Abstract
Background
No prior study has evaluated financial relationships of investigators with pharmaceutical manufacturers for basic science. An example of the importance and impact of such relationships is in the evaluation of erythropoietin receptors’(EpoRs) effects on cancer cell lines, since studies have reported increased mortality when cancer patients receive erythropoiesis stimulating agents (ESAs).
Purpose
To assess the disclosed association that exist between pharmaceutical industry support and EpoRs effects on solid cancer cell lines.
Data Sources
MEDLINE and EMBASE (1988- July 2008) and two EpoR conferences sponsored by the National Institutes of Health.
Study Selection
All publications investigating EpoRs that met inclusion criteria were identified and included.
Data Extraction
Data were extracted on detection of EpoRs, presence of erythropoietin-induced signaling events, presence of erythropoietin-induced changes in cellular function, nature of qualitative conclusions, and sources of funding for all 74 studies.
Data Synthesis
In comparison to studies of academic investigators with no disclosed funding support from ESA manufacturers (n=64), the studies from academic investigators with funding support from ESA manufacturers (n= 7) and the laboratories directed by investigators employed by ESA manufacturers (n=3) were both less likely to identify: EpoR presence on solid tumor cells; erythropoietin-induced signaling events; erythropoietin-induced changes in cellular function; and less likely to conclude that their research had identified potentially harmful effects of erythropoietin on cancer cells. Additionally, presentations from industry-based investigator teams at NIH conferences were less likely to report EpoRs on cancer cell lines, downstream effects of erythropoietin, and cell proliferation and migration effects following EpoR administration.
Conclusion
Financial conflicts of interest impact the outcomes and presentation of basic science research data as well as publications.
INTRODUCTION
United States Senator Charles Grassley recently initiated conflict of interest probes of clinical investigators at Harvard Medical School, Columbia University, and Emory University.(1) These investigations focus on payments from pharmaceutical manufacturers to key opinion leaders. Grassley and Senator Charles Schumer introduced the Physician Payments Sunshine Act that requires transparency in relationships with pharmaceutical manufacturers. These actions stem from long-standing observations that clinical investigators who have financial support from pharmaceutical manufacturers are less likely than other clinical researchers to criticize the safety, effectiveness, or cost-effectiveness of drugs and devices distributed by sponsoring manufacturers (2–4) and more likely to endorse novel and less proven treatments.(5) A related and previously unanswered question concerns financial conflicts of interest for basic scientists.
Prior studies provide some insight on academic-industrial relationships in the life sciences. In one review of relationships between academic institutions and industry, Blumenthal reported that these relationships facilitated technology transfer.(6) However, there is less scrutiny of academic conflicts of interest involving non-clinical research than clinical research. Moreover, partly due to less federal level regulatory requirements for disclosure of conflicts and relatively minimal monitoring or oversight of research conduct, academic institutions have embraced industrial sponsorship of basic science research and placed relatively less emphasis on disclosure, management, and monitoring of these relationships. In a survey of life science researchers, Blumenthal et al. reported that faculty members with industrial research support were at least as productive academically as those without such support and were more productive commercially, although they were more likely to restrict their communication with colleagues.(7) Bekelman et al. reported that financial relationships among industry, scientific investigators, and academic institutions were widespread, and that industry sponsorship was associated with pro-industry conclusions.(8) While Bekelman et al. primarily focused on clinical investigators with conflicts of interest, the study also noted that both clinical and basic science investigators with conflicts reported an unwillingness to publish certain findings, restrictions on publication efforts, or shifting research emphasis based on commercial considerations.(8) The use of ESAs in cancer patients represents a prime example of a hotly pursued basic science question that may ultimately have a large impact on the pharmaceutical companies as well as on clinical practice.
Recently, basic science studies have raised concern over the safety of administering erythropoiesis stimulating agents (ESAs) to cancer patients. Preclinical studies demonstrated erythropoietin receptors (EpoRs) on cell lines derived from solid tumors.(9, 10) Concern that EpoR signaling on tumor cells might contribute to tumor progression remained theoretical until 2003 when clinical trials identified increased tumor progression and mortality among ESA-treated head and neck cancer and breast cancer patients.(11, 12) Furthermore, a follow-up to the head and neck trial demonstrated that increased mortality with ESA administration was noted only in patients whose tumors expressed EpoRs, while patients lacking the receptor experienced decreased mortality with ESA treatment.(13) Safety concerns grew when six additional trials demonstrated increased mortality risks and four studies found increased tumor progression among ESA-treated patients.(14) These findings support the recent FDA labeling change that states ESAs are not indicated for cancer patients undergoing chemotherapy regimens with curative intent.(15, 16)
Basic science findings for EpoRs provide an opportunity to evaluate whether financial conflicts of interest might affect basic science research. Interpreting findings from investigations of EpoRs conducted by basic science investigators who do or do not have financial support from pharmaceutical manufacturers is central to evaluating this concern. Herein, we reviewed findings reported by academic investigators with or without pharmaceutical support and investigators employed by ESA manufacturers on the effects of ESAs on tumor cells, xenografts, and human cancers.
METHODS
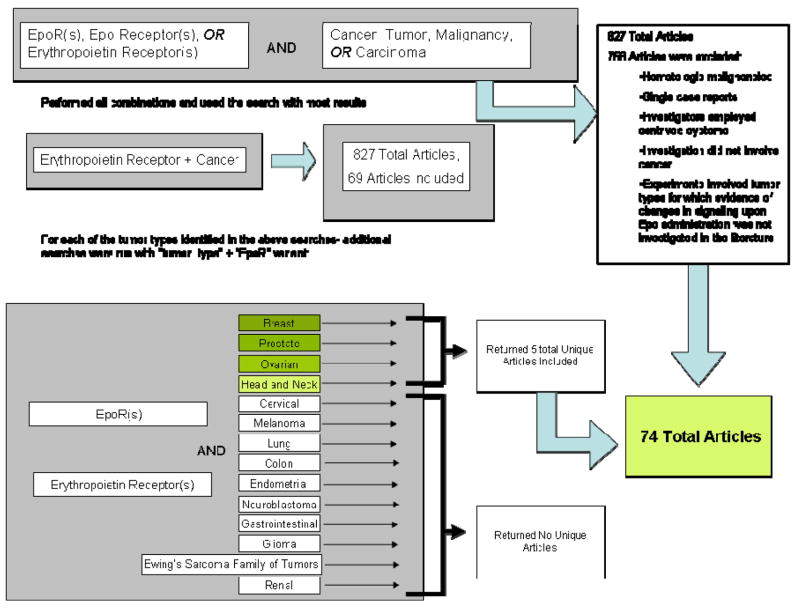
We searched the MEDLINE and EMBASE (1988- July 2008) databases to identify research articles investigating EpoRs in solid tumors (Keywords: (Erythropoietin (Epo), erythropoietin receptor (EpoR)) AND (cancer, tumor, malignancy, carcinoma)). All publications investigating EpoRs were identified. Editorials, reviews, investigations concerning hematologic malignancies, articles that did not investigate presence of EpoRs, and studies that used artificially constructed EpoR complexes were excluded. Moreover, studies concerning tumor types for which ESA-stimulated signaling events were not investigated elsewhere in the literature were also excluded. Seventy-four articles were included in this study (Figure 1).
Figure 1.
Search methods.
Data were abstracted on detection of EpoRs, presence of erythropoietin-induced signaling events, presence of erythropoietin-induced changes in cellular function, nature of qualitative conclusions, and sources of funding. Information on EpoR detection included: detection method, types of antibodies, negative and positive controls, band size, and location in the cell. For signaling events, information was obtained on positive and negative controls, and assessments of erythropoietin-induced changes in signaling proteins Janus-activated- kinase-2 (JAK2), signal transducer and activator of transcription-5 (STAT5), the Bcl-2 (B-cell lymphoma 2), family of proteins (including pro-apoptotic Bax, anti-apoptotic Bcl-2 and Bcl-xL), phosphoinositide 3-kinase (PI3K), Akt, Nuclear factor-kappa B (NF-κB), mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase-1 (ERK1), jun N-terminal kinase (JNK), Jagged-1, Notch-1, vascular endothelial growth factorm (VEGF), platelet derived growth factor-b (PDGF). For cellular function studies, information was obtained on erythropoietin-induced changes in cell survival, invasiveness, migration, apoptosis, hypoxia-inducible factor, EpoR expression, angiogenesis, tumor oxygenation, chemosensitization, proliferation, and growth in tumor samples, cancer cell lines or xenografted cancers. Conflicts of interest were abstracted through conflict statements and funding disclosures included in the publications. For publications that did not include funding acknowledgments as a matter of course, journal editors and first and last authors were queried regarding funding sources.
Analyses
All seventy-four articles were divided according to funding source. The first group, investigator teams with no conflicts of interest, included investigators with no disclosed funding support from ESA manufacturers. The second group, industry based investigator teams with conflicts of interest, included studies where greater than 75% of co-authors were employees of ESA manufacturers. The third group, academic based investigator teams with conflicts of interest, included studies from academic investigators who received funding support from ESA manufacturers (operationally defined as research grant support or academic investigators who received honoraria from ESA manufacturers, were consultants to ESA manufacturers, or who collaborated in the research study with active engagement in the research by investigators employed by ESA manufacturers).
Qualitative conclusions were scored as: unfavorable, neutral, favorable, or not present. Studies that explicitly reported potential adverse effects of ESAs in cancer patients were scored as “unfavorable.” Studies that explicitly emphasized potential therapeutic value of ESAs in cancer patients were scored as “favorable.” Studies that presented both favorable and unfavorable information and did not validate one over the other were scored as “neutral.” Studies which made no qualitative conclusions were scored as “not present”
The Chi-square statistical test was used to compare differences in study results according to funding source.
RESULTS
Our literature review of EpoR in solid tumors identified 64 investigations from academic investigators who indicated no financial support from ESA manufacturers, three from industry-based investigators employed by ESA manufacturers, and seven from academic-based investigators who received support from ESA manufacturers (including three academically-associated studies that included co-investigators employed by ESA manufacturers). Prior to 2003 (the year when the first two trials identifying increased rates of tumor progression and death with ESA administration to cancer patients were published), one-third of studies on EpoRs in the solid tumor setting from academic-based investigators who indicated no financial conflicts of interest had been published, two-thirds of such studies from academic-based investigators with financial conflicts of interest had been published, and none of the studies were from industry-based investigators had been reported.
Published basic science findings
Group I: Reports from academic investigative teams who did not receive financial support from ESA manufacturers
Sixty-four studies from investigators without financial support from ESA manufacturers predominantly reported results in support of potentially harmful effects of erythropoietin on solid tumors (Table 1). Fifty-seven studies investigated that EpoR was present on cancer cells. All of these studies positively identified EpoRs (Table 1). To detect EpoR protein, investigators employed immunohistochemistry, immunocytochemistry, and radiolabeled ligands. Reverse transcriptase PCR and real-time PCR were used to detect EpoR DNA. Most EpoR bands were approximately 66 kiloDaltons in weight (range from 59 to 110 kiloDaltons). Twenty-two studies evaluated EpoR in malignant and paired benign tissues; 20 identified EpoRs in malignant cells and reported either no EpoRs on paired benign tissue, or EpoR levels lower than in the malignant counterpart.(9, 17–35) Two studies identified EpoR in both samples: one did not report on comparative levels(36) and the second reported similar levels in brain tissue.(37) Of 34 studies evaluating signaling events in response to the administration of erythropoietin, 31 identified these events (Table 1). Signaling proteins investigated included JAK2 pathways (three of three studies identified activated JAK2(29, 30, 38) and six of seven demonstrated inhibition of erythropoietin-induced downstream effects with concurrent administration of JAK2 inhibitor, (30, 38–43) PI3-kinase (five studies, all demonstrated inhibition of erythropoietin-induced downstream effects with concurrent administration of PI3K inhibitor)(10, 37, 43–45), and MAP-kinase (one of two studies identified activated MAPK (46)’ (24) and one study demonstrated inhibition of erythropoietin-induced downstream effects with concurrent administration of MAPK inhibitor(39)). Forty studies evaluated cell function and regulation changes following erythropoietin administration, including mitogenic effects (13 studies, 10 identified effects) (9, 26, 37, 43, 47–52) (32, 46, 53), invasiveness and migration (five studies, all identified effects)(29, 30, 38, 39, 42), angiogenesis (five studies, two identified effects)(51, 52, 54, 55), growth (18 studies, 9 identified effects) (37, 38, 43, 47, 49–52, 56) (10, 32, 46, 48, 53, 57–60) and cytoprotective effects (19 studies, 12 identified effects). (10, 18, 24, 26, 27, 29, 41, 44, 45, 61, 62) (40, 46, 50, 54, 55, 57, 63) Other investigations evaluated the effects of erythropoiesis stimulating agents on chemotherapy effectiveness, including tumor protection (14 studies- 8 reported protection (9, 18, 24, 26, 27, 29, 41, 62), six reported no effects (10, 24, 46, 50, 57, 62), and two reported increased chemotherapy efficacy (40, 54), some studies reported different results within their manuscript and thus were counted twice) and effects of erythropoietin on apoptosis (four studies, all reported reduced apoptosis).(44, 61, 62, 64) These studies utilized primary tumor samples and established tumor cell lines including cancers of the breast (17 studies), cervix (9 studies), neuroblastoma (7 studies), kidney (6 studies), ovary (7 studies), and prostate (7 studies) (Table 1).
Table 1.
List of studies included in comprehensive review. Tumor type investigated, findings regarding presence of erythropoietin receptors, ESA induced changes in signaling, ESA-induced changes in cellular function, qualitative conclusions and descriptions of conflicts of interest are summarized.
| Study: First Author, Year of Publication, Journal | Report Detection of EpoR | Report Epo- Induced Signaling Events | Report Epo- Induced Harmful Cellular Function Changes | Qualitative Conclusions | Description of Financial Conflict of Interest | |
|---|---|---|---|---|---|---|
| Tumor Type: Breast | ||||||
| Acs 2004 Cancer Letters(64) | Yes | Yes | Yes | No Statement | None | |
| Acs 2002 Cancer(19) | Yes | Not Investigated | Not Investigated | Harmful | None | |
| Arcasoy 2002 Laboratory Investigation(20) | Yes | Yes | Yes | No Statement | None | |
| Blackwell 2003 Cancer Research(68) | Not Investigated | Not Investigated | No | Beneficial | Research Grant from ESA manufacturer and 2 of 8 authors are employees | |
| Gewirtz 2006 Clinical Cancer Research(57) | Yes | Yes | No | Neutral | None | |
| Hardee 2006 Molecular Cancer Therapeutics(10) | Not Investigated | Yes | Yes | Neutral | None | |
| Hardee 2007 PLoS ONE(52) | Not Investigated | Yes | Yes | No Statement | None | |
| Lester 2005 The Journal of Biological Chemistry(42) | Not Investigated | Yes | Yes | Harmful | None | |
| LaMontagne 2006 Molecular Cancer Therapeutics(67) | Yes | No | No | Neutral | Research is supported by grant from Johnson and Johnson and 5 of 7 authors are employees of J&J | |
| Pelekanou 2007 Cancer Epidemiology Biomarkers and Prevention(31) | Yes | Not Investigated | Not Investigated | Neutral | None | |
| Phillips 2007 Neoplasia(43) | Yes | Yes | Yes | Neutral | None | |
| Wincewicz 2007 Folia Histochem Cytobiol(78) | Yes | Not Investigated | Not Investigated | No Statement | None | |
| Tumor Type: Lung | ||||||
| Brown 2007 Stem Cells(69) | No | Not Investigated | Not Investigated | Neutral | 1 of 8 authors is “Johnson and Johnson funded”, however has a university affiliation. The authors disclosed “no conflicts of interest” | |
| Dagnon 2005 Clinical Cancer Research(79) | Yes | Not Investigated | Not Investigated | Harmful | None | |
| Dunlop 2007 Stem Cells(80) | Yes | Not Investigated | Not Investigated | Harmful | None | |
| Dunlop 2006 Neuro-degenerative Diseases(48) | Yes | Yes | Not Investigated | No Statement | None | |
| Kayser 1992 Zentralbl Pathol(81) | Yes | Not Investigated | Not Investigated | No Statement | None | |
| Saintigny 2007 Clinical Cancer Research(82) | Yes | Not Investigated | Not Investigated | Harmful | None | |
| Shannon 2005 British Journal of Cancer(54) | Yes | No | No | Beneficial | None | |
| Tumor Type: Prostate | ||||||
| Arcasoy 2005 Modern Pathology(21) | Yes | Not Investigated | Not Investigated | Harmful | None | |
| Feldman 2006 Prostate(49) | Yes | Yes | Yes | Harmful | None | |
| Zhou 2008 Prostate Cancer and Prostatic Disease(35) | Yes | Not Checked | Not Checked | Neutral | None | |
| Tumor Type: Cervical | ||||||
| Acs 2003 American Journal of Pathology(18) | Yes | Yes | Yes | Harmful | None | |
| Hamadmad 2008 Journal of Pharmacology and Experimental Therapeutics(39) | Not Investigated | Yes | Yes | Harmful | None | |
| Leo 2006 Clin Cancer Res(83) | Yes | Not Investigated | Not Investigated | Neutral | None | |
| Shenouda 2006 International Journal of Gynecologic Cancer(34) | Yes | Not Investigated | Not Investigated | No Statement | None | |
| Tumor Type: Endometrial | ||||||
| Acs 2004 Cancer (17) | Yes | Not Investigated | Not Investigated | Harmful | None | |
| Tumor Type: Ovarian | ||||||
| Hale 2006 Gynecologic Oncology(74) | Yes | Not Investigated | No | Beneficial | Research was supported by research grants from Ortho Biotech Clinical Affairs as well as Mary Kay Ash foundation | |
| Jeong 2008 International Journal of Cancer(59) | Yes | Yes | Yes | Harmful | None | |
| McBroom 2005 Gynecologic Oncology(27) | Yes | Not Investigated | Yes | Neutral | None | |
| Silver 1999 Gynecologic Oncology(73) | Not Investigated | Not Investigated | No | Beneficial | “Was supported by a research grant by orthobiotech” | |
| Solar 2008 International Journal of Cancer(62) | Yes | Yes | Yes | Harmful | None | |
| Tumor Type: Glioma | ||||||
| Mittelbronn 2007 Neuropathology and Applied Neurobiology(28) | Yes | Not Investigated | Not Investigated | No Statement | None | |
| Pinel 2004 International Journal of Radiation Oncology Biology, Physics(55) | Not Investigated | Not Investigated | No | Beneficial | None | |
| Stuben 2003 Strahlentherapie und Onkologie(63) | Not Investigated | Not Investigated | No | Beneficial | None | |
| Yin 2007 International Journal of Oncology(37) | Yes | Yes | Yes | Neutral | None | |
| Tumor Type: Head and Neck | ||||||
| Arcasoy 2005 Clinical Cancer Research(84) | Yes | Not Investigated | Not Investigated | Harmful | None | |
| Hoogsteen 2005 Radiotherapy Oncology(85) | Yes | Not Investigated | Not Investigated | No Statement | None | |
| Kjellen 2006 Acta Oto-Laryngologica(60) | Yes | Not Investigated | Yes | Harmful | None | |
| Lai 2005 Oncogene(38) | Yes | Yes | Yes | Harmful | None | |
| Winter 2005 Clinical Cancer Research(36) | Yes | Not Investigated | Not Investigated | No Statement | None | |
| Tumor Type: Neuroblastoma | ||||||
| Assandri 1999 Journal of Physiology(86) | Yes | Not Investigated | Not Investigated | No Statement | None | |
| Pregi 2006 Biochimica et Biophysica Acta Mol cell res(44) | Yes | Yes | Yes | No Statement | None | |
| Ribatti 2007 Histopathology(87) | Yes | Not Investigated | Not Investigated | No Statement | None | |
| Rossler 2004 Journal of Cellular Biochemistry(53) | Yes | Not Investigated | No | Neutral | None | |
| Sartelet 2007 Cancer(32) | Yes | Not Investigated | No | Beneficial | None | |
| Um 2007 Cellular Signaling(61) | Yes | Yes | Yes | No Statement | None | |
| Wollman 1996 Life Sciences(70) | Yes | Not Investigated | No | No Statement | Research support from Boheringer Mannhiem-Roche Germany | |
| Tumor Type: Renal | ||||||
| Carvalho 2005 Oncogene(40) | Yes | Yes | Not Investigated | Beneficial | None | |
| Gong 2006 Cancer Biology & Therapy(23) | Yes | Not Investigated | Not Investigated | No Statement | None | |
| Lee 2005 Clinical Cancer Research(25) | Yes | Not Investigated | Not Investigated | No Statement | None | |
| Li 2007 Cancer Biology & Therapy(26) | Yes | Yes | Yes | Harmful | None | |
| Liu 2004 Oncogene(46) | Yes | Yes | No | Neutral | None | |
| Westenfelder 2000 Kidney International(47) | Yes | Not Investigated | Yes | Harmful | None | |
| Tumor Type: Pancreatic | ||||||
| Bose 2008 Am J Physiol Cell Physiol(56) | Yes | Yes | Yes | No Statement | None | |
| Tumor Type: Melanoma | ||||||
| Kumar 2006 Melanoma Research(45) | Yes | Yes | Yes | Harmful | None | |
| Kumar 2005 American Journal of Pathology(24) | Yes | Yes | Yes | Harmful | None | |
| Selzer 2000 Melanoma Research(33) | Yes | Not Investigated | Not Investigated | No Statement | None | |
| Tumor Type: Mesothelioma | ||||||
| Palumbo 2008 Cancer Chemotherapeutics Pharmacology(50) | Yes | Yes | Yes | Neutral | None | |
| Study: First Author, Year of Publication, Journal | Tumor Type | Report Detection of EpoR | Report Epo-Induced Signaling Events | Report Epo- Induced Harmful Cellular Function Changes | Qualitative Conclusions | Description of Financial Conflict of Interest |
| Tumor Type: Multiple Types | ||||||
| Acs 2001 Cancer Research(9) | Breast, Lung, Cervical, Ovarian, Neuroblastoma, Glioma | Yes | Yes | Yes | Harmful | None |
| Arcasoy 2003 Biochemical and Biophysical Research Communications(88) | Prostate, Lung, Ovarian, Breast | Yes | Not Investigated | Not Investigated | No Statement | None |
| Batra 2003 Laboratory Investigation(22) | Neuroblastoma, Ewing’s Sarcoma, Breast, Gglimoa | Yes | Yes | Not Investigated | Harmful | None |
| Belenkov 2004 Molecular Cancer Therapeutics(41) | Cervical, Glioma | Yes | Yes | Yes | Harmful | None |
| Elliot 2006 Blood(65) | Breast, Renal, Neuroblastoma, Cervical | No | Not Investigated | Not Investigated | No Statement | The manuscript notes: “From Amgen and Whitehead Institute for Biomedical Research” 9 of 10 authors are employees of Amgen |
| Hardee 2005 British Journal of Cancer(58) | Breast, Head & Neck | Yes | Not Investigated | No | Neutral | None |
| Laugsch 2008 International Journal of Cancer(71) | Cervical, Neuroblastoma, Breast, Renal | No | No | No | No Statement | The last author discloses Honoraria and Consultancies to Amgen, Roche, Shire and Ortho Biotech. (On a2007 publication the same author also claims stock holdings for these companies) |
| Lonnroth 2008 Med Oncol(89) | Melanoma, Breast, Pancreatic | Yes | Not Investigated | Not Investigated | Beneficial | None |
| Mohyeldin 2007 Journal of Neurosurgery(29) | Prostate, Glimoa, Breast | Yes | Yes | Yes | Harmful | None |
| Mohyeldin 2005 Neoplasia(30) | Head & Neck, Prostate | Yes | Yes | Yes | Harmful | None |
| Sinclair 2008 British Journal of Cancer(66) | Prostate, Lung, Ovarian, Cervical, Melanoma, Glioma, Nerublastoma, Renal, Head & Neck, Breast | No | Not Investigated | Not Investigated | 9 of 11 authors are from Amgen | |
| Westphal 2002 Tumori(72) | Prostate, Renal, Neuroblastoma, Cervical, Breast, Melanoma, Pancreatic | Yes | No | No | Neutral | 1 of 8 authors is an employee of Roche. No disclosure as to any funding, company related or otherwise. |
| Yasuda 2001 British Journal of Cancer(90) | Ovarian, Endometrial, Cervical | Yes | Not Investigated | Yes | No Statement | None |
| Yasuda 2003 Carcinogenesis(51) | Prostate, Cervical, Lung, Melanoma, Glioma, Breast, Pancreatic | Yes | Yes | Yes | No Statement | None |
| Yasuda 2002 Carcinogenesis(91) | Ovarian, Endometrial, Cervical | Yes | Yes | Yes | No Statement | None |
No formatting: No authors presented at NIDDK or NIH Meeting Sept 2008
Bold: At least ONE author presented at NIDDK or NIH Meeting Sept 2008
White: Authors classified as academics with no conflicts of interest (n= 64)
Light Orange: Authors classified as academics with conflicts of interest (n= 7)
Dark Orange: Authors classified as employed by ESA manufacturers (n= 3)
Group II: Reports from industry-based investigative teams
Three studies from investigators employed by ESA manufacturers reported findings concerning presence of EpoR in tumor cells. One investigated erythropoietin-induced growth in tumor cells.(65–67) Elliot et al. investigated the specificity of commercially available antibodies used in EpoR studies. These antibodies identified proteins with weights larger than the expected molecular weight of EpoR. To validate this discrepancy, the authors performed protein sequence analysis on the proteins detected by the antibodies and found an abundance of non-EpoR proteins, including heat shock protein 70 (a protein commonly found in more aggressive tumors).(65) The M20 antibody was an exception to this non-specificity, and accurately detected EpoR in immunoblotting, but not in immunnohistochemical, studies. The authors conclude that caution should be used when interpreting results of studies using common antibodies. In a second study, Sinclair et al.(66) reported gene amplification of EpoR loci in solid tumor samples occurred at a frequency similar to that of non-oncogenes; EpoR transcript levels in tumors and tumor cell lines were low in comparison to bone marrow and equivalent to or lower than those reported for normal tissues of tumor origin and when EpoR mRNA was detected, it was not on the cell surface.(66) Investigators employed by another ESA manufacturer evaluated effects of erythropoietin administration alone or in combination with anticancer therapy on breast cancer cell lines.(67) Immunoblotting, flow cytometry, and immunohistochemistry evaluations identified cytosolic EpoR expression. Tumor growth assessments in breast cancer xenograft models found no evidence of migration, proliferation, or activation of mitogen-activated protein kinase and AKT following erythropoietin treatment and that treatment with erythropoietin alone or with paclitaxel resulted in equivalent tumor burdens compared with vehicle-based controls.
Group III: Reports from investigative teams comprised of academics where one or more co-authors disclosed funding support from ESA manufacturers
Seven studies were reported by academic investigators who had funding support from ESA manufacturers.(68–73) One study identified mRNA EpoR expression in neuroblastoma cell lines; erythropoietin administration did not induce tumor proliferation of the cell lines.(70) Using tissue microarrays, Brown et al. reported that preabsorption of C20 antibodies with synthetic heat shock protein peptides resulted in suppression of cytoplasmic staining in formalin fixed non-small cell lung cancer tissues.(69) Also, Western blots identified three components of which one was lost after C20 pre-absorption and one (the putative EpoR component) was retained. Laugsch et al. investigated by real-time RT-PCR, immunofluorescence microscopy, Western blotting, and cell growth analysis whether several human cancer cell lines possessed functional EpoRs.(71) These researchers detected EpoR mRNA in all cell lines, although neither hypoxia nor erythropoietin treatment altered EpoR mRNA expression. Four commercial antibodies cross-reacted with several proteins. Depending on the antibody used, EpoR was localized to the plasma membrane, the cytoplasm, or the nucleus. Experiments with small interfering RNA showed that EpoR protein was not expressed by tumor cells except for UT7/erythropoietin leukemia cells, which served as an EpoR positive control line, and by cells transfected with the human EpoR gene. The authors reported that erythropoietin increased signaling and proliferation in the UT7 control line and did not result in activation of signaling proteins or increased cell proliferation in tumor cells.(71) Westphal et al. reported EpoR and protein expression in various tumor cell lines using RT-PCR, Western blots, and immocytochemistry, although erythropoietin treatment did not result in increased proliferation of EpoR-positive tumor cell lines.(72) In aggregate, these findings were similar to findings from ESA manufacturers, raising concern over the significance of studies from investigators without pharmaceutical support who reported on EpoR expression in malignancies.
Three studies identified off-target effects that could improve tumor responsiveness to chemotherapy and radiation therapy- increased tumor oxygenation, cis-platinum sensitization, or angiogenesis inhibition following erythropoietin exposure.(68, 73, 74) Silver et al. demonstrated cis-platin sensitizing effects of erythropoietin on human ovarian cancer xenografts in mice.(73) Blackwell et al. found that hypoxic measurements were lower in non-anemic mice that received erythropoietin after tumor implantation versus mice with tumors that received erythropoietin before tumor implantation or that received placebo.(68) These findings suggested that erythropoietin administration might improve tumor oxygenation independent of hemoglobin effects. Hale et al. found that in SK-OV-3 ovarian cancer cell lines, erythropoietin-treatment decreased hypoxia-induced HIF-1-alpha protein levels and VEGF transcription, with no effect on cell growth and in MCF-7 breast cancer cell lines, erythropoietin inhibited HIF-1-alpha signaling.(74) These findings suggested that erythropoietin may have anti-angiogenic properties.
Groups I, II, and III: A Comparison
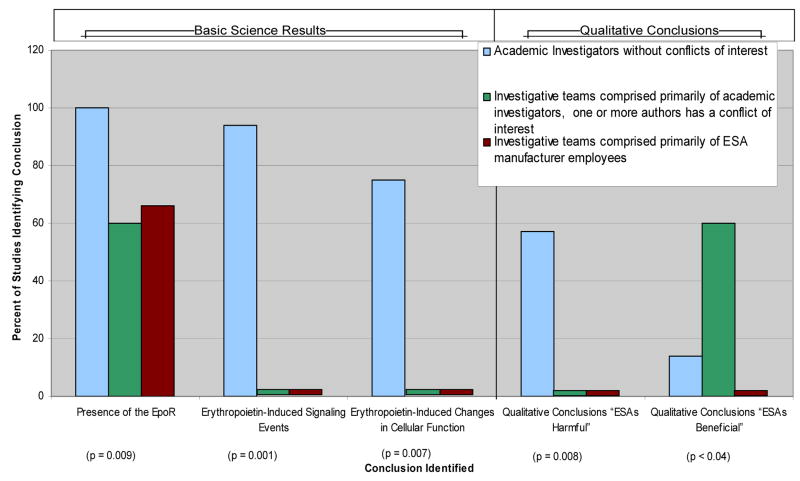
The findings differed according to whether the studies were reported by academic investigators who did not receive financial support from ESA manufacturers, academic investigators who received financial support from ESA manufacturers, and investigators employed by ESA manufacturers with respect to several fundamental issues: EpoR presence on solid tumor cells (100%, 60%, and 67%, respectively, (p<0.04); erythropoietin-induced signaling events (94%, 0%, and 0%, respectively), p=0.001); and erythropoietin-induced changes in cellular function (57%, 0%, and 0%, respectively; p=0.007) (Figure 2). Qualitative statements about clinical implications were included in 42 reports from investigators who did not have funding from ESA manufacturers, five reports from investigators who had received funding support from ESA manufacturers, and two reports from investigators who directed laboratories supported by ESA manufacturers. Among these studies, statements concluding that the investigations had identified potentially harmful effects of erythropoietin on cancer cells were included in 57% of reports from academic investigators who did not have funding from ESA manufacturers, 0% of the reports from academic investigators who had received financial support from ESA manufacturers, and 0% of the reports from investigators who directed laboratories supported by ESA manufacturers (p=0.008). In contrast, statements indicating that the findings identified potentially beneficial anti-tumor effects of ESAs were included in 14% of reports from academic investigators who did not have funding from ESA manufacturers, 0% of reports from investigators employed by ESA manufacturers, and 60% of reports from academic investigators who had received financial support from ESA manufacturers (p<0.04).
Figure 2.
Percentage of published studies identifying presence of EpoR, erythropoietin-induced changes in signaling, erythropoietin-induced changes in cellular function and qualitative conclusions by conflict of interest type.
NIH Conferences on EpoRs
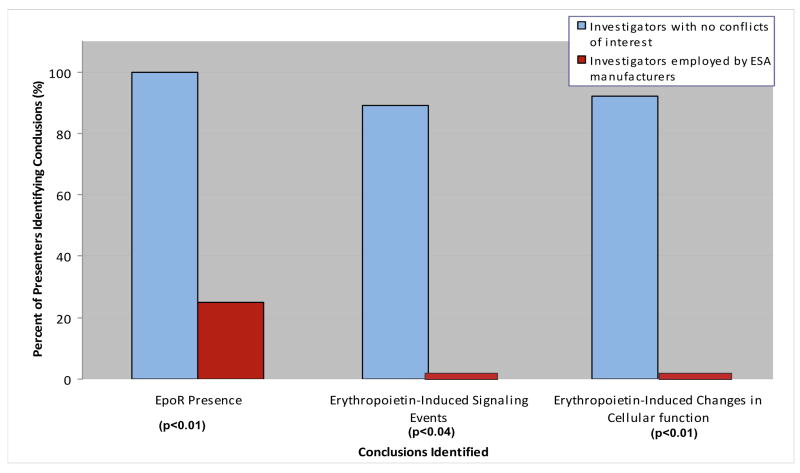
Workshops on EpoRs have been convened by the National Cancer Institute in 2007 and the National Institutes of Diabetes and Diseases of the Kidney (NIDDK) in 2008.(75, 76) Participants included 12 investigators from academic institutions who reported EpoR findings and four investigators employed by ESA manufacturers.(Figure 3). Academic investigators were co-authors for 27 of the 64 published manuscripts on EpoRs from academic investigators who did not have financial support from ESA manufacturers and one of the seven published manuscripts on EpoRs reported by academic investigators who had received financial support from ESA manufacturers. Investigators employed by ESA manufacturers were authors for all three published manuscripts on EpoRs conducted at the basic science laboratories of ESA manufacturers. Presentations reported on cell lines established from lung, head and neck, melanoma, ovarian, brain, cervical, and breast cancers, xenograft animal models, or in one case, findings from clinical specimens. These investigations identified EpoR mRNA and protein on various tumor cells and demonstrated that erythropoietin/EpoR signaling axis activation in cancer cells involved PI3K-Akt, JAK-STAT and NF-kB. Tumor cells could utilize the erythropoietin/EpoR-signaling axis in autocrine or paracrine fashion and recruitment of exogenous erythropoietin increased proliferation, anti-apoptosis, invasion, chemotherapy resistance, and angiogenesis. In contrast, investigators employed by ESA manufacturers reported that their investigations of tumor cell lines had identified a protein band corresponding to heat shock protein-70, and not EpoRs and that their investigations of animal models did not identify erythropoietin-induced angiogenesis or tumor promotion. Overall, in comparison to presentations from investigators employed by ESA manufacturers, presentations from academic investigators were more likely to report EpoRs on cancer cell lines (100% versus 25%; p<0.01), downstream effects of erythropoietin (88% versus 0%; p<0.04), and cell proliferation and migration effects following EpoR administration (91% versus 0%; p<0.01) (Table 2).
Figure 3.
Percentage of research presentations at National Cancer Institute and the National Institute of Diabetes and Digestive and Kidney Diseases national meetings identifying presence of EpoR, erythropoietin-induced changes in signaling, and erythropoietin-induced changes in cellular function by conflict of interest type.
Table 2.
Summary of presentations given at NCI and NIDDK* meetings regarding presence of erythropoietin receptors, erythropoietin-induced changes in signaling, erythropoietin-induced -induced changes in cellular function, qualitative conclusions and descriptions of conflicts of interest are summarized.
| Presenter | Report Detection of EpoR | Report Erythropoietin-Induced Signaling Events | Report Erythropoietin-Induced Harmful Cellular Function Changes | Presenter Affiliation | Participation at NIDDK or NCI Meeting* | Number of Papers Included in Review Co- Authored by Presenter** |
|---|---|---|---|---|---|---|
| Presenters with Academic Affiliations (n= 12) | ||||||
| Acs, G | Yes | Not Investigated | Yes | University of Pennsylvania | NCI | 10 |
| Arcasoy, MO | Yes | Yes | Yes | Duke University | NIDDK, NCI | 6 |
| Blau, CA | Yes | Not Investigated | Yes | University of Washington | NIDDK | 0 |
| Gewirtz, D | Not Investigated | No | No | Virginia Commonwealth University | NCI | 0 |
| Hardee, ME | Not Investigated | Yes | Yes | Memorial Sloan-Kettering Cancer Center | NCI | 3 |
| Henke, M | Yes | Not Investigated | Yes | University of Freiburg | NCI | 0 |
| Jeong, JY | Yes | Not Investigated | Yes | Harvard University | NIDDK | 2 |
| Lai, SY | Yes | Yes | Yes | University of Pittsburgh | NCI | 2 |
| Lappin, TRJ | Not Investigated | Yes | Yes | Queen’s University Belfast | NIDDK | 3 |
| Lodish, HF | Yes | Yes | Not Investigated | Massachusetts Institute of Technology | NIDDK | 1 |
| Sytkowski, AJ | Yes | Yes | Yes | Harvard University | NIDDK | 3 |
| Xu, X | Yes | Yes | Yes | University of Pennsylvania | NIDDK, NCI | 4 |
| Presenters with ESA Manufacturer Affiliation (n= 4) | ||||||
| Begley, CG | No | No | No | AMGEN | NIDDK, NCI | 1 |
| Elliot, S | No | No | No | AMGEN | NIDDK | 2 |
| Farrell, FX | Yes | No | No | Centocor | NCI | 2 |
| Sinclair, AM | No | Not Investigated | No | AMGEN | NIDDK | 2 |
NIDDK refers to the Workshop on Erythropoietin Receptor (Epo-R) Expression and Function in Non-Hematopoietic Tissues hosted by the National Institute of Diabetes and Digestive and Kidney Diseases in Bethesda, MD (Sep 8–9, 2008). NCI refers to Erythropoietic Stimulating Agents and Tumor Growth Workshop hosted by National Cancer Institute in Rockville, MD (Dec 18–19, 2007).
Based on the 74 articles on EpoRs included in Table 1A.
Discussion
The difference in findings for EpoR investigations reported by investigators with and without financial support from ESA manufacturers provides empirical evidence that conflicts of interest exist in the basic science setting that impact outcomes. This observation is unexpected as it runs counter to the popular belief that the scientific process is reproducible and protects against variable outcomes of laboratory studies. In interpreting our findings, several factors should be considered.
Three areas of disagreement for studies of EpoR have been debated. At the level of protein detection, investigators employed by one ESA manufacturer noted that the C20 antibody, commonly used in academic investigators’ studies, identified bands representing proteins between 64 and 68 kiloDaltons in size. Academic investigators reported that degradation of these EpoRs could result in identification of a 59 kiloDalton EpoR band (the size of the band noted in reports from Elliot et al.). Academic investigators noted that one report published by the ESA manufacturers of western blot analyses with a less commonly evaluated antibody (M-20) confirmed the presence of EpoRs in breast, cervical and brain tumor cells. Also, several studies reported by academic investigators reported functional EpoRs on cancer cells with methods that did not involve antibodies. A second difference centers around the level of changes in cellular function of erythropoietin exposed cancer cells that should be considered biologically significant. Investigators employed by ESA manufacturers operationally defined a 2-fold difference in cellular function between tumor cells and control cells to be biologically significant. Conversely, academic investigators did not establish a threshold level, positing that reproducible, statistically significant differences could have clinical implications. A third issue concerned the choice of positive and negative controls. Investigators employed by ESA manufacturers considered the carrier protein included in proprietary formulations of ESAs to be the appropriate negative control. Academic investigators noted that while they did not use specific proprietary compounds as controls, adequate negative controls, such as the addition of soluble EpoR to the medium, had been employed in their studies.
Recommendations for adjudication of the methodological differences vary. An investigator employed by an ESA manufacturer suggested that academic and ESA manufacturers should develop a consensus statement on the types of positive and negative controls and characteristics of reagents used in studies of erythropoietin and EpoRs.(77) Academic researchers countered that the peer-review system is the usual venue for quality control, and adopting a single set of study conditions might result in laboratory costs too expensive for academic laboratories. ESA manufacturers offered to provide $5 million to the NIH Foundation to support a Request for Application for basic science studies of EpoRs in tumor cells. The Board of Scientific Advisors of the NIH raised ethical concerns over accepting these funds.
While studies have identified conflict of interest concerns with clinical studies evaluating efficacy, safety, and cost-effectiveness of pharmaceuticals, conflicts in basic science studies may be more worrisome. Basic science manuscripts from investigators who have received financial support from pharmaceutical manufacturers undergo corporate review to maximally protect disclosure of proprietary information. Inclusion of proprietary information might be dis-allowed by the industry sponsor. Also, unlike in clinical research, in basic science research, some manuscripts are more commonly authored solely by investigators employed by pharmaceutical manufacturers.(5) Moreover, the pharmaceutical manufacturers, as sponsors, often decide which investigations may be submitted for peer-review and often influence the decision on the targeted journal for publication.
We conclude that financial conflict of interest considerations impact the interpretation of basic science studies in presentation and publications. As many universities have established basic science research partnerships with pharmaceutical manufacturers, transparency in these collaborations is paramount to allow for continued free exchange of scientific knowledge.
Acknowledgments
The authors have no conflicts of interest to report. Dr. Charles L Bennett had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. This paper was supported in part from a grant from the US National Cancer Institute (1 R01 CA125077-01A1 (CLB)).
Footnotes
A portion of this paper was presented orally at the annual conference of the American Society of Hematology in San Francisco in December 2008.
Conflict of Interest and Author Contribution Statements:
Charles L. Bennett MD PhD MPP: I declare that I participated in the planning, writing, and literature review for this paper and that I have seen and approved the final version. I have no conflicts of interests to disclose.
Simone N. Boyle BA: I declare that I participated in the planning, writing, and literature review for this paper and that I have seen and approved the final version. I have no conflicts of interests to disclose.
Adam Kuykendal MD: I declare that I participated in the writing and literature review for this paper and that I have seen and approved the final version. I have no conflicts of interests to disclose.
Matthew J. Fisher BA: I declare that I participated in the writing and literature review for this paper and that I have seen and approved the final version. I have no conflicts of interests to disclose.
Athena T. Samaras BA: I declare that I participated in the writing and literature review for this paper and that I have seen and approved the final version. I have no conflicts of interests to disclose.
Sara E. Barnato MD: I declare that I participated in the writing and literature review for this paper and that I have seen and approved the final version. I have no conflicts of interests to disclose.
Robin L. Wagner BS: I declare that I participated in the literature review for this paper and that I have seen and approved the final version. I have no conflicts of interests to disclose.
Carolyn E. Goldstein BA: I declare that I participated in the literature review for this paper and that I have seen and approved the final version. I have no conflicts of interests to disclose.
Jacob Tallman: I declare that I participated in the literature review for this paper and that I have seen and approved the final version. I have no conflicts of interests to disclose.
Hidayatullah G. Munshi MD: I declare that I participated in the planning, writing, and literature review for this paper and that I have seen and approved the final version. I have no conflicts of interests to disclose.
Stephen Y. Lai MD PhD: I declare that I participated in the planning, writing, and literature review for this paper and that I have seen and approved the final version. I have no conflicts of interests to disclose.
Michael Henke MD: I declare that I participated in the planning and writing for this paper and that I have seen and approved the final version. I have no conflicts of interests to disclose.
References
- 1.Kaiser J. Conflicts of interest. Cardiologists come under the glare of a Senate inquiry. Science. 2008;322(5901):513. doi: 10.1126/science.322.5901.513. [DOI] [PubMed] [Google Scholar]
- 2.Lurie P, Almeida CM, Stine N, Stine AR, Wolfe SM. Financial conflict of interest disclosure and voting patterns at Food and Drug Administration Drug Advisory Committee meetings. Jama. 2006;295(16):1921–8. doi: 10.1001/jama.295.16.1921. [DOI] [PubMed] [Google Scholar]
- 3.Stelfox HT, Chua G, O’Rourke K, Detsky AS. Conflict of interest in the debate over calcium-channel antagonists. N Engl J Med. 1998;338(2):101–6. doi: 10.1056/NEJM199801083380206. [DOI] [PubMed] [Google Scholar]
- 4.Friedberg M, Saffran B, Stinson TJ, Nelson W, Bennett CL. Evaluation of conflict of interest in economic analyses of new drugs used in oncology. Jama. 1999;282(15):1453–7. doi: 10.1001/jama.282.15.1453. [DOI] [PubMed] [Google Scholar]
- 5.Booth CM, Cescon DW, Wang L, Tannock IF, Krzyzanowska MK. Evolution of the Randomized Controlled Trial in Oncology Over Three Decades. J Clin Oncol. 2008;26(33):5458. doi: 10.1200/JCO.2008.16.5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blumenthal D. Academic-industrial relationships in the life sciences. N Engl J Med. 2003;349(25):2452–9. doi: 10.1056/NEJMhpr035460. [DOI] [PubMed] [Google Scholar]
- 7.Blumenthal D, Campbell EG, Causino N, Louis KS. Participation of life-science faculty in research relationships with industry. N Engl J Med. 1996;335(23):1734–9. doi: 10.1056/NEJM199612053352305. [DOI] [PubMed] [Google Scholar]
- 8.Bekelman JE, Li Y, Gross CP. Scope and impact of financial conflicts of interest in biomedical research: a systematic review. Jama. 2003;289(4):454–65. doi: 10.1001/jama.289.4.454. [DOI] [PubMed] [Google Scholar]
- 9.Acs G, Acs P, Beckwith SM, Pitts RL, Clements E, Wong K, et al. Erythropoietin and erythropoietin receptor expression in human cancer. Cancer Res. 2001;61(9):3561–5. [PubMed] [Google Scholar]
- 10.Hardee ME, Rabbani ZN, Arcasoy MO, Kirkpatrick JP, Vujaskovic Z, Dewhirst MW, et al. Erythropoietin inhibits apoptosis in breast cancer cells via an Akt-dependent pathway without modulating in vivo chemosensitivity. Mol Cancer Ther. 2006;5(2):356–61. doi: 10.1158/1535-7163.MCT-05-0196. [DOI] [PubMed] [Google Scholar]
- 11.Henke M, Laszig R, Rube C, Schafer U, Haase KD, Schilcher B, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet. 2003;362(9392):1255–60. doi: 10.1016/S0140-6736(03)14567-9. [DOI] [PubMed] [Google Scholar]
- 12.Leyland-Jones B, Semiglazov V, Pawlicki M, Pienkowski T, Tjulandin S, Manikhas G, et al. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: a survival study. J Clin Oncol. 2005;23(25):5960–72. doi: 10.1200/JCO.2005.06.150. [DOI] [PubMed] [Google Scholar]
- 13.Henke M, Mattern D, Pepe M, Bezay C, Weissenberger C, Werner M, et al. Do erythropoietin receptors on cancer cells explain unexpected clinical findings? J Clin Oncol. 2006;24(29):4708–13. doi: 10.1200/JCO.2006.06.2737. Erratum in: J Clin Oncol. 2007 Apr 10;25(11):1457. [DOI] [PubMed] [Google Scholar]
- 14.Bennett CL, Silver SM, Djulbegovic B, Samaras AT, Blau CA, Gleason KJ, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA. 2008;299(8):914–24. doi: 10.1001/jama.299.8.914. [DOI] [PubMed] [Google Scholar]
- 15.FDA Complete Response and Labeling Change Order, Epogen. July 30, 2008. Vol. 2008.
- 16.FDA Complete Response and Labeling Change Order, Aranesp. July 31, 2008. Vol. 2008.
- 17.Acs G, Xu X, Chu C, Acs P, Verma A. Prognostic significance of erythropoietin expression in human endometrial carcinoma. Cancer. 2004;100(11):2376–86. doi: 10.1002/cncr.20244. [DOI] [PubMed] [Google Scholar]
- 18.Acs G, Zhang PJ, McGrath CM, Acs P, McBroom J, Mohyeldin A, et al. Hypoxia-inducible erythropoietin signaling in squamous dysplasia and squamous cell carcinoma of the uterine cervix and its potential role in cervical carcinogenesis and tumor progression. Am J Pathol. 2003;162(6):1789–806. doi: 10.1016/S0002-9440(10)64314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Acs G, Zhang PJ, Rebbeck TR, Acs P, Verma A. Immunohistochemical expression of erythropoietin and erythropoietin receptor in breast carcinoma. Cancer. 2002;95(5):969–81. doi: 10.1002/cncr.10787. [DOI] [PubMed] [Google Scholar]
- 20.Arcasoy MO, Amin K, Karayal AF, Chou SC, Raleigh JA, Varia MA, et al. Functional significance of erythropoietin receptor expression in breast cancer. Lab Invest. 2002;82(7):911–8. doi: 10.1097/01.lab.0000020415.72863.40. [DOI] [PubMed] [Google Scholar]
- 21.Arcasoy MO, Amin K, Vollmer RT, Jiang X, Demark-Wahnefried W, Haroon ZA. Erythropoietin and erythropoietin receptor expression in human prostate cancer. Mod Pathol. 2005;18(3):421–30. doi: 10.1038/modpathol.3800288. [DOI] [PubMed] [Google Scholar]
- 22.Batra S, Perelman N, Luck LR, Shimada H, Malik P. Pediatric tumor cells express erythropoietin and a functional erythropoietin receptor that promotes angiogenesis and tumor cell survival. Lab Invest. 2003;83(10):1477–87. doi: 10.1097/01.lab.0000090156.94795.48. [DOI] [PubMed] [Google Scholar]
- 23.Gong K, Zhang N, Zhang Z, Na Y. Coexpression of erythopoietin and erythopoietin receptor in sporadic clear cell renal cell carcinoma. Cancer Biol Ther. 2006;5(6):582–5. doi: 10.4161/cbt.5.6.2709. [DOI] [PubMed] [Google Scholar]
- 24.Kumar SM, Acs G, Fang D, Herlyn M, Elder DE, Xu X. Functional erythropoietin autocrine loop in melanoma. Am J Pathol. 2005;166(3):823–30. doi: 10.1016/S0002-9440(10)62303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee YS, Vortmeyer AO, Lubensky IA, Vogel TW, Ikejiri B, Ferlicot S, et al. Coexpression of erythropoietin and erythropoietin receptor in von Hippel-Lindau disease-associated renal cysts and renal cell carcinoma. Clin Cancer Res. 2005;11(3):1059–64. [PubMed] [Google Scholar]
- 26.Li J, Vesey DA, Johnson DW, Gobe G. Erythropoietin reduces cisplatin-induced apoptosis in renal carcinoma cells via a PKC dependent pathway. Cancer Biol Ther. 2007;6(12):1944–50. doi: 10.4161/cbt.6.12.4975. [DOI] [PubMed] [Google Scholar]
- 27.McBroom JW, Acs G, Rose GS, Krivak TC, Mohyeldin A, Verma A. Erythropoietin receptor function and expression in epithelial ovarian carcinoma. Gynecol Oncol. 2005;99(3):571–7. doi: 10.1016/j.ygyno.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 28.Mittelbronn M, Capper D, Bunz B, Dietz K, Goeppert B, Ajaaj R, et al. De novo erythropoietin receptor (EPO-R) expression in human neoplastic glial cells decreases with grade of malignancy but is favourably associated with patient survival. Neuropathol Appl Neurobiol. 2007;33(3):299–307. doi: 10.1111/j.1365-2990.2006.00820.x. [DOI] [PubMed] [Google Scholar]
- 29.Mohyeldin A, Dalgard CL, Lu H, McFate T, Tait AS, Patel VC, et al. Survival and invasiveness of astrocytomas promoted by erythropoietin. J Neurosurg. 2007;106(2):338–50. doi: 10.3171/jns.2007.106.2.338. [DOI] [PubMed] [Google Scholar]
- 30.Mohyeldin A, Lu H, Dalgard C, Lai SY, Cohen N, Acs G, et al. Erythropoietin signaling promotes invasiveness of human head and neck squamous cell carcinoma. Neoplasia. 2005;7(5):537–43. doi: 10.1593/neo.04685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pelekanou V, Kampa M, Kafousi M, Dambaki K, Darivianaki K, Vrekoussis T, et al. Erythropoietin and its receptor in breast cancer: correlation with steroid receptors and outcome. Cancer Epidemiol Biomarkers Prev. 2007;16(10):2016–23. doi: 10.1158/1055-9965.EPI-06-1023. [DOI] [PubMed] [Google Scholar]
- 32.Sartelet H, Fabre M, Castaing M, Bosq J, Racu I, Lagonotte E, et al. Expression of erythropoietin and its receptor in neuroblastomas. Cancer. 2007;110(5):1096–106. doi: 10.1002/cncr.22879. [DOI] [PubMed] [Google Scholar]
- 33.Selzer E, Wacheck V, Kodym R, Schlagbauer-Wadl H, Schlegel W, Pehamberger H, et al. Erythropoietin receptor expression in human melanoma cells. Melanoma Res. 2000;10(5):421–6. doi: 10.1097/00008390-200010000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Shenouda G, Mehio A, Souhami L, Duclos M, Portelance L, Belenkov A, et al. Erythropoietin receptor expression in biopsy specimens from patients with uterine cervix squamous cell carcinoma. Int J Gynecol Cancer. 2006;16(2):752–6. doi: 10.1111/j.1525-1438.2006.00357.x. [DOI] [PubMed] [Google Scholar]
- 35.Zhou T, Xu C, He M, Sun Y. Upregulation of erythropoietin receptor in human prostate carcinoma and high-grade prostatic intraepithelial neoplasia. Prostate Cancer Prostatic Dis. 2008;11(2):143–7. doi: 10.1038/sj.pcan.4500995. [DOI] [PubMed] [Google Scholar]
- 36.Winter SC, Shah KA, Campo L, Turley H, Leek R, Corbridge RJ, et al. Relation of erythropoietin and erythropoietin receptor expression to hypoxia and anemia in head and neck squamous cell carcinoma. Clin Cancer Res. 2005;11(21):7614–20. doi: 10.1158/1078-0432.CCR-05-1097. [DOI] [PubMed] [Google Scholar]
- 37.Yin D, Kawabata H, Tcherniamtchouk O, Huynh T, Black KL, Koeffler HP. Glioblastoma multiforme cells: expression of erythropoietin receptor and response to erythropoietin. Int J Oncol. 2007;31(5):1193–8. [PubMed] [Google Scholar]
- 38.Lai SY, Childs EE, Xi S, Coppelli FM, Gooding WE, Wells A, et al. Erythropoietin-mediated activation of JAK-STAT signaling contributes to cellular invasion in head and neck squamous cell carcinoma. Oncogene. 2005;24(27):4442–9. doi: 10.1038/sj.onc.1208635. [DOI] [PubMed] [Google Scholar]
- 39.Hamadmad SN, Hohl RJ. Erythropoietin stimulates cancer cell migration and activates RhoA protein through a mitogen-activated protein kinase/extracellular signal-regulated kinase-dependent mechanism. J Pharmacol Exp Ther. 2008;324(3):1227–33. doi: 10.1124/jpet.107.129643. [DOI] [PubMed] [Google Scholar]
- 40.Carvalho G, Lefaucheur C, Cherbonnier C, Metivier D, Chapel A, Pallardy M, et al. Chemosensitization by erythropoietin through inhibition of the NF-kappaB rescue pathway. Oncogene. 2005;24(5):737–45. doi: 10.1038/sj.onc.1208205. [DOI] [PubMed] [Google Scholar]
- 41.Belenkov AI, Shenouda G, Rizhevskaya E, Cournoyer D, Belzile JP, Souhami L, et al. Erythropoietin induces cancer cell resistance to ionizing radiation and to cisplatin. Mol Cancer Ther. 2004;3(12):1525–32. [PubMed] [Google Scholar]
- 42.Lester RD, Jo M, Campana WM, Gonias SL. Erythropoietin promotes MCF-7 breast cancer cell migration by an ERK/mitogen-activated protein kinase-dependent pathway and is primarily responsible for the increase in migration observed in hypoxia. J Biol Chem. 2005;280(47):39273–7. doi: 10.1074/jbc.M509446200. [DOI] [PubMed] [Google Scholar]
- 43.Phillips TM, Kim K, Vlashi E, McBride WH, Pajonk F. Effects of recombinant erythropoietin on breast cancer-initiating cells. Neoplasia. 2007;9(12):1122–9. doi: 10.1593/neo.07694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pregi N, Vittori D, Perez G, Leiros CP, Nesse A. Effect of erythropoietin on staurosporine-induced apoptosis and differentiation of SH-SY5Y neuroblastoma cells. Biochim Biophys Acta. 2006;1763(2):238–46. doi: 10.1016/j.bbamcr.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 45.Kumar SM, Yu H, Fong D, Acs G, Xu X. Erythropoietin activates the phosphoinositide 3-kinase/Akt pathway in human melanoma cells. Melanoma Res. 2006;16(4):275–83. doi: 10.1097/01.cmr.0000222594.60611.c3. [DOI] [PubMed] [Google Scholar]
- 46.Liu WM, Powles T, Shamash J, Propper D, Oliver T, Joel S. Effect of haemopoietic growth factors on cancer cell lines and their role in chemosensitivity. Oncogene. 2004;23(4):981–90. doi: 10.1038/sj.onc.1207294. [DOI] [PubMed] [Google Scholar]
- 47.Westenfelder C, Baranowski RL. Erythropoietin stimulates proliferation of human renal carcinoma cells. Kidney Int. 2000;58(2):647–57. doi: 10.1046/j.1523-1755.2000.00211.x. [DOI] [PubMed] [Google Scholar]
- 48.Dunlop EA, Percy MJ, Boland MP, Maxwell AP, Lappin TR. Induction of signalling in non-erythroid cells by pharmacological levels of erythropoietin. Neurodegener Dis. 2006;3(1–2):94–100. doi: 10.1159/000092099. [DOI] [PubMed] [Google Scholar]
- 49.Feldman L, Wang Y, Rhim JS, Bhattacharya N, Loda M, Sytkowski AJ. Erythropoietin stimulates growth and STAT5 phosphorylation in human prostate epithelial and prostate cancer cells. Prostate. 2006;66(2):135–45. doi: 10.1002/pros.20310. [DOI] [PubMed] [Google Scholar]
- 50.Palumbo C, Battisti S, Carbone D, Albonici L, Alimandi M, Bei R, et al. Recombinant erythropoietin differently affects proliferation of mesothelioma cells but not sensitivity to cisplatin and pemetrexed. Cancer Chemother Pharmacol. 2008;61(5):893–901. doi: 10.1007/s00280-007-0608-3. [DOI] [PubMed] [Google Scholar]
- 51.Yasuda Y, Fujita Y, Matsuo T, Koinuma S, Hara S, Tazaki A, et al. Erythropoietin regulates tumour growth of human malignancies. Carcinogenesis. 2003;24(6):1021–9. doi: 10.1093/carcin/bgg060. [DOI] [PubMed] [Google Scholar]
- 52.Hardee ME, Cao Y, Fu P, Jiang X, Zhao Y, Rabbani ZN, et al. Erythropoietin blockade inhibits the induction of tumor angiogenesis and progression. PLoS ONE. 2007;2(6):e549. doi: 10.1371/journal.pone.0000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rossler J, Stolze I, Frede S, Freitag P, Schweigerer L, Havers W, et al. Hypoxia-induced erythropoietin expression in human neuroblastoma requires a methylation free HIF-1 binding site. J Cell Biochem. 2004;93(1):153–61. doi: 10.1002/jcb.20133. [DOI] [PubMed] [Google Scholar]
- 54.Shannon AM, Bouchier-Hayes DJ, Condron CM, Toomey D. Correction of anaemia through the use of darbepoetin alfa improves chemotherapeutic outcome in a murine model of Lewis lung carcinoma. Br J Cancer. 2005;93(2):224–32. doi: 10.1038/sj.bjc.6602685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pinel S, Barberi-Heyob M, Cohen-Jonathan E, Merlin JL, Delmas C, Plenat F, et al. Erythropoietin-induced reduction of hypoxia before and during fractionated irradiation contributes to improvement of radioresponse in human glioma xenografts. Int J Radiat Oncol Biol Phys. 2004;59(1):250–9. doi: 10.1016/j.ijrobp.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 56.Bose C, Udupa KB. Erythropoietin enhancement of rat pancreatic tumor cell proliferation requires the activation of ERK and JNK signals. Am J Physiol Cell Physiol. 2008;295(2):C394–405. doi: 10.1152/ajpcell.00423.2007. [DOI] [PubMed] [Google Scholar]
- 57.Gewirtz DA, Di X, Walker TD, Sawyer ST. Erythropoietin fails to interfere with the antiproliferative and cytotoxic effects of antitumor drugs. Clin Cancer Res. 2006;12(7 Pt 1):2232–8. doi: 10.1158/1078-0432.CCR-05-2287. [DOI] [PubMed] [Google Scholar]
- 58.Hardee ME, Kirkpatrick JP, Shan S, Snyder SA, Vujaskovic Z, Rabbani ZN, et al. Human recombinant erythropoietin (rEpo) has no effect on tumour growth or angiogenesis. Br J Cancer. 2005;93(12):1350–5. doi: 10.1038/sj.bjc.6602846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jeong JY, Feldman L, Solar P, Szenajch J, Sytkowski AJ. Characterization of erythropoietin receptor and erythropoietin expression and function in human ovarian cancer cells. Int J Cancer. 2008;122(2):274–80. doi: 10.1002/ijc.23068. [DOI] [PubMed] [Google Scholar]
- 60.Kjellen E, Sasaki Y, Kjellstrom J, Zackrisson B, Wennerberg J. Recombinant erythropoietin beta enhances growth of xenografted human squamous cell carcinoma of the head and neck after surgical trauma. Acta Otolaryngol. 2006;126(5):545–7. doi: 10.1080/00016480500437427. [DOI] [PubMed] [Google Scholar]
- 61.Um M, Gross AW, Lodish HF. A “classical” homodimeric erythropoietin receptor is essential for the antiapoptotic effects of erythropoietin on differentiated neuroblastoma SH-SY5Y and pheochromocytoma PC-12 cells. Cell Signal. 2007;19(3):634–45. doi: 10.1016/j.cellsig.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 62.Solar P, Feldman L, Jeong JY, Busingye JR, Sytkowski AJ. Erythropoietin treatment of human ovarian cancer cells results in enhanced signaling and a paclitaxel-resistant phenotype. Int J Cancer. 2008;122(2):281–8. doi: 10.1002/ijc.23071. [DOI] [PubMed] [Google Scholar]
- 63.Stuben G, Thews O, Pottgen C, Knuhmann K, Sack H, Stuschke M, et al. Impact of anemia prevention by recombinant human erythropoietin on the sensitivity of xenografted glioblastomas to fractionated irradiation. Strahlenther Onkol. 2003;179(9):620–5. doi: 10.1007/s00066-003-1110-4. [DOI] [PubMed] [Google Scholar]
- 64.Acs G, Chen M, Xu X, Acs P, Verma A, Koch CJ. Autocrine erythropoietin signaling inhibits hypoxia-induced apoptosis in human breast carcinoma cells. Cancer Lett. 2004;214(2):243–51. doi: 10.1016/j.canlet.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 65.Elliott S, Busse L, Bass MB, Lu H, Sarosi I, Sinclair AM, et al. Anti-Epo receptor antibodies do not predict Epo receptor expression. Blood. 2006;107(5):1892–5. doi: 10.1182/blood-2005-10-4066. [DOI] [PubMed] [Google Scholar]
- 66.Sinclair AM, Rogers N, Busse L, Archibeque I, Brown W, Kassner PD, et al. Erythropoietin receptor transcription is neither elevated nor predictive of surface expression in human tumour cells. Br J Cancer. 2008;98(6):1059–67. doi: 10.1038/sj.bjc.6604220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.LaMontagne KR, Butler J, Marshall DJ, Tullai J, Gechtman Z, Hall C, et al. Recombinant epoetins do not stimulate tumor growth in erythropoietin receptor-positive breast carcinoma models. Mol Cancer Ther. 2006;5(2):347–55. doi: 10.1158/1535-7163.MCT-05-0203. [DOI] [PubMed] [Google Scholar]
- 68.Blackwell KL, Kirkpatrick JP, Snyder SA, Broadwater G, Farrell F, Jolliffe L, et al. Human recombinant erythropoietin significantly improves tumor oxygenation independent of its effects on hemoglobin. Cancer Res. 2003;63(19):6162–5. [PubMed] [Google Scholar]
- 69.Brown WM, Maxwell P, Graham AN, Yakkundi A, Dunlop EA, Shi Z, et al. Erythropoietin receptor expression in non-small cell lung carcinoma: a question of antibody specificity. Stem Cells. 2007;25(3):718–22. doi: 10.1634/stemcells.2006-0687. [DOI] [PubMed] [Google Scholar]
- 70.Wollman Y, Westphal G, Blum M, Simantov R, Blumberg S, Peer G, et al. The effect of human recombinant erythropoietin on the growth of a human neuroblastoma cell line. Life Sci. 1996;59(4):315–22. doi: 10.1016/0024-3205(96)00300-1. [DOI] [PubMed] [Google Scholar]
- 71.Laugsch M, Metzen E, Svensson T, Depping R, Jelkmann W. Lack of functional erythropoietin receptors of cancer cell lines. Int J Cancer. 2008;122(5):1005–11. doi: 10.1002/ijc.23201. [DOI] [PubMed] [Google Scholar]
- 72.Westphal G, Niederberger E, Blum C, Wollman Y, Knoch TA, Rebel W, et al. Erythropoietin and G-CSF receptors in human tumor cells: expression and aspects regarding functionality. Tumori. 2002;88(2):150–9. doi: 10.1177/030089160208800214. [DOI] [PubMed] [Google Scholar]
- 73.Silver DF, Piver MS. Effects of recombinant human erythropoietin on the antitumor effect of cisplatin in SCID mice bearing human ovarian cancer: A possible oxygen effect. Gynecol Oncol. 1999;73(2):280–4. doi: 10.1006/gyno.1999.5368. [DOI] [PubMed] [Google Scholar]
- 74.Hale SA, Wong C, Lounsbury KM. Erythropoietin disrupts hypoxia-inducible factor signaling in ovarian cancer cells. Gynecol Oncol. 2006;100(1):14–9. doi: 10.1016/j.ygyno.2005.08.056. [DOI] [PubMed] [Google Scholar]
- 75.Erythropoietic Stimulating Agents and Tumor Growth Workshop. Rockville, MD: 2007. [Google Scholar]
- 76.Willicombe M, Cunningham J. Nephrogenic Systemic Fibrosis: A Sufficient Reason to Avoid Gadolinium-Based Contrast in All Patients with Renal Impairment? Semin Dial. 2008 doi: 10.1111/j.1525-139X.2007.00400.x. [DOI] [PubMed] [Google Scholar]
- 77.Erythropoietic Stimulating Agents and Tumor Growth Workshop Transcript. The National Cancer Institute; Rockville, Maryland: 2008. [December 18–19, 2007]. [Google Scholar]
- 78.Wincewicz A, Sulkowska M, Koda M, Lesniewicz T, Kanczuga-Koda L, Sulkowski S. STAT3, HIF-1alpha, EPO and EPOR - signaling proteins in human primary ductal breast cancers. Folia Histochem Cytobiol. 2007;45(2):81–6. [PubMed] [Google Scholar]
- 79.Dagnon K, Pacary E, Commo F, Antoine M, Bernaudin M, Bernaudin JF, et al. Expression of erythropoietin and erythropoietin receptor in non-small cell lung carcinomas. Clin Cancer Res. 2005;11(3):993–9. [PubMed] [Google Scholar]
- 80.Dunlop EA, Maxwell AP, Lappin TR. Impaired downregulation following erythropoietin receptor activation in non-small cell lung carcinoma. Stem Cells. 2007;25(2):380–4. doi: 10.1634/stemcells.2006-0452. [DOI] [PubMed] [Google Scholar]
- 81.Kayser K, Gabius HJ. Analysis of expression of erythropoietin-binding sites in human lung carcinoma by the biotinylated ligand. Zentralbl Pathol. 1992;138(4):266–70. [PubMed] [Google Scholar]
- 82.Saintigny P, Besse B, Callard P, Vergnaud AC, Czernichow S, Colombat M, et al. Erythropoietin and erythropoietin receptor coexpression is associated with poor survival in stage I non-small cell lung cancer. Clin Cancer Res. 2007;13(16):4825–31. doi: 10.1158/1078-0432.CCR-06-3061. [DOI] [PubMed] [Google Scholar]
- 83.Leo C, Horn LC, Rauscher C, Hentschel B, Liebmann A, Hildebrandt G, et al. Expression of erythropoietin and erythropoietin receptor in cervical cancer and relationship to survival, hypoxia, and apoptosis. Clin Cancer Res. 2006;12(23):6894–900. doi: 10.1158/1078-0432.CCR-06-1285. [DOI] [PubMed] [Google Scholar]
- 84.Arcasoy MO, Amin K, Chou SC, Haroon ZA, Varia M, Raleigh JA. Erythropoietin and erythropoietin receptor expression in head and neck cancer: relationship to tumor hypoxia. Clin Cancer Res. 2005;11(1):20–7. [PubMed] [Google Scholar]
- 85.Hoogsteen IJ, Peeters WJ, Marres HA, Rijken PF, van den Hoogen FJ, van der Kogel AJ, et al. Erythropoietin receptor is not a surrogate marker for tumor hypoxia and does not correlate with survival in head and neck squamous cell carcinomas. Radiother Oncol. 2005;76(2):213–8. doi: 10.1016/j.radonc.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 86.Assandri R, Egger M, Gassmann M, Niggli E, Bauer C, Forster I, et al. Erythropoietin modulates intracellular calcium in a human neuroblastoma cell line. J Physiol. 1999;516 (Pt 2):343–52. doi: 10.1111/j.1469-7793.1999.0343v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ribatti D, Marzullo A, Gentile A, Longo V, Nico B, Vacca A, et al. Erythropoietin/erythropoietin-receptor system is involved in angiogenesis in human hepatocellular carcinoma. Histopathology. 2007;50(5):591–6. doi: 10.1111/j.1365-2559.2007.02654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arcasoy MO, Jiang X, Haroon ZA. Expression of erythropoietin receptor splice variants in human cancer. Biochem Biophys Res Commun. 2003;307(4):999–1007. doi: 10.1016/s0006-291x(03)01303-2. [DOI] [PubMed] [Google Scholar]
- 89.Lonnroth C, Svensson M, Wang W, Korner U, Daneryd P, Nilsson O, et al. Survival and erythropoietin receptor protein in tumours from patients randomly treated with rhEPO for palliative care. Med Oncol. 2008;25(1):22–9. doi: 10.1007/s12032-007-9001-7. [DOI] [PubMed] [Google Scholar]
- 90.Yasuda Y, Musha T, Tanaka H, Fujita Y, Fujita H, Utsumi H, et al. Inhibition of erythropoietin signalling destroys xenografts of ovarian and uterine cancers in nude mice. Br J Cancer. 2001;84(6):836–43. doi: 10.1054/bjoc.2000.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yasuda Y, Fujita Y, Masuda S, Musha T, Ueda K, Tanaka H, et al. Erythropoietin is involved in growth and angiogenesis in malignant tumours of female reproductive organs. Carcinogenesis. 2002;23(11):1797–805. doi: 10.1093/carcin/23.11.1797. [DOI] [PubMed] [Google Scholar]