considered to be statistically significant at P < 0.05.
RESULTS
Characteristics of the subjects
All LTs were ABO type compatible. Among the 223 cases of HCC undergoing LTs, 135 were beyond the Milan criteria. During the period between January 2001 and December 2006, we performed 502 LTs in total. As 44.4% of the 502 patients had HCC, HCC was considered a major indication for LT in our center. These patients were followed for 46.31 mo on average (range, 0.03-118.25 mo). The one-, three- and five-year cumulative survival of HCC patients after LT was 78.9%, 53.2% and 46.4%, respectively. The one-, three- and five-year HCC recurrence-free survival after LT was 77.4%, 52.6% and 45.8%, respectively. Of the HCC patients in our study, the proportion meeting the Hangzhou and Fudan criteria was significantly higher than the proportion meeting the Milan criteria (64.6% vs 39.5%; 52.0% vs 39.5%, P < 0.05). Moreover, the proportion meeting the Hangzhou criteria was also significantly higher than the proportion meeting other criteria (P < 0.01). Demographic and clinical characteristics are summarized in Table 1.
Univariate analysis
A univariate analysis was conducted using the Cox proportional hazards model. This analysis showed that the predictors significantly affecting long-term survival included age, gender, history of variceal bleeding, retransplantation, total bilirubin (TB), international normalized ratio, AFP, intraoperative blood loss, LT technique (classic or piggyback), biliary reconstruction pattern and four HCC LT criteria; the P values of which were all < 0.05.
Constructing MHCAT based on different criteria
The characteristics presented in the plurality and the lowest value was used as the reference group for qualitative and quantitative data, respectively. In particular, HCC meeting the indication criteria was used as a reference.
All risk factors that were significant in the univariate analysis were entered into the Cox proportional hazard model and the final MHCAT was built using a backward selection procedure. Intraoperative blood loss, retransplantation and AFP level were common significant predictors for survival of five years in HCC patients after LT with reference to Milan, UCSF, and Shanghai Fudan criteria, whereas in MHCAT based on the Hangzhou criteria, TB, intraoperative blood loss and retransplantation were independent predictors (Table 2). ROC curves were generated for the MHCAT scoring system (Figure 1). The area under the ROC curves for MHCAT based on the Milan, UCSF, Fudan and Hangzhou criteria was 0.818 (95%CI: 0.763-0.872), 0.824 (95%CI: 0.771-0.878), 0.811 (95%CI: 0.755-0.867) and 0.773 (95%CI: 0.711-0.835), respectively. These high areas under the ROC values, of which there was no significant difference using the Kruskal and Wallis test (P > 0.05), showed that MHCAT had a good performance in predicting five-year post-transplant survival of HCC patients. The MHCAT cut-off value with reference to the Milan, UCSF, Fudan and Hangzhou criteria was 1.749 (sensitivity 0.821; specificity 0.320), 1.714 (sensitivity 0.813; specificity 0.300), 1.152 (sensitivity 0.754; specificity 0.250) and 1.295 (sensitivity 0.642; specificity 0.130), respectively. Patients were divided into high-risk or low-risk groups according to these four cut-off values. Of 223 HCC cases, the number of cases in the low-risk group with reference to MHCAT based on the Milan, UCSF, Fudan and Hangzhou criteria was 91, 93, 104 and 121, respectively. There were more HCC patients in the low-risk group under the Hangzhou criteria that under the Milan and UCSF criteria (P < 0.05). Irrespective of the criteria adopted, Kaplan-Meier analysis showed a significantly higher long-term survival in low-risk patients compared with high-risk patients (Figure 2, P < 0.001).
Table 2.
Long-term survival model for hepatocellular carcinoma patients after liver transplantation
| Variables | Regression coefficient | Regression coefficient SE | P value | Hazard ratio | 95%CI |
| Milan1 | |||||
| AFP (logevalue) | 0.093 | 0.031 | 0.003 | 1.097 | 1.032-1.167 |
| Intraoperative blood loss | 0.038 | 0.009 | < 0.001 | 1.039 | 1.021-1.057 |
| Retransplantation | 1.429 | 0.522 | 0.006 | 4.173 | 1.501-11.604 |
| Criteria exceeded | 1.504 | 0.253 | < 0.001 | 4.500 | 2.741-7.386 |
| University of California San Francisco2 | |||||
| AFP (logevalue) | 0.090 | 0.031 | 0.003 | 1.094 | 1.030-1.161 |
| Intraoperative blood loss | 0.038 | 0.009 | < 0.001 | 1.039 | 1.022-1.057 |
| Retransplantation | 1.373 | 0.522 | 0.009 | 3.947 | 1.419-10.976 |
| Criteria exceeded | 1.555 | 0.242 | < 0.001 | 4.737 | 2.950-7.607 |
| Shanghai Fudan3 | |||||
| AFP (logevalue) | 0.091 | 0.031 | 0.003 | 1.096 | 1.031-1.165 |
| Intraoperative blood loss | 0.034 | 0.008 | < 0.001 | 1.035 | 1.018-1.052 |
| Retransplantation | 1.296 | 0.523 | 0.013 | 3.654 | 1.311-10.182 |
| Criteria exceeded | 1.361 | 0.213 | < 0.001 | 3.899 | 2.566-5.924 |
| Hangzhou4 | |||||
| TB (logevalue) | 0.186 | 0.094 | 0.049 | 1.204 | 1.001-1.449 |
| Intraoperative blood loss | 0.020 | 0.010 | 0.046 | 1.020 | 1.000-1.040 |
| Retransplantation | 1.312 | 0.520 | 0.012 | 3.715 | 1.340-10.295 |
| Criteria exceeded | 1.520 | 0.190 | < 0.001 | 4.570 | 3.149-6.633 |
MHCAT (Milan) = 0.093 × LnAFP + 0.038 × IBL + 1.429 × Re (Re = 0 without retransplantation; Re = 1 with retransplantation) + 1.504 × Cri (Cri = 0 within criteria; Cri = 1 exceeding criteria);
MHCAT (University of California San Francisco) = 0.090 × LnAFP + 0.038*IBL + 1.373 × Re (Re = 0 without retransplantation; Re = 1 with retransplantation) + 1.555 × Cri (Cri = 0 within criteria; Cri = 1 exceeding criteria);
MHCAT (Fudan) = 0.091 × LnAFP +0.034 × IBL + 1.296 × Re (Re = 0 without retransplantation; Re = 1 with retransplantation) + 1.361 × Cri (Cri = 0 within criteria; Cri = 1 exceeding criteria);
MHCAT (Hangzhou) = 0.186 × LnTB + 0.020 × IBL + 1.312 × Re (Re = 0 without retransplantation; Re = 1 with retransplantation) + 1.520 × Cri (Cri = 0 within criteria; Cri = 1 exceeding criteria). TB: Total bilirubin; AFP: Alfa-fetoprotein.
Figure 1.
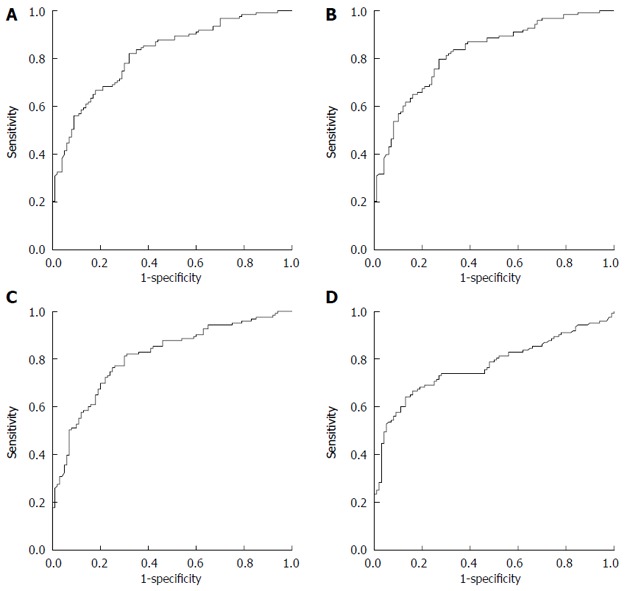
Receiver operating curve for model to predict long-term survival of hepatocellular carcinoma patients after liver transplantation scoring system. A: Milan criteria; B: University of California San Francisco criteria; C: Shanghai Fudan criteria; D: Hangzhou criteria.
Figure 2.
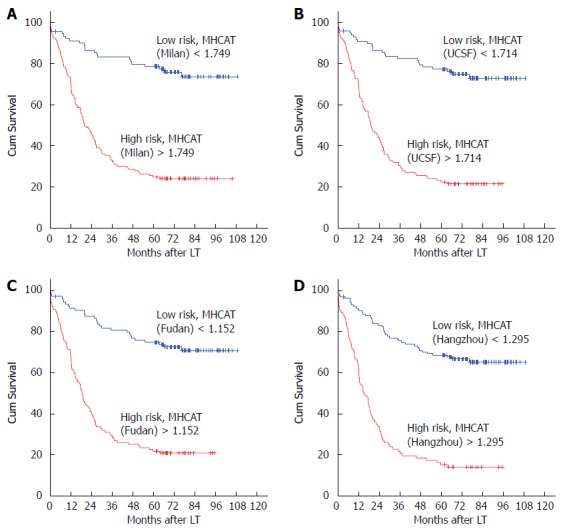
Survival analysis based on cut-off values for the model to predict long-term survival of hepatocellular carcinoma patients after liver transplantation scoring system (P < 0.01 for all). A: Milan criteria; B: University of California San Francisco criteria; C: Shanghai Fudan criteria; D: Hangzhou criteria.
DISCUSSION
Over the past decade, the gap in LT expertise between developing and developed countries has significantly narrowed. With a total number of LTs of more than 26000 cases, China is now second only to the United States. However, the five-year survival after LT in China is significantly lower than that in the United States and Europe (60.5% vs 73.7% and 60.5% vs 73.0%, respectively)[1-3]. However, the difference in survival of patients with benign end-stage liver diseases is smaller: 73.2% in China, 74.1% in America and 73.2% in Europe. A low curative effect is mainly responsible for the low survival rate of HCC patients in China as compared with those in the United States and Europe (49.7% vs 67.5% and 49.7% vs 64.0%, respectively). In China, 65.7% of HCC patients exceeded the Milan criteria before transplantation, which inevitably adversely affected their long-term survival. A multicenter evaluation showed that allocation strategies and different regions could also affect long-term survival after LT[10]. The MHCAT was built with reference to the four most representative HCC LT criteria, using accurate HCC patient data from a single center in China with a follow-up of at least six years. This model may help clinicians determine which candidates with HCC should receive LT.
LT produces excellent results in HCC patients within the Milan criteria. These recipients showed a five-year survival of up to 70% after LT and HCC recurrence was lower than 10%[4]. In recent years, some groups have argued that the Milan criteria are too restrictive and exclude some HCC patients from LT despite the possibility of benefit. Apart from the four criteria included in our study, there are many other criteria for HCC LT, such as the Up-to-Seven criteria, Pittsburgh criteria, and Navarra criteria[11]. However, we considered that the Milan, UCSF, Fudan and Hangzhou criteria best represented the HCC LT criteria. The Milan criteria are now the most widely accepted criteria, recommended by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. The Milan criteria are also the basis of other expanded criteria and the standard reference in studies related to HCC LT criteria. The UCSF criteria are the first expanded criteria and the most studied, involving the largest number of HCC patients. In China, 65.7% of HCC patients exceeded the Milan criteria before transplantation, which is quite different from the United States and European countries. Therefore, we believe that it was reasonable to include criteria based on HCC patients in China in the MHCAT study. The Fudan criteria were chosen because they were proposed according to multicenter data. Moreover, the Hangzhou criteria first included the AFP level and Edmondson staging, which were quite different from other criteria based on tumor morphology.
Several studies have shown that prognostic factors for HCC LT include not only tumor load, such as number of lesions, size and vascular invasion, but also characteristics of the biological activities in the tumor, such as the level and dynamic change in AFP, tumor progression after transcatheter arterial chemoembolization, as well as tumor recurrence after hepatectomy[6,12-14]. Our study on MHCAT also found that the pre-transplant AFP level was an independent prognostic factor, and other factors, such as intraoperative blood loss, retransplantation and TB, may play critical roles in the long-term survival of HCC patients. Therefore, the allocation system based on the model for end-stage liver disease (MELD) has its defects when giving additional priority to HCC patients, as it cannot take all these factors into account. Consequently, it might give rise to controversy and ethical concerns when considering the rights of other recipients.
The goal of LT, regardless of the underlying disease, is to provide liver recipients with the maximum benefit possible from limited resources of donated organs in a fair, ethical, and cost-effective manner. Rules for the distribution of donor organs are closely supervised by all stakeholders involved in LT. Thus, survival models, especially for HCC patients, which may offset the disadvantages of the MELD scoring system, have been an area of research focus. A national conference on liver allocation to patients with HCC in the United States achieved a general consensus for the development of a calculated continuous HCC priority scoring system for ranking HCC candidates on the waiting list[15]. The scoring system devised by Rana et al[16], in which the most significant risk factors were previous transplantation and life support before transplant, could accurately predict the three-month survival following LT. The study by Weismüller et al[17] found that age, pre-transplant creatinine and cholinesterase were predictors of one-year survival after LT. Schaubel et al[18] evaluated a benefit-based survival system for allocating deceased-donor livers to chronic liver failure patients. They recommended that the proposed score based on the difference in five-year predicted mean lifetime should be used for guiding liver allocation. All the models discussed above are built on the data obtained from the entire population of recipients or patients with benign end-stage liver disease without considering HCC patients independently. To construct a prediction model for HCC patients usually requires a long period of follow-up work, as HCC patients may survive for a time even with tumor recurrence. An analysis of rank correlations between benefit scores using different follow-up time points showed that the favorable time point should be three years or more[18]. The 2010 International Consensus Conference on LT for HCC accepted five years as the time point for survival assessment[5]. Thus, all living recipients in our study were followed for at least six years after LT, taking full account of the influence of tumor recurrence on long-term survival.
The UCSF criteria and the Fudan criteria are characteristic of a homogeneous extension of the Milan criteria boundary. Therefore, MHCAT based on these three criteria showed identical risk factors, such as AFP level, intraoperative blood loss and retransplantation. The Hangzhou criteria are somewhat different from the above three criteria when AFP is included. However, intraoperative blood loss and retransplantation were still significant predictors in MHCAT based on the Hangzhou criteria. The current HCC LT criteria focus more on morphological rather than biological factors. The other prognostic factors in MHCAT may reflect the biological characteristics of HCC. A number of studies on hepatectomy revealed that intraoperative blood loss was a predictive factor of HCC recurrence and cancer-related death[19-21]. However, the mechanism of the relationship between excessive blood loss and poor oncological outcomes has not been clearly identified. Potential reasons included tumor spillage and hematogenous spread during surgery, hypoperfusion and impaired oxygen delivery to vital organs and the introduction of some cytokines due to hemorrhagic shock. As the reason for retransplantation in our study was HCC recurrence, intraoperative blood loss and retransplantation in MHCAT may indicate the effect of circulating tumor cells (CTC) on HCC recurrence and metastasis. More intraoperative blood loss leads to an elevation in CTC level, whereas CTC homing in the graft may induce HCC recurrence, especially in the immunosuppressive state after LT. Therefore, CTC can serve as a potential icebreaker for HCC biological invasiveness.
In conclusion, we established a criteria-specific model for predicting long-term survival of HCC patients after LT, in which intraoperative blood loss, AFP level, retransplantation, TB, together with different indications for LT, may significantly affect the long-term survival of these recipients. The limitation of MHCAT lies in the data collected from our sole center, and this survival-prediction model may be statistically different among the four HCC LT criteria when it is applied in more centers. Therefore, MHCAT requires further evaluation in multicenter studies to optimize the current HCC LT criteria, which may facilitate pre-transplant clinical management, outcome prediction and decision-making.
ACKNOWLEDGMENTS
We highly appreciate Jian-Min Zhang, Professor of English at Zhejiang University, China, for his English editing of the paper.
COMMENTS
Background
With a total number of liver transplantation (LT)s of more than 26000 cases, China is now second only to the United States. However, the five-year survival after LT in China is significantly lower than that in the United States and Europe, which is mainly responsible for the low survival rate of hepatocellular carcinoma (HCC) patients in China. Multicenter evaluations showed that allocation strategies and different regions could also affect long-term survival after LT. Therefore, it is of significant value to explore the risk factors for long-term survival of LT recipients with HCC in China.
Research frontiers
This study was conducted to build a model to predict long-term survival of HCC patients after LT with reference to different criteria and peri-transplant risk factors.
Innovations and breakthroughs
Currently, the HCC LT criteria are mainly based on tumor morphology, and less on biological characteristics. By combining all potential risk factors, model to predict long-term survival of hepatocellular carcinoma patients after liver transplantation (MHCAT) provides a more effective tool for survival prediction of HCC patients.
Applications
The MHCAT was built with reference to the four most representative HCC LT criteria, using accurate HCC patient data from a single center in China with a follow-up of at least six years. This model may help clinicians determine which candidates with HCC, especially in China, should receive LT.
Terminology
MHCAT refers to a criteria-specific long-term survival prediction model for hepatocellular carcinoma patients after liver transplantation, which was built using the Cox proportional hazards model with multivariate analysis.
Peer review
This manuscript describes a model to predict long-term survival of HCC patients after liver transplantation. The study design is scientifically sound and the findings are of potential clinical significance.
Footnotes
Supported by the Foundation of Shanghai Science and Technology Commission NO. 134119a7300 and Shanghai Changzheng Hospital Foundation for Young Scientists NO. 2012CZQN08 and NO. 2012CZQN01
P- Reviewer: Hassan M, Tang KF S- Editor: Gou SX L- Editor: Webster JR E- Editor: Zhang DN
References
- 1.Adam R, Karam V, Delvart V, O’Grady J, Mirza D, Klempnauer J, Castaing D, Neuhaus P, Jamieson N, Salizzoni M, et al. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR) J Hepatol. 2012;57:675–688. doi: 10.1016/j.jhep.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 2.Kim WR, Stock PG, Smith JM, Heimbach JK, Skeans MA, Edwards EB, Harper AM, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2011 Annual Data Report: liver. Am J Transplant. 2013;13 Suppl 1:73–102. doi: 10.1111/ajt.12021. [DOI] [PubMed] [Google Scholar]
- 3.China Liver Transplant Registry. CLTR 2011 Annual Scientific Report. 2013-05-03, cited 2013-12-27. Available from: http://www.cltr.org/pages/datainfo/datainfo_apparatus.jsp?subType=11.
- 4.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 5.Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11–e22. doi: 10.1016/S1470-2045(11)70175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7:2587–2596. doi: 10.1111/j.1600-6143.2007.01965.x. [DOI] [PubMed] [Google Scholar]
- 7.Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 8.Zheng SS, Xu X, Wu J, Chen J, Wang WL, Zhang M, Liang TB, Wu LM. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation. 2008;85:1726–1732. doi: 10.1097/TP.0b013e31816b67e4. [DOI] [PubMed] [Google Scholar]
- 9.Fan J, Yang GS, Fu ZR, Peng ZH, Xia Q, Peng CH, Qian JM, Zhou J, Xu Y, Qiu SJ, et al. Liver transplantation outcomes in 1,078 hepatocellular carcinoma patients: a multi-center experience in Shanghai, China. J Cancer Res Clin Oncol. 2009;135:1403–1412. doi: 10.1007/s00432-009-0584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weismüller TJ, Fikatas P, Schmidt J, Barreiros AP, Otto G, Beckebaum S, Paul A, Scherer MN, Schmidt HH, Schlitt HJ, et al. Multicentric evaluation of model for end-stage liver disease-based allocation and survival after liver transplantation in Germany--limitations of the ‘sickest first’-concept. Transpl Int. 2011;24:91–99. doi: 10.1111/j.1432-2277.2010.01161.x. [DOI] [PubMed] [Google Scholar]
- 11.Silva MF, Sherman M. Criteria for liver transplantation for HCC: what should the limits be? J Hepatol. 2011;55:1137–1147. doi: 10.1016/j.jhep.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Shetty K, Timmins K, Brensinger C, Furth EE, Rattan S, Sun W, Rosen M, Soulen M, Shaked A, Reddy KR, et al. Liver transplantation for hepatocellular carcinoma validation of present selection criteria in predicting outcome. Liver Transpl. 2004;10:911–918. doi: 10.1002/lt.20140. [DOI] [PubMed] [Google Scholar]
- 13.Vibert E, Azoulay D, Hoti E, Iacopinelli S, Samuel D, Salloum C, Lemoine A, Bismuth H, Castaing D, Adam R. Progression of alphafetoprotein before liver transplantation for hepatocellular carcinoma in cirrhotic patients: a critical factor. Am J Transplant. 2010;10:129–137. doi: 10.1111/j.1600-6143.2009.02750.x. [DOI] [PubMed] [Google Scholar]
- 14.Ravaioli M, Grazi GL, Ercolani G, Fiorentino M, Cescon M, Golfieri R, Trevisani F, Grigioni WF, Bolondi L, Pinna AD. Partial necrosis on hepatocellular carcinoma nodules facilitates tumor recurrence after liver transplantation. Transplantation. 2004;78:1780–1786. doi: 10.1097/01.tp.0000145892.97114.ee. [DOI] [PubMed] [Google Scholar]
- 15.Pomfret EA, Washburn K, Wald C, Nalesnik MA, Douglas D, Russo M, Roberts J, Reich DJ, Schwartz ME, Mieles L, et al. Report of a national conference on liver allocation in patients with hepatocellular carcinoma in the United States. Liver Transpl. 2010;16:262–278. doi: 10.1002/lt.21999. [DOI] [PubMed] [Google Scholar]
- 16.Rana A, Hardy MA, Halazun KJ, Woodland DC, Ratner LE, Samstein B, Guarrera JV, Brown RS, Emond JC. Survival outcomes following liver transplantation (SOFT) score: a novel method to predict patient survival following liver transplantation. Am J Transplant. 2008;8:2537–2546. doi: 10.1111/j.1600-6143.2008.02400.x. [DOI] [PubMed] [Google Scholar]
- 17.Weismüller TJ, Prokein J, Becker T, Barg-Hock H, Klempnauer J, Manns MP, Strassburg CP. Prediction of survival after liver transplantation by pre-transplant parameters. Scand J Gastroenterol. 2008;43:736–746. doi: 10.1080/00365520801932944. [DOI] [PubMed] [Google Scholar]
- 18.Schaubel DE, Guidinger MK, Biggins SW, Kalbfleisch JD, Pomfret EA, Sharma P, Merion RM. Survival benefit-based deceased-donor liver allocation. Am J Transplant. 2009;9:970–981. doi: 10.1111/j.1600-6143.2009.02571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sasaki K, Matsuda M, Ohkura Y, Kawamura Y, Inoue M, Hashimoto M, Ikeda K, Kumada H, Watanabe G. Factors associated with early cancer-related death after curative hepatectomy for solitary small hepatocellular carcinoma without macroscopic vascular invasion. J Hepatobiliary Pancreat Sci. 2014;21:142–147. doi: 10.1002/jhbp.13. [DOI] [PubMed] [Google Scholar]
- 20.Katz SC, Shia J, Liau KH, Gonen M, Ruo L, Jarnagin WR, Fong Y, D’Angelica MI, Blumgart LH, Dematteo RP. Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg. 2009;249:617–623. doi: 10.1097/SLA.0b013e31819ed22f. [DOI] [PubMed] [Google Scholar]
- 21.Taketomi A, Toshima T, Kitagawa D, Motomura T, Takeishi K, Mano Y, Kayashima H, Sugimachi K, Aishima S, Yamashita Y, et al. Predictors of extrahepatic recurrence after curative hepatectomy for hepatocellular carcinoma. Ann Surg Oncol. 2010;17:2740–2746. doi: 10.1245/s10434-010-1076-2. [DOI] [PubMed] [Google Scholar]


