Abstract
Fluorine-18-labeled steroid receptor tracers, 16α-[18F]fluoroestradiol (FES), [18F]fluoro furanyl norprogesterone (FFNP), and 16β-[18F]fluoro-5α-dihydrotestosterone (FDHT), are important imaging tools for studies of breast and prostate cancers using positron emission tomography (PET). The automated production of these ligands with high specific activity (SA) as radiopharmaceuticals requires modification and optimization of the currently reported methods. [18F]FES with high SA was synthesized in over 60% radiochemical yield (RCY) at the end of synthesis (EOS) using a small amount of precursor (1) (as low as 0.3 mg) and 1 M H2SO4 for deprotection of the intermediate (2). [18F]FFNP was synthesized in up to 77% RCY at EOS using the triflate precursor (4) at room temperature or in 25% RCY using the mesylate precursor (6) at 65°C. Both methods are highly reproducible and afford high SA. [18F]FDHT was synthesized by radiofluoride incorporation at room temperature, reduction with NaBH4, and deprotection with HCl/acetone, giving [18F]FDHT in up to 75% yield (RCY). All of these methods can be easily translated to automated production. The information provided here will aid in the development of automated production of these steroid receptor tracers with high or improved yields, optimal SA, and ease of processing for research and clinical use.
Keywords: steroid receptor ligand, FES, FFNP, FDHT, PET, radiopharmaceuticals
Introduction
For decades, the steroid receptors, estrogen receptor (ER), progesterone receptor (PR), and androgen receptor (AR), have been the targets of diagnostic molecular imaging in breast and prostate cancers using positron emission tomography (PET), for the purpose of staging disease and monitoring tumor drug exposure and response to therapies.1–6 Among the fluorine-18-labeled steroid receptor ligands, 16α-[18F]fluoroestradiol ([18F] FES) has been studied extensively7,8 as an ER tracer in breast cancer; 16β-[18F]fluoro-5α-dihydrotestosterone ([18F]FDHT), an AR tracer, has also been found useful for imaging prostate cancer in both the research and clinical settings.9–13 Most recently, [18F] fluoro-19-norprogesterone-16α,17α-endo-furanyl acetal ([18F] fluoro furanyl norprogesterone or [18F]FFNP) has demonstrated its potential for imaging PR in breast cancer patients14 and for predicting response to endocrine therapy at an early stage in small animal models of breast cancer.15
16α-[18F]fluoroestradiol has been used worldwide as a PET radiopharmaceutical for research and clinical applications, partially because of the improved radiosynthesis of FES using a cyclic sulfate precursor.16 Recently, an alternative synthesis of [18F]FES using di-methoxymethyl-protected nosylate precursor was reported, aiming to avoid the difficult hydrolysis of the bisulfate intermediate.17 The original radiosynthesis of [18F]FDHT was reported in 1992, using LiAlH4, liquid–liquid extraction, normal phase HPLC purification, and so on, which are not amenable to automated production of radiopharmaceuticals. Because of this, the use of FDHT has been limited to only a few sites. [18F]FFNP was first reported in 1995,18 but only after an efficient route to make an FFNP precursor was reported in 2002 did clinical trials with FFNP become feasible.14 However, the radiosynthesis of [18F]FFNP is still challenging and requires optimization for adaptation to automated production (refer to Figure 1 for structures and reaction scheme).
Figure 1.
Schemes for the radiosyntheses of [18F]FES (3), [18F]FFNP(5), and [18F]FDHT (10).
Because of the unique value of PET for in vivo molecular imaging of various disease states, there is an increasing interest in using FES, FFNP, and FDHT in research and in clinical studies of breast and prostate cancers. The radiosynthesis of 18F-labeled steroid receptor ligands as radiopharmaceuticals is demanding because the imaging target of each of these radiotracers is a high affinity, limited capacity, specific ligand-binding receptor, which means that high specific activity (SA) and high effective SA (ESA) are required for these radiolabeled ligands to be useful in PET imaging.19
In this paper, the optimization of the radiosyntheses of FES, FFNP, and FDHT are described with the aim of providing the information needed to develop automated syntheses of these steroid receptor ligands that will operate simply and reproducibly and produce final products in good yield and with high purity and high SA.
Experimental
General
All chemical reagents were obtained from standard commercial sources and used without further purification. FES precursor (1) and standard (3) were purchased from ABX (Germany) or Futurechem (Korea). FFNP precursor (4 and 6) and standard (5) were synthesized according to literature with some modifications.18,20,21 FDHT precursor (7) and standard (10) were custom-synthesized according to the literature.22 [18F]Fluoride was produced at Washington University by the 18O(p, n)18F reaction through proton irradiation of enriched (95%) [18O] water in the RDS 111 cyclotron. High performance liquid chromatography (HPLC) was performed with an ultraviolet (UV) detector and a well-scintillation NaI (Tl) detector and associated electronics for radioactivity detection. A Phenomenex Luna C18 250 × 10-mm 5-μm semipreparative column and an Alltech Altima C18 250 × 4.6-mm 10-μm column were used for preparative purification and for postsynthesis analysis of chemical and radiochemical purity and SA, respectively. The following solvents were used as mobile phases: acetonitrile (A), ammonium formate buffer (0.1 M, pH 4.5) (B), water (C), and potassium phosphate monobasic solution (0.05 M) (D). The HPLC conditions are as follows: FES (38% A/ 62% B with a flow at 4 mL/min and UV detection at 280 nm for purification and 54% A/46% C with a flow at 2 mL/min and UV detection at 280 nm for analysis); FFNP (54% A/46% C with a flow at 4 mL/min and UV detection at 254 nm for purification and 60% A/40% C with a flow at 2 mL/min and UV detection at 254 nm for analysis); and FDHT (44% A/56% D with a flow at 2 mL/min and UV detection at 215 nm for purification and 55% A/45% C with a flow at 2 mL/min and UV detection at 215 nm for purification). Radio-TLC was accomplished using a Bioscan AR-2000 imaging scanner (Bioscan, Inc., Washington DC).
General procedure of drying [18F]fluoride
[18F]Fluoride in [18O]water (e.g., 50 mCi/~100 μL) was transferred into a BD Vacutainer (5 mL, glass, no additives) or a Pyrex tube with a screw cap (10 mL) containing known amount of K2CO3/Kryptofix 222 (K222), and then, the activity was dried by azeotropic distillation at 105°C using MeCN (3 × 1 mL) under a gentle flow of N2 gas, which was used to remove vapor and to prevent condensation of moisture on the upper part of the tube. When the drying was nearly complete, the last solvent residue (~100 μL) was carefully removed under a gentle a flow of N2 at a lower temperature (~85°C) to avoid overdrying of the activity.
Radiosynthesis of [18F]FES (3)
A stock solution of FES precursor (1) (0.5 mg, 1.27 μmol) in MeCN (0.5 mL) was added to the activity, previously dried with K2CO3 (0.5 mg, 3.62 μmol) and K222 (2.8 mg, 7.44 μmol) as described before. The reaction mixture was heated at 105°C for 7 min, and then, a solution of H2SO4 (1 M, 100 μL) and MeCN (400 μL) was added. The heating continued for 10 more minutes for deprotection. At room temperature, a solution of ammonium formate (0.1 M, pH 6.5, 2 mL) was added to the reaction mixture for HPLC injection via a 0.45-μm Nylon filter. [18F]FES was collected at 26–27 min, and the radioactive fractions were diluted with water (50 mL). The dilution material was passed through a C18 Sep-Pak (Waters, Classic or Plus) under pressure or under vacuum, and then, the Sep-Pak was rinsed with water (10 mL). The final dose of [18F]FES was prepared by sequentially eluting the radioactivity from the Sep-Pak with ethanol (1 mL) and saline (10 mL). For animal dosing, the radioactivity was eluted with ethanol in portions (0.2 mL). The portion containing the most of the activity was used to prepare the animal dose.
Radiosynthesis of [18F]FFNP (5)
Using a triflate precursor (4)
A solution of an FFNP triflate precursor (4) (2.0 mg, 3.58 μmol) in MeCN (1.0 mL) was added to the activity, previously dried with K2CO3 (0.5 mg, 3.62 μmol) and K222 (2.8 mg, 7.44 μmol) as described before. The reaction mixture was shaken or stirred at room temperature for 10 min, and then, water (1.5 mL) was added to the reaction mixture for HPLC injection via a 0.45-μm Nylon filter. [18F]FFFNP was collected at 26–27 min. The final [18F]FFNP dose was prepared using the solid phase extraction (SPE) method described previously for [18F]FES.
Using a mesylate precursor (6)
A solution of an FFNP mesylate precursor (6) (1.8 mg, 3.57 μmol) in MeCN (0.6 mL) was added to the activity, previously dried with K2CO3 (0.4 mg, 2.90 μmol) and K222 (2.8 mg, 7.44 μmol) as described before, and then heated at 65°C for 10 min. After the temperature was quickly lowered to room temperature, water (1.5 mL) was added to the reaction mixture for HPLC injection via a 0.45-μm Nylon filter. The final [18F]FFNP dose was prepared as described previously.
Radiosynthesis of [18F]FDHT (10)
A solution of an FDHT precursor (7) (1.8 mg, 3.75 μmol) in MeCN (0.5 mL) was added at room temperature to the activity, previously dried with K2CO3 (0.5 mg, 3.62 μmol) and K222 (2.8 mg, 7.44 μmol) as described before. The reaction mixture was shaken or stirred at room temperature for 10 min, and then, a solution of NaBH4 (5 mg) in ethanol (0.5 mL) was added for reduction. After 10 min at room temperature, acetone (0.5 mL) was added to quench excess NaBH4 before the deprotection with acid by the following two methods. Solution method: A solution of HCl (4 N, 0.5 mL) was added, and then, the reaction mixture was heated at 50°C for 10 min. The reaction mixture was diluted with water (10 mL), and the diluted mixture passed a C18 Sep-Pak (Waters, Classic), which was further rinsed with water (10 mL). The final radioactive product was sequentially eluted from the Sep-Pak (backwards) with MeCN (1 mL) and water (2 mL) for HPLC injection; C18 Sep-Pak method: The reaction mixture was diluted with water (10 mL), and the diluted mixture passed a C18 Sep-Pak (Waters, Classic), which was rinsed twice with water (2 × 10 mL). A solution of HCl (6 N, 1 mL) with acetone (0.1 mL) was loaded onto the Sep-Pak. After 5 min at room temperature, the Sep-Pak was rinsed with water (2 × 10 mL), and the final radioactive product was sequentially eluted from the Sep-Pak (backwards) with MeCN (1 mL) and water (2 mL) for HPLC injection. [18F]FDHT was collected at 23– 24 min. The final [18F]FDHT dose was prepared using the SPE method described previously for [18F]FES.
Results and discussion
General consideration of optimizations
Specific activity and ESA are arguably critical parameters in the radiolabeling of steroid receptor ligands for use as radio-pharmaceuticals.23 It was reported that the standardized uptake valueof[18F]FESovertherangeofSAvaluesfrom500–18,000 mCi/ μMwas almost same in clinical trials.24 However, much higher dose per weight could be injected for small animal imaging studies, and also, significantly lower ESA than SA was reported for [18F]FES.25,26 Therefore, it is ideal to maintain SA and ESA as high as possible for the production of steroid receptor radiopharmaceutical ligands.
For the manual synthesis, [18F]fluoride in target [18O]water without QMA treatment was used not only to avoid the potential deterioration of SA by QMA treatment27,28 but also to provide a better control of the amount of K2CO3 used, which is critical for the radiolabeling of base-labile precursors. When QMA treatment is preferred for automated production using a large amount of radioactivity, the amount of base can be minimized by using commercially available minute QMA columns.29 Minimum amounts of precursors were also used for improved yields and purification of final products. The HPLC purification was carefully optimized by using a strong buffer mobile phase, increasing the retention time, and using modern, relatively fresh HPLC columns dedicated to purification of radiopharmaceutical preparations. In addition, prior to each use, the HPLC column and the synthesis module were rinsed thoroughly with 80% MeCN/water and MeCN, respectively, to avoid cross contamination from earlier productions.
Radiosynthesis of [18F]FES (3)
Automated synthesis of [18F]FES for clinical use has been reported by several sites worldwide.24,30–33 The reported synthesis protocols are similar, using 2 mg of a cyclic sulfate precursor (1), large amount of K2CO3/K222, and HCl for acid hydrolysis, with variable yields and SA of the final product. The ring-opening reaction using the cyclic sulfate precursor (1) is highly efficient.16 Therefore, we explored minimizing the amount of precursor for better purification. As shown in Table 1, when only 0.3 mg of precursor and 0.3 mg K2CO3 were used, the labeling reaction still afforded high incorporation yields, comparable to these of using up to 1 mg precursor. When 2 N HCl was used for hydrolysis, the results were variable. Very recently, the inefficiency of the HCl hydrolysis was noted in the literature, and this leads to the development of an alternative synthetic route of [18F]FES.17 When 1 M H2SO4, reported in the original literature,16 was used for the hydrolysis, the yield was highly reproducible. Before HPLC purification, the strong acid, H2SO4, was converted to formic acid by interaction with ammonium formate.
Table 1.
Radiosythesis of [18F]FES
| Substrate (mg) | K2CO3/K222 (mg) | Hydrolysis method | Incorporation yielda (%) | Isolated yieldb (%) |
|---|---|---|---|---|
| 1.0 | 1.0/5.6 | 2N HCl (100 μL) | 88 (n = 1) | NDc |
| 0.5 | 0.5/2.8 | 2N HCl (100 μL) | 71.7 ± 14.2 (n = 3) | NDc |
| 0.5 | 0.5/2.8 | 1 M H2SO4 (100 μL) | 90.7 ± 0.7 (n = 3) | 68 (n = 1) |
| 0.4 | 0.4/2.3 | 1 M H2SO4 (100 μL) | 89 ± 8.5 (n = 2) | 66.3 ± 6.1 (n = 3) |
| 0.3 | 0.3/1.7 | 1 M H2SO4 (100 μL) | 86.8 ± 6.6 (n = 6) | 65.2 ± 7.6 (n = 8) |
Determined by radio-TLC (silica/20% MeOH in dichloromethane)
Reported yield after HPLC purification (decay corrected) from 50mCi [18F]fluoride
ND: not determined.
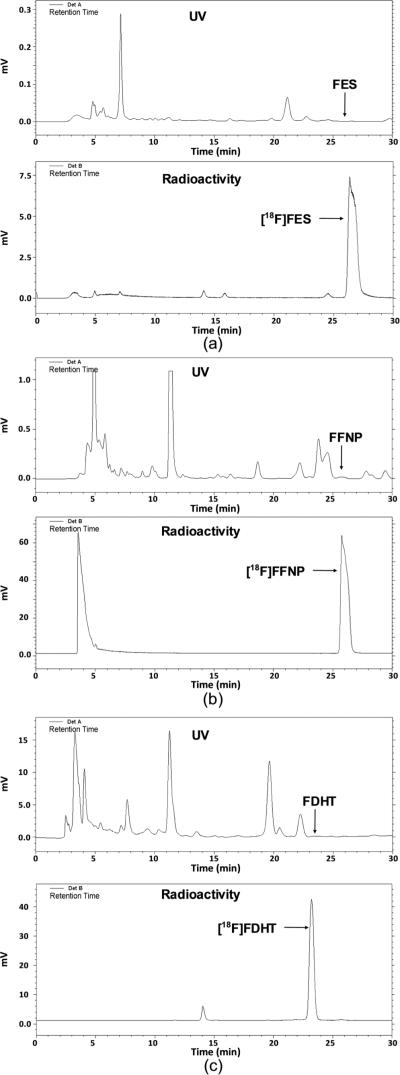
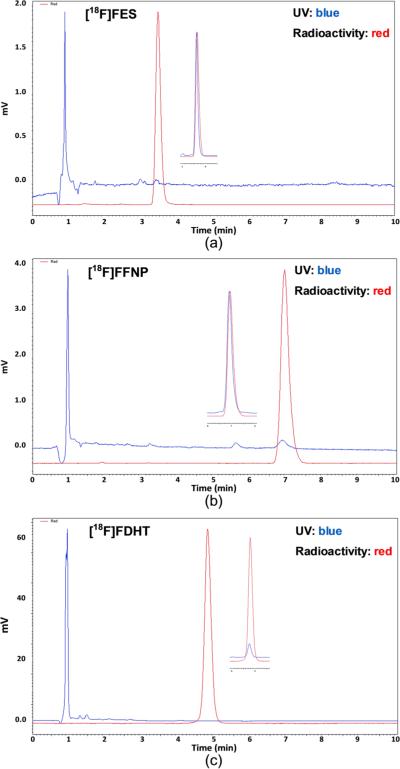
As shown in Figure 2a, there were no major mass peaks eluting around FES, which is ideal for achieving high purity of FES, and [18F]FES was also the major radioactivity peak in the HPLC chromatograph. Starting with 50 mCi [18F]fluoride, yields up to 70% (isolated, decay corrected) of [18F]FES were obtained using only 0.3 mg substrate within 90 min, with SA ranging from 2000 to 9000 mCi/μmol (Figure 3a). The dose of [18F]FES in 10% ethanol/saline was stable for hours, even at a high concentration of 20 mCi/mL, indicating that radiolysis of the dose was minimal.
Figure 2.
Semipreparative HPLC chromatographs of [18F]FES (a), [18F]FFNP from the triflate precursor (4) (b), and [18F]FDHT (c).
Figure 3.
Analytical HPLC chromatographs of [18F]FES (a), [18F]FFNP (b), and [18F] FDHT (c) (inset: coinjection of reference compounds).
Radiosynthesis of [18F]FFNP (5)
Originally, [18F]FFNP was synthesized using the triflate precursor (4) with Bu4NOH in THF at room temperature, but only 2–13% decay-corrected yields were obtained.18 For clinical use, an improved method using a mesylate precursor (6) and K2CO3/K222 by semiautomation was developed to afford [18F]FFNP in up to 15% nondecay-corrected yields. The low yields and low reproducibility of the labeling appear to result from the instability of the precursors and FFNP under typical labeling conditions. Because of FDA regulations, a method for automated synthesis of [18F]FFNP for clinical use is needed.
It was found that the triflate precursor (4) is much more stable than expected when 4 was purified without aqueous workup. Therefore, the labeling of FFNP using the triflate precursor (4) was revisited using K2CO3/K222 in MeCN at room temperature (Table 2). Within 5 min, the incorporation reached a plateau of over 80% yield, with the complete consumption of the triflate precursor, according to HPLC analysis. Starting with 50 mCi [18F]fluoride, up to 77% yield (isolated, decay corrected) of [18F] FFNP with SA ranging from 1300 to 8500 mCi/μmol was obtained in 90 min (refer to Figures 2b and 3b for HPLC chromatographs). Thus, the triflate precursor (4) appears to be the most promising one for the automated synthesis of [18F]FFNP, but we further explored the stability and utility of the mesylate precursor (6) for the automated production of FFNP.
Table 2.
Radiosynthesis of [18F]FFNP using the triflate precursor (4)
| Entry | 4/MeCN (mg/mL) | K2CO3/K222 (mg/mg) | Incorporation yielda/time (%/min) | Yieldc (%) | SA mCi/μol | ||
|---|---|---|---|---|---|---|---|
| 1 | 1.5/0.7 | 0.5/2.8 | / | 75.7/5 | 83.7/10b | 65 | 8465 |
| 2 | 1.8/0.4 | 0.5/2.8 | / | 84.5/5 | 76.1/10b | 77 | 1476 |
| 3 | 1.8/0.6 | 0.5/2.8 | / | 62.6/5 | 57.5/10b | 50 | 7948 |
| 4 | 1.8/0.6 | 0.5/2.8 | / | 74.2/5 | / | 53.9 | 5222 |
| 5 | 1.8/1.0 | 1/5.6 | / | 58/5 | 58.2/10b | NDd | NDd |
| 6 | 2.0/1.0 | 0.5/2.8 | 65.5/3 | 84.6/6 | 84.8/10b | 72.7 | 1322 |
| 7 | 2.0/1.0 | 0.5/2.8 | 65.3/3 | 83.0/6 | 79.4/10b | 77 | (4636)e |
| 8 | 2.0/1.0 | 0.5/2.8 | / | 83.3/5 | 79.5/10b | 75 | (4656)e |
Incorporation yield by radio-TLC (silica/ethyl acetate)
After 1.5 mL water was added to the reaction mixture
Isolated yield after HPLC purification (decay corrected) starting from 50mCi [18F]fluoride, except for entry 5
ND: not determined
Decay corrected to 2 hr after end of bombardment.
The results using the mesylate precursor (6) are shown in Table 3. Between 60 and 70°C, the incorporation yields were 65% at 5 min and remained the same at 10 min, suggesting that [18F]FFNP was stable under such conditions. At higher and lower temperatures, the yields were lower. The ratio of precursor 6 to K2CO3 is also critical for achieving high yields. Slightly less than 1 equivalent of K2CO3 (0.8 equivalent) appears to afford the best yield, while 0.5 and 1.2 equivalents resulted in lower yields. Other bases (Bu4NOH and KHCO3) were tested, but they did not afford good results. The optimized condition as shown in Table 3 (entries 2 and 4) was used to synthesize [18F]FFNP at a larger scale (~50 mCi in 100–400 μL target water) using the mesylate precursor (6); the incorporation yields were up to 45%, and the isolated yield was around 25%. The lower yields in larger scale reactions might be because of less complete drying of the fluoride ion when starting from large amounts of target water.
Table 3.
Optimization of [18F]FFNP labeling using the mesylate precursor (6)
| Entry | Precursor (mg) | Base (mg) | Solvent (ML) | Temperature (°C) | Incorporation yielda (%) |
||
|---|---|---|---|---|---|---|---|
| 5 min | 10 min | 15 min | |||||
| 1 | 1.8 | K2CO3/K222 0.4/2.8 | MeCN (600) | 50 | 45.8 | 52.8 | / |
| 2 | 1.8 | K2CO3/K222 0.4/2.8 | MeCN (600) | 63 | 64.9 | 65.2 | / |
| 3 | 1.8 | K2CO3/K222 0.6/3.4 | MeCN (600) | 70 | 43.7 | 43.6 | / |
| 4 | 1.8 | K2CO3/K222 0.4/2.8 | MeCN (600) | 70 | 65.1 | 62.5 | / |
| 5 | 1.8 | K2CO3/K222 0.25/1.4 | MeCN (600) | 70 | 46.7 | 45.0 | / |
| 6 | 1.8 | K2CO3/K222 0.25/1.4 | MeCN (300) | 74 | 28 | / | / |
| 7 | 1.8 | K2CO3/K222 0.25/1.4 | MeCN (600) | 80 | 18 | / | / |
| 8 | 1.8 | K2CO3/K222 0.4/2.8 | MeCN (600) | 81 | 14, 29 | / | / |
| 9 | 1.8 | Bu4NOH 3.57 μL/1 M | MeCN (600) | 70 | 7.6 | 7.3 | / |
| 10 | 1.9 | KHCO3/K222 0.41/2.8 | MeCN (500) | 80 | 18.8 (4min) | 24.1 (8min) | 26.9 (105°C/4min) |
Determined by radio-TLC (silica/ethyl acetate).
The dryness of [18F]fluoride, which is related to the drying temperature, drying cycles using acetonitrile, and the potential condensation of moisture in the reaction vessel, proved to be critical for the success of the labeling reaction of FFNP using either triflate or mesylate precursors. It was found that the optimal fluoride ion drying temperature for use in FFNP labeling was around 105°C. Interestingly, under the optimal conditions (105°C drying, 60–70°C heating, and 6 in 0.6 mL MeCN), the major by-products were very lipophilic compounds, which are eluted only with 80% acetonitrile/ water. Because of the formation of these lipophilic compounds, it is critical that the HPLC column is thoroughly cleaned up before each [18F]FFNP production in order to avoid cross contamination by these late-eluting materials. The yields using mesylate precursor 6 are not as high as those using triflate precursor 4, but the stability of 6 is preferable for the distribution of this precursor to different sites for radiosynthesis. Under optimized conditions, the mesylate precursor can easily provide FFNP in yields sufficient for research and clinical use.
Radiosynthesis of [18F]FDHT (10)
16β-[18F]fluoro-5α-dihydrotestosterone (10) was originally radiosynthesized using the triflate precursor (7) with Bu4NOH in THF at 55°C.10 The reported protocol used LiAlH4 as the reducing agent at [C0]78°C, liquid–liquid extraction, and normal phase HPLC for purification.10,11 None of these conditions and processing are easily adaptable to automated production. Recently, an automated synthesis has been reported using NaBH4 as the reducing agent.34 The success of using NaBH4 as the reducing agent prompted us to explore the labeling reaction for automated production of [18F] FDHT with the aim of achieving simplicity, high yield, and high purity. Because of the high reactivity of the triflate precursor (7), the incorporation reaction was carried out at room temperature using K2CO3/K222 in MeCN to afford up to 90% incorporation yield. Unlike LiAlH4, NaBH4 is a very mild reducing agent, so the reduction of intermediate 8 can be carried out at room temperature by adding a solution of NaBH4 in ethanol directly to the previous mixture without removing MeCN. It took 10 min to complete the reduction at room temperature. However, if the large excess of NaBH4 was not completely destroyed after the reduction is complete, it can further reduce the deprotected ketone produced during the acid deprotection step, resulting in significant decomposition of [18F]FDHT during the deprotection step. Thus, to quench NaBH4 and to protect the ketone formed during the deprotection, acetone was added before the addition of acid.
Two methods were developed for the acid deprotection: a method in which the deprotection was carried out in a solution coupled with an SPE using a C18 Sep-Pak and an SPE method in which the acid deprotection was carried out in a C18 Sep-Pak, followed by rinsing with water. The purpose of SPE is to remove HCl and boron residues before HPLC purification in order to achieve better purification. The semipreparative HPLC chromatographs are shown in Figure 2c. The minor radioactive peak at 14 min is most likely the product from the reduction of [18F]FDHT by residual NaBH4. The reductions by LiAlH4 at –78°C and by NaBH4 at room temperature were compared, and both reducing agents afforded identical HPLC chromatographs. Under this condition, [18F]FDHT was obtained in up to 75% overall yield (isolated, decay corrected) at the end of synthesis with high radiochemical purity. No UV peaks corresponding to FDHT were observed at 215 nm with very concentrated dose (6 mCi/mL), indicating high SA of [18F]FDHT (Figure 3c).
Conclusions
The radiosyntheses of steroid receptor ligands, [18F]FES, [18F] FFNP, and [18F]FDHT, have been optimized giving high or improved yields, optimized SA and purities, and easy processing to obtain material for PET imaging experiments and clinical use. The information provided here will aid in the development of the automated production of these steroid receptor ligands as radiopharmaceuticals for research and clinical studies.
Acknowledgement
We are grateful for the support of this research by research and training grants from the Department of Energy (DE-SC0005434, DE-SC0002032, and DEFG02-84ER-60218), the National Institutes of Health (CA25836), and cyclotron facility in Washington University School of Medicine. We thank Robert Dennett and Brian Wingbermuehle for the production of [18F]fluoride and Christopher Bognar for discussion about automated productions.
Footnotes
Additional supporting information may be found in the online version of this article at the publisher's web site.
Conflict of interest
The authors did not report any conflict of interest.
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher's web site.
References
- 1.Jadvar H. AJR Am. J. Roentgenol. 2012;199:278. doi: 10.2214/AJR.12.8816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Den Bossche B, Van de Wiele C. J. Clin. Oncol. 2004;22:3593. doi: 10.1200/JCO.2004.10.216. [DOI] [PubMed] [Google Scholar]
- 3.Currin E, Linden HM, Mankoff DA. Curr. Breast Cancer Rep. 2011;3:205. doi: 10.1007/s12609-011-0053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jonson SD, Welch MJ, Nucl QJ. Med. 1998;42:8. [PubMed] [Google Scholar]
- 5.Oliveira MC, Neto C, Ribeiro Morais G, Thiemann T. Curr. Med. Chem. 2013;20:222. doi: 10.2174/092986713804806658. [DOI] [PubMed] [Google Scholar]
- 6.Linden HM, Kurland BF, Peterson LM, Schubert EK, Gralow JR, Specht JM, Ellis GK, Lawton TJ, Livingston RB, Petra PH, Link JM, Krohn KA, Mankoff DA. Clin. Cancer Res. 2011;17:4799. doi: 10.1158/1078-0432.CCR-10-3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jonson SD, Bonasera TA, Dehdashti F, Cristel ME, Katzenellenbogen JA, Welch MJ. Nucl. Med. Biol. 1999;26:123. doi: 10.1016/s0969-8051(98)00079-1. [DOI] [PubMed] [Google Scholar]
- 8.Mortimer JE, Dehdashti F, Siegel BA, Katzenellenbogen JA, Fracasso P, Welch MJ. Clin. Cancer Res. 1996;2:933. [PubMed] [Google Scholar]
- 9.Zanzonico PB, Finn R, Pentlow KS, Erdi Y, Beattie B, Akhurst T, Squire O, Morris M, Scher H, McCarthy T, Welch M, Larson SM, Humm JL. J. Nucl. Med. 2004;45:1966. [PubMed] [Google Scholar]
- 10.Liu A, Dence CS, Welch MJ, Katzenellenbogen JA. J. Nucl. Med. 1992;33:724. [PubMed] [Google Scholar]
- 11.Larson SM, Morris M, Gunther I, Beattie B, Humm JL, Akhurst TA, Finn RD, Erdi Y, Pentlow K, Dyke J, Squire O, Bornmann W, McCarthy T, Welch M, Scher H. J. Nucl. Med. 2004;45:366. [PubMed] [Google Scholar]
- 12.Dehdashti F, Picus J, Michalski JM, Dence CS, Siegel BA, Katzenellenbogen JA, Welch MJ. Eur. J. Nucl. Med. Mol. Imaging. 2005;32:344. doi: 10.1007/s00259-005-1764-5. [DOI] [PubMed] [Google Scholar]
- 13.Beattie BJ, Smith-Jones PM, Jhanwar YS, Schoder H, Schmidtlein CR, Morris MJ, Zanzonico P, Squire O, Meirelles GS, Finn R, Namavari M, Cai S, Scher HI, Larson SM, Humm JL. J. Nucl. Med. 2010;51:183. doi: 10.2967/jnumed.109.066159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dehdashti F, Laforest R, Gao F, Aft RL, Dence CS, Zhou D, Shoghi KI, Siegel BA, Katzenellenbogen JA, Welch MJ. J. Nucl. Med. 2012;53:363. doi: 10.2967/jnumed.111.098319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fowler AM, Chan SR, Sharp TL, Fettig NM, Zhou D, Dence CS, Carlson KE, Jeyakumar M, Katzenellenbogen JA, Schreiber RD, Welch MJ. J. Nucl. Med. 2012;53:1119. doi: 10.2967/jnumed.112.103465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berridge MS, Franceschini MP, Rosenfeld E, Tewson TJ. J. Org. Chem. 1990;55:1211. [Google Scholar]
- 17.Kil HS, Cho HY, Lee SJ, Oh SJ, Chi DY. J. Label. Compd. Radiopharm. 2013;56:619. doi: 10.1002/jlcr.3076. [DOI] [PubMed] [Google Scholar]
- 18.Buckman BO, Bonasera TA, Kirschbaum KS, Welch MJ, Katzenellenbogen JA. J. Med. Chem. 1995;38:328. doi: 10.1021/jm00002a014. [DOI] [PubMed] [Google Scholar]
- 19.Senderoff SG, McElvany KD, Carlson KE, Heiman DF, Katzenellenbogen JA, Welch MJ. Int. J. Appl. Radiat. Isot. 1982;33:545. doi: 10.1016/0020-708x(82)90010-2. [DOI] [PubMed] [Google Scholar]
- 20.Zhou D, Carlson KE, Katzenellenbogen JA, Welch MJ. J. Med. Chem. 2006;49:4737. doi: 10.1021/jm060348q. [DOI] [PubMed] [Google Scholar]
- 21.Vijaykumar D, Mao W, Kirschbaum KS, Katzenellenbogen JA. J. Org. Chem. 2002;67:4904. doi: 10.1021/jo020190r. [DOI] [PubMed] [Google Scholar]
- 22.Liu A, Carlson KE, Katzenellenbogen JA. J. Med. Chem. 1992;35:2113. doi: 10.1021/jm00089a024. [DOI] [PubMed] [Google Scholar]
- 23.Lapi SE, Welch MJ. Nucl. Med. Biol. 2013;40:314. doi: 10.1016/j.nucmedbio.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Dixit M, Shi J, Wei L, Afari G, Bhattacharyya S. Int. J. Mol. Imaging. 2013;2013:10. doi: 10.1155/2013/278607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beauregard J-M, Croteau E, Ahmed N, Ouellette R, van Lier JE, Bénard F. Nucl. Med. Biol. 2007;34:325. doi: 10.1016/j.nucmedbio.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Kiesewetter DO, Kilbourn MR, Landvatter SW, Heiman DF, Katzenellenbogen JA, Welch MJ. J. Nucl. Med. 1984;25:1212. [PubMed] [Google Scholar]
- 27.Gao M, Wang M, Mock BH, Glick-Wilson BE, Yoder KK, Hutchins GD, Zheng Q-H. Appl. Radiat. Isot. 2010;68:1079. doi: 10.1016/j.apradiso.2009.09.071. [DOI] [PubMed] [Google Scholar]
- 28.Lu S, Giamis AM, Pike VW. Curr. Radiopharm. 2009;2:49. doi: 10.2174/1874471010902010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moon BS, Park JH, Lee HJ, Kim JS, Kil HS, Lee BS, Chi DY, Lee BC, Kim YK, Kim SE. Appl. Radiat. Isot. 2010;68:2279. doi: 10.1016/j.apradiso.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 30.Kumar P, Mercer J, Doerkson C, Tonkin K, McEwan AJ. J. Pharm. Pharm. Sci. 2007;10:256 s. [PubMed] [Google Scholar]
- 31.Mori T, Kasamatsu S, Mosdzianowski C, Welch MJ, Yonekura Y, Fujibayashi Y. Nucl. Med. Biol. 2006;33:281. doi: 10.1016/j.nucmedbio.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Oh SJ, Chi DY, Mosdzianowski C, Kil HS, Ryu JS, Moon DH. Appl. Radiat. Isot. 2007;65:676. doi: 10.1016/j.apradiso.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 33.Romer J, Fuchtner F, Steinbach J, Johannsen B. Nucl. Med. Biol. 1999;26:473. doi: 10.1016/s0969-8051(98)00098-5. [DOI] [PubMed] [Google Scholar]
- 34.Mori T, Kiyono Y, Asai T, Yoshii Y, Okazawa H, Dence C, Welch M, Fujibayashi Y. J Nucl. Med. Meeting. Abstr. 2010;51:1525. [Google Scholar]