Abstract
BACKGROUND
Obesity and obstructive sleep apnea tend to coexist and are associated with inflammation, insulin resistance, dyslipidemia, and high blood pressure, but their causal relation to these abnormalities is unclear.
METHODS
We randomly assigned 181 patients with obesity, moderate-to-severe obstructive sleep apnea, and serum levels of C-reactive protein (CRP) greater than 1.0 mg per liter to receive treatment with continuous positive airway pressure (CPAP), a weight-loss intervention, or CPAP plus a weight-loss intervention for 24 weeks. We assessed the incremental effect of the combined interventions over each one alone on the CRP level (the primary end point), insulin sensitivity, lipid levels, and blood pressure.
RESULTS
Among the 146 participants for whom there were follow-up data, those assigned to weight loss only and those assigned to the combined interventions had reductions in CRP levels, insulin resistance, and serum triglyceride levels. None of these changes were observed in the group receiving CPAP alone. Blood pressure was reduced in all three groups. No significant incremental effect on CRP levels was found for the combined interventions as compared with either weight loss or CPAP alone. Reductions in insulin resistance and serum triglyceride levels were greater in the combined-intervention group than in the group receiving CPAP only, but there were no significant differences in these values between the combined-intervention group and the weight-loss group. In per-protocol analyses, which included 90 participants who met prespecified criteria for adherence, the combined interventions resulted in a larger reduction in systolic blood pressure and mean arterial pressure than did either CPAP or weight loss alone.
CONCLUSIONS
In adults with obesity and obstructive sleep apnea, CPAP combined with a weight-loss intervention did not reduce CRP levels more than either intervention alone. In secondary analyses, weight loss provided an incremental reduction in insulin resistance and serum triglyceride levels when combined with CPAP. In addition, adherence to a regimen of weight loss and CPAP may result in incremental reductions in blood pressure as compared with either intervention alone.
AVAILABLE CLINICAL DATA DERIVED largely from observational studies link obstructive sleep apnea1 to proatherosclerotic risk factors, including insulin resistance,2 dyslipidemia, hypertension,3 and inflammation.4 Obesity and obstructive sleep apnea are strongly associated.5-8 Like obstructive sleep apnea, obesity is linked to insulin resistance,6 dyslipidemia,9 hypertension,9,10 and inflammation.10 However, the relative causal roles that obstructive sleep apnea and obesity play in these abnormalities is unclear.6,11,12 The interrelationships between obesity and obstructive sleep apnea are complex and bi-directional, and they cannot be confidently discerned in observational studies. Randomized trials have shown the beneficial effects of weight loss on cardiovascular risk factors. However, even modest reductions in body weight are associated with changes in obstructive sleep apnea, with a 10% reduction in body weight predicting an approximate change of 26 to 32% in the apnea–hypopnea index (AHI).13 Previous trials assessing the effects of weight loss on cardiovascular risk factors have neither assessed the effect of sleep-disordered breathing nor included a controlled intervention for obstructive sleep apnea. Conversely, trials of continuous positive airway pressure (CPAP) therapy have not included a control intervention for obesity. Furthermore, the incremental benefit of a weight-loss intervention plus CPAP as compared with each intervention alone in reducing cardiovascular risk factors is unknown. We evaluated the incremental effect of CPAP combined with a weight-loss intervention over the effect of each intervention alone on subclinical inflammation, insulin resistance, dyslipidemia, and blood pressure in patients with obesity and obstructive sleep apnea.
METHODS
STUDY DESIGN
In this randomized, parallel-group, 24-week trial, we compared the effects of CPAP, weight loss, or both CPAP and weight loss in adults with obesity (body-mass index [the weight in kilograms divided by the square of the height in meters], ≥30), moderate-to-severe obstructive sleep apnea (AHI, ≥15 apnea or hypopnea events per hour), and a serum level of C-reactive protein (CRP) greater than 1.0 mg per liter. Detailed criteria for inclusion and exclusion are provided in Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org.
Potential participants were screened with the use of a home-based sleep monitor (ApneaLink, ResMed) for 1 or 2 nights. If this test yielded an AHI score of 10 or more events per hour, we performed 12-channel diagnostic polysomnography in the sleep laboratory for a full night. Patients with a polysomnogram that showed an AHI of 15 or more events per hour were randomly assigned to a study group. Randomization was conducted with a permuted-block design, with stratification according to sex, status with respect to statin use, and enrollment site (the Hospital of the University of Pennsylvania or the Philadelphia Veterans Affairs Medical Center).
The study was approved by the institutional review boards of the University of Pennsylvania and the Philadelphia Veterans Affairs Medical Center. Participants provided written informed consent. ApneaLink devices and CPAP machines were provided at no cost by ResMed, which had no role in study design, data accrual or analysis, or manuscript preparation. The first and last authors vouch for the accuracy and completeness of the data and for the fidelity of the study to the protocol.
INTERVENTIONS
In the CPAP and combined-intervention groups, participants underwent an overnight in-laboratory sleep study to allow for individual calibration of the CPAP therapy each participant would receive. Nightly CPAP therapy was provided thereafter through a fixed-pressure or autoadjusting CPAP device (ResMed). Adherence to CPAP therapy was monitored weekly by means of a wireless router attached to the CPAP device (ResTraxx, ResMed).
Participants in the weight-loss group and those in the combined-intervention group had individual weekly counseling sessions. The goals for caloric intake were set at 1200 to 1500 kcal per day for participants weighing less than 114 kg and at 1500 to 1800 kcal per day for those weighing 114 kg or more. Dietary composition was aligned with recommendations from the National Cholesterol Education Program (NCEP). Self-selected foods within the framework of the NCEP diet were prescribed for the first 2 weeks. For weeks 3 to 19, a more structured diet was prescribed, including two to three liquid-meal replacements per day.14 Unsupervised exercise was initiated at week 4, starting with four 15-minute weekly sessions that increased progressively to four 50-minute weekly sessions by week 15. This target for level of activity was chosen because of its association with long-term maintenance of weight loss. Cognitive-behavioral strategies, including self-monitoring, goal setting, stimulus control, problem solving (to address problems with adherence to recommendations on diet and exercise), and relapse prevention, were used to facilitate and maintain weight loss.15
STUDY ASSESSMENTS AND END POINTS
Assessments were performed at baseline and at 8 and 24 weeks after the initiation of therapy. Serum levels of CRP and lipoproteins were measured after an overnight fast. Insulin sensitivity was assessed with the use of the frequently sampled intravenous glucose-tolerance test, which allowed for calculation of the insulin sensitivity index with Bergman’s minimal model.16 (Analytic methods are described in detail in the Supplementary Appendix.)
The primary end point was the serum CRP level. Secondary end points included insulin sensitivity and atherogenic dyslipidemia (determined by measuring serum levels of triglycerides, high-density lipoprotein [HDL] cholesterol, low-density lipoprotein [LDL] cholesterol, and LDL-particle concentration). Exploratory end points included systolic blood pressure, mean arterial pressure, pulse pressure, and HDL-particle concentration.
STATISTICAL ANALYSIS
Our primary analysis was based on a modified intention-to-treat population, defined for the purposes of the study as all participants who were randomly assigned to a study group and for whom there was at least one observation after randomization. Additional per-protocol analyses were performed to investigate the causal relation between obstructive sleep apnea or obesity and underlying metabolic abnormalities. These analyses were based on the principle that any incremental benefit of weight loss combined with effective CPAP treatment, as compared with the benefit of effective CPAP treatment alone, can be attributed to effects of obesity that are independent of the effects of obstructive sleep apnea. Conversely, any incremental benefit of effective CPAP therapy combined with weight loss, as compared with weight loss alone, can be attributed to effects of obstructive sleep apnea that are independent of the effects of obesity. For these assumptions to be valid, the estimation of between-group differences requires actual reductions in body weight and obstructive sleep apnea. Accordingly, our per-protocol analyses were restricted to participants who met minimum requirements for weight loss (at least 5% of baseline weight) and adherence to CPAP therapy (use for an average of at least 4 hours per night on at least 70% of the total number of nights). On the basis of previous studies evaluating the effects of CPAP therapy alone17 and weight loss alone18 on CRP levels, we powered the trial to detect standardized between-group differences in the change from the baseline CRP level of at least 0.53 mg per liter in the modified intention-to-treat population and at least 0.79 mg per liter in the per-protocol population, with 90% power, allowing for a type I error rate of 0.05.
The effects of the interventions on end points were analyzed with the use of general linear mixed models, with all measurements available at 24 weeks used to estimate intervention effects.19 Restricted maximum-likelihood estimation was used, and an unstructured covariance matrix was specified to adjust for within-participant clustering resulting from the repeated-measures design. Individual measures of growth were modeled as a function of randomly assigned group, time, and the interaction between group and time.
The primary comparisons of interest were the 24-week change in the CRP level among participants in the modified intention-to-treat population who were assigned to the combined intervention as compared with the changes among those assigned to CPAP alone and those assigned to weight loss alone. To account for two primary comparisons, a Bonferroni-adjusted significance level was set at 0.025 for each comparison. All other analyses were considered secondary, with a nominal significance level of 0.05. Accordingly, any nominally significant P values in secondary analyses should be interpreted conservatively, given an increased type I error rate introduced by multiple comparisons. Considering the number of comparisons made for a total of 10 end points, up to two significant tests of incremental benefit (P<0.05) would be expected on the basis of chance alone in each of the study populations analyzed (the modified intention-to-treat and per-protocol samples). Although this study was not designed to compare the effects of CPAP alone and weight loss alone, we also provide the results of exploratory analyses for these pairwise comparisons. Data on the CRP level were log-transformed because of their skewed distribution. Analyses were performed with SAS software, version 9.2 (SAS Institute).
RESULTS
STUDY PARTICIPANTS
A total of 544 persons were screened and 181 underwent randomization (Fig. 1). Baseline characteristics were similar among the three groups (Table 1). Of the 181 participants, 35 dropped out of the trial before the follow-up visit at 8 weeks, and an additional 10 participants dropped out after that visit; a total of 136 participants completed the study. The modified intention-to-treat analyses included the 146 participants who underwent at least one assessment for the study end points after the initiation of therapy.
Figure 1. Numbers of Patients Who Were Screened, Randomly Assigned to a Study Group, and Included in Analyses.
AHI denotes apnea–hypopnea index, BDI Beck Depression Inventory (in which scores range from 0 to 63, with higher scores indicating more severe depression), BMI body-mass index (the weight in kilograms divided by the square of the height in meters), CPAP continuous positive airway pressure, and CRP C-reactive protein. To convert the values for glucose to millimoles per liter, multiply by 0.05551.
Table 1.
Baseline Characteristics of the Study Participants.*
| Characteristic | Weight Loss (N = 61) |
CPAP (N = 58) |
CPAP and Weight Loss (N = 62) |
P Value |
|---|---|---|---|---|
| Age — yr | 48.3 | 49.8 | 49.0 | 0.75 |
| Male sex — no. (%) | 36 (59) | 35 (60.3) | 33 (53.2) | 0.70 |
| Race — no. (%)† | 0.95 | |||
| White | 33 (54) | 34 (59.6) | 36 (59.0) | |
| Black | 25 (41) | 23 (40.4) | 25 (41.0) | |
| Mixed or other | 3 (5) | 1 (1.72) | 1 (1.61) | |
| Height — cm | 172.5±10 | 170.2±14.2 | 165.9±23 | 0.09 |
| Weight — kg | 114.5±21.9 | 115.1±21.7 | 111.5±28.1 | 0.68 |
| Body-mass index‡ | 38.1±5.8 | 39.8±7.1 | 38.4±6.4 | 0.29 |
| Cholesterol — mg/dl | ||||
| Total | 188.1±46.1 | 196.4±46 | 177.1±44.4 | 0.07 |
| HDL | 43.1±11.7 | 43.1±10.6 | 40±12.6 | 0.23 |
| LDL | 116.7±35 | 123.8±31.1 | 107±32 | 0.02 |
| Cholesterol particle size — nm | ||||
| LDL | 20.5 (0.73) | 20.5 (0.71) | 20.5 (0.65) | 0.96 |
| HDL | 8.7 (0.32) | 8.8 (0.41) | 8.7 (0.31) | 0.62 |
| Triglycerides — mg/dl | 130±72.3 | 133.1±63.4 | 145.3±98.5 | 0.53 |
| Hypertension — no. (%) | 25 (41) | 26 (45) | 24 (39) | 0.79 |
| Insulin sensitivity index§ | 1.4±0.82 | 1.2±1.03 | 1.4±1.14 | 0.63 |
| Apoprotein — mg/dl | ||||
| A-I | 120.6 (31.2) | 125.7 (25.86) | 118.7 (32.69) | 0.45 |
| B | 87.3 (29.98) | 92.5 (26.23) | 85.1 (24.06) | 0.33 |
| High-sensitivity C-reactive protein — mg/liter | 0.74 | |||
| Median | 4.4 | 4.7 | 4.3 | |
| Interquartile range | 1.9–8.5 | 2.5–8.2 | 2.1–9.3 | |
| Blood pressure — mm Hg | ||||
| Systolic | 126.5±10.02 | 129.9±14.87 | 123±19.91 | 0.053 |
| Diastolic | 78.9±7.5 | 80.1±8.98 | 76.8±13.71 | 0.22 |
| AHI — events/hr¶ | 39.7±20.3 | 41.2±20.96 | 47.1±26.86 | 0.17 |
| Oxygen desaturation index — no. of events/hr | ||||
| >3% drop from baseline | 22.6±18.8 | 25.5±22.1 | 27.6±25.7 | 0.47 |
| >4% drop from baseline | 18.3±17.4 | 20.7±20.4 | 23.3±24.7 | 0.44 |
| Sleep time with Spo2 <90% — % | 5.0±8.4 | 6.9±13.8 | 8.7±15.6 | 0.29 |
| Mean Spo2 during sleep — % | ||||
| Nadir | 78±14.8 | 76.7±11.1 | 73.7±20.7 | 0.34 |
| Mean | 94.8±1.8 | 94.7±2.3 | 94.1±2.4 | 0.17 |
| Arousal index — no. of arousals/hr of sleep | 31.7±15.8 | 37±20.5 | 39.6±20.1 | 0.07 |
| Score on Epworth Sleepiness Scale∥ | 9.3±4.3 | 9.8±4.6 | 8.9±4.8 | 0.55 |
| Current or former smoker — no. (%) | 21 (34) | 20 (35) | 12 (19) | 0.11 |
| Medication use — no. (%) | ||||
| Statin | 12 (20) | 12 (21) | 13 (21) | 0.99 |
| Antihypertensive medication | 25 (41) | 24 (41) | 21 (34) | 0.63 |
Plus–minus values are means ±SD. Spo2 denotes oxygen saturation level as measured by pulse oximetry. To convert values for low-density lipoprotein (LDL), high-density lipoprotein (HDL), and total cholesterol to millimoles per liter, multiply by 0.02586. To convert values for triglycerides to millimoles per liter, multiply by 0.01129.
Race was self-reported.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
The insulin sensitivity index was calculated with the use of values from frequently sampled glucose tolerance tests. Higher levels indicate greater insulin sensitivity.
The apnea–hypopnea index (AHI) is the number of apnea and hypopnea episodes per hours of sleep time.
Scores on the Epworth Sleepiness Scale range from 0.0 to 24.0, with higher scores indicating more daytime sleepiness.
The decline in body weight was similar in the weight-loss and combined-intervention groups (6.8 kg and 7.0 kg, respectively); there was no discernible decline in body weight in the CPAP group (Fig. S1A in the Supplementary Appendix). The average duration of CPAP use was 4.0 hours per night, with no significant differences between the CPAP and combined-intervention groups. Among all three groups, the adherence criteria for inclusion in the per-protocol analyses were met by 90 participants: 39 in the CPAP group, 27 in the weight-loss group, and 24 in the combined-intervention group. (Table S2 in the Supplementary Appendix shows the baseline characteristics of participants who met the prespecified adherence criteria.)
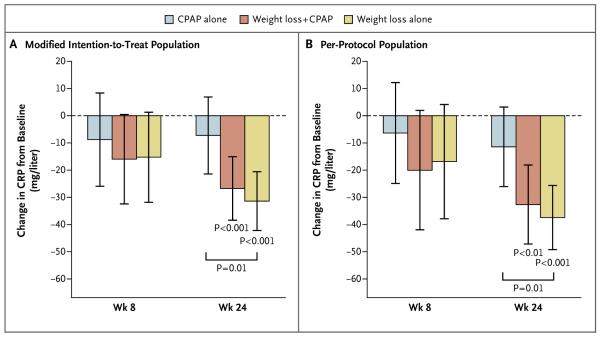
CRP LEVELS
The combined interventions did not have a significant incremental effect on CRP levels, as compared with either weight loss alone or CPAP alone, in either the modified intention-to-treat population (Fig. 2A) or the per-protocol population (Fig. 2B). In both the modified intention-to-treat population and the per-protocol population, the latter comprising participants who met prespecified adherence criteria, the CRP level was significantly reduced at 24 weeks in the weight-loss and combined-intervention groups but not in the CPAP group, and the reduction in the CRP level was greater in the weight-loss group than in the CPAP group.
Figure 2. Changes from Baseline in CRP in the Modified Intention-to-Treat and Per-Protocol Populations.
Panel A shows changes in CRP levels in the modified intention-to-treat population, and Panel B shows changes in CRP levels in the per-protocol population. The per-protocol population consisted of participants who met prespecified adherence criteria. I bars represent 95% confidence intervals. P values without brackets are for the change from baseline in each group. P values with brackets are for between-group differences at week 24.
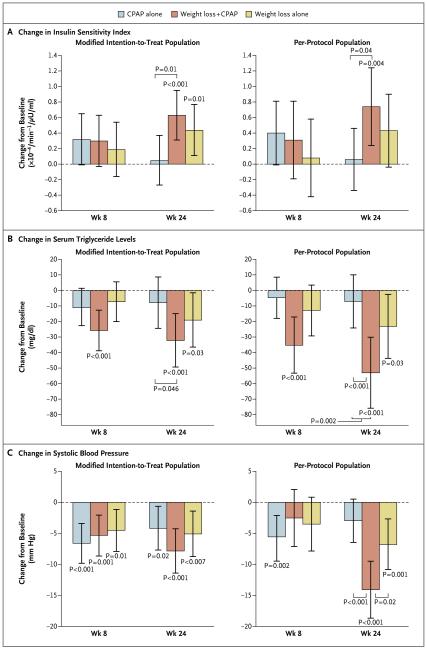
INSULIN SENSITIVITY
In the modified intention-to-treat analyses, insulin sensitivity increased in the weight-loss and combined-intervention groups at 24 weeks but not in the CPAP group. The increase in insulin sensitivity was significantly greater in the combined-intervention group than in the CPAP group (Fig. 3A), but the difference between the combined-intervention group and the weight-loss group was not significant. The results of the per-protocol analyses were similar (Fig. 3A).
Figure 3 (facing page). Changes from Baseline in the Insulin Sensitivity Index, Serum Triglyceride Levels, and Systolic Blood Pressure in the Modified-Intention-to-Treat and Per-Protocol Populations.
Panel A shows changes in the insulin sensitivity index, Panel B changes in serum triglyceride levels, and Panel C changes in systolic blood pressure. I bars represent 95% confidence intervals. P values without brackets are for the change from baseline in each group. P values with brackets are for between-group differences at week 24. To convert the values for triglycerides to millimoles per liter, multiply by 0.01129.
DYSLIPIDEMIA
In the modified intention-to-treat population, the reduction in serum triglyceride levels at 24 weeks was greater in the combined-intervention group than in the CPAP group; there was no significant difference in the change in triglyceride levels between the combined-intervention group and the weight-loss group (Fig. 3B). The results were similar in the per-protocol population (Fig. 3B). Serum triglyceride levels were significantly reduced at 24 weeks in the weight-loss and combined-intervention groups but not in the CPAP group.
Changes in LDL cholesterol levels at 24 weeks did not differ significantly among the three study groups in either the modified intention-to-treat population or the per-protocol population (Fig. S1B in the Supplementary Appendix). In the modified intention-to-treat population, a reduction in LDL cholesterol levels was observed in the weight-loss group. In the per-protocol population, LDL cholesterol levels were reduced in the combined-intervention group and the weight-loss group but not in the CPAP group. Changes in HDL cholesterol levels from baseline to 24 weeks were similar among the three study groups (Fig. S1C in the Supplementary Appendix). There was no significant change in HDL cholesterol levels at 24 weeks in any of the study groups. The findings for LDL-particle and HDL-particle concentrations (Fig. S1D and S1E in the Supplementary Appendix) were similar to those for LDL and HDL cholesterol levels, respectively.
BLOOD PRESSURE
In the modified intention-to-treat population, systolic blood pressure was reduced at 24 weeks in all three study groups (Fig. 3C), with no significant between-group differences. In the per-protocol population, the reduction in systolic blood pressure at 24 weeks was greater in the combined-intervention group (14.1 mm Hg) than in the weight-loss group (6.8 mm Hg) and the CPAP group (3.0 mm Hg) (Fig. 3C). Mean arterial pressure also decreased in all three study groups in the modified intention-to-treat population, with no significant between-group differences (Fig. S2A in the Supplementary Appendix). In the per-protocol population, the reduction in mean arterial pressure was significantly greater in the combined-intervention group than in either the weight-loss group or the CPAP group. In the modified intention-to-treat population, pulse pressure was significantly reduced at 24 weeks only in the combined-intervention group, and there were no significant between-group differences (Fig. S2B in the Supplementary Appendix). In the per-protocol population, the reduction in pulse pressure was greater in the combined-intervention group and weight-loss groups than in the CPAP group.
SENSITIVITY ANALYSES
We performed sensitivity analyses in which the baseline value was carried forward for participants in the modified intention-to-treat population who had missing follow-up data because of early withdrawal from the study (Table S4 in the Supplementary Appendix). These analyses showed within-group changes that were less pronounced than those observed in the primary analysis, but the trends were similar.
ADVERSE EVENTS
Nasal or sinus congestion, nostril irritation, or other upper respiratory symptoms were reported in 10 participants in the CPAP group, 9 participants in the weight-loss group, and 10 participants in the combined-intervention group. Other adverse events were much less common (Table S5 in the Supplementary Appendix). No serious adverse events occurred that were related to the study interventions or to study participation.
DISCUSSION
In this randomized trial, CPAP combined with weight loss did not have a significant incremental effect on CRP levels, as compared with either CPAP alone or a weight-loss intervention alone. Secondary analyses, which should be interpreted conservatively, showed that weight loss had an incremental effect on insulin resistance and serum triglyceride levels, as compared with CPAP, but no significant incremental effects on these end points were observed with combination therapy as compared with the weight-loss intervention alone. In exploratory analyses, the combination of CPAP and the weight-loss intervention was associated with a larger reduction in blood pressure than was either intervention alone among participants who adhered to the therapeutic regimen.
As reported in previous studies, weight loss significantly reduced CRP levels, insulin resistance, dyslipidemia, and blood pressure.20-22 In contrast, CPAP therapy did not have a significant effect on CRP level, insulin sensitivity, or dyslipidemia, even among participants who adhered to the therapy. Cross-sectional observational studies4,23 and prospective observational studies24-27 have yielded conflicting results regarding the role of obstructive sleep apnea in inflammation, but the two largest observational studies did not show a reduction in CRP levels after 9 to 12 months of CPAP therapy in patients with obstructive sleep apnea.26,27 Similarly, short-term observational studies28,29 have suggested an improvement in insulin resistance with CPAP therapy30; however, a crossover trial,31 in which insulin resistance was measured with the use of homeostatic model assessment, an indirect index of hepatic insulin sensitivity, did not show changes in insulin resistance after 6 to 12 weeks of CPAP therapy, even among participants who adhered to the treatment regimen. Using the frequently sampled intravenous glucose-tolerance test, which measures whole-body insulin sensitivity,32 we found that CPAP monotherapy did not improve insulin sensitivity or enhance the improvement in insulin sensitivity associated with weight loss. In contrast, weight loss, with or without CPAP, increased insulin sensitivity. CPAP monotherapy for 24 weeks also did not improve dyslipidemia in our trial, a finding that is consistent with the results of a recent randomized trial, which showed no significant changes in total cholesterol levels after 24 weeks of CPAP therapy.33
Unlike the other cardiovascular risk factors we assessed, blood pressure decreased in the CPAP, weight-loss, and combined-intervention groups. In analyses including only participants who met the prespecified adherence criteria, a larger reduction was seen in the combined-intervention group than in either the weight-loss group or the CPAP group. Despite the causal relationship between obstructive sleep apnea and hypertension that has been reported in animal models and the epidemiologic association34 between obstructive sleep apnea and hypertension in humans, improvements in blood pressure with CPAP therapy in clinical studies have been absent or remarkably small,35-37 estimated at approximately 1.3 to 3.0 mm Hg in systolic or diastolic blood pressure. However, to the degree that obstructive sleep apnea and CPAP activate similar or overlapping pathophysiological pathways leading to hypertension, it was not possible to clearly separate their direct effects without the inclusion of randomly assigned CPAP and weight-loss interventions, alone and in combination. Our findings suggest that both obstructive sleep apnea and obesity have an independent causal relation to hypertension.
Our study has limitations. We did not include a sham CPAP intervention. However, both sham CPAP and the absence of treatment for obstructive sleep apnea are considered to be adequate controls for an active CPAP intervention.38 In addition, sham CPAP is not a perfect placebo, since it may result in significant reductions in the number of apnea events, increases in the number of hypopnea events, and a small impairment in sleep quality.39 We did not include any group in which no therapy was implemented because of ethical considerations and because its inclusion was not needed to test our hypotheses. Our 24-week attrition rate was high (25%). We did not assess ambulatory blood pressure. Finally, our findings cannot be extended to populations with diabetes mellitus or mild obstructive sleep apnea because of our exclusion criteria.
In conclusion, we found that CPAP therapy combined with a weight-loss intervention did not have a significant incremental effect on CRP levels, as compared with either intervention alone. The weight-loss intervention combined with CPAP therapy had an incremental effect on insulin resistance and serum triglyceride levels, as compared with CPAP alone, but no significant incremental effects were detected for combination therapy as compared with the weight-loss intervention alone, even among participants who adhered to the therapeutic regimen. In an analysis that included only participants who adhered to the regimen, the combined interventions resulted in a larger reduction in blood pressure than either CPAP or weight loss alone. Our study shows that a weight-loss intervention is effective as a central component of the strategies used to improve the cardiovascular risk-factor profile in patients with obesity and obstructive sleep apnea.
Supplementary Material
Acknowledgments
Funded by the National Heart, Lung, and Blood Institute; ClinicalTrials.gov number, NCT0371293.
Supported by grants from the National Heart, Lung, and Blood Institute (HL-R01080076, to Dr. Chirinos; and P01 HL094307, to Dr. Pack).
We thank Dr. Frederick F. Samaha, who led the design of this trial and its initial implementation but who died unexpectedly, and the members of the data safety and monitoring board (Arshed Quyyumi, M.D., chair; and Robert Basner, M.D., Atul Malhotra, M.D., and Diane Catellier, Ph.D.).
Footnotes
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Dr. Wadden reports receiving fees for serving on advisory boards for Novo Nordisk, Nutrisystem, and Orexigen, consulting fees from Boehringer Ingelheim, and grant support from Weight Watchers, Novo Nordisk, and Nutrisystem. Dr. Foster reports receiving fees for serving on advisory boards for ConAgra Foods, Tate and Lyle, and UnitedHealth Group and reports being an employee of Weight Watchers. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670–6. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 3.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study: Sleep Heart Health Study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. Erratum, JAMA 2002;288:1985. [DOI] [PubMed] [Google Scholar]
- 4.Shamsuzzaman AS, Winnicki M, Lanfranchi P, et al. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105:2462–4. doi: 10.1161/01.cir.0000018948.95175.03. [DOI] [PubMed] [Google Scholar]
- 5.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360:237–45. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 6.Pack AI, Gislason T. Obstructive sleep apnea and cardiovascular disease: a perspective and future directions. Prog Cardiovasc Dis. 2009;51:434–51. doi: 10.1016/j.pcad.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Shinohara E, Kihara S, Yamashita S, et al. Visceral fat accumulation as an important risk factor for obstructive sleep apnoea syndrome in obese subjects. J Intern Med. 1997;241:11–8. doi: 10.1046/j.1365-2796.1997.63889000.x. [DOI] [PubMed] [Google Scholar]
- 8.Grunstein R, Wilcox I, Yang TS, Gould Y, Hedner J. Snoring and sleep apnoea in men: association with central obesity and hypertension. Int J Obes Relat Metab Disord. 1993;17:533–40. [PubMed] [Google Scholar]
- 9.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 10.Shamsuzzaman ASM, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290:1906–14. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- 11.Tasali E, Ip MS. Obstructive sleep apnea and metabolic syndrome: alterations in glucose metabolism and inf lammation. Proc Am Thorac Soc. 2008;5:207–17. doi: 10.1513/pats.200708-139MG. [DOI] [PubMed] [Google Scholar]
- 12.McNicholas WT, Bonsigore MR. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J. 2007;29:156–78. doi: 10.1183/09031936.00027406. Erratum, Eur Respir J 2007;29:614. [DOI] [PubMed] [Google Scholar]
- 13.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–21. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 14.Wadden TA, Volger S, Sarwer DB, et al. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med. 2011;365:1969–79. doi: 10.1056/NEJMoa1109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wadden TA, Webb VL, Moran CH, Bailer BA. Lifestyle modification for obesity: new developments in diet, physical activity, and behavior therapy. Circulation. 2012;125:1157–70. doi: 10.1161/CIRCULATIONAHA.111.039453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergman RN, Prager R, Volund A, Olefsky JM. Equivalence of the insulin sensitivity index in man derived by the minimal model method and the euglycemic glucose clamp. J Clin Invest. 1987;79:790–800. doi: 10.1172/JCI112886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yokoe T, Minoguchi K, Matsuo H, et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107:1129–34. doi: 10.1161/01.cir.0000052627.99976.18. [DOI] [PubMed] [Google Scholar]
- 18.Seshadri P, Iqbal N, Stern L, et al. A randomized study comparing the effects of a low-carbohydrate diet and a conventional diet on lipoprotein subfractions and C-reactive protein levels in patients with severe obesity. Am J Med. 2004;117:398–405. doi: 10.1016/j.amjmed.2004.04.009. Erratum, Am J Med 2006;119:191. [DOI] [PubMed] [Google Scholar]
- 19.Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. J Educ Behav Stat. 1998;23:323–55. [Google Scholar]
- 20.Horvath K, Jeitler K, Siering U, et al. Long-term effects of weight-reducing interventions in hypertensive patients: systematic review and meta-analysis. Arch Intern Med. 2008;168:571–80. doi: 10.1001/archinte.168.6.571. [DOI] [PubMed] [Google Scholar]
- 21.Samaha FF, Iqbal N, Seshadri P, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003;348:2074–81. doi: 10.1056/NEJMoa022637. [DOI] [PubMed] [Google Scholar]
- 22.Nicklas BJ, Ambrosius W, Messier SP, et al. Diet-induced weight loss, exercise, and chronic inf lammation in older, obese adults: a randomized controlled clinical trial. Am J Clin Nutr. 2004;79:544–51. doi: 10.1093/ajcn/79.4.544. [DOI] [PubMed] [Google Scholar]
- 23.Sharma SK, Mishra HK, Sharma H, et al. Obesity, and not obstructive sleep apnea, is responsible for increased serum hs-CRP levels in patients with sleep-disordered breathing in Delhi. Sleep Med. 2008;9:149–56. doi: 10.1016/j.sleep.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Ishida K, Kato M, Kato Y, et al. Appropriate use of nasal continuous positive airway pressure decreases elevated C-reactive protein in patients with obstructive sleep apnea. Chest. 2009;136:125–9. doi: 10.1378/chest.08-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patruno V, Aiolfi S, Costantino G, et al. Fixed and autoadjusting continuous positive airway pressure treatments are not similar in reducing cardiovascular risk factors in patients with obstructive sleep apnea. Chest. 2007;131:1393–9. doi: 10.1378/chest.06-2192. [DOI] [PubMed] [Google Scholar]
- 26.Colish J, Walker JR, Elmayergi N, et al. Obstructive sleep apnea: effects of continuous positive airway pressure on cardiac remodeling as assessed by cardiac biomarkers, echocardiography, and cardiac MRI. Chest. 2012;141:674–81. doi: 10.1378/chest.11-0615. [DOI] [PubMed] [Google Scholar]
- 27.Akashiba T, Akahoshi T, Kawahara S, Majima T, Horie T. Effects of long-term nasal continuous positive airway pressure on C-reactive protein in patients with obstructive sleep apnea syndrome. Intern Med. 2005;44:899–900. doi: 10.2169/internalmedicine.44.899. [DOI] [PubMed] [Google Scholar]
- 28.Dorkova Z, Petrasova D, Molcanyiova A, Popovnakova M, Tkacova R. Effects of continuous positive airway pressure on cardiovascular risk profile in patients with severe obstructive sleep apnea and metabolic syndrome. Chest. 2008;134:686–92. doi: 10.1378/chest.08-0556. [DOI] [PubMed] [Google Scholar]
- 29.Harsch IA, Schahin SP, Radespiel-Tröger M, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2004;169:156–62. doi: 10.1164/rccm.200302-206OC. [DOI] [PubMed] [Google Scholar]
- 30.Yang D, Liu Z, Yang H, Luo Q. Effects of continuous positive airway pressure on glycemic control and insulin resistance in patients with obstructive sleep apnea: a meta-analysis. Sleep Breath. 2013;17:33–8. doi: 10.1007/s11325-012-0680-8. [DOI] [PubMed] [Google Scholar]
- 31.Coughlin SR, Mawdsley L, Mugarza JA, Wilding JP, Calverley PM. Cardiovascular and metabolic effects of CPAP in obese males with OSA. Eur Respir J. 2007;29:720–7. doi: 10.1183/09031936.00043306. [DOI] [PubMed] [Google Scholar]
- 32.Bergman RN. Orchestration of glucose homeostasis: from a small acorn to the California oak. Diabetes. 2007;56:1489–501. doi: 10.2337/db07-9903. [DOI] [PubMed] [Google Scholar]
- 33.Craig SE, Kohler M, Nicoll D, et al. Continuous positive airway pressure improves sleepiness but not calculated vascular risk in patients with minimally symptomatic obstructive sleep apnoea: the MOSAIC randomised controlled trial. Thorax. 2012;67:1090–6. doi: 10.1136/thoraxjnl-2012-202178. [DOI] [PubMed] [Google Scholar]
- 34.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 35.Haentjens P, Van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167:757–64. doi: 10.1001/archinte.167.8.757. [DOI] [PubMed] [Google Scholar]
- 36.Bazzano LA, Khan Z, Reynolds K, He J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension. 2007;50:417–23. doi: 10.1161/HYPERTENSIONAHA.106.085175. [DOI] [PubMed] [Google Scholar]
- 37.Barbé F, Durán-Cantolla J, Capote F, et al. Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;181:718–26. doi: 10.1164/rccm.200901-0050OC. [DOI] [PubMed] [Google Scholar]
- 38.Brown DL, Anderson CS, Chervin RD, et al. Ethical issues in the conduct of clinical trials in obstructive sleep apnea. J Clin Sleep Med. 2011;7:103–8. [PMC free article] [PubMed] [Google Scholar]
- 39.Rodway GW, Weaver TE, Mancini C, et al. Evaluation of sham-CPAP as a placebo in CPAP intervention studies. Sleep. 2010;33:260–6. doi: 10.1093/sleep/33.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.