Abstract
Accelerated ovarian failure (AOF) can be induced in young mice with low doses of 4-vinylcyclohexene diepoxide (VCD), modeling the hormone changes observed across menopause. We assessed markers of synaptic plasticity in the hippocampus, anxiety-like behavior, and spatial learning longitudinally at 4 time points across the AOF model: premenopause, early perimenopause, late perimenopause, and postmenopause (POST). As others have shown, VCD administration decreased ovarian follicle counts and increased acyclicity as the model progressed to POST but with no impact on organ or body weights. The morphology of Iba1 immunoreactive microglia did not differ between vehicle- and VCD-administered mice. Hippocampal postsynaptic density 95 levels were minimally altered across the AOF model but decreased at POST in CA3b 24 hours after exogenous estradiol benzoate (EB). In contrast, hippocampal phosphorylated AKT levels transiently decreased in premenopause but increased at POST after 24 hours of EB in select subregions. Electron microscopy revealed fewer estrogen receptor α containing dendritic spines and terminals in CA1 stratum radiatum at POST. mRNA levels of most brain-derived neurotrophic factor exons (except V and VI) were lower in POST compared with ovariectomized mice. Exon V was sensitive to 24 hours of EB administration in POST-VCD. Anxiety-like behavior was unaffected at any menopause phase. Spatial learning was unaffected in all groups, but POST-VCD mice performed below chance. Our results suggest that the AOF model is suitable for longitudinal studies of neurobiological changes across the menopause transition in mice. Our findings also point to complex interactions between estrogen receptors and pathways involved in synaptic plasticity.
The menopause transition is associated with a wide range of physiological, psychological, and neurobiological changes in women. Estrogens are important for cognitive processes (1–3), and diminished cognitive performance is associated with decreased ovarian function (3–5). Greater cognitive difficulties within the first year after menstrual cessation (6) suggests that the transition from late perimenopause (LATE) to early postmenopause (POST) constitutes a highly labile period. Effectively modeling this transition in rodents is important to understanding the neurobiological mechanisms of these changes.
The accelerated ovarian failure (AOF) mouse model allows for longitudinal study across menopause phases and recapitulation the hormonal milieu and menopause transition (for review and mechanism, see Ref. 7). Intact aging and ovariectomy (OVX) models fail to accurately recapitulate human menopause, and neither allows for a longitudinal study of neurobiological changes during the menopause transition (7). In the AOF model, ovarian follicles are depleted via 15-day ip administration of 4-vinylcyclohexene diepoxide (VCD), which selectively eliminates primary ovarian follicles (8) (see Supplemental Methods) while maintaining ovarian tissue and its steroidigenic capacity (9). After VCD, mature follicles deplete with normal estrous cycles (10, 11), resulting in acyclicity and eventual ovarian senescence (9). Previous work in the AOF model not only established the validity of menopause induction (hormone levels and acyclicity) (10) and predictable and reproducible time points corresponding to putative perimenopause and POST (9, 12) but also demonstrated differences between OVX and AOF models (13, 14) (see Supplemental Methods).
Endogenous fluctuations (15–18) and exogenous administration of ovarian steroids (19–21), particularly estrogens, modulate hippocampal levels of postsynaptic density 95 (PSD-95), phosphorylated AKT (pAKT), estrogen receptor (ER)α, and brain-derived neurotrophic factor (BDNF). Although the hippocampal-dependent object placement (OP) spatial learning task is not affected by estrous cycle stage (16), estradiol administration improves task performance in OVX females (19), suggesting a contribution of estrogen signaling to task success.
Although behavioral cognitive changes have been compared in rodents in OVX and AOF models (13, 14, 22), these changes have not been examined longitudinally across all menopause phases. Thus, we examined markers of synaptic plasticity in the hippocampus and assessed anxiety behavior and spatial learning across the AOF model at 4 time points: premenopause (PRE), early perimenopause (EARLY), LATE, and POST. We hypothesized that markers of synaptic plasticity and spatial learning would be negatively affected by loss of endogenous estrogen particularly at LATE and POST. Given that PSD-95, pAKT, and ERα respond to nongenomic acting doses of exogenous estradiol, VCD mice at POST were administered estradiol benzoate (EB) 24 hours before the collection of brains and organs. We hypothesized that EB administration would alter hippocampal levels of PSD-95 and pAKT. The results of our study suggest that the AOF model is suitable for longitudinal studies of neurobiological changes across the menopause transition in mice.
Materials and Methods
For details see Supplemental Methods.
Animals
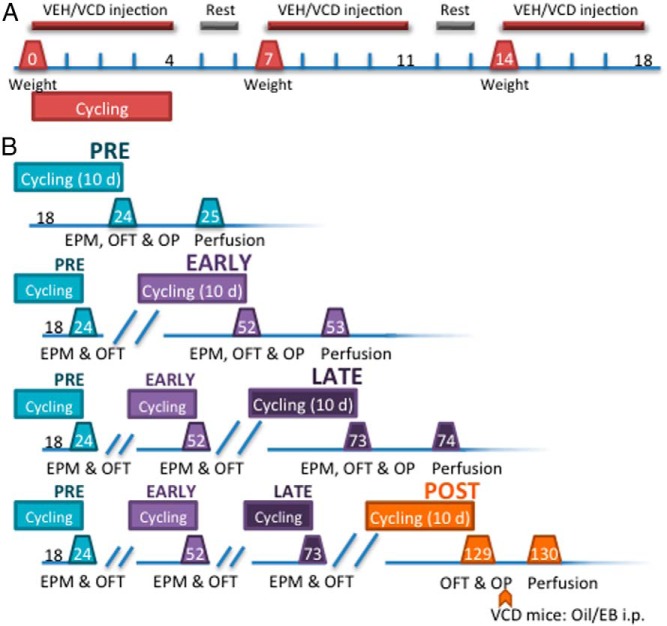
Experiments were approved by The Rockefeller University and Weill Cornell Medical College Institutional Animal Care and Use Committees and conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Intact, postnatal day 50 female mice (n = 100) were injected ip with vehicle (VEH) (0.5% dimethyl sulfoxide (DMSO) in sesame oil) or VCD (130 mg/kg) 5 sequential days per week for 3 weeks (Figure 1A). Vaginal smears were acquired as previously described (23) for the first 5 injection days to verify regular cyclicity (Figure 1A). At each time point (PRE, EARLY, LATE, and POST), behavioral testing was preceded by 10 days of vaginal smears. Mice were considered acyclic if there were fewer than 2 days of proestrus in the 10-day period (24). All mice underwent cycling and behavior at each time point but were perfused (12 VCD and 12 VEH) only at their assigned terminal time point (Figure 1B). At POST, VCD mice received a single injection (ip) of sesame oil or EB (0.25 mg/kg) 2 weeks after the OP task (below) and 24 hours before perfusion (21, 25); VEH animals were not injected, but cycle stage was determined.
Figure 1.
Induction of AOF and longitudinal experimental design. The AOF mouse model allows for longitudinal study across menopause phases. Ovarian follicles are depleted via 15-day administration of VCD. Timelines represent the days after the injections began (ie, d 0 is the first day of injections). A, Induction of AOF. Mice were weighed weekly and administered VEH or VCD 5 days per week for 3 weeks. Mice were cycled daily through the first week of injections. B, Longitudinal experimental design for examination of estrogen signaling and behavior in across the AOF model. Before each time point mice were cycled for 10 days. EPM and OFT occurred at each time point, but OP only occurred at the terminal time point. After behavioral testing at POST, VCD mice were injected with either oil or EB 24 hours before perfusion. There were 9–13 animals in each group at the terminal time points (PRE: VEH, VCD = 11; EARLY: VEH = 13, VCD = 12; LATE: VEH, VCD = 10; POST: VEH = 11, VCD+Oil = 6, VCD+EB = 8).
To determine the effect of acute or chronic exposure to VCD on inflammation, we examined microglial activation in 2 groups of 2-month-old female mice (n = 3/group): 1) single administration of VEH or VCD and 2) 3 week administration (above) of VEH or VCD. For comparison, a third group was administered 1 injection (ip) of saline or lipopolysaccharide (LPS) (50 μg/kg; n = 2/group). Brains were perfusion fixed as previously described (26) 24 (acute; LPS) or 48 (chronic) hours after last injection. Areas of interest were processed for ionized calcium adapter binding molecule 1 (Iba1) immunoperoxidase.
To compare the effect of ovarian hormones on BDNF transcript levels in AOF with a typical surgical model of hormone loss, postnatal day 50 mice were injected ip with VEH (0.5% dimethyl sulfoxide (DMSO) in sesame oil) or VCD (130 mg/kg) 5 sequential days per week for 3 weeks (see Figure 1A); at POST (127 d after injection), mice were administered oil or EB (0.25 mg/kg). To compare with surgical menopause, 3-month-old female and male mice were OVX or orchidectomized (ORX), respectively, and 10 days after gonadectomy, they were administered oil or EB (0.25 mg/kg). All mice were rapidly decapitated 24 hours after oil/EB administration, and the hippocampus was processed for quantitative real-time RT-PCR (qRT-PCR) as described below.
Determining menopause phase
PRE occurred at postinjection day 24, EARLY at day 52, LATE at day 73, and POST at day 127 (Figure 1B). These time points are referred to as “menopause phase.” “Menopause status” refers to VEH or VCD status at each time point.
Behavioral paradigms
The elevated plus maze (EPM) and the open field test (OFT) were used to monitor anxiety-like behavior. OP measured spatial learning at the terminal time point (Figure 1B). The time spent investigating the new location is reported as a percentage of the total time spent investigating objects at either location.
Brain histology
Mice were deeply anesthetized with sodium pentobarbital (150 mg/kg, ip) and perfused through the ascending aorta as previously described (26). At the time of brain removal, liver, spleen, adrenal glands, uterus, and ovaries (Supplemental Figure 1) were weighed and histologically processed.
qRT-PCR preparation and analysis
Total RNA isolation from the dorsal hippocampus was performed using RNeasy Lipid Tissue Mini kit and QIAcube (QIAGEN) in accordance with the manufacturer's instructions. Total RNA (0.5 μg) was reverse transcribed at 25°C for 10 minutes, 37°C for 120 minutes, and 85°C for 5 minutes using a High-Capacity cDNA Reverse Transcription kit (Applied Biosystems). The qRT-PCR was performed using an Applied Biosystems 7900HT Sequence Detection System v2.3 using the SYBR Green PCR Master Mix. Primers are indicated in Supplemental Table 1.
Light microscopic immunohistochemistry
Specificity was determined for all antibodies (see Supplemental Methods). Coronal sections were processed for peroxidase immunohistochemistry as previously described (26).
To identify microglia, coronal sections from dorsal hippocampus, paraventricular nucleus of the hypothalamus (PVN), subfornical organ (SFO), nucleus of the solitary tract (NTS), and area postrema (AP) were immunolabeled for Iba1 (7, 26). For quantitative densitometry, coronal dorsal hippocampal sections (Supplemental Figure 2A) were immunolabeled for either PSD-95 or pAKT.
For densitometry, regions of interest were photographed on a Nikon Eclipse 80i microscope using NIH Image 1.50 software, and relative optical density (ROD) was determined as previously described (16, 27). Regions of interest included: cornu ammonis (CA) 1 stratum oriens (SO), stratum radiatum (SR), stratum lacunosum-moleculare (SLM), and pyramidal cell layer (PCL); CA3 SO, SR, and stratum lucidum (SLu); dentate gyrus (DG), molecular layer (ML), hilus (Hil), and granule cell layer (GCL). For each condition (VEH and VCD) at each menopause phase (PRE, EARLY, LATE, and POST), 1 brain section per animal (n = 6–12 per group) was analyzed.
Electron microscopic (EM) immunohistochemistry
Coronal dorsal hippocampal sections were immunolabeled for ERα (gift of Dr Hayashi, 1:10 000) and prepared for EM as previously described (26). Sections were examined on a CM10 electron microscope. Profiles were identified by defined morphological criteria (15–18, 28) in CA1 near and distal SR and CA3b SLu (Supplemental Figure 2, B and C). “Unknown profiles” could not be positively classified.
Data analysis
Statistical analyses were performed using JMP 8 (SAS Institute). Significant differences were defined as P < .05. VEH and VCD mice were compared within each menopause phase (PRE, EARLY, and LATE) using the Student's t test. One-way ANOVA was used for more than 2 experimental groups, and Tukey's HSD post hoc test was used to analyze significant main effects. Two-way ANOVA was used to analyze mRNA levels, because the experiment used a 3 × 2 design. The Holm-Sidak multiple comparison test was used as a post hoc analysis of significant main effects.
Results
Accelerated ovarian follicle depletion and acyclicity
To verify the ovotoxic effects of VCD, primary and mature ovarian follicles (Supplemental Figure 1) were counted in hematoxylin and eosin-stained ovaries from VEH and VCD mice at each time point (Supplemental Table 2). VCD significantly reduced primary follicle counts (PRE: t16 = 3.9, P = .0016; EARLY: t22 = 5.3, P < .0001; LATE: t18 = 5.0, P < .0001; POST: t22 = 3.2, P = .0042) (Supplemental Table 2) and mature follicle counts at all time points except PRE (EARLY: t22 = 5.8, P < .0001; LATE: t18 = 2.4, P = .024; POST: t22 = 2.5, P = .022) (Supplemental Table 2) relative to VEH.
Increased acyclicity was evident at LATE and POST time points (Supplemental Table 2). Only 50% of LATE-VCD were cycling normally, whereas over 90% of LATE-VEH demonstrated normal cyclicity (Supplemental Table 2). By POST, all VCD mice but 1 were acyclic, compared with acyclicity in 27% of VEH mice (Supplemental Table 2). Uterine weights of VEH and VCD mice, including POST-VCD mice administered EB, were not significantly different (Supplemental Table 3).
VCD administration is not associated with somatic or neural toxicity
Consistent with others (10, 12, 29–33), VEH and VCD mice did not differ in body or spleen weights (Supplemental Table 4). Liver and spleen sections were histologically unremarkable (data not shown). LATE-VCD mice had significantly higher adrenal weights than LATE-VEH (t18 = −2.4, P = .028; PRE, EARLY, and POST, P > .05) (Supplemental Table 4).
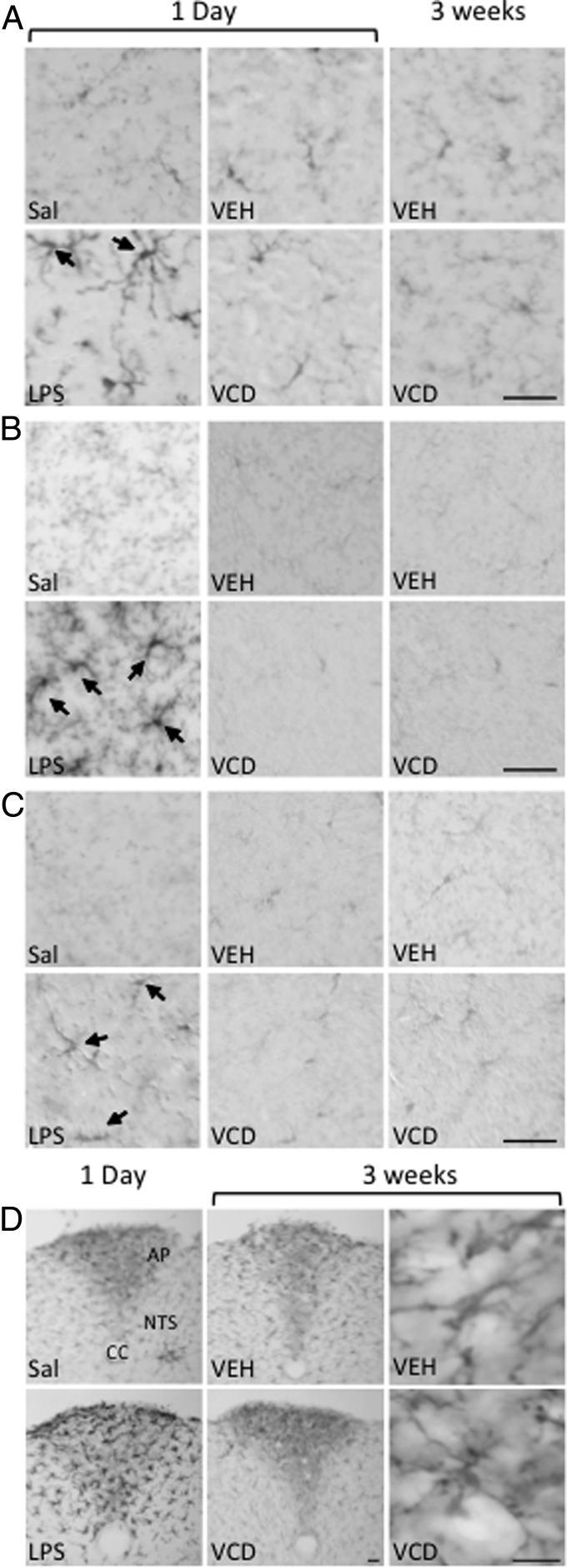
Activated microglia contribute to neuron damage after exposure to environmental and neural toxins (for review, see Refs. 24, 34). To verify that VCD administration would not elicit a neuroimmune response, Iba1-immunoreactivity (ir) was examined in brain regions inside (PVN, hippocampus, and NTS) and outside (SFO and AP) the blood-brain barrier, after acute or chronic (15 d) administration of VCD compared with 1-day post-LPS, which is known to activate microglia in the hippocampus (29, 35). As expected, Iba1-ir was found in cells with thick processes, indicative of activated microglia in all brain regions from the LPS-, but not saline-, injected mice (Figure 2, A–D; SFO not shown). However, activated Iba1-labeled microglia were not seen in PVN (Figure 2A), hippocampus (Figure 2, B and C), NTS and AP (Figure 2D), and other regions, such as cortex and SFO (data not shown), after acute/chronic VEH or VCD.
Figure 2.
VCD administration does not increase Iba1-ir. Although acute LPS administration resulted in an increase in activated microglia (arrows) in all brain regions, neither acute (1 d) nor chronic (3 wk) administration of VEH or VCD resulted in any alteration of Iba1-ir relative to saline-injected mice in the PVN (A) or the SR (B) or ML (C) of the hippocampus. D, In the AP, there was no difference between saline and chronic VEH or VCD. Scale bar, 50 μm. CC, central canal.
PSD-95 levels were minimally altered across menopause phases
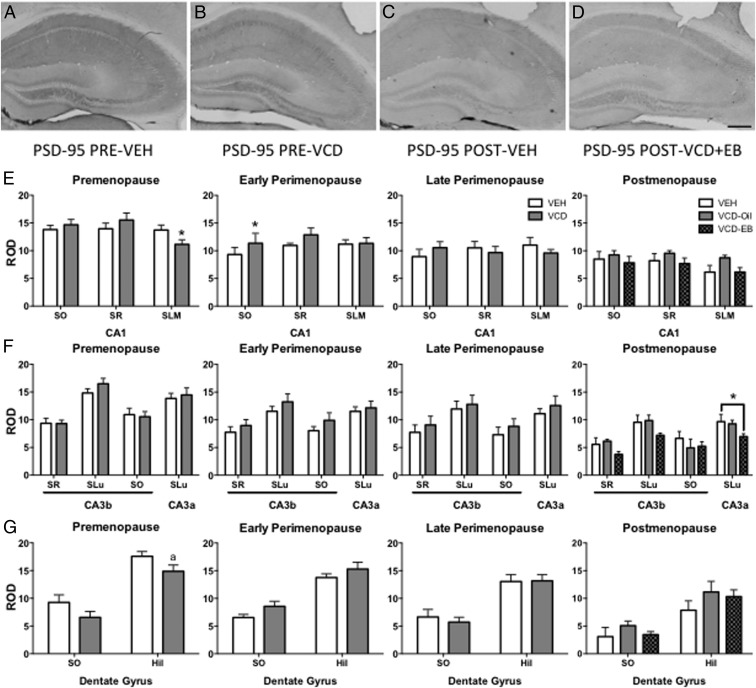
PSD-95 contributes to spine maturation (29, 36) and responds to exogenous EB (19, 29) and estrous fluctuations (16, 24) and is affected in other models of menopause (16, 19, 21). PSD-95-ir was observed in all hippocampal layers except for PCL and GCL (Figure 3, A–D).
Figure 3.
Hippocampal PSD-95 does not change across the menopause phases but is responsive to EB at POST. In the hippocampus, PSD-95-ir is found in all layers except for the PCL and GCL (A–D). Representative examples of PSD95-ir in PRE-VEH (A), PRE-VCD (B), POST-VEH (C), and POST-VCD+EB (D). In CA1, VCD mice showed significant decrease in PSD-95-ir density relative to VEH only at PRE in the SLM (E). In the DG, there was no significant difference in PSD-95-ir density at any menopause time point (F). There was a significant difference in PSD-95-ir density only in CA3a with significantly lower levels in POST-VCD+EB vs POST-VEH mice. There was no significant difference between POST-VEH and POST-VCD+Oil mice (G). Scale bar, 200 μm. *, P < .05; a P = .059.
At PRE, PSD-95-ir was significantly decreased in VCD relative to VEH in CA1 SLM (t16 = 2.2, P = .044) (Figure 3E), nearly significant in Hil (t16 = 2.0, P = .059) (Figure 3G), but was not significant in CA1 SO and SR, CA3b SO, SR, SLu, CA3a SLu, or DG ML. At EARLY, the only region with a significant difference was CA1 SO (t14 = −2.2, P = .049) with greater PSD-95-ir in VCD than VEH.
PSD-95-ir ROD did not differ significantly between VEH and VCD mice in any hippocampal region at LATE. In POST, one-way ANOVA showed a significant main effect of EB administration on PSD-95-ir ROD in CA3a (F2,15 = 3.9, P = .045) (Figure 3F). Post hoc analysis indicated that PSD-95-ir ROD in VCD+EB mice was significantly lower than VEH mice (P = .036). No significant differences were seen in either PSD-95-ir ROD between VEH and VCD+Oil mice, nor in the main effects of EB in CA1, CA3b, or DG (Figure 3, E–G).
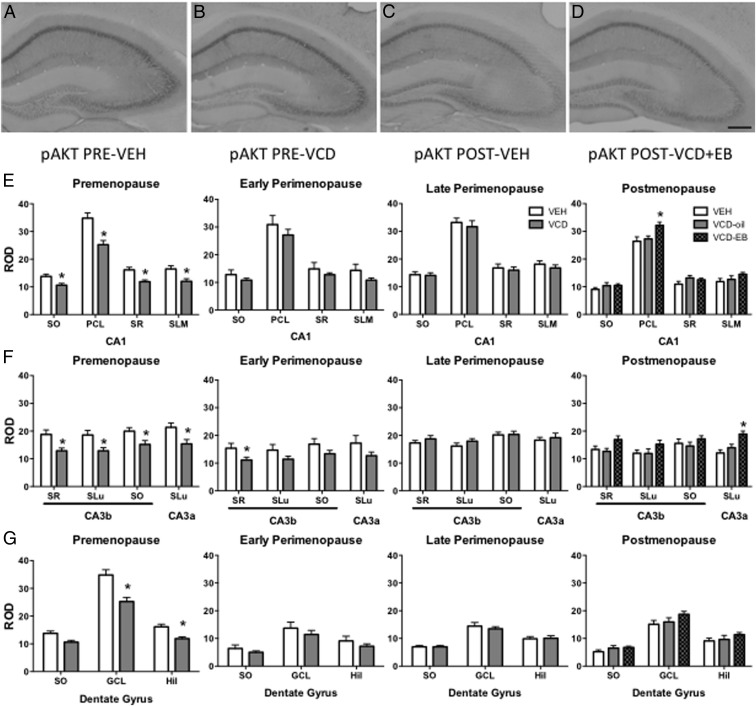
AOF pAKT levels transiently decrease in PRE and are responsive to EB in POST
pAKT is activated by estradiol and is important for spine formation and cognition (10, 16, 37). pAKT-ir was observed throughout the hippocampus in pyramidal and granule cell bodies and processes (Figure 4, A–D). pAKT-ir ROD was significantly decreased in PRE-VCD relative to PRE-VEH in CA1 (SO: t18 = 3.0, P = .009; PCL: t18 = 3.9, P = .001; SR: t18 = 3.7, P = .002; SLM: t18 = 2.9, P = .010), CA3b (SR: t18 = 3.0, P = .009; SLu: t18 = 2.7, P = .014; SO: t18 = 2.5, P = .021), CA3a (t18 = 2.6, P = .018), and DG (GCL: t18 = 2.8, P = .013; Hil: t18 = 2.6, P = .018) (Figure 4, E–G). By EARLY, VCD pAKT-ir ROD was only significantly lower in CA3b SR (t21 = 2.2, P = .044) (Figure 4G). There was no difference in pAKT-ir ROD between VEH and VCD mice in any other region or layer or LATE (Figure 4, E–G).
Figure 4.
Hippocampal pAKT-ir transiently decreases PRE but is responsive to EB at POST. In the hippocampus, pAKT-ir is found in cell bodies and processes of pyramidal and granule cells (A–D). Representative examples of pAKT-ir in PRE-VEH (A), PRE-VCD (B), POST-VEH (C), and POST-VCD+EB (D). In all layers of CA1, pAKT-ir density was significantly decreased in VCD mice only at PRE. Levels of pAKT-ir were only increased in POST-VCD+EB in the PCL (E). In DG, pAKT-ir density was only significantly decreased in VCD mice at PRE and only in GCL and Hil, with no effect on any layer of POST-VCD+Oil or EB (F). pAKT-ir density was significantly decreased in PRE-VCD mice in all CA3 layers, and in CA3b SR EARLY, but there was no significant difference in any layer LATE. pAKT-ir density was significantly greater in POST-VCD+EB only in CA3a (G). Scale bar, 200 μm. *, P < .05.
At POST, one-way ANOVA of pAKT-ir ROD showed a significant main effect of EB only in CA1 PCL (F2,18 = 4.9, P = .022) (Figure 4A) and CA3a SLu (F2,18 = 9.3, P = .002) (Figure 4C). Post hoc analysis revealed significantly greater pAKT-ir ROD in VCD+EB mice than VEH in CA1 PCL (P = .022) (Figure 4E), and in CA3a, pAKT-ir ROD was greater in VCD+EB mice than in either VEH (P = .002) or VCD+Oil (P = .036) (Figure 4F). Although differences in pAKT-ir ROD approached significance in CA3b SR (F2,18 = 3.1, P = .072) (Figure 4F), it was not significantly different in any other hippocampal regions (Figure 4, E and F)
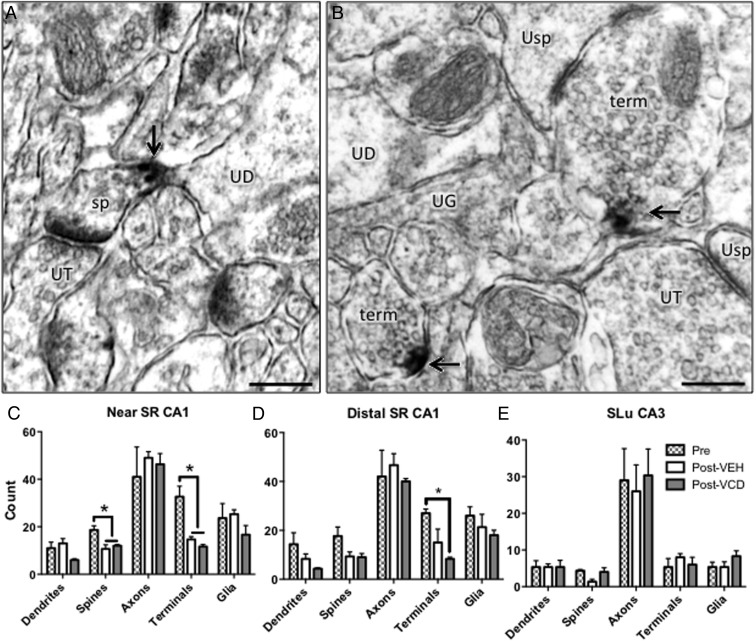
Decrease in number of ERα-ir-labeled terminals by POST in CA1
In rodents, extranuclear ERα-ir occurs in neuronal and glial profiles throughout the hippocampus (18, 38). Synaptic and spinous extranuclear ERα is sensitive to changes in estrogens and aging (18, 39). Here, we used quantitative immuno-EM to determine whether menopause phase or status altered the number of dendrites, spines (Figure 5A), axons, terminals (Figure 5B), and glia containing ERα-ir. Because PSD-95 and pAKT were still responsive to EB in POST-VCD mice, we focused our ERα study on PRE and POST time points. ERα-ir containing profiles were counted in the near (Figure 5C) and distal (Figure 5D) CA1 SR and in CA3 SLu (Figure 5E) in PRE-VEH, POST-VEH, and POST-VCD+Oil, because extranuclear ERα has a role regulating synaptic plasticity in these regions (40). In the near SR, one-way ANOVA revealed a main effect of menopause status on the number of ERα-containing spines (F2,8 = 8.4, P = .018) and terminals (F2,8 = 18, P = .003) (Figure 5C). Post hoc analysis revealed significantly more ERα-labeled spines and terminals in the PRE-VEH group than in either POST-VEH (spines, P = .020; terminals, P = .0076) or POST-VCD (spines, P = .043; terminals, P = .0036). In near SR, menopause status did not have a significant main effect on the number or ERα-containing dendrites, axons, or glia.
Figure 5.
ERα-ir greatest at PRE but is not affected by menopause status at POST. ERα-ir was observed in dendrites, spines (sp), axons, terminals (te), and glia in the SR of CA1 and SLu of CA3 as shown in representative examples of from PRE-VEH (A), POST-VEH (B), and POST-VCD+Oil (C). In the near SR of CA1, post hoc analysis revealed that the number of spines containing ERα-ir was significantly greater in the PRE-VEH group relative to POST-VEH, with no significant difference between VEH- and POST-VCD groups (D). In the distal SR of CA1, by post hoc analysis, the number of ERα-containing terminals was significantly greater in the PRE-VEH group relative to POST-VCD+Oil, again with no significant difference between VEH- and POST-VCD groups (E). In the SLu of CA3, there was no main effect of menopause phase in the CA3-SLu as analyzed by one-way ANOVA (F). Scale bar, 250 nm. *, P < .05. UD, unlabeled dendrite; UT, unlabeled terminal; UG, unlabeled glia, Usp, unlabeled spine.
In distal SR, there was a significant main effect of menopause phase only on the number of ERα-labeled terminals (F2,8 = 7.9, P = .021) (Figure 5D). Post hoc analysis revealed a significantly higher number of ERα-labeled terminals in PRE-VEH than in POST-VCD mice (P = .018) (Figure 5D). There was no significant effect of menopause phase in distal SR dendrites, spines, axons, or glia, nor in CA3-SLu (Figure 5E).
Reduced expression of BDNF exon mRNA in VCD but not OVX or ORX
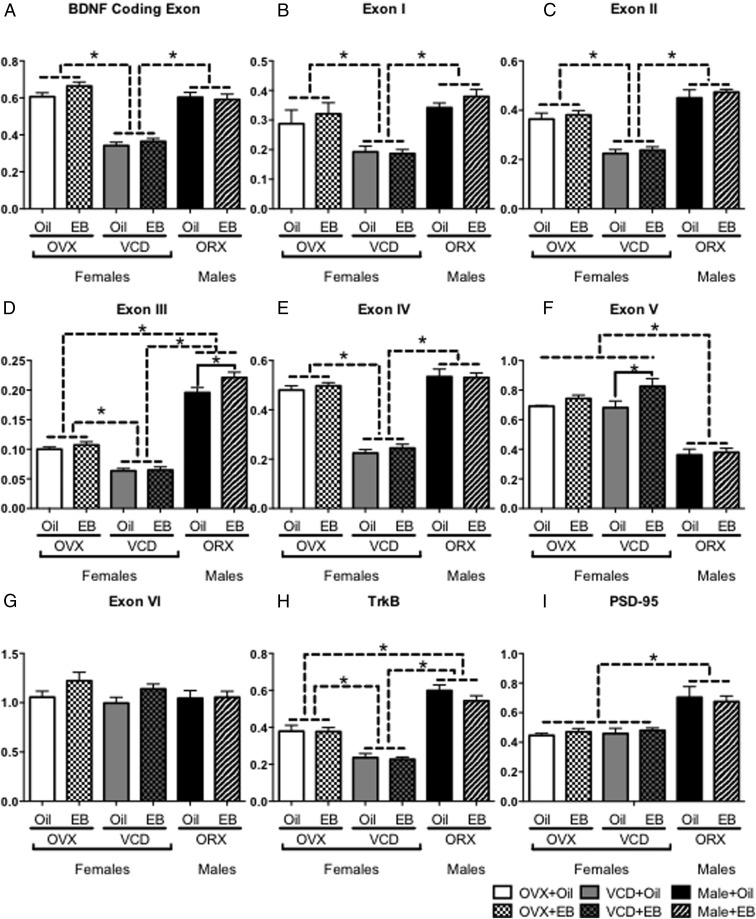
BDNF has a role in cognition and spatial learning (41, 42). With a putative estrogen response element (43), BDNF levels and function have demonstrated estrogen sensitivity (15, 44). Activation of extranuclear ERs induces BDNF signaling in CA1, resulting in neuroprotective effects (45). Exogenous EB in OVX mice increases BDNF mRNA levels (46). To compare how VCD affected levels of BDNF mRNA, POST-VCD, OVX, and ORX mice were administered exogenous oil or EB 24 hours before brain collection. To determine whether there was a differential response to EB in the 3 manipulations of gonadal tissue, we used qRT-PCR primers to the protein-coding exon and exons I–VI of BDNF, as well as tyrosine kinase B (TrkB), and PSD-95. Results in each exon were analyzed with two-way ANOVA.
There was a significant main effect of gonadal manipulation on the mRNA levels of the BDNF protein coding exon (F2,35 = 96.0, P < .0001), exon I (F2,35 = 17, P < .0001), exon II (F2,35 = 67, P < .0001), and exon IV (F2,35 = 150, P < .0001) (Figure 6, A–C and E). However, there was no significant main effect of hormone manipulation and no significant interaction between gonadal and hormone manipulations on any exons. Post hoc analysis for simple effects of gonadal manipulation revealed that mRNA levels of these exons (coding, I, II, and IV) were significantly lower in VCD than either OVX (P < .0001) or ORX (P < .0001), but there was no significant difference in levels between OVX and ORX .
Figure 6.
Reduced expression of BDNF exon mRNA in VCD but not OVX or ORX. OVX, VCD, and ORX mice were administered either oil or EB 24 hours before brain removal and mRNA expression of the BDNF coding exon (A), and nonprotein coding exons I (B), II (C), III (D), IV (E), V (F), VI (G) as well as TrkB (H), and PSD-95 (I), was measured by qRT-PCR. Levels of mRNA were lowest in VCD mice in the BDNF coding exon, as well as exons I, II, III, IV, and TrkB (A–D and H, respectively). Only in exon V did administration of EB significantly increase mRNA expression over oil administration (F). There was no difference in mRNA expression between OVX and VCD mice in exon V (F), VI (G), and PSD-95 (I).
In exons III and V (Figure 6, D and F), there was a significant main effect of gonadal manipulations (exon III: F2,35 = 324, P < .0001; exon V: F2,35 = 55.3, P < .0001) and a significant main effect of hormone manipulation (exon III: F1,35 = 5.50, P = .0249; exon V: F1,35 = 4.37, P = .0365) but no significant interaction between gonadal and hormone manipulations. In exon III, post hoc analysis of simple effects of gonadal manipulation revealed significantly lower mRNA levels in OVX (P < .0001) than ORX, but levels of exon III mRNA in VCD mice were significantly lower than in either OVX or ORX. Post hoc analysis of hormone manipulation simple effects revealed that EB administration significantly increased exon III mRNA only in the ORX group (P = .0319). In contrast, post hoc analysis of simple effects of gonadal manipulation in exon V revealed significantly lower levels of mRNA in ORX than in either OVX (P < .0001) or VCD (P < .0001). Post hoc analysis of hormone manipulation simple effects showed that unlike any other exon measured, EB administration increased exon V mRNA only in VCD (P = .0192).
In exon VI (Figure 6G), there were no significant main effects of either gonadal or hormone manipulations, nor was there a significant interaction between gonadal and hormone manipulations.
For TrkB mRNA levels (Figure 6H), there was a significant main effect of gonadal manipulation (F2,35 = 94.6, P < .0001) but no significant main effect of hormone manipulation and no significant interaction between gonadal and hormone manipulations. Post hoc analysis of simple effects of gonadal manipulation revealed that in addition to significantly lower TrkB mRNA levels in VCD mice than in either OVX or ORX, TrkB mRNA levels in OVX were significantly lower (P < .0001) than in ORX.
Two-way ANOVA of PSD-95 (Figure 6I) revealed a significant main effect of gonadal manipulation (F2,35 = 28.3, P < .0001) but no significant main effect of hormone manipulation and no significant interaction between gonadal and hormonal manipulations. Post hoc analysis of simple effects of gonadal manipulation revealed that PSD-95 mRNA levels were significantly greater (P < .0001) in ORX than in either OVX or VCD.
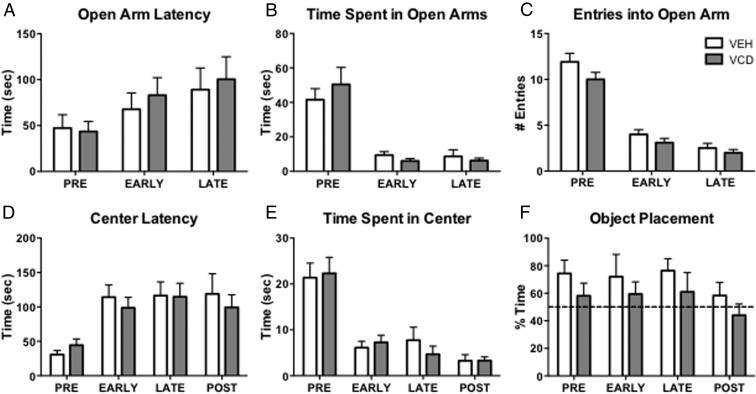
Cognitive changes not observed until POST
Mice were tested on the EPM and OFT at each menopause phase (Figure 1B). On the EPM, VEH and VCD mice did not differ significantly at any tested time point on their latency to enter open arms (Figure 7A), the amount of total time spent in the open arms (Figure 7B), or the number of entries into the open arms (Figure 7C). The OFT did not indicate any effect of VEH or VCD at any time point in the latency to enter the center (Figure 7D) or the total time spent in the center (Figure 7E).
Figure 7.
OP and behavior. Measures of anxiety-like behavior such as latency to enter (A), time spent (B), and entries (C) into open arms of the EPM did not differ between VEH and VCD mice at any time point. In the OFT, there was no significant difference in latency to enter the center (D) or time spent in the center (E). Unlike VEH and VCD mice at other time points, POST-VCD mice spent less than 50% (dotted line) of the time exploring the object in the new location (F).
OP has been shown to be sensitive to exogenous estradiol administration (19, 47, 48). Although there were no significant differences between VEH and VCD mice in the investigation of the new location (time spent investigating the new location as a percentage of total time) (Figure 7F), only at POST did the mean of VCD mice fall below 50% (Figure 7F).
Discussion
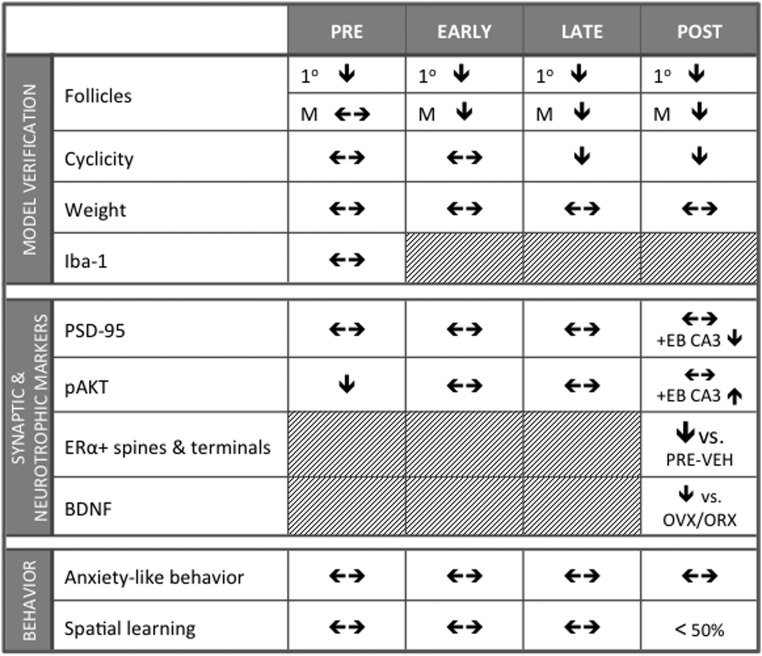
This is the first study to assess markers of pathways germane to synaptic plasticity, spatial learning, and anxiety-like behavior in the hippocampus longitudinally across the AOF model. Our results not only demonstrate that the AOF mouse model is suitable for longitudinal studies of neurobiological changes across the menopause transition, particularly from EARLY to POST time points, but also suggest complex interactions between ERs and pathways involved in synaptic plasticity (Figure 8).
Figure 8.
Summary of findings across menopause phases. Administration of VCD reduced number of primary and mature follicles and increased acyclicity in LATE and POST, but there was no effect of administration on organ or body weights. Microglial activation was not affected by VCD administration. Synaptic and neurotrophic markers were responsive to nongenomic acting doses of EB at POST in AOF mice. Anxiety-like behavior and spatial learning were largely unaffected by menopause phase or menopause status. M, mature.
Acyclicity and toxicology across menopause phases
Our findings of follicle loss and acyclicity with VCD concur with other studies. Because primary follicles are the direct targets of VCD (8, 49, 50), mature follicles decrease only after primordial and primary follicle pools have been sufficiently depleted (30, 51). Thus, the onset of acyclicity follows with the decrease in the mature follicle pool (30, 51). Although we found no difference in uterine weights between VEH and VCD mice, weight variations, consistent with other studies, were equal across menopause phase and status (52) (for a summary of findings, see Figure 8).
As others have shown, we did not observe any overall effect of VCD administration on body or organ weights (31–33). Although adrenal weights of LATE-VCD were significantly greater than LATE-VEH, no other organ weights differed between VEH and VCD at any other time point. Importantly, neither acute nor chronic VCD administration elicited a neuroimmune response. Our results suggest that VCD does not have any direct effects on the brain and does not activate microglia in regions outside the blood-brain barrier.
Transient decrease in pAKT levels in AOF mice at PRE are not accompanied by behavioral changes
The transient decrease in pAKT-ir throughout the hippocampus and in PSD-95-ir in CA1 SLM is likely not hormonally mediated, as it occurs at PRE, a time point is not characterized by decreased mature follicle count or abnormal cyclicity. Although VEH and VCD experienced identical handling and experimental manipulations, VCD mice may be more prone to negative effects of injections and handling. Stress decreases levels of hippocampal pAKT and PSD-95 (53). The apical dendritic tufts of CA1-SLM may be particularly sensitive (54), which may account for the decrease in PSD-95-ir at PRE only occurring in the SLM of CA1.
The continued depression of pAKT-ir in CA3b at EARLY may be due to the vulnerability of this region to stress (55). Despite altered pAKT levels, there were no significant differences in EPM, OFT, or OP performance between PRE- or EARLY-VEH or VCD mice. Thus, the temporal proximity to the injection period the PRE time point is not best suited for neurobiological and behavioral investigations.
POST PSD-95, pAKT, and ERα
Based on work in cycling and OVX mice, we hypothesized that PSD-95 and pAKT levels would be lower in VCD than in VEH mice. Yet we observed no significant difference between VEH and VCD+Oil groups. Given that PSD-95 and pAKT levels vary across the estrous cycle, we thought that these markers would be susceptible to the distinct, subtle hormonal changes characteristic of AOF. However, such observations may have been obscured by the dynamics of hippocampal spine maturation in response to estrogen, or subtle changes in ERs through the menopause transition (39). Unlike rats, estrogen administration in OVX mice does not increase total dendritic spine density but does increase the number of the mature, better-established mushroom-shaped spines (19). Thus, levels of PSD-95 and pAKT may not be reflecting spine number but rather the maturation of existing spines.
Estrogens differentially affect ERα and ERβ (21, 56–58), and activation of these receptors have distinct effects on levels of PSD-95 and pAKT (21) and spine density (59). POST-VCD mice were still responsive to the rapid effects of EB as measured by PSD-95- and pAKT-ir. Although the EB-associated decline in PSD-95 levels seems unexpected, rapid EB administration in mice constitutively lacking ERβ showed a trend for decreased levels of PSD-95 in CA3a, perhaps reflecting changing receptor populations (21). The increase in pAKT levels in CA1 and CA3a in POST-VCD+EB mice is consistent with previous findings of rapid, nongenomic EB effects in OVX female mice and rats (21, 60). Estrogens have been shown to activate pAKT via ERα (61, 62), and the expression of pAKT has been shown to change over the course of the estrous cycle and in response to exogenous estrogens, in a manner dependent on ERs (19, 60, 63). Although we observed no difference in the number of ERα-labeled neuronal profiles in CA3 SLu, this does not rule out changes in other receptor populations, eg, ERβ or G protein-coupled receptor 1, that may be influencing the rapid response of PSD-95 to EB (59). Indeed, recent work suggests that ERα and ERβ interact with levels of ovarian hormones, responding with transcriptional changes to maintain hippocampal function (57).
POST BDNF exons
BDNF levels fluctuate over the estrous cycle, decrease after OVX, and impact learning and memory (15, 44, 45). Decreased levels of BDNF exon mRNA in POST-VCD mice relative to OVX and ORX groups may reflect another difference between AOF and OVX models. Previous work has demonstrated the differences between AOF and OVX models of menopause on both cognition and response to EB (13, 14). Several studies demonstrating that increased duration of ovarian hormone deprivation decreases responsivity to estrogens more than aging (64, 65) might suggest that levels of mRNA in BDNF exons I, II, and IV were lower in POST-VCD than OVX because of the greater duration of hormone deprivation. However, no BDNF exons were responsive to EB in OVX mice, and yet it was in POST-VCD mice that levels of exon V mRNA were increased 24 hours after EB. Thus, intact, steroidigenic ovarian tissue (9) may be important for neurotrophic responses to menopause. Previous work suggests that an increase in BDNF transcript follows EB administration only in OVX ERα, but not ERβ, knockout mice (16). Thus, transitional menopause and the presence of intact, reproductively senescent ovarian tissue may affect ERs differently than OVX.
Complex behavior in POST
With the complexity of hormonal and synaptic plasticity pathways, in combination with our longitudinal approach, it is not surprising that there were not striking behavioral differences between VEH and VCD groups. Handling is known to have significant effects on mice (66) and can alter behavioral performance due to habituation or masking (67). To monitor cyclicity, mice were handled for 10 days before time points. By virtue of the longitudinal design, POST mice experienced the most handling of all groups. Handling may have affected VEH and VCD mice very differently, thus obscuring any behavioral differences specifically due to the transition to menopause. In recent studies of OVX mice, handling had the same effect in both oil- and EB-treated animals as EB alone (ie, without handling) (E.M.W., T.A.V.K., T.A.M., unpublished data). Thus, changes in cognitive behavior in models of menopause in mice may parallel observations in the human literature in their nuance and complexity.
Stress and life events may significantly contribute to cognitive dysfunction in menopause (68, 69). Indeed, in postmenopausal women, coadministration of 17β-estradiol and corticosterone attenuated the positive cognitive effects of 17β-estradiol alone (70). Thus, menopause phase and other risk factors (stress, genetics, etc) may interact to influence cognitive changes in menopause.
Functional considerations
Human studies of menopause involve longitudinal testing on memory and cognitive tasks, resulting in substantial task learning effects that confound and obscure menopause-related changes in cognitive function and performance (71). The degree to which neurobiological mechanisms can be assayed in humans is limited. The AOF model is poised to be of great utility as a rodent model that can be used longitudinally to probe mechanisms underlying not only cognitive dysfunctions but also other physiological changes across the menopause transition.
Research in both humans and rodents supports an emerging link between perimenopause, cognitive deficits, and cardiovascular dysfunction. Vasomotor symptoms, such as hot flashes and night sweats, begin during perimenopause, and hot flash frequency correlates with poor verbal memory (72) and increased risk of cardiovascular disease (73, 74). Although women have lower incidence of hypertension compared with men at younger ages, this reverses through the menopause transition (75). Neurobiological mechanisms underlying neural regulation of blood pressure and cognition have yet to be fully elucidated, and the AOF model will allow for the longitudinal investigation of potential mechanisms.
Acknowledgments
We thank Dr Steven Einheber of Hunter College, City University of New York, for his advice and assistance examining histopathology and Nawreen Rahman for her contribution to the Iba1 immunohistochemistry.
This work was supported by National Institutes of Health Grants AG039850, AG16765 (to E.M.W. and T.A.M.), DA08259, HL098351, and HL096571 (to T.A.M.), and T32 DA007274 (to T.A.V.K.) and by The Rockefeller Center for Clinical and Translational Science Grant 5ULI RR024243 (to E.M.W.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AOF
- accelerated ovarian failure
- AP
- area postrema
- BDNF
- brain-derived neurotrophic factor
- CA
- cornu ammonis region
- DG
- dentate gyrus
- EARLY
- early perimenopause
- EB
- estradiol benzoate
- EM
- electron microscopy
- EPM
- elevated plus maze
- ER
- estrogen receptor
- GCL
- granule cell layer
- Hil
- hilus
- Iba1
- ionized calcium binding adapter molecule 1
- ir
- immunoreactivity
- LATE
- late perimenopause
- LPS
- lipopolysaccharide
- ML
- molecular layer
- NTS
- nucleus of the solitary tract
- OFT
- open field test
- OP
- object placement
- ORX
- orchidectomy
- OVX
- ovariectomy
- pAKT
- phosphorylated AKT
- PCL
- pyramidal cell layer
- POST
- early postmenopause
- PRE
- premenopause
- PSD-95
- postsynaptic density 95
- PVN
- paraventricular nucleus of the hypothalamus
- qRT-PCR
- quantitative real-time RT-PCR
- ROD
- relative optical density
- SFO
- subfornical organ
- SLM
- stratum lacunosum-moleculare
- SLu
- lucidum
- SO
- stratum oriens
- SR
- stratum radiatum
- TrkB
- tyrosine kinase B
- VCD
- 4-vinylcyclohexene diepoxide
- VEH
- vehicle.
References
- 1. Sandstrom NJ, Williams CL. Spatial memory retention is enhanced by acute and continuous estradiol replacement. Horm Behav. 2004;45:128–135 [DOI] [PubMed] [Google Scholar]
- 2. Protopopescu X, Pan H, Altemus M, et al. Orbitofrontal cortex activity related to emotional processing changes across the menstrual cycle. Proc Natl Acad Sci USA. 2005;102:16060–16065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Craig MC, Brammer M, Maki PM, et al. The interactive effect of acute ovarian suppression and the cholinergic system on visuospatial working memory in young women. Psychoneuroendocrinology. 2010;35:987–1000 [DOI] [PubMed] [Google Scholar]
- 4. Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008;29:219–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dumas JA, Kutz AM, Naylor MR, Johnson JV, Newhouse PA. Increased memory load-related frontal activation after estradiol treatment in postmenopausal women. Horm Behav. 2010;58:929–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weber MT, Rubin LH, Maki PM. Cognition in perimenopause: the effect of transition stage. Menopause. 2013;20:511–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van Kempen TA, Milner TA, Waters EM. Accelerated ovarian failure: a novel, chemically induced animal model of menopause. Brain Res. 2011;1379:176–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Keating AF, Fernandez SM, Mark-Kappeler CJ, Sen N, Sipes IG, Hoyer PB. Inhibition of PIK3 signaling pathway members by the ovotoxicant 4-vinylcyclohexene diepoxide in rats. Biol Reprod. 2011;84:743–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mayer LP, Devine PJ, Dyer CA, Hoyer PB. The follicle-deplete mouse ovary produces androgen. Biol Reprod. 2004;71:130–138 [DOI] [PubMed] [Google Scholar]
- 10. Lohff JC, Christian PJ, Marion SL, Hoyer PB. Effect of duration of dosing on onset of ovarian failure in a chemical-induced mouse model of perimenopause. Menopause. 2006;13:482–488 [DOI] [PubMed] [Google Scholar]
- 11. Mayer LP, Pearsall NA, Christian PJ, et al. Long-term effects of ovarian follicular depletion in rats by 4-vinylcyclohexene diepoxide. Reprod Toxicol. 2002;16:775–781 [DOI] [PubMed] [Google Scholar]
- 12. Lohff JC, Christian PJ, Marion SL, Arrandale A, Hoyer PB. Characterization of cyclicity and hormonal profile with impending ovarian failure in a novel chemical-induced mouse model of perimenopause. Comp Med. 2005;55:523–527 [PubMed] [Google Scholar]
- 13. Acosta JI, Mayer L, Talboom JS, et al. Transitional versus surgical menopause in a rodent model: etiology of ovarian hormone loss impacts memory and the acetylcholine system. Endocrinology. 2009;150:4248–4259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Acosta JI, Mayer LP, Braden BB, Nonnenmacher S, Mennenga SE, Bimonte-Nelson HA. The cognitive effects of conjugated equine estrogens depend on whether menopause etiology is transitional or surgical. Endocrinology. 2010;151:3795–3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ. Hippocampal excitability increases during the estrous cycle in the rat: a potential role for brain-derived neurotrophic factor. J Neurosci. 2003;23:11641–11652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spencer JL, Waters EM, Milner TA, McEwen BS. Estrous cycle regulates activation of hippocampal Akt, LIM kinase, and neurotrophin receptors in C57BL/6 mice. Neuroscience. 2008;155:1106–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Spencer-Segal JL, Waters EM, Bath KG, Chao MV, McEwen BS, Milner TA. Distribution of phosphorylated TrkB receptor in the mouse hippocampal formation depends on sex and estrous cycle stage. J Neurosci. 2011;31:6780–6790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mitterling KL, Spencer JL, Dziedzic N, et al. Cellular and subcellular localization of estrogen and progestin receptor immunoreactivities in the mouse hippocampus. J Comp Neurol. 2010;518:2729–2743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li C, Brake WG, Romeo RD, et al. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci USA. 2004;101:2185–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Waters EM, Yildirim M, Janssen WG, et al. Estrogen and aging affect the synaptic distribution of estrogen receptor β-immunoreactivity in the CA1 region of female rat hippocampus. Brain Res. 2011;1379:86–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spencer-Segal JL, Tsuda MC, Mattei L, et al. Estradiol acts via estrogen receptors α and β on pathways important for synaptic plasticity in the mouse hippocampal formation. Neuroscience. 2012;202:131–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Golub MS, Germann SL, Mercer M, et al. Behavioral consequences of ovarian atrophy and estrogen replacement in the APPswe mouse. Neurobiol Aging. 2008;29:1512–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Byers SL, Wiles MV, Dunn SL, Taft RA. Mouse estrous cycle identification tool and images. PLoS ONE. 2012;7:e35538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80:84–97 [DOI] [PubMed] [Google Scholar]
- 25. Frick KM, Fernandez SM, Bennett JC, Prange-Kiel J, MacLusky NJ, Leranth C. Behavioral training interferes with the ability of gonadal hormones to increase CA1 spine synapse density in ovariectomized female rats. Eur J Neurosci. 2004;19:3026–3032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Milner TA, Waters EM, Robinson DC, Pierce JP. Degenerating processes identified by electron microscopic immunocytochemical methods. Methods Mol Biol. 2011;793:23–59 [DOI] [PubMed] [Google Scholar]
- 27. Pierce JP, Kurucz OS, Milner TA. Morphometry of a peptidergic transmitter system: dynorphin B-like immunoreactivity in the rat hippocampal mossy fiber pathway before and after seizures. Hippocampus. 1999;9:255–276 [DOI] [PubMed] [Google Scholar]
- 28. Peters A, Palay SL, Webster HD. The Fine Structure of the Nervous System. New York, NY: Oxford University Press; 1991 [Google Scholar]
- 29. McLean AC, Valenzuela N, Fai S, Bennett SAL. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J Vis Exp. 2012:e4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Flaws JA, Doerr JK, Sipes IG, Hoyer PB. Destruction of preantral follicles in adult rats by 4-vinyl-1-cyclohexene diepoxide. Reprod Toxicol. 1994;8:509–514 [DOI] [PubMed] [Google Scholar]
- 31. Haas JR, Christian PJ, Hoyer PB. Effects of impending ovarian failure induced by 4-vinylcyclohexene diepoxide on fertility in C57BL/6 female mice. Comp Med. 2007;57:443–449 [PubMed] [Google Scholar]
- 32. Wright LE, Christian PJ, Rivera Z, et al. Comparison of skeletal effects of ovariectomy versus chemically induced ovarian failure in mice. J Bone Miner Res. 2008;23:1296–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sahambi SK, Visser JA, Themmen AP, Mayer LP, Devine PJ. Correlation of serum anti-Müllerian hormone with accelerated follicle loss following 4-vinylcyclohexene diepoxide-induced follicle loss in mice. Reprod Toxicol. 2008;26:116–122 [DOI] [PubMed] [Google Scholar]
- 34. Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69 [DOI] [PubMed] [Google Scholar]
- 35. Chung DW, Yoo KY, Hwang IK, et al. Systemic administration of lipopolysaccharide induces cyclooxygenase-2 immunoreactivity in endothelium and increases microglia in the mouse hippocampus. Cell Mol Neurobiol. 2010;30:531–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368 [PubMed] [Google Scholar]
- 37. Liu J, Bisschop PH, Eggels L, et al. Intrahypothalamic estradiol modulates hypothalamus-pituitary-adrenal-axis activity in female rats. Endocrinology. 2012;153:3337–3344 [DOI] [PubMed] [Google Scholar]
- 38. Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal α estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–371 [PubMed] [Google Scholar]
- 39. Adams MM, Fink SE, Shah RA, et al. Estrogen and aging affect the subcellular distribution of estrogen receptor-α in the hippocampus of female rats. J Neurosci. 2002;22:3608–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hart SA, Snyder MA, Smejkalova T, Woolley CS. Estrogen mobilizes a subset of estrogen receptor-α-immunoreactive vesicles in inhibitory presynaptic boutons in hippocampal CA1. J Neurosci. 2007;27:2102–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J Neurosci. 2000;20:7116–7121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Scharfman HE, Maclusky NJ. Differential regulation of BDNF, synaptic plasticity and sprouting in the hippocampal mossy fiber pathway of male and female rats. Neuropharmacology. 2014;76(pt C):696–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sohrabji F, Miranda RC, Toran-Allerand CD. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc Natl Acad Sci USA. 1995;92:11110–11114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Scharfman HE, Hintz TM, Gomez J, et al. Changes in hippocampal function of ovariectomized rats after sequential low doses of estradiol to simulate the preovulatory estrogen surge. Eur J Neurosci. 2007;26:2595–2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yang L-C, Zhang Q-G, Zhou C-F, et al. Extranuclear estrogen receptors mediate the neuroprotective effects of estrogen in the rat hippocampus. PLoS ONE. 2010;5:e9851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhou J, Zhang H, Cohen RS, Pandey SC. Effects of estrogen treatment on expression of brain-derived neurotrophic factor and cAMP response element-binding protein expression and phosphorylation in rat amygdaloid and hippocampal structures. Neuroendocrinology. 2005;81:294–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res. 1998;57:1–9 [DOI] [PubMed] [Google Scholar]
- 48. Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844 [DOI] [PubMed] [Google Scholar]
- 49. Shrivastava K, Gonzalez P, Acarin L. The immune inhibitory complex CD200/CD200R is developmentally regulated in the mouse brain. J Comp Neurol. 2012;520:2657–2675 [DOI] [PubMed] [Google Scholar]
- 50. Keating AF, Sen N, Sipes IG, Hoyer PB. Dual protective role for glutathione S-transferase class pi against VCD-induced ovotoxicity in the rat ovary. Toxicol Appl Pharmacol. 2010;247:71–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Keating AF, J Mark C, Sen N, Sipes IG, Hoyer PB. Effect of phosphatidylinositol-3 kinase inhibition on ovotoxicity caused by 4-vinylcyclohexene diepoxide and 7, 12-dimethylbenz[a]anthracene in neonatal rat ovaries. Toxicol Appl Pharmacol. 2009;241:127–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Barton HA, Andersen ME, Allen BC. Dose-response characteristics of uterine responses in rats exposed to estrogen agonists. Regul Toxicol Pharmacol. 1998;28:133–149 [DOI] [PubMed] [Google Scholar]
- 53. Fang ZH, Lee CH, Seo MK, et al. Effect of treadmill exercise on the BDNF-mediated pathway in the hippocampus of stressed rats. Neurosci Res. 2013;76:187–194 [DOI] [PubMed] [Google Scholar]
- 54. Donohue HS, Gabbott PL, Davies HA, et al. Chronic restraint stress induces changes in synapse morphology in stratum lacunosum-moleculare CA1 rat hippocampus: a stereological and three-dimensional ultrastructural study. Neuroscience. 2006;140:597–606 [DOI] [PubMed] [Google Scholar]
- 55. Pierce JP, Kelter DT, McEwen BS, Waters EM, Milner TA. Hippocampal mossy fiber leu-enkephalin immunoreactivity in female rats is significantly altered following both acute and chronic stress. J Chem Neuroanat. 2014;55:9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Van Kempen TA, Kahlid S, Gonzalez AD, et al. Sex and estrogen receptor expression influence opioid peptide levels in the mouse hippocampal mossy fiber pathway. Neurosci Lett. 2013;552:66–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Han X, Aenlle KK, Bean LA, et al. Role of estrogen receptor α and β in preserving hippocampal function during aging. J Neurosci. 2013;33:2671–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Waters EM, Mitterling K, Spencer JL, Mazid S, McEwen BS, Milner TA. Estrogen receptor α and β specific agonists regulate expression of synaptic proteins in rat hippocampus. Brain Res. 2009;1290:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Phan A, Lancaster KE, Armstrong JN, MacLusky NJ, Choleris E. Rapid effects of estrogen receptor α and β selective agonists on learning and dendritic spines in female mice. Endocrinology. 2011;152:1492–1502 [DOI] [PubMed] [Google Scholar]
- 60. Yildirim M, Janssen WG, Lou WY, et al. Effects of estrogen and aging on the synaptic distribution of phosphorylated Akt-immunoreactivity in the CA1 region of the female rat hippocampus. Brain Res. 2011;1379:98–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. McEwen B, Akama K, Alves S, et al. Tracking the estrogen receptor in neurons: implications for estrogen-induced synapse formation. Proc Natl Acad Sci USA. 2001;98:7093–7100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Znamensky V, Akama KT, McEwen BS, Milner TA. Estrogen levels regulate the subcellular distribution of phosphorylated Akt in hippocampal CA1 dendrites. J Neurosci. 2003;23:2340–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Akama KT, McEwen BS. Estrogen stimulates postsynaptic density-95 rapid protein synthesis via the Akt/protein kinase B pathway. J Neurosci. 2003;23:2333–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Smith CC, Vedder LC, Nelson AR, Bredemann TM, McMahon LL. Duration of estrogen deprivation, not chronological age, prevents estrogen's ability to enhance hippocampal synaptic physiology. Proc Natl Acad Sci USA. 2010;107:19543–19548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhang QG, Wang RM, Scott E, et al. Hypersensitivity of the hippocampal CA3 region to stress-induced neurodegeneration and amyloidogenesis in a rat model of surgical menopause. Brain. 2013;136:1432–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Longordo F, Fan J, Steimer T, Kopp C, Lüthi A. Do mice habituate to “gentle handling?” A comparison of resting behavior, corticosterone levels and synaptic function in handled and undisturbed C57BL/6J mice. Sleep. 2011;34:679–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gerlai R. Behavioral tests of hippocampal function: simple paradigms complex problems. Behav Brain Res. 2001;125:269–277 [DOI] [PubMed] [Google Scholar]
- 68. Woods NF, Mitchell ES. Symptom interference with work and relationships during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women's Health Study. Menopause. 2011;18:654–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Weber MT, Mapstone M, Staskiewicz J, Maki PM. Reconciling subjective memory complaints with objective memory performance in the menopausal transition. Menopause. 2012;19:735–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Baker LD, Asthana S, Cholerton BA, et al. Cognitive response to estradiol in postmenopausal women is modified by high cortisol. Neurobiol Aging. 2012;33:829.e9–.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Greendale GA, Derby CA, Maki PM. Perimenopause and cognition. Obstet Gynecol Clin North Am. 2011;38:519–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Maki PM, Drogos LL, Rubin LH, Banuvar S, Shulman LP, Geller SE. Objective hot flashes are negatively related to verbal memory performance in midlife women. Menopause. 2008;15:848–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lambrinoudaki I, Augoulea A, Armeni E, et al. Menopausal symptoms are associated with subclinical atherosclerosis in healthy recently postmenopausal women. Climacteric. 2012;15:350–357 [DOI] [PubMed] [Google Scholar]
- 74. Hitchcock CL, Elliott TG, Norman EG, Stajic V, Teede H, Prior JC. Hot flushes and night sweats differ in associations with cardiovascular markers in healthy early postmenopausal women. Menopause. 2012;19:1208–1214 [DOI] [PubMed] [Google Scholar]
- 75. Maranon R, Reckelhoff JF. Sex and gender differences in control of blood pressure. Clin Sci. 2013;125:311–318 [DOI] [PMC free article] [PubMed] [Google Scholar]