Abstract
Objectives
Abnormalities in nitric oxide (NO) bioavailability have been reported in African Americans. Whether there are differences in endothelium-derived hyperpolarizing factor (EDHF) in addition to NO between African Americans and whites, and how these affect physiologic vasodilation remains unknown. We hypothesized that the bioavailability of vascular NO and EDHF, at rest and with pharmacologic and physiologic vasodilation, varies between white and African Americans.
Approach and Results
In 74 white and 86 African American subjects without known cardiovascular disease risk factors, forearm blood flow (FBF) was measured using plethysmography at rest and during inhibition of NO with NG-monomethyl-L-arginine (L-NMMA) and/or of K+Ca channels (EDHF) with tetraethylammonium (TEA). The reduction in resting FBF was greater with L-NMMA (p=0.019) and similar with TEA in whites compared to African Americans. Vasodilation with bradykinin, acetylcholine, and sodium nitroprusside was lower in African Americans compared to whites (all p<0.0001). Inhibition with L-NMMA was greater in whites compared to African Americans with bradykinin, acetylcholine, and exercise. Inhibition with TEA was lower in African Americans with bradykinin, but greater during exercise and with acetylcholine.
Conclusions
The contribution to both resting and stimulus-mediated vasodilator tone of NO is greater in whites compared to African Americans. EDHF partly compensates for the reduced NO release in exercise and acetylcholine-mediated vasodilation in African Americans. Preserved EDHF but reduced NO bioavailability and sensitivity characterizes the vasculature in healthy African Americans.
Keywords: vasodilation, exercise, African American, endothelial function, endothelium-derived hyperpolarizing factor, nitric oxide
Introduction
Endothelial dysfunction predisposes to the development of hypertension and atherosclerosis that together inflict significant morbidity and mortality. African Americans (AA) suffer from a higher incidence of premature stroke and myocardial infarction when compared to white counterparts.1 Although these disparities are often attributed to a higher prevalence of cardiovascular risk factors, and socioeconomic burden, underlying endothelial abnormalities may play a crucial underlying role.2-4 Abnormalities in nitric oxide (NO) bioavailability in the microcirculation and conductance vessels, reduced sensitivity of exogenous NO, increased endothelin bioavailability, and structural abnormalities in the vascular wall have all been observed in AAs, but the results have been controversial.5-16
The endothelium contributes to the maintenance of vasodilator tone by releasing NO, prostacyclin, and endothelium derived hyperpolarizing factors (EDHF).17-20 NO contributes to resting vasodilator tone,21 and to physiologic vasodilation during exercise,18 and its bioavailability is significantly impaired in individuals with cardiovascular risk factors.21-23 We have demonstrated an important contribution of EDHF via activation of K+Ca channels to (1) resting vascular tone, (2) vasodilation in response to the endothelium-dependent agonist, bradykinin, and (3) exercise-induced vasodilation in the healthy human forearm vasculature.24, 25 Whether racial differences in EDHF exist remains unknown. We hypothesized that there is a differential racial contribution of the two endogenous agonists, NO and EDHF to vasodilator tone at rest and to vasodilation during pharmacologic stimulation and with exercise.
Methods
Materials and Methods are available in the online-only Data Supplement
Results
Baseline Characteristics
AA subjects had a greater body mass index and whites had a higher fasting glucose level, but the remaining characteristics were similar (Table 1). There were no significant changes in the FBF and FVR in the non-infused arm and mean arterial pressure and heart rate remained unchanged in both groups throughout the study period.
Table 1. Characteristics of Study Subjects.
| African American (n=86) | Caucasians (n=74) | |
|---|---|---|
| Age, years | 39±11 | 40±13 |
| Male, n (%) | 46 (53.5) | 38 (51.4) |
| Systolic BP, mmHg | 118±13 | 117±11 |
| Diastolic BP, mmHg | 72±10 | 69±12 |
| Body Mass Index, kg/m2 | 28.8±6 | 26.8±5* |
| Creatinine, mg/dL | 0.9±0.2 | 0.9±0.2 |
| Glomerular Filtration Rate, ml/min | 100.6±17.7 | 101.1±19.0 |
| Glucose, mg/dL | 85±10 | 89±10* |
| Triglycerides, mg/dL | 102±49 | 127±109 |
| High Density Lipoprotein, mg/dL | 55±13 | 54±13 |
| Low Density Lipoprotein, mg/dL | 126±39 | 122±43 |
Glomerular Filtration Rate calculated using Cockcrauft-Gault equation. Data are mean ± SD.
Indicates a statistically significant (p < 0.05; t test) difference between groups.
Contribution of NO and K+Ca channel activation to resting vasodilator tone
Effect of L-NMMA on resting forearm vascular tone
Resting FBF and FVR were similar in AA and white subjects. NO blockade with L-NMMA reduced resting FBF and increased FVR in both groups, but the 21.3% reduction in FBF (p=0.019) and 35% increase in FVR (p=0.023) in AA were both lower than in whites (32.1% decrease in FBF and 54% increase in FVR), indicating reduced contribution of NO to resting vasodilator tone in AAs (Figure 2a).
Figure 2.
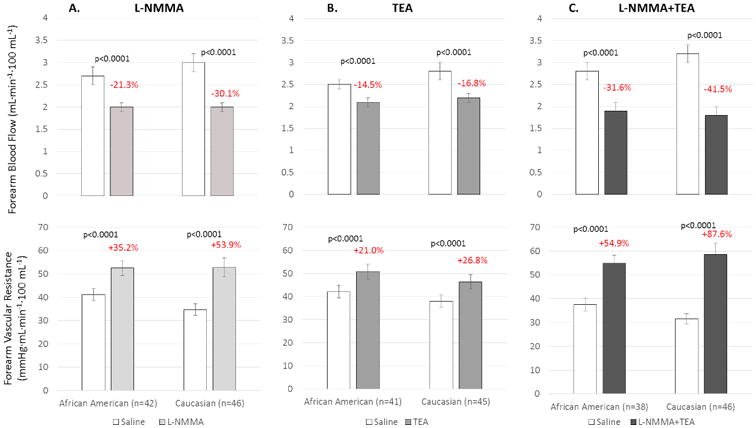
Change in resting FBF and FVR in response to (a) L-NMMA in 42 healthy AA and 46 white subjects, (b) in response to TEA in 41 healthy AA and 45 white subjects, and (c) to combined L-NMMA and TEA infusions in 38 healthy AA and 46 white subjects. Data is shown as mean ± SEM.
Effect of TEA on resting forearm vascular tone
Blockade of K+Ca channels with TEA decreased resting FBF and increased FVR in both subsets and the magnitude of change was similar in whites and AAs, indicating similar contribution of K+Ca channel activation to resting vasodilator tone in AAs and whites (Figure 2b).
Effect of L-NMMA combined with TEA on resting forearm vascular tone
Combined blockade of NO and K+Ca channels with L-NMMA and TEA had an additive effect in both subsets and resulted in significantly greater reduction in FBF and increase in FVR than with either antagonist alone (Figure 2c). The reduction in FBF (p=0.003) and increase in FVR (p=0.006) was greater in whites than in AAs, indicating that there is increased contribution to resting vasodilator tone of NO and EDHF together in whites compared to AAs.
Bradykinin-stimulated vasodilation
Bradykinin infusion resulted in dose-dependent vasodilation in both ethnic subsets, however, the response was attenuated in AA compared to white subjects, with a 27.3% (p<0.0001) lower mean FBF and 32% (p<0.0001) greater mean FVR in AAs (Figure 3a).
Figure 3.
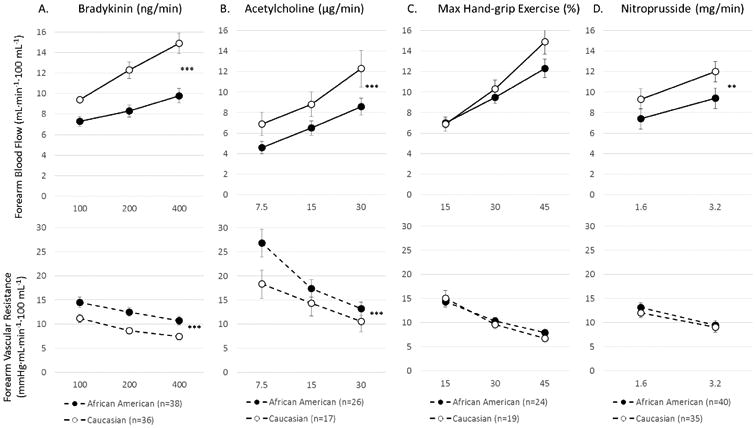
Forearm vasodilator response to (a) bradykinin, (b) acetylcholine, (c) hand-grip exercise, and (d) sodium nitroprusside in healthy African American and Caucasian subjects. Data shown as mean + SEM. Data shown as mean + SEM. *p<0.05; **p<0.01; ***p<0.001
Effects of L-NMMA on bradykinin-mediated vascular responses
Bradykinin-induced dilation was attenuated with L-NMMA in both ethnic subsets, however, the reduction in FBF (−32% vs −26%, p=0.012) and the increase in FVR (78% vs 45%, p=0.002) was greater in whites than in AAs, respectively (Figure 4a), indicating reduced contribution of NO to bradykinin-mediated microvascular dilation in AA subjects.
Figure 4.
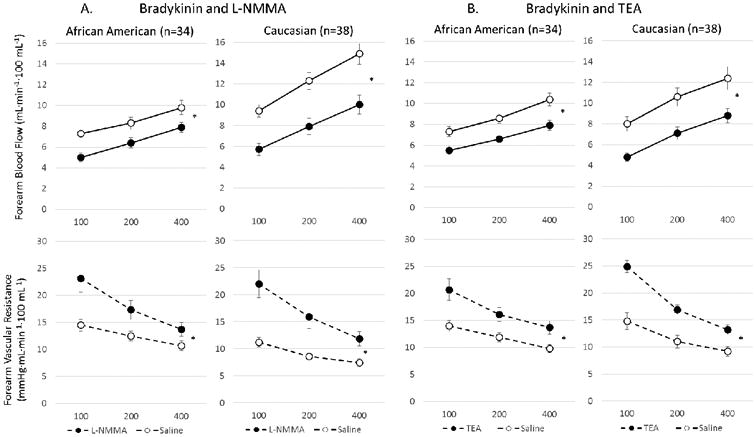
Vasodilation with bradykinin before and after inhibition with (a) L-NMMA or (b) TEA in African American and Caucasian subjects. Change in FBF and FVR in response to bradykinin after inhibition with (a) L-NMMA in AA (n=34) and white (n=38) subjects, and (b) with TEA in AA (n=32) and white (n=28) subjects. Data is shown as mean ± SEM. *p<0.0001
Effects of TEA on bradykinin-mediated vascular responses
Bradykinin-induced dilation was attenuated with TEA in both ethnic subsets, however, the reduction in FBF (−31% vs −22%, p<0.0001) and the increase in FVR (40% vs 32%, p=0.008) was greater in whites than in AAs (figure 4b), indicating a decreased contribution of K+Ca channel activation to bradykinin-stimulated vasodilation in AAs compared to whites.
Effects of combined infusions of L-NMMA and TEA on bradykinin-mediated vascular responses
After combined infusion of L-NMMA and TEA, bradykinin-induced vasodilation was inhibited additively and equally in both subsets; in AA, 45% (p<0.0001) mean reduction in FBF and 94% (p<0.0001) increase in mean FVR; and in whites, 57%, p<0.0001 lower FBF and 155%, p=0.0001 higher FVR.
Acetylcholine-stimulated vasodilation
Acetylcholine infusion produced dose-dependent vasodilation in both ethnic subsets, however, the response was attenuated in AA compared to whites with a 34% (p<0.0001) lower mean FBF and 66% (p<0.0001) greater mean FVR in AAs (Figure 3b).
Effects of L-NMMA on acetylcholine-mediated vascular responses
Acetylcholine-induced vasodilation was significantly inhibited by L-NMMA in both ethnic subsets, however, the reduction in FBF (−36% vs −20%, p=0.042) and the increase in FVR (81% vs 39%, p=0.004) were greater in whites than in AAs, indicating greater contribution of NO to acetylcholine-mediated dilation in white compared to AA subjects (Figure 5a).
Figure 5.
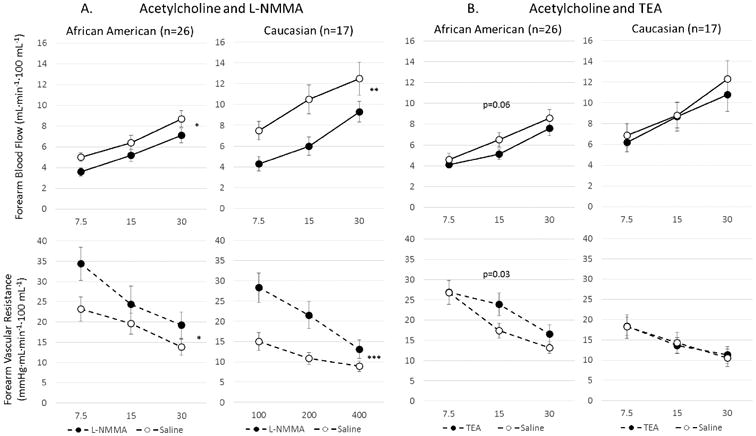
Vasodilation with acetylcholine before and after inhibition with L-NMMA or TEA in African Americans and Caucasians subjects. Change in FBF and FVR in response to acetylcholine after inhibition with (a) L-NMMA in AA (n=26) and white (n=17) subjects, and (b) after inhibition with TEA in African American (n=26) and Caucasian (n=17) subjects. Data is shown as mean ± SEM. *p<0.01; **p<0.001; ***p<0.0001
Effects of TEA on acetylcholine-mediated vascular responses
Acetylcholine-induced vasodilation was inhibited in AAs with TEA (−15%, p=0.06 reduction in FBF and 14%, p=0.03 increase in FVR) but remained unchanged in whites (Figure 5B), indicating an important contribution of K+Ca channel activation to acetylcholine-stimulated vasodilation in AAs and none in whites.
Exercise-induced vasodilation
Intermittent handgrip exercise resulted in progressive vasodilation that was not significantly different between the two ethnic groups (p=0.15), although at the two higher exercise levels, there was a trend toward a lower increase in FBF in AA (−11%, p<0.06) (Figure 3c).
Effects of L-NMMA on exercise-induced vasodilation
Exercise-induced vasodilation was significantly inhibited by L-NMMA in both ethnic subsets, however, the reduction in FBF was greater in whites than in AAs (−21% vs −9% (p<0.0001), indicating decreased contribution of NO to exercise-induced vasodilation in AAs (Figure 6a).
Figure 6.
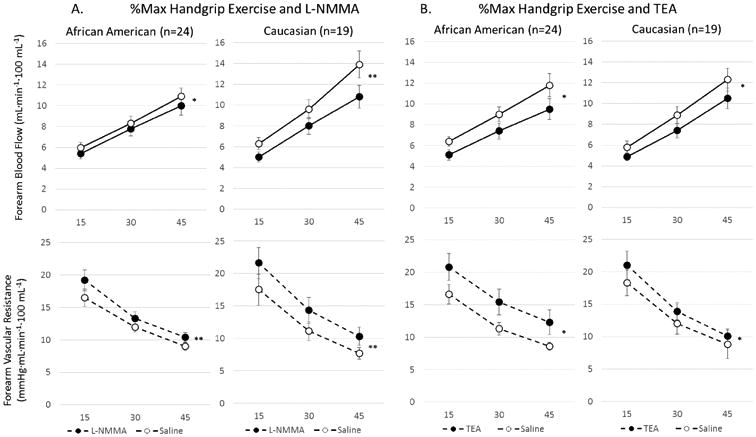
Vasodilation with forearm exercise before and after inhibition with (a) L-NMMA or (b) TEA in African American and Caucasian subjects. Change in FBF and FVR in response to increasing handgrip exercise (15, 30 and 45% of maximal handgrip) alone and (a) after L-NMMA, 16μmol/min) in AA (n=24) and Caucasian (n=19) subjects, and (b) after TEA in AA (n=24) and Caucasian (n=19) subjects. Data shown as mean ± SEM. *p=0.01; **p<0.0001
Effects of TEA on exercise-induced vasodilation
Exercise-induced vasodilation was significantly inhibited by TEA in both ethnic subsets, however, the reduction in FBF was greater in AAs than in whites (−20% vs −16%, p=0.013), indicating greater contribution of K+Ca channel activation to exercise-stimulated vasodilation in AAs (Figure 6b). Moreover, in AA subjects, exercise-induced vasodilation was attenuated to a greater extent with TEA than with L-NMMA (−20% vs −9%, p<0.0001 reduction in FBF, respectively), indicating that K+Ca channel activation and not NO is the predominant mechanism of exercise-induced vasodilation in AAs.
Effect of combined infusions of L-NMMA and TEA on exercise-induced vasodilation
Exercise-induced vasodilation was attenuated in an additive manner in both ethnic subsets with similar reductions in FBF in AAs and whites (−26% and −33%, p=0,56, respectively).
Sodium nitroprusside-induced vasodilation
Sodium nitroprusside produced vasodilation in both ethnic subsets, however, the response was attenuated in AAs compared to whites (−22%, p=0.0002 lower mean FBF and 12%, p=0.0015 greater mean FVR) (Figure 3d). This indicates reduced sensitivity of the smooth muscle cells to NO in AAs. Importantly, L-NMMA and TEA did not alter the vasodilator responses to sodium nitroprusside in either group.
Discussion
In the most comprehensive comparative investigation of microvascular function in a healthy bi-racial population to date, we investigated the relative contributions of NO and EDHF to basal vasodilator tone, to agonist-stimulated endothelium-dependent and -independent vasodilation, and to physiologic vasodilation. Important novel findings are: 1) there is greater contribution of NO than of EDHF to resting vasodilator tone in white compared to AA subjects, with similar EDHF bioavailability in both races; 2) there is reduced endothelium-dependent vasodilation with both acetylcholine and bradykinin in AA compared to white subjects, and this is secondary to reduced contribution of both NO and EDHF to bradykinin-mediated dilation, and to reduced contribution of NO to acetylcholine-mediated dilation in AA. EDHF partly compensates for the reduced NO release with acetylcholine; 3) there is reduced contribution of NO and relatively increased contribution of EDHF to exercise-induced vaodilation in AA compared to white subjects; and 4) there is reduced sensitivity of the vascular smooth muscle to NO, demonstrable as a reduced responses to sodium nitroprusside in AAs compared to whites.
Contribution of NO and EDHF to resting vasodilator tone
We have demonstrated an important contribution of both NO and EDHF, measured as TEA-inhibitable K+Ca channel activation, to resting forearm microvascular dilator tone in both ethnic subsets.26-28 There were no differences in the contribution of EDHF whether TEA was administered before or after L-NMMA was given. Although resting FBF was similar in the two groups, AAs had reduced contribution of NO, demonstrated by the decreased response to L-NMMA, and preserved contribution of EDHF in comparison to white subjects. Conductance vessel NO bioavailability has been reported to be either lower,5, 10, 14 or not different in AAs compared to whites.9 This is the first demonstration of reduced tonic basal NO activity in the microcirculation of healthy AA subjects, and may provide an explanation for the proportionally higher risk and prevalence of hypertension and cardiovascular disease in this group.1 Whether other endogenous vasodilators such as prostacyclin, adenosine, carbon monoxide etc. are differentially bioavailable in AA compared to White subjects needs to be studied.
Contribution of NO and EDHF to bradykinin-stimulated vasodilation
Not only did we confirm that bradykinin-mediated vasodilation is attenuated in AAs, but show that this is due to lower contribution of NO and EDHF.8, 15 Bradykinin is a potent endogenous vasodilator that stimulates NO, prostaglandins, and EDHF release by stimulating endothelial B2 receptors. We confirmed that bradykinin-mediated vasodilation is both NO- and EDHF-mediated, 22, 24, 26, 29-31 but whether their reduced contribution in the AA vasculature is due to enhanced metabolism need further study.15
Contribution of NO and EDHF to acetylcholine-stimulated vasodilation
Previous studies have reported either impaired15, 16 or intact11 endothelial responses to muscarinic receptor stimulation with methacholine or acetylcholine in healthy AAs compared to whites. We recently demonstrated that acetylcholine-mediated vasodilation is largely due to stimulation of release of NO, and unlike bradykinin, acetylcholine does not stimulate EDHF release, a finding consistent with previous reports.27, 32 Now we demonstrate that not only is acetylcholine-mediated vasodilation depressed in AAs compared to whites, but that there is some compensatory increase in release of EDHF with acetylcholine from the AA microvasculature, a finding also observed in experimental models.33, 34 These findings in healthy AAs are similar to those observed in patients with multiple risk factors.24
Contribution of NO and EDHF to exercise-induced vasodilation
We have recently shown that both NO and K+Ca channel activation (EDHF) contribute individually and in concert to exercise-induced microvascular vasodilation.25 Here, we demonstrate that the contribution to exercise-induced forearm microvascular dilation of NO is lower, and of EDHF is greater, in AAs compared to white subjects. Thus, even in healthy AAs, EDHF is the predominant mechanism responsible for exercise-induced forearm vasodilation, whereas NO is the predominant endothelium-dependent agonist in white subjects. These abnormalities are similar to the reduced mental stress-induced vasodilation we previously reported in normotensive AAs.35
Mechanisms underlying vascular dysfunction in AAs
Oxidative stress from upregulation of superoxide anions and its effects on NO metabolism and endothelial nitric oxide synthase uncoupling and inhibition, play a central role in risk factor-mediated vascular pathophysiology.36 Decreased NO bioavailability and increased production of peroxynitrite has been demonstrated in isolated human umbilical vein endothelial cells obtained from AAs,37 and we have also demonstrated increased systemic oxidative stress measured as higher oxidized aminothiol levels and increased endothelin activity in AAs compared to whites which provides an explanation for the observed reduced NO bioavailability.38, 39 Treatment with nebivolol appears to normalize the increased free radical production in cells from AA and restores NO bioavailability to that observed in cells from Whites.40 Other studies have demonstrated higher asymmetric dimethyarginine in AA compared to Whites.41
Differences in endothelium-independent vasodilation
We confirmed previous findings of reduced microvascular dilatory responses to sodium nitroprusside, an NO donor, in AAs, likely due to decreased responsiveness of the vascular smooth muscle to NO.42 Our previous findings of reduced cyclic nucleotide-mediated responses in AA forearm microcirculation involving both the adenylate cyclase and guanylate cyclase systems suggest either a generalized decrease in responsiveness of multiple vasodilator systems or involvement of a common distal step in the vasodilating pathways.16, 42 Modulation of cytosolic calcium concentration is one potential final pathway for regulating smooth muscle tone, and racial differences in its regulation may be one mechanism.42 Paradoxically, an enhanced response of conductance vessels to sublingual nitroglycerin was observed in AAs.9
Limitations
Although we found that the vasoconstrictor response to L-NMMA was lower in AAs, we did not examine effects of other non-specific vasoconstrictors to investigate whether this is a reflection of reduced sensitivity of the vascular smooth muscle to vasoconstrictors. However, the fact that the constrictor response to TEA was similar to whites suggests that the response to L-NMMA is specific for reduced NO bioavailability. The reduced sensitivity to exogenous NO (sodium nitroprusside) complicates the interpretation of the reduced dilator responses observed with acetylcholine and bradykinin in AAs. However, because basal NO and the contribution of NO during exercise is lower in AAs, it is likely that in addition to reduced sensitivity, there is also an endothelial defect in NO release in AAs.
L-NMMA and TEA are competitive inhibitors and thus our results may underestimate the physiological contribution of both NO and K+Ca channels to vasodilation. Our investigation was conducted on a background of cyclooxygenase inhibition because of its negligible contribution in the healthy human circulation.43, 44 Although we employed intermittent isometric handgrip exercise, other muscle beds and different intensities of exercise may need to be explored in future studies. Using plethysmography, we have assessed the microcirculatory responses and not the conductance vessel changes in this study, however, it is known that the contribution of EDHF is less in conductance vessels than in the microvessels.45
Significance
Although NO and EDHF contribute to resting blood flow, to agonist-mediated vasodilation, and and to vasodilation during exercise, the contribution of NO is reduced and of EDHF is preserved or increased in the microcirculation of healthy AAs with these stimuli. Preserved EDHF but reduced NO bioavailability in AAs may underlie their increased risk of hypertension and cardiovascular disease and have crucial therapeutic implications that need to be explored.
Supplementary Material
Figure 1.
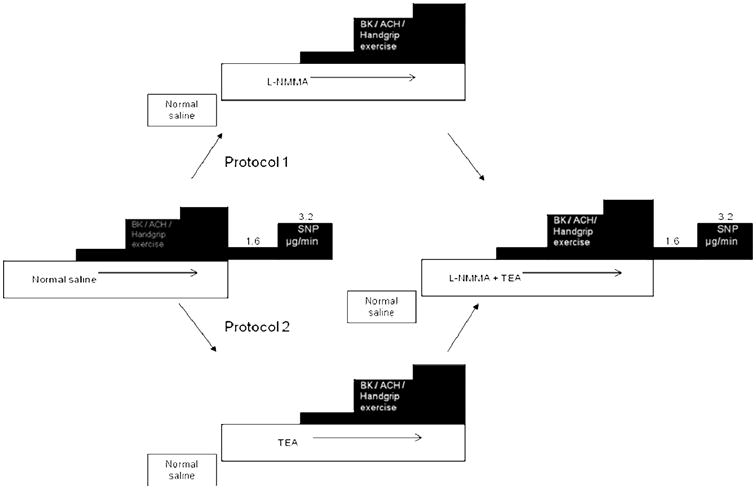
Aspirin (975mg) was administered 1hour prior to commencement of the study. In separate protocols, FBF measurements were performed after either intra-arterial infusions of bradykinin (100, 200 and 400 ng/min), or intra-arterial acetylcholine (7.5, 15 and 30 μg/min), or handgrip exercise (15%, 30% and 45% of maximum grip strength) followed by endothelium-independent vasodilation with sodium nitroprusside (1.6, 3.2 μg/min). Measurements were repeated after NO blockade with L-NMMA, K+Ca channel blockade with TEA, and after combined blockade with L-NMMA and TEA.
Acknowledgments
none
Funding sources: National Institutes of Health Research Grant RO1 HL79115, and in part by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award no UL1TR000454 and the British Cardiovascular Society Research Fellowship, and the National Blood Foundation
Abbreviations
- AA
African American
- EDHF
Endothelium-Derived Hyperpolarizing Factor
- NO
Nitric Oxide
Footnotes
Disclosures: none
Clinical Trial Registration Information: http://clinicaltrials.gov/, Identifier: NCT00166166
Contributor Information
Muhiddin A Ozkor, Division of Cardiology, Department of Medicine, Emory University School of Medicine, Atlanta, GA.
Ayaz M Rahman, Division of Cardiology, Department of Medicine, Emory University School of Medicine, Atlanta, GA.
Jonathan R Murrow, Division of Cardiology, Department of Medicine, Emory University School of Medicine, Atlanta, GA.
Nino Kavtaradze, Division of Cardiology, Department of Medicine, Emory University School of Medicine, Atlanta, GA.
Ji Lin, Rollins School of Public Health, Emory University, Atlanta, GA.
Amita Manatunga, Rollins School of Public Health, Emory University, Atlanta, GA.
Salim Hayek, Division of Cardiology, Department of Medicine, Emory University School of Medicine, Atlanta, GA.
Arshed A Quyyumi, Division of Cardiology, Department of Medicine, Emory University School of Medicine, Atlanta, GA.
References
- 1.Feinstein M, Ning H, Kang J, Bertoni A, Carnethon M, Lloyd-Jones DM. Racial differences in risks for first cardiovascular events and noncardiovascular death: The atherosclerosis risk in communities study, the cardiovascular health study, and the multi-ethnic study of atherosclerosis. Circulation. 2012;126:50–59. doi: 10.1161/CIRCULATIONAHA.111.057232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The sixth report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med. 1997;157:2413–2446. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 3.Ferdinand KC, Clark LT. The epidemic of diabetes mellitus and the metabolic syndrome in african americans. Rev Cardiovasc Med. 2004;5(Suppl 3):S28–33. [PubMed] [Google Scholar]
- 4.McBean AM, Li S, Gilbertson DT, Collins AJ. Differences in diabetes prevalence, incidence, and mortality among the elderly of four racial/ethnic groups: Whites, blacks, hispanics, and asians. Diabetes Care. 2004;27:2317–2324. doi: 10.2337/diacare.27.10.2317. [DOI] [PubMed] [Google Scholar]
- 5.Campia U, Choucair WK, Bryant MB, Waclawiw MA, Cardillo C, Panza JA. Reduced endothelium-dependent and -independent dilation of conductance arteries in african americans. Journal of the American College of Cardiology. 2002;40:754–760. doi: 10.1016/s0735-1097(02)02015-6. [DOI] [PubMed] [Google Scholar]
- 6.D'Agostino RB, Jr, Burke G, O'Leary D, Rewers M, Selby J, Savage PJ, Saad MF, Bergman RN, Howard G, Wagenknecht L, Haffner SM. Ethnic differences in carotid wall thickness. The insulin resistance atherosclerosis study. Stroke. 1996;27:1744–1749. doi: 10.1161/01.str.27.10.1744. [DOI] [PubMed] [Google Scholar]
- 7.Evans RR, Phillips BG, Singh G, Bauman JL, Gulati A. Racial and gender differences in endothelin-1. Am J Cardiol. 1996;78:486–488. doi: 10.1016/s0002-9149(96)00344-x. [DOI] [PubMed] [Google Scholar]
- 8.Gainer JV, Stein CM, Neal T, Vaughan DE, Brown NJ. Interactive effect of ethnicity and ace insertion/deletion polymorphism on vascular reactivity. Hypertension. 2001;37:46–51. doi: 10.1161/01.hyp.37.1.46. [DOI] [PubMed] [Google Scholar]
- 9.Gokce N, Holbrook M, Duffy SJ, Demissie S, Cupples LA, Biegelsen E, Keaney JF, Jr, Loscalzo J, Vita JA. Effects of race and hypertension on flow-mediated and nitroglycerin-mediated dilation of the brachial artery. Hypertension. 2001;38:1349–1354. doi: 10.1161/hy1201.096575. [DOI] [PubMed] [Google Scholar]
- 10.Jones DS, Andrawis NS, Abernethy DR. Impaired endothelial-dependent forearm vascular relaxation in black americans. Clin Pharmacol Ther. 1999;65:408–412. doi: 10.1016/S0009-9236(99)70135-9. [DOI] [PubMed] [Google Scholar]
- 11.Kahn DF, Duffy SJ, Tomasian D, Holbrook M, Rescorl L, Russell J, Gokce N, Loscalzo J, Vita JA. Effects of black race on forearm resistance vessel function. Hypertension. 2002;40:195–201. doi: 10.1161/01.hyp.0000024571.69634.ed. [DOI] [PubMed] [Google Scholar]
- 12.Lang CC, Stein CM, Brown RM, Deegan R, Nelson R, He HB, Wood M, Wood AJ. Attenuation of isoproterenol-mediated vasodilatation in blacks. N Engl J Med. 1995;333:155–160. doi: 10.1056/NEJM199507203330304. [DOI] [PubMed] [Google Scholar]
- 13.Lange LA, Bowden DW, Langefeld CD, Wagenknecht LE, Carr JJ, Rich SS, Riley WA, Freedman BI. Heritability of carotid artery intima-medial thickness in type 2 diabetes. Stroke. 2002;33:1876–1881. doi: 10.1161/01.str.0000019909.71547.aa. [DOI] [PubMed] [Google Scholar]
- 14.Perregaux D, Chaudhuri A, Rao S, Airen A, Wilson M, Sung BH, Dandona P. Brachial vascular reactivity in blacks. Hypertension. 2000;36:866–871. doi: 10.1161/01.hyp.36.5.866. [DOI] [PubMed] [Google Scholar]
- 15.Rosenbaum DA, Pretorius M, Gainer JV, Byrne D, Murphey LJ, Painter CA, Vaughan DE, Brown NJ. Ethnicity affects vasodilation, but not endothelial tissue plasminogen activator release, in response to bradykinin. Arterioscler Thromb Vasc Biol. 2002;22:1023–1028. doi: 10.1161/01.atv.0000017704.45007.1d. [DOI] [PubMed] [Google Scholar]
- 16.Stein CM, Lang CC, Nelson R, Brown M, Wood AJ. Vasodilation in black americans: Attenuated nitric oxide-mediated responses. Clin Pharmacol Ther. 1997;62:436–443. doi: 10.1016/S0009-9236(97)90122-3. [DOI] [PubMed] [Google Scholar]
- 17.Quyyumi AA, Dakak N, Andrews NP, Gilligan DM, Panza JA, Cannon RO., 3rd Contribution of nitric oxide to metabolic coronary vasodilation in the human heart. Circulation. 1995;92:320–326. doi: 10.1161/01.cir.92.3.320. [DOI] [PubMed] [Google Scholar]
- 18.Gilligan DM, Panza JA, Kilcoyne CM, Waclawiw MA, Casino PR, Quyyumi AA. Contribution of endothelium-derived nitric oxide to exercise-induced vasodilation. Circulation. 1994;90:2853–2858. doi: 10.1161/01.cir.90.6.2853. [DOI] [PubMed] [Google Scholar]
- 19.Joannides R, Richard V, Haefeli WE, Linder L, Luscher TF, Thuillez C. Role of basal and stimulated release of nitric oxide in the regulation of radial artery caliber in humans. Hypertension. 1995;26:327–331. doi: 10.1161/01.hyp.26.2.327. [DOI] [PubMed] [Google Scholar]
- 20.Mullen MJ, Kharbanda RK, Cross J, Donald AE, Taylor M, Vallance P, Deanfield JE, MacAllister RJ. Heterogenous nature of flow-mediated dilatation in human conduit arteries in vivo: Relevance to endothelial dysfunction in hypercholesterolemia. Circ Res. 2001;88:145–151. doi: 10.1161/01.res.88.2.145. [DOI] [PubMed] [Google Scholar]
- 21.Panza JA, Casino PR, Kilcoyne CM, Quyyumi AA. Role of endothelium-derived nitric oxide in the abnormal endothelium-dependent vascular relaxation of patients with essential hypertension. Circulation. 1993;87:1468–1474. doi: 10.1161/01.cir.87.5.1468. [DOI] [PubMed] [Google Scholar]
- 22.Panza JA, Garcia CE, Kilcoyne CM, Quyyumi AA, Cannon RO., 3rd Impaired endothelium-dependent vasodilation in patients with essential hypertension. Evidence that nitric oxide abnormality is not localized to a single signal transduction pathway. Circulation. 1995;91:1732–1738. doi: 10.1161/01.cir.91.6.1732. [DOI] [PubMed] [Google Scholar]
- 23.Quyyumi AA, Dakak N, Mulcahy D, Andrews NP, Husain S, Panza JA, Cannon RO., 3rd Nitric oxide activity in the atherosclerotic human coronary circulation. J Am Coll Cardiol. 1997;29:308–317. doi: 10.1016/s0735-1097(96)00472-x. [DOI] [PubMed] [Google Scholar]
- 24.Ozkor MA, Murrow JR, Rahman AM, Kavtaradze N, Lin J, Manatunga A, Quyyumi AA. Endothelium-derived hyperpolarizing factor determines resting and stimulated forearm vasodilator tone in health and in disease. Circulation. 2011;123:2244–2253. doi: 10.1161/CIRCULATIONAHA.110.990317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozkor MA, Kavtaradze N, Murrow J, Jorgensen J, Pohlel K, Aznaouridis K, Matanunga A, Quyyumi AA. The contribution of endothelium-derived hyperpolarizing factor and nitric oxide to exercise-induced vasodilation. 57th Annual Scientific Session of the American-College-of-Cardiology. 2008:A279–A280. [Google Scholar]
- 26.Honing ML, Smits P, Morrison PJ, Rabelink TJ. Bradykinin-induced vasodilation of human forearm resistance vessels is primarily mediated by endothelium-dependent hyperpolarization. Hypertension. 2000;35:1314–1318. doi: 10.1161/01.hyp.35.6.1314. [DOI] [PubMed] [Google Scholar]
- 27.Inokuchi K, Hirooka Y, Shimokawa H, Sakai K, Kishi T, Ito K, Kimura Y, Takeshita A. Role of endothelium-derived hyperpolarizing factor in human forearm circulation. Hypertension. 2003;42:919–924. doi: 10.1161/01.HYP.0000097548.92665.16. [DOI] [PubMed] [Google Scholar]
- 28.Bellien J, Iacob M, Gutierrez L, Isabelle M, Lahary A, Thuillez C, Joannides R. Crucial role of no and endothelium-derived hyperpolarizing factor in human sustained conduit artery flow-mediated dilatation. Hypertension. 2006;48:1088–1094. doi: 10.1161/01.HYP.0000246672.72188.bd. [DOI] [PubMed] [Google Scholar]
- 29.Cockcroft JR, Chowienczyk PJ, Brett SE, Bender N, Ritter JM. Inhibition of bradykinin-induced vasodilation in human forearm vasculature by icatibant, a potent b2-receptor antagonist. Br J Clin Pharmacol. 1994;38:317–321. doi: 10.1111/j.1365-2125.1994.tb04360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Kane KP, Webb DJ, Collier JG, Vallance PJ. Local l-ng-monomethyl-arginine attenuates the vasodilator action of bradykinin in the human forearm. Br J Clin Pharmacol. 1994;38:311–315. doi: 10.1111/j.1365-2125.1994.tb04359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taddei S, Ghiadoni L, Virdis A, Buralli S, Salvetti A. Vasodilation to bradykinin is mediated by an ouabain-sensitive pathway as a compensatory mechanism for impaired nitric oxide availability in essential hypertensive patients. Circulation. 1999;100:1400–1405. doi: 10.1161/01.cir.100.13.1400. [DOI] [PubMed] [Google Scholar]
- 32.Halcox JP, Narayanan S, Cramer-Joyce L, Mincemoyer R, Quyyumi AA. Characterization of endothelium-derived hyperpolarizing factor in the human forearm microcirculation. American Journal of Physiology - Heart & Circulatory Physiology. 2001;280:H2470–2477. doi: 10.1152/ajpheart.2001.280.6.H2470. [DOI] [PubMed] [Google Scholar]
- 33.Komori K, Lorenz RR, Vanhoutte PM. Nitric oxide, ach, and electrical and mechanical properties of canine arterial smooth muscle. Am J Physiol. 1988;255:H207–212. doi: 10.1152/ajpheart.1988.255.1.H207. [DOI] [PubMed] [Google Scholar]
- 34.Parkington HC, Tare M, Tonta MA, Coleman HA. Stretch revealed three components in the hyperpolarization of guinea-pig coronary artery in response to acetylcholine. J Physiol. 1993;465:459–476. doi: 10.1113/jphysiol.1993.sp019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cardillo C, Kilcoyne CM, Cannon RO, III, Panza JA. Racial differences in nitric oxide–mediated vasodilator response to mental stress in the forearm circulation. Hypertension. 1998;31:1235–1239. doi: 10.1161/01.hyp.31.6.1235. [DOI] [PubMed] [Google Scholar]
- 36.Huk I, Nanobashvili J, Neumayer C, Punz A, Mueller M, Afkhampour K, Mittlboeck M, Losert U, Polterauer P, Roth E, Patton S, Malinski T. L-arginine treatment alters the kinetics of nitric oxide and superoxide release and reduces ischemia/reperfusion injury in skeletal muscle. Circulation. 1997;96:667–675. doi: 10.1161/01.cir.96.2.667. [DOI] [PubMed] [Google Scholar]
- 37.Kalinowski L, Dobrucki IT, Malinski T. Race-specific differences in endothelial function: Predisposition of african americans to vascular diseases. Circulation. 2004;109:2511–2517. doi: 10.1161/01.CIR.0000129087.81352.7A. [DOI] [PubMed] [Google Scholar]
- 38.Morris AA, Zhao L, Patel RS, Jones DP, Ahmed Y, Stoyanova N, Gibbons GH, Vaccarino V, Din-Dzietham R, Quyyumi AA. Differences in systemic oxidative stress based on race and the metabolic syndrome: The morehouse and emory team up to eliminate health disparities (meta-health) study. Metabolic syndrome and related disorders. 2012;10:252–259. doi: 10.1089/met.2011.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campia U, Cardillo C, Panza JA. Ethnic differences in the vasoconstrictor activity of endogenous endothelin-1 in hypertensive patients. Circulation. 2004;109:3191–3195. doi: 10.1161/01.CIR.0000130590.24107.D3. [DOI] [PubMed] [Google Scholar]
- 40.Mason RP, Kalinowski L, Jacob RF, Jacoby AM, Malinski T. Nebivolol reduces nitroxidative stress and restores nitric oxide bioavailability in endothelium of black americans. Circulation. 2005;112:3795–3801. doi: 10.1161/CIRCULATIONAHA.105.556233. [DOI] [PubMed] [Google Scholar]
- 41.Melikian N, Wheatcroft SB, Ogah OS, Murphy C, Chowienczyk PJ, Wierzbicki AS, Sanders TA, Jiang B, Duncan ER, Shah AM, Kearney MT. Asymmetric dimethylarginine and reduced nitric oxide bioavailability in young black african men. Hypertension. 2007;49:873–877. doi: 10.1161/01.HYP.0000258405.25330.80. [DOI] [PubMed] [Google Scholar]
- 42.Cardillo C, Kilcoyne CM, Cannon RO, III, Panza JA. Attenuation of cyclic nucleotide–mediated smooth muscle relaxation in blacks as a cause of racial differences in vasodilator function. Circulation. 1999;99:90–95. doi: 10.1161/01.cir.99.1.90. [DOI] [PubMed] [Google Scholar]
- 43.Duffy SJ, New G, Tran BT, Harper RW, Meredith IT. Relative contribution of vasodilator prostanoids and no to metabolic vasodilation in the human forearm. Am J Physiol. 1999;276:H663–670. doi: 10.1152/ajpheart.1999.276.2.H663. [DOI] [PubMed] [Google Scholar]
- 44.Quyyumi AA. Effects of aspirin on endothelial dysfunction in atherosclerosis. The American Journal of Cardiology. 1998;82:31S–33S. doi: 10.1016/s0002-9149(98)00673-0. [DOI] [PubMed] [Google Scholar]
- 45.Urakami-Harasawa L, Shimokawa H, Nakashima M, Egashira K, Takeshita A. Importance of endothelium-derived hyperpolarizing factor in human arteries. J Clin Invest. 1997;100:2793–2799. doi: 10.1172/JCI119826. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


