Abstract
Prenatal testosterone (T) excess disrupts ovarian cyclicity and increases circulating estradiol levels as well as follicular recruitment and persistence culminating in multifollicular ovary similar to women with polycystic ovary syndrome. We tested whether prenatal T excess, by androgenic or estrogenic action, disrupts the steroid biosynthetic machinery in sheep in a cell-, follicle stage-, age-, and treatment-specific manner consistent with the ovarian disruptions and increased estradiol release. Impact of T/dihydrotestosterone (DHT) treatments from days 30–90 of gestation on steroidogenic acute regulatory protein, 3β-hydroxysteroid dehydrogenase, cytochrome P-450 17α-hydroxylase/C17, 20-lyase (CYP17A1), and cytochrome P-450 aromatase (CYP19A1) were examined on fetal day 90, 140 and 10 months (postpubertal), and 21 months (adult, no DHT group) of age by immunohistochemistry. All 4 markers changed in a cell-, follicle stage-, and age-specific manner. Both treatments increased steroidogenic acute regulatory protein expression in preantral follicles of postpubertal and adult females. Effects of prenatal T and DHT on 3β-hydroxysteroid dehydrogenase differed in a follicle- and age-specific manner. CYP17A1 was reduced in the theca interna of antral follicles by T, but not DHT, in 10- and 21-month-old females. CYP19A1 was reduced by both T and DHT at all ages barring an increase on fetal day 140. Reduced granulosa CYP19A1 and thecal CYP17A1 in adults likely disrupt the intrafollicular androgen/estrogen balance contributing to follicular persistence. The reduced thecal CYP17A1 expression suggests that the hyperandrogenic ovarian phenotype may originate from increased enzyme activity or alternatively via a different isoform of CYP17. The reduced CYP19A1 in antral follicles of adults indicates that the increased circulating estradiol release likely arises from the increased number of persisting follicles.
Inappropriate activation of the reproductive system by exposure to excess steroid hormones or environmental chemicals with steroidogenic/antisteroidogenic potential is a major concern, especially in the female (1–3). Developing fetuses have the likelihood of getting exposed to excess steroids through their mother. This can stem from failed contraception and continued exposure to contraceptive steroids (4, 5), unintended exposure to environmental compounds with steroidogenic or antisteroidogenic potential (2, 6–9), or reproductive pathologies, such as polycystic ovary syndrome (PCOS) (10, 11) and congenital adrenal hyperplasia (1). Supportive of inappropriate exposure, an earlier cordocentesis study carried out during midgestation found testosterone (T) levels to be in the male range in 40% of female fetuses (12). An increase in androgen levels in amniotic fluid of diabetic pregnancies (13) and manifestation of features of androgen excess (hirsutism, ovarian theca-lutein cysts, and theca cell hyperplasia) in female stillbirth offspring of diabetic mothers (14) have also been reported.
Considerable evidence exists linking adult pathologies to inappropriate steroid exposure during development. Several animal models have evolved (15–17) to address the contribution of excess steroids in the developmental origin of PCOS, a major infertility disorder in the female (18–20). Studies in rats, sheep, and monkey have found that female fetuses exposed to excess T during development manifest features characteristic of women with PCOS (15–17). Because T can be aromatized to estrogen, comparative studies of T, dihydrotestosterone (DHT) (a nonaromatizable androgen), or T plus an androgen antagonist have been undertaken (3, 17, 21) in sheep to address the relative contribution of androgen and estrogen in programming the various disruptions at the reproductive neuroendocrine, ovarian, and metabolic levels. These studies have found that increased follicular persistence is mediated by estrogenic actions of T. Consistent with this premise, fetal sampling found that fetuses of gestational T-treated animals were getting exposed to excess estradiol (22), suggestive of potential disruption in ovarian steroidogenic pathways. Studies with adult sheep have found prenatal T-treated animals are characterized by increased estradiol levels (23), and manifest features of androgen excess, namely enhanced follicular recruitment and follicular persistence (24, 25), suggestive of disrupted steroid signaling. Although extensive studies carried out from fetal to adult life found that ovarian androgen and estrogen receptor expression are disrupted in a stage- and time-specific manner in prenatal T-treated sheep (26), the developmental impact of prenatal T excess on steroid biosynthetic pathway remains to be elucidated.
Steroidogenic enzymes orchestrate biosynthesis of various steroids from cholesterol (27, 28). Synthesis of all steroid hormones starts with the conversion of cholesterol to pregnenolone. The steroidogenic acute regulatory protein (STAR) initiates the process of steroidogenesis by transporting cholesterol from the outer to the inner mitochondrial membrane, where cholesterol side-chain cleavage enzyme catalyzes the conversion of cholesterol to pregnenolone (28). Pregnenolone is converted to progesterone by 3β-hydroxysteroid dehydrogenase (HSD3B). Pregnenolone and progesterone serve as the precursors for androgen and estrogen biosynthesis. The focus of this study is on 4 key mediators of steroid biosynthetic pathway, namely STAR, HSD3B, cytochrome P-450 17α-hydroxylase/C17, 20-lyase (CYP17A1), a key enzyme that regulates androgen synthesis (catalyzes the conversion of pregnenolone and progesterone into the androgen precursors dehydroepiandrosterone and androstenedione, respectively) and hence a significant factor in the expression of hyperandrogenism, and cytochrome P-450 aromatase (CYP19A1), key mediator of estrogen biosynthesis that regulates aromatization of androgens into estrogens. Understanding developmental disruptions in expression of these key mediators of steroidogenesis is key to our understanding of pathologic consequences stemming from steroid deficiencies or excess and in developing intervention strategies to overcoming them. Using prenatal T- and DHT-treated female sheep as model systems, this study tested the hypothesis that prenatal androgen and/or estrogen excess disrupts the steroid biosynthetic machinery in a follicle stage- and age-specific manner consistent with the ovarian disruptions seen in these females.
Materials and Methods
Breeding and prenatal treatment
Institutional Animal Care and Use Committee of the University of Michigan approved all procedures used in this study. All procedures used follow the National Research Council's Guide for the Care and Use of Laboratory Animals. Gestational treatments (T and DHT) and the dietary regimen of the breeder sheep and lambs have been previously published (29). Breeder sheep diet consisted of 2.6–2.9 (Mcal/kg) of digestible energy based on Nutrient Requirements of Small Ruminants, National Research Council requirements throughout pregnancy. For generation of prenatal T- and DHT-treated female sheep, 100 mg of T propionate (1.2 mg/kg; Sigma-Aldrich Corp) or 100 mg of DHT propionate (Steraloids, Inc) were suspended in cottonseed oil (Sigma-Aldrich Corp) and injected im to pregnant sheep twice weekly from days 30 to 90 of gestation. The T and DHT doses used in this study are the same doses used in earlier investigations (3, 17, 21, 23–25). This mode of T delivery resulted in oligo-/anovulation, functional hyperandrogenism, reduced sensitivity to progesterone negative feedback, multifollicular ovarian morphology, and insulin resistance, features similar to those seen in women with PCOS. The concentrations of T achieved in maternal and fetal circulation with this mode of T delivery are in the range of adult males and control fetal males, respectively (22). To enable confirmation of androgenic regulation and to parallel doses used in our earlier studies documenting offspring outcomes (3, 17, 21, 23–25), DHT concentrations were set to be the same as T, in spite of it being more potent androgen. At the chosen doses, both T and DHT lead to complete virilization of the female fetus.
Ovaries from all 3 treatment groups (control, prenatal T, and DHT treated) were collected at fetal day 90, 140 and 22 weeks (prepubertal), 10 months (end of first breeding season), and 21 months of age (end of second breeding season). For the 10- and 21-month-old postpubertal females, ovaries were collected during the presumptive follicular phase after administration of prostaglandin F2α, twice 11 days apart. Sample size ranged between 5 and 8 for each treatment group and each developmental time point. Due to insufficient number of DHT-treated females born, a 21-month-old prenatal DHT-treated group was not available to study. Details of euthanasia and ovary collection/processing have been previously described (24, 26). Only 1 ovary from each animal was used in this study. Developmental changes in ovarian follicular distribution (24), expression of androgen/estrogen receptors (26), metabolic mediators (30), and proliferative/apoptotic markers (31) from the same sets of ovaries have been published thus allowing integration of findings.
Immunohistochemistry
Antigen/antibody specificity
Basic Local Alignment Search Tool (BLAST software; http://www.ncbi.nlm.nih.gov/BLAST) was used to determine the peptide location and the homology between the target peptide of each antibody and the ovine protein being tested and confirm antigen specificity. Sheep ovarian tissue extracts were separated in SDS-PAGE (15% resolving gel), as previously described (26, 30, 31), for testing specificity of the primary antibodies used in this study (Table 1). After transfer of proteins onto nitrocellulose membranes (Amersham), membranes were blocked for 1 hour in 2% nonfat milk in Tris-buffered saline containing 0.05% Tween 20 (Sigma-Aldrich Corp) followed by incubation overnight at 4°C with specific primary antibodies (Table 1). Membranes were then washed and treated for 1 hour with antirabbit or antimouse IgG peroxidase (Amersham) 1:1000 secondary peroxidase-conjugated antibody. Visualization of immunopositive bands was done with the aid of a chemiluminescent detection kit (ECL-Plus; GE-Amersham).
Table 1.
Antibodies Used for Immunohistochemistry and Western Blotting
| Peptide Target | Antigen Sequence | Name of Antibody | Manufacturer, Catalog Number, or Name of Providing the Antibody | Species Raised In | Dilution |
|---|---|---|---|---|---|
| STAR | Recombinant full-length rat steroidogenic acute regulatory protein | Anti-StAR | Abcam (ab 3343) | Rabbit polyclonal | IH, 1:100 |
| WB, 1:100 | |||||
| HSD3B | Recombinant human type II 3β-HSD | R1484 | Ian Mason, University of Edinburgh | Rabbit polyclonal | IH, 1:1000 |
| WB, 1:3500 | |||||
| CYP17A1 | Human CYP17A1 | C.R. Parker Jr, University of Alabama at Birmingham | Mouse monoclonal | IH, 1:100 | |
| WB, 1:200 | |||||
| CYP19A1 | Recombinant full-length human aromatase | Antiaromatase | Affinity Bioreagents (PA1-21398) | Rabbit polyclonal | IH, 1:100 |
| WB, 1:200 |
IH, immunohistochemistry; WB, Western blotting.
Streptavidin-biotin immunoperoxidase method was used for immunohistochemistry following previously described procedures (26, 30, 31). After deparaffinization of sections, retrieval of antigen was accomplished by heating the slides with the sections in a microwave except for HSD3B. Blocking of endogenous peroxidase was carried out with 3% H2O2 in methanol. Nonspecific binding was prevented using 10% (vol/vol) normal goat serum. Slides with ovarian sections were incubated with primary antibodies (Table 1) at 4°C for 18 hours. Detection was achieved with an universal biotinylated secondary antibody and streptavidin-peroxidase solution (CytoScan, Avidin-Biotin Detection Systems; Cell Marque) with 3.3-diaminobenzidine (Dako) as chromogen. After counterstaining with Mayer's hematoxylin, ovarian sections were dehydrated and mounted. To validate endogenous peroxidase activity blockade, some sections were incubated with 3.3-diaminobenzidine alone. Specificity of the secondary antibodies used was determined by replacing the primary antibodies with nonimmune serum. To accommodate the large number of sections, multiple series of histological processing were undertaken. Serial sections of ovaries from nonexperimental animals were included with each series to facilitate normalization across series. For each marker, 2 sections (1/3 and 2/3 into the ovary to avoid overlap) were used for immunohistochemical quantification. Each immunohistochemical series included randomly selected slides with ovarian sections from different ages and treatments. Follicle classes (primordial, primary, small preantral, large preantral, and antral follicles) were defined using previously established criteria (32).
Image analysis
Image analysis was performed as per methods described earlier (26, 30, 31). All growing follicles in both sections were analyzed (ranged between 8 and 15 for each follicular class). Only healthy follicles without pycnotic nucleus (indicative of atresia) were used in this analysis. In view of the large number of primordial follicles that existed in each section, the slides were scanned left to right from the top, and the first 20 primordial follicles that showed no overlap with neighboring follicles were imaged. All the analyzed images in antral follicles covered the entire granulosa layer from antrum to theca. Ten images of cortical stromal tissue (random locations) were analyzed from each ovary.
Image Pro-Plus 3.0.1 system (Media Cybernetics) was used to perform image analysis following procedures detailed earlier (26, 30, 31). Images were digitized with an Olympus C5060 digital camera mounted on a conventional light microscope (Olympus BH-2; Olympus Co). The average density (% of immunopositive area) was calculated from percentage of total area evaluated through color segmentation analysis, which extracts objects by locating all objects of a specific color (brown stain). Densitometric values were normalized against nonexperimental control ovarian sections included with each series. The percentage of immunopositive area was calculated for each follicular compartment (granulosa, theca interna, and theca externa) as well as the stroma. Observers were blinded to treatment while processing sections. It should be noted because image analyses were optimized for each protein, quantitative comparison across proteins is not possible.
Statistical analyses
The average density (% of immunopositive area) of each follicular compartment (granulosa and theca) within a follicle class was first calculated, and a group mean across follicles was obtained for each follicle class for each animal. Statistical comparisons were carried out using a statistical software package (SPSS 11.0 for Windows; SPSS, Inc). Treatment outcomes were compared by ANOVAs, followed by Duncan's multiple range tests. P < .05 was considered significant. Results are expressed as mean ± SEM.
Results
Antibody specificity
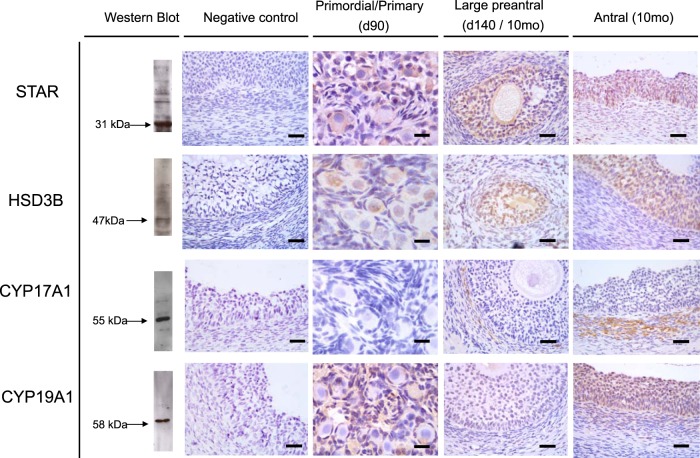
Figure 1 shows Western blotting recognition of proteins in ovarian homogenate and immunohistochemical localization of the 4 proteins in ovarian sections. Western blot analysis revealed positive bands of appropriate sizes for each of the protein studied (Figure 1, left). The STAR, HSD3B, CYP17A1, and CYP19A1 antibodies detected a single band at 31, 47, 55, and 58 kDa, respectively. No staining was observed in the absence of the primary antibodies (Figure 1, negative control). Immunostaining of the 4 markers in primordial/primary (from fetal d 90), large preantral (from fetal d 140 or postpubertal 10 mo old), and antral follicles (10 mo old) of control females are shown in Figure 1. All proteins had cytoplasmic localization.
Figure 1.
Representative images of STAR, HSD3B, CYP17A1, and CYP19A1 immunostaining in primordial/primary, preantral, and antral follicles are shown. Verification of antibody specificity by Western blot analyses of ovarian homogenate and negative controls for immunostaining demonstrating the specificity of the antibody are shown on the left 2 columns, respectively.
Age-related changes in ovarian expression of STAR, HSD3B, CYP17A1, and CYP19A1
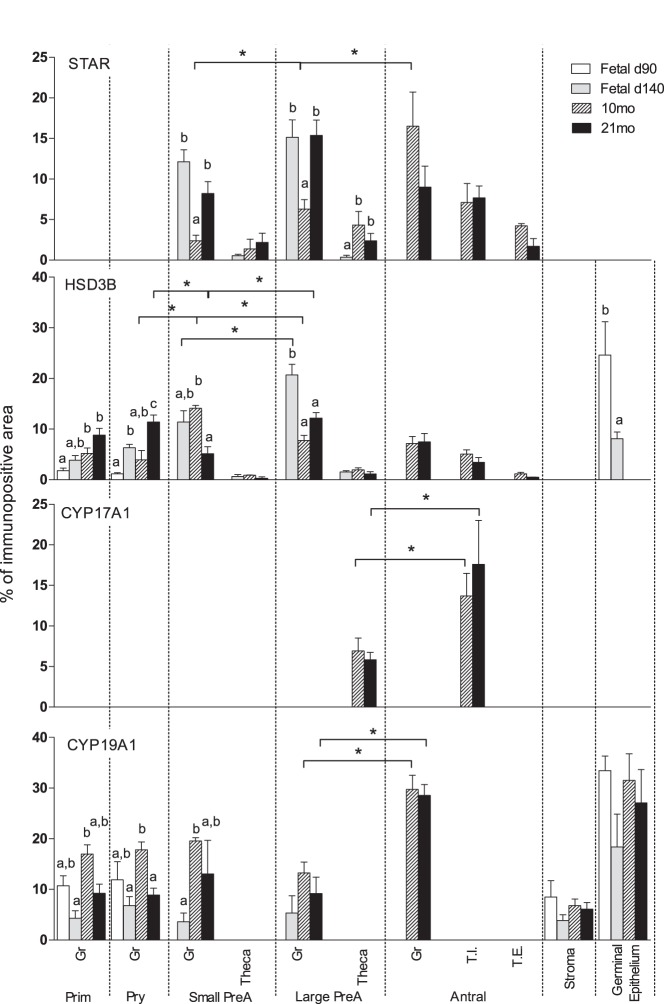
STAR protein expression was below the limit of detection in primordial and primary follicles at any of the ages studied (Figure 2). STAR expression was found in all classes of follicles starting at the preantral stage, with both granulosa and theca cells expressing STAR protein (Figures 1 and 2). Expression of STAR was higher in granulosa of preantral follicles of fetal day 140- and 21-month-old ovaries compared with 10-month-old females. In 10-month-old animals, the granulosa cell STAR expression increased from small preantral to large preantral to antral follicle (Figure 2). This was not the case with 21-month-old animals. STAR expression was lower in theca cells compared with expression in granulosa cells and did not differ across ages.
Figure 2.
Relative expression (measured as % of immunopositive area) of STAR, HSD3B, CYP17A1, and CYP19A1 in ovaries of control day 90 and 140 fetuses, 10- and 21-month-old sheep showing follicle stage and age specific changes. Significant differences across follicle classes within each age are shown by asterisks. Significant differences across age groups within each follicular class category are shown by differing letters. Prim, primordial; Pry, primary; Small PreA, small preantral; Large PreA, large preantral; Gr, granulosa; T.I., theca interna; T.E., theca externa.
HSD3B protein was localized in granulosa and theca cells of follicular compartment and the germinal epithelium (Figure 1). In primordial and primary follicles, granulosa cell HSD3B protein expression increased with advancing age. HSD3B protein expression differed among follicular classes in an age-specific manner. In fetal day 140 ovaries, HSB3B expression increased from small prenatal to large preantral follicle stages. HSD3B protein levels increased from primary to small preantral and decreased from small preantral to large preantral in ovaries from 10-month-old animals. At 21 months, HSD3B protein expression decreased from primary to small prenatal and again increasing at large preantral stage. HSD3B was also expressed in germinal epithelium of fetal ovaries, with expression being higher at day 90 compared with day 140 (P < .05) (Figure 2).
CYP17A1 was expressed only in theca cells of large preantral follicles and antral follicles of 10- and 21-month-old animals. Expression level CYP17A1 was higher in theca interna cells of antral follicles relative to theca cells of large preantral follicles at both ages (Figure 2). Theca externa of antral follicles did not express CYP17A1. The relatively few preantral and antral follicles present in fetal day 140 ovaries (24) precluded quantification of CYP17A1 at this time point. Only primordial and primary follicles are present in fetal day 90 ovaries (24).
CYP19A1 was expressed in granulosa, stroma, and germinal epithelium but not in the theca cells (Figure 1). Higher expression of CYP19A1 was observed in primordial, primary, and small preantral follicles of 10-month-old animals (postpubertal) compared with day 140 fetuses. In 10- and 21-month-old females, higher expression of CYP19A1 was present in granulosa cells of antral follicles compared with the large preantral follicles (P < .05) (Figure 2).
Effects of prenatal T and DHT treatment
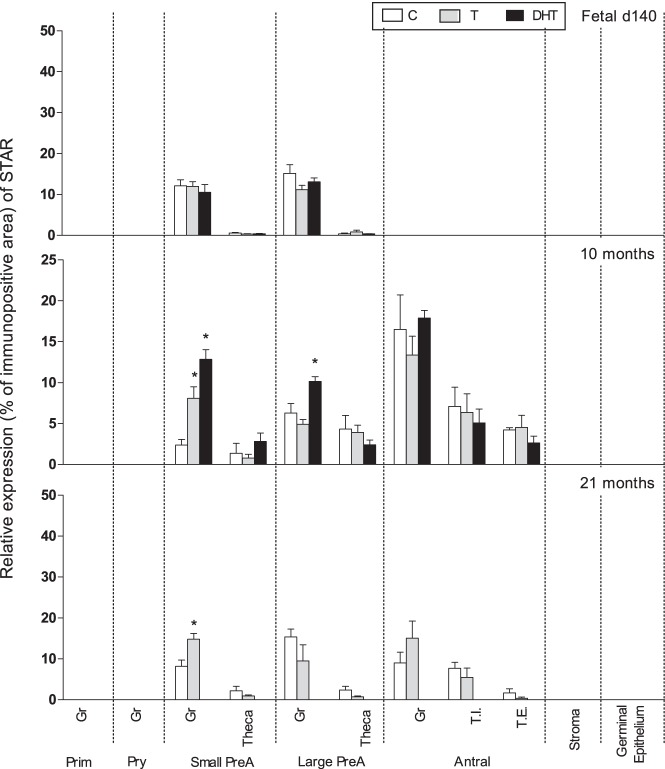
Expression level of STAR in preantral follicle was not impacted by prenatal T or DHT treatment at fetal day 140. Prenatal T treatment increased (P < .05) STAR expression in granulosa but not theca cells of small preantral follicles of 10- and 21-month-old animals. Prenatal DHT but not T treatment increased (P < .05) STAR expression in granulosa cells of large preantral follicles of 10-month-old animals (Figures 1 and 3).
Figure 3.
Relative expression (measured as % of immunopositive area) of STAR in ovaries of control, prenatal T-, and prenatal DHT-treated day 140 fetuses, 22-week-, 10-month-, and 21-month-old sheep. STAR expression was too low for quantification in fetal day 90 ovaries. Significant treatment effect within each ovarian compartment at each age is indicated by asterisks. Prim, primordial; Pry, primary; Small PreA, small preantral; Large PreA, large preantral; Gr, granulosa; T.I., theca interna; T.E., theca externa.
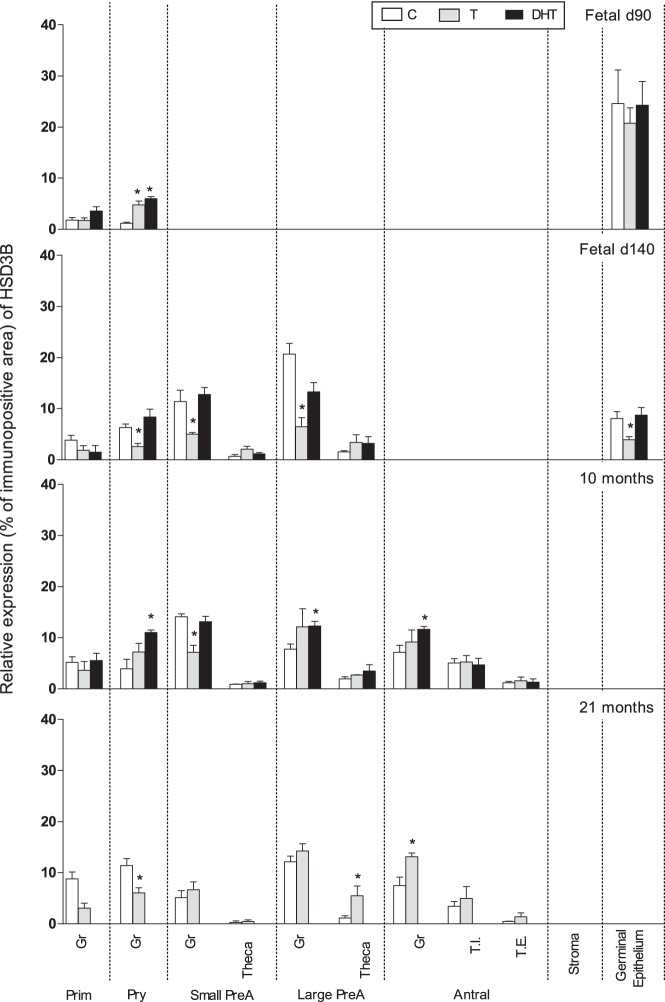
On fetal day 90, prenatal treatment with T or DHT increased (P < .05) HSD3B expression in granulosa of primary but not primordial follicles (Figure 4). In contrast, on fetal day 140, HSD3B expression was reduced in primary, small preantral, and large preantral follicles from prenatal T-treated, but not DHT-treated, ovaries. A similar direction of changes was also evident at the germinal epithelium level with prenatal T treatment decreasing and DHT having no effect. In 10-month-old animals, prenatal DHT treatment increased (P < .05) the expression of HSD3B in granulosa cells of primary, large preantral, and antral follicles. Prenatal T treatment reduced HSD3B expression in the granulosa cells of small preantral follicles. Although a numerical increase was evident in other follicular classes parallel with the increase seen in DHT-treated animals, this did not reach statistical significance. In 21-month-old prenatal T-treated females, levels of HSD3B expression was lower in the granulosa cells of primary follicles but higher in granulosa cells of large antral follicles, compared with the corresponding controls (Figures 1 and 4).
Figure 4.
Relative expression (measured as % of immunopositive area) of HSD3B in ovaries of control, prenatal T-, and prenatal DHT-treated day 90 and 140 fetuses, 22-week-, 10-month-, and 21-month-old sheep. Significant treatment effect within each ovarian compartment at each age is indicated by asterisks. Prim, primordial; Pry, primary; Small PreA, small preantral; Large PreA, large preantral; Gr, granulosa; T.I., theca interna; T.E., theca externa.
CYP17A1 expression was reduced by prenatal T treatment in theca interna of antral follicles in both 10- and 21-month-old females (P < .05) (Figures 1 and 5). CYP17A1 expression levels in prenatal DHT-treated animals did not differ from controls at 10 months of age.
Figure 5.
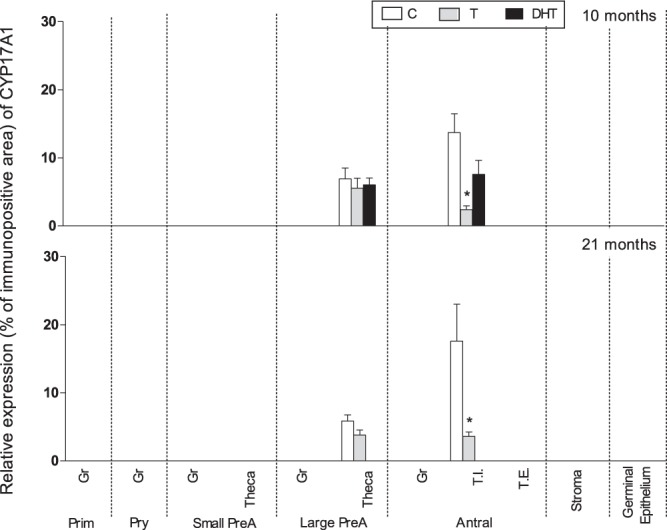
Relative expression (measured as % of immunopositive area) of CYP17A1 in ovaries of control, prenatal T-, and prenatal DHT-treated 10- and 21-month-old sheep. Due to restricted localization of CYP17A1 in theca cells and absence of preantral and antral follicles in fetal day 90 ovaries and the few preantral and antral follicles present in day 140 ovaries, only data from 10- and 21-month-old ovaries are shown. Significant treatment effect within each ovarian compartment at each age is indicated by asterisks. Prim, primordial; Pry, primary; Small PreA, small preantral; Large PreA, large preantral; Gr, granulosa; T.I., theca interna; T.E., theca externa.
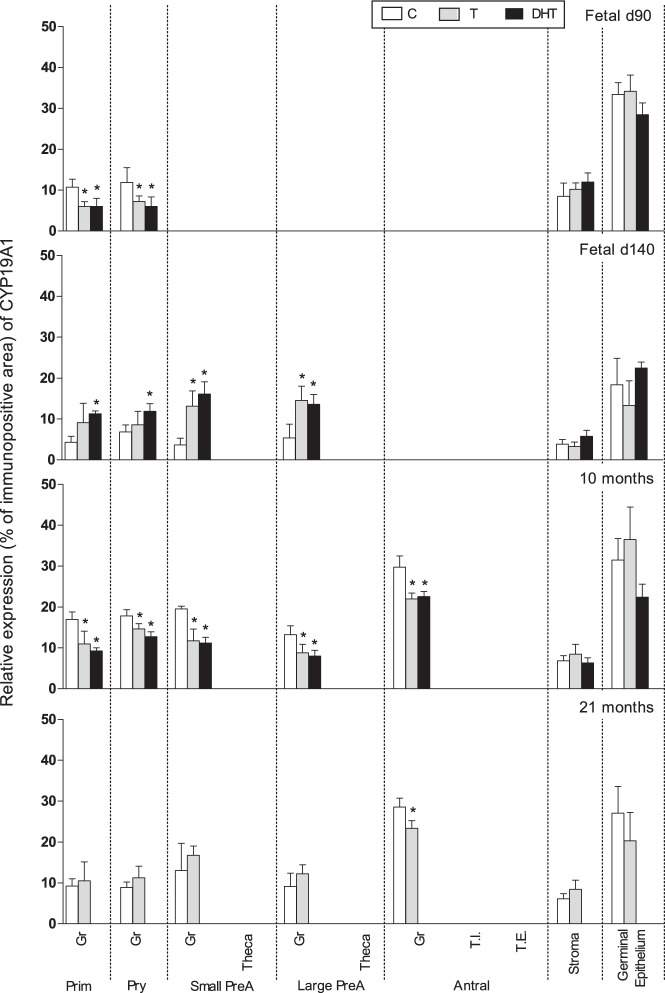
Prenatal T and DHT treatment decreased (P < .05) CYP19A1 expression in granulosa of primordial (fetal d 90, 10 mo), primary (fetal d 90, 10 mo), small and large preantral (10 mo), and antral (10- and 21-mo-old animals). Paradoxically, at fetal day 140, prenatal T and DHT treatment increased CYP19A1 expression in primordial (DHT only), primary (DHT only), and small and large preantral follicles (Figures 1 and 6). CYP19A1 was expressed in germinal epithelium and stroma (low expression) but not altered by prenatal T or DHT treatment.
Figure 6.
Relative expression (measured as % of immunopositive area) of CYP19A1 in ovaries of control, prenatal T-, and prenatal DHT-treated day 90 and 140 fetuses, 22-week-, 10-month-, and 21-month-old sheep. Significant treatment effect within each ovarian compartment at each age is indicated by asterisks. Prim, primordial; Pry, primary; Small PreA, small preantral; Large PreA, large preantral; Gr, granulosa; T.I., theca interna; T.E., theca externa.
Discussion
Ovarian steroidogenesis is key to optimal ovarian follicular growth, differentiation, and function. Synthesis of specific steroids in ovarian follicle is dependent on a distinct pattern of expression of steroid metabolizing enzymes. Both theca and granulosa cells work in concert in the production of androgens and estrogens. LH stimulates theca cells to produce androstenedione, which gets aromatized to estradiol via aromatase in the granulosa cells. High levels of HSD3B and CYP17A1 are expressed in theca cells and are needed for androstenedione production. Granulosa cells do not express CYP17A1 and are characterized by high levels of CYP19A1 expression. Altered enzyme expression in either compartment leads to aberrant steroidogenesis culminating in a spectrum of reproductive disorders. Findings from this study provide evidence in support of disrupted ovarian steroidogenesis in prenatal T-treated sheep and indicate that ovarian and reproductive disruptions evidenced in prenatal T-treated sheep are at least in part mediated by alterations in the developmental ontogeny of key steroidogenic enzymes. Furthermore, comparative studies with DHT indicate that some of this regulation is mediated likely via androgenic actions of T while others by estrogenic actions, due to aromatization of T to estradiol. The relevance of these findings, because they relate to ovarian and cyclic disruptions seen in prenatal T-treated sheep and translational significance to human pathology, are discussed below.
Developmental ontogeny of key steroidogenic enzymes
In sheep, the fetal ovary is steroidogenically active at midgestation. Fetal day 75 ovaries produce detectable levels of progesterone, androstenedione, T, and estradiol, as well as express STAR, HSD3B, CYP17A, and CYP19A, key mediators of steroidogenesis (33). During follicular differentiation, follicles show cell- and follicle stage-specific expression of steroidogenic enzymes. Findings from the present study showing tissue-specific expression of CYP17A in theca cells, CYP19A in granulosa cells, and HSD3B in both cell types, parallel expression patterns of steroidogenic enzymes seen in rat (34, 35), cow (36–38), pig (39), and human (40, 41).
The findings that both theca and granulosa cells express STAR, a key acute regulator of steroidogenesis, is consistent with earlier findings in sheep (42). Although quantifiable protein levels of STAR were evident only from the small preantral stage in the present study, an earlier study reported STAR mRNA expression as early as the primordial stage (42). Expression of HSD3B protein in all classes of follicles in both granulosa and theca cells is in agreement with findings from an earlier mRNA/protein expression study (42). The finding that CYP17A1, the dual functional enzyme possessing both 17α-hydroxylase and 17, 20-lyase activities, is not expressed in fetal day 90 primordial and primary follicles is consistent with CYP17A1 expression being limited to theca cells, which are evident only from preantral stage (43); ovarian morphometric studies found that preantral follicles are not present on day 90 of fetal development (24). Furthermore, consistent with the theca being the androgen producing compartment in the follicle, CYP17A1 expression was evident only in the theca of large preantral follicles and theca interna of antral follicles of 10- and 21-month-old follicles. There were too few preantral/antral follicles to undertake this quantification in fetal day 140 ovaries. In contrast, the finding that aromatase protein was detected in all classes of follicles beginning with primordial is not in agreement with earlier studies where aromatase (42) expression was found only in follicles greater than 3 mm. Findings from in vitro studies (44) showing that follicles 0.5 mm in diameter are capable of producing estradiol is inconsistent with aromatase expression being limited to follicles greater than 3 mm. As such, the discrepancy between present study and earlier study in CYP19A1 expression may relate to sensitivity of the detection system used. Overall, these findings provide compelling evidence in support of follicle and age-specific changes in STAR, HSD3B, and CYP19A1 expressions. Although such detailed developmental comparison was not undertaken in earlier studies, one study reported similar levels of expression of STAR, HSD3B, and CYP19A1 in follicles recovered from neonates and adults (42).
Impact of prenatal T excess on steroidogenic enzymes
STAR and HSD3B
The finding that prenatal T excess increased STAR and HSD3B expression in granulosa cells of preantral and antral follicles agrees to some extent with findings from other hyperandrogenic models. Increased expression of STAR and HSD3B was evident in ovarian lysates of rodents treated prenatally from days 16 to 19 of gestation with 5 mg but not 2 mg of T (45), although one cannot determine whether this increase is at the theca or granulosa cell level. Increased expression of STAR and HSD3B protein was also evident in both theca and granulosa cells of rats that were made hyperandrogenic with letrozol treatment beginning at 8 weeks of age (46). Paradoxically, treatment initiated early at day 29 of life yielded opposite outcome, namely a decrease in ovarian STAR and HSD3B expression (47). As opposed to lack of change in STAR protein expression in the theca cells in this study, an increase in STAR mRNA was evident in theca cells (granulosa cells not looked at) of follicles more than 3 mm obtained from sheep treated prenatally from days 60 to 90 of gestation (48). Differences in outcome among various studies may relate to whether mRNA or protein is being measured, time of treatment, and whether organizational or activational effects are being studied. Treatments initiated before completion of ovarian differentiation are likely to result in permanent alterations (organizational effects), whereas those initiated after completion of ovarian differentiation lead to activational effects that disappear after cessation of treatment. Alternatively, the differences among studies may be a function of when during their life span the ovaries were studied and the prevailing hormonal milieu. For instance, HSD3B expression in the present study was found to be increased in theca cells of 21- but not 10-month-old animals. Another possibility may relate to the type of follicles being studied; in the present study, HSD3B was found to be decreased in granulosa cells of primary follicles of prenatal T-treated animals but increased in granulosa cells of antral follicles.
CYP17A1
Contrary to our expectation, CYP17A1 expression was reduced in theca interna of antral follicles from both 10- and 21-month-old animals. Reduction in CYP17A1 mRNA expression was also found in day 90 fetal ovaries treated with T from 60 to 90 days of gestation (49). However, origin of CYP17A1 in fetal ovaries is unlikely to be of theca cell origin, because preantral follicles are not present in day 90 fetuses (24, 50).
Because CYP17A1 is responsible for androgen production in the ovary, the reduction in CYP17A1 is inconsistent with the hyperandrogenic ovarian phenotype, namely enhanced follicular recruitment, persistence, and multifollicular ovarian phenotype (24, 25, 51, 52). The increased androgen receptor expression evident in granulosa cells of prenatal T-treated sheep (26), also supportive of functional hyperandrogenism, indicates that alternative mechanisms potentiating the hyperandrogenic condition are in place. One possibility is that the reduction in CYP17A1 may be compensated for by activation of a novel CYP17A1 isoform discovered in sheep (53). Another likely possibility is that although expression level of enzyme is down, activity of enzyme may be increased. This may result from increased cytochrome b5 in prenatal T-treated females, which stimulates only the 17, 20-lyase activity (54). A similar paradox was also reported within letrozol-treated rats, where an increase in androgen production was evident in the face of reduced mRNA expression of several genes involved in steroidogenesis except for CYP17A1 (47).
Conceivably, an androgen-induced feedback inhibition of steroidogenic enzyme expression may be operational at the autocrine/paracrine level to regulate expression of CYP17A1 in a follicular stage-specific manner. In support of this, LH-induced androstenedione release from follicles obtained from prenatal T-treated sheep was found to be reduced when follicles less than 3 mm in size were tested but increased in follicles more than 4 mm in size, relative to size-matched control follicles (48). An increase in CYP17A1 mRNA expression was evident only in theca of estrogenic follicles more than 3 mm in size but not in nonestrogenic follicles (48). The lack of polycystic ovarian adult phenotype after this prenatal window of exposure (d 60–102) makes it hard to extrapolate these findings (48) to the current study.
CYP19A1
The reduced CYP19A1 protein expression in granulosa cells of primordial and primary follicles seen in this study at day 90 may be a direct consequence of T treatment, which encompasses the period between days 30 and 90 of gestation. A decline in mRNA level of CYP19A1 was also found in fetal day 90 ovaries exposed to excess T only from day 60–90 of gestation (49). Consistent with the potential for T having a direct impact, a decrease in CYP19A1 was not evident in granulosa cells of primordial and primary follicles of fetal day 140 fetuses, collected 50 days after cessation of androgen exposure. If any, an increase in CYP19A1 was evident in granulosa cells of preantral follicles at fetal day 140, suggestive of potential escape from feedback inhibition from androgen treatment. The contrasting findings of increased CYP19A1 mRNA expression seen in fetal day 65 ovaries in our earlier study (55) vs the decrease in CYP19A1 protein at fetal day 90 in the present study may be a function of remodeling of follicular population; between days 75 and 90, 75% of germ cells die in the sheep fetal ovary and primordial follicle development gets completed (50) or that mRNA and protein expression do not undergo parallel changes as is often the case.
The decrease in CYP19A1 evident in late follicular phase ovaries of 10-month postpubertal female is consistent with follicular developmental arrest. A reduction in aromatase protein expression was also evident in follicles of a dehydroepiandrosterone-induced hyperandrogenic rat model (56). The reduced aromatase expression in 10- and 21-month-old animals is inconsistent with the increased preovulatory estradiol release found in these animals (23); the increased estradiol release may therefore result from increased number of follicles contributing to their production.
Androgenic vs estrogenic contribution to disruptions in steroidogenic enzymes
The fact that both T and DHT increase STAR expression in small preantral follicles of 10-month-old animals is indicative of androgenic mediation. Paradoxically, the increased STAR expression in prenatal DHT- but not T-treated animals in large preantral follicles suggests that pubertal hormonal changes may modulate such expression. The increase in HSD3B in primary follicles at day 90 of both T and DHT, a time point when they are being treated, supports direct androgenic mediation. In contrast, the findings that HSD3B expression is reduced in prenatal T-treated, but not DHT-treated, animals in fetal day 140 ovaries support programming via estrogenic not androgenic action. Reduced expression of CYP17A1 in theca cells of T, but not DHT, animals is also supportive of estrogenic programming of this trait. The finding of increased estradiol in prenatal T-treated female fetuses during T treatment period (22) provides a means for such estrogenic mediation. In contrast, a similar direction of change in expression of CYP19A1 at all life stages in the T- and DHT-treated animals indicates that this aspect is programmed by androgenic actions of T. The sum effect of the androgenic and estrogenic programming contributes to follicular persistence/arrest (3, 17, 25, 52) via disruption of intrafollicular milieu, namely disruption of steroid biosynthetic machinery (this study), steroid receptor expression (26), antimullerian hormone (57), adiponectin (30), and apoptotic balance (31) within ovarian follicles.
Translational relevance
Because the reproductive and metabolic attributes of prenatal T-treated sheep mimic attributes of women with PCOS (17), it is important to compare ovarian expression of steroidogenic enzymes in this model with that in PCOS women. Increased expression of STAR and HSD3B found in the present study is consistent with the mRNA changes seen in PCOS ovaries (58). The finding that CYP17A1 enzyme expression was down-regulated, and not up-regulated, in theca interna in the present study differs from findings from PCOS women, where an increase in CYP17A1 mRNA or activity has been reported (59–63). One study, which examined immunohistochemical localization of CYP17A1 from archived tissues, found increased density of CYP17A1 in antral follicles of ovulatory and anovulatory women with polycystic ovaries (hyperandrogenic status unknown) (64). These differences may be a function of the size class of follicles examined. Human PCOS studies used 3- to 5-mm antral follicles (59), periovulatory follicle after hyperstimulation (62), or cultured theca cells after passage (63). In contrast, antral follicles of all sizes were included in the present study (size classes cannot be determined from ovarian sections). Another possibility is that the regulation occurs at the activity level and hence missed, because the phosphorylated form of CYP17A1 was not quantified in this study. In terms of PCOS etiology, existing evidence fails to support variants of CYP17 contributing to the pathogenesis of PCOS (65). The decreased granulosa cell production of CYP19A1 is, however, consistent with what has been found in PCOS women (66).
The power of this study is that it identifies several lines of investigation that are difficult to pursue in humans, such as determining relationship between protein expression and activity, cotreating with androgen/estrogen antagonist to determine relative contribution of androgen/estrogen and thus helping in developing prevention strategies. In summary, studies carried out in this investigation provide evidence that prenatal T excess leads to disruptions in steroidogenic biosynthetic machinery at the ovarian level and that these disruptions are programmed partly via androgenic programming and partly via estrogenic programming. The reduced theca cell CYP17A1 and granulosa cell CYP19A1 expression suggests that alternative mechanisms must be in place to account for the hyperandrogenic phenotype and the increased estradiol release seen in this model. The findings are of translational relevance in understanding reproductive disorders of ovarian origin.
Acknowledgments
We thank Mr Douglas Doop for his help with generation of the experimental lambs, expert animal care, and facility management; Dr Mohan Manikkam, Dr Teresa Steckler, Ms Olga Astapova, Ms Carol Herkimer, and Mr James Lee for assistance with prenatal steroid treatment and help during collection of ovaries; Dr Almudena Veiga-Lopez for help with ovarian sectioning and her critique of the manuscript; and staff members of the Laboratory of Cellular Biology (Facultad de Ciencias Veterinarias–Univerisdad Nacional del Litoral) for their technical support during immunohistochemistry. We also thank Professor I.J. Mason for the HSD3B antibody and Dr C.R. Parker Jr for the CYP17A1 antibody.
This work was supported by National Institute of Health Grant P01-HD44232 (to V.P.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CYP17A1
- cytochrome P-450 17α-hydroxylase/C17, 20-lyase
- CYP19A1
- cytochrome P-450 aromatase
- DHT
- dihydrotestosterone
- HSD3B
- 3β-hydroxysteroid dehydrogenase
- PCOS
- polycystic ovary syndrome
- STAR
- steroidogenic acute regulatory protein.
References
- 1. White PC. Steroid 11 β-hydroxylase deficiency and related disorders. Endocrinol Metab Clin North Am. 2001;30:61–79, vi [DOI] [PubMed] [Google Scholar]
- 2. McLachlan JA, Simpson E, Martin M. Endocrine disrupters and female reproductive health. Best Pract Res Clin Endocrinol Metab. 2006;20:63–75 [DOI] [PubMed] [Google Scholar]
- 3. Padmanabhan V, Veiga-Lopez A. Developmental origin of reproductive and metabolic dysfunctions: androgenic versus estrogenic reprogramming. Semin Reprod Med. 2011;29:173–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sibeko S, Baxter C, Yende N, Karim QA, Karim SS. Contraceptive choices, pregnancy rates, and outcomes in a microbicide trial. Obstet Gynecol. 2011;118:895–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jellesen R, Strandberg-Larsen K, et al. Maternal use of oral contraceptives and risk of fetal death. Paediatr Perinat Epidemiol. 2008;22:334–340 [DOI] [PubMed] [Google Scholar]
- 6. Tefre de Renzy-Martin K, Frederiksen H, Christensen J, et al. Current exposure of 200 pregnant Danish women to phthalates, parabens and phenols. Reproduction. 2014;147:443–453 [DOI] [PubMed] [Google Scholar]
- 7. Hogg K, Price EM, Hanna CW, Robinson WP. Prenatal and perinatal environmental influences on the human fetal and placental epigenome. Clin Pharmacol Ther. 2012;92:716–726 [DOI] [PubMed] [Google Scholar]
- 8. Swan SH. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ Res. 2008;108:177–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ranjit N, Siefert K, Padmanabhan V. Bisphenol-A and disparities in birth outcomes: a review and directions for future research. J Perinatol. 2010;30:2–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sir-Petermann T, Maliqueo M, Angel B, Lara HE, Pérez-Bravo F, Recabarren SE. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod. 2002;17:2573–2579 [DOI] [PubMed] [Google Scholar]
- 11. Cattrall FR, Vollenhoven BJ, Weston GC. Anatomical evidence for in utero androgen exposure in women with polycystic ovary syndrome. Fertil Steril. 2005;84:1689–1692 [DOI] [PubMed] [Google Scholar]
- 12. Beck-Peccoz P, Padmanabhan V, Baggiani AM, et al. Maturation of hypothalamic-pituitary-gonadal function in normal human fetuses: circulating levels of gonadotropins, their common α-subunit and free testosterone, and discrepancy between immunological and biological activities of circulating follicle-stimulating hormone. J Clin Endocrinol Metab. 1991;73:525–532 [DOI] [PubMed] [Google Scholar]
- 13. Barbieri RL, Saltzman DH, Torday JS, Randall RW, Frigoletto FD, Ryan KJ. Elevated concentrations of the β-subunit of human chorionic gonadotropin and testosterone in amniotic fluid of gestations of diabetic mothers. Am J Obstet Gynecol. 1986;154:1039–1043 [DOI] [PubMed] [Google Scholar]
- 14. Driscoll SG, Benirschke K, Curtis GW. Neonatal deaths among infants of diabetic mothers. Postmortem findings in ninety-five infants. Am J Dis Child. 1960;100:818–835 [DOI] [PubMed] [Google Scholar]
- 15. Abbott DH, Nicol LE, Levine JE, Xu N, Goodarzi MO, Dumesic DA. Nonhuman primate models of polycystic ovary syndrome. Mol Cell Endocrinol. 2013;373:21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McNeilly AS, Duncan WC. Rodent models of polycystic ovary syndrome. Mol Cell Endocrinol. 2013;373:2–7 [DOI] [PubMed] [Google Scholar]
- 17. Padmanabhan V, Veiga-Lopez A. Sheep models of polycystic ovary syndrome phenotype. Mol Cell Endocrinol. 2013;373:8–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hart R, Hickey M, Franks S. Definitions, prevalence and symptoms of polycystic ovaries and polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2004;18:671–683 [DOI] [PubMed] [Google Scholar]
- 19. Dunaif A, Thomas A. Current concepts in the polycystic ovary syndrome. Annu Rev Med. 2001;52:401–419 [DOI] [PubMed] [Google Scholar]
- 20. Hampton T. NIH panel: name change, new priorities advised for polycystic ovary syndrome. JAMA. 2013;309:386. [DOI] [PubMed] [Google Scholar]
- 21. Padmanabhan V, Veiga-Lopez A. Animal models of the polycystic ovary syndrome phenotype. Steroids. 2013;78:734–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Veiga-Lopez A, Steckler TL, Abbott DH, et al. Developmental programming: impact of excess prenatal testosterone on intra-uterine fetal endocrine milieu and growth in sheep. Biol Reprod. 2011;84:87–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Veiga-Lopez A, Ye W, Phillips DJ, Herkimer C, Knight PG, Padmanabhan V. Developmental programming: deficits in reproductive hormone dynamics and ovulatory outcomes in prenatal, testosterone-treated sheep. Biol Reprod. 2008;78:636–647 [DOI] [PubMed] [Google Scholar]
- 24. Smith P, Steckler TL, Veiga-Lopez A, Padmanabhan V. Developmental programming: differential effects of prenatal testosterone and dihydrotestosterone on follicular recruitment, depletion of follicular reserve, and ovarian morphology in sheep. Biol Reprod. 2009;80:726–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manikkam M, Steckler TL, Welch KB, Inskeep EK, Padmanabhan V. Fetal programming: prenatal testosterone treatment leads to follicular persistence/luteal defects; partial restoration of ovarian function by cyclic progesterone treatment. Endocrinology. 2006;147:1997–2007 [DOI] [PubMed] [Google Scholar]
- 26. Ortega HH, Salvetti NR, Padmanabhan V. Developmental programming: prenatal androgen excess disrupts ovarian steroid receptor balance. Reproduction. 2009;137:865–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rodgers RJ. Steroidogenic cytochrome P450 enzymes and ovarian steroidogenesis. Reprod Fertil Dev. 1990;2:153–163 [DOI] [PubMed] [Google Scholar]
- 28. Jamnongjit M, Hammes SR. Ovarian steroids: the good, the bad, and the signals that raise them. Cell Cycle. 2006;5:1178–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Manikkam M, Crespi EJ, Doop DD, et al. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology. 2004;145:790–798 [DOI] [PubMed] [Google Scholar]
- 30. Ortega HH, Rey F, Velazquez MM, Padmanabhan V. Developmental programming: effect of prenatal steroid excess on intraovarian components of insulin signaling pathway and related proteins in sheep. Biol Reprod. 2010;82:1065–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Salvetti NR, Ortega HH, Veiga-Lopez A, Padmanabhan V. Developmental programming: impact of prenatal testosterone excess on ovarian cell proliferation and apoptotic factors in sheep. Biol Reprod. 2012;87:221–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lundy T, Smith P, O'Connell A, Hudson NL, McNatty KP. Populations of granulosa cells in small follicles of the sheep ovary. J Reprod Fertil. 1999;115:251–262 [DOI] [PubMed] [Google Scholar]
- 33. Quirke LD, Juengel JL, Tisdall DJ, Lun S, Heath DA, McNatty KP. Ontogeny of steroidogenesis in the fetal sheep gonad. Biol Reprod. 2001;65:216–228 [DOI] [PubMed] [Google Scholar]
- 34. Zlotkin T, Farkash Y, Orly J. Cell-specific expression of immunoreactive cholesterol side-chain cleavage cytochrome P-450 during follicular development in the rat ovary. Endocrinology. 1986;119:2809–2820 [DOI] [PubMed] [Google Scholar]
- 35. Ishimura K, Yoshinaga-Hirabayashi T, Tsuri H, Fujita H, Osawa Y. Further immunocytochemical study on the localization of aromatase in the ovary of rats and mice. Histochemistry. 1989;90:413–416 [DOI] [PubMed] [Google Scholar]
- 36. Rodgers RJ, Rodgers HF, Hall PF, Waterman MR, Simpson ER. Immunolocalization of cholesterol side-chain cleavage cytochrome P-450 an 17 α-hydroxylase cytochrome P-450 in bovine ovarian follicles. J Reprod Fertil. 1986;78:627–638 [DOI] [PubMed] [Google Scholar]
- 37. Zhong C, Ishimura K, Yoshinaga-Hirabayashi T, Fujita H, Kitawaki J, Osawa Y. Immunocytochemical study on the localization of aromatase in the ovary of golden hamster, guinea pig and cow. Acta Histochem Cytochem. 1989;22:501–507 [Google Scholar]
- 38. Xu Z, Garverick HA, Smith GW, Smith MF, Hamilton SA, Youngquist RS. Expression of messenger ribonucleic acid encoding cytochrome P450 side-chain cleavage, cytochrome p450 17 α-hydroxylase, and cytochrome P450 aromatase in bovine follicles during the first follicular wave. Endocrinology. 1995;136:981–989 [DOI] [PubMed] [Google Scholar]
- 39. Meduri G, Vu Hai MT, Jolivet A, et al. Comparison of cellular distribution of LH receptors and steroidogenic enzymes in the porcine ovary. J Endocrinol. 1996;148:435–446 [DOI] [PubMed] [Google Scholar]
- 40. Sasano H, Okamoto M, Mason JI, et al. Immunolocalization of aromatase, 17 α-hydroxylase and side-chain-cleavage cytochromes P-450 in the human ovary. J Reprod Fertil. 1989;85:163–169 [DOI] [PubMed] [Google Scholar]
- 41. Tamura T, Kitawaki J, Yamamoto T, et al. Immunohistochemical localization of 17 α-hydroxylase/C17-20 lyase and aromatase cytochrome P-450 in the human ovary during the menstrual cycle. J Endocrinol. 1992;135:589–595 [DOI] [PubMed] [Google Scholar]
- 42. Logan KA, Juengel JL, McNatty KP. Onset of steroidogenic enzyme gene expression during ovarian follicular development in sheep. Biol Reprod. 2002;66:906–916 [DOI] [PubMed] [Google Scholar]
- 43. McNatty KP, Reader K, Smith P, Heath DA, Juengel JL. Control of ovarian follicular development to the gonadotrophin-dependent phase: a 2006 perspective. Soc Reprod Fertil Suppl. 2007;64:55–68 [DOI] [PubMed] [Google Scholar]
- 44. McNatty KP, Kieboom LE, McDiarmid J, Heath DA, Lun S. Adenosine cyclic 3′,5′-monophosphate and steroid production by small ovarian follicles from Booroola ewes with and without a fecundity gene. J Reprod Fertil. 1986;76:471–480 [DOI] [PubMed] [Google Scholar]
- 45. Amalfi S, Velez LM, Heber MF, et al. Prenatal hyperandrogenization induces metabolic and endocrine alterations which depend on the levels of testosterone exposure. PloS ONE. 2012;7:e37658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zurvarra FM, Salvetti NR, Mason JI, Velazquez MM, Alfaro NS, Ortega HH. Disruption in the expression and immunolocalisation of steroid receptors and steroidogenic enzymes in letrozole-induced polycystic ovaries in rat. Reprod Fertil Dev. 2009;21:827–839 [DOI] [PubMed] [Google Scholar]
- 47. Ortega I, Sokalska A, Villanueva JA, et al. Letrozole increases ovarian growth and Cyp17a1 gene expression in the rat ovary. Fertil Steril. 2013;99:889–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hogg K, Young JM, Oliver EM, Souza CJ, McNeilly AS, Duncan WC. Enhanced thecal androgen production is prenatally programmed in an ovine model of polycystic ovary syndrome. Endocrinology. 2012;153:450–461 [DOI] [PubMed] [Google Scholar]
- 49. Hogg K, McNeilly AS, Duncan WC. Prenatal androgen exposure leads to alterations in gene and protein expression in the ovine fetal ovary. Endocrinology. 2011;152:2048–2059 [DOI] [PubMed] [Google Scholar]
- 50. Sawyer HR, Smith P, Heath DA, Juengel JL, Wakefield SJ, McNatty KP. Formation of ovarian follicles during fetal development in sheep. Biol Reprod. 2002;66:1134–1150 [DOI] [PubMed] [Google Scholar]
- 51. West C, Foster DL, Evans NP, Robinson J, Padmanabhan V. Intra-follicular activin availability is altered in prenatally-androgenized lambs. Mol Cell Endocrinol. 2001;185:51–59 [DOI] [PubMed] [Google Scholar]
- 52. Steckler T, Manikkam M, Inskeep EK, Padmanabhan V. Developmental programming: follicular persistence in prenatal testosterone-treated sheep is not programmed by androgenic actions of testosterone. Endocrinology. 2007;148:3532–3540 [DOI] [PubMed] [Google Scholar]
- 53. Hough D, Cloete SW, Storbeck K, Swart AC, Swart P. Cortisol production in sheep is influenced by the functional expression of two cytochrome P450 17α-hydroxylase/17,20-lyase (CYP17) isoforms. J Anim Sci. 2013;91:1193–1206 [DOI] [PubMed] [Google Scholar]
- 54. Akhtar MK, Kelly SL, Kaderbhai MA. Cytochrome b(5) modulation of 17α hydroxylase and 17-20 lyase (CYP17) activities in steroidogenesis. J Endocrinol. 2005;187:267–274 [DOI] [PubMed] [Google Scholar]
- 55. Luense LJ, Veiga-Lopez A, Padmanabhan V, Christenson LK. Developmental programming: gestational testosterone treatment alters fetal ovarian gene expression. Endocrinology. 2011;152:4974–4983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lin W, Song ZJ, Sun WM, et al. [Expression of steroidogenic enzymes in the rat model of polycystic ovary syndrome]. Sheng Li Xue Bao. 2013;65:171–177 [PubMed] [Google Scholar]
- 57. Veiga-Lopez A, Ye W, Padmanabhan V. Developmental programming: prenatal testosterone excess disrupts anti-Müllerian hormone expression in preantral and antral follicles. Fertil Steril. 2012;97:748–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jakimiuk AJ, Weitsman SR, Navab A, Magoffin DA. Luteinizing hormone receptor, steroidogenesis acute regulatory protein, and steroidogenic enzyme messenger ribonucleic acids are overexpressed in thecal and granulosa cells from polycystic ovaries. J Clin Endocrinol Metab. 2001;86:1318–1323 [DOI] [PubMed] [Google Scholar]
- 59. Wickenheisser JK, Velen L, Nelson-Degrave VL, McAllister JM. Dysregulation of cytochrome P450 17α-hydroxylase messenger ribonucleic acid stability in theca cells isolated from women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:1720–1727 [DOI] [PubMed] [Google Scholar]
- 60. Nelson VL, Qin KN, Rosenfield RL, et al. The biochemical basis for increased testosterone production in theca cells propagated from patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2001;86:5925–5933 [DOI] [PubMed] [Google Scholar]
- 61. Daneshmand S, Weitsman SR, Navab A, Jakimiuk AJ, Magoffin DA. Overexpression of theca-cell messenger RNA in polycystic ovary syndrome does not correlate with polymorphisms in the cholesterol side-chain cleavage and 17α-hydroxylase/C(17-20) lyase promoters. Fertil Steril. 2002;77:274–280 [DOI] [PubMed] [Google Scholar]
- 62. Sander VA, Hapon MB, Sícaro L, Lombardi EP, Jahn GA, Motta AB. Alterations of folliculogenesis in women with polycystic ovary syndrome. Steroid Biochem Mol Biol. 2011;124:58–64 [DOI] [PubMed] [Google Scholar]
- 63. Wickenheisser JK, Nelson-DeGrave VL, McAllister JM. Human ovarian theca cells in culture. Trends Endocrinol Metab. 2006;17:65–71 [DOI] [PubMed] [Google Scholar]
- 64. Comim FV, Teerds K, Hardy K, Franks S. Increased protein expression of LHCG receptor and 17α-hydroxylase/17-20-lyase in human polycystic ovaries. Human Reproduction. 2013;28:3086–3092 [DOI] [PubMed] [Google Scholar]
- 65. Chua AK, Azziz R, Goodarzi MO. Association study of CYP17 and HSD11B1 in polycystic ovary syndrome utilizing comprehensive gene coverage. Mol Hum Reprod. 2012;18:320–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jakimiuk AJ, Weitsman SR, Brzechffa PR, Magoffin DA. Aromatase mRNA expression in individual follicles from polycystic ovaries. Mol Hum Reprod. 1998;4:1–8 [DOI] [PubMed] [Google Scholar]