Abstract
Repeated administration of the long-acting glucagon-like peptide 1 receptor agonist exendin-4 (EX-4) has been shown to reduce food intake and body weight and do so without a rebound increase in food intake after treatment termination. The current study examines the neural mechanisms underlying these actions. After 6 weeks of maintenance on a standard chow or a high-fat (HF) diet, male Sprague Dawley rats were treated with EX-4 (3.2 μg/kg, ip, twice a day) or vehicle for 9 consecutive days. Food intake and body weight (BW) were monitored daily. Expression of the genes for the hypothalamic arcuate nucleus (ARC) peptides proopiomelanocortin (POMC), neuropeptide Y (NPY), and agouti gene-related protein was determined. Expression of the dopamine precursor tyrosine hydroxylase (TH) gene in the ventral tegmental area and genes for dopamine receptors 1 (D1R) and dopamine receptor 2 in the nucleus accumbens were also determined. Pair-fed groups were included to control for the effects of reduced food intake and BW. Treatment with EX-4 significantly decreased food intake and BW over the 9-day period in both the standard chow and HF groups. HF feeding decreased POMC without changing NPY/agouti gene-related protein gene expression in the ARC. Treatment with EX-4 increased POMC and decreased NPY expression independent of the reduction of food intake and BW. Mesolimbic TH and D1R gene expression were decreased significantly in chronic HF diet-fed rats, and these changes were reversed in both EX-4 and pair-fed conditions. These results suggest a role for increased POMC and decreased NPY expression in the ARC in the effects of EX-4 on food intake and BW. Our findings also suggest that EX-4 induced the recovery of mesolimbic TH and D1R expression in HF diet-fed rats may be secondary to HF intake reduction and/or weight loss.
The worldwide obesity epidemic has prompted an urgent need to increase the understanding of the neurobiological mechanisms underlying overeating and to develop new effective treatments for obesity and eating disorders (1–3). Eating beyond immediate metabolic need is a key component of weight gain and subsequent obesity. High-calorie palatable foods can reinforce their own intake by increasing the pleasure associated with eating (4). Therefore, effective future obesity treatments should ideally impact not only the mechanisms underlying hunger and satiety but also the mechanisms mediating food reward.
The neurobiology underlying the controls of both homeostatic feeding and food reward is emerging. Peptides and hormones from the gastrointestinal track and from adipose tissues are now known to impact feeding behavior and energy homeostasis (5–9). These signals interact directly or indirectly with hypothalamic and hindbrain systems in the regulation of energy balance (10). Furthermore, dopaminergic projections from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) play important roles in the rewarding aspects of ingestive behaviors (4, 11, 12). Although energy intake is mediated by multiple mechanisms, compounds that can affect energy homeostasis and food reward simultaneously may provide promising antiobesity outcomes.
Glucagon-like peptide 1 (7–36; GLP-1) is a 30-amino acid peptide that is secreted from L cells in the distal intestine in response to the ingestion of nutrients. GLP-1 stimulates insulin while inhibiting glucagon secretion, enhancing the disposal of glucose. GLP-1 has also been demonstrated to alter gastrointestinal function and decrease food intake (13, 14).
Endogenous GLP-1 is rapidly degraded by dipeptidyl peptidase-4 (DPP-IV) and thus is not an ideal pharmacological agent for studying the actions of GLP-1 signaling in reducing food intake or improving glucose metabolism. However, a number of long acting GLP-1 analogs that are resistant to degradation by DPP-IV has been identified. Exendin-4 (EX-4) is a 39-amino acid incretin mimetic initially isolated from the saliva of the Gila monster that shares 53% homology and similar function to GLP-1 and is resistant to degradation by DPP-IV (15). EX-4 (marketed as Byetta) is a Food and Drug Administration-approved drug for the treatment of type 2 diabetes (16). Clinical research has indicated that diabetic subjects treated with EX-4 show not only improvement of glucose metabolism but also substantial weight loss (17). The mechanisms underlying the beneficial effects of EX-4 on obesity, however, are still not clear (18–21).
Centrally, GLP-1 is produced exclusively by neurons in the nucleus of the solitary tract (22–24). These neurons project to multiple forebrain GLP-1 receptor (GLP-1R) expressing regions including areas mediating both energy homeostasis and reward (25). To date, substantial research has demonstrated that central injections of GLP-1 or GLP-1 analogs such as EX-4 can decrease food intake (26–28) and activate brain circuits such as the arcuate nucleus (ARC) and paraventricular nucleus of the hypothalamus (29) that are involved in metabolic control. Recent findings that the administration of GLP-1 or EX-4 into reward-mediating mesolimbic areas, eg, the VTA and NAc (24, 30, 31), results in reductions in food intake have led to the suggestion that GLP-1 signaling plays a role in food reward/motivation.
Given the potential of EX-4 to cross the blood-brain barrier and gain access to brain parenchyma (32), it is of considerable interest to determine the potential relative roles of hypothalamic homeostatic and mesolimbic reward systems in the reductions of food intake produced by peripheral EX-4 administration. In the present study, we first tested how repeated twice-daily peripheral administration of EX-4 affects energy balance in rats with differences in body weight (BW) as a consequence of chronic feeding with a standard chow (SC) or a palatable high-fat (HF) diet. After 9 days of EX-4 treatments, the expression of both homeostatic [neuropeptide Y (NPY), agouti gene-related protein (AgRP), and proopiomelanocortin (POMC)] and dopaminergic genes [tyrosine hydroxylase (TH), dopamine receptors 1 and 2 (D1R and D2R)] were examined. To assess whether EX-4-induced differences in behavior or physiological measures were a direct effect of EX-4 or secondary to decreases in food intake or body weight, we included both SC and HF diet pair-fed groups whose intakes were limited to that of those receiving the EX-4 treatment.
Materials and Methods
Animals
Adult male Sprague Dawley rats (Harlan Laboratories) weighing 250–275 g (n = 48) were housed individually under standard conditions (12 h light, 12 h dark cycle, lights on at 10:00 pm, 50%–60% humidity, and 22–25°C). During the period of habituation to housing, rats were maintained with ad libitum access to SC (Teklad Global 18% protein rodent diet, Harlan Laboratories). All animal procedures were approved by the Institutional Animal Care and Use Committee at Johns Hopkins University and conformed to the guidelines of the National Institutes of Health.
Drugs
EX-4 was purchased from BACHEM (7000921; Bachem Americas, Inc), dissolved in sterile water and stored at −80°C. EX-4 was taken to room temperature immediately prior to the ip injections. The dose used here (3.2 μg/kg) was chosen based on our previous study (33). This is a moderate dose that results in approximately 12% reduction of 20 hours intake without producing significant changes in the BW. To produce larger effects on intake and BW and to use strategies that resemble clinical use of EX-4, rats received this dose twice a day.
Food intake and BW testing
After a period of habituation to the laboratory (22 d), half the rats were randomly assigned to be maintained on SC (n = 24), and the other half were switched to the HF diet (n = 24) (D12492; Research Diets, Inc). Food intake and BW were measured daily. After 6 weeks of dietary pretreatment, injections with either EX-4 or vehicle were initiated. Eight rats from each diet group received EX-4 and eight received vehicle injections, resulting in four experimental groups: HF+EX-4; HF+VEHICLE; SC+EX-4; and SC+VEHICLE. The injections were performed twice a day: once at the beginning of the dark cycle (10:00 am) and once 8 hours into the dark cycle (6:00 pm) for 9 consecutive days. During the 9 days of the injections, food was removed 2 hours prior to the onset of the dark cycle and returned to the cages at lights out. Food intake was measured at1 hour, 2 hours, 4 hours, and 8 hours after lights off on the second, fourth, sixth, and ninth treatment days. The total food intake, water intake, and body mass were measured daily.
The remaining eight rats per diet group were used to control for effects secondary to a decrease in food intake and/or body weight caused by EX-4. They were food restricted to match the average amount of food eaten by the HF+EX-4 and SC+EX-4 groups during the 9 days of drug administration. Pair-fed rats fed with the HF diet are referred to as HF-PAIR FED, and rats fed with SC diet are referred to as SC-PAIR FED. The pair-fed animals did not receive any injections. During the period of pair feeding, any remaining food was removed 2 hours before the onset of the dark cycle and the day's ration given at lights out.
On the 10th day, after 9 consecutive days of injections, food was again removed 2 hours before the onset of the dark cycle, the rats received injections, and food was returned at lights out. The rats were then killed by decapitation 2 hours after lights out. Immediately before decapitation, blood glucose levels were measured using a 3-μL drop of blood obtained by nicking the tail vein and a One Touch Ultra link blood glucose meter (One Touch Ultra 2; Life Scan, Inc). Trunk blood was collected in EDTA-treated tubes. The blood was then centrifuged and the plasma was stored at −80°C. Brains were removed and snap frozen in isopentane on dry ice and stored at −80°C to be used later for analysis of gene expression.
Radioimmunoassay
The plasma levels of leptin and insulin were measured once at the end of the experiment using a rat leptin or total insulin RIA kits (Millipore) following the manufacturer's protocols.
RNA isolation
Coronal brain sections of 500 μm each were cut with a cryostat and thawed briefly on a precleaned glass slide. Tissue punches were then taken using a blunt 19-G needle and were immediately frozen on dry ice in ribonuclease-free microcentrifuge tubes. Punches for NAc, ARC, and VTA were taken from sections at +0.88 mm, −3.20 mm, and −6.40 mm from bregma, respectively. Total RNA was extracted from the samples using a TRIzol reagent (Invitrogen) following the manufacturer's protocol. The RNA quantity and quality was determined with a NanoDrop spectrophotometer (ND-1000; Thermo Fischer Scientific). Finally, 1 μL of a ribonuclease inhibitor was added into each sample. All samples were then stored at −80°C.
Reverse transcription-polymerase chain reaction
The QuantiTect reverse transcription kit (QIAGEN) was used to generate cDNA for quantitative real-time PCR. Negative reverse transcription samples were used to control for possible contamination of genomic DNA. All reactions were carried out in triplicate using 1× Taqman master mix (Applied Biosystems), 1× Taqman probes for each gene (POMC, NPY, AgRP, D1R, D2R, and TH), and 2 μg of cDNA template in a total volume of 20 μL. Real-time reactions were performed on an Applied Biosystems 7900HT Fast real-time PCR system with standard PCR conditions (50°C for 2 min; 95°C for 10 min; and 95°C for 15 sec and 60°C for 1 min for 40 cycles). Each set of triplicates was checked to ensure that the cycle threshold (Ct) values were all within 1 Ct of one another. To determine relative expression values, the −δδCt method (Applied Biosystems) was used, in which triplicate Ct values for each tissue sample were averaged and subtracted from those derived from the housekeeping gene β-actin. The average Ct difference for the control group was subtracted from those of the test samples, and the resulting −δδCt values were raised to a power of 2 to determine normalized relative expression.
Statistical analysis
All data are presented as mean ± SEM. All behavioral parameters were analyzed by a repeated-measures ANOVA followed by a post hoc Fisher least significant differences test as appropriate or a Student t test in which only two conditions were compared. Factors for three-way ANOVAs include the following: diet (HF vs SC), treatment (vehicle, EX-4, and pair fed) and time (days), whereas two-way ANOVAs include only the first two factors. All statistical analyses were conducted using Statistica (version 7.1). Differences were considered significant at P < .05.
Results
BW and food intake
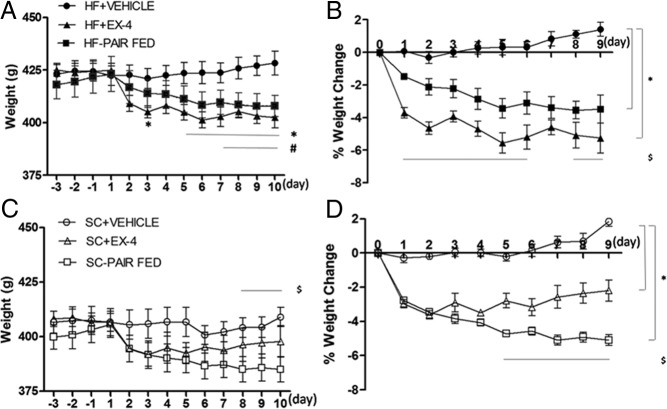
After 6 weeks, rats exposed to the HF diet weighed significantly more than those exposed to SC (HF diet, 421.85 ± 2.54 g vs SC diet, 405.01 ± 3.51 g, P < .01). Three-way repeated-measures ANOVA revealed significant effects of diet (F1,41 = 17.2, P < .001), time (F12, 492 = 65.6, P < .001), time × treatment (F24, 492 = 24.2, P < .001), and diet × treatment × time (F24, 492 = 3.0, P < .001). By the end of the 9 days of injections, the HF+EX-4 group weighed significantly less than the HF+VEHICLE group (428.4 ± 5.7 g, P < .01) and also weighed less than their pre-EX-4 baseline (pre-EX-4, 424.9 ± 3.2 g vs post-EX-4, 402.6 ± 4.9, P < .01 (Figure 1A). Similarly, the BW of the SC+EX-4 group decreased relative to their pre-EX-4 baseline (pre-EX-4, 405.8 ± 5.9 g vs post-EX-4, 397.8 ± 7.2 g, post hoc P < .001). Although SC+EX-4 weighed less than the SC+VEHICLE group (409.0 ± 4.2 g, Figure 1C) by the end of the EX-4 treatment, the difference did not reach a significant level (post hoc P > .1). Although the SC rats weighed less than the HF rats prior to EX-4 treatment, the BW of HF+EX-4 and SC+EX-4 did not differ by the end of 10 days of EX-4 injections (post hoc P > .5). Furthermore, restricting food intake in the PAIR-FED groups to the same amount consumed by EX-4-treated rats reduced BW significantly in both chow- (P < .001) and HF (P < .002)-fed conditions. Post hoc tests revealed that SC-PAIR FED (385.0 ± 5.8 g) weighed significantly less than the SC+VEHICLE and HF-PAIR FED (407.9 ± 5.1 g) by the end of 10 days of restriction (P < .007).
Figure 1.
Daily BW (A and C) and percentage weight change (B and D) in rats fed with a SC or a HF diet in response to EX-4, vehicle, or paired-fed treatments. Data represent mean ± SEM. A, Both EX-4 and paired-fed groups reduced BW in the HF feeding condition. *, P < .05 from HF+EX-4/HF+VEHICLE; #, P < .05 from HF-PAIR FED/HF+VEHICLE. B, The magnitude of BW reduction is more with EX-4 than with paired-fed treatment in HF feeding condition. *, P < .05 from HF+EX-4/HF+VEHICLE and HF-PAIR FAD/HF+VEHICLE; $, P < .05 from HF+EX-4/HF-PAIR FED. C, Paired fed but not EX-4 significantly reduced body weight in SC feeding condition. $, P < .05 from SC-PAIR FED/SC+VEHICLE. D, The magnitude of BW reduction is greater with paired-fed than with EX-4 treatment in SC feeding condition. *, P < .05 from SC+EX-4/SC+VEHICLE and SC-PAIR FED/SC+VEHICLE; $, P < .05 from SC+EX-4/SC-PAIR FED.
The magnitude of weight loss by EX-4 treatment or food restriction differed, depending on the diet exposure. Three-way ANOVA comparisons of percentage weight change revealed significant effects of treatment (F2, 41 = 73.0, P < .001), diet × treatment (F2, 41 = 9.7, P < .001), time (F8, 328 = 5.7, P < .001), treatment × time (F16, 328 = 12.1, P < .001), and diet × treatment × time (F16, 328 = 2.1, P < .008). These results are consistent with the absolute BW results in the fact that EX-4 treatment reduces BW in both feeding conditions (Figure 1, B and D). HF diet-fed rats lost significantly more weight than did the SC rats after long-term EX-4 treatment (HF+EX-4, −5.3% ± 0.9% vs SC+EX-4, −2.2% ± 0.6%, P < .001). Although restricting food intake can also reduce weight, the percentage BW analysis indicates that the food restriction effect is more pronounced in the SC feeding condition. That is, SC-PAIR FED lost more percentage weight than did the HF-PAIR FED throughout the 10 days of the restriction period (post hoc P < .05). Finally, EX-4 produced more percentage weight loss than did the food restriction in the HF feeding condition (HF+EX-4 vs HF-PAIR FED, −5.3% ± 0.9% vs −3.5% ± 0.9%, post hoc P < .007), whereas the opposite effects were found in SC feeding condition (SC+EX-4 vs SC-PAIR FED, −2.2% ± 0.6% vs −5.1% ± 0.3%, post hoc P < .001).
EX-4 reduced daily food intake persistently throughout the entire treatment period in HF rats but only for part of the treatment period in the SC rats. Three-way repeated-measures ANOVA revealed significant effects of diet (F1,28 = 14.3, P < .001), treatment (F1,28 = 51.2, P < .001), time (F11, 308 = 9.2, P < .001), time × treatment (F11, 308 = 4.7, P < .001) and diet × time (F11, 308 = 2.3, P = .02). In the HF feeding condition, EX-4 treatment significantly reduced food intake as compared with either HF+EX-4 baseline or the HF+VEHICLE on each day (Figure 2A, d 1 to d 9, P < .05). On the other hand, EX-4 injections significantly decreased daily total food intake only on days 1, 2, 4, and 6 as compared with either SC+EX-4 baseline or the corresponding day of SC+VEHICLE in SC feeding condition (Figure 2B, P < .04). There were no effects of Ex-4 treatment on water intake in either the SC or HF conditions (data not shown).
Figure 2.
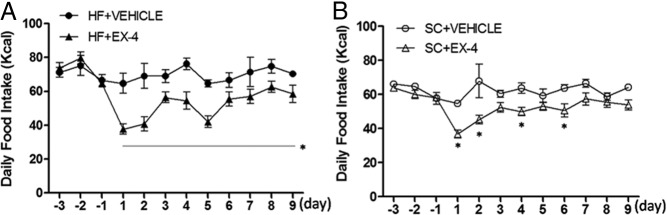
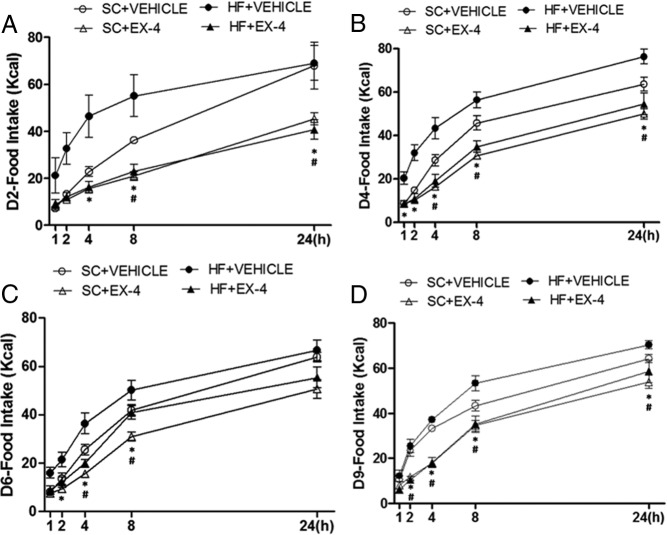
Daily food intake in rats fed with a HF diet (A) or a SC diet (B) in responding to EX-4 or vehicle treatments. Data represent mean ± SEM. A, EX-4 reduced daily food intake persistently throughout the treatment period with HF feeding. B, EX-4 reduced daily food intake during the first few but not the last 3 days of treatment with SC feeding.*, P < .05from HF+EX-4/HF+VEHICLE and SC+EX-4/SC+VEHICLE.
To further elucidate the effects of EX-4 on food intake, 1 hour, 2 hours,4 hours, 8 hours, and 24 hours of food intake was measured on days 2, 4, 6, and 9 (Figure 3, A–D) and were analyzed with a three-way ANOVA. The results indicate a significant effect of EX-4 treatment on each day (15.0 < F < 41.3, P < .001). There was a diet effect on days 4 and 6 (F = 11.2 and 7.6, P < .02). In general, EX-4 began to reduce food intake at earlier time points in HF than in SC feeding condition. Post hoc tests revealed that EX-4 treatment in HF feeding significantly reduced food intake beginning at 1 hour on day 4 and at 2 hours for the rest of the days measured. For the SC feeding condition, EX-4 significantly reduced food intake, beginning at 2 hours on day 9, 4 hours on days 4 and 6, and 8 hours on day 9.
Figure 3.
Food intake at1 hour, 2 hours, 4 hours, 8 hours, and 24 hours after the first injection of EX-4 or vehicle on the second (A), fourth (B), sixth (C). and ninth (D) days of the b.i.d. treatment. Data represent mean ± SEM. Overall, EX-4 reduced food intake beginning at an earlier time point in the HF than in the SC feeding condition. *, P < .05 from HF+EX-4/HF+VEHICLE; #, P < .05 from SC+EX-4/SC+VEHICLE.
Blood glucose, plasma leptin, and insulin levels
Rats were killed on the 10th day of injections after 2 hours of access to food and blood glucose was immediately measured. There were no differences in blood glucose between groups (data not shown). Results for plasma levels of leptin are shown in Figure 4A. A two-way ANOVA revealed significant effects of diet (F1,42 = 11.7, P < .002) and treatment (F2,42 = 9.1, P < .001) but not diet × treatment (F2, 42 = 2.3, P = .12). Plasma leptin was significantly elevated with chronic HF feeding (P < .002). For SC rats, neither EX-4 treatment nor food restriction changed plasma leptin levels because SC+EX-4 and SC-PAIR FED had similar levels to SC+VEHICLE rats (post hoc P > .1). Under the HF feeding condition, leptin level was decreased after 9 days of EX-4 treatment, but this reduction did not reach a statistical significance (post hoc P = .08). On the other hand, HF-PAIR FED had significantly lower levels of plasma leptin than both HF+VEHICLE and HF+EX-4 (post hoc P < .004).
Figure 4.
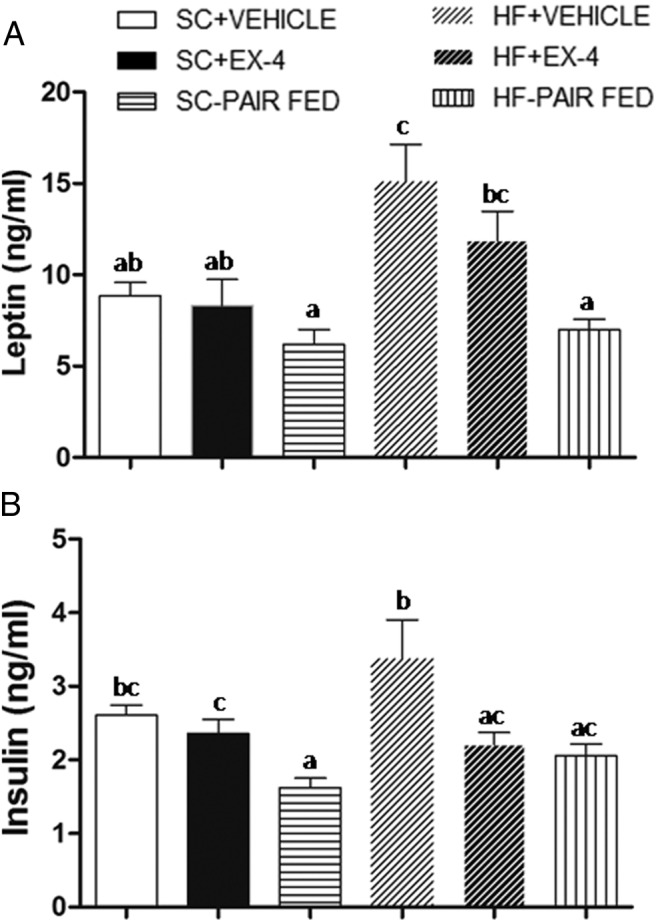
Plasma leptin (A) and insulin (B) levels at the end of vehicle, EX-4, or pair-fed treatments. Data represent mean ± SEM. In each panel, groups that share the same letter are not significantly different from each other after obtaining a significant main effect by a two-way ANOVA. A, HF feeding increased plasma leptin levels, and food restriction by paired fed can significantly reduce this high plasma leptin. B, Plasma insulin was elevated due to chronic HF feeding. EX-4 treatment and food restriction in HF diet-fed rats both normalized plasma levels of insulin.
Results for plasma levels of insulin are shown in Figure 4B. A two-way ANOVA revealed a significant effect of treatment (F2,42 = 10.4, P < .001). The diet (F1,42 = 2.5, P = .12) and diet × treatment (F2,42 = 1.5, P = .23) effects were not significant. HF feeding increased plasma insulin, but this increase was not significant (post hoc SC+VEHICLE vs HF+VEHICLE P = .06). After long-term EX-4 injections, plasma insulin decreased significantly in the HF (post hoc VEHICLE vs EX-4, P < .04) but not in the SC (post hoc VEHICLE vs EX-4, P = .48) feeding condition. Finally, food restriction significantly reduced the plasma levels of insulin in both SC and HF feeding condition as post hoc tests comparing VEHICLE and PAIR FED revealed significant effects in both conditions (P < .007).
NPY, POMC, and AgRP mRNA expression in the ARC of the hypothalamus
To gain an understanding of the neural mechanisms underlying the effects of EX-4 on food intake, we examined the gene expressions of the anorexigneic and orexigenic signaling peptides POMC, AgRP, and NPY in the ARC. The results are shown in Figure 5. A two-way ANOVA revealed a significant effect of treatment (F2,40 = 6.2, P < .005) but not diet (F1,40 = 3.3, P = .08) or diet × treatment (F2,40 = 0.3, P = .77) in POMC mRNA expression. The expression of POMC was increased in both groups receiving EX-4. Unlike EX-4 treatment, restricting food intake by pair feeding did not lead to increased POMC expression. For NPY expression, a two-way ANOVA revealed a significant effect of treatment (F2,40 = 5.1, P < .02) but not diet (F1,40 = 0.0, P = .94) or diet × treatment (F2, 40 = 0.15, P = .86). That is, EX-4 treatment decreased NPY expression in both SC and HF feeding conditions. Conversely, pair feeding increased NPY expressions in both diet conditions. There were no significant changes in gene expression of AgRP: treatment (F2,40 = 2.2, P = .12), diet (F1,40 = 0.6, P = .44), or diet × treatment (F2, 40 = 0.1, P = .92).
Figure 5.
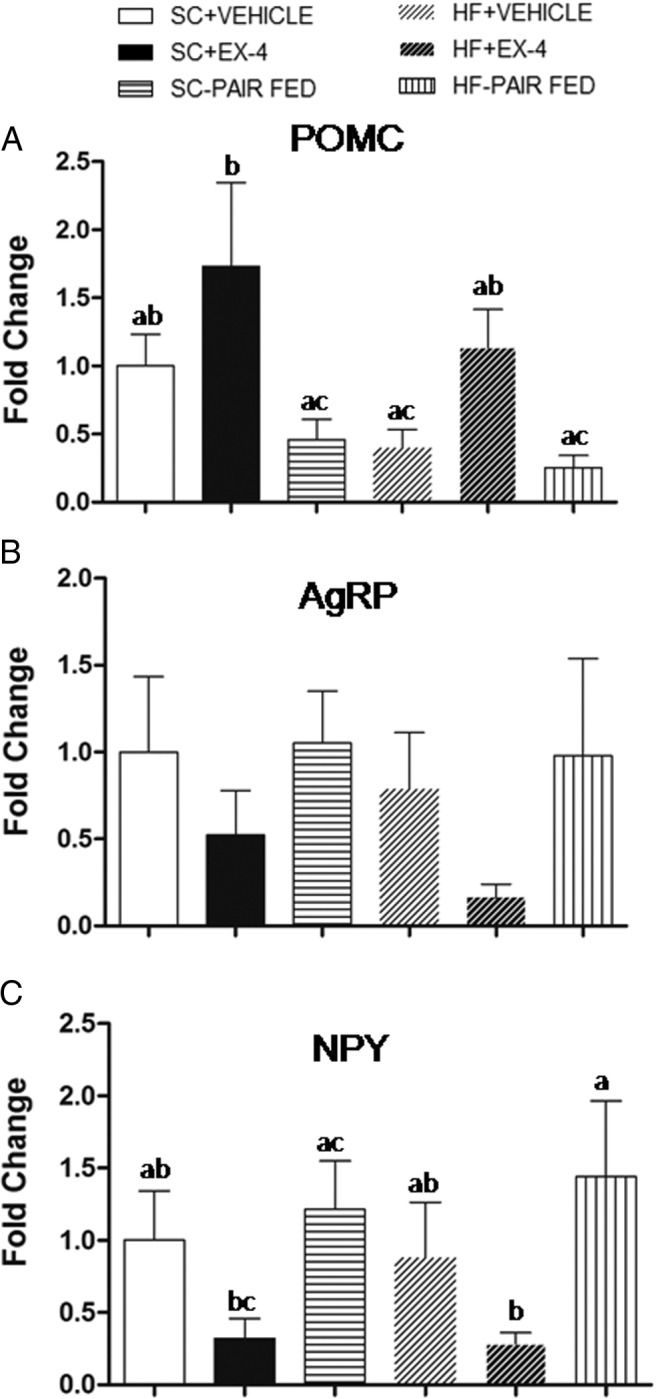
Gene expression of POMC (A), AgRP (B), and NPY (C) in the ARC after long-term treatments of EX-4, vehicle, or paired fed. In either the SC or HF feeding condition, chronic EX-4 but not paired-fed treatment increased POMC and decreased NPY expression. Neither treatment was found to produce a significant alteration of AgRP gene expression. Values are mean ± SEM. In each panel, groups that share the same letter are not significantly different from each other after obtaining a significant main effect by two-way ANOVA.
Expression levels of genes involved in dopaminergic neurotransmission
We hypothesized that EX-4 injection might also alter the expression of central nervous system neurotransmitters/neuropeptides that are involved in the processing of reward. We focused on the dopamine system by measuring the gene expression of TH in the VTA and D1R and D2R in the NAc. Results for these gene expressions are shown in Figure 6. The results revealed a significant effects of diet (F1, 37 = 6.8, P < .02) but not of treatment (F2, 37 = 0.5, P = .64) or diet × treatment (F2,37 = 0.8, P = .45). This is evident as a significant reduction in the expression of TH in the VTA with HF feeding. Neither EX-4 nor the food restriction altered the TH expression profile in the SC feeding condition. Both Ex-4 and food restriction appeared to increase the TH expression in HF feeding, but these elevations did not reach statistical significance (HF+VEHICLE vs HF+EX-4, P = .64; HF+VEHICLE vs HF-PAIR FED, P = .75).
Figure 6.
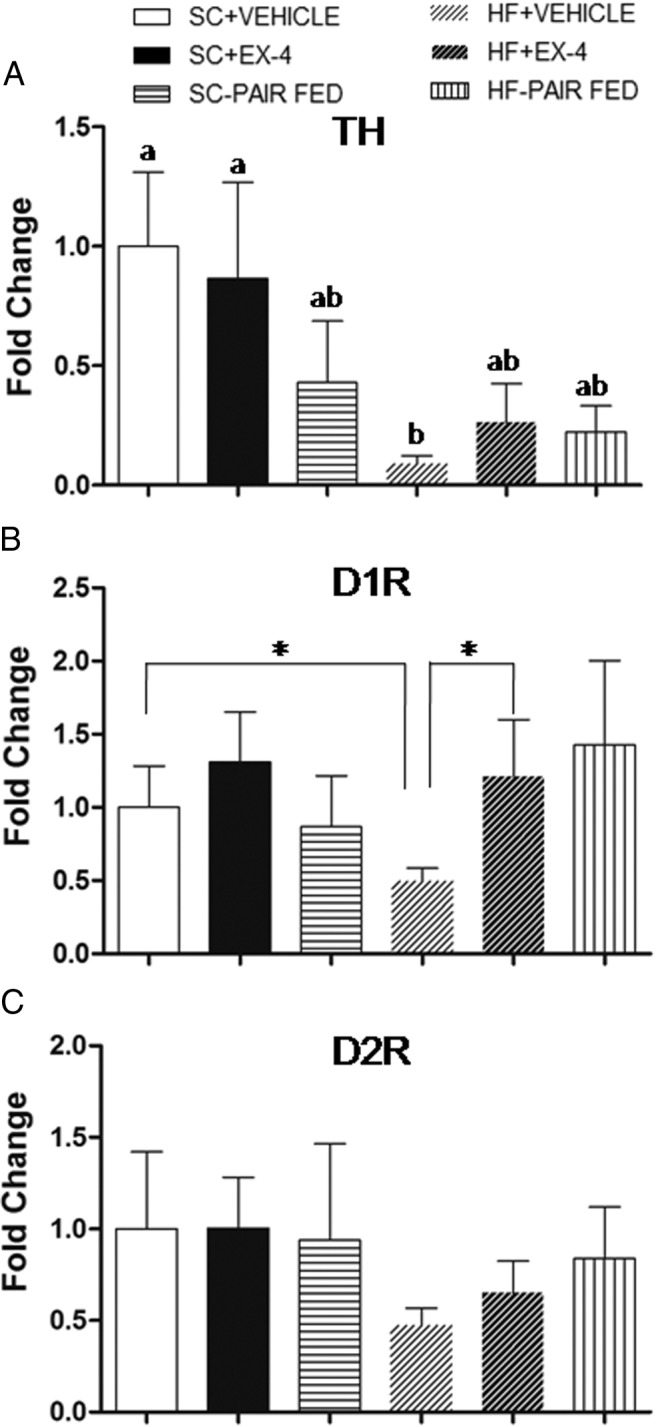
Gene expression of TH in the VTA (A) and D1R (B) and D2R (C) in the NAc after long-term treatments of EX-4, vehicle, or paired fed. A, Chronic HF feeding greatly reduced TH gene expression in the VTA. Both EX-4 and food restriction appeared to elevate this reduction in TH gene expression, but the effect was not statistically significant. Values are mean ± SEM. In each panel, groups that share the same letter are not significantly different from each other after obtaining a significant main effect by two-way ANOVA. B, Chronic HF feeding reduced gene expression of D1R in the NAc. This reduction was reversed by long-term EX-4 treatment. Chronic food restriction by paired fed appeared to produce similar result, but the effect was not statistically significant. *, Student t test between the two groups (P < .05). C, Neither treatment was found to produce significant alteration of D2R gene expression in the NAc.
Although the overall two-way ANOVA did not reveal any significant effects (details not presented), gene expression patterns of D1R and D2R in the NAc were similar to those of TH in the VTA. Chronic HF feeding decreased D1R expression (SC+VEHICLE vs HF+VEHICLE, t test, t = −4.30, P < .002). Both EX-4 and food restriction normalized D1R expression to the level of the SC feeding condition. The effect by EX-4 is supported by a t test comparing HF-VEHICLE and HF+EX-4 (t = −2.80, P < .02). Although D2R expression appeared to be decreased with chronic HF feeding, a t test comparing SC+VEHICLE and HF+VEHICLE was not significant. Similarly, D2R expression seemed to be increased in EX-4- and food restriction-treated HF fed rats, but these results were not significant. Finally, either EX-4 or food restriction did not change the expression of D1R or D2R in the SC feeding condition.
Discussion
The ability of GLP-1R agonists to suppress food intake leading to weight loss has been recognized for more than a decade. Nevertheless, the neural mechanisms by which peripherally administered GLP-1R agonists reduce food intake and body weight have remained largely unknown. This study aimed to investigate whether the beneficial effects by the GLP-1R agonist EX-4 are produced via neural circuits involved in not only energy homeostasis but also in the rewarding aspect of feeding. Data from this study indicate that peripheral administration of EX-4 significantly decreases food intake and BW in both chow- and HF diet-fed rats. Plasma levels of leptin and insulin were decreased after 9 days EX-4 injections in HF but not chow-fed rats, suggesting that EX-4 may improve insulin and leptin sensitivity in diet-induced obese status.
We extend these findings by showing that the expression of homeostasis and reward-related genes was altered by HF feeding, and such changes were reversed by long-term EX-4 treatment. That is, 6 weeks of HF feeding significantly reduced mRNA expression of POMC in the ARC, TH in the VTA, and D1R in the NAc. Long-term treatment of EX-4 changed homeostatic POMC and NPY gene expression similarly in chow- and HF-fed rats. These changes, however, were not seen in food-restricted (pair fed) controls. Moreover, the effects on the expression of dopaminergic genes differed in the two diet conditions. Long-term EX-4 or food restriction did not affect dopaminergic gene expression in the SC fed rats. In contrast, EX-4 and food restriction treatment both reversed the reduced expression of TH in the VTA and D1R in the NAc in HF diet-fed rats. These results provide evidence to support the notion that the activation of GLP-1 signaling reduces food intake and BW via neural mechanisms mediating the energy homeostasis. The results, however, suggest that peripheral EX-4 treatment does not directly modulate food reward/motivation via changes in the gene expression in the mesolimbic dopamine system. Because similar effects on TH and D1R gene expression were produced by EX-4 and food restriction in the HF diet-fed rats, it is likely that the effects on TH and D1R is secondary to the reduced food intake and/or weight loss.
Whether the alterations in hypothalamic and dopaminergic gene expression are a direct effect of EX-4 at these sites cannot be determined from these experiments. Although GLP-1 receptors are expressed in the hypothalamus, VTA, and NAc (25) and EX-4 has been shown to cross the blood-brain barrier (32), EX-4 was peripherally administered and there are multiple peripheral actions of EX-4 that could contribute to these central changes.
GLP-1R agonists have been reported to produce persisting malaise or visceral illness in rats (33–35). Such negative physiological effects in mice, nonhuman primates, and humans, however, do not appear to be as common and persistent. A detailed review on this issue can be found in our previous papers (33, 35). Although to what extent the aversive effects contribute to hypophagic responses and weight loss cannot be easily determined, previous reports indicate that the threshold dose of EX-4 to reduce food intake is lower than that for malaise in mice (36) and rats (37, 38). Our previous study (35) also demonstrated that a dose of LiCl that produces a similar degree of conditioned food aversion, unlike EX-4, does not subsequently reduce daily total food intake and body weight. Furthermore, the present results that similar dopamine gene expression profiles in EX-4-treated and pair-fed rats suggest that such changes in gene expression are not likely secondary to the adverse effects of EX-4. Overall, these data suggest that the effects on food intake, weight, and gene expression are not solely results of the aversive effects of EX-4.
The results of EX-4 on decreasing food intake and body weight are consistent with both preclinical and clinical reports. It has been shown that this GLP-1 analog has long-term efficacy for sustaining weight loss in adults with type 2 diabetes or a body mass index of 30–40 kg/m−2 (17, 39). Multiple animal studies have also revealed that body weight suppression by EX-4 treatment is maintained throughout the treatment period (26, 37, 40). Furthermore, our prior studies with nonhuman primates revealed no postdrug rebound in intake. That is, intakes return to baseline post-EX-4 treatment but a compensatory increase in intake does not occur (7, 41). In the current study, the food intake reduction effect persisted longer in HF-fed than chow-fed rats. Similar results have been demonstrated using both EX-4 and another long-acting GLP-1 analog liraglutide (42). A study by Hayes et al (26), however, showed that chow intake was decreased persistently and significantly throughout 7 days of EX-4 treatments. Although the experimental subjects (Sprague Dawley rats) and drug dose [3 μg/kg, twice a day (b.i.d.)] are similar between the two studies, the source of the rats was different and this may have contributed to the different outcomes.
The fact that body weight loss in EX-4-treated rats sustained, even when food intake was no longer significantly suppressed, draws attention to the potential role of GLP-1 signaling in the energy expenditure/metabolic rate. Studies have demonstrated that acute treatment of GLP-1 or its analog either increases (43–45) or decreases (46, 47) energy expenditure. These studies with acute treatments indicate that GLP-1's role in energy expenditure could differ, depending on factors such as the route of administration and the index of metabolic rate measured. The results of this study suggest that the effects of long-term GLP-1R or activation on energy expenditure are complex. Although both HF diet- and SC pair-fed rats decreased body weight, the relative amount of weight loss in comparison with the EX-4 treated rats revealed a complex relationship between EX-4 and diet. In the chow-fed group, the magnitude of percentage weight loss was larger in pair-fed than in EX-4-treated rats. Conversely, EX-4-treated HF diet-fed rats lost a greater percentage of body weight than did their pair-fed controls. This suggests that long-term treatment of EX-4 has effects on energy expenditure that depend on the maintenance diet. Given these results and the reported mixed effects of acute GLP-1R activation on energy expenditure, further studies are necessary to elucidate how the GLP-1 system is involved in mediating energy expenditure.
Circulating concentrations of insulin and leptin vary in proportion to body mass and, particularly, to adiposity. Thus, plasma levels of the two hormones are significantly increased in obese subjects and decrease with weight loss (48, 49). The present study showed that plasma leptin was elevated in the HF diet-fed rats. Although the HF diet-fed rats were significantly heavier than the chow-fed ones, such differences in weight do not appear to have been sufficient to produce a significantly higher plasma insulin level. Long-term treatment with EX-4 did not alter either leptin or insulin levels in the chow-fed rats. In contrast, EX-4 injections in HF diet-fed rats decreased plasma leptin and insulin to the levels of chow-fed controls, suggesting that the EX-4 treatment resulted in a reduction in fat mass (body composition was not measured). Because EX-4 as a GLP-1 analog can induce the release of insulin, the result that EX-4 injections significantly reduced plasma insulin in HF diet-fed rats is intriguing. This result could be due to the effects of long-term EX-4 treatment on body weight and resulting decreased insulin level or increased insulin sensitivity in the HF feeding condition. Finally, pair feeding produced similar effects to those of EX-4 treatments, suggesting that the effects of the EX-4 on insulin and leptin levels may have been indirect and secondary to the decreases in the food intake and body weight.
The ARC is a critical brain nucleus for the regulation of energy balance. Important roles for neurons expressing either NPY/AgRP or POMC in the control of feeding and body weight have been demonstrated. Prior work has shown that the gene expression of NPY/AgRP and POMC changes in correspondence with the energy state. NPY and AgRP mRNA expression is increased in negative energy balance state, ie, after acute or chronic food restriction (50–52). Reports on the effects of negative energy balance on POMC expression in the ARC have been less consistent. That is, unchanged (53) or decreased expression of POMC have both been found in response to fasting. Effects of exposure to HF or high-energy diets on orexigenic and anorexigenic gene expression can be counterintuitive. Increased/unchanged NPY/AgRP or decreased POMC gene expression in the ARC has been found in response to acute or chronic exposure to a highly palatable, high-energy diet (54). The results of this study reflect this paradoxical POMC response because mRNA expression of POMC in the ARC was greatly reduced after 6 weeks of HF diet consumption. Consistent with most previous reports, NPY/AgRP expression after such HF experience was not significantly different from that found in the chow controls. Although leptin resistance and alteration in the extent of AgRP innervation of POMC neurons in response to a HF diet (55) could be a possible contributor to the paradoxical NPY/AgRP and POMC responses mentioned above, further studies are required to elucidate the underlying mechanisms.
Treatment with EX-4 altered NPY/AgRP and POMC expression in the ARC differently from the simple food restriction that occurred with pair feeding. The GLP-1R is expressed throughout the brain including in forebrain areas that play roles in mediating food intake and reward. Acute EX-4-induced anorexia has been shown to be accompanied by activation of GLP-1R-expressing regions including the ARC, paraventricular nucleus, and dorsomedial nucleus of hypothalamus as assessed by c-Fos. Furthermore, double-labeling immunohistochemistry indicated that central EX-4 significantly activated POMC and NPY neurons in the ARC (56). In this study, we found that long-term EX-4 injection increased POMC and decreased NPY expression in both HF diet-fed rats and SC-fed rats. This response pattern was different from what we found in the respective pair-fed controls. Pair feeding did not alter NPY or POMC expression in SC pair-fed rats but resulted in significantly decreased POMC expression in the HF pair-fed rats. The differential effects of EX-4 and pair feeding on POMC and NPY expression provide strong support that EX-4 directly alters signaling in the ARC to change POMC and NPY gene expression to an anorexigenic profile. Such anorexigenic gene expression profile provides a potential explanation for the lack of compensatory increases in food intake after EX-4 treatment mentioned above. EX-4-induced the alteration of phosphatidylinositol 3-kinase/protein kinase B signaling is a likely candidate involved in the gene expression pattern reported here because it has been shown to be involved in the hindbrain GLP-1R-mediated suppression of food intake (57).
Although the role of GLP-1R activation in reducing food intake is well established, the notion that GLP-1R activation can mediate the rewarding/motivational aspect of ingestive behavior is a more recent concept. This idea is supported by reports that GLP-1 neurons in the nucleus of the solitary tract project to the VTA and NAc (24, 31), that GLP-1R are expressed in these regions (25), and that local administration of GLP-1 agonists reduces food intake (58, 59). Our finding that chronic HF feeding decreases the gene expression of TH in the VTA as well as of D1R in the NAc is consistent with previous reports (60–62). Chronic treatment (9 d b.i.d.) with EX-4 did not influence the dopaminergic gene expression profile in the chow-fed rats but was able to partially or fully rescue the down-regulation of mesolimbic dopaminergic gene expression produced by HF feeding. That is, TH expression in the VTA was increased (although not returned to the level of chow fed controls), and the expression of D1R was fully reversed to that of the chow-fed controls. Because similar gene expression patterns were seen in the brains of the HF diet pair-fed rats, it is likely that the recovery of dopaminergic gene expression pattern after long-term EX-4 treatment is secondary to the intake reduction or weight loss induced by EX-4. These results do not support the concept that peripheral EX-4 directly alters the expression of TH and mesolimbic dopamine system to affect food reward. Nevertheless, a local injection of EX-4 has been shown to decrease food reward (63). Whether peripherally administered EX-4 accesses these sites or whether local injections are mimicking the actions of a central GLP-1 circuit remains to be determined.
In conclusion, our data illustrate that long-term of EX-4 treatment decreases food intake and BW in both chow-fed and HF-fed rats. These effects are associated with increased expression of POMC while decreasing NPY expression in the ARC. These data provide further evidence that chronic GLP-1R activation may produce the sustained suppression of food intake and BW via directly affecting hypothalamic circuits underlying energy balance. Our findings also suggest that long-term EX-4 treatment may partially or fully rescue the HF-induced down-regulation of dopaminergic genes in the VTA and NAc as a secondary effect to HF intake reduction.
Acknowledgments
We thank Dr Yada Treesukosol, Gretha Boersma, and Lin Li for their technical advice and assistance with this study.
Y.Y. received an Oversea Funding from Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology in China. T.H.M. has received funding from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-19302 (to T.H.M).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AgRP
- agouti gene-related protein
- ARC
- arcuate nucleus
- b.i.d.
- twice a day
- BW
- body weight
- Ct
- cycle threshold
- D1R
- dopamine receptor 1
- D2R
- dopamine receptor 2
- DPP-IV
- degraded by dipeptidyl peptidase-4
- EX-4
- exendin-4
- GLP-1
- glucagon-like peptide 1
- GLP-1R
- GLP-1 receptor
- HF
- high fat
- NAc
- nucleus accumbens
- NPY
- neuropeptide Y
- NST
- nucleus of the solitary tract
- POMC
- proopiomelanocortin
- PVN
- paraventricular nucleus
- SC
- standard chow
- TH
- tyrosine hydroxylase
- VTA
- ventral tegmental area.
References
- 1. Zimmermann-Belsing T, Feldt-Rasmussen U. Obesity: the new worldwide epidemic threat to general health and our complete lack of effective treatment. Endocrinology. 2004;145(4):1501–1502 [DOI] [PubMed] [Google Scholar]
- 2. Smith DG, Robbins TW. The neurobiological underpinnings of obesity and binge eating: a rationale for adopting the food addiction model. Biol Psychiatry. 2013;73(9):804–810 [DOI] [PubMed] [Google Scholar]
- 3. Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obes Rev. 2013;14(1):2–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86(5):773–795 [DOI] [PubMed] [Google Scholar]
- 5. Healy JE, Bateman JL, Ostrom CE, Florant GL. Peripheral ghrelin stimulates feeding behavior and positive energy balance in a sciurid hibernator. Horm Behav. 2011;59(4):512–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moran TH, Shnayder L, Hostetler AM, McHugh PR. Pylorectomy reduces the satiety action of cholecystokinin. Am J Physiol. 1988;255(6 Pt 2):R1059–R1063 [DOI] [PubMed] [Google Scholar]
- 7. Scott KA, Moran TH. The GLP-1 agonist exendin-4 reduces food intake in nonhuman primates through changes in meal size. Am J Physiol Regul Integr Comp Physiol. 2007;293(3):R983–R987 [DOI] [PubMed] [Google Scholar]
- 8. Sun B, Purcell RH, Terrillion CE, Yan J, Moran TH, Tamashiro KL. Maternal high-fat diet during gestation or suckling differentially affects offspring leptin sensitivity and obesity. Diabetes. 2012;61(11):2833–2841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kinzig KP, Coughlin JW, Redgrave GW, Moran TH, Guarda AS. Insulin, glucose, and pancreatic polypeptide responses to a test meal in restricting type anorexia nervosa before and after weight restoration. Am J Physiol Endocrinol Metab. 2007;292(5):E1441–E1446 [DOI] [PubMed] [Google Scholar]
- 10. Sainsbury A, Zhang L. Role of the hypothalamus in the neuroendocrine regulation of body weight and composition during energy deficit. Obes Rev. 2012;13(3):234–257 [DOI] [PubMed] [Google Scholar]
- 11. Hardman CA, Herbert VM, Brunstrom JM, Munafo MR, Rogers PJ. Dopamine and food reward: effects of acute tyrosine/phenylalanine depletion on appetite. Physiol Behav. 2012;105(5):1202–1207 [DOI] [PubMed] [Google Scholar]
- 12. Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci. 2011;15(1):37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Enc FY, Imeryuz N, Akin L, et al. Inhibition of gastric emptying by acarbose is correlated with GLP-1 response and accompanied by CCK release. Am J Physiol Gastrointest Liver Physiol. 2001;281(3):G752–G763 [DOI] [PubMed] [Google Scholar]
- 14. Gulpinar MA, Bozkurt A, Coskun T, Ulusoy NB, Yegen BC. Glucagon-like peptide (GLP-1) is involved in the central modulation of fecal output in rats. Am J Physiol Gastrointest Liver Physiol. 2000;278(6):G924–G929 [DOI] [PubMed] [Google Scholar]
- 15. Thorens B, Porret A, Buhler L, Deng SP, Morel P, Widmann C. Cloning and functional expression of the human islet GLP-1 receptor. Demonstration that exendin-4 is an agonist and exendin-(9–39) an antagonist of the receptor. Diabetes. 1993;42(11):1678–1682 [DOI] [PubMed] [Google Scholar]
- 16. Bond A. Exenatide (Byetta) as a novel treatment option for type 2 diabetes mellitus. Proc (Bayl Univ Med Cent). 2006;19(3):281–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Astrup A, Carraro R, Finer N, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obesity. 2012;36(6):843–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Elfers CT, Simmons JH, Roth CL. Glucagon-like peptide-1 agonist exendin-4 leads to reduction of weight and caloric intake in a rat model of hypothalamic obesity. Horm Res Paediatr. 2012;78(1):47–53 [DOI] [PubMed] [Google Scholar]
- 19. Primeaux SD, Barnes MJ, Braymer HD, Bray GA. Sensitivity to the satiating effects of exendin 4 is decreased in obesity-prone Osborne-Mendel rats compared to obesity-resistant S5B/Pl rats. Int J Obes. 2010;34(9):1427–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Di Pasquale G, Dicembrini I, Raimondi L, et al. Sustained exendin-4 secretion through gene therapy targeting salivary glands in two different rodent models of obesity/type 2 diabetes. PloS One. 2012;7(7):e40074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Duca FA, Sakar Y, Covasa M. Combination of obesity and high-fat feeding diminishes sensitivity to GLP-1R agonist exendin-4. Diabetes. 2013;62(7):2410–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vrang N, Larsen PJ. Preproglucagon derived peptides GLP-1, GLP-2 and oxyntomodulin in the CNS: role of peripherally secreted and centrally produced peptides. Prog Neurobiol. 2010;92(3):442–462 [DOI] [PubMed] [Google Scholar]
- 23. Tang-Christensen M, Cowley MA. GLP-1 analogs: satiety without malaise? Am J Physiol Regulat Integr Comp Physiol. 2007;293(3):R981–R982 [DOI] [PubMed] [Google Scholar]
- 24. Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology. 2012;153(2):647–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403(2):261–280 [DOI] [PubMed] [Google Scholar]
- 26. Hayes MR, Kanoski SE, Alhadeff AL, Grill HJ. Comparative effects of the long-acting GLP-1 receptor ligands, liraglutide and exendin-4, on food intake and body weight suppression in rats. Obesity. 2011;19(7):1342–1349 [DOI] [PubMed] [Google Scholar]
- 27. Scott MM, Williams KW, Rossi J, Lee CE, Elmquist JK. Leptin receptor expression in hindbrain Glp-1 neurons regulates food intake and energy balance in mice. J Clin Invest. 2011;121(6):2413–2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Donahey JC, van Dijk G, Woods SC, Seeley RJ. Intraventricular GLP-1 reduces short- but not long-term food intake or body weight in lean and obese rats. Brain Res. 1998;779(1–2):75–83 [DOI] [PubMed] [Google Scholar]
- 29. Tolppanen AM, Lavikainen P, Solomon A, et al. History of medically treated diabetes and risk of Alzheimer disease in a nationwide case-control study. Diabetes Care. 2013;36(7):2015–2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dossat AM, Diaz R, Gallo L, Panagos A, Kay K, Williams DL. Nucleus accumbens GLP-1 receptors influence meal size and palatability. Am J Physiol Endocrinol Metab. 2013;304(12):E1314–E1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dossat AM, Lilly N, Kay K, Williams DL. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci. 2011;31(41):14453–14457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kastin AJ, Akerstrom V, Pan W. Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J Mol Neurosci. 2002;18(1–2):7–14 [DOI] [PubMed] [Google Scholar]
- 33. Liang NC, Bello NT, Moran TH. Additive feeding inhibitory and aversive effects of naltrexone and exendin-4 combinations. Int J Obes. 2013;37(2):272–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kanoski SE, Rupprecht LE, Fortin SM, De Jonghe BC, Hayes MR. The role of nausea in food intake and body weight suppression by peripheral GLP-1 receptor agonists, exendin-4 and liraglutide. Neuropharmacology. 2012;62(5–6):1916–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liang NC, Smith ME, Moran TH. Palatable food avoidance and acceptance learning with different stressors in female rats. Neuroscience. 2013;235:149–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL. Peripheral exendin-4 and peptide YY(3–36) synergistically reduce food intake through different mechanisms in mice. Endocrinology. 2005;146(9):3748–3756 [DOI] [PubMed] [Google Scholar]
- 37. Mack CM, Moore CX, Jodka CM, et al. Antiobesity action of peripheral exenatide (exendin-4) in rodents: effects on food intake, body weight, metabolic status and side-effect measures. Int J Obes. 2006;30(9):1332–1340 [DOI] [PubMed] [Google Scholar]
- 38. Baraboi ED, St-Pierre DH, Shooner J, Timofeeva E, Richard D. Brain activation following peripheral administration of the GLP-1 receptor agonist exendin-4. Am J Physiol Regul Integr Comp Physiol. 2011;301(4):R1011–R1024 [DOI] [PubMed] [Google Scholar]
- 39. Nayak UA, Govindan J, Baskar V, Kalupahana D, Singh BM. Exenatide therapy in insulin-treated type 2 diabetes and obesity. QJM. 2010;103(9):687–694 [DOI] [PubMed] [Google Scholar]
- 40. Szayna M, Doyle ME, Betkey JA, et al. Exendin-4 decelerates food intake, weight gain, and fat deposition in Zucker rats. Endocrinology. 2000;141(6):1936–1941 [DOI] [PubMed] [Google Scholar]
- 41. Bello NT, Kemm MH, Ofeldt EM, Moran TH. Dose combinations of exendin-4 and salmon calcitonin produce additive and synergistic reductions in food intake in nonhuman primates. Am J Physiol Regul Integr Comp Physiol. 2010;299(3):R945–R952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mul JD, Begg DP, Barrera JG, et al. High-fat diet changes the temporal profile of GLP-1 receptor-mediated hypophagia in rats. Am J Physiol Regul Integr Comp Physiol. 2013;305(1):R68–R77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Osaka T, Endo M, Yamakawa M, Inoue S. Energy expenditure by intravenous administration of glucagon-like peptide-1 mediated by the lower brainstem and sympathoadrenal system. Peptides. 2005;26(9):1623–1631 [DOI] [PubMed] [Google Scholar]
- 44. Lockie SH, Heppner KM, Chaudhary N, et al. Direct control of brown adipose tissue thermogenesis by central nervous system glucagon-like peptide-1 receptor signaling. Diabetes. 2012;61(11):2753–2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yamamoto H, Lee CE, Marcus JN, et al. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest. 2002;110(1):43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Baggio LL, Huang Q, Brown TJ, Drucker DJ. Oxyntomodulin and glucagon-like peptide-1 differentially regulate murine food intake and energy expenditure. Gastroenterology. 2004;127(2):546–558 [DOI] [PubMed] [Google Scholar]
- 47. Hayes MR, Skibicka KP, Grill HJ. Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology. 2008;149(8):4059–4068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Flier JS. Hormone resistance in diabetes and obesity: insulin, leptin, and FGF21. Yale J Biol Med. 2012;85(3):405–414 [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang XJ, Wang YQ, Long Y, et al. Alteration of sweet taste in high-fat diet induced obese rats after 4 weeks treatment with exenatide. Peptides. 2013;47:115–123 [DOI] [PubMed] [Google Scholar]
- 50. Brady LS, Smith MA, Gold PW, Herkenham M. Altered expression of hypothalamic neuropeptide mRNAs in food-restricted and food-deprived rats. Neuroendocrinology. 1990;52(5):441–447 [DOI] [PubMed] [Google Scholar]
- 51. Bi S, Robinson BM, Moran TH. Acute food deprivation and chronic food restriction differentially affect hypothalamic NPY mRNA expression. Am J Physiol Regul Integr Comp Physiol. 2003;285(5):R1030–R1036 [DOI] [PubMed] [Google Scholar]
- 52. de Rijke CE, Hillebrand JJ, Verhagen LA, Roeling TA, Adan RA. Hypothalamic neuropeptide expression following chronic food restriction in sedentary and wheel-running rats. J Mol Endocrinol. 2005;35(2):381–390 [DOI] [PubMed] [Google Scholar]
- 53. Bertile F, Oudart H, Criscuolo F, Maho YL, Raclot T. Hypothalamic gene expression in long-term fasted rats: relationship with body fat. Biochem Biophys Res Commun. 2003;303(4):1106–1113 [DOI] [PubMed] [Google Scholar]
- 54. van den Heuvel JK, van Rozen AJ, Adan RA, la Fleur SE. An overview on how components of the melanocortin system respond to different high energy diets. Eur J Pharmacol. 2011;660(1):207–212 [DOI] [PubMed] [Google Scholar]
- 55. Newton AJ, Hess S, Paeger L, et al. AgRP innervation onto POMC neurons increases with age and is accelerated with chronic high-fat feeding in male mice. Endocrinology. 2013;154(1):172–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dalvi PS, Nazarians-Armavil A, Purser MJ, Belsham DD. Glucagon-like peptide-1 receptor agonist, exendin-4, regulates feeding-associated neuropeptides in hypothalamic neurons in vivo and in vitro. Endocrinology. 2012;153(5):2208–2222 [DOI] [PubMed] [Google Scholar]
- 57. Rupprecht LE, Mietlicki-Baase EG, Zimmer DJ, McGrath LE, Olivos DR, Hayes MR. Hindbrain GLP-1 receptor-mediated suppression of food intake requires a PI3K-dependent decrease in phosphorylation of membrane-bound Akt. Am J Physiol Endocrinol Metab. 2013;305(6):E751–E759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Riederer P, Bartl J, Laux G, Grunblatt E. Diabetes type II: a risk factor for depression-Parkinson-Alzheimer? Neurotox Res. 2011;19(2):253–265 [DOI] [PubMed] [Google Scholar]
- 59. Irie F, Fitzpatrick AL, Lopez OL, et al. Enhanced risk for Alzheimer disease in persons with type 2 diabetes and APOE epsilon4: the Cardiovascular Health Study Cognition Study. Arch Neurol. 2008;65(1):89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vucetic Z, Carlin JL, Totoki K, Reyes TM. Epigenetic dysregulation of the dopamine system in diet-induced obesity. J Neurochem. 2012;120(6):891–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li Y, South T, Han M, Chen J, Wang R, Huang XF. High-fat diet decreases tyrosine hydroxylase mRNA expression irrespective of obesity susceptibility in mice. Brain Res. 2009;1268:181–189 [DOI] [PubMed] [Google Scholar]
- 62. Alsio J, Olszewski PK, Norback AH, et al. Dopamine D1 receptor gene expression decreases in the nucleus accumbens upon long-term exposure to palatable food and differs depending on diet-induced obesity phenotype in rats. Neuroscience. 2010;171(3):779–787 [DOI] [PubMed] [Google Scholar]
- 63. Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci. 2012;32(14):4812–4820 [DOI] [PMC free article] [PubMed] [Google Scholar]