Abstract
Objectives
To study the prevalence and mechanisms underlying right ventricular (RV) dyssynchrony in Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy (ARVD/C) using tissue Doppler echocardiography (TDE).
Background
ARVD/C is characterized by fibrofatty replacement of RV myocardium and RV dilatation. These pathologic changes may result in electromechanical dyssynchrony.
Methods
Electrocardiography, conventional and TDE was performed in 52 ARVD/C patients fulfilling Task Force criteria and 25 controls. RV end-diastolic and end-systolic areas, RV fractional area change (RVFAC), and left ventricular (LV) volumes and function were assessed. Mechanical synchrony was assessed by measuring differences in time-to-peak systolic velocity (TSV) between the RV free wall, ventricular septum and LV lateral wall. RV dyssynchrony was defined as the difference in TSV between the RV free wall and the ventricular septum, >2 SD above the mean value for controls.
Results
Mean difference in RV TSV was higher in ARVD/C compared to controls (55 ± 34 ms vs. 26 ± 15 ms, p<0.001). Significant RV dyssynchrony was not noted in any of the controls. Based on a cut-off value of 56 ms, significant RV dyssynchrony was present in 26 ARVD/C patients (50%). Patients with RV dyssynchrony had larger RV end-diastolic area (22 ± 5 vs. 19 ± 4 cm2, p=0.02), and lower RVFAC (29 ± 8 vs. 34 ± 8%, p=0.03) compared to ARVD/C patients without RV dyssynchrony. No differences in QRS duration, LV volumes and function were present between the two groups.
Conclusions
RV dyssynchrony may occur in up to 50% of ARVD/C patients, and is associated with RV remodeling. This finding may have therapeutic and prognostic implications in ARVD/C.
Keywords: Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy, Echocardiography, Ventricular dyssynchrony
Introduction
Arrhythmogenic right ventricular dysplasia cardiomyopathy (ARVD/C) is an inherited disease characterized by fibrofatty replacement of right ventricular (RV) myocardium (1). The diagnosis is established based on the presence of a conglomeration of factors (2,3). Other than ventricular arrhythmias, ARVD/C results in progressive RV dilatation and systolic dysfunction leading to heart failure (4,5).
Ventricular electro-mechanical delay (or mechanical dyssynchrony) has been well described in left ventricular (LV) failure and has formed the basis of cardiac resynchronization therapy leading to significant improvements in symptoms, functional capacity and survival in heart failure patients (6). Although RV mechanical dyssynchrony has been described in pulmonary hypertension (7), there are no data on whether a primary RV cardiomyopathy such as ARVD/C is associated with mechanical dyssynchrony. Tissue Doppler (TDE) and strain (SE) echocardiography have emerged as the predominant means of evaluating ventricular mechanics (8,9).
Several components of the ARVD/C disease process could potentially lead to the development of RV mechanical dyssynchrony. Fibrofatty infiltration could involve the RV conduction system resulting in electrical and electro-mechanical delays. Similar to LV failure, RV dilatation and dysfunction may cause dyssynchrony. Lastly, other factors such as pulmonary pressures and LV involvement may influence RV mechanical properties. Importantly, ventricular electro-mechanical dyssynchrony has prognostic and therapeutic implications (10,11).
Accordingly, the aims of this study were to determine the prevalence of mechanical dyssynchrony in a large cohort of ARVD/C patients and to better elucidate the factors influencing RV mechanics in ARVD/C.
Methods
Study population and protocol
This study was approved by the institutional review board with written informed consent obtained in all subjects. The study population comprised 52 ARVD/C patients with diagnosis confirmed by Task Force criteria (2) and 25 control subjects. All control subjects were healthy volunteers, recruited on campus, with no history of medical illness, not on any cardioactive medications, who had a normal echo Doppler examination (18 men, 7 women; mean age 32 ± 6 years). All patients underwent a detailed history and physical examination, 12 lead electrocardiogram (ECG), signal averaged ECG, conventional echocardiography and TDE/SE.
Echocardiography
Conventional and TDE/SE images were acquired from at least 3 consecutive heart beats and digitally stored for off-line analysis using a Vivid 7 ultrasound machine (GE Healthcare, Waukesha, WI). Offline analysis was performed using EchoPAC PC version 6.1 (GE Healthcare). During image acquisition, special care was taken to acquire accurate images of the RV free wall. Off-plane images of the RV were acquired to maximize visualization of RV morphology.
The RV outflow tract dimension was measured in the parasternal short-axis view at the level of the aortic valve plane (12). In addition, RV end-diastolic area (RVEDA) and RV end-systolic area (RVESA) were measured by tracing the RV endocardial border on the apical 4-chamber view and RV fractional area change (RVFAC) was calculated as a measure of RV systolic function using the following equation: RVFAC = (RVEDA - RVESA)/ RVEDA × 100% (12). Biplane LV end-diastolic and -systolic volumes were assessed from the apical 2- and 4-chamber images, and LV ejection fraction was calculated using the biplane Simpson's formula (13).
Tissue Doppler/strain echocardiography
Standard apical 4-chamber images and narrow-angle-sector images were acquired for tissue Doppler and strain analysis. Adjustments to the sector width were made to visualize one myocardial wall at a time (RV free wall, interventricular septum, LV lateral wall), in order to obtain an optimal alignment between the wall and the ultrasound beam, and to maximize frame rates (mean frame rate 253 ± 46 frames/s). The gain settings, filters and pulse repetition frequency were adjusted to optimize color saturation and to avoid aliasing.
Off-line analysis was performed by placing the Doppler sample at the basal segment of the RV free wall, interventricular septum and LV lateral wall, as previously described (14). Semi-automated tissue tracking was used to maintain the sample area within the region of interest throughout the cardiac cycle. Peak systolic tissue velocity of each segment was obtained and averaged from 3 cardiac cycles. For peak systolic strain analysis, an offset (strain) distance of 12 mm was used; for all segments the time-to-peak systolic strain was similarly assessed. Off-line analyses were performed by two observers, blinded to the results of the echocardiographic RV function analysis.
Ventricular dyssynchrony
For the assessment of ventricular dyssynchrony, the time from the onset of the QRS complex to the peak systolic tissue velocity of different segments was measured (TSV). The difference between the TSV of the septum and the TSV of the RV free wall was calculated as an indicator of RV dyssynchrony. Significant RV dyssynchrony was defined as a septal to RV free wall TSV delay exceeding 2 standard deviation (SD) above the mean value for the control group.
Similarly, for LV dyssynchrony the difference in TSV between the septum and the LV lateral wall was calculated. A value > 2 SD above the mean value derived from the control group, was used as a cut-off value for the presence of significant LV dyssynchrony. Finally, interventricular dyssynchrony was calculated as the difference in TSV between the RV free wall and the LV lateral wall. The cut-off value for significant interventricular dyssynchrony was defined similar to RV and LV dyssynchrony.
Statistical analysis
Continuous data are presented as mean ± SD; categorical data are presented as frequencies and percentages. Differences between the ARVD/C patients and the controls, and between the ARVD/C patients with and without ventricular dyssynchrony, were evaluated using unpaired student t test (continuous variables), or Chi-square tests (dichotomous variables). Differences in continuous variables between controls and ARVD/C patients with and without ventricular dyssynchrony were evaluated with oneway ANOVA. Correlations between echocardiographic variables and the extent of RV dyssynchrony were assessed with Pearson's correlation test.
Inter- and intra-observer variability for the assessment of TSV of the RV free wall and the interventricular septum and RV dyssynchrony were assessed using Bland-Altman analysis, in 10 random ARVD/C patients that were analyzed by two independent observers (inter-observer variability) and by a single observer at two different time points (intra-observer variability); mean differences ± SD and 95% confidence intervals are reported. In addition, kappa statistic was used to assess the inter- and intra-observer variability for the classification of the presence or absence of RV dyssynchrony.
All statistical analyses were performed using SPSS software (version 12.0, SPSS Inc. Chicago, Illinois). All statistical tests were two-sided, and a p-value <0.05 was considered statistically significant.
Results
Baseline characteristics of the 52 ARVD/C patients are summarized in Table 1. In none of the patients, symptoms of right-sided heart failure were present. Right ventricular areas (RVEDA and RVESA) were higher and RVFAC significantly lower in ARVD/C compared to the controls (Table 2). There were no significant inter-group differences in LV volumes and function. Peak systolic velocities and strain values in the interventricular septum and the LV lateral wall were comparable between the ARVD/C patients and controls (Table 2). In contrast, RV free wall peak systolic velocity (7.4 ± 2.1 vs. 9.9 ± 1.2 cm/s, p<0.001) and RV free wall peak systolic strain (-19 ± 7 vs. -25 ± 9%, p=0.002) were significantly lower in ARVD/C patients compared to controls, respectively.
Table 1. Baseline characteristics.
| ARVD/C patients (n=52) | |
|---|---|
| Age, yrs | 41 ± 12 |
| Gender, M/F | 22 / 30 |
| Symptomatic, n (%) | 45 (87) |
| Syncope, n (%) | 13 (25) |
| Palpitations, n (%) | 18 (35) |
| Ventricular tachycardia, n (%) | 14 (27) |
| Other symptoms, n (%) | 9 (17) |
| Implantable cardioverter-defibrillator, n (%) | 45 (87) |
| Filtered QRS duration, ms | 131 ± 36 |
| Right bundle branch block, n (%) | 10 (19) |
| Epsilon waves, n (%) | 0 (0) |
| T wave inversion in right precordial leads, n (%) | 39 (75) |
| RV systolic pressure, mmHg | 29 ± 6 |
RV = right ventricular
Table 2. Echocardiographic data.
| Variable | Controls (n=25) |
ARVD/C patients (n=52) |
P value |
|---|---|---|---|
| RVOT diameter (cm) | 2.6 ± 0.2 | 2.9 ± 0.4 | 0.001 |
| RVEDA (cm2) | 17 ± 3 | 20 ± 5 | <0.001 |
| RVESA (cm2) | 9 ± 2 | 14 ± 4 | <0.001 |
| RVFAC (%) | 44 ± 7 | 32 ± 8 | <0.001 |
| LVEDV (ml) | 108 ± 31 | 104 ± 27 | 0.5 |
| LVESV (ml) | 45 ± 14 | 45 ± 14 | 0.9 |
| LVEF (%) | 59 ± 5 | 57 ± 5 | 0.1 |
| Septum | |||
| Peak systolic velocity (cm/s) | 5.9 ± 1.1 | 5.4 ± 1.1 | 0.1 |
| Peak systolic strain (%) | -24 ± 6 | -21 ± 7 | 0.1 |
| RV free wall | |||
| Peak systolic velocity (cm/s) | 9.9 ± 1.2 | 7.4 ± 2.1 | <0.001 |
| Peak systolic strain (%) | -25 ± 9 | -19 ± 7 | 0.002 |
| LV lateral wall | |||
| Peak systolic velocity (cm/s) | 7.0 ± 2.1 | 6.5 ± 1.4 | 0.2 |
| Peak systolic strain (%) | -18 ± 8 | -18 ± 6 | 0.2 |
ARVD/C = arrhythmogenic right ventricular dysplasia/cardiomyopathy; LV = left ventricular; LVEDV = left ventricular end-diastolic volume; LVEF = left ventricular ejection fraction; LVESV = left ventricular end-systolic volume; RV = right ventricular; RVEDA = right ventricular end-diastolic area; LVESA = right ventricular end-systolic area; RVFAC = right ventricular fractional area change; RVOT = right ventricular outflow tract
Ventricular dyssynchrony
In all subjects, echocardiographic images were of sufficient quality to assess time-to-peak systolic velocity. Mean TSV of the septum and the RV free wall in the ARVD/C patients was 159 ± 40 ms and 210 ± 42 ms, respectively. In the controls, mean TSV of the septum and the RV free wall was 135 ± 39 ms and 160 ± 33 ms, respectively. Mean time-to-peak strain of the septum and RV free wall was 387 ± 67 ms and 434 ± 73 ms in the ARVD/C patients and 345 ± 88 ms and 368 ± 75 ms in the controls.
The mean difference in TSV between the septum and the RV free wall, representing RV dyssynchrony, was 55 ± 34 ms in the ARVD/C patients, and 26 ± 15 ms in the controls (p<0.001). Based on a cut-off value of ≥56 ms, significant RV dyssynchrony was present in 26 ARVD/C patients (50%). In these patients, mean RV dyssynchrony was 84 ± 20 ms, whereas it was 26 ± 16 ms in the remaining patients (p<0.001). An example of a patient with significant RV dyssynchrony is shown in Figure 1.
Figure 1. Example of an ARVD/C patient with significant RV dyssynchrony.
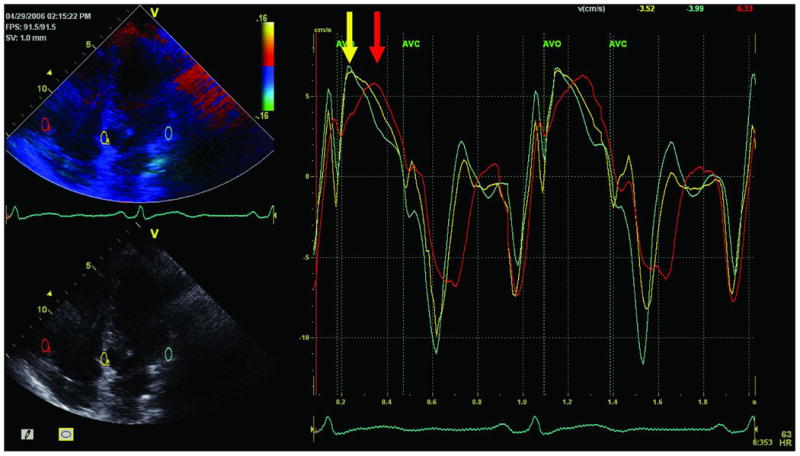
Samples are placed at the basal parts of the septum (yellow curve), RV free wall (red curve) and LV lateral wall (green curve). In this patient, a significant delay between the septum and the RV free wall was present (110 ms), indicated by the yellow and red arrows.
Mean TSV for the LV lateral wall in the ARVD/C patients and the controls was 171 ± 47 ms and 155 ± 47 ms, respectively. Mean time-to-peak strain of the LV lateral wall was 398 ± 70 ms in the ARVD/C patients and 370 ± 86 ms in the controls. There was no significant difference in LV dyssynchrony between the ARVD/C patients and the controls (21 ± 18 ms vs. 22 ± 19 ms, p=0.7). Using a cut-off value of ≥60 ms (>2 SD of the controls), 2 ARVD/C patients (4%) demonstrated significant LV dyssynchrony.
Interventricular dyssynchrony, calculated as the difference in TSV between the RV free wall and the LV lateral wall, was 53 ± 36 ms in the ARVD/C patients and 21 ± 15 ms in the controls (p<0.001). Based on a cut-off value of ≥51 ms (>2 SD of the controls), significant interventricular dyssynchrony was present in 22 patients (42%) with ARVD/C. In these patients, mean interventricular dyssynchrony was 88 ± 17 ms, whereas it was 27 ± 21 ms in the remaining patients (p<0.001). In 19 of the 26 patients with RV dyssynchrony (73%), significant interventricular dyssynchrony was present. Conversely, in 23 of the 26 patients without RV dyssynchrony (88%), no significant interventricular dyssynchrony was present.
Factors influencing right ventricular dyssynchrony
We examined several morphologic and functional factors that could potentially impact RV mechanical synchrony. These included 1) Electrocardiographic: presence of RV conduction abnormalities as typified by QRS duration and presence of right bundle branch block; 2) Morphologic: RV volumes and LV volumes; 3) Functional: RV function and LV function. To study these factors, ARVD/C patients were divided into those with RV dyssynchrony (n=26) and those without RV dyssynchrony (n= 26).
No differences in RV conduction abnormalities, evaluated by signal averaged and surface ECG, were noted between the two groups: filtered QRS duration on signal averaged ECG was similar in ARVD/C patients with versus those without RV dyssynchrony (134 ± 41 ms vs. 128 ± 32 ms, respectively; p=0.6). No difference in the prevalence of T wave inversion in right precordial leads was noted between the two groups (with RV dyssynchrony n=18; without RV dyssynchrony n=21, p=0.5). Similarly, there were no differences in the prevalence of right bundle branch block noted in 5 patients (19%) with RV dyssynchrony and in 5 patients (19%) without RV dyssynchrony (p=1.0). In addition, there was no difference in the number of patients with documented ventricular tachycardia at baseline between the group with and the group without RV dyssynchrony (10 patients vs. 4 patients, p=0.1).
Compared to patients without RV dyssynchrony, the patients with RV dyssynchrony had larger RVEDA (Table 3), and a lower RVFAC (Figure 2). No significant differences in LV volumes, function, and peak systolic velocities and peak systolic strain were noted between patients with and without RV dyssynchrony. In contrast, peak systolic strain of the RV free wall was significantly decreased in patients with RV dyssynchrony, compared with patients without RV dyssynchrony (Figure 2).
Table 3. Echocardiographic data in ARVD/C patients with and without right ventricular dyssynchrony.
| Variable | Without RV dyssynchrony (n=26) |
With RV dyssynchrony (n=26) |
P value |
|---|---|---|---|
| RVOT diameter (cm) | 2.9 ± 0.4 | 2.9 ± 0.4 | 0.9 |
| RVEDA (cm2) | 19 ± 4 | 22 ± 5 | 0.02 |
| RVESA (cm2) | 12 ± 3 | 16 ± 5 | 0.005 |
| RVFAC (%) | 34 ± 8 | 29 ± 8 | 0.03 |
| LVEDV (ml) | 104 ± 28 | 103 ± 26 | 1.0 |
| LVESV (ml) | 45 ± 13 | 45 ± 15 | 1.0 |
| LVEF (%) | 57 ± 4 | 57 ± 6 | 0.8 |
| Septum | |||
| Peak systolic velocity (cm/s) | 5.4 ± 1.3 | 5.4 ± 1.0 | 1.0 |
| Peak systolic strain (%) | -21 ± 7 | -22 ± 7 | 0.8 |
| RV free wall | |||
| Peak systolic velocity (cm/s) | 7.4 ± 2.5 | 7.3 ± 1.7 | 0.9 |
| Peak systolic strain (%) | -22 ± 7 | -16 ± 6 | 0.001 |
| LV lateral wall | |||
| Peak systolic velocity (cm/s) | 6.4 ± 1.5 | 6.6 ± 1.4 | 0.5 |
| Peak systolic strain (%) | -19 ± 7 | -16 ± 5 | 0.1 |
Abbreviations as in Table 2.
Figure 2. RVFAC and RV peak systolic strain in controls and ARVD/C patients.
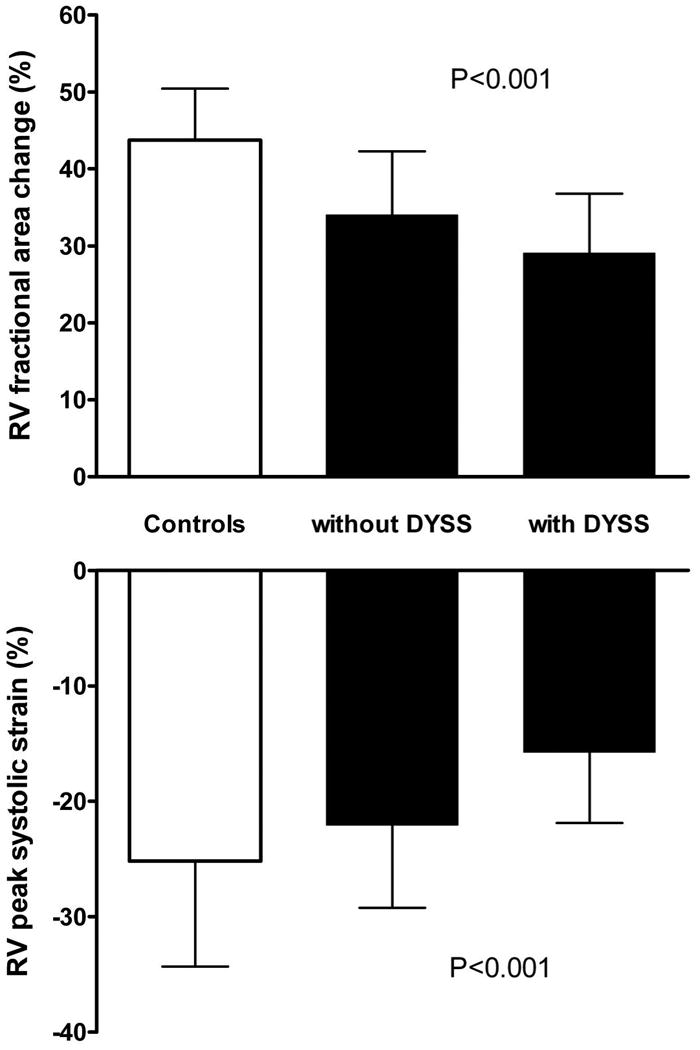
Right ventricular fractional area change (upper panel) and RV peak systolic strain (lower panel) in the 25 controls, 26 ARVD/C patients without RV dyssynchrony and 26 ARVD/C patients with RV dyssynchrony. Both RV fractional area change and RV peak systolic strain were significantly decreased in the ARVD/C patients with RV dyssynchrony.
A modest, but significant correlation was found between FAC and RV dyssynchrony (r=-0.38, p=0.001), and between RVEDA and RV dyssynchrony (r=0.38, p=0.001). In addition, a modest, but significant correlation was found between peak systolic strain of the RV free wall and RV dyssynchrony (r=0.40, p<0.001).
Reproducibility of RV dyssynchrony
The intra-observer and inter-observer variability for time-to-peak systolic velocity for the RV free wall were 1.0 ± 16.6 ms (95% CI -31.6 to 33.6), and 0 ± 26.7 ms (95% CI -52.3 to 52.3), respectively. The intra-observer and inter-observer variability for RV dyssynchrony were 0 ± 18.9 ms (95% CI -36.9 to 36.9) and -5.0 ± 29.5 ms (95% CI -62.9 to 52.9), respectively. For the classification of the presence or absence of RV dyssynchrony, an excellent agreement was noted between the two observers (κ=0.80) and within the same observer (κ=1.0).
Discussion
We present a previously unreported finding of significant ventricular mechanical dyssynchrony in patients with a primary RV cardiomyopathy, ARVD/C. In a relatively large cohort of ARVD/C patients we demonstrate RV dyssynchrony in 50% and interventricular dyssynchrony in 42% of the patients. Patients with RV dyssynchrony had larger RV volumes and lower RV function compared to controls.
Right ventricular dyssynchrony
The presence of LV and interventricular dyssynchrony has been studied in a broad spectrum of clinical settings (9). In contrast, RV dyssynchrony has not been studied extensively. The presence of RV dyssynchrony was first reported by Lopez-Candales et al. in 20 patients with pulmonary hypertension (7). Using time-to-peak strain between the septum and RV free wall, RV dyssynchrony was found to be more pronounced in patients with pulmonary hypertension as compared to controls (92 ± 78 ms vs. 11 ± 23 ms, p<0.001). In contrast, there were no differences in LV dyssynchrony between the two groups (7). Similarly, intra- and inter-ventricular dyssynchrony was examined in 34 patients with LV systolic heart failure, mean LV ejection fraction 22 ± 7% (56% with non-ischemic cardiomyopathy) (15). Mean RV dyssynchrony was 59 ± 45 ms and mean LV dyssynchrony was 80 ± 62 ms.
In a larger unselected cohort of patients with a primary RV cardiomyopathy (ARVD/C), we report for the first time the occurrence of significant RV and interventricular mechanical dyssynchrony. As opposed to previous studies, dyssynchrony in this population occurred in the absence of confounding factors such as pulmonary hypertension and LV failure. Our data also established a cutoff value for mechanical dyssynchrony in the RV using 25 healthy controls. Interestingly, our cutoff value of 56 ms is close to the previously reported cut-off values for LV dyssynchrony (16).
Factors influencing RV dyssynchrony in ARVD/C
The presence of RV dyssynchrony is not surprising given previous and recent knowledge about the pathophysiology of ARVD/C. Recent data on potential causal genes suggest that most mutations involve genes that encode desmosomal proteins and include but are not limited to desmoplakin, plakophilin 2 and desmoglein (17-19). Thus ARVD/C is considered a desmopathy that is likely associated with abnormal cell to cell coupling, both electrically and mechanically, providing the substrate for the RV dyssynchrony.
Akin to LV dysfunction, electrical conduction abnormalities in the RV could be associated with mechanical delays. However, in our cohort we found no differences in QRS duration and/or the presence of right bundle branch block between patients with and without RV dyssynchrony. Although in general the presence of mechanical dyssynchrony is related to intra-ventricular conduction abnormalities, substantial LV ventricular dyssynchrony has been previously demonstrated in the absence of QRS prolongation (20,21). Thus ARVD/C may be another example of dyssynchrony with a narrow QRS. Another potential explanation may be that in ARVD/C, ventricular dyssynchrony is more related to regional and heterogeneous abnormalities in conduction and contractility, not evident on a surface ECG (22).
In contrast to the lack of association between electrocardiographic abnormalities and dyssynchrony, RV morphology and function appeared to be related to RV dyssynchrony. Larger RVEDA and RVESA were noted in the patients with RV dyssynchrony. However, this relationship was not as strong as previously reported in patients with pulmonary hypertension (r=0.70, p<0.001 between RVEDA and RV dyssynchrony) (23). One potential reason for a weaker relationship could be the difference in pathology. ARVD/C is a patchy infiltrative process with regional dilatation while pulmonary hypertension (pressure overload) affects the RV globally and is more likely to cause uniform chamber dilatation in the load-sensitive RV (24).
Similar to dyssynchrony associated with LV failure (20,25), our data indicate a relationship between RV function, as determined by RVFAC and RV peak systolic strain, and RV dyssynchrony in ARVD/C. These findings are also in line with previous studies in patients with pulmonary hypertension (7,23) and systolic heart failure (15). Finally, fibrofatty infiltration in ARVD/C could involve the conduction system and thereby introduce electro-mechanical delays resulting in dyssynchrony. Similar relationships have been examined in ischemic cardiomyopathy where significant amounts of fibrosis result in the presence of mechanical dyssynchrony (22).
Our findings present several incremental points of knowledge concerning ARVD/C that could be potentially used for prognostic and therapeutic purposes. In patients with LV failure, the presence of significant ventricular dyssynchrony is associated with a worse prognosis (10). Dyssynchrony in ARVD/C may similarly predict worse clinical outcomes. Serial monitoring of RV dyssynchrony may identify patients at higher risk and deserving of aggressive therapy. Cardiac resynchronization therapy has improved symptoms and survival in dyssynchronous left heart failure (8,26). The presence of significant RV or interventricular dyssynchrony may introduce the possibility of resynchronization therapy for right sided failure in patients with ARVD/C who would otherwise be transplant candidates. However, more prospective studies are needed to further elucidate the clinical implications of the presence of RV dyssynchrony in ARVD/C.
Limitations
The mean age of the control group was lower than the ARVD/C patients. This may affect the definition of RV dyssynchrony for the ARVD/C patients. However, it has been demonstrated that ventricular dyssynchrony does not depend on age (27). In addition, LV dyssynchrony was comparable between the controls and the ARVD/C patients in the present study. Lastly, we strictly selected healthy normal controls since a previous definition for RV dyssynchrony was not available. Older controls tended to have medical conditions such as hypertension and diabetes, whose effects on RV dyssynchrony are unclear and were therefore excluded from the normal group. Larger studies with the power to assess the influence of other co-morbidities should ideally include an age-matched control group.
Furthermore, in the present study only TDE was used to define interventricular dyssynchrony. Interventricular dyssynchrony, calculated as the time difference between RV and LV pre-ejection intervals may have also provided additional information. However, RV outflow Doppler was not consistently performed in a fair number of subjects and we are unable to assess this parameter in our population.
Duration of disease is likely an important factor in the development of RV dyssynchrony in ARVD/C. However, determining the onset and duration of disease in this relatively asymptomatic group is challenging. We are therefore unable to evaluate its influence on RV dyssynchrony.
Similarly, the extent of fibrofatty infiltration may be an important factor in the pathogenesis of RV dyssynchrony in ARVD/C patients. In a small subset of patients enrolled in the present study, who also had clinical magnetic resonance imaging, we found no correlation between the extent of fibrofatty infiltration (as assessed by gadolinium enhancement) and RV dyssynchrony. These data were not presented due to the small sample size and lack of statistical power to offer reliable conclusions.
Finally, although the present study is the first observational study that demonstrates the presence of RV dyssynchrony in ARVD/C patients, unfortunately, this cross-sectional analysis does not provide insights into the clinical significance of the presence of RV dyssynchrony, and its exact role in ARVD/C management remains unclear. However, our findings prompt larger longitudinal studies to evaluate the influence of dyssynchrony on diagnosis, treatment and prognostication of ARVD/C patients including prediction of clinical outcomes such as heart failure, potential for arrhythmias and response to treatment. In particular, future studies may allow a more systematic assessment of several important factors including but not limited to duration of disease and genotype.
Conclusions
Significant RV dyssynchrony may occur in up to 50% of ARVD/C patients and is associated with RV remodeling and dysfunction rather than electrocardiographic abnormalities. This finding may have therapeutic and prognostic implications in ARVD/C.
Acknowledgments
Funding / Disclosures: This work was supported by funds from the Bogle Foundation, National Institutes of Health (HL65594 and AG22554), The Netherlands Heart Foundation, Leids Universiteits Fonds and Foundation ‘De Drie Lichten.’
Dr. Abraham receives honoraria and research grants from GE Healthcare. Dr. Bax receives grants from Medtronic, Biotronik, BMS medical imaging, St. Jude Medical, GE Healthcare and Edwards Lifesciences.
Abbreviations
- ARVD/C
arrhythmogenic right ventricular dysplasia/cardiomyopathy
- ECG
electrocardiogram
- LV
left ventricle / ventricular
- RV
right ventricle / ventricular
- RVEDA
right ventricular end-diastolic area
- RVESA
right ventricular end-systolic area
- RVFAC
right ventricular fractional area change
- SD
standard deviation
- SE
strain echocardiography
- TDE
tissue Doppler echocardiography
- Tsv
time-to-peak systolic velocity
Footnotes
Drs. Tops and Prakasa contributed equally to this manuscript.
All other authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Corrado D, Basso C, Thiene G, et al. Spectrum of clinicopathologic manifestations of arrhythmogenic right ventricular cardiomyopathy/dysplasia: a multicenter study. J Am Coll Cardiol. 1997;30:1512–20. doi: 10.1016/s0735-1097(97)00332-x. [DOI] [PubMed] [Google Scholar]
- 2.McKenna WJ, Thiene G, Nava A, et al. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br Heart J. 1994;71:215–8. doi: 10.1136/hrt.71.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kies P, Bootsma M, Bax J, Schalij MJ, van der Wall EE. Arrhythmogenic right ventricular dysplasia/cardiomyopathy: screening, diagnosis, and treatment. Heart Rhythm. 2006;3:225–34. doi: 10.1016/j.hrthm.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 4.Dalal D, Nasir K, Bomma C, et al. Arrhythmogenic right ventricular dysplasia: a United States experience. Circulation. 2005;112:3823–32. doi: 10.1161/CIRCULATIONAHA.105.542266. [DOI] [PubMed] [Google Scholar]
- 5.Hulot JS, Jouven X, Empana JP, Frank R, Fontaine G. Natural history and risk stratification of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circulation. 2004;110:1879–84. doi: 10.1161/01.CIR.0000143375.93288.82. [DOI] [PubMed] [Google Scholar]
- 6.Bax JJ, Ansalone G, Breithardt OA, et al. Echocardiographic evaluation of cardiac resynchronization therapy: ready for routine clinical use? A critical appraisal. J Am Coll Cardiol. 2004;44:1–9. doi: 10.1016/j.jacc.2004.02.055. [DOI] [PubMed] [Google Scholar]
- 7.Lopez-Candales A, Dohi K, Bazaz R, Edelman K. Relation of right ventricular free wall mechanical delay to right ventricular dysfunction as determined by tissue Doppler imaging. Am J Cardiol. 2005;96:602–6. doi: 10.1016/j.amjcard.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 8.Bax JJ, Abraham T, Barold SS, et al. Cardiac resynchronization therapy: Part 1--issues before device implantation. J Am Coll Cardiol. 2005;46:2153–67. doi: 10.1016/j.jacc.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Abraham TP, Dimaano VL, Liang HY. Role of tissue Doppler and strain echocardiography in current clinical practice. Circulation. 2007;116:2597–609. doi: 10.1161/CIRCULATIONAHA.106.647172. [DOI] [PubMed] [Google Scholar]
- 10.Bader H, Garrigue S, Lafitte S, et al. Intra-left ventricular electromechanical asynchrony. A new independent predictor of severe cardiac events in heart failure patients. J Am Coll Cardiol. 2004;43:248–56. doi: 10.1016/j.jacc.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 11.Gorcsan J, III, Abraham T, Agler DA, et al. Echocardiography for cardiac resynchronization therapy: recommendations for performance and reporting--a report from the American Society of Echocardiography Dyssynchrony Writing Group endorsed by the Heart Rhythm Society. J Am Soc Echocardiogr. 2008;21:191–213. doi: 10.1016/j.echo.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Lang RM, Bierig M, Devereux RB, et al. Recommendations for Chamber Quantification: A Report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, Developed in Conjunction with the European Association of Echocardiography, a Branch of the European Society of Cardiology. Journal of the American Society of Echocardiography. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–67. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 14.Prakasa KR, Wang J, Tandri H, et al. Utility of tissue Doppler and strain echocardiography in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Am J Cardiol. 2007;100:507–12. doi: 10.1016/j.amjcard.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 15.Rajagopalan N, Dohi K, Simon MA, et al. Right ventricular dyssynchrony in heart failure: a tissue Doppler imaging study. J Card Fail. 2006;12:263–7. doi: 10.1016/j.cardfail.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Bax JJ, Bleeker GB, Marwick TH, et al. Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol. 2004;44:1834–40. doi: 10.1016/j.jacc.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Rampazzo A, Nava A, Malacrida S, et al. Mutation in human desmoplakin domain binding to plakoglobin causes a dominant form of arrhythmogenic right ventricular cardiomyopathy. Am J Hum Genet. 2002;71:1200–6. doi: 10.1086/344208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerull B, Heuser A, Wichter T, et al. Mutations in the desmosomal protein plakophilin-2 are common in arrhythmogenic right ventricular cardiomyopathy. Nat Genet. 2004;36:1162–4. doi: 10.1038/ng1461. [DOI] [PubMed] [Google Scholar]
- 19.Pilichou K, Nava A, Basso C, et al. Mutations in desmoglein-2 gene are associated with arrhythmogenic right ventricular cardiomyopathy. Circulation. 2006;113:1171–9. doi: 10.1161/CIRCULATIONAHA.105.583674. [DOI] [PubMed] [Google Scholar]
- 20.Ghio S, Constantin C, Klersy C, et al. Interventricular and intraventricular dyssynchrony are common in heart failure patients, regardless of QRS duration. Eur Heart J. 2004;25:571–8. doi: 10.1016/j.ehj.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 21.Bleeker GB, Schalij MJ, Molhoek SG, et al. Relationship between QRS duration and left ventricular dyssynchrony in patients with end-stage heart failure. J Cardiovasc Electrophysiol. 2004;15:544–9. doi: 10.1046/j.1540-8167.2004.03604.x. [DOI] [PubMed] [Google Scholar]
- 22.Kass DA. An epidemic of dyssynchrony: but what does it mean? J Am Coll Cardiol. 2008;51:12–7. doi: 10.1016/j.jacc.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Candales A, Dohi K, Rajagopalan N, et al. Right ventricular dyssynchrony in patients with pulmonary hypertension is associated with disease severity and functional class. Cardiovasc Ultrasound. 2005;3:23. doi: 10.1186/1476-7120-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haddad F, Doyle R, Murphy DJ, Hunt SA. Right ventricular function in cardiovascular disease, part II: pathophysiology, clinical importance, and management of right ventricular failure. Circulation. 2008;117:1717–31. doi: 10.1161/CIRCULATIONAHA.107.653584. [DOI] [PubMed] [Google Scholar]
- 25.Ghio S, Freemantle N, Serio A, et al. Baseline echocardiographic characteristics of heart failure patients enrolled in a large European multicentre trial (CArdiac REsynchronisation Heart Failure study) Eur J Echocardiogr. 2006;7:373–8. doi: 10.1016/j.euje.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Bradley DJ, Bradley EA, Baughman KL, et al. Cardiac resynchronization and death from progressive heart failure: a meta-analysis of randomized controlled trials. JAMA. 2003;289:730–40. doi: 10.1001/jama.289.6.730. [DOI] [PubMed] [Google Scholar]
- 27.Ng AC, Tran dT, Newman M, et al. Left ventricular longitudinal and radial synchrony and their determinants in healthy subjects. J Am Soc Echocardiogr. 2008;21:1042–8. doi: 10.1016/j.echo.2008.05.002. [DOI] [PubMed] [Google Scholar]


