Abstract
In many species, including mice, maternal responsiveness is experience-dependent and permanent, lasting for long periods (months to years). We have shown that after brief exposures to pups, virgin female mice continue to respond maternally toward pups for at least one month. Administration of a histone deacetylase inhibitor (HDACi) reduces the amount of maternal experience required to affect maternal behavior and gene expression. In this set of studies, we examined the epigenetic mechanisms that underlie these motivated behaviors. We assessed whether the effects of HDACi persisted 1 month after the initial experience (in the absence of continued pup experience or HDACi treatment) and whether the maintenance of maternal memory was associated with stable changes in gene expression. Using chromatin immunoprecipitation, we examined whether Esr2 and Oxt gene expression might be mediated by recruitment of the histone acetyltransferase cAMP response element binding protein (CBP) to their promoter regions after maternal memory consolidation. We report that HDACi treatment induced long-lasting changes in maternal responsiveness. Maternal learning was associated with increased recruitment of CBP to the Esr2 and Oxt gene promoters during the consolidation of maternal memory as well as a persistent increase in estrogen receptor-β (Esr2) mRNA and decreased expression of the de novo DNA methyltransferase Dnmt3a within the medial preoptic area. The consolidation of the maternal experience may involve the CBP recruitment and stable changes in gene expression, which maintain increased maternal responsiveness for long periods of time.
For all mammals, the first few days, weeks, months, or years of life represent a time when survival depends on the quality of care received from the mother. Typically, the transition into motherhood is precipitated by the hormonal events of gestation and birth, which function, in part, to decrease avoidance and increase attraction to infants, thereby facilitating mother-infant interactions (1). It is the experience of interacting with infants, however, that sustains maternal responding (2). Research in multiple species, including humans, has shown that initial mother-infant experiences induce long-lasting changes in subsequent maternal responsiveness that persist for months and can affect maternal care of subsequent offspring even in the absence of continued interaction with infants or hormonal exposure (3–12). Therefore, the experience of interacting with the infant is consolidated, as in any learning experience, such that subsequent interactions with infants are facilitated.
Maternal experiences are likely consolidated in the neural circuits that regulate maternal responsiveness (13–16). The medial preoptic area (MPOA) of the hypothalamus plays a central role in these circuits, integrating hormonal and pup-related information from all sensory modalities and regulating maternal behavior responses (17). As naïve females transition into motherhood, the MPOA undergoes a variety of changes, many of which are specifically related to interaction with pups. For example, alterations in gene transcription, steroid and peptide hormone signaling, neuronal activity to pups, or pup-related cues occur during initial mother-infant interactions in rats and mice (18–30). Structural changes, such as dendritic branching and astrocyte remodeling, within the MPOA also materialize (31–33). Thus, MPOA neurons exhibit phenotypic plasticity or sensory experience-driven alterations in the physical properties that support functional changes in MPOA responses toward pups. Some or all of these changes might contribute to the elevated c-Fos response in MPOA neurons during the recall of maternal memory (16, 34). Considering that maternal responses do not become MPOA independent, it seems that the MPOA is a key site of plasticity. However, relatively little is known about the molecular mechanisms that mediate the stable representation of maternal memory within the MPOA.
Like other forms of learning and memory, maternal learning and memory requires cAMP response element binding protein (CREB)-mediated gene transcription and new protein synthesis (35). The activation of phosphorylated CREB, which is restricted to MPOA neurons during mother-infant interactions, may be a critical component of the molecular mechanism underlying the consolidation of maternal experience, as CREB is a neuronal marker for neurons encoding fear memories (36). We have recently shown that CREB binding protein (CBP), a coactivator of CREB, is up-regulated during maternal experience consolidation (37). Importantly, CBP also acts as a histone acetylatransferase adding acetyl groups to histone proteins in gene promoters bound by CREB, and functionally supporting the consolidation of memory (38). We have hypothesized that this molecular mechanism may be involved in the consolidation of maternal experience, because administration of a histone deacetyltransferase inhibitor (HDACi), which increased CBP activity, reduced the amount of maternal learning required to consolidate maternal memory (37). HDACi treatment was also associated with increased transcription of some of the genes that have found to be altered by mother-infant interactions in the MPOA. Recent work has shown that alterations in gene transcription can overlap between postpartum and virgin rodents interacting with infants, indicating that these changes depend on pup interaction and not hormone stimulation (24, 30). We have shown that two steroid and peptide hormone genes, estrogen receptor beta (Esr2), oxytocin (Oxt), can be potentiated in virgin mice by maternal experiences alone. Considering that maternal experiences are consolidated, regardless of hormone stimulation, we have focused on how these two genes might be involved in the consolidation of maternal experience and the stable representation of maternal memory within the MPOA.
Here we test the hypothesis that maternal memory consolidation involves epigenetic regulation of Esr2 and Oxt and that stable changes in the expression of these genes are associated with long-lasting changes in maternal care. First, we established that HDACi treatment supports the consolidation of subthreshold maternal experiences into a long-lasting maternal memory. Second, we asked whether the long-lasting change in maternal care was associated with a stable change in the expression of the two genes that may be epigenetically regulated during maternal experience consolidation: Esr2 and Oxt. Because the maintenance and consolidation of memory has been linked to alterations in genes that affect DNA methylation, we also examined DNA methyltransferase (DNMT) gene expression (39–41). Finally, as a first step to understanding how epigenetic regulation of Esr2 and Oxt in the MPOA might support the stabilization of maternal memory, we examined whether HDACi treatment drives CBP recruitment to the Esr2 and Oxt gene promoters during maternal memory consolidation.
Materials and Methods
Subjects and drug treatment
All mice were C57BL/6J virgin nulliparous females (60–100 d of age), naive to pups (except for their own littermates). Sodium butyrate (SB; Sigma-Aldrich) was dissolved in sterile water and administered at a dose of 8 mg/mL in the drinking water (n = 8) (37, 42). Control mice received standard drinking water (n = 7). Oral administration of SB at this dose increases histone acetylation in the hypothalamus (including MPOA) (43). Drinking water containing SB was available ad libitum for a total of 72 hours: beginning 24 hours prior to the first 2-hour pup-experience and ending 24 hours after the second 2-hour experience (Figure 1a). Daily drinking was monitored for all animals. A separate group of C57BL/6J mice, drinking normal water, served as foster dams that provided stimulus pups. Mice were housed on a 12-hour reverse light cycle (lights off at 12:00 am) and given food (diet number 7912; Harlan Tekland) and water ad libitum. Behavioral testing began one hour after lights off under dim red light. All procedures were in compliance with the University of Virginia Animal Care and Use Committee.
Figure 1.
A, Time line of the experimental design. The HDACi, sodium butyrate, was administered in the drinking water for a total of 72 hours. Subthreshold maternal experience occurred on 2 consecutive days for 2 hours daily in the dam's home cage. Pup retrieval was measured in a novel T-maze 24 hours, and 1 month, later. Mice received no additional interaction with pups, or HDACi, during the 1-month interim. B, Females that received HDACi (n = 8) retrieved more pups at both times than females that did not get HDACi (n = 7). C, Females that received HDACi spent less time grouping the pups than females that did not get HDACi. D, HDACi treated females were faster to begin pup retrieval at both time points as compared with females that did not receive HDACi. E, HDACi-treated females were faster to retrieve all pups at both time points as compared with females that did not receive HDACi. F, Median change (in seconds) to initiate pup retrieval from test 1 (24 h) to test 2 (1 mo). A positive score denotes an improvement over time (P < .01). Error bars indicate interquartile range. HDACi-treated females were faster to initiate retrieval the second time they were tested, whereas females that did not receive HDACi retrieved at the same rate in both tests. G, No effect of treatment or time on latency to investigate pups. In all panels except F, the means and SEMs are given. Gray bars represent the group that received HDACi and the white bars represent the control drinking normal water. *, Significantly different from corresponding control group (P < .05); **, significant main effect of time (P < .05).
Maternal experience paradigm
All mice were exposed to a subthreshold maternal experience, which consisted of interacting with stimulus pups for 2 h/d for 2 consecutive days, which has previously been found to have no facilitatory effects on maternal memory (37). Briefly, at the start of each 2-hour experience, three stimulus pups (1–7 d old, mixed sex) were scattered in the cage. All females retrieved the three pups within the first 15 minutes. At the end of the 2-hour experience, pups were removed and returned to lactating donor females. Due to random distribution of the pups, it is unlikely that females were exposed to the same pups more than once.
T-maze pup retrieval testing
The walls and floors of the T-maze apparatus (67.3 × 11.4 × 8.3 cm) were clear plexiglas upon which a removable plexiglas top was fitted. The vertical runway measured 48.3 cm in length and opened into a horizontal runway that measured 67.3 cm in length. A goal box (11.4 cm × 12.7 cm) was attached to the end of the vertical runway, which could be closed off from the rest of the T-maze by a clear plexiglas guillotine door. Three stimulus pups were placed at equal intervals in the horizontal arm of the plexiglas T-maze. Mice were tested for retrieval of pups in the T-maze 24 hours, and 1 month, after the last 2-hour pup experience. Each female was placed into the goal box of the T-maze with her nest material for a 10-minute habituation period. Next, the plexiglas door was removed and the 15-minute pup retrieval test began. Latencies to emerge from the goal box (all four paws), investigate the first pup, and retrieve each pup to the goal box were recorded. The test ended after 15 minutes, or when the female had retrieved all three pups to the goal box (whichever occurred first). The total time spent grouping the pups together was measured as the time (in seconds) from first pup retrieval to last pup retrieval. Females that did not retrieve pups during the test were assigned a latency of 900 seconds for statistical purposes.
Quantification of mRNA by real time PCR
After the final T-maze pup retrieval test, which occurred 1 month after maternal experience, gene expression was examined as described previously (ns = 6–8) (37). Briefly, mice were briefly anesthetized with isoflourane and euthanized by cervical dislocation. Brains were immediately removed, frozen and later sectioned (120 μm) on a cryostat, and mounted onto slides. The MPOA (bregma 0.26 to −0.58) was dissected out using a tissue punch (blunted 16.5 gauge needle). Total RNA was isolated using an RNeasy lipid tissue minikit (QIAGEN) according to the manufacturer's protocol. A NanoVue spectrophotometer (GE Life Sciences) was used to determine the quality (260/280 > 1.8) and quantity of the RNA. Gene expression was not examined in one sample from the control group due to insufficient RNA quantity and quality (<5 ng/μL, 260/280 = 1.09). The cDNA templates were prepared using an AffinityScript cDNA synthesis kit (Agilent Technologies) according to the manufacturer's protocol. Quantitative real-time PCR (qPCR) was performed using the ABI StepOnePlus real-time PCR system.
The following TaqMan gene expression assays from (Applied Biosystems) were used to detect PCR products of interest: CREB binding protein (Crebbp, Mm01342452_m1), estrogen receptor-β (Esr2, Mm00599821_m1), oxytocin (Oxt, Mm00726655_S1), and oxytocin receptor (Oxtr, Mm01182684_m1). All samples were normalized to β2-microglobulin (Mm00437762_m1). Oligonucleotide primers for Dnmt1, Dnmt3a, Dnmt3b, ACTB (β-actin), and B2M (β2-microglobulin) were designed and validated (efficiencies between 90% and 103%) as previously described (see Table 1) (44). All samples were normalized to ACTB or B2M. There were no significant differences in the expression of endogenous control genes between treatment groups. For TaqMan- and SYBR Green-based detection method, target and endogenous control genes were measured in triplicate for each cDNA sample during each real-time run to avoid intersample variance. For SYBR Green-based quantitative RT-PCR, a no-reverse transcriptase reaction was run in parallel to the cDNA synthesis samples to control for contamination and genomic amplification. The melting temperatures were monitored to ensure that there was only one peak. All genes of interest were analyzed with StepOne software (Life Technologies) using the comparative cycle threshold method.
Table 1.
Primer Sequences for Syber Green qPCR Assays
| Primer | Sequence (5′-3′) |
|---|---|
| Dnmt1 forward | CCGCAGGCGGCTAAAGACTT |
| Dnmt1 reverse | GCTCCCGTTGGCGGGACAAC |
| Dnmt3a forward | GAGGGAACTGAGACCCCAC |
| Dnmt3a reverse | CTGGAAGGTGAGTCTTGGCA |
| Dnmt3b forward | AGCGGGTATGAGGAGTGCAT |
| DNMT3b reverse | GGGAGCATCCTTCGTGTCTG |
| β2-Microglobulin forward | GGCTCACACTGAATTCACCCCCAC |
| β2-Microglobulin reverse | ACATGTCTCGATCCCAGTAGACGGT |
| β-Actin forward | GCCACCAGTTCGCCATGGAT |
| β-Actin reverse | TCTGGGCCTCGTCACCCACATA |
| Esr2 forward | AGAGGATGACTGTGAAGTGGC |
| Esr2 reverse | AGAGATCTCAGGCACTCCCG |
| Oxt forward | AGTTAGAAGAGCTGAGGTGCAT |
| Oxt reverse | AGGCTAGAGAGACTTGGGAGG |
Chromatin immunoprecipitation (ChIP) followed by real-time qPCR
To examine whether changes in Esr2 or Oxt expression might be mediated by recruitment of the histone acetyltransferase, CBP, to their promoter regions during maternal memory consolidation, we used another cohort of mice. Four groups of females were used (n = 6/group): virgin females naïve to pups and HDACi treatment, virgin females treated with HDACi, virgin females with suboptimal maternal experience, and virgin females treated with HDACi during suboptimal experience with pups. Twenty-four hours after the last experience (pup or no pup), brain tissue was prepared as described above. For each ChIP, bilateral MPOA punches were pooled from six mice. Independent duplicate samples were immunoprecipitated with a ChIP-grade antibody that recognizes CBP (Abcam catalog number ab10489). An IgG antibody (antimouse) was run as a control (Sigma; catalog number I-5381). Resulting immunoprecipitated DNA and total (input) genomic DNA were run in triplicate for real time qPCR detection. A ChIP assay was performed according to a published protocol with some modifications (45).
Brain tissues were fixed with 1% formaldehyde to covalently cross-link DNA with associated proteins for 5 minutes at room temperature. The cross-linking reaction was stopped with the addition of glycine to a final concentration of 0.125 M. After cross-linking, chromatin was extracted by detergent lysis and subsequently sheared by sonication. Extraction consisted of homogenizing tissue in sodium dodecyl sulfate (SDS) lysis buffer (5% SDS; 50 mM Trsi-HCL, pH 6.8; 10 mM EDTA, pH 8.0) with the addition of proteinase inhibitors and 0.2 M phenylmethylsulfonyl fluoride (PMSF). Homogenates were incubated on ice for 15 minutes, vortexing every 5 minutes, and centrifuged at 4000 × g for 5 minutes. The supernatant was decanted and ChIP dilution buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris-HCL, 167 mΜ NaCl) with proteinase inhibitors and 0.2 M PMSF was added to each pellet. Chromatin extracts were sheared to 400–600 bp with 22 cycles of the Diagenode Biorupter 300 (30 second cycles with 30 sec of rest between each cycle). After sonication, samples were centrifuged at 10 000 × g for 10 minutes at 4°C and supernatant was divided into three aliquots. Twenty microliters of preimmunoprecipitated lysate were saved from each aliquot as input for later normalization. The chromatin solution was precleared with Dynabeads Protein G Beads (Invitrogen) for 30 minutes at 4°C to remove nonspecific debris and immunoprecipitated overnight at 4°C with 3 μL of antibody directed against CBP or 5 μL of nonimmune mouse IgG. After immunoprecipitation, the DNA-histone complex was collected with 40 μL of Dynabeads Protein G Beads for 2 hours at 4°C. The beads were sequentially washed with low salt, high salt, and LiCl and washed twice with 10 mM Tris (pH 8) per 1 mM EDTA buffers. The DNA-protein complex was then eluted from the beads with 400 μL elution buffer (1% SDS, 5 mM EDTA, 50 mM Tris-HCL, 500 mM NaCl, plus proteinase inhibitors and 0.2 M PMSF). The DNA and protein were dissociated at 65°C for 2 hours. Proteins were digested using proteinase K treatment for 1 hour at 45°C. The DNA associated with CBP was extracted with phenol/chloroform/isoamyl alcohol (25:24:1, pH 8.0), precipitated with 100% ethanol, then resuspended in 30 μL of PCR-grade water.
The quality and quantity of DNA were determined using a NanoDrop spectrophotometer (Thermo Scientific). DNA was amplified using SYBR green fast mix (Applied Biosystems) using 5 ng of template and 0.25 μM concentration of each primer. The primer sequences designed to target the mouse Esr2 and Oxt promoters are listed in Table 1. Primers were designed to target the promoter region (<500 bp upstream from the transcription start site). Primers were validated for efficiency using genomic DNA. Omission of the chromatin sample was used as a control. Melting temperatures were monitored to ensure that there was only one peak. Cycle threshold values were normalized to input DNA and enrichment of CBP was calculated relative to mock (IGg) immunoprecipitation. Fold change values are expressed as relative to naïve controls.
Statistical analysis
All data were analyzed using GraphPad Prism 5 software (GraphPad Inc). Data that violated assumptions of normality and/or homogeneity of variance were analyzed by nonparametric statistics. Pup retrieval was analyzed by a mixed two-way ANOVA (treatment × time) with repeated measures on the second factor, followed by Bonferroni post hoc tests for planned comparisons between treatment groups at each time point. To examine changes in the initiation of pup retrieval from the first to the second T-maze test, difference scores were calculated for each animal by subtracting the latency to retrieve the first pup 1 month after experience from latency to retrieve the first pup 24 hours after experience. Therefore, a positive difference score reflects an increase in responsiveness and a negative difference score reflects a decrease in responsiveness. A Wilcoxon signed rank test against the hypothetical value of 0 (no change) was used to determine whether each treatment group had a significant change in retrieval speed. Student's t tests or one way/Kruskal-Wallis ANOVAs was used to determine group differences in relative quantification of mRNA or DNA. One sample from each group was identified as an outlier by the Grubb's test and was removed from statistical analysis. Correlations between gene expression and pup retrieval on the T-maze were calculated using a Pearson correlation matrix and linear regression to specifically examine whether the impact of HDACi treatment on correlations. For all data significance was set at P < .05, and two-tailed tests were used.
Results
Long-lasting effects of histone deacetyltransferase (HDAC) inhibition on maternal experience-dependent maternal care
In support of our hypothesis that maternal experience is consolidated into a long-lasting maternal memory via epigenetic mechanisms, we found that HDACi administration allowed subthreshold maternal experience to be consolidated into a maternal memory that persisted for up to 1 month later after administration. Virgin female mice treated with an HDACi during a subthreshold maternal experience retrieved more pups on the T-maze as compared with controls. In addition, females that ingested HDACi were faster to retrieve and group pups on the T-maze 24 hours after pup experience, and these effects persisted 1 month later (P < .05, Figure 1, B–E). HDACi treated females also showed an increase in maternal responsiveness from the first to second T-maze test. Females treated with HDACi initiated pup retrieval faster (median = 233 sec, P < .01) 1 month after the pup experience and HDACi treatment, whereas water-treated females showed no change (median 0, Figure 1F). There were no significant effects of HDACi treatment or time on latency to investigate the first pup in the maze (Figure 1G, P ≥ .3).
Long-lasting effects of HDAC inhibition and maternal experience on gene expression in the MPOA
Analysis of gene expression in the MPOA 1 month after initial maternal experience revealed a significant effect of previous HDACi treatment on the expression of some of the genes associated with pup retrieval in the T-maze 24 hours after maternal experience (37). Female mice treated with an HDACi during the experience phase showed a significant increase in Esr2 expression 1 month later, and a significant decrease in Oxt expression relative to controls (P < .05; Figure 2B). There were no significant differences in the expression of Crebbp or Oxtr between treatment groups (P ≥ .4). Recent evidence indicates that the consolidation and maintenance of certain experience-dependent behavioral changes depend on DNA methylation and are linked to dynamic alterations in DNMT expression; thus, we determined whether the expression of DNMTs (Dnmt1, Dnmt3a, Dnmt3b) were altered 1 month after maternal experience (39, 40, 46–49). HDACi facilitation of maternal experience was associated with a decrease in the de novo DNA methyltransferase, Dnmt3a (P < .05). In support of the hypothesis that long-lasting changes in maternal care are linked with a persistent up-regulation of estrogen receptor-β, Esr2 mRNA expression was significantly negatively correlated with latency to initiate and complete pup retrieval in the T-maze 1 month after maternal experience (P < .05, Figure 2, C and D).
Figure 2.
A, Time line of the experimental design. The HDACi, sodium butyrate, was administered in the drinking water for a total of 72 hours. Subthreshold maternal experience occurred on 2 consecutive days for 2 hours daily in the dam's home cage. Pup retrieval was measured in a novel T-maze 24 hours, and 1 month, later. Mice received no additional interaction with pups, or HDACi, during the 1-month interim. B, Analysis of gene expression by RT-qPCR in the MPOA 1 month after the last maternal experience. Data are means and SEMs. Group effects on Esr2, Oxt, and Dnmt3a expression in HDACi-treated (n = 7–8) vs control females (n = 6). *, Significantly different from corresponding control group, for each gene (P < .05). C, Latency to retrieve the first pup in the maze was negatively correlated with Esr2 expression (R2 = −0.60, P < .03, Ns = 6–8) D, Latency to retrieve the last pup in the maze was negatively correlated with Esr2 expression (R2 = −0.73, P < .01, Ns = 6–8).
The consolidation of maternal experience may involve recruitment of CBP to the Esr2 and Oxt promoters
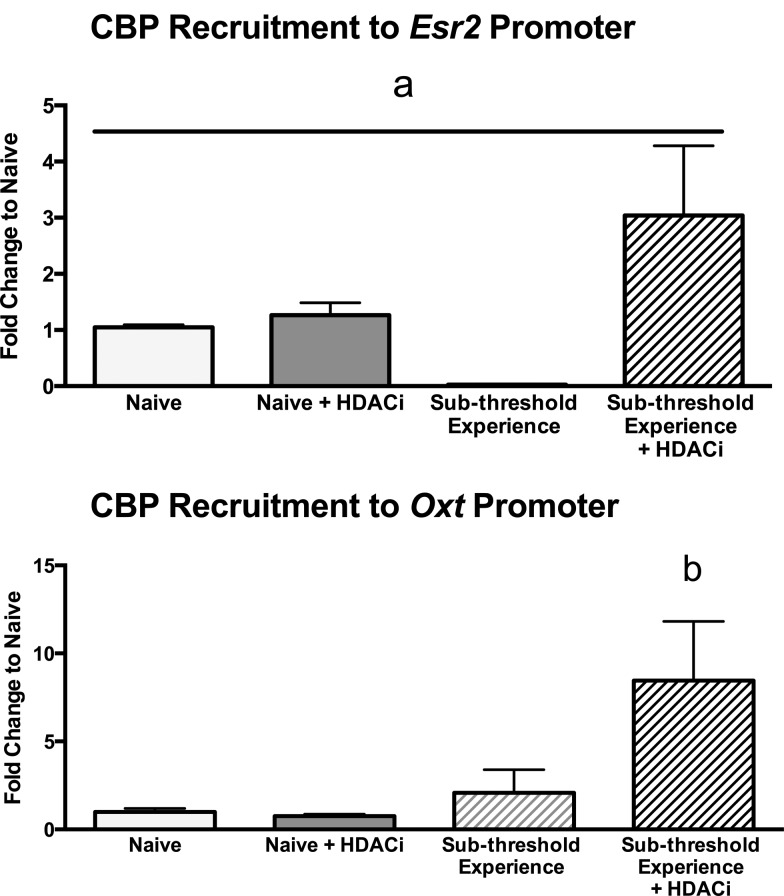
We have previously reported that 24 hours after the pup experience, HDACi amplified the expression of genes associated with optimal maternal experience in the MPOA in suboptimally experienced virgin females (Crebbp, Esr2, and Oxt) (37). Because CBP functions as a transcriptional coactivator of both CREB and ERβ as well as a histone acetylatransferase, and given that activation of intracellular signaling cascades that turn on CREB can increase the expression of both Esr2 and Oxt, we hypothesized that the change in Crebbp gene expression would reflect an increased activation and recruitment of CBP to the Esr2 and Oxt promoters during the consolidation of maternal experience. To test this hypothesis, we used ChIP followed by qPCR to determine the extent to which maternal experience-associated increases in gene expression are driven by recruitment of the CBP. ChIP analyses of the Esr2 and Oxt promoters indicated that CBP recruitment to both the Esr2 and Oxt promoters was significantly different among treatment groups (P < .05; Figure 3). Post hoc analyses reveal a significant difference between females treated with HDACi during subthreshold pup experience and all other groups in CBP recruitment to the promoter of Oxt gene (P < .05).
Figure 3.
A, Time line of the experimental design. The HDACi, sodium butyrate, was administered in the drinking water for a total of 72 hours. Subthreshold maternal experience occurred on 2 consecutive days for 2 hours daily in the dam's home cage. B, Data are normalized to naïve group and expressed as medians, with error bars representing interquartile range. a, Significant difference between medians, Kruskal Wallis (H = 6.167, P < .05). C, b, Significant difference between females treated with HDACi during subthreshold pup experience and all other groups (F3,12 = 4.018, P < .05). Data are expressed as mean ± SEM.
Discussion
The present data provide evidence for epigenetic regulation of the consolidation of maternal experience and maintenance of maternal memory. We have previously reported that alterations in gene expression occur during the consolidation of maternal experience (37). The present data are the first to uncover alterations in gene expression that are associated with the maintenance of maternal memory. Furthermore, these results suggest that one mechanism through which HDACi allows brief, subthreshold maternal experience to induce lasting changes in maternal behavior and gene expression involves recruitment of CBP to the promoter regions of Esr2 and Oxt genes. Thus, the present data show that the epigenetic mechanisms sustain maternal memory in the absence of continued maternal experience, for at least 1 month.
Dynamic fluctuations in pregnancy hormones, which synchronize the onset of maternal responses with birth, also function to facilitate the consolidation of maternal memory. Although it is clear pregnancy hormone stimulation is not required for maternal experiences to be consolidated into lasting maternal memory, the critical question of what molecular mechanisms specifically support the consolidation of female-infant interactions has not been resolved. Thus, here we have experimentally teased apart effects of social experience alone on gene expression in the MPOA. We have hypothesized that hormonal and experiential stimulation of maternal behavior share common molecular mechanisms (50). For example, CBP is a coactivator of estrogen receptors. Dimerization of estrogen receptor-β upon ligand binding recruits CBP and drives the expression of the estrogen-responsive genes (51–55). Thus, increased activation of estrogen receptor-β could drive the expression of the Oxt, which contains an estrogen response element that seems to be driven by estrogen receptor-β exclusively (51). Estrogen receptor-β and oxytocin proteins are coexpressed in neurons of the paraventricular nucleus of the hypothalamus (56). Recent data indicate that oxytocin activity is required for the anxiolytic effects of an estrogen receptor-β agonist, suggesting that these two genes may interact to regulate anxiety-like behavior. The present data, which indicate that CBP is recruited to the promoter region of Esr2 and Oxt in maternally experienced virgin mice, support the idea that increased activation of these genes may play an important role in the consolidation of maternal experience in the MPOA. However, the extent to which Esr2 directly drives Oxt expression is not clear at this time.
Experience-driven changes in estrogen receptor expression are known to be associated with maternal responsiveness. For example, early-life experiences can induce stable patterns of estrogen receptors (α and β) and oxytocin receptor expression via epigenetic mechanisms (28, 57, 58). We have previously found that experiences in adulthood, which also impact maternal responsiveness, can alter the expression of Esr2 and Oxt (but not estrogen receptor-α and oxytocin receptor) (37). Here we report that the alterations induced by maternal experience persist for up to 1 month. We speculate that CBP recruitment to the Esr2 promoter may be involved in the persistent up-regulation of Esr2 expression 1 month after maternal experience consolidation, whereas CBP recruitment to the Oxt promoter may reflect a transient regulation of Oxt during maternal experience consolidation because Oxt expression was no longer up-regulated one month later. This finding is in agreement with the idea that oxytocin is involved in the onset of maternal behavior but not required for its maintenance (59). Furthermore, the increase in Oxt expression in control mice with subthreshold levels of experience compared with HDACi-treated females may be related to the brief interaction with pups on the T-maze. These control mice have yet to reach the threshold level of experience necessary to promote pup retrieval in the T-maze and therefore may still be in the process of consolidating maternal experiences (60). Perhaps one way that HDACi treatment maintains behavioral and gene expression changes is by setting into motion a new pattern in the expression of transcriptional or translational regulatory genes that can maintain maternal memory (41).
It is becoming increasingly clear that memory consolidation involves chromatin remodeling and HDAC inhibitors have been instrumental in these investigations (38, 61–67). Memories consolidated during HDACi treatment as well as the associated gene expression changes are maintained for long periods of time (41, 68–70). There is good evidence that certain HDAC enzymes (classes I and IIa) are involved in memory consolidation. Administration of HDACi drugs that affect classes I and IIa HDACS, such as valproic acid, trichostatin A, suberoylanilide hydroxamic acid, and sodium butyrate, have all been found to enhance learning and memory. When directly compared, the behavioral effects of different HDACi drugs are not significantly different (71, 72). Thus, we speculate that administration of valproic acid, trichostatin A, or suberoylanilide hydroxamic acid would produce similar effects on maternal learning as sodium butyrate. Specifically, class I HDACs 1–3, 8 have been found to act as memory consolidation break enzymes by interfering with CREB-mediated gene transcription and/or CBP-mediated histone acetylation (73–75). Future work will need to examine the effects of selective class I HDACi drug MS275 on maternal learning (72) as well as the extent to which maternal memory is associated with long-term changes in HDAC expression. Despite the fact that sodium butyrate can inhibit eight of the 12 HDAC enzymes, it should be noted that only a small percentage of genes seem to be affected (74, 76). Instead of globally altering gene transcription, HDAC inhibition seems to affect active loci exclusively (64, 76). Specifically, HDACi treatment is frequently reported to affect estrogen receptor and estrogen-responsive genes as well as genes whose protein products modify chromatin, such as Crebbp, Dnmt1, Dnmt3a, and Dnmt3b (77, 78). Furthermore, despite the frequent use of systemic administration, we and others find that HDACi effects on learning and memory are not associated with a decrease in anxiety or a general increase in exploration (37, 43, 79).
The mechanisms through which HDACi facilitates memory consolidation are currently being elucidated. It is clear that facilitatory effects of HDACi on memory consolidation require CBP-mediated histone acetylation (38, 80). Recent reports have also emphasized the role of DNA methylation in memory consolidation (81). DNA methylation is mediated by DNA methyltransferases, enzymes that catalyze the transfer of methyl groups to cytosine residues followed by guanine. Dnmt3a and Dnmt3b are de novo methylators, which methylate previously unmethylated cytosines. Dnmt1 recognizes hemimethylated DNA and functions to stabilize methyl marks ensuring that they are not lost over time. Because alterations in DNMT gene expression may affect the methylation state of genes that facilitate or suppress the consolidation of a learning experience, it can be difficult to interpret global changes in DNMT expression (82). For example, an increase in Dnmt3a gene expression is associated with estradiol-facilitated memory consolidation (39). In contrast, a decrease in Dnmt3a mRNA expression is associated with cocaine reward (83). Other work has suggested that Dnmt3a mRNA expression is dynamically altered after social experience: increased at 4 hours and decreased at 24 hours (47). Alterations in DNMT activity are best interpreted with respect to the specific genes that are affected. However, in the studies discussed above, as well as the present study, it is not clear which genes are directly affected by alterations in Dnmt3a activity or expression.
Like other forms of memory, maternal memory is associated with both transient and persistent changes in gene expression (41). The facilitatory effect of HDACi on the consolidation of maternal memory is associated with a long-lasting down-regulation in Dnmt3a expression and a long-lasting up-regulation in Esr2 expression. Considering that HDACi treatment is associated with increased access of transcriptional machinery to regulatory regions of genes, it is logical that Dnmt3a expression, which would oppose this effect, is down-regulated. Although we have not examined DNA methylation here, we speculate that decreases in Dnmt3a activity may be related with the increase in Esr2 expression. In support of this idea, HDACi and inhibitors of DNA methylation have synergistic effects on Esr2 gene expression in vitro (77). However, note that these data must be interpreted with some caution until we know where in the genome Dnmt3a recruitment is depleted and there is a reduction in methylation.
Together the results of the present study indicate that the combination of HDACi and maternal experience results in a prolonged increase of maternal responsiveness and Esr2 expression, which may be mediated by CBP recruitment to the Esr2 gene promoter during the consolidation of maternal experience and maintained by alterations in Dnmt3a activity 1 month later. These data are a first step toward resolving the mechanism through which maternal experience induces long-lasting changes in gene expression within the MPOA.
Acknowledgments
We thank A. Ryalls and S. Shetty for their outstanding technical assistance.
This work was supported by National Institutes of Health Grants T32 DK007646 (to D.S.S.) and R01 MH057759.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- CBP
- CREB binding protein
- ChIP
- chromatin immunoprecipitation
- CREB
- cAMP response element binding protein
- DNMT
- DNA methyltransferase
- HDAC
- histone deacetyltransferase
- HDACi
- HDAC inhibitor
- MPOA
- medial preoptic area
- PMSF
- phenylmethylsulfonyl fluoride
- qPCR
- quantitative PCR
- SB
- sodium butyrate
- SDS
- sodium dodecyl sulfate.
References
- 1. Numan M, Insel TR. The Neurobiology of Parental Behavior. New York: Springer-Verlag; 2003 [Google Scholar]
- 2. Fleming AS, O'Day DH, Kraemer GW. Neurobiology of mother-infant interactions: experience and central nervous system plasticity across development and generations. Neurosci Biobehav Rev. 1999;23(5):673–685 [DOI] [PubMed] [Google Scholar]
- 3. Orpen BG, Fleming AS. Experience with pups sustains maternal responding in postpartum rats. Physiol Behav. 1987;40(1):47–54 [DOI] [PubMed] [Google Scholar]
- 4. O'Connor S, Vietze PM, Sherrod KB, Sandler HM, Altemeier WA., 3rd Reduced incidence of parenting inadequacy following rooming-in. Pediatrics. 1980;66(2):176–182 [PubMed] [Google Scholar]
- 5. Li M, Fleming AS. Differential involvement of nucleus accumbens shell and core subregions in maternal memory in postpartum female rats. Behav Neurosci. 2003;117(3):426–445 [DOI] [PubMed] [Google Scholar]
- 6. Klaus MH, Jerauld R, Kreger NC, McAlpine W, Steffa M, Kennel JH. Maternal attachment. Importance of the first post-partum days. N Engl J Med. 1972;286(9):460–463 [DOI] [PubMed] [Google Scholar]
- 7. Kennell JH, Klaus MH. Bonding: recent observations that alter perinatal care. Pediatr Rev. 1998;19(1):4–12 [PubMed] [Google Scholar]
- 8. Fleming AS, Korsmit M, Deller M. Rat pups are potent reinforcers to the maternal animal: effects of experience, parity, hormones, and dopamine function. Psychobiology. 1994;22:44–53 [Google Scholar]
- 9. Erlandsson K, Dsilna A, Fagerberg I, Christensson K. Skin-to-skin care with the father after cesarean birth and its effect on newborn crying and prefeeding behavior. Birth. 2007;34(2):105–114 [DOI] [PubMed] [Google Scholar]
- 10. Bystrova K, Ivanova V, Edhborg M, et al. Early contact versus separation: effects on mother-infant interaction one year later. Birth. 2009;36(2):97–109 [DOI] [PubMed] [Google Scholar]
- 11. Buranasin B. The effects of rooming-in on the success of breastfeeding and the decline in abandonment of children. Asia Pac J Public Health. 1991;5(3):217–220 [DOI] [PubMed] [Google Scholar]
- 12. Bridges RS. Long-term effects of pregnancy and parturition upon maternal responsiveness in the rat. Physiol Behav. 1975;14(3):245–249 [DOI] [PubMed] [Google Scholar]
- 13. Olazábal DE, Pereira M, Agrati D, et al. Flexibility and adaptation of the neural substrate that supports maternal behavior in mammals. Neurosci Biobehav Rev. 2013;37(8):1875–1892 [DOI] [PubMed] [Google Scholar]
- 14. Kinsley CH, Lambert KG. Reproduction-induced neuroplasticity: natural behavioural and neuronal alterations associated with the production and care of offspring. J Neuroendocrinol. 2008;20(4):515–525 [DOI] [PubMed] [Google Scholar]
- 15. Kim P, Leckman JF, Mayes LC, Feldman R, Wang X, Swain JE. The plasticity of human maternal brain: longitudinal changes in brain anatomy during the early postpartum period. Behav Neurosci. 2010;124(5):695–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fleming AS, Korsmit M. Plasticity in the maternal circuit: effects of maternal experience on Fos-Lir in hypothalamic, limbic, and cortical structures in the postpartum rat. Behav Neurosci. 1996;110(3):567–582 [DOI] [PubMed] [Google Scholar]
- 17. Numan M. Hypothalamic neural circuits regulating maternal responsiveness toward infants. Behav Cogn Neurosci Rev. 2006;5(4):163–190 [DOI] [PubMed] [Google Scholar]
- 18. Pérez-Laso C, Rubio S, Martin JL, Gomez F, Segovia S, Del Cerro MC. Differential regional brain responses to induced maternal behavior in rats measured by cytochrome oxidase immunohistochemistry. Behav Brain Res. 2011;223(2):293–296 [DOI] [PubMed] [Google Scholar]
- 19. Numan M, Roach JK, del Cerro MC, et al. Expression of intracellular progesterone receptors in rat brain during different reproductive states, and involvement in maternal behavior. Brain Res. 1999;830(2):358–371 [DOI] [PubMed] [Google Scholar]
- 20. Numan M, Numan MJ, Marzella SR, Palumbo A. Expression of c-fos, fos B, and egr-1 in the medial preoptic area and bed nucleus of the stria terminalis during maternal behavior in rats. Brain Res. 1998;792(2):348–352 [DOI] [PubMed] [Google Scholar]
- 21. Numan M, Numan MJ. Expression of Fos-like immunoreactivity in the preoptic area of maternally behaving virgin and postpartum rats. Behav Neurosci. 1994;108(2):379–394 [DOI] [PubMed] [Google Scholar]
- 22. Meddle SL, Bishop VR, Gkoumassi E, van Leeuwen FW, Douglas AJ. Dynamic changes in oxytocin receptor expression and activation at parturition in the rat brain. Endocrinology. 2007;148(10):5095–5104 [DOI] [PubMed] [Google Scholar]
- 23. Mann PE, Bridges RS. Prolactin receptor gene expression in the forebrain of pregnant and lactating rats. Brain Res Mol Brain Res. 2002;105(1–2):136–145 [DOI] [PubMed] [Google Scholar]
- 24. Kuroda KO, Meaney MJ, Uetani N, Fortin Y, Ponton A, Kato T. ERK-FosB signaling in dorsal MPOA neurons plays a major role in the initiation of parental behavior in mice. Mol Cell Neurosci. 2007;36(2):121–131 [DOI] [PubMed] [Google Scholar]
- 25. Gammie SC, Hasen NS, Awad TA, et al. Gene array profiling of large hypothalamic CNS regions in lactating and randomly cycling virgin mice. Brain Res Mol Brain Res. 2005;139(2):201–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ehret G, Buckenmaier J. Estrogen-receptor occurrence in the female mouse brain: effects of maternal experience, ovariectomy, estrogen and anosmia. J Physiol Paris. 1994;88(5):315–329 [DOI] [PubMed] [Google Scholar]
- 27. Del Cerro MC, Perez Izquierdo MA, Rosenblatt JS, Johnson BM, Pacheco P, Komisaruk BR. Brain 2-deoxyglucose levels related to maternal behavior-inducing stimuli in the rat. Brain Res. 1995;696(1–2):213–220 [DOI] [PubMed] [Google Scholar]
- 28. Champagne FA, Weaver IC, Diorio J, Sharma S, Meaney MJ. Natural variations in maternal care are associated with estrogen receptor α expression and estrogen sensitivity in the medial preoptic area. Endocrinology. 2003;144(11):4720–4724 [DOI] [PubMed] [Google Scholar]
- 29. Byrnes EM, Babb JA, Bridges RS. Differential expression of oestrogen receptor α following reproductive experience in young and middle-aged female rats. J Neuroendocrinol. 2009;21(6):550–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Akbari EM, Shams S, Belay HT, et al. The effects of parity and maternal behavior on gene expression in the medial preoptic area and the medial amygdala in postpartum and virgin female rats: a microarray study. Behav Neurosci. 2013;127(6):913–922 [DOI] [PubMed] [Google Scholar]
- 31. Shams S, Pawluski JL, Chatterjee-Chakraborty M, Oatley H, Mastroianni A, Fleming AS. Dendritic morphology in the striatum and hypothalamus differentially exhibits experience-dependent changes in response to maternal care and early social isolation. Behav Brain Res. 2012;233(1):79–89 [DOI] [PubMed] [Google Scholar]
- 32. Salmaso N, Cossette MP, Woodside B. Pregnancy and maternal behavior induce changes in glia, glutamate and its metabolism within the cingulate cortex. PLoS One. 2011;6(9):e23529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Featherstone RE, Fleming AS, Ivy GO. Plasticity in the maternal circuit: effects of experience and partum condition on brain astrocyte number in female rats. Behav Neurosci. 2000;114(1):158–172 [DOI] [PubMed] [Google Scholar]
- 34. Fleming AS, Walsh C. Neuropsychology of maternal behavior in the rat: c-fos expression during mother-litter interactions. Psychoneuroendocrinology. 1994;19(5–7):429–443 [DOI] [PubMed] [Google Scholar]
- 35. Jin SH, Blendy JA, Thomas SA. Cyclic AMP response element-binding protein is required for normal maternal nurturing behavior. Neuroscience. 2005;133(3):647–655 [DOI] [PubMed] [Google Scholar]
- 36. Kim J, Kwon JT, Kim HS, Josselyn SA, Han JH. Memory recall and modifications by activating neurons with elevated CREB. Nat Neurosci. 2014;17(1):65–72 [DOI] [PubMed] [Google Scholar]
- 37. Stolzenberg DS, Stevens JS, Rissman EF. Experience-facilitated improvements in pup retrieval; evidence for an epigenetic effect. Horm Behav. 2012;62(2):128–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Korzus E, Rosenfeld MG, Mayford M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron. 2004;42(6):961–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhao Z, Fan L, Frick KM. Epigenetic alterations regulate estradiol-induced enhancement of memory consolidation. Proc Natl Acad Sci USA. 2010;107(12):5605–5610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Monsey MS, Ota KT, Akingbade IF, Hong ES, Schafe GE. Epigenetic alterations are critical for fear memory consolidation and synaptic plasticity in the lateral amygdala. PLoS One. 2011;6(5):e19958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Miller CA, Gavin CF, White JA, et al. Cortical DNA methylation maintains remote memory. Nat Neurosci. 2010;13(6):664–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Minamiyama M, Katsuno M, Adachi H, et al. Sodium butyrate ameliorates phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Hum Mol Genet. 2004;13(11):1183–1192 [DOI] [PubMed] [Google Scholar]
- 43. Bonthuis PJ, Patteson JK, Rissman EF. Acquisition of sexual receptivity: roles of chromatin acetylation, estrogen receptor-α, and ovarian hormones. Endocrinology. 2011;152(8):3172–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wolstenholme JT, Taylor JA, Shetty SR, Edwards M, Connelly JJ, Rissman EF. Gestational exposure to low dose bisphenol a alters social behavior in juvenile mice. PLoS One. 2011;6(9):e25448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Braveman MW, Chen-Plotkin AS, Yohrling GJ, Cha JH. Chromatin immunoprecipitation technique for study of transcriptional dysregulation in intact mouse brain. Methods Mol Biol. 2004;277:261–276 [DOI] [PubMed] [Google Scholar]
- 46. Day JJ, Sweatt JD. DNA methylation and memory formation. Nat Neurosci. 2010;13(11):1319–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. LaPlant Q, Vialou V, Covington HE, 3rd, et al. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat Neurosci. 2010;13(9):1137–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Feng J, Chang H, Li E, Fan G. Dynamic expression of de novo DNA methyltransferases Dnmt3a and Dnmt3b in the central nervous system. J Neurosci Res. 2005;79(6):734–746 [DOI] [PubMed] [Google Scholar]
- 49. Feng J, Zhou Y, Campbell SL, et al. Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat Neurosci. 2010;13(4):423–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stolzenberg DS, Numan M. Hypothalamic interaction with the mesolimbic DA system in the control of the maternal and sexual behaviors in rats. Neurosci Biobehav Rev. 2011;35(3):826–847 [DOI] [PubMed] [Google Scholar]
- 51. Patisaul HB, Scordalakes EM, Young LJ, Rissman EF. Oxytocin, but not oxytocin receptor, is regulated by oestrogen receptor β in the female mouse hypothalamus. J Neuroendocrinol. 2003;15(8):787–793 [DOI] [PubMed] [Google Scholar]
- 52. Edwards DP. The role of coactivators and corepressors in the biology and mechanism of action of steroid hormone receptors. J Mammary Gland Biol Neoplasia. 2000;5(3):307–324 [DOI] [PubMed] [Google Scholar]
- 53. Duong V, Licznar A, Margueron R, et al. ERα and ERβ expression and transcriptional activity are differentially regulated by HDAC inhibitors. Oncogene. 2006;25(12):1799–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cheung E, Schwabish MA, Kraus WL. Chromatin exposes intrinsic differences in the transcriptional activities of estrogen receptors α and β. EMBO J. 2003;22(3):600–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sharma D, Handa RJ, Uht RM. The ERβ ligand 5α-androstane, 3β,17β-diol (3β-diol) regulates hypothalamic oxytocin (Oxt) gene expression. Endocrinology. 2012;153(5):2353–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kudwa AE, MicGivern RF, Handa RJ. Estrogen receptor β and oxytocin interact to modulate anxiety-like behavior and neuroendocrine stress reactivity in adult male and female rats. Physiol Behav. 2014;129(22):287–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Peña CJ, Neugut YD, Champagne FA. Developmental timing of the effects of maternal care on gene expression and epigenetic regulation of hormone receptor levels in female rats. Endocrinology. 2013;154(11):4340–4351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Champagne FA, Weaver IC, Diorio J, Dymov S, Szyf M, Meaney MJ. Maternal care associated with methylation of the estrogen receptor-α1b promoter and estrogen receptor-α expression in the medial preoptic area of female offspring. Endocrinology. 2006;147(6):2909–2915 [DOI] [PubMed] [Google Scholar]
- 59. Fahrbach SE, Morrell JI, Pfaff DW. Possible role for endogenous oxytocin in estrogen-facilitated maternal behavior in rats. Neuroendocrinology. 1985;40(6):526–532 [DOI] [PubMed] [Google Scholar]
- 60. Lopatina O, Inzhutova A, Pichugina YA, Okamoto H, Salmina AB, Higashida H. Reproductive experience affects parental retrieval behaviour associated with increased plasma oxytocin levels in wild-type and CD38-knockout mice. J Neuroendocrinol. 2011;23(11):1125–1133 [DOI] [PubMed] [Google Scholar]
- 61. Yeh SH, Lin CH, Gean PW. Acetylation of nuclear factor-κB in rat amygdala improves long-term but not short-term retention of fear memory. Mol Pharmacol. 2004;65(5):1286–1292 [DOI] [PubMed] [Google Scholar]
- 62. Yeh KY, Pu HF, Wu CH, Tai MY, Tsai YF. Different subregions of the medial preoptic area are separately involved in the regulation of copulation and sexual incentive motivation in male rats: a behavioral and morphological study. Behav Brain Res. 2009;205(1):219–225 [DOI] [PubMed] [Google Scholar]
- 63. Alarcón JM, Malleret G, Touzani K, et al. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron. 2004;42(6):947–959 [DOI] [PubMed] [Google Scholar]
- 64. Vecsey CG, Hawk JD, Lattal KM, et al. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J Neurosci. 2007;27(23):6128–6140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Malvaez M, Sanchis-Segura C, Vo D, Lattal KM, Wood MA. Modulation of chromatin modification facilitates extinction of cocaine-induced conditioned place preference. Biol Psychiatry. 2010;67(1):36–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Roozendaal B, Hernandez A, Cabrera SM, et al. Membrane-associated glucocorticoid activity is necessary for modulation of long-term memory via chromatin modification. J Neurosci. 2010;30(14):5037–5046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Levenson JM, O'Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279(39):40545–40559 [DOI] [PubMed] [Google Scholar]
- 68. Stefanko DP, Barrett RM, Ly AR, Reolon GK, Wood MA. Modulation of long-term memory for object recognition via HDAC inhibition. Proc Natl Acad Sci USA. 2009;106(23):9447–9452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Intlekofer KA, Berchtold NC, Malvaez M, et al. Exercise and sodium butyrate transform a subthreshold learning event into long-term memory via a brain-derived neurotrophic factor-dependent mechanism. Neuropsychopharmacology. 2013;38(10):2027–2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dagnas M, Guillou JL, Prevot T, Mons N. HDAC inhibition facilitates the switch between memory systems in young but not aged mice. J Neurosci. 2013;33(5):1954–1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kilgore M, Miller CA, Fass DM, et al. Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer's disease. Neuropsychopharmacology. 2010;35(4):870–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hawk JD, Florian C, Abel T. Post-training intrahippocampal inhibition of class I histone deacetylases enhances long-term object-location memory. Learn Mem. 2011;18(6):367–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Canettieri G, Morantte I, Guzman E, et al. Attenuation of a phosphorylation-dependent activator by an HDAC-PP1 complex. Nat Struct Biol. 2003;10(3):175–181 [DOI] [PubMed] [Google Scholar]
- 74. Morris MJ, Monteggia LM. Unique functional roles for class I class II histone deacetylases in central nervous system development and function. Int J Dev Neurosci. 2013;31(6):370–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gao J, Siddoway B, Huang Q, Xia H. Inactivation of CREB mediated gene transcription by HDAC8 bound protein phosphatase. Biochem Biophys Res Commun. 2009;379(1):1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lopez-Atalaya JP, Ito S, Valor LM, Benito E, Barco A. Genomic targets, and histone acetylation and gene expression profiling of neural HDAC inhibition. Nucleic Acids Res. 2013;41(17):8072–8084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Walton TJ, Li G, Seth R, McArdle SE, Bishop MC, Rees RC. DNA demethylation and histone deacetylation inhibition co-operate to re-express estrogen receptor β and induce apoptosis in prostate cancer cell-lines. Prostate. 2008;68(2):210–222 [DOI] [PubMed] [Google Scholar]
- 78. Xiong Y, Dowdy SC, Podratz KC, et al. Histone deacetylase inhibitors decrease DNA methyltransferase-3B messenger RNA stability and down-regulate de novo DNA methyltransferase activity in human endometrial cells. Cancer Res. 2005;65(7):2684–2689 [DOI] [PubMed] [Google Scholar]
- 79. Covington HE, 3rd, Maze I, LaPlant QC, et al. Antidepressant actions of histone deacetylase inhibitors. J Neurosci. 2009;29(37):11451–11460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Korzus E. Manipulating the brain with epigenetics. Nat Neurosci. 2010;13(4):405–406 [DOI] [PubMed] [Google Scholar]
- 81. Day JJ, Sweatt JD. Cognitive neuroepigenetics: a role for epigenetic mechanisms in learning and memory. Neurobiol Learn Mem. 2011;96(1):2–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007;53(6):857–869 [DOI] [PubMed] [Google Scholar]
- 83. Laplant Q, Nestler EJ. CRACKing the histone code: cocaine's effects on chromatin structure and function. Horm Behav. 2010;59(3):321–330 [DOI] [PMC free article] [PubMed] [Google Scholar]