Abstract
Leukocyte transendothelial migration (TEM; diapedesis) is a critical event in immune surveillance and inflammation. Most TEM occurs at endothelial cell borders (paracellular). However, there is indirect evidence to suggest that at the tight junctions of the blood-brain barrier (BBB), leukocytes migrate directly through the endothelial cell body (transcellular). Why leukocytes migrate through the endothelial cell body rather than the cell borders is unknown. To test the hypothesis that the tightness of endothelial cell junctions influences the pathway of diapedesis, we developed an in vitro model of the BBB that possessed ten-fold higher electrical resistance than standard culture conditions and strongly expressed the BBB tight junction proteins claudin-5 and claudin-3. We found that paracellular TEM was still the predominant pathway (≥98%) and TEM was dependent on PECAM-1 and CD99. We show that endothelial tight junctions expressing claudin-5 are dynamic and undergo rapid remodeling during TEM. Membrane from the endothelial lateral border recycling compartment (LBRC) is mobilized to the exact site of tight junction remodeling. This preserves the endothelial barrier by sealing the intercellular gaps with membrane and engaging the migrating leukocyte with unligated adhesion molecules (PECAM-1 and CD99) as it crosses the cell border. These findings provide new insights into leukocyte-endothelial interactions at the BBB and suggest that tight junctions are more dynamic than previously appreciated.
Keywords: Transendothelial migration, endothelial cell junctions, leukocyte, endothelium, Blood-brain barrier, PECAM-1, CD31, CD99
Introduction
The brain is under continuous immune surveillance by circulating leukocytes, which patrol this specialized organ to detect and eliminate potential infectious and damaging agents (1–3). When left unabated, however, leukocyte recruitment into the brain is a prominent pathologic feature in many neurological diseases, including cerebral infection (4, 5), stroke (6–9), trauma (10–12), multiple sclerosis (13, 14) and certain neurodegenerative disorders (15). These disorders cause serious long-term neurological deficits and significant morbidity and mortality worldwide. Homeostasis within the CNS is largely maintained by a unique microvasculature called the blood-brain barrier (BBB) (16). The BBB is formed by highly specialized endothelial cells (EC) that are interconnected by complex and continuous tight junctions. These promote normal brain physiological function by restricting the entry of ions, macromolecules and noxious blood borne agents (17). Astrocytes, which are in close apposition to the cerebral vasculature, are crucial inducers of the BBB phenotype and help facilitate tight junction protein expression and maintenance through contact-dependent mechanisms and by releasing soluble factors (18).
Leukocytes have been speculated to take the “path of least resistance” across the endothelium (19); therefore, the relative tightness of the endothelium may significantly influence the pathway of diapedesis (transendothelial migration, TEM). Leukocytes have been reported to cross endothelium in vitro and in vivo using paracellular and transcellular pathways (20–22). The mechanisms underlying the leukocyte’s decision to migrate through the endothelial cell body rather than the cell borders are unknown. Indirect evidence has suggested that at the BBB, leukocytes migrate transcellularly through the endothelial cell body (23, 24). We hypothesized that the tightness of endothelial junctions and the ability of leukocytes to breach them might dictate the pathway of TEM. Endothelial cells in vitro form monolayers of low resistance, and paracellular TEM predominates even under cytokine-activated conditions (25). However, if junctions are very tight, such as at the BBB, transcellular migration across the EC body at a thin point may be easier rather than having to disrupt and reform the complex three-dimensional interactions of the tight junctions.
Whether leukocytes primarily cross the BBB paracellularly at cell borders through tight junctions or directly through the EC body transcellularly to reach the brain remains unclear (26–28). Furthermore, if leukocytes migrate paracellularly, whether and how tight junctions are remodeled at the BBB is not known. This is a critical issue, as inflammation and BBB dysfunction are at the root of most CNS pathologies (29, 30). Understanding the molecular mechanisms governing the route of TEM in the CNS is fundamental to developing better reagents to modulate pathologic immune responses or enhance host protective mechanisms in neuroinflammatory diseases. To test the hypothesis that transcellular migration would be more common if endothelial cell junctions were tighter, we constructed a simplified and robust cell culture model of the human BBB by treating human umbilical vein endothelial cells (HUVEC) with astrocyte conditioned medium (ACM) supplemented with agents that raise intracellular cAMP. This culture system was sufficient to induce BBB characteristics significantly enhancing the barrier function to ions and small molecules and inducing the expression of BBB tight junction proteins. We report that paracellular diapedesis is the prevalent pathway utilized by monocytes and neutrophils at the BBB in vitro and that similar molecules and mechanisms regulate TEM under these conditions as in low resistance endothelium. More important, we report the novel finding that endothelial tight junctions are remarkably dynamic and undergo rapid remodeling to accommodate the passage of leukocytes across EC borders without perturbing the endothelial barrier. Junctional remodeling and barrier function preservation were facilitated by the recruitment of membrane from the lateral border recycling compartment (LBRC), a novel endothelial specific compartment containing PECAM-1 and CD99 that we have previously shown to be essential for TEM (25, 31). We conclude that rapid remodeling of tight junctions during paracellular TEM is a tightly regulated process and endothelial tight junctions are more dynamic than previously appreciated.
Materials and Methods
All experimental protocols and procedures involving human subjects and materials were approved by the Institutional Review Board of Northwestern University Feinberg School of Medicine (Chicago, IL).
Reagents
The mAbs hec1 (anti-VE-cadherin), hec7 (anti-PECAM-1), and hec2 (anti-CD99) were produced from hybridomas generated in the laboratory as previously described (32). P1.1 (non-blocking anti-PECAM mAb) was column purified on protein A-Sepharose (GE Healthcare) from ascites provided by P.J. Newman (Blood Center of Wisconsin, Milwaukee, WI) (33). Fab fragments of P1.1 were cut using papain (Thermo Fisher Scientific), followed by column purification on protein A-Sepharose. Purity of Fab fragments was confirmed by SDS-PAGE. Unlabeled and Dylight 549 conjugated goat-anti-mouse F(ab’)2 antibodies used for targeted recycling experiments of PECAM-1 were purchased from Jackson ImmunoResearch laboratories. Antibodies for immunofluorescence were conjugated to DyLight 488, DyLight 550 or Alexa Fluor 650 according to the manufacturers protocol (Thermo Fisher Scientific). Alexa Fluor 488 conjugated monoclonal antibodies against occludin and claudin-5 were purchased from Life Technologies (Invitrogen). Polyclonal antibody against claudin-3 was purchased from Life Technologies (Invitrogen). 8-(4-chlorophenylthio(CPT)-cAMP was purchased from Sigma Aldrich and phosphodiesterase inhibitor type IV RO-20–1724 was purchased from Tocris Bioscience.
Cells
HUVECs were isolated from fresh human umbilical cords as previously described (32). HUVEC for standard culture conditions were grown in M199 medium (Invitrogen) supplemented with 20% heat-inactivated normal human serum and 100U/ml penicillin-streptomycin at 37° C in a humidified atmosphere of 5% CO2. HUVECs at passage two were cultured on thick hydrated collagen type 1 (Vitrogen, Cohesion Technologies) gels set in 96 well plates coated with 5 µg/ml fibronectin. hCMEC/D3 brain endothelial cells were obtained as a gift from Dr. Babette Weksler (Weill Cornell Medical College). TY10 brain endothelial cells generated by Dr. Takashi Kanda (Yamaguchi University Graduate School of Medicine) were obtained as a gift from Dr. Richard Ransohoff (Cleveland Clinic Lerner Research Institute). Primary human astrocytes were purchased from ScienCell Research Laboratories. Peripheral blood mononuclear cells (PBMC) were isolated from healthy volunteers by density gradient centrifugation in Ficoll-Pague (GE Healthcare) as previously described (34). Briefly, blood drawn from healthy volunteers was immediately mixed with 10 mmol/L final concentration of EDTA and an equal volume of HBSS (Mediatech Inc., Herndon, VA) and then layered over Ficoll-Paque density gradient medium (GE Healthcare Biosciences AB, Uppsala, Sweden). After centrifugation, the upper plasma layer was collected into fresh tubes and the PBMCs at the interface were collected into a separate tube, diluted with HBSS and centrifuged at 1500 RPM for 10 minutes. The cell pellet was resuspended in the spun plasma and centrifuged at 1200 RPM for 5 minutes. The resulting pellet was washed 2–3 times with HBSS containing 0.1% human serum albumin (HSA; Grifols Biologicals Inc., Los Angeles, CA) via resuspension and centrifugation. The final cell pellet was resuspended in M199 containing 0.1% HSA. Neutrophils were isolated and prepared from healthy volunteers as previously described (34).
Human BBB model culture conditions
Inducible HUVEC Astrocyte Conditioned Media BBB Model
Astrocytes were grown on Poly-D-lysine coated tissue culture dishes and used until passage 10. ACM was collected and treated as previously described (35–37). Briefly, astrocyte cultures were grown at 37°C in a humidified atmosphere of 5% CO2 and were fed with fresh astrocyte complete medium (ScienCell Research Laboratories) containing low concentration of fetal bovine serum (2%). After ~48h when confluent, the medium was collected, sterile filtered (0.2 µm), centrifuged and diluted 1:1 with HUVEC media and either used immediately or stored at −80°C. HUVEC used for the BBB model were cultured until confluence in ACM. Once confluent, ACM was then supplemented with 17.5 µM RO-20–1724 (Tocris Bioscience) and 250 µM 8-(4-chlorophenylthio(CPT)-cAMP (Sigma Aldrich) for 24h. After 24h the monolayers were extensively washed immediately prior to experimentation. In some experiments, TNF was added to some monolayers to activate endothelial cells. TNF treatment was 20 ng/ml for 4 h, except for Supplemental Fig. 2, where treatments are described in the figure legend.
hCMEC/D3 and TY10
hCMEC/D3 were cultured as previously described (38) at 37° C in a humidified atmosphere of 5% CO2. Briefly, hCMEC/D3 were grown in endothelial basal medium-2 (EBM®-2; Lonza) that was supplemented with basic-fibroblast growth factor (1 ng/ml), hydrocortisone (1.4 µM), ascorbic acid (5 µg/ml), chemically defined lipid concentrate (1/100 dilution), 5% FBS, and 1% Penicillin-Streptomycin. TY10 (39) were grown in EBM®-2 containing 20% heat inactivated FBS, 0.6% Antibiotic-Antimycotic solution (Sigma Aldrich), and supplemented with EGM®-2 SingleQuots® (except for GA1000) at 33° C. TY10 were grown at 33° C for the growth phase and are then transferred to 37° C for 1–2 days when confluent to arrest growth and induce differentiation.
Characterization of Barrier Function
Endothelial barrier function was measured by 2 assays: 1) recording transendothelial electrical resistance (TEER) and 2) monitoring permeability to FITC conjugated-dextran (20 kDa, Sigma Aldrich). Briefly, the tightness of cell junctions was quantitated by measuring the EC junctions’ ability to restrict paracellular flux of small ions via analysis of TEER. Endothelial cells were plated on 3 µm Transwell® filter inserts (Corning) that were pre-coated with collagen type I diluted 1:50 in 60% Ethanol and then overlaid with 5 µg/ml fibronectin. For each filter, the electrical resistance was measured using an electrical resistance system containing a current-passing and voltage-measuring electrode (World Precision Instruments, Inc, New Haven, CT). Resistances of blank filters were subtracted from those filters containing cells. Final resistance calculations were corrected for filter surface area.
For permeability assays, endothelial cells were cultured on hydrated fibrillar collagen gels as described above. Monolayers were washed with PBS and 100 µg/ml 20kDa FITC-Dextran was added to each well. Monolayers were then incubated at 37° C in 5% CO2 for 1h. After 1h, monolayers were then washed extensively and the relative fluorescence intensity of FITC-dextran that had passed across the monolayers into the collagen gels was measured by a FilterMax™ F5 microplate reader (Molecular Devices, LLC, Sunnyvale, California). Indicated results are the mean fluorescence intensity of at least 3 independent experiments with replicates of six for each condition. In some experiments measuring barrier function during TEM, monocytes were added (2×105 per well) at the same time as FITC-Dextran and permeability was measured as described.
Transendothelial Migration Assay
Quantitative endpoint TEM assays were performed as previously described (40). In brief, freshly isolated PBMC were resuspended in M199 medium containing 0.1% human serum albumin and added to monolayers at 2×105 cells/well and then warmed for 1h at 37° C in a CO2 incubator for TEM to take place. As previously published (34), only monocytes and not lymphocytes transmigrate within this time frame. In experiments determining the dependence of TEM on PECAM-1 or CD99, PBMCs (4 × 106 cell/mL) were mixed 1:1 with either non-blocking control mAb (hec1; anti-VE-cadherin) or blocking anti-PECAM-1 (hec7) or anti-CD99 (hec2) mAb to a final concentration of 20 µg/ml. Then 100 µl was added to the appropriate wells. At the end of the incubation, monolayers were washed twice with 1 mmol/L EDTA (Sigma Aldrich) in HBSS followed by 2–3 washes with PBS containing Ca+2 and Mg+2. The monolayers and leukocytes were then fixed in freshly prepared 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (Electron Microscopy Sciences) pH 7.4. Before quantitative analysis, the monolayers were stained with modified Wright-Geimsa (Protocol Hema3; Fisher Diagnostics, Middletown, VA) and mounted onto glass slides for visualization. Imaging was performed with a Zeiss Ultraphot microscope with Nomarski optics and a SPOT Insight Color CCD (Diagnostic Instruments, Inc., Sterling Heights, MI). TEM was analyzed by manually counting at least 100 cells per collagen gel and noting their position relative to the endothelial monolayer (above or below, defining above as any monocyte having most of the cell body on or above the focal plate of the endothelial cell nuclei). Cell adhesion was quantified as the mean of the number of leukocytes associated with each field.
Immunofluorescence microscopy
To visualize the distribution of adhesion molecules, adherens junctions and tight junctions and their behavior during leukocyte TEM, leukocytes were allowed to TEM for 10 min across BBB or standard control HUVEC monolayers. They were then rapidly washed and fixed in freshly prepared 4% paraformaldehyde on ice as previously described (25). Blocking buffer (PBS + 2% FBS, 2% FCS and 2% species specific normal serum) was added for 1h at room temperature. Specific antibodies for each adhesion molecule and junctional protein were directly conjugated to Alexa Fluor dyes and incubated with monolayers at final concentration of 20 µg/ml for 1h at room temperature. In experiments visualizing tight junction proteins, cells were permeabilized after fixation with 0.2% Triton X-100 (Sigma Aldrich) for 10 min and incubated with Alexa Fluor 488 labeled claudin-5. Images and stacks of optical sections were collected with a spinning-disk confocal microscope using an Ultraview VoX imaging system (Ultraview, Waltham, MA) equipped with a Yokogawa CSU-1 spinning disk (Yokogawa Electric Company, Tokyo, Japan). Images were acquired through a 40x oil immersion objective using Volocity software version 6.2.1 (Perkin Elmer, Waltham, MA) and subsequently processed and analyzed using ImageJ. The figures show representative optical sections through the region of interest or projections of the whole stack using the maximum intensity method.
Quantification of Transcellular and Paracellular Diapedesis
Monolayers were cultured as described above with or without TNF (20 ng/ml) activation for 4h before each experiment. For some experiments, 200 ng/ml CCL2 was added to the monolayers for 30 min and then washed extensively prior to experimentation as previously described (41). 2×105 monocytes in cold M199 containing 0.1% human serum albumin were added to each well and cells were allowed to settle for 15 minutes on ice at 4° C. Monolayers were then warmed for 10 min at 37° C in a CO2 incubator. Cells were rapidly washed with ice cold PBS and fixed in ice cold 4% paraformaldehyde, and then stained for VE-cadherin to label the cell junction and CD18 to label monocytes. Fixed samples were examined by spinning-disk confocal microscopy. Image stacks were obtained by spinning disk confocal microscopy and were analyzed for quantification of diapedesis. Monocytes crossing the endothelium at the cell junction with an intercellular gap in VE-cadherin were scored as paracellular, and those crossing through the endothelial cell body at a distance of 2 µm from the cell junction not associated with gap in VE-cadherin were scored as transcellular. Total monocytes associated with the endothelial cells in each field (i.e. monocytes on top and under endothelium plus those in the act of diapedesis) were counted for each experiment. Percent TEM was calculated by dividing the total number of cells in the act of diapedesis by the total number of monocytes in each field (25).
Targeted Recycling Experiments
Targeted recycling experiments were performed as previously described (25, 31) and performed with resting or TNF-activated monolayers. Briefly, endothelial cells plated on collagen gels were incubated with non-blocking Fab fragment P1.1 for 1h at 37° C to label total PECAM-1. Monolayers were subsequently washed, chilled and incubated with an excess of unlabeled goat-anti-mouse F(ab’)2 IgG for 1h on ice at 4° C to saturate primary antibody bound to surface PECAM-1. Cells were then washed and M199 containing Alexa Fluor 594 conjugated goat-anti-mouse F(ab’)2 IgG and 2×105 monocytes were added to the endothelial monolayer. Cells were kept on ice for 15 minutes to allow for leukocytes to settle and then warmed at 37° C for 10 min in a CO2 incubator. Monolayers were subsequently washed and fixed on ice in freshly prepared 4% paraformaldehyde for 10 minutes at room temperature. Images were obtained, processed and percent targeted recycling was quantified as previously described (25, 31).
Quantification of Adherens and Tight Junction Gaps During TEM
3D confocal imaging of leukocytes fixed in the act of TEM was performed as described above. Immunofluorescence staining was used to identify changes in junctional staining distribution of adherens junction VE-cadherin and tight junction claudin-5 with respect to monocytes stained with CD18. The number and location of monocytes extending pseudopods in the act of TEM associated with or without disruption in staining of VE-cadherin and/or claudin-5 was quantitated. A monocyte in the act of TEM was considered to be associated with an observed staining disruption if it was located at the cell junction and the gap in VE-cadherin or claudin-5 was at least 2 microns in width. In some experiments, transmigrating leukocytes associated with gap in VE-cadherin or claudin-5 and recycled LBRC were quantified. Data were normalized to the total number of monocytes per field.
Statistical Analysis
All experiments with quantification were performed independently at least 3 times with a minimum of 3 replicates for each sample within each experiment. For the TEM assay, the values for the replicates were averaged together within each experiment. The average and standard deviation of these averages are shown in the figures. Group differences were tested with one-way ANOVA (or two-way ANOVA when appropriate) followed by the Tukey multiple comparision post-hoc test to calculate P values and determine that the means are statistically and significantly different from each other. Differences between two groups were analyzed with non-parametric Mann-Whitney U-test. Statistical analyses were done using GraphPad Prism software (GraphPad, San Diego, CA).
Results
Establishment of a cell culture model of the human blood-brain barrier
To investigate the relationship between the tightness of EC junctions and the route of diapedesis in vitro, we sought to simulate the BBB by generating monolayers of tight endothelium with BBB features. Brain endothelial cells, irrespective of their species, lose many of their BBB properties in vitro (42, 43). Pharmacologically increasing levels of intracellular cAMP in combination with ACM enhances endothelial barrier function in vitro and induces expression of BBB markers in brain endothelial cells and non-cerebral peripheral vascular endothelial cells (35, 36, 44). Therefore, we attempted to establish a human BBB model by culturing HUVEC in ACM supplemented with the cAMP analog 8-(4-chlorophenylthio(CPT)-cAMP and phosphodiesterase inhibitor RO-20–1724, known agents that raise intracellular cAMP (35, 36). HUVEC grown under standard culture conditions in which they do not express organized tight junctions (45, 46) were used as a control for all experiments.
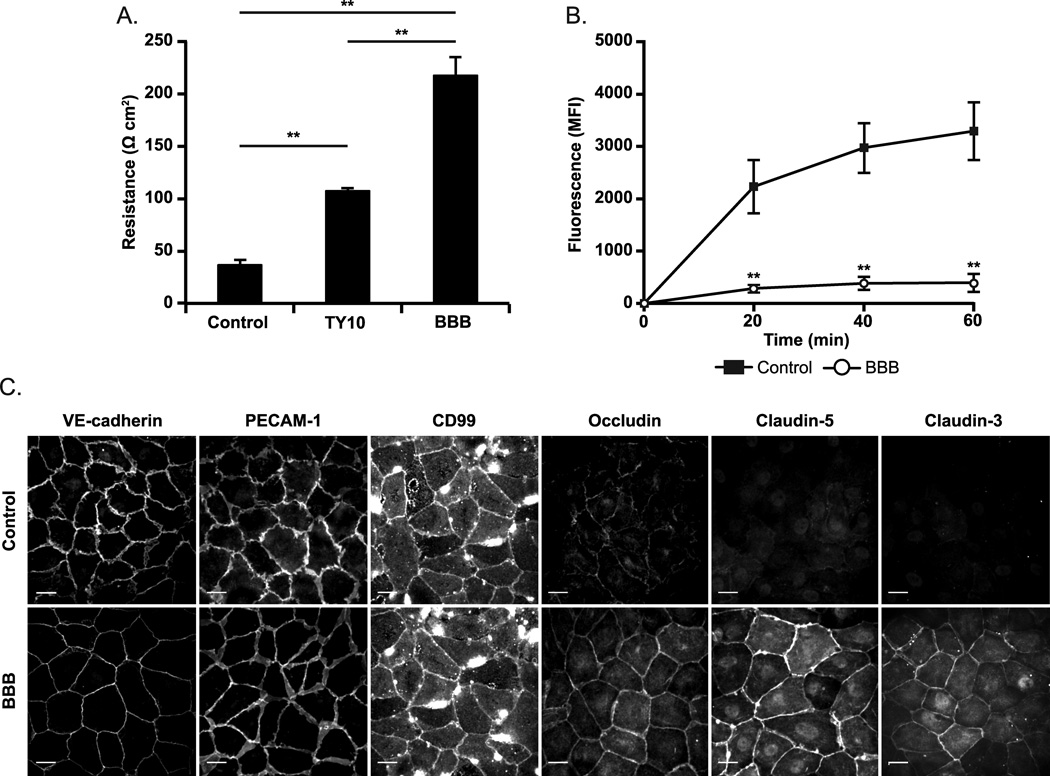
We first performed functional studies to assay the barrier function of our in vitro model by measuring TEER and permeability to 20 kDa FITC-dextran. As demonstrated in Figure 1A, HUVEC grown under BBB culture conditions displayed electrical resistances up to 250 ohm-cm2, nearly 10 times greater than standard culture controls (p < 0.0001). In fact, this TEER is as high as has been reported for human endothelial cells of any kind in vitro (47–49). In addition, we used two stable human BBB brain endothelial cell lines, TY10 and hCMEC/D3, grown in special medium that is reported to allow them to maintain tight junction characteristics and BBB properties (38, 39). Although these cell lines formed junctions that had higher electrical resistance than conventional endothelial cells (Figure 1A and hCMEC/D3 data not shown), neither were as high as our HUVEC-ACM model. Permeability studies supported our findings with TEER (Figure 1B). Our BBB culture system was 10 times more restrictive to permeability of 20 kDa FITC-dextran across its junctions than standard HUVEC controls. In addition, our HUVEC-ACM model behaves physiologically and can be disrupted by sufficient stimulation with TNF, a proinflammatory cytokine and inducer of vascular permeability that is commonly associated with neuroinflammation (Supplemental Figure 1).
Figure 1. Establishment of a cell culture based model of the human BBB.
HUVEC acquire BBB like properties when cultured with ACM and agents that raise intracellular cAMP. In this and subsequent figures, EC monolayers so treated are designated “BBB.” (A-B) Barrier function was assayed by measuring (A) transendothelial electrical resistance and (B) permeability to 20 kDa FITC-dextran. (C) Confluent BBB and control monolayers were stained for endothelial junction markers VE-cadherin, PECAM-1 and CD99 and for BBB associated tight junction proteins occludin and claudin-5. HUVEC cultured in ACM with cAMP modulators upregulate BBB associated tight junction proteins including occludin and claudin-5 compared to standard controls. All images taken using same exposure settings. Scale bar = 20 microns. Data represent the mean of at least three replicates from at least three independent experiments. Error bars represent the Standard Deviation. **p < 0.0001 (one-way ANOVA, followed by Tukey test).
We next examined the induction of the BBB phenotype by determining the expression and localization of BBB-associated tight junction proteins occludin, claudin-5, and claudin-3. In addition, we examined endothelial adhesion and signaling molecules known to be involved in TEM that are also expressed at brain EC junctions, including PECAM-1, CD99 and Vascular Endothelial (VE)-cadherin (50). As depicted in Figure 1C, our BBB model exhibits continuous staining of PECAM-1, CD99, and VE-cadherin at EC borders similar to standard HUVEC controls. However, expression of BBB associated tight junction proteins occludin, claudin-5, and claudin-3 are upregulated and display continuous junctional staining under our BBB culture conditions compared to control. These results taken together with our functional data confirm that it is possible to induce HUVEC with many of the essential features that characterize BBB vasculature by including normal inducers of BBB differentiation (i.e. ACM) and through elevation of intracellular levels of the secondary messenger cAMP. Since our culture conditions rendered our endothelial cells quantitatively (TEER, permeability) and qualitatively (induction of BBB-restricted claudin-5) similar to BBB endothelium, we will refer to these conditions as BBB hereafter, realizing that the BBB in vivo is more complex.
Rate and extent of TEM does not differ between the BBB model and HUVEC
We next compared the rate and extent of TEM between BBB and standard controls using a quantitative endpoint in vitro assay of TEM. This assay can distinguish adherent leukocytes bound to the apical surface of the endothelial monolayer from diapedesis of leukocytes across the endothelium and subsequent migration across the subendothelial basal lamina (34, 51). Quantitative TEM assays were conducted with monocytes from whole PBMC for 15, 30 and 60 minutes with resting and TNF-activated monolayers (Figure 2A). Note that the TNF treatment used in these experiments (20 ng/ml for 4h) activates endothelial cells to promote TEM, but does not drastically increase permeability (See supplemental Fig. 1). In addition, when using whole PBMC, we have previously reported (34, 51) that under these conditions only monocytes and not lymphocytes transmigrate efficiently within this time frame. To our surprise, neither the rate nor the extent of monocyte transmigration differed between BBB and control for both resting and cytokine activated conditions for all time points assessed. We also observed no differences in adhesion between BBB and control (Figure 2B). These observations demonstrate that BBB monolayers support TEM to the same extent as conventional endothelium. Thus, the ability of leukocytes to migrate across the endothelium is not affected by the tightness of the endothelial barrier under these conditions in vitro.
Figure 2. Rate and extent of TEM does not differ between BBB model and standard culture conditions.
Primary human monocytes were isolated from healthy donors and quantitative endpoint TEM assays (A-C) were conducted for 15, 30 and 60 minutes across unstimulated or TNF activated monolayers. (D-f) The number of monocytes that had adhered to the monolayer was scored for multiple high power fields in each monolayer. There was neither a defect in monocyte adhesion nor a difference in the rate and extent of TEM across the BBB model compared to standard HUVEC control for any of the indicated time points (one-way ANOVA followed by Tukey test). Data represent the mean and standard deviation of at least six replicates from three independent experiments.
Paracellular TEM is the predominant pathway across the BBB
Although there was no difference in the rate and extent of monocyte TEM across BBB and control HUVEC monolayers, this endpoint assay does not distinguish the pathway utilized to cross the monolayer (i.e. paracellular or transcellular). Therefore, the leukocytes may have exploited different pathways for TEM across the endothelium. To discern the pathway of TEM across BBB and standard monolayers, we performed 3D confocal imaging of monocytes fixed in the act of TEM. This short time point assay is designed to catch leukocytes in the act of TEM and effectively discern the route of TEM by acquiring confocal images through a series of Z-planes (25). Highly and/or directly activated leukocytes have been reported to have an increased propensity to migrate transcellularly (25, 41, 52) As such, resting and TNF-activated monolayers in addition to TNF-activated monolayers pre-incubated with apically placed CCL2 (monocyte chemotactic protein-1, MCP-1) were used for this assay. Apical application of CCL2 works by directly activating monocytes without providing a chemotactic gradient (25, 41). Monocytes were first allowed to settle on the monolayers for 15 minutes on ice before warming for 10 minutes at 37°C to initiate and synchronize TEM. Cells were then rapidly washed and fixed on ice. Cells were then immunostained with monoclonal antibodies (mAb) against VE-cadherin to label the EC junctions and CD18 to specifically label the leukocytes. Monocytes crossing at the EC border were scored as paracellular, while those crossing the monolayer greater than 2µm away from an intact cell junction were scored as transcellular. This is assay is distinct from Figure 2, in which cells were not allowed to settle before warming for TEM to proceed. Because TEM was stopped early, only 50% of the monocytes had transmigrated by that time (Figure 3A). There was no difference in total TEM across monolayers that received TNF, chemokine or untreated controls (Figure 3A). Contrary to our hypothesis, we observed ≥ 98% paracellular diapedesis across the BBB, the same extent as under standard culture conditions (Figure 3B). Monocytes were observed to transmigrate at both tricellular and bicellular junctions. However, 60% of monocytes were observed to transmigrate at tricellular junctions compared to 40% at bicellular junctions (Supplemental Figure 2). Similar to our previous reports in vitro (25), transcellular TEM was rarely observed under standard activation conditions. These results were also reproduced using human neutrophils (not shown). Furthermore, similar observations were made for TEM across TY10 human brain EC line (Figure 3) and hCMEC/D3 (not shown), suggesting that this is not a phenomenon limited to HUVEC. Directly activating the monocytes with apically placed CCL2 increased the proportion of transcellular TEM equally (up to 15% in some experiments) under both standard and BBB conditions (Figure 3C). Thus, the route of TEM is not altered by the tightness of endothelial junctions under these conditions in vitro, but can be influenced by the activation state of the leukocytes.
Figure 3. Paracellular TEM is the predominant pathway across the BBB in vitro.
Monocytes were allowed to transmigrate for 10 minutes at 37° C across resting, TNF activated, or monolayers pre-incubated for 30 minutes with apically placed CCL2 (200 ng/ml). (A) There was no significant difference in the extent of total TEM among the different treatment conditions. (B) The vast majority of TEM occurred via the paracellular pathway for all conditions tested. (C) The proportion of transcellular TEM could be increased only by direct activation of the monocyte with apically placed CCL-2. Here the data in (B) are normalized to the total TEM that took place. (D) Monocytes were allowed to transmigrate across TY10 monolayer as described in A. (E) Paracellular TEM predominated across TY10 monolayer just as observed in B. Data for BBB represent the mean of hundreds of TEM events recorded in 5 independent experiments. Data for TY10 represent the mean of hundreds of TEM events recorded in 3 independent experiments. Error bars represent standard deviation. A-C **p < 0.0001 (two-way ANOVA followed by Tukey test). D Mann-Whitney U-test. E one-way ANOVA followed by Tukey test.
TEM across the BBB requires PECAM-1 and CD99
PECAM-1 and CD99 have both been shown to be required for efficient monocyte and neutrophil diapedesis in peripheral tissues (40, 53–58), however their role in regulating diapedesis across tight endothelium and the BBB has not yet been assessed and may be different. To examine the role of PECAM-1 and CD99 for monocyte TEM at the BBB in vitro, we performed quantitative endpoint assays in the presence of blocking mAb against PECAM-1, CD99 or non-blocking control mAb against VE-cadherin. TEM was unaffected in control conditions and proceeded to a maximal 90%. However, disrupting PECAM-1 and CD99 function significantly blocked TEM down to 20% and 15% respectively (Figure 4A). None of the mAb treatments had a significant effect on the total number of monocytes able to adhere to the monolayers (not shown), suggesting that our observations are specifically due to the ability of anti-PECAM-1 and anti-CD99 mAb to block TEM. Moreover, these results were not restricted to our HUVEC-BBB model and were confirmed using the human BBB cell line TY10. Monocyte TEM across TY10 monolayers was reduced down to ~25% using either anti-PECAM-1 or anti-CD99 mAb (Figure 4B). Taken together, these data support a role for PECAM-1 and CD99 at the BBB for monocyte diapedesis.
Figure 4. TEM is dependent on PECAM-1 and CD99 across the BBB in vitro.
Transmigration across the human BBB model can be blocked by anti-PECAM-1 or anti-CD99 treatment. Endothelial monolayers were cultured and treated as described in the Materials and Methods. Prior to the TEM assay, monocytes were re-suspended in M199 containing anti-PECAM-1, anti-CD99 or control non-blocking anti-VE-cadherin mAb’s at 20 µg/ml. TEM proceeded for 1h at 37° C. (A) Disrupting PECAM-1 and CD99 function blocked transmigration equally under BBB and standard culture conditions. (B) Anti-PECAM-1 and anti-CD99 mAb also blocked TEM across TY10 brain endothelial cells, similarly to our BBB model. Data represent six replicates from three independent experiments. Error bars indicate the standard deviation. **p< 0.0001 compared to non-blocking VE-cad control mAb (two-way ANOVA followed by Tukey test).
Paracellular TEM is associated with adherens junction remodeling
VE-cadherin is the major adhesion molecule comprising the adherens junction and is expressed in both brain and peripheral endothelium (59). VE-cadherin is responsible for maintaining endothelial barrier function in peripheral endothelium, acting as a “gatekeeper” for the passage of both macromolecules and leukocytes (60). In vitro studies have shown that VE-cadherin is transiently removed from the site of transmigration at cell-cell junctions, forming a de novo gap in VE-cadherin expression along the endothelial plasma membrane in contact with the leukocyte (61, 62). The gap reseals within minutes once the leukocyte has completed TEM (61). Preventing adherens junction gap formation blocks TEM (63, 64). This suggests that structural components of the junction must move out of the way to accommodate paracellular TEM. Because we found paracellular TEM was the predominant pathway across high resistance BBB endothelium, we investigated whether TEM uses a similar mechanism under our BBB culture conditions in vitro. As in Figure 3, because TEM was stopped early to catch monocytes in the act of TEM, only about half the total monocytes had begun migrating by the end of the 10 minute assay. However, virtually every monocyte undergoing diapedesis was associated with de novo gap in VE-cadherin staining (≥ 95%) in BBB and standard control culture conditions as well as TY10 brain endothelium (Figure 5). These gaps are resealed once the leukocyte has completed TEM (not shown). Since VE-cadherin defines the cell borders and transcellular migration takes place without disrupting VE-cadherin (25), the direct observation of VE-cadherin staining gaps during TEM in our BBB model further confirms our finding that monocytes are truly migrating paracellularly rather than transcellularly close to the junctions. Our observations demonstrate that TEM in high resistance BBB endothelium follows a similar pathway as in conventional endothelium, with formation of gaps in VE-cadherin distribution.
Figure 5. Paracellular TEM across the BBB is associated with adherens junction gap formation.
(A, C) 3D confocal images were taken of monocytes fixed in the act of TEM to discern the pathway of TEM as described in Materials and Methods. The majority of TEM occurred paracellularly at EC borders in all experimental conditions. (A, C) Remodeling of adherens junction VE-cadherin occurred selectively at sites of paracellular TEM in all culture conditions, including TY10 cells (C). The adherens junction is re-established once TEM is completed (not shown). Orthogonal (XZ) projection demonstrates monocytes are crossing the endothelial cell border in the act of TEM. (B, D) Quantitation of percent TEM and TEM associated with VE-cadherin gaps as shown in A and C. Nearly every transmigrating monocyte is associated with a de novo gap in adherens junction VE-cadherin. Scale bar indicates 10 microns. Data represent the mean of hundreds of TEM events from 5 experiments. Error bars are standard deviation.
Paracellular TEM is associated with tight junction remodeling
Tight junctions rather than adherens junctions represent the major functional and structural components of the BBB. We postulated that tight junctions would have to remodel within minutes for paracellular TEM, forming focal gaps to allow leukocytes passage at the cell border and then reseal once TEM is completed, similar to VE-cadherin. To date, tight junction remodeling has not been reported on such a rapid time scale. To examine the behavior and dynamics of BBB associated tight junctions during TEM, we performed 3D confocal imaging of monocytes fixed and immunostained in the act of TEM as earlier described. We examined the BBB tight junction protein claudin-5, because claudin-5 is an endothelial specific tight junction protein that is exclusively enriched at the BBB (65, 66) and is a major determinant of BBB integrity (67). As depicted in Figure 6, monocytes undergoing paracellular TEM were associated with focal de-novo gaps in claudin-5 staining. These gaps appeared concurrently with gaps in VE-cadherin distribution. Virtually every TEM event with a gap in VE-cadherin also displayed de novo gap formation in claudin-5. The error bars displayed in Figure 6B represent variation in percent TEM among experiments, rather than variation occurring in TEM events with gaps in either VE-cadherin or claudin-5 alone. Gaps in VE-cadherin and claudin-5 resealed after monocytes completed TEM (Figure 6A). As noted above, this is a short time point assay (10 minutes); therefore, resealing of the adherens and tight junctions must occur rapidly and within minutes following completion of leukocyte TEM across the junction. These data suggest that claudin-5 is dynamic and “moves out of the way” similar to the adherens junction VE-cadherin to accommodate diapedesis across cell borders at the BBB in vitro.
Figure 6. Paracellular TEM across the BBB is associated with claudin-5 gap formation.
(A) Upper panels 3D confocal images were taken of monocytes fixed in the act of TEM. Monolayers were immunostained with VE-cadherin (red) and claudin-5 (green) to demarcate adherens junction and tight junction, respectively. The majority of TEM occurred paracellularly at EC borders in all experimental conditions. De novo gap formation of adherens junction VE-cadherin and claudin-5 (arrows) coincided at sites of paracellular monocyte (CD18, blue) TEM under BBB conditions. Claudin-5 staining was not observed in control monolayers under standard culture conditions (not shown). Orthogonal (XZ) projection demonstrates leukocytes in the act of TEM. Lower panels: A representative leukocyte that has just crossed the endothelium (see orthogonal projection in merged image). VE-cadherin and claudin-5 gaps have already resealed at this point. (B) Quantitation of A, showing that the majority of TEM is associated with focal VE-cadherin and claudin-5 remodeling. Scale bar = 10 microns. Data are the means and standard deviation from 3 independent experiments.
Membrane from the LBRC is recruited to the site of adherens and tight junction remodeling
A large fraction of PECAM-1 and CD99 have been shown to reside in the LBRC, an interconnected vesicular-membrane compartment in endothelial cells that is contiguous with the plasma membrane at cell borders (25, 31). Membrane constitutively cycles between this compartment and the surface at cell borders (31). During para- and transcellular migration, PECAM-1 and CD99-bearing membrane from the LBRC is actively recruited to surround leukocytes at the site of TEM in a process known as targeted recycling (TR) (25, 31). We have previously shown that TR requires leukocyte-endothelial homophilic PECAM-1 interactions, intact microtubules and kinesin molecular motors (21). We next investigated how LBRC membrane dynamics were coordinated with VE-cadherin and claudin-5 gap formation during paracellular TEM across our in vitro BBB model. Figure 7 shows Z-series confocal projections of images in which monocytes are crossing the endothelium at the cell-cell borders, as shown in the XZ insets. Gaps in VE-cadherin and claudin-5 distribution were observed at cell borders exclusively at sites of paracellular TEM. The LBRC was recruited to surround monocytes undergoing diapedesis at the cell border and was detected specifically at the sites of intercellular VE-cadherin and claudin-5 gaps where monocytes were actively transmigrating paracellularly (Figure 7A and 7C respectively). Greater than 95% of monocytes migrating paracellularly were associated with both TR of membrane from the LBRC and gaps in VE-cadherin (Figure 7B) or claudin-5 (Figure 7D). Monocytes adherent but not in the act of TEM were not associated with either TR of the LBRC or gap formation of the adherens or tight junction (not shown). These data suggest coordinated movement of LBRC membrane with the remodeling of adherens and tight junction during paracellular diapedesis at the BBB in vitro.
Figure 7. Membrane from the LBRC is targeted to the site of adherens and tight junction gap formation during paracellular TEM at the BBB in vitro.
(A, C) Targeted recycling assay depicting targeted trafficking of membrane from the LBRC (red) to surround monocytes undergoing TEM at EC borders at the exact location of adherens and tight gap formation. Staining of recycled LBRC (red) is observed around monocytes (CD18, blue) migrating at cell borders where there is an associated gap in adherens junction VE-cadherin (green) and (C) claudin-5 (green). Orthogonal (XZ) projection demonstrates monocytes are in the act of TEM. (B,D) Quantitation of A and C, showing percent TEM and that the majority of TEM is associated with adherens junction and tight remodeling and TR of the LBRC. Scale bar = 10 microns. Data are representative images from 3 independent experiments. Quantitation of transendothelial migration, targeted recycling, VE-cadherin and claudin-5 gap formation are means and standard deviation derived from 3 independent experiments.
Barrier function is maintained during paracellular diapedesis
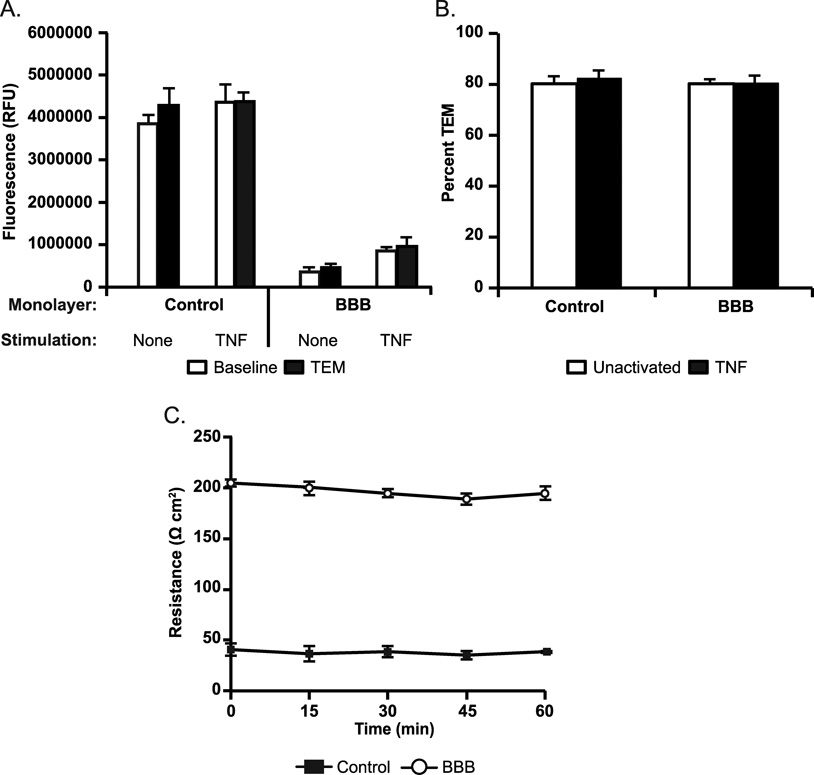
We next investigated endothelial barrier function during TEM. During paracellular TEM, endothelial junctions must presumably be breached, as evidenced by gap formation in both adherens and tight junctions (Figures 5–7). However, junctional integrity must be maintained within the brain vasculature during ongoing leukocyte trafficking that has been demonstrated to take place during routine immune surveillance or inflammation to maintain proper neuronal function (3, 68). Therefore, we speculated that TR of the LBRC and the dynamic opening and closing of endothelial adherens and tight junctions during diapedesis must be highly coordinated and tightly regulated. To address this question, we measured the baseline integrity of endothelial monolayers under basal and TNF stimulated conditions compared to monolayer integrity during monocyte TEM by recording permeability to 20 kDa FITC-dextran. We observed no significant increase in permeability to FITC-dextran during TEM in both BBB and standard culture conditions (Figure 8A) compared to baseline permeability conditions. Both resting and TNF-activated monolayers displayed greater than 80% monocyte TEM (Figure 8B). In addition, we performed monocyte TEM assays using Transwell® filters and recorded TEER every 15 minutes over the duration of 1 hour. Consistent with our permeability data, there was no significant decrease in TEER throughout the duration of the TEM assay (Figure 8C). These data demonstrate that TEM is a tightly regulated process and show that junctional integrity is preserved at the BBB during TEM.
Figure 8. Barrier function is preserved during TEM across the BBB in vitro.
Permeability to FITC-Dextran 20 kDa was measured during TEM to determine if endothelial barrier function is lost during TEM to support paracellular TEM (A). Monocytes (~1:1 ratio to EC) were added to unactivated or TNF activated BBB or HVEC monolayers and TEM was allowed to proceed for 1h at 37° C. Monolayers without monocytes were used as controls to determine baseline permeability. After 1h, monolayers were washed extensively and fluorescence was measured using a microplate reader at the end of the assay. (B) After recording fluorescence, monolayers were fixed and percent TEM was quantitated as described in the Materials and Methods. Data represent the mean of at least three replicates from at least three separate experiments. Error bars indicate standard deviation from the mean. (C) TEER was recorded every 15 minutes during monocyte TEM over the course of 1h to further assay and confirm the regulation of barrier function during TEM. There was no significant decrease in TEER over the course of TEM for either BBB or standard HUVEC controls. One representative experiment of three with a minimum of three replicates is shown.
Discussion
We hypothesized that the relative tightness of endothelial junctions may significantly influence whether a leukocyte transmigrates paracellularly or transcellularly. Therefore, we established high resistance endothelium with features of the BBB (Figure 1 and Supplemental Figure 1) to study the relationship between the pathway of diapedesis and the tightness of endothelial junctions. We demonstrate that paracellular diapedesis is the primary pathway utilized by monocytes at the BBB in vitro (Figure 3). PECAM-1 and CD99 were required for efficient monocyte TEM (Figure 4). The role of CD99 in transmigration of leukocytes across tight endothelial cells has not been previously studied. We also report the novel finding that tight junctions displayed remarkable plasticity and were rapidly remodeled during paracellular TEM (Figure 5 and Figure 6). Targeted trafficking of the LBRC was coordinated with remodeling of EC junctions at sites of TEM (Figure 7). Rapid remodeling of tight junctions was reversible and a tightly regulated process as barrier function was maintained during TEM (Figure 8).
The generation of a reliable human cell-based BBB model optimized for barrier tightness is a prerequisite for better understanding the molecular regulation of diapedesis and for the development of therapeutic agents that block it. We adapted our standard culture system to generate a simplified cell culture model of the human BBB. Using HUVEC for the BBB model presents several advantages because 1) they are of human origin, 2) easy to obtain, isolate and maintain, 3) form monolayers in culture, 4) can be passaged for several days without losing their phenotype, and most importantly, as we report here 5) can acquire BBB-like properties when treated with ACM. Thus, our BBB culture conditions were sufficient to bestow HUVEC with many features germane to the specialized endothelium of the BBB, including greater than 10-fold increased barrier function to ions and small molecules (Figure 1A–B) and the expression of hallmark BBB tight junction proteins occludin, claudin-5 and claudin-3 (Figure 1C). Obviously, however, our model—like all models—is imperfect. It lacks astrocytes and the three-dimensional aspects of true vasculature. The TEER values of our BBB model still remain significantly less than the BBB in vivo (69), although it is one of the tightest human BBB models to date (47–49). Nonetheless, the purpose of our study was not to make a better BBB model but to make an in vitro transmigration system with tight junctions reminiscent of the BBB to determine whether the restrictiveness of the tight junction affected the path of leukocyte transmigration.
Burns et al. cultured EC on glutaraldehyde fixed extracellular matrix and found that these EC made tighter junctions (although their TEER was 1.5 fold higher than controls compared to 10 fold higher TEER in our system). They reported that neutrophil TEM occurs primarily at tricellular corners, where they observed discontinuities at these regions in both VE-cadherin and tight junctions (37). The authors speculated that these “pores” provide a potential opening for PMN acting as a gateway to traverse the endothelium. However, in our current BBB experimental system, and similar to Luscinskas and colleagues (61), tricellular and bicellular borders not associated with leukocytes were intact and we only observed gaps in VE-cadherin staining when associated with actively transmigrating monocytes. This suggests that junctional remodeling and gap formation during TEM is an active process.
These observations may also help explain how EC junction remodeling can occur during diapedesis while still maintaining the vascular barrier (Figure 8). Our data demonstrate that targeted recycling of the LBRC facilitates remodeling of the tight junction by displacing the tight junction laterally and sealing the intercellular gaps with membrane. Targeted recycling of the LBRC is dependent on PECAM-PECAM interactions (31). Therefore, we favor the hypothesis that when monocyte PECAM-1 homophilic interaction with endothelial PECAM-1 recruits the LBRC to the monocyte at the site of TEM, this membrane then facilitates the remodeling of the junction locally by displacing the tight junction laterally. This is consistent with Luscinskas and colleagues (61), who have demonstrated the VE-cadherin is pushed laterally to accommodate paracellular TEM. VE-cadherin expression at the site where TEM occurred is then quickly reestablished within minutes when TEM is completed. The additional membrane provided by the LBRC can seal the de novo gaps while tightly engaging the leukocyte with homophilic adhesion molecules such as PECAM-1 and CD99 as it passes between endothelial junctions. This would prevent plasma leakage and thereby maintain the endothelial barrier. We speculate that since both adherens junction proteins and tight junctions are attached to actin filaments, and these junctions are intermixed in endothelial cells (70–72), similar mechanisms allow for tight junctions and adherens junctions to undergo lateral movement in the plane of the membrane.
In conclusion, we report that, within the limits tested, the relative tightness of BBB endothelial cell junctions does not influence the pathway of TEM in vitro. Leukocyte TEM at the BBB in vitro is a complex process that requires complex coordination of diverse signaling events, including PECAM-1, CD99 and targeted recycling of the LBRC. These events all converge to facilitate the remodeling of adherens and tight junctions to allow for the passage of the migrating leukocytes at cell borders. The LBRC serves as a novel endothelial cell mechanism to promote diapedesis and maintain vascular integrity. The degree of cross talk between adherens and tight junctions is undoubtedly more complex than previously appreciated. Future studies geared toward understanding the interconnection between these pathways will bring us closer to novel and selective therapies that can regulate inflammation.
Supplementary Material
Acknowledgments
We wish to thank Clifford D. Carpenter for excellent technical assistance. We thank Dr. Babette Weksler for providing the hCMEC/D3 cell line. We thank Dr. Deyu Feng for use of the microplate reader. We thank Dr. Yukio Takeshita for technical assistance with TY10 cell line. We also thank Dr. David Sullivan, Dr. Birgit Obermeier, Evan Weber and Richard Watson for thoughtful and helpful review of the manuscript.
This work was supported by NIH T32 AI7476-17 to R.C. Winger and by NIH grants R37 HL064774 and R01 HL046849 to W.A. Muller.
Abbreviations used in this article
- TEM
Transendothelial Migration
- HUVEC
Human Vein Endothelial Cells
- EC
Endothelial Cell
- BBB
blood-brain barrier
- ACM
Astrocyte conditioned media
- VE-cadherin
Vascular Endothelial-cadherin
- LBRC
Lateral Border Recycling Compartment
- TR
Targeted Recycling
- TEER
Transendothelial Electrical Resistance
- mAb
Monoclonal antibody
References
- 1.Ousman SS, Kubes P. Immune surveillance in the central nervous system. Nature neuroscience. 2012;15:1096–1101. doi: 10.1038/nn.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hickey WF. Basic principles of immunological surveillance of the normal central nervous system. Glia. 2001;36:118–124. doi: 10.1002/glia.1101. [DOI] [PubMed] [Google Scholar]
- 3.Hickey WF. Leukocyte traffic in the central nervous system: the participants and their roles. Semin Immunol. 1999;11:125–137. doi: 10.1006/smim.1999.0168. [DOI] [PubMed] [Google Scholar]
- 4.Koedel U, Klein M, Pfister HW. New understandings on the pathophysiology of bacterial meningitis. Current opinion in infectious diseases. 2010;23:217–223. doi: 10.1097/QCO.0b013e328337f49e. [DOI] [PubMed] [Google Scholar]
- 5.Roberts TK, Buckner CM, Berman JW. Leukocyte transmigration across the blood-brain barrier: perspectives on neuroAIDS. Frontiers in bioscience. 2010;15:478–536. doi: 10.2741/3631. [DOI] [PubMed] [Google Scholar]
- 6.Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol. 2010;87:779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kochanek PM, Hallenbeck JM. Polymorphonuclear leukocytes and monocytes/macrophages in the pathogenesis of cerebral ischemia and stroke. Stroke; a journal of cerebral circulation. 1992;23:1367–1379. doi: 10.1161/01.str.23.9.1367. [DOI] [PubMed] [Google Scholar]
- 9.Yilmaz G, Granger DN. Cell adhesion molecules and ischemic stroke. Neurological research. 2008;30:783–793. doi: 10.1179/174313208X341085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shechter R, Miller O, Yovel G, Rosenzweig N, London A, Ruckh J, Kim KW, Klein E, Kalchenko V, Bendel P, Lira SA, Jung S, Schwartz M. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity. 2013;38:555–569. doi: 10.1016/j.immuni.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwarzmaier SM, Zimmermann R, McGarry NB, Trabold R, Kim SW, Plesnila N. In vivo temporal and spatial profile of leukocyte adhesion and migration after experimental traumatic brain injury in mice. Journal of neuroinflammation. 2013;10:32. doi: 10.1186/1742-2094-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feuerstein GZ, Wang X, Barone FC. Inflammatory gene expression in cerebral ischemia and trauma. Potential new therapeutic targets. Ann N Y Acad Sci. 1997;825:179–193. doi: 10.1111/j.1749-6632.1997.tb48428.x. [DOI] [PubMed] [Google Scholar]
- 13.Greenwood J, Heasman SJ, Alvarez JI, Prat A, Lyck R, Engelhardt B. Review: leucocyte-endothelial cell crosstalk at the blood-brain barrier: a prerequisite for successful immune cell entry to the brain. Neuropathology and applied neurobiology. 2011;37:24–39. doi: 10.1111/j.1365-2990.2010.01140.x. [DOI] [PubMed] [Google Scholar]
- 14.Awad AM, Stuve O. Immunopathogenesis of multiple sclerosis: new insights and therapeutic implications. Continuum (Minneap Minn) 2010;16:166–180. doi: 10.1212/01.CON.0000389940.92283.aa. [DOI] [PubMed] [Google Scholar]
- 15.Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154–169. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daneman R. The blood-brain barrier in health and disease. Annals of neurology. 2012;72:648–672. doi: 10.1002/ana.23648. [DOI] [PubMed] [Google Scholar]
- 17.Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19:1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nature reviews. Neuroscience. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 19.Lossinsky AS, Shivers RR. Structural pathways for macromolecular and cellular transport across the blood-brain barrier during inflammatory conditions. Review. Histol Histopathol. 2004;19:535–564. doi: 10.14670/HH-19.535. [DOI] [PubMed] [Google Scholar]
- 20.Muller WA. Migration of leukocytes across endothelial junctions: Some concepts and controversies. Microcirculation. 2001;8:181–193. doi: 10.1038/sj/mn/7800078. [DOI] [PubMed] [Google Scholar]
- 21.Muller WA. Mechanisms of leukocyte transendothelial migration. Annu Rev Pathol. 2011;6:323–344. doi: 10.1146/annurev-pathol-011110-130224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carman CV, Springer TA. Trans-cellular migration: cell-cell contacts get intimate. Curr Opin Cell Biol. 2008;20:533–540. doi: 10.1016/j.ceb.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolburg H, Wolburg-Buchholz K, Engelhardt B. Diapedesis of mononuclear cells across cerebral venules during experimental autoimmune encephalomyelitis leaves tight junctions intact. Acta Neuropathol. 2005;109:181–190. doi: 10.1007/s00401-004-0928-x. [DOI] [PubMed] [Google Scholar]
- 24.von Wedel-Parlow M, Schrot S, Lemmen J, Treeratanapiboon L, Wegener J, Galla HJ. Neutrophils cross the BBB primarily on transcellular pathways: an in vitro study. Brain research. 2011;1367:62–76. doi: 10.1016/j.brainres.2010.09.076. [DOI] [PubMed] [Google Scholar]
- 25.Mamdouh Z, Mikhailov A, Muller WA. Transcellular migration of leukocytes is mediated by the endothelial lateral border recycling compartment. J Exp Med. 2009;206:2795–2808. doi: 10.1084/jem.20082745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raine CS, Cannella B, Duijvestijn AM, Cross AH. Homing to central nervous system vasculature by antigen-specific lymphocytes. II. Lymphocyte/endothelial cell adhesion during the initial stages of autoimmune demyelination. Lab Invest. 1990;63:476–489. [PubMed] [Google Scholar]
- 27.Cross AH, Raine CS. Central nervous system endothelial cell-polymorphonuclear cell interactions during autoimmune demyelination. Am J Pathol. 1991;139:1401–1409. [PMC free article] [PubMed] [Google Scholar]
- 28.Lampert P. Electron microscopic studies on ordinary and hyperacute experimental allergic encephalomyelitis. Acta Neuropathol. 1967;9:99–126. doi: 10.1007/BF00691436. [DOI] [PubMed] [Google Scholar]
- 29.Alvarez JI, Cayrol R, Prat A. Disruption of central nervous system barriers in multiple sclerosis. Biochim Biophys Acta. 2011;1812:252–264. doi: 10.1016/j.bbadis.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 30.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 31.Mamdouh Z, Chen X, Pierini LM, Maxfield FR, Muller WA. Targeted recycling of PECAM from endothelial surface-connected compartments during diapedesis. Nature. 2003;421:748–753. doi: 10.1038/nature01300. [DOI] [PubMed] [Google Scholar]
- 32.Muller WA, Ratti CM, McDonnell SL, Cohn ZA. A human endothelial cell-restricted, externally disposed plasmalemmal protein enriched in intercellular junctions. J Exp Med. 1989;170:399–414. doi: 10.1084/jem.170.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liao F, Huynh HK, Eiroa A, Greene T, Polizzi E, Muller WA. Migration of monocytes across endothelium and passage through extracellular matrix involve separate molecular domains of PECAM-1. J Exp Med. 1995;182:1337–1343. doi: 10.1084/jem.182.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muller WA, Weigl S. Monocyte-selective transendothelial migration: Dissection of the binding and transmigration phases by an in vitro assay. J Exp Med. 1992;176:819–828. doi: 10.1084/jem.176.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaillard PJ, Voorwinden LH, Nielsen JL, Ivanov A, Atsumi R, Engman H, Ringbom C, Boer AGde, Breimer DD. Establishment and functional characterization of an in vitro model of the blood-brain barrier, comprising a co-culture of brain capillary endothelial cells and astrocytes. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. 2001;12:215–222. doi: 10.1016/s0928-0987(00)00123-8. [DOI] [PubMed] [Google Scholar]
- 36.Rubin LL, Hall DE, Porter S, Barbu K, Cannon C, Horner HC, Janatpour M, Liaw CW, Manning K, Morales J, et al. A cell culture model of the blood-brain barrier. J Cell Biol. 1991;115:1725–1735. doi: 10.1083/jcb.115.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burns AR, Walker DC, Brown ES, Thurmon LT, Bowden RA, Keese CR, Simon SI, Entman ML, Smith CW. Neutrophil transendothelial migration is independent of tight junctions and occurs preferentially at tricellular corners. J Immunol. 1997;159:2893–2903. [PubMed] [Google Scholar]
- 38.Weksler BB, Subileau EA, Perriere N, Charneau P, Holloway K, Leveque M, Tricoire-Leignel H, Nicotra A, Bourdoulous S, Turowski P, Male DK, Roux F, Greenwood J, Romero IA, Couraud PO. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005;19:1872–1874. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]
- 39.Sano Y, Kashiwamura Y, Masaaki A, Dieu L-H, Huwyler J, Shimizu F, Haruki H, Maeda T, Saito K, Tasaki A, Kanda T. A stable human brain microvascular endothelial cell line retaining its barrier-specific nature, independent of the passage number. Clin Exp Neuroimmunol. 2013;4:92–103. [Google Scholar]
- 40.Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993;178:449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol. 2004;167:377–388. doi: 10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubin LL. The blood-brain barrier in and out of cell culture. Current opinion in neurobiology. 1991;1:360–363. doi: 10.1016/0959-4388(91)90053-a. [DOI] [PubMed] [Google Scholar]
- 43.Calabria AR, Shusta EV. A genomic comparison of in vivo and in vitro brain microvascular endothelial cells. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2008;28:135–148. doi: 10.1038/sj.jcbfm.9600518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prat A, Biernacki K, Wosik K, Antel JP. Glial cell influence on the human blood-brain barrier. Glia. 2001;36:145–155. doi: 10.1002/glia.1104. [DOI] [PubMed] [Google Scholar]
- 45.Tio S, Deenen M, Marani E. Astrocyte-mediated induction of alkaline phosphatase activity in human umbilical cord vein endothelium: an in vitro model. European journal of morphology. 1990;28:289–300. [PubMed] [Google Scholar]
- 46.Elgjo RF, Henriksen T, Evensen SA. Ultrastructural identification of umbilical cord vein endothelium in situ and in culture. Cell and tissue research. 1975;162:49–59. doi: 10.1007/BF00223261. [DOI] [PubMed] [Google Scholar]
- 47.Eigenmann DE, Xue G, Kim KS, Moses AV, Hamburger M, Oufir M. Comparative study of four immortalized human brain capillary endothelial cell lines, hCMEC/D3, hBMEC, TY10, and BB19, and optimization of culture conditions, for an in vitro blood-brain barrier model for drug permeability studies. Fluids and barriers of the CNS. 2013;10:33. doi: 10.1186/2045-8118-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reichel A, Begley DJ, Abbott NJ. An overview of in vitro techniques for blood-brain barrier studies. Methods Mol Med. 2003;89:307–324. doi: 10.1385/1-59259-419-0:307. [DOI] [PubMed] [Google Scholar]
- 49.Forster C, Burek M, Romero IA, Weksler B, Couraud PO, Drenckhahn D. Differential effects of hydrocortisone and TNFalpha on tight junction proteins in an in vitro model of the human blood-brain barrier. J Physiol. 2008;586:1937–1949. doi: 10.1113/jphysiol.2007.146852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coisne C, Engelhardt B. Tight junctions in brain barriers during central nervous system inflammation. Antioxidants & redox signaling. 2011;15:1285–1303. doi: 10.1089/ars.2011.3929. [DOI] [PubMed] [Google Scholar]
- 51.Muller WA, Luscinskas FW. Assays of transendothelial migration in vitro. Methods Enzymol. 2008;443:155–176. doi: 10.1016/S0076-6879(08)02009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feng D, Nagy JA, Pyne K, Dvorak HF, Dvorak AM. Neutrophils emigrate from venules by a transendothelial cell pathway in response to FMLP. J Exp Med. 1998;187:903–915. doi: 10.1084/jem.187.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schenkel AR, Chew TW, Muller WA. Platelet endothelial cell adhesion molecule deficiency or blockade significantly reduces leukocyte emigration in a majority of mouse strains. J Immunol. 2004;173:6403–6408. doi: 10.4049/jimmunol.173.10.6403. [DOI] [PubMed] [Google Scholar]
- 54.Muller WA. The role of PECAM-1 (CD31) in leukocyte emigration: studies in vitro and in vivo. J Leukoc Biol. 1995;57:523–528. doi: 10.1002/jlb.57.4.523. [DOI] [PubMed] [Google Scholar]
- 55.Bogen S, Pak J, Garifallou M, Deng X, Muller WA. Monoclonal antibody to murine PECAM-1 [CD31] blocks acute inflammation in vivo. J Exp Med. 1994;179:1059–1064. doi: 10.1084/jem.179.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dufour EM, Deroche A, Bae Y, Muller WA. CD99 is essential for leukocyte diapedesis in vivo. Cell Commun Adhes. 2008;15:351–363. doi: 10.1080/15419060802442191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lou O, Alcaide P, Luscinskas FW, Muller WA. CD99 is a key mediator of the transendothelial migration of neutrophils. J Immunol. 2007;178:1136–1143. doi: 10.4049/jimmunol.178.2.1136. [DOI] [PubMed] [Google Scholar]
- 58.Schenkel AR, Mamdouh Z, Chen X, Liebman RM, Muller WA. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat Immunol. 2002;3:143–150. doi: 10.1038/ni749. [DOI] [PubMed] [Google Scholar]
- 59.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- 60.Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci. 2008;121:2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- 61.Shaw SK, Bamba PS, Perkins BN, Luscinskas FW. Real-time imaging of vascular endothelial-cadherin during leukocyte transmigration across endothelium. J Immunol. 2001;167:2323–2330. doi: 10.4049/jimmunol.167.4.2323. [DOI] [PubMed] [Google Scholar]
- 62.Allport JR, Muller WA, Luscinskas FW. Monocytes induce reversible focal changes in vascular endothelial cadherin complex during transendothelial migration under flow. J Cell Biol. 2000;148:203–216. doi: 10.1083/jcb.148.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alcaide P, Newton G, Auerbach S, Sehrawat S, Mayadas TN, Golan DE, Yacono P, Vincent P, Kowalczyk A, Luscinskas FW. p120-Catenin regulates leukocyte transmigration through an effect on VE-cadherin phosphorylation. Blood. 2008;112:2770–2779. doi: 10.1182/blood-2008-03-147181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Allingham MJ, Buul JDvan, Burridge K. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J Immunol. 2007;179:4053–4064. doi: 10.4049/jimmunol.179.6.4053. [DOI] [PubMed] [Google Scholar]
- 65.Morita K, Furuse M, Fujimoto K, Tsukita S. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc. Natl. Acad. Sci. USA. 1999;96:511–516. doi: 10.1073/pnas.96.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morita K, Sasaki H, Furuse M, Tsukita S. Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol. 1999;147:185–194. doi: 10.1083/jcb.147.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cramer EB. The ability of leukocytes to cross the tight junctions. Boca Raton: CRC Press; 1992. [Google Scholar]
- 69.Butt AM. Effect of inflammatory agents on electrical resistance across the blood-brain barrier in pial microvessels of anaesthetized rats. Brain research. 1995;696:145–150. doi: 10.1016/0006-8993(95)00811-4. [DOI] [PubMed] [Google Scholar]
- 70.Vorbrodt AW, Dobrogowska DH. Molecular anatomy of interendothelial junctions in human blood-brain barrier microvessels. Folia histochemica et cytobiologica / Polish Academy of Sciences, Polish Histochemical and Cytochemical Society. 2004;42:67–75. [PubMed] [Google Scholar]
- 71.Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5:261–270. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 72.Schulze C, Firth JA. Immunohistochemical localization of adherens junction components in blood-brain barrier microvessels of the rat. J Cell Sci. 1993;104(Pt 3):773–782. doi: 10.1242/jcs.104.3.773. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.