Abstract
Oxidative stress contributes to the development of pulmonary hypertension in experimental models, but this association in humans is unknown. We investigated the relationship between pulmonary artery systolic pressure measured by echocardiography and plasma aminothiol oxidative stress markers, with the hypothesis that oxidative stress will be higher in those with pulmonary hypertension. A group of 347 patients aged 65±12 years from the Emory Cardiovascular Biobank underwent echocardiographic assessment of left ventricular ejection fraction and pulmonary artery systolic pressure. Plasma aminothiols, cysteine, its oxidized form, cystine; glutathione, and its oxidized disulphide (GSSG) were measured and the redox potentials (Eh) of cysteine/cystine and glutathione/GSSG couples were calculated. Non-normally distributed variables were log transformed (Ln). Univariate predictors of pulmonary artery systolic pressure included age (p<0.001), gender (p=0.002), mitral regurgitation (p<0.001), left ventricular ejection fraction (p<0.001), left atrial size (p< 0.001), diabetes (p=0.03), Plasma Ln cystine (β=9.53, p<0.001), Ln glutathione (β =-5.4, p=0.002), and Eh glutathione (β =0.21, p=0.001). A multivariate linear regression model adjusting for all confounding variables demonstrated that Ln cystine (β=6.56, p=0.007), mitral regurgitation (β= 4.52, P<0.001), statin use (β =-3.39, p=0.03), left ventricular ejection fraction (β=-0.26, p=0.003), and age (β=0.17, p=0.003) were independent predictors of pulmonary artery systolic pressure. For each 1% increase in plasma cystine, pulmonary artery systolic pressure increased by 16%. This association persisted in the subgroup with preserved left ventricular ejection fraction (≥50%) and no significant mitral regurgitation.
Whether treatment of oxidative stress will improve pulmonary hypertension requires further study.
Keywords: Pulmonary hypertension, pulmonary artery systolic pressure, cystine, oxidative stress, plasma aminothiols
Introduction
Pulmonary hypertension (PH) is defined as persistent elevation of mean pulmonary artery pressure above 25 mmHg.1 Although Group 1 PH is a rare and progressive life-threatening disease, mild to moderate PH secondary to a variety of cardiac and pulmonary disorders is far more common.2, 3 Underlying pathophysiologic changes include pulmonary arterial smooth muscle proliferation, endothelial dysfunction, oxidative stress (OS), and inflammation.4 OS is also associated with aging and several chronic ailments including cardiovascular disease (CVD),5 diabetes,6 and chronic pulmonary disease.7 In experimental models, OS characterized by increased levels of reactive oxygen species, contributes to the development of PH and subsequent right ventricular remodeling.8-11 Similarly, increased intracellular calcium flux, mediated by higher OS, leads to smooth muscle contraction and higher pulmonary artery systolic pressure (PASP),12 and improvement of OS decreases PASP and improves right ventricular dysfunction.4, 13-15 However, few human studies have explored the link between OS and PH.16, 17
Aminothiol compounds play a crucial role in redox signaling and can be quantified in plasma to assess OS burden in vivo.18 Of these, cysteine constitutes the major extracellular thiol pool which reacts readily with oxidants to form its disulphide cystine which is an abundant and sensitive indicator of systemic oxidant burden.19 Intra-cellularly, glutathione is a major antioxidant that helps eliminate peroxides and maintains redox state.20 The Nernst equation may be used to calculate the redox potentials of both the glutathione and cysteine pools. Increased OS, measured as lower levels of glutathione and/or higher levels of cystine, or a more oxidized redox potential, is associated with CVD risk factors, CVD, subclinical vascular disease, and importantly with adverse outcomes.21-28
To investigate the association of plasma aminothiols with PASP, we measured plasma levels of aminothiols and estimated PASP using surface echocardiography, with the hypothesis that increased OS, characterized as a higher cystine level will be associated with increased PASP in humans.
Methods
Study participants were recruited as part of the Emory Cardiovascular Biobank, consisting of 347 patients aged between 53 and 77 years enrolled prior to undergoing cardiac catheterization for confirmed or suspected CAD across three Emory Healthcare sites between 2004 and 2008. Subjects with connective tissue disease, HIV infection, liver disease, congenital heart disease, sickle cell disease, or other systemic inflammatory conditions were excluded. Patients' demographic characteristics, medical history, and behavioral (lifestyle) habits were collected by interview. Risk factor prevalence was determined by physician diagnosis and/or treatment for hypertension, hyperlipidemia, and diabetes. Smoking was classified as non-smoker or “ever smoked” if there was a lifetime history of smoking at least 100 cigarettes. Medical records were reviewed to confirm self-reported history of cardiovascular or pulmonary diseases. The study was approved by the Emory University Institutional Review Committee and all subjects provided informed consent.
Each subject underwent transthoracic echocardiographic assessment of left ventricular ejection fraction (LVEF), mitral regurgitation (MR), left atrial (LA) size, and estimation of PASP. Echocardiographic measurements were all performed according to the American Society of Echocardiography recommendations.29 PASP was estimated using the velocity of the tricuspid regurgitant jet based on the simplified Bernouli equation (PASP=4VTR2 + right atrial pressure) where VTR is the peak velocity of the tricuspid regurgitant jet measured by continuous-wave Doppler.30 Estimation of the severity of MR was performed by assessment of vena contracta using color Doppler echocardiography with values <0.3 mm corresponding to mild MR; 0.3 to 0.69 mm as moderate MR, and >0.7 mm as severe MR by personnel who were blinded to the OS data. Severity of coronary artery disease was assessed by the Gensini score with two operators who independently evaluated all coronary angiograms. This score uses a point system for the degree of luminal narrowing along with a multiplier for specific coronary tree locations, thereby weighting each lesion score for prognostic significance. The total of the lesion scores is summed to give a final Gensini score.31
Measurement of plasma aminothiol levels
Plasma levels of cysteine, its oxidized form, cystine, glutathione, and its oxidized disulphide (GSSG) were measured and the ratios of cysteine/cystine and glutathione/GSSG were calculated and expressed as redox potentials, Eh cystine and Eh glutathione, respectively, where a more oxidized value has a more positive numeric value. Detailed methods for the measurements of aminothiols in plasma have been described previously. 24, 26, 32, 33 Briefly, samples were collected directly into specially prepared tubes containing a preservative to retard auto-oxidation, centrifuged, and the supernatant frozen at -80°C, which shows no significant loss for 1 year. Analyses by high performance liquid chromatography were performed after dansyl derivatization on a 3-aminopropyl column with fluorescence detection. Metabolites were identified by co-elution with standards and quantified by integration relative to the internal standards, with validation relative to external standards. Issues of sample collection, stability, analysis, and standardization have been extensively studied, and the method has been used in several clinical studies.34
Statistical Analysis
Mean±standard deviation (SD) and proportions are used to describe continuous and dichotomous variables, respectively. Spearman correlation test was used to determine bivariate correlations between aminothiols and PASP. Kolmogorov-Smirinov test was used to determine normality. Where data was not normally distributed, natural logarithm (Log) transformation was performed. Multivariate linear regression including all variables with P≤0.20 in univariate analyses were used to determine independent predictors of PASP. Regression coefficients are presented as point estimates with 95% confidence intervals. Binary logistic regression was performed to investigate independent predictors of pulmonary hypertension as defined by PASP≥50 mmHg. Only p values ≤ 0.05 were considered significant. Analyses were performed using the SPSS 20.0 software (SPSS Inc., USA).
Results
A total of 347 subjects undergoing coronary angiography, 61% male, mean age 65±12 years, with and without coronary artery disease were enrolled (Table 1). Subjects had multiple risk factors, 15% presented with acute myocardial infarction, and 5% had a history of chronic lung disease.
Table 1. Baseline Characteristics.
| Predictors | All (N=347) |
|---|---|
| Age (years) | 65±12 |
| Male Gender (%) | 61 |
| African American (%) | 16 |
| BMI (kg/m2) | 26±11 |
| Hypertension (%) | 71 |
| Diabetes (%) | 34 |
| Acute Myocardial Infarction | 15 |
| Smoking (%) | 12 |
| Pulmonary Disease (%) | 5 |
| Statin Use (%) | 70 |
| Beta-Blocker Use (%) | 71 |
| Mitral Regurgitation (%) | 84 |
| -Mild | 66 |
| -Moderate | 23 |
| -Severe | 11 |
| LVEF (%) | 50±15 |
| LA Size (cm) | 4.2±0.8 |
| LV hypertrophy (%) | 15 |
| Gensini score | 52±70 |
| Cystine (μM) | 107.2±33.7 |
| Cysteine (μM) | 13.4±5.8 |
| Glutathione (μM) | 1.27±0.62 |
| GSSG (μM) | 0.03±0.03 |
| Eh Glutathione (mV) | -136.1±11.7 |
| Eh Cysteine (mV) | -75.3±9.1 |
| CRP (mg/L) | 9±17.5 |
Data presented as Mean±SD. BMI: Body Mass Index, CRP: C - reactive protein, LVEF: Left Ventricular Ejection Fraction, MI: Myocardial Infarction, LA: Left atrium.
Relationship of plasma amoinothiols with cardiovascular risk factors
Plasma cystine, glutathione, and Eh glutathione significantly correlated with age (r=0.32, p<0.0001; r=-0.12, p=0.02; and r=0.13, p=0.01, respectively). Diabetics had significantly higher levels of cystine (117±40 μM vs. 102±29 μM, p=0.00008) and lower glutathione (1.18±0.6 μM vs. 1.3±0.6 μM, p=0.04) compared to non-diabetics. History of hypertension was associated with higher cystine (112±36 μM vs. 94±22 μM, p=0.00001). We observed no difference in plasma aminothiol levels between males and females.
Plasma cystine level correlated with glutathione (r=-0.13, p=0.01), cysteine (r=0.39, p<0.001), and Eh glutathione (r=0.17, p=0.001) levels. Plasma aminothiols did not correlate with serum C-reactive protein (CRP) levels (r=-0.01, p=0.77 for cystine).
Relationship between aminothiol markers of OS and PASP
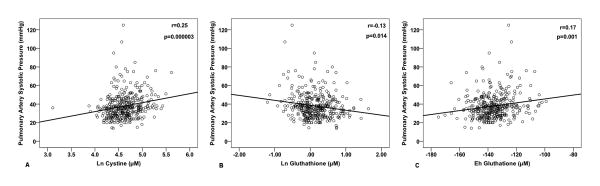
Using linear regression, univariate predictors of PASP included Ln cystine (p<0.001), Ln glutathione (p=0.002), and Eh glutathione (p=0.001) with Ln cysteine (p=0.06) trending towards significance, Table 2, Figure 1. Additionally, PASP was higher in females (p=0.005), in those with MR (P<0.001) with a trend towards higher PASP in diabetics (p=0.06). Other univariate correlates of PASP were age (p<0.001), LA size (p<0.001) and LVEF (p<0.001), Table 2.
Table 2. Univariate and Multivariate Correlates of PASP.
| Characteristics | Univariate Linear Regression | Multivariate Linear Regression | ||
|---|---|---|---|---|
|
|
|
|||
| Beta | P value | Beta | P value | |
| Age | 0.27 | <0.001 | 0.19 | 0.003 |
| Male Gender | -4.38 | 0.005 | -1.94 | 0.16 |
| African American | 1.22 | 0.55 | -- | -- |
| Hypertension | 0.40 | 0.81 | -- | -- |
| Diabetes | 2.94 | 0.06 | 1.46 | 0.32 |
| BMI (kg/m2) | -0.007 | 0.92 | -- | -- |
| Acute MI | -0.68 | 0.74 | -- | -- |
| Smoking | -2.26 | 0.33 | -- | -- |
| Pulmonary Disease | 0.33 | 0.92 | -- | -- |
| Statin Use | -1.92 | 0.20 | -3.12 | 0.03 |
| Beta-Blocker Use | 0.47 | 0.76 | -- | -- |
| LA Size | 4.71 | <0.001 | 1.41 | NS |
| Mitral Regurgitation | 5.02 | <0.001 | 4.52 | <0.001 |
| LVEF | -0.26 | <0.001 | -0.12 | 0.003 |
| Gensini score | 0.015 | 0.18 | 0.004 | NS |
| CRP (mg/L) | 0.06 | 0.18 | 0.01 | NS |
| Ln Cystine (μM) | 9.53 | <0.001 | 6.56 | 0.007 |
| Ln Cysteine (μM) | 3.76 | 0.06 | -- | -- |
| Ln Glutathione (μM) | -5.43 | 0.002 | -0.30 | NS |
| Ln GSSG (μM) | 0.175 | 0.87 | -- | -- |
| Eh Cysteine (mV) | -0.03 | 0.664 | -- | -- |
| Eh Glutathione (mV) | 0.219 | 0.001 | 0.06 | NS |
BMI: Body Mass Index, CRP: C - reactive protein, LA: Left atrium LVEF: Left Ventricular Ejection Fraction, MI: Myocardial Infarction
Figure 1.
Relationship between aminothiol levels and PASP. Panels A, B, and C demonstrate bivariate correlations of PASP with Ln cysteine, Ln glutathione, and Eh glutathione respectively.
A multivariate linear regression model that included age, gender, diabetes, statin use, presence of MR, LVEF, LA size, Gensini score, CRP as well as Ln cystine, Ln glutathione, and Eh glutathione, revealed Ln cystine (p=0.007), MR (p<0.001), statin use (p=0.03), LVEF (p=0.003), and age (p=0.003) as independent predictors of PASP, Table 2. Ln cysteine was not included in the multivariate model given its high collinearity with Ln cystine.
Relationship between PH and markers of OS
When divided into groups with normal PASP ≤25 mmHg, those with PASP ≥50 mmHg, and the intermediate group of PASP 26-49 mmHg, there was a graded increase in PASP with greater OS, Figure 2. In a multivariate analysis of correlates of PASP ≥ 50 mmHg that included age, gender, diabetes, statin use, MR, LVEF, LA size, Gensini score, Ln glutathione, Eh gluthatione, and CRP, Ln cystine was again a significant predictor (β=1.86, p=0.028).
Figure 2.
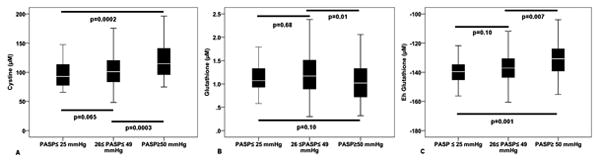
Differences in aminothiol levels in subjects with normal PASP (≤25 mmHg), and mild or moderate PH.
Subgroup Analysis
Association of OS markers with PASP in patients with normal LVEF and insignificant MR
In order to investigate the effects of OS on PASP without the confounding effects of low LVEF and MR that may also be associated with OS, we analyzed patients with normal LVEF (≥50%) and those free of moderate or severe MR. In this subset of 217 subjects, a multivariate linear regression model controlling for age, gender, diabetes, statin use, LA size, Gensini, CRP as well as Ln cystine, Ln glutathione, and Eh glutathione, revealed only Ln cystine as an independent OS marker predicting PASP (β=6.82, p=0.009). Moreover, Ln cystine was significantly higher in patients with PASP ≥ 50 mmHg compared to those with PASP between 26-49 mmHg and those with PASP ≤ 25 mmHg (p=0.003).
Association of OS markers with PASP in patients without chronic lung disease
In order to investigate whether the observed association of OS markers exists even in those without a diagnosis of chronic lung disease, we excluded these subjects (N=19, 5%). Linear regression model adjusting for the aforementioned variables revealed an independent association between Ln cystine and PASP (β=5.6, p=0.03) in the remaining subjects.
Discussion
Herein, we report that a higher level of plasma aminothiols, representing non-free radical OS burden, is associated with higher PASP assessed by echocardiography, independent of other clinical and echocardiographic risk factors including MR and left ventricular dysfunction. Every 1% increase in plasma cystine level was associated with a 16% increase in PASP. To our knowledge this is the first study in humans to explore the relationship between readily measurable plasma markers of OS and PASP.
Inflammatory and oxidative changes are present in the lung vasculature in PH and patients with systemic inflammatory conditions such as lupus or scleroderma are at risk for PH.35 Patients with established PH have up-regulation of inflammatory mediators such as intracellular adhesion molecule-1 (ICAM-1) and endothelial leukocyte adhesion molecule-1 (ELAM-1),36 and anti-inflammatory therapy has been invariably associated with decreases in PASP in experimental models.37
The putative role of OS in the pathophysiology of PH is supported by experimental data. Renin over-expressing rats have higher intrapulmonary NADPH oxidase activity and higher right ventricular systolic pressure.11 Conversely, reduction in NADPH oxidase activity in the gp91phox knockout mice decreases hypoxia-induced generation of reactive oxygen species and abolishes pathophysiological changes in the pulmonary artery.38 Furthermore, xanthine oxidase-mediated increase in reactive oxygen species expression in neonatal rats exposed to chronic hypoxia has been shown to result in PH.39 In lung tissues from patients with pulmonary arterial hypertension, increased nitrotyrosine and 8-hydroxyguanosine activities have been noted.16 Finally, in a murine model of PH, lower superoxide dismutase (SOD2) activity was reported,40 findings similar to those observed in lung tissue harvested from patients with PH at autopsy. Increased proliferation of pulmonary artery smooth muscle cells was ameliorated by SOD2 augmentation.40 These data along with our findings of an independent association of OS markers with PASP, even in early stages of PH, suggests a pathophysiologic role for OS in the development of human PH.
Plasma aminothiols are reliable measures of systemic oxidative burden, with glutathione representing intracellular and the cysteine/cystine pools reflecting extracellular oxidative burden.18 While we did not observe any significant independent association with glutathione or its redox potential Eh glutathione, the oxidized disulfide cystine, was independently associated with PASP.
We have previously shown that aminothiol markers of OS are associated with cardiovascular risk factors including hypertension, endothelial dysfunction, increased systemic arterial stiffness, increased carotid wall thickness, even in relatively healthy subjects without risk factors, and with worse long term outcomes.23-26, 35-42 This study extends our findings to hypertension in the pulmonary circulation. To ensure that risk factors for atherosclerosis were not responsible for our observed association, we controlled for all known and measured risk factors including medications, and found that the association of PASP with OS persisted. Similarly, we adjusted for presence of significant MR, left ventricular dysfunction, and significant coronary artery disease, all known to be associated with PH. Thus, even in the subset with absence of significant MR and left ventricular dysfunction, plasma cystine was a predictor of PASP independent of other risk factors including systemic inflammation, measured as plasma CRP level. A PASP of 38 mmHg is considered the best discriminatory cut-off value corresponding to mean pulmonary artery pressure of 25 mmHg for diagnosis of PH.43 We chose a higher cutoff of 50 mmHg in this study to further reduce the potential of false positive high PASP readings with echocardiography. We found that plasma cystine is significantly higher in those with PASP ≥ 50 mmHg than those with PASP ≤ 25 and PASP between 26-49 mmHg.
Despite a large body of evidence on the association of OS with PH especially in animal models, it remains uncertain whether antioxidant therapy would be a useful therapeutic modality in patients with PH.44 This may have been partly secondary to the lack of well-designed randomized controlled clinical trials with therapies targeted to OS pathways as almost all studies to date have investigated the effect of non-targeted antioxidant compounds in experimental models of PH with overall mixed results.45, 46
Future studies need to validate our results and investigate whether plasma cystine can predict PH as well as be used to monitor the severity of the disease.
Strengths and limitations
This is the first study to demonstrate an independent association between readily available plasma markers of systemic OS with PASP. A limitation of our study is the potential inaccuracy in measuring PASP with echocardiography. However, it remains the most widely used method for non-invasive screening of PH that is highly correlated with direct pressure measurements during right heart catheterization with a pooled sensitivity and specificity of 83% and 72%, respectively.43, 47, 48 We may have underestimated the prevalence of chronic lung disease as this diagnosis was obtained from the history and chart review. Noneheless, the independent association between PASP and cystine persisted even after excluding subjects with chronic lung disease. We acknowledge that findings from this study might not be generalizable to all subcategories of PH.49
Conclusion
Increased plasma OS burden, estimated as increased circulating levels of cystine is associated with increased PASP.
Perspectives
In this study for the first time we have shown that novel plasma aminothiol markers of OS are associated with PASP independent of traditional cardiovascular risk factors as well as presence of chronic lung disease, significant MR, and depressed left ventricular systolic function. Whether treatment of PH can be monitored using aminothiol markers and whether targeted treatment of OS that normalizes aminothiol levels will improve PH requires further investigation. Future studies are also needed to investigate the association of these aminothiol markers with PASP in different subcategories of PH.
Novelty and Significance.
What is New?
To our knowledge this is the first study in humans to investigate the association between readily measurable plasma markers of systemic OS and PH. Every 1% increase in plasma cystine level was associated with a 16% increase in PASP.
What is Relevant?
Whether these measurements can be used to titrate therapy and whether anti-oxidants that normalize aminothiol levels will be therapeutic for PH needs to be investigated.
Summary
In summary, plasma cystine is associated with PASP independent of other risk factors and even in those with normal left ventricular systolic function and no significant MR.
Acknowledgments
We would like to thank the members of the Emory Cardiovascular Biobank Team, Emory Clinical Cardiovascular Research Institute (ECCRI), Emory Clinical Biomarkers Laboratory, and Atlanta Clinical and Translational Science Institute for recruitment of participants, compilation of data, and preparation of samples.
Funding: Funding for collection and management of samples was received from the Robert W. Woodruff Health Sciences Center Fund (Atlanta, GA), Emory Heart and Vascular Center (Atlanta, GA), Katz Family Foundation, and in part from NIH grant UL1 RR02008. Sample measurements were performed at the Emory Clinical Biomarkers Laboratory.
Abbreviations and Acronyms
- PH
Pulmonary Hypertension
- PASP
Pulmonary Artery Systolic Pressure
- LVEF
Left Ventricular Ejection Fraction
- MR
Mitral Regurgitation
- LA
Left Atrium
- CRP
C-Reactive Protein
- GSSG
Oxidized Glutathione
- Eh Cystine
Redox potential for cystine
- Eh Glutathione
Redox potential for glutathione
Footnotes
Conflict of Interests/Disclosures: None
References
- 1.Rubin LJ. Primary pulmonary hypertension. New England Journal of Medicine. 1997;336:111–117. doi: 10.1056/NEJM199701093360207. [DOI] [PubMed] [Google Scholar]
- 2.Crosswhite P, Sun Z. Nitric oxide, oxidative stress and inflammation in pulmonary arterial hypertension. J Hypertens. 28:201–212. doi: 10.1097/HJH.0b013e328332bcdb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strange G, Playford D, Stewart S, Deague JA, Nelson H, Kent A, Gabbay E. Pulmonary hypertension: Prevalence and mortality in the armadale echocardiography cohort. Heart. 2012;98:1805–1811. doi: 10.1136/heartjnl-2012-301992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Csiszar A, Labinskyy N, Olson S, Pinto JT, Gupte S, Wu JM, Hu F, Ballabh P, Podlutsky A, Losonczy G, de Cabo R, Mathew R, Wolin MS, Ungvari Z. Resveratrol prevents monocrotaline-induced pulmonary hypertension in rats. Hypertension. 2009;54:668–675. doi: 10.1161/HYPERTENSIONAHA.109.133397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 6.Bonnefont-Rousselot D. The role of antioxidant micronutrients in the prevention of diabetic complications. Treat Endocrinol. 2004;3:41–52. doi: 10.2165/00024677-200403010-00005. [DOI] [PubMed] [Google Scholar]
- 7.Cantin AM. Potential for antioxidant therapy of cystic fibrosis. Curr Opin Pulm Med. 2004;10:531–536. doi: 10.1097/01.mcp.0000138997.29276.a1. [DOI] [PubMed] [Google Scholar]
- 8.Farahmand F, Hill MF, Singal PK. Antioxidant and oxidative stress changes in experimental cor pulmonale. Mol Cell Biochem. 2004;260:21–29. doi: 10.1023/b:mcbi.0000026047.48534.50. [DOI] [PubMed] [Google Scholar]
- 9.Redout EM, Wagner MJ, Zuidwijk MJ, Boer C, Musters RJ, van Hardeveld C, Paulus WJ, Simonides WS. Right-ventricular failure is associated with increased mitochondrial complex ii activity and production of reactive oxygen species. Cardiovasc Res. 2007;75:770–781. doi: 10.1016/j.cardiores.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Cai H, Griendling KK, Harrison DG. The vascular nad(p)h oxidases as therapeutic targets in cardiovascular diseases. Trends in Pharmacological Sciences. 2003;24:471–478. doi: 10.1016/S0165-6147(03)00233-5. [DOI] [PubMed] [Google Scholar]
- 11.DeMarco VG, Habibi J, Whaley-Connell AT, Schneider RI, Heller RL, Bosanquet JP, Hayden MR, Delcour K, Cooper SA, Andresen BT, Sowers JR, Dellsperger KC. Oxidative stress contributes to pulmonary hypertension in the transgenic (mren2)27 rat. American Journal of Physiology - Heart & Circulatory Physiology. 2008;294:H2659–2668. doi: 10.1152/ajpheart.00953.2007. [DOI] [PubMed] [Google Scholar]
- 12.Gupte SA, Wolin MS. Oxidant and redox signaling in vascular oxygen sensing: Implications for systemic and pulmonary hypertension. Antioxidants & Redox Signaling. 2008;10:1137–1152. doi: 10.1089/ars.2007.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeMarco VG, Habibi J, Whaley-Connell AT, Schneider RI, Sowers JR, Andresen BT, Gutweiler AA, Ma L, Johnson MS, Ferrario CM, Dellsperger KC. Rosuvastatin ameliorates the development of pulmonary arterial hypertension in the transgenic (mren2)27 rat. American Journal of Physiology - Heart & Circulatory Physiology. 2009;297:H1128–1139. doi: 10.1152/ajpheart.00048.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redout EM, van der Toorn A, Zuidwijk MJ, van de Kolk CW, van Echteld CJ, Musters RJ, van Hardeveld C, Paulus WJ, Simonides WS. Antioxidant treatment attenuates pulmonary arterial hypertension-induced heart failure. Am J Physiol Heart Circ Physiol. 2010;298:8. doi: 10.1152/ajpheart.00097.2009. [DOI] [PubMed] [Google Scholar]
- 15.Lakshminrusimha S, Russell JA, Wedgwood S, Gugino SF, Kazzaz JA, Davis JM, Steinhorn RH. Superoxide dismutase improves oxygenation and reduces oxidation in neonatal pulmonary hypertension. Am J Respir Crit Care Med. 2006;174:1370–1377. doi: 10.1164/rccm.200605-676OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowers R, Cool C, Murphy RC, Tuder RM, Hopken MW, Flores SC, Voelkel NF. Oxidative stress in severe pulmonary hypertension. Am J Respir Crit Care Med. 2004;169:764–769. doi: 10.1164/rccm.200301-147OC. [DOI] [PubMed] [Google Scholar]
- 17.Cracowski JL, Cracowski C, Bessard G, Pepin JL, Bessard J, Schwebel C, Stanke-Labesque F, Pison C. Increased lipid peroxidation in patients with pulmonary hypertension. Am J Respir Crit Care Med. 2001;164:1038–1042. doi: 10.1164/ajrccm.164.6.2104033. [DOI] [PubMed] [Google Scholar]
- 18.Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 19.Jones DP, Carlson JL, Mody VC, Cai J, Lynn MJ, Sternberg P. Redox state of glutathione in human plasma. Free Radic Biol Med. 2000;28:625–635. doi: 10.1016/s0891-5849(99)00275-0. [DOI] [PubMed] [Google Scholar]
- 20.Buettner GR. The pecking order of free radicals and antioxidants: Lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys. 1993;300:535–543. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- 21.Moriarty SE, Shah JH, Lynn M, Jiang S, Openo K, Jones DP, Sternberg P. Oxidation of glutathione and cysteine in human plasma associated with smoking. Free Radic Biol Med. 2003;35:1582–1588. doi: 10.1016/j.freeradbiomed.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Cardona F, Tunez I, Tasset I, Montilla P, Collantes E, Tinahones FJ. Fat overload aggravates oxidative stress in patients with the metabolic syndrome. European Journal of Clinical Investigation. 2008;38:510–515. doi: 10.1111/j.1365-2362.2008.01959.x. [DOI] [PubMed] [Google Scholar]
- 23.Ashfaq S, Abramson JL, Jones DP, Rhodes SD, Weintraub WS, Hooper WC, Vaccarino V, Harrison DG, Quyyumi AA. The relationship between plasma levels of oxidized and reduced thiols and early atherosclerosis in healthy adults. J Am Coll Cardiol. 2006;47:1005–1011. doi: 10.1016/j.jacc.2005.09.063. [DOI] [PubMed] [Google Scholar]
- 24.Ashfaq S, Abramson JL, Jones DP, Rhodes SD, Weintraub WS, Hooper WC, Vaccarino V, Alexander RW, Harrison DG, Quyyumi AA. Endothelial function and aminothiol biomarkers of oxidative stress in healthy adults. Hypertension. 2008;52:80–85. doi: 10.1161/HYPERTENSIONAHA.107.097386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris AA, Zhao L, Patel RS, Jones DP, Ahmed Y, Stoyanova N, Gibbons GH, Vaccarino V, Din-Dzietham R, Quyyumi AA. Differences in systemic oxidative stress based on race and the metabolic syndrome: The morehouse and emory team up to eliminate health disparities (meta-health) study. Metabolic syndrome and related disorders. 2012;10:252–259. doi: 10.1089/met.2011.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel RS, Al Mheid I, Morris AA, Ahmed Y, Kavtaradze N, Ali S, Dabhadkar K, Brigham K, Hooper WC, Alexander RW, Jones DP, Quyyumi AA. Oxidative stress is associated with impaired arterial elasticity. Atherosclerosis. 2011;218:90–95. doi: 10.1016/j.atherosclerosis.2011.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel RS, Veledar E, Patel RB, Sher S, Arshad S, Clements S, Douglas J, Morris D, Rab ST, Samady H, Alexander RW, Vaccarino V, Zafari AM, Jones DP, Quyyumi AA. Abstract 1138: The oxidized disulphide cystine predicts adverse long term cardiovascular outcomes. Circulation. 2009;120:S454. [Google Scholar]
- 28.Mills BJ, Weiss MM, Lang CA, Liu MC, Ziegler C. Blood glutathione and cysteine changes in cardiovascular disease. Journal of Laboratory and Clinical Medicine. 2000;135:396–401. doi: 10.1067/mlc.2000.105976. [DOI] [PubMed] [Google Scholar]
- 29.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: A report from the american society of echocardiography's guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the european association of echocardiography, a branch of the european society of cardiology. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984;70:657–662. doi: 10.1161/01.cir.70.4.657. [DOI] [PubMed] [Google Scholar]
- 31.Gensini G. Coronary arteriography. New York, NY: Futura Publishing Co; 1975. [Google Scholar]
- 32.Jones DP, Mody VC, Jr, Carlson JL, Lynn MJ, Sternberg P., Jr Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radical Biology & Medicine. 2002;33:1290–1300. doi: 10.1016/s0891-5849(02)01040-7. [DOI] [PubMed] [Google Scholar]
- 33.Jones DP, Liang Y. Measuring the poise of thiol/disulfide couples in vivo. Free Radic Biol Med. 2009;47:1329–1338. doi: 10.1016/j.freeradbiomed.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samiec PS, Drews-Botsch C, Flagg EW, Kurtz JC, Sternberg P, Jr, Reed RL, Jones DP. Glutathione in human plasma: Decline in association with aging, age-related macular degeneration, and diabetes. Free Radic Biol Med. 1998;24:699–704. doi: 10.1016/s0891-5849(97)00286-4. [DOI] [PubMed] [Google Scholar]
- 35.Dorfmuller P, Perros F, Balabanian K, Humbert M. Inflammation in pulmonary arterial hypertension. Eur Respir J. 2003;22:358–363. doi: 10.1183/09031936.03.00038903. [DOI] [PubMed] [Google Scholar]
- 36.Okawa-Takatsuji M, Aotsuka S, Fujinami M, Uwatoko S, Kinoshita M, Sumiya M. Up-regulation of intercellular adhesion molecule-1 (icam-1), endothelial leucocyte adhesion molecule-1 (elam-1) and class ii mhc molecules on pulmonary artery endothelial cells by antibodies against u1-ribonucleoprotein. Clin Exp Immunol. 1999;116:174–180. doi: 10.1046/j.1365-2249.1999.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voelkel NF, Tuder RM, Bridges J, Arend WP. Interleukin-1 receptor antagonist treatment reduces pulmonary hypertension generated in rats by monocrotaline. Am J Respir Cell Mol Biol. 1994;11:664–675. doi: 10.1165/ajrcmb.11.6.7946395. [DOI] [PubMed] [Google Scholar]
- 38.Liu JQ, Zelko IN, Erbynn EM, Sham JS, Folz RJ. Hypoxic pulmonary hypertension: Role of superoxide and nadph oxidase (gp91phox) Am J Physiol Lung Cell Mol Physiol. 2006;290:5. doi: 10.1152/ajplung.00135.2005. [DOI] [PubMed] [Google Scholar]
- 39.Jankov RP, Kantores C, Pan J, Belik J. Contribution of xanthine oxidase-derived superoxide to chronic hypoxic pulmonary hypertension in neonatal rats. Am J Physiol Lung Cell Mol Physiol. 2008;294:14. doi: 10.1152/ajplung.00166.2007. [DOI] [PubMed] [Google Scholar]
- 40.Archer SL, Marsboom G, Kim GH, Zhang HJ, Toth PT, Svensson EC, Dyck JR, Gomberg-Maitland M, Thebaud B, Husain AN, Cipriani N, Rehman J. Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: A basis for excessive cell proliferation and a new therapeutic target. Circulation. 2010;121:2661–2671. doi: 10.1161/CIRCULATIONAHA.109.916098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Go YM, Jones DP. Intracellular proatherogenic events and cell adhesion modulated by extracellular thiol/disulfide redox state. Circulation. 2005;111:2973–2980. doi: 10.1161/CIRCULATIONAHA.104.515155. [DOI] [PubMed] [Google Scholar]
- 42.Azumi H, Inoue N, Ohashi Y, Terashima M, Mori T, Fujita H, Awano K, Kobayashi K, Maeda K, Hata K, Shinke T, Kobayashi S, Hirata K, Kawashima S, Itabe H, Hayashi Y, Imajoh-Ohmi S, Itoh H, Yokoyama M. Superoxide generation in directional coronary atherectomy specimens of patients with angina pectoris: Important role of nad(p)h oxidase. Arteriosclerosis, thrombosis, and vascular biology. 2002;22:1838–1844. doi: 10.1161/01.atv.0000037101.40667.62. [DOI] [PubMed] [Google Scholar]
- 43.Lafitte S, Pillois X, Reant P, Picard F, Arsac F, Dijos M, Coste P, Dos Santos P, Roudaut R. Estimation of pulmonary pressures and diagnosis of pulmonary hypertension by doppler echocardiography: A retrospective comparison of routine echocardiography and invasive hemodynamics. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2013;26:457–463. doi: 10.1016/j.echo.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki YJ, Steinhorn RH, Gladwin MT. Antioxidant therapy for the treatment of pulmonary hypertension. Antioxid Redox Signal. 2013;18:1723–1726. doi: 10.1089/ars.2013.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lorenzoni AG, Ruiz-Feria CA. Effects of vitamin e and l-arginine on cardiopulmonary function and ascites parameters in broiler chickens reared under subnormal temperatures. Poultry science. 2006;85:2241–2250. doi: 10.1093/ps/85.12.2241. [DOI] [PubMed] [Google Scholar]
- 46.Wong CM, Bansal G, Pavlickova L, Marcocci L, Suzuki YJ. Reactive oxygen species and antioxidants in pulmonary hypertension. Antioxid Redox Signal. 2013;18:1789–1796. doi: 10.1089/ars.2012.4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lindqvist P, Soderberg S, Gonzalez MC, Tossavainen E, Henein MY. Echocardiography based estimation of pulmonary vascular resistance in patients with pulmonary hypertension: A simultaneous doppler echocardiography and cardiac catheterization study. European journal of echocardiography : the journal of the Working Group on Echocardiography of the European Society of Cardiology. 2011;12:961–966. doi: 10.1093/ejechocard/jer222. [DOI] [PubMed] [Google Scholar]
- 48.Janda S, Shahidi N, Gin K, Swiston J. Diagnostic accuracy of echocardiography for pulmonary hypertension: A systematic review and meta-analysis. Heart. 2011;97:612–622. doi: 10.1136/hrt.2010.212084. [DOI] [PubMed] [Google Scholar]
- 49.Simonneau G, Galie N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, Gibbs S, Lebrec D, Speich R, Beghetti M, Rich S, Fishman A. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43:5S–12S. doi: 10.1016/j.jacc.2004.02.037. [DOI] [PubMed] [Google Scholar]