Abstract
AIM: To investigate clinical, endoscopic and pathological characteristics of drug-induced esophagitis.
METHODS: Data for patients diagnosed with drug-induced esophagitis from April 2002 to May 2013 was reviewed. Patients diagnosed with malignancy, viral or fungal esophagitis were excluded. Clinical, endoscopic and pathological characteristics of patients diagnosed with drug-induced esophagitis were analyzed.
RESULTS: Seventy-eight patients were diagnosed with drug-induced esophagitis. Their mean age was 43.9 ± 18.9 years and 35.9% were male. Common symptoms were chest pain (71.8%), odynophagia (38.5%) and dysphagia (29.5%). The endoscopic location was in the middle third of esophagus in 78.2%. Endoscopic findings were ulcer (82.1%), erosion (17.9%), ulcer with bleeding (24.4%), coating with drug material (5.1%), impacted pill fragments (3.8%) and stricture (2.6%). Kissing ulcers were observed in 43.6%. The main causative agents were antibiotics and non-steroidal anti-inflammatory drugs. All the patients were treated with proton pump inhibitors (PPIs) or sucralfate, and the causative drugs were discontinued. Nineteen patients with drug-induced esophagitis were followed up with endoscopy and revealed normal findings, scars or healing ulcers.
CONCLUSION: Drug-induced esophagitis mainly presents as chest pain, odynophagia and dysphagia, and may be successfully treated with PPIs and discontinuation of the causative drug. Kissing ulcers were observed in 43.6%.
Keywords: Drug, Esophagitis, Endoscopy, Pathology, Symptoms, Kissing ulcers
Core tip: This study investigated the clinical characteristics of drug-induced esophagitis, such as the main symptoms, common endoscopic findings and main causative agents. Uniquely, kissing ulcers were observed in 43.6% of drug-induced esophagitis, which is a higher rate than in the previous reports. This might be helpful in diagnosing this rare disease. To the best of our knowledge, the present study is the first to compare the histopathological features between drug-induced esophagitis group and reflux esophagitis group.
INTRODUCTION
To date, hundreds of drugs have been reported to cause drug-induced esophagitis. However, many clinicians do not recognize this as a cause of chest pain or odynophagia. The majority of the patients usually report self-limited symptoms, so this diagnosis is often underestimated[1]. However, lack of awareness of drug-induced esophagitis can lead to persistent exposure to causative drugs, resulting in severe complications[2-4]. Patients who are not initially and accurately diagnosed with drug-induced esophagitis may suffer from unnecessary work-up or extensive diagnostic evaluation for chest symptoms. To avoid these undesirable situations, awareness of this disease must be improved. Nonetheless, most of the studies on drug-induced esophagitis are case reports or reviews of case reports, which provide limited understanding of this disease. The purpose of this study was to investigate the clinical and endoscopic characteristics of drug-induced esophagitis.
MATERIALS AND METHODS
Study population
The data for 78 patients diagnosed with drug-induced esophagitis between April 2002 and May 2013 was reviewed and analyzed from four university hospitals. Patients with a definite history of taking medicines and with acute esophageal symptoms (odynophagia, dysphagia and chest pain) of less than two weeks were included in the drug-induced esophagitis group. Demographic features, clinical history, endoscopic findings and histopathological features were obtained by reviewing electronic medical records at each hospital. Patients with malignancy, viral or fungal esophagitis, esophageal varix, and corrosive esophageal injury were excluded. Patients with esophageal reflux symptoms that were persistent for greater than two weeks were also excluded. To compare their histopathology with the drug-induced esophagitis group, 19 patients with endoscopic evidence of reflux esophagitis (grade A to D according to the Los Angeles classification) and gastrointestinal symptoms were selected and included in the reflux esophagitis group[5]. The Institutional Review Board of Seoul National University Boramae Hospital approved the study, which was performed in accordance with the ethical guidelines of the Declaration of Helsinki.
Statistical analysis
SPSS version 18.0 software (IBM, Chicago, IL, United States) was used for statistical analysis. Continuous data were tested for the normality assumption using the Kolmogorov-Smirnov test. Normally distributed variables were described using the mean and SD. Descriptive data were shown as mean ± SD, number of patients and percentage. Categorical variables were analyzed between groups using the χ2 test. All results were considered statistically significant when P values were less than 0.05 (two-tailed).
RESULTS
Demographic findings and clinical symptoms
Among 78 patients with drug-induced esophagitis, 35.9% (n = 28) were males and 64.1% (n = 50) were females. Their mean age was 43.9 ± 18.9 years (mean ± SD, range 16-84).
Common symptoms were chest pain (n = 56, 71.8%), odynophagia (n = 30, 38.5%), dysphagia (n = 23, 29.5%) and vomiting (n = 6, 7.7%). Two patients had melena (n = 2, 2.6%) caused by esophageal bleeding (Table 1).
Table 1.
Demographic features and clinical symptoms of patients diagnosed with drug-induced esophagitis n (%)
| Characteristics | ||
| Age(yr) | mean ± SD | 43.9 ± 18.9 |
| Sex | Male/female | 28/50 |
| Symptom | Chest pain | 56 (71.8) |
| Odynophagia | 30 (38.5) | |
| Dysphagia | 23 (29.5) | |
| Vomiting | 6 (7.7) | |
| Melena | 2 (2.6) |
Endoscopic findings
78.2% (61/78) of the endoscopic location of drug-induced esophagitis was in the middle third of the esophagus. Endoscopic findings in the esophagus were ulcers (n = 64, 82.1%), erosions (n = 14, 17.9%), ulcer with bleeding (n = 19, 24.4%), coating with drug material (n = 4, 5.1%), impacted pill fragments (n = 3, 3.8%) and stricture (n = 2, 2.6%). Thirty-four cases (43.6%) showed kissing ulcers (ulcers facing each other) (Figure 1, Table 2).
Figure 1.
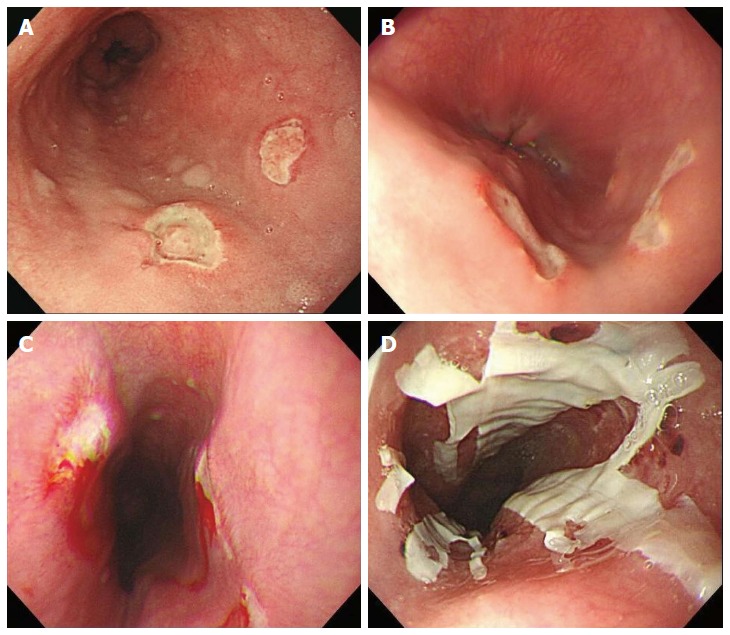
Endoscopic findings of drug-induced esophagitis. A: Typical kissing ulcers in the middle third of esophagus; B: Another typical kissing ulcer; C: Kissing ulcers with spontaneous bleeding; D: Coating with drug material.
Table 2.
Endoscopic features of patients diagnosed with drug-induced esophagitis
| Feature | n (%) | |
| Location | Proximal | 3 (3.8) |
| Middle | 61 (78.2) | |
| Distal | 14 (17.9) | |
| Endoscopic findings | Ulcers | 64 (82.1) |
| Bleeding | 19 (24.4) | |
| Erosions | 14 (17.9) | |
| Coating | 4 (5.1) | |
| Pill | 3 (3.8) | |
| Stricture | 2 (2.6) | |
| Kissing ulcers | 34 (43.6) |
Causative agents
Causative agents were antibiotics (doxycycline, amoxicillin, ciprofloxacin, metronidazole, sultamicillin tosylate and rifaximin) in 28 patients (35.9%), non-steroidal anti-inflammatory drug (as) (aspirin, aceclofenac) in 27 patients (34.6%), anti-hypertensive drugs (amlodipine, ramipril) in nine patients (11.5%), acetaminophen in seven patients (9.0%), oral hypoglycemic agents (glimepiride) in four patients (5.1%), bisphosphonates (alendronate, ibandronate) in four patients (5.1%), ascorbic acid in 2 patients (2.6%), warfarin in 2 patients (2.6%) and other drugs (tiropramide, pinaverium bromide, mosapride, esomeprazole) in 4 patients (Table 3). The proportion of antibiotics as a cause of drug-induced esophagitis was higher among the younger group (< 45 years) than in the elderly group (≥ 45 years, 47.6% vs 22.2%, P = 0.02, χ2 test). The proportion of NSAID as a cause of drug-induced esophagitis showed no significant differences between the two age groups (28.6% vs 41.7%, P = 0.226, χ2 test) (Table 4).
Table 3.
Causative drugs of patients diagnosed with drug-induced esophagitis
| Drug | n (%) |
| Antibiotics | 28 (35.9) |
| NSAID | 27 (34.6) |
| Anti-hypertensive | 9 (11.5) |
| Acetaminophen | 7 (9.0) |
| Oral hypoglycemic | 4 (5.1) |
| Bisphosphonate | 4 (5.1) |
| Ascorbic acid | 2 (2.6) |
| Warfarin | 2 (2.6) |
| Other drugs | 4 (5.1) |
NSAID: Non-steroidal anti-inflammatory drug.
Table 4.
Proportion of antibiotics and non-steroidal anti-inflammatory drugs between both age groups
|
Age |
Total | P value | ||
| < 45 yr | ≥ 45 yr | |||
| Antibiotics | 0.020 | |||
| (+) | 20 | 8 | 28 | |
| (-) | 22 | 28 | 50 | |
| Total | 42 | 36 | 78 | |
| NSAID | ||||
| (+) | 12 | 15 | 27 | 0.226 |
| (-) | 30 | 21 | 51 | |
| Total | 42 | 36 | 78 | |
NSAID: Non-steroidal anti-inflammatory drug.
Pathological findings
In 17 cases (21.8%), endoscopic biopsy was performed to evaluate the pathological finding of the esophageal lesion. Pathological findings were evaluated between the drug-induced esophagitis group and the reflux esophagitis (RE) group. There were no significant differences in basal cell hyperplasia (P = 0.559), papillary elongation (P = 0.086), dilated intercellular spaces (P = 0.175), and cell vacuolization (P = 0.074) between the two groups (Table 5).
Table 5.
Pathological findings of drug-induced esophagitis group and reflux esophagitis group n (%)
| Drug-induced esophagitis | Reflux esophagitis | P value | |
| (n = 17) | (n = 19) | ||
| Basal cell hyperplasia | 6 (35.3) | 5 (26.3) | 0.559 |
| Papillary elongation | 5 (29.4) | 11 (57.9) | 0.086 |
| Dilated intercellular spaces | 11 (64.7) | 8 (42.1) | 0.175 |
| Cell vacuolization | 13 (76.5) | 9 (47.4) | 0.074 |
Treatment and follow up
All of the patients were treated with proton pump inhibitors (PPIs) or sucralfate and the causative drugs were discontinued. Nineteen patients (24.4%) with drug-induced esophagitis were followed up with endoscopy after 2 d-2 mo, where they revealed normal findings or well-healed scars in the esophagus in all but two patients who still had healing ulcers. The remaining 59 patients (75.6%) had no symptoms during follow up and did not undergo follow up endoscopy or were lost during follow up.
DISCUSSION
If impacted pill fragments are present in the esophagus during the endoscopic examination of a symptomatic patient, a clear diagnosis can be made. However, impacted pill fragments are rarely found. Pathological findings, such as brown-black crystals for iron, and basophilic crystals for Kayexalate, are known to aid in diagnosing drug-induced esophagitis. Mitotic arrest is also a pathological finding helpful in diagnosing drug-induced esophagitis caused by taxol or colchicines. Other than these reported rare cases, diagnosing drug-induced esophagitis is based on the clinical history and endoscopic findings. Many cases reporting drug-induced esophagitis were identified. However, other than case reports, there were very few studies addressing the characteristics of drug-induced esophagitis[6,7]. Higuchi et al[8] reported that the etiologies of esophageal ulcers included RE in 65.9%, drug-induced esophagitis in 22.7% and the others (viral, fungal etc.) in 11.4%. When esophageal ulcers are encountered during endoscopy, reflux esophagitis or drug-induced esophagitis should first be considered, given that there is no clinical suspicion of other diseases (i.e., viral/fungal esophagitis, Levin tube injury, Crohn’s disease, or radiation injury). Higuchi et al[8] also reported that 91.4% of RE-induced esophageal ulcers were located in the lower esophagus and 80% of drug-induced esophageal ulcers were located in the middle portion of the esophagus. Other studies also found that lesions of drug-induced esophagitis were frequently located in the middle third of esophagus[6,7]. The middle third of the esophagus is subject to compression by the aortic arch or enlarged left atrium; therefore, drug-induced esophagitis is commonly located in the mid-esophagus[9]. Therefore, with the location of esophageal ulcers, RE can be differentiated from drug-induced ulcers in many cases. Typical reflux esophagitis patients often have persistent reflux symptoms and patients with drug-induced esophagitis, in general, have abrupt-onset chest symptoms. According to Kirkendall, the typical drug-induced esophagitis patient presents with the sudden onset of odynophagia, dysphagia or retrosternal pain[10]. Based on this report, the study of Abid et al[6] was performed with patients who experienced acute onset of esophageal symptoms of less than 3 d duration. According to Boyce, symptoms of drug-induced esophagitis can develop within hours to 10 d after medication[11]. After being lodged in the esophagus, injurious pills release noxious contents damaging the esophageal wall[10]. Thus, it is postulated that this damage of esophageal wall gives rise to the abrupt-onset symptoms of drug-induced esophagitis. Patients with drug-induced esophagitis often have a history of medication in the recumbent position or before going to sleep, with no or little water[10,12]. In our study, patients with a definite history of taking medicines and with acute esophageal symptoms of less than two weeks were included.
As eosinophilic infiltration is frequently found in the distal esophagus of reflux esophagitis; mid-to-proximal esophagus is recommended for tissue biopsy of eosinophilic esophagitis[13]. The location of the lesions in eosinophilic esophagitis is similar to drug-induced esophagitis and eosinophilic infiltration is also commonly found in drug-induced esophageal lesions[14]. Therefore, a differential diagnosis between eosinophilic esophagitis and drug-induced esophagitis can be unclear. Though most patients with eosinophilic esophagitis have abnormal endoscopic findings, endoscopic changes alone are inadequate for the diagnosis of eosinophilic esophagitis[15]. The differentiation between eosinophilic esophagitis and drug-induced esophagitis needs to be a clinicopathological diagnosis, which requires clinical findings and pathological criteria for a diagnosis[16]. In differentiating the diagnosis of eosinophilic esophagitis and reflux esophagitis, endoscopic findings and clinical response to medication of reflux esophagitis can be useful[14]. There are some studies on histological parameters for the differential diagnosis of eosinophilic esophagitis and reflux esophagitis[14,17]. Our study attempted to find pathological clues that can differentiate drug-induced esophagitis from reflux esophagitis; however, there were no significant differences of basal cell hyperplasia (P = 0.559), papillary elongation (P = 0.086), dilated intercellular spaces (P = 0.175) and cell vacuolization (P = 0.074) between the two groups. To the best of our knowledge, the present study is the first study to compare the histopathological features between a drug-induced esophagitis group and a reflux esophagitis group.
There are reports that drug-induced esophagitis is predominantly found among elderly patients, as they are more likely to spend time in the recumbent position, consume more medications, including alendronate or non-steroidal anti-inflammatory drugs (NSAIDs), have more esophageal motility problems or cardiac enlargement with mid-esophagus compression, and are less aware of the drug instructions[11]. A study showed that the esophageal transit time was significantly longer in elderly subjects than in younger subjects[18]. However in our study, the proportion of antibiotics use was higher in younger group than in elderly group. According to the literature, antibiotics were the commonest or second commonest cause of drug-induced esophagitis[6,9]. In our study, antibiotics were the commonest causative drugs. In contrast to NSAIDs, anti-hypertensive drugs and bisphosphonates, which are frequently prescribed for elderly patients, antibiotics are commonly prescribed in young patients to treat acne, urinary tract infections or pelvic inflammatory disease[11]. Our study showed that the predominant causative drugs were different between age groups. Previous reports showed that drug-induced esophagitis was more prevalent among women than among men[1,6]. In this study, 64.1% were females, which was consistent with previous reports.
Our study showed that the common symptoms were chest pain, odynophagia and dysphagia. Many of these patients reported multiple symptoms, such as odynophagia with concurrent chest pain. Zografos et al[1] showed that the main symptoms caused by drug-induced esophagitis were chest pain (60%), odynophagia (50%), and dysphagia (40%). 78.2% of endoscopic locations of drug-induced esophagitis were found in the middle third of esophagus, which was consistent with previous studies[6,8]. In thirty-four cases (43.6%), there were kissing ulcers (ulcers facing each other). Kissing ulcers were also reported in esophageal injury other than drug-induced esophagitis[19]. Therefore, kissing ulcers alone cannot confirm drug-induced esophagitis. However, we showed that kissing ulcers were observed in drug-induced esophagitis more frequently than the previously reported studies[6]. Patients with longer esophageal symptoms were included in our study; therefore, the duration of esophageal exposure to causative agents may be longer. This may have contributed to the formation of kissing ulcers. A clinical study on drug-induced esophagitis showed that kissing ulcers occupied 7.6%, which is lower than in our study[6]. In Higuchi’s study, active bleeding was noted in 45% of drug-induced esophageal ulcers, which is higher than the 24.4% in our study[8]. This difference can be explained by the difference in the proportion of patients taking NSAIDs (65% vs 34.6%). Notably, the study of Higuchi et al[8] included only esophageal ulcers, whereas our study included shallow esophageal erosions, as well as esophageal ulcers. From these results, drug-induced esophagitis should also be considered as a cause of upper gastrointestinal bleeding. Two cases with esophageal stricture were also identified, both of which had dysphagia symptoms and were associated with NSAID use. It has been reported that NSAIDs were associated with an increased risk of reflux esophagitis and esophageal strictures[20]. In patients with reflux esophagitis, one should be careful in prescribing NSAIDs. It was reported that pill fragment impaction was associated with esophageal stricture[21]. Here, we observed three cases of impacted pill fragments with no definite esophageal stricture.
For patients with drug-induced esophagitis, oral sucralfate and PPIs are frequently administered, and the offending drugs are discontinued[6]. In our study, 19 patients (24.4%) with drug-induced esophagitis were treated with oral sucralfate, PPI and quitting drugs. These patients were then, followed up with endoscopy after 2 d-2 mo; where most of them revealed normal findings or well-healed scars in the esophagus, and only two patients still had healing ulcers. Once the offending drug is discontinued, oral sucralfate and PPI are thought to be sufficient for the treatment of drug-induced esophagitis. Intramural esophageal hematoma with drug-induced esophagitis was also reported to have a favorable outcome after a conservative treatment[22]. In contrast, it has been reported that endoscopic intervention was necessary to treat complications of drug-induced esophagitis[23].
If a medication history and chronology of acute esophageal symptoms strongly suggest it, diagnosing drug-induced esophagitis is not so difficult, even without endoscopic examination[11]. However, the diagnosis of drug-induced esophagitis can be more easily confirmed with the appropriate endoscopic findings. Additionally, helpful findings, such as pill fragments or residues can be observed at the sites of injury, making the diagnosis clear[24]. Malignancy and viral or fungal esophagitis can also be ruled out using endoscopy.
This study is a retrospective observational study, and lacks a control group. Therefore, it is difficult to measure the significance of the descriptive results. However, from the results of our study with 78 subjects, the clinical characteristics such as main symptoms, common endoscopic findings (ulcers in the middle third of esophagus) and main causative agents could be identified. A unique finding in this study was that kissing ulcers were observed in 43.6% of the patients diagnosed with drug-induced esophagitis, which might be helpful in diagnosing this rare disease.
In conclusion, drug-induced esophagitis mainly presented as chest pain, odynophagia and dysphagia, and was successfully treated with PPIs and the discontinuation of the causative drug. Kissing ulcers were observed in 43.6% of the patients diagnosed with drug-induced esophagitis. It is important to be mindful of the possibility of drug-induced esophagitis in patients with acute esophageal symptoms. With an accurate diagnosis, patients will be able to avoid unnecessary work-up or fatal complications.
COMMENTS
Background
Drug-induced esophagitis is a rare disease, and the likelihood of this diagnosis is often underestimated. Lack of awareness of drug-induced esophagitis can lead to severe complications or unnecessary work-up.
Research frontiers
Most studies on drug-induced esophagitis are case reports or reviews of case reports, and large-scale studies are rare. In this study, the authors investigated the clinical and endoscopic characteristics of drug-induced esophagitis in a multi-center setting.
Innovations and breakthroughs
A unique finding was that kissing ulcers were observed in 43.6% of the patients diagnosed with drug-induced esophagitis, which might aid in diagnosing this rare disease. This study is also the first study to compare the histopathological features between a drug-induced esophagitis group and a reflux esophagitis group.
Applications
Clinical characteristics such as symptoms, common endoscopic findings and main causative agents were identified. The main symptoms were chest pain, odynophagia, and dysphagia. Common endoscopic findings were ulcers in the middle third of esophagus; kissing ulcers were frequently observed. These findings could be helpful in the diagnosis of drug-induced esophagitis.
Terminology
Drug-induced esophagitis is a clinical problem caused by esophageal damage associated with the ingestion of certain drugs. Kissing ulcers are ulcers facing each other, which is a common finding in drug-induced esophagitis, though it is not pathognomonic. Non-steroidal anti-inflammatory drugs are drugs, including aspirin and ibuprofen, which are used for reducing inflammation and pain in various diseases. Proton pump inhibitors are drugs that irreversibly inhibit proton pump function and are the most potent gastric acid-suppressing agents in clinical use.
Peer review
This is a very interesting observational study on the clinical, endoscopic and pathological characteristics of drug-induced esophagitis. From the results of this study, practitioners can identify the features of drug-induced esophagitis and also get help in diagnosing patients with drug-induced esophagitis.
Footnotes
P- Reviewer: Hashimoto N S- Editor: Ding Y L- Editor: Stewart GJ E- Editor: Ma S
References
- 1.Zografos GN, Georgiadou D, Thomas D, Kaltsas G, Digalakis M. Drug-induced esophagitis. Dis Esophagus. 2009;22:633–637. doi: 10.1111/j.1442-2050.2009.00972.x. [DOI] [PubMed] [Google Scholar]
- 2.Cummin AR, Hangartner JR. Oesophago-atrial fistula: a side effect of tetracycline? J R Soc Med. 1990;83:745–746. doi: 10.1177/014107689008301122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henry JG, Shinner JJ, Martino JH, Cimino LE. Fatal esophageal and bronchial artery ulceration caused by solid potassium chloride. Pediatr Cardiol. 1983;4:251–252. doi: 10.1007/BF02242266. [DOI] [PubMed] [Google Scholar]
- 4.Yamaoka K, Takenawa H, Tajiri K, Yamane M, Kadowaki K, Marumo F, Sato C. A case of esophageal perforation due to a pill-induced ulcer successfully treated with conservative measures. Am J Gastroenterol. 1996;91:1044–1045. [PubMed] [Google Scholar]
- 5.Armstrong D, Bennett JR, Blum AL, Dent J, De Dombal FT, Galmiche JP, Lundell L, Margulies M, Richter JE, Spechler SJ, et al. The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology. 1996;111:85–92. doi: 10.1053/gast.1996.v111.pm8698230. [DOI] [PubMed] [Google Scholar]
- 6.Abid S, Mumtaz K, Jafri W, Hamid S, Abbas Z, Shah HA, Khan AH. Pill-induced esophageal injury: endoscopic features and clinical outcomes. Endoscopy. 2005;37:740–744. doi: 10.1055/s-2005-870129. [DOI] [PubMed] [Google Scholar]
- 7.McCord GS, Clouse RE. Pill-induced esophageal strictures: clinical features and risk factors for development. Am J Med. 1990;88:512–518. doi: 10.1016/0002-9343(90)90431-c. [DOI] [PubMed] [Google Scholar]
- 8.Higuchi D, Sugawa C, Shah SH, Tokioka S, Lucas CE. Etiology, treatment, and outcome of esophageal ulcers: a 10-year experience in an urban emergency hospital. J Gastrointest Surg. 2003;7:836–842. doi: 10.1007/s11605-003-0027-7. [DOI] [PubMed] [Google Scholar]
- 9.Jaspersen D. Drug-induced oesophageal disorders: pathogenesis, incidence, prevention and management. Drug Saf. 2000;22:237–249. doi: 10.2165/00002018-200022030-00007. [DOI] [PubMed] [Google Scholar]
- 10.Kikendall JW. Pill esophagitis. J Clin Gastroenterol. 1999;28:298–305. doi: 10.1097/00004836-199906000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Boyce HW. Drug-induced esophageal damage: diseases of medical progress. Gastrointest Endosc. 1998;47:547–550. doi: 10.1016/s0016-5107(98)70264-0. [DOI] [PubMed] [Google Scholar]
- 12.Kikendall JW, Friedman AC, Oyewole MA, Fleischer D, Johnson LF. Pill-induced esophageal injury. Case reports and review of the medical literature. Dig Dis Sci. 1983;28:174–182. doi: 10.1007/BF01315148. [DOI] [PubMed] [Google Scholar]
- 13.Prasad GA, Talley NJ, Romero Y, Arora AS, Kryzer LA, Smyrk TC, Alexander JA. Prevalence and predictive factors of eosinophilic esophagitis in patients presenting with dysphagia: a prospective study. Am J Gastroenterol. 2007;102:2627–2632. doi: 10.1111/j.1572-0241.2007.01512.x. [DOI] [PubMed] [Google Scholar]
- 14.Mueller S, Aigner T, Neureiter D, Stolte M. Eosinophil infiltration and degranulation in oesophageal mucosa from adult patients with eosinophilic oesophagitis: a retrospective and comparative study on pathological biopsy. J Clin Pathol. 2006;59:1175–1180. doi: 10.1136/jcp.2005.031922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, Burks AW, Chehade M, Collins MH, Dellon ES, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20.e6; quiz 21-22. doi: 10.1016/j.jaci.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 16.Read AJ, Pandolfino JE. Biomechanics of esophageal function in eosinophilic esophagitis. J Neurogastroenterol Motil. 2012;18:357–364. doi: 10.5056/jnm.2012.18.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mueller S, Neureiter D, Aigner T, Stolte M. Comparison of histological parameters for the diagnosis of eosinophilic oesophagitis versus gastro-oesophageal reflux disease on oesophageal biopsy material. Histopathology. 2008;53:676–684. doi: 10.1111/j.1365-2559.2008.03187.x. [DOI] [PubMed] [Google Scholar]
- 18.Hey H, Jørgensen F, Sørensen K, Hasselbalch H, Wamberg T. Oesophageal transit of six commonly used tablets and capsules. Br Med J (Clin Res Ed) 1982;285:1717–1719. doi: 10.1136/bmj.285.6356.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim GB, Jeong JJ, Park S, Ko JE, Ko SH, Kang HM, Lee GS. A large symmetrical esophageal ulcer caused by thermal and compressive injury from a solid foodstuff known as ‘Song-Pyen’. Korean J Med. 2012;82:589–593. [Google Scholar]
- 20.El-Serag HB, Sonnenberg A. Association of esophagitis and esophageal strictures with diseases treated with nonsteroidal anti-inflammatory drugs. Am J Gastroenterol. 1997;92:52–56. [PubMed] [Google Scholar]
- 21.Kirsch M. Pill-induced esophageal obstruction: discovery of a peptic stricture. South Med J. 1997;90:861–862. doi: 10.1097/00007611-199708000-00021. [DOI] [PubMed] [Google Scholar]
- 22.Lin IT, Bair MJ, Chen HL, Wu CH. Pill-related esophageal intramural hematoma and dissection. Gastrointest Endosc. 2010;72:432–443; discussion 433. doi: 10.1016/j.gie.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Park HW, Kim SJ, Park JW, Shin WG, Kim KH, Jang MK, Lee JH, Kim HY, Kim HS. Pill-related esophageal intramural dissection treated by an endoscopic procedure. Gastrointest Endosc. 2011;74:1422–1424. doi: 10.1016/j.gie.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Chen Z, Scudiere JR, Montgomery E. Medication-induced upper gastrointestinal tract injury. J Clin Pathol. 2009;62:113–119. doi: 10.1136/jcp.2008.058263. [DOI] [PubMed] [Google Scholar]


