Abstract
The adrenal hormone corticosterone (CORT) acts on brain to mediate physiology and behavior. In songbirds, behavioral effects of CORT vary across species, environmental conditions, and life history stage, with several mechanisms proposed to account for these divergent results. Although blood CORT levels are well characterized, few studies measure CORT within the brain itself. Here we used in vivo microdialysis to measure CORT in two regions of the zebra finch brain, the hippocampus (HP) and caudal nidopallium (cNp). Our results show that we can successfully measure physiological levels of CORT in brain within 15- to 30-minute intervals of dialysate collection. Moreover, we found that levels in the cNp were generally lower than levels in the HP. Surprisingly, whereas plasma CORT levels increased in response to a standard stressor, no stress-induced surge was detected in the HP or cNp. In addition, although a diel CORT rhythm was observed in plasma, the rhythm in brain was attenuated and only observed when levels were integrated over a 4-hour time period. Regional differences in brain CORT levels were reflected in local mRNA expression levels of the CORT-inactivating enzyme 11β-hydroxysteroid dehydrogenase type 2 with levels elevated in the cNp relative to the HP. Region-specific CORT metabolism may therefore play a role in buffering the brain from CORT fluctuations.
The steroid hormone corticosterone (CORT) crucially regulates numerous physiological and behavioral processes. Traditionally thought of as a stress hormone, CORT is synthesized in the adrenals and is elevated in the bloodstream in response to endogenous or exogenous perturbations. Although critical for survival during a stressful event, CORT is also continuously released at baseline levels during unstressed conditions to aid in the maintenance of homeostasis (1, 2). Across many vertebrates, baseline circulating CORT fluctuates on a diel basis (3). Therefore, in addition to being critical for the response to stress, CORT is an important mediator of numerous circadian-related physiological functions.
Behavioral effects of elevated circulating CORT have been studied extensively (4). In birds, CORT supplementation can lead to increased activity levels and feeding (5–7). Elevated CORT can also trigger irruptive migration and cessation of breeding and induce other behavioral modifications (1). The influence of CORT on avian behavior is by no means straightforward, however. CORT effects vary from study to study [eg, effects of CORT on nestling begging (6, 8–10)] and can vary within a species [eg, as a function of photoperiod (11, 12)]. Such variation could arise via differential modulation of CORT levels in the brain. If true, then measurement of plasma levels alone would not provide information sufficient to understand the interplay between CORT and behavior. Indeed, despite the abundance of data available on plasma CORT levels and correlations with physiology and behavior, less is known about how CORT fluctuates within the brain to activate, maintain, or suppress its various target neural circuits.
Circulating steroid hormones are thought to readily diffuse across the blood brain barrier (13) to bind neural nuclear and membrane receptors (14, 15). Once in the brain, some steroids are metabolized into inactive or more potent products or products that function along divergent signaling pathways (16, 17). This may also be the case for CORT in the songbird brain. For example, Newman and Soma (18) extracted CORT from brain tissue collected from song sparrows (Melospiza melodia) during multiple life-history stages (breeding, molt, and nonbreeding). In breeding and nonbreeding birds, CORT was readily detected in the hippocampus (HP), and levels were elevated when birds were subjected to a standard restraint stressor. In contrast, during molt, CORT was undetectable in the HP after, but not before, the restraint stressor. The authors suggested that differential regulation of CORT, perhaps through inactivation by 11β-hydroxysteroid dehydrogenase type 2 (11βHSD2), might have accounted for the divergence between blood and brain levels. However, the dynamics of 11βHSD2 activity in birds are not well known because this enzyme was only recently identified and explored in the songbird brain (19).
To evaluate the relationship between brain and circulating CORT levels, we used in vivo microdialysis in the zebra finch brain. Microdialysis has been used successfully for the measurement of CORT in the rodent brain (eg, Reference 20), and we have adapted our procedures from those used to measure neuroestrogens in zebra finches (21–23). The objectives of the current study were 4-fold. First, we sought to validate the use of in vivo microdialysis for the measurement of CORT in the songbird brain. Second, we used in vivo microdialysis to compare CORT levels in two brain regions in which CORT levels may differ: the HP, a region rich in CORT receptors that participates in negative feedback of the hypothalamic-pituitary-adrenal axis (24, 25) and which is crucial for CORT-sensitive spatial memory function (26–29), and the caudal nidopallium (cNp), in which glucocorticoid receptor abundance is low (30) and thus, CORT action is expected to be limited. Third, we investigated whether naturally occurring fluctuations in circulating CORT produce concurrent changes in HP and cNp CORT. Thus, we measured neural CORT levels at multiple time points over the daily cycle and subjected individuals to a standard restraint stressor known to elevate plasma levels within minutes. Finally, we previously identified 11βHSD2 expression in adult and young zebra finch brain (19); we therefore asked whether the region-specific brain CORT levels observed during microdialysis were mirrored by corresponding changes in 11βHSD2 mRNA expression. We also investigated regional differences in expression of the gene encoding p-glycoprotein (ABCB1), a blood-brain barrier protein hypothesized to regulate CORT access to brain (13).
Materials and Methods
Study species
We used adult male zebra finches (>90 d of age) obtained from our colony at University of California, Los Angeles. Birds were maintained on a 14-hour light, 10-hour dark light cycle and fed a diet of seed supplemented with vitamins, hard-boiled egg, and lettuce. Food, water, and grit were available ad libitum, both in the aviary and during testing (see below). All experimental procedures were approved by the University of California, Los Angeles Chancellor's Animal Research Committee.
Surgery
Cannula implantation surgery proceeded as described previously (21). The left HP (n = 8 birds) was targeted using the following coordinates: 1.5 mm rostral from the bifurcation of the midsagittal sinus and 0.5 mm lateral from the midline. A guide cannula (CMA7, CMA Microdialysis) was lowered at a 60° angle from horizontal until the bottom of the cannula just touched the surface of the brain and then fixed in place with dental cement. Individuals assigned to the control region group (n = 7 birds) received an implant targeting field L within the cNp at the following coordinates: 1.2 mm rostral, 1.5 mm lateral, and 0.00 mm ventral [the end of the cannula sat on the surface of the brain (31)].
Microdialysis
As described previously (21, 22), birds were housed in a specialized chamber during microdialysis. FEP tubing ran from a Harvard Apparatus PHD 2000 infusion pump into a cage within a sound-proof chamber. A dual-channel, quartz-lined swivel (Instech) was attached to the top of the cage, and a 3.5-in. horizontal lever arm (Instech) allowed for vertical and horizontal motion of the animal. Tubing was rinsed with ethanol, water, and Dulbecco's PBS (dPBS; Sigma) prior to probe implantation. The dead volume of the tubing was 1.2 μL per 100 mm (CMA) and the temporal delay this produced was considered in the data analysis (see Figure 4 legend).
Figure 4.
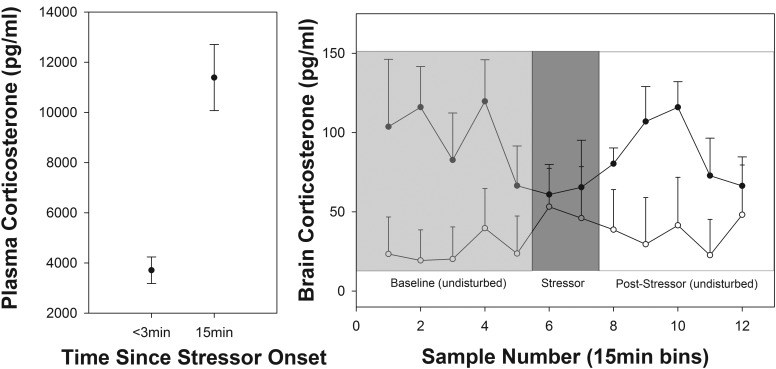
Plasma and brain CORT levels in response to a standardized restraint stressor. Left panel, Plasma CORT levels at the start (baseline) and after 15 minutes of restraint. Right panel, Mean HP (dark circles; n = 8) and cNp (open circles; n = 6) CORT levels over the course of the restraint stressor. Points represent 15 minutes of sample collection. Four baseline samples were collected prior to the start of the stress series. Note that the dead volume of the tubing was approximately 18 μL and, due to an oversight, was not taken into account. However, given that each 15 minutes of collection yielded approximately 15 μL of sample, the actual time at which the stress response could be expected to be observed in the cerebrospinal fluid was adjusted to the next 15-minute sample after the actual start of blood sampling (ie, sample 6 instead of sample 5).
Five days after surgery, a CMA7 microdialysis probe (CMA; 1 mm cuprophane membrane) was implanted under light isoflurane anesthesia and secured with cyanoacrylate. The bird was connected to the microdialysis system under constant perfusion with dPBS. Flow was verified at 1–4 μL/min and then reduced to 0.5 μL/min for the overnight period. Once the sample collection began the next morning, the flow rate was maintained at 1 μL/min. Samples were collected in a refrigerated autosampler (CMA470) for the duration of the experimental timeline.
Validations
Use of microdialysis to measure CORT was first validated in vitro (see Supplemental Figure 1). To confirm that we could apply these procedures in vivo, birds with a microdialysis probe in the HP received an im (pectoralis) CORT injection at one of the following concentrations: vehicle (oil; 10–20 μL), 5 μg (10 μL), 10 μg (20 μL), or 20 μg (40 μL). Crystalline CORT (Sigma) was dissolved in 1 mL of 100% ethanol and then mixed with 9 mL peanut oil. A small blood sample (<70 μL) was taken 1.5 hours after injection to verify CORT elevation in the plasma. Four hours later, each bird received another CORT injection, followed again by a blood sample 1.5 hours later. Therefore, each bird received two injections, the order and dosage of which was determined randomly. Dialysate was collected every 30 minutes on a continuous basis throughout the entire procedure.
Experiments
The timeline of all experiments is presented in Table 1 and described below.
Table 1.
Timeline of Experiments
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 |
|---|---|---|---|---|
| Move bird into sound-proof chamber | Implant probe and start microdialysis | Start microdialysis collection (diel experiment) | Continue microdialysis collection (diel experiment) | Conduct stress series; conduct in vivo validation (HP birds only) |
Cannula implantation surgery took place 5 days prior to probe implantation.
Diel variation in brain CORT
The morning after probe implantation, baseline sample collection was initiated at a flow rate of 1 μL/min every 30 minutes for a total of 48 hours. After collection, each vial was capped and frozen at −20°C until assay.
To establish the nature of the diel pattern in the circulation, a separate set of male zebra finches (n = 18) was sampled for baseline plasma CORT in the absence of microdialysis. These individuals were also singly housed in sound-proof chambers. Birds were separated into two groups with one group (n = 9) sampled at 2:00 am with subsequent sampling at 8-hour intervals (10:00 am and 6:00 pm). Similarly, the second group (n = 9) was sampled at 6:00 am, followed by samplings at 2:00 and 10:00 pm. The 8-hour interval between successive samples was used to allow circulating levels to return to baseline after the stress of blood sampling (eg, Reference 32). Blood samples were obtained via brachial venipuncture (<70 μL) within 3 minutes of opening the chamber door.
Blood and brain CORT during a stressor
On the third full day of microdialysis, birds were subjected to a restraint stress protocol with dialysate samples collected every 15 minutes before, during, and after the stressor. Starting at 6:00 am, four baseline (undisturbed) samples were collected, at which point the chamber was opened and a small baseline blood sample was taken from the brachial vein within 3 minutes of disturbance. The bird was placed in an opaque cloth bag and sampled again 15 minutes later (stress induced blood sample). All blood samples were kept on ice until centrifugation at 10 000 rpm for 10 minutes to separate the plasma from the red blood cells. The plasma portion was removed and frozen at −20°C for later measurement of CORT.
Histology
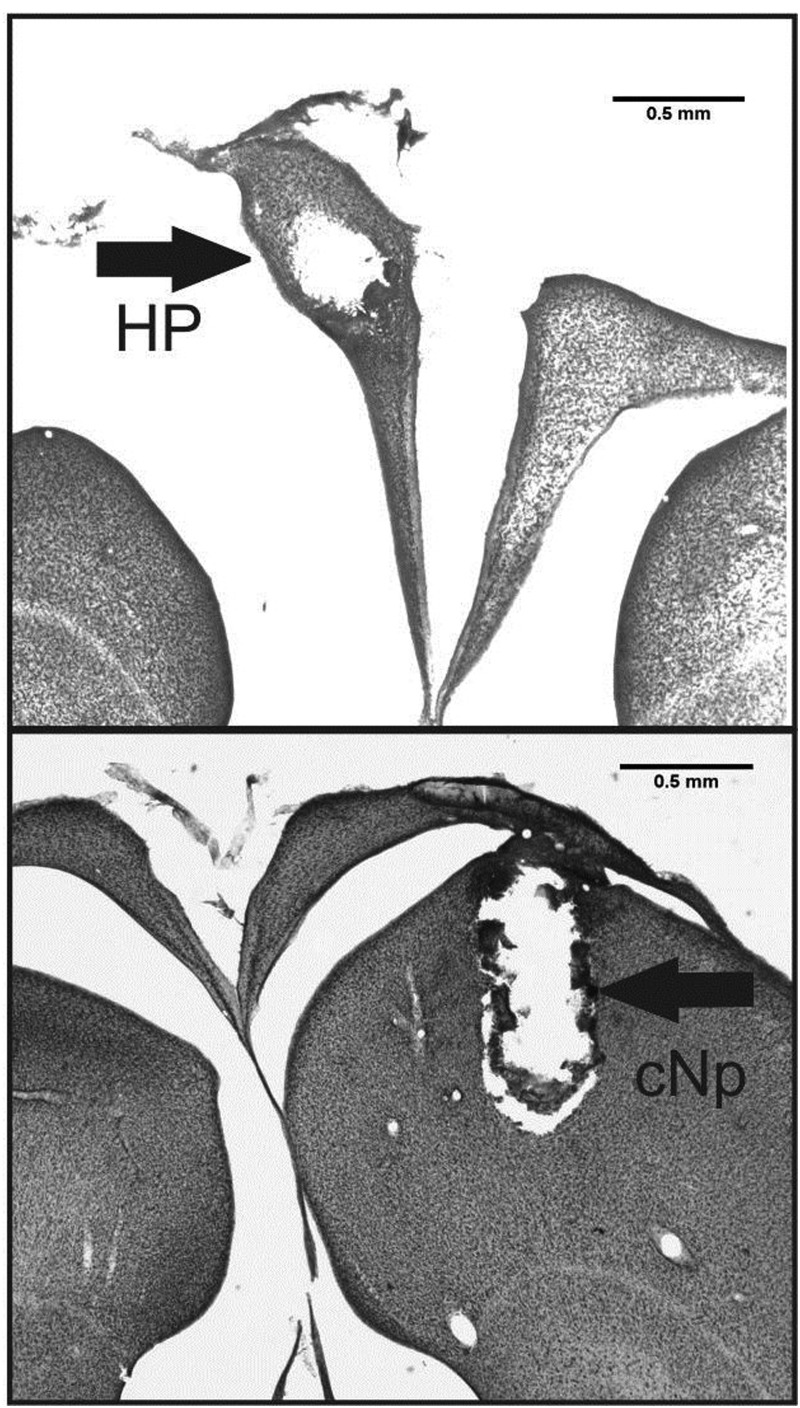
At the completion of sampling, each bird was euthanized with isoflurane and immediately perfused through the heart with PBS followed by 10% neutral buffered formalin. The brain was removed from the skull and postfixed in 20% sucrose in formalin overnight at 4°C. After the postfixation, the brain was frozen in optimal cutting temperature compound (Tissue-Tek) and the region encompassing the damage caused by the microdialysis probe sectioned on a cryostat (Leica) at 50 μM. Sections were mounted onto Superfrost Slides (Fisher), dried overnight, thionin stained, and coverslipped using Permount (Sigma). Probes aimed at cNp were all found to be adjacent to the auditory field L. For HP probes, although most the probe penetration remained fully within the HP, in some birds the tip extended into the adjacent ventricle or dorsal telencephalon. Nevertheless, in these cases, the vast majority of the dialysis membrane was contained within the HP itself (Figure 1).
Figure 1.
Representative coronal sections from an individual with a microdialysis probe in the left HP (top panel) and an individual with a microdialysis probe in the cNp (bottom panel). Arrows point to the damage caused by the microdialysis probe (Note: the HP becomes separated from the underlying nidopallium at the lateral ventricle when tissue is slide mounted).
CORT measurement
Brain and plasma CORT were assessed using a commercially available enzyme immunoassay kit (Cayman Chemical). Both brain and plasma CORT assays were validated prior to use to ensure lack of assay interference (see Supplemental Materials for details).
For brain CORT assays, samples were run at a 1:2 or 1:4 dilution, depending on the volume collected. The mean intraassay coefficient of variation (CV) was 2.6% and the mean interassay CV was 11.7%. One to two wells with dPBS alone (blanks) were included in each plate to control for background; background values were averaged and subtracted from each sample prior to analysis. All nondetectable samples (lower than the lowest standard on the curve) were set to zero. Plasma samples were run at a 1:40 dilution to eliminate interference; the mean intraassay CV was 2.0% and the interassay CV was 3.8%.
11βHSD2 and ABCB1 expression
We investigated differences in 11βHSD2 and ABCB1 mRNA expression between the HP and cNp of a separate set of zebra finches (n = 5 per sex). After rapid decapitation, brains were immediately removed and placed on a cold petri dish resting on wet ice. The HP and cNp were dissected as follows: following the protocol of Saldanha et al (33), we made two parasagittal cuts approximately 1 mm from the midline on each side. The anterior portion of the brain was removed using a razor blade. Using a pair of watchmaker forceps, the HP was gently peeled off the remaining dorsal surface of the brain between the midline and the parasagittal cut. The left HP was frozen immediately on dry ice (to match the side on which microdialysis was performed). To obtain the cNp, a cut approximately 2 mm long, 1 mm wide, and 1 mm deep was made just lateral to the region in which the HP had already been removed (designed to match the cNp microdialysis probe location). Tissue was taken from both sides and pooled into one sample to ensure adequate sample volume. All samples were frozen at −80°C until further processing.
RNA was extracted using the RNeasy minikit (QIAGEN). After visual inspection of RNA integrity with gel electrophoresis and Nanodrop assessment of concentration and A260/280 (Thermo Scientific), 600 ng RNA was reverse transcribed into cDNA using Reverse Transcriptase II (Promega). The resultant cDNA was subjected to quantitative PCR following established protocols (eg, Reference 34) to determine abundance of 11βHSD2 (forward: CCCGGCTACTACAAAACAGG; reverse: CATGAACTGGATGAACTGCTG) and ABCB1 (forward: CGACCAGAAGTCAAAATCCTG; reverse: CAACAGTGCTCTTTCCACAGC) mRNA relative to glyceraldehyde-3-phosphate dehydrogenase (GAPdH; forward: TGACCTGCCGTCTGGAAAA; reverse: CCATCAGCAGCAGCCTTCA). We have used the housekeeping gene GAPdH previously in several quantitative PCR studies of songbird gene expression (eg, Reference 34) and verified its utility here by observing that cycle threshold (CT) values do not differ between samples taken from the two brain regions of interest (left HP and cNp). Primers were designed from predicted gene sequences annotated in the zebra finch genome. Dissociation curves were inspected to ensure lack of contamination, and reaction efficiencies were between 90% and 110%. Relative δCT values were generated for each sample using the equation [1000 × (2-[CT 11βHSD2 − CT GAPdH])].
Statistics
Plasma and brain CORT data were analyzed using linear mixed models, with bird identity nested within the assay number as a random factor (which accounts for the repeated sampling of individuals and interplate variation). The effect of CORT injections on plasma and brain CORT was assessed by including time relative to the injection (for brain CORT only), injection dosage, and time of injection (brain only; first or second injection for a given bird) as fixed factors.
The diel pattern of circulating CORT was assessed by including time of blood collection as a fixed factor. To assess the diel brain pattern, two consecutive 30-minute samples were averaged to obtain CORT measures at each of the six time points throughout the day in which blood samples were taken from the group of birds not used in microdialysis. In a second analysis, brain CORT values were divided into 4-hour bins with start times set to match the timing of each blood sample. We calculated the area under the curve for each bin using GraphPad Prism 6 and used this as a measure of integrated CORT. This integrated measure has been used previously to assess patterns of CORT secretion in the brain (35). The brain region of the probe implantation (HP or cNp) was included as a fixed factor in both analyses.
The effect of the restraint stressor on both plasma and brain CORT was assessed by including sample number and brain region as fixed factors. In a second analysis, we calculated the area under the curve for the five baseline brain samples and five of the seven stress-induced brain samples for each individual using GraphPad Prism 6 (integrated CORT). The first and the last of the seven stress-induced samples were eliminated to allow comparison of integrated CORT between the two time periods using a paired t test. Because only 29.2% of samples from the control area (cNp) were detectable whereas most samples (91.7%) from the HP were detectable, we calculated only the integrated CORT for HP samples. In addition, we used linear regression to test the relationship during the stressor between percentage change in circulating CORT and HP-integrated CORT.
Differences in 11βHSD2 and ABCB1 expression between the left HP and cNp were assessed with mixed models, with sex and region as fixed factors and bird identity as a random factor.
All analyses were performed using IBM SPSS Statistics 22. Where appropriate, post hoc comparisons were made using Fisher's least significant difference test. Data are presented as means ± 1 SE.
Results
In vitro validation
In vitro CORT recovery of a 10 ng/mL solution decreased as a function of flow rate (0.5 to 2 μL/min). We therefore determined that a flow rate of 1 μL/min, with an approximately 5% recovery, was optimal for obtaining an adequate fluid volume for assay. This level of recovery is comparable with that obtained previously in microdialysis studies of the brain (eg, References 21 and 36). Furthermore, most samples obtained from brain under these conditions were detectable within the enzyme immunoassay we used. In a second in vitro test, CORT recoveries were not affected by concentrations between 1 and 5 ng/mL, a physiologically relevant range for zebra finch plasma (see Supplemental Materials for details on both validations). Thus, fluctuations in endogenous CORT concentration should not affect recovery. Overall, these validation procedures indicate that the microdialysis probe and flow rate chosen for use in the zebra finch HP reliably detect physiological levels of CORT.
In vivo validation
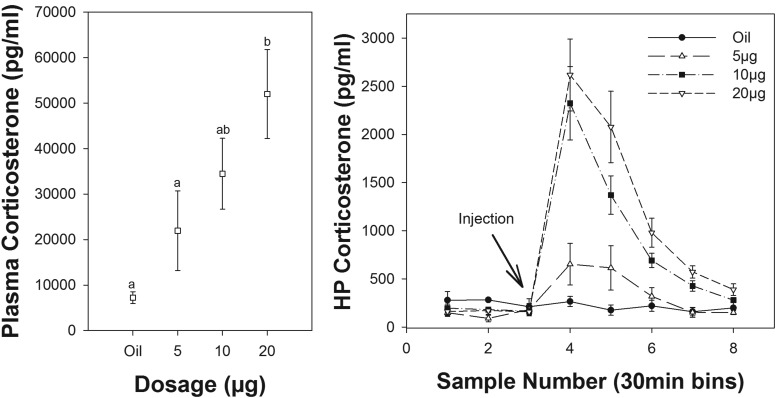
Intramuscular injection of CORT led to a significant dose-dependent increase in plasma CORT (F3,5.9 = 9.0; P = .013; Figure 2), well within the range of CORT normally detected in passerine birds (37–41). Specifically, a CORT injection of 20 μg produced a significant plasma elevation over the 5-μg dose or oil injection alone (P = .008 and P = .005, respectively), although doses of 5 and 10 μg did not lead to a significant elevation over oil injection (P = .29 and P = 0.07, respectively). All other comparisons were nonsignificant (all P > .07).
Figure 2.
Effect of CORT injections on HP (right panel) and plasma (left panel) CORT (n = 8). Each time point in the right panel represents 30 minutes of sample collection. Samples 1–2 are baseline levels prior to injection at the start of sampling period 3. Letters above symbols indicate significance.
HP CORT was significantly influenced by time relative to the injection (F7,73.7 = 31.6; P < .0001) and dosage (F3,31.2 = 12.3; P < .0001), and there was a significant interaction between time and dosage (F21,73.6 = 4.3; P < .0001; Figure 2). Within the oil-injected group, there was no change in CORT before, during, or after the injection (F7,73.9 = 0.43; P = .880). All samples within the oil-injected group were detectable with mean values between 150 and 300 pg/mL throughout. Within the 5-, 10-, and 20-μg dosage groups, the effect of time was significant (F7,73.5 = 8.7; P < .0001, F7, 73.5 = 20.0, P < .0001; and F7,73.5 = 27.3, P < .0001, respectively). Within these groups, CORT levels were significantly increased in the brain after injection; 10- and 20-μg injections produced peak levels of approximately 2–3ng/mL, whereas 5-μg injections elevated HP CORT to approximately 700 pg/mL. Brain levels did not reflect a doubling of the injected dosage, which likely reflects differences in metabolism, excretion, or saturation of buffering mechanisms (see Discussion) at higher dosages. There was no effect of the time of injection (first or second of the day; F1,72.8 = 0.57; P = .45), nor was there any interaction between the time of injection and the dosage (F3,8.6 = 1.63; P = .254) or the time of injection and sample number (F7,64.9 = 0.44; P = .873).
Diel variation in plasma and hippocampal CORT
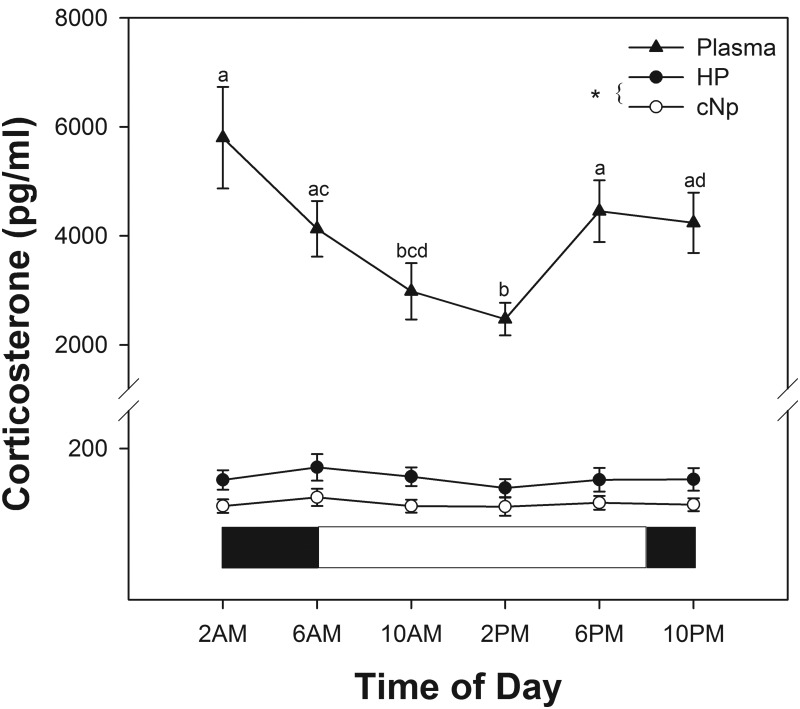
There was a significant diel rhythm in baseline plasma CORT (F5,35.4 = 5.10; P = .001). Plasma CORT levels were highest during the evening and night hours and lowest at midday (Figure 3).
Figure 3.
Baseline brain CORT (HP and cNp; n = 8 and n = 7, respectively) and plasma CORT (n = 9 per sampling period) across the daily cycle (24 h). Brain CORT represents raw CORT levels averaged across two successive 30-minute bins. White and black bars below data indicate light and dark periods, respectively. Letters above plasma CORT symbols indicate significance (P < .05). *, A significant overall difference between HP and cNp CORT.
When averaged into 1-hour bins and compared at the same times periods as those used for blood sampling, concentrations of CORT were lower in the cNp than in the HP (F1,24.9 = 7.2; P = .013), but there was no detectable diel rhythm in either brain region (day 2 of sampling; F5,53.7 = 1.7; P = .158; Figure 3). When 4-hour integrated CORT levels were assessed, cNp levels remained lower than HP levels (F1,13 = 5.2; P = .04), but we detected a significant diel rhythm in baseline brain CORT irrespective of brain region (F5,70 = 3.0; P = .016). Four-hour integrated CORT levels beginning at 2:00, 6:00, and 10:00 am were significantly higher than levels during the 4-hour period beginning at 10 pm (all P < .04). In addition, CORT during the 6:00 am-10:00 am bin was significantly higher than CORT during the 2:00 pm-6:00 pm bin (P = .01). Despite the difficulty inherent in comparing plasma samples from one time point with integrated brain CORT levels, the latter difference parallels the difference in circulating CORT; however, the brain CORT pattern of lowest levels in the evening-night hours (10:00 pm to 2:00 am) does not mirror the circulation, in which the lowest levels were obtained midday (2:00 pm).
Comparison of blood and brain CORT during a stressor
Baseline CORT in birds undergoing a stressor during microdialysis was comparable with previously published baseline levels in zebra finches (mean = 3.7 ng/mL in this study; mean = ∼3.5ng/mL in Reference 42). Restraint stress induced a significant rise in plasma CORT to a mean level of 11ng/mL (F1,14 = 43.3; P < .0001; Figure 4). Plasma CORT did not differ between birds with probes in the two brain regions (F1,13 = 3.3; P = .091), nor did the stress response differ between birds with probes in the two locations (F1,13 = 0.91; P = .358).
Although mean CORT levels were elevated in the HP compared with the cNp, the differences did not reach significance (F1,11.9 = 3.38; P = .091). Moreover, and in contrast to the results from the plasma, CORT levels in both brain regions did not change significantly between successive 15-minute sampling periods before, during, or after the restraint stressor (F11,140.9 = 1.08; P = .379; Figure 4). The interaction between sample number and brain region was also nonsignificant (F11,129.9 = 0.89; P = .553). When integrated CORT levels were analyzed, there was also no change in HP CORT between baseline and stress-induced bins (paired t = 0.57; P = .585). In addition, there was no correlation between percentage change in circulating CORT and in HP integrated CORT, although this relationship tended toward significance (F1,6 = 3.86; P = .097).
11βHSD2 and ABCB1 expression
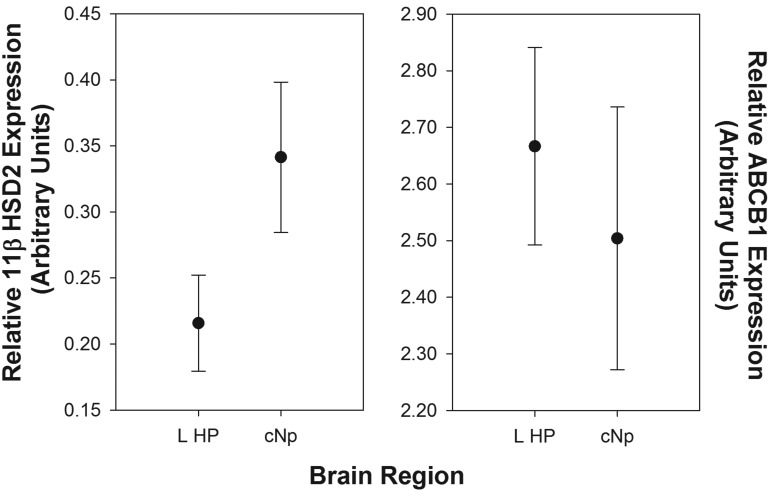
Relative expression of 11βHSD2 was higher in the cNp than the left HP (F1,8.26 = 9.67; P = .014; Figure 5), whereas expression did not differ between males and females (F1,7.97 = 0.60; P = .463). There was no interaction between brain region and sex (F1,7.32 = 0.01; P = .914). ABCB1 expression did not differ between the cNp and the left HP (F1,8.12 = 1.45; P = .262), sexes (F1,7.88 = 4.05; P = .08), or as a function of the sex by region interaction (F1,7.03 = 1.26; P = .298).
Figure 5.
Relative expression levels of 11βHSD2 (left panel) and ABCB1 (right panel) in the left hippocampus (L HP; n = 9) and cNp (n = 10) of male and female zebra finches. The values represent the δCT measurements calculated with reference to the housekeeping gene GAPdH. Note the difference in scale between the left and right panels. 11β11HSD2 levels were significantly higher in the cNp than the L HP.
Discussion
The complex interplay between circulating and brain glucocorticoids in the context of stress-physiology, behavior, and cognition is gaining considerable interest within the field of behavioral endocrinology. In this study, we show that in vivo microdialysis within discrete brain regions can be used successfully to quantify CORT in the brain of small songbirds. More interestingly, we present evidence of local CORT regulation as CORT levels were not uniform across brain regions and these differences were mirrored by expression of the CORT-metabolizing enzyme 11βHSD2. In support of a role for CORT regulation in the songbird brain, we found that across modes of CORT secretion (baseline as well as stress induced), brain CORT, both within the HP and a control region, did not directly parallel natural fluctuations found within the circulation.
We identified a regional difference in free CORT levels in brain such that higher CORT levels were found in the HP compared with the cNp (Figure 3). Local metabolism of CORT is one possible explanation for this result. We previously reported that 11βHSD2 is present in the zebra finch telencephalon, cerebellum, hypothalamus, and optic tectum (19), and in the current study, we identified a regional difference in 11βHSD2 mRNA inversely related to the difference in CORT. Specifically, higher levels of 11βHSD2 mRNA were observed in the cNp, the region with lower CORT, than in the HP. This suggests that 11βHSD2 reduces local concentrations of CORT in the brain, which could aid in protection from drastic CORT fluctuations under certain circumstances. The function of elevated 11βHSD2 mRNA in the cNp and the reduced CORT relative to the HP is unknown. Because GR is less abundant in the cNp than in the HP, it is possible that CORT is metabolized to reduce potential nonspecific CORT effects. Alternatively, it is possible that elevated 11βHSD2 in this region may provide protection against CORT acting on possible mineralocorticoid or membrane receptors (30, 43). Interestingly, although region-specific CORT differences have been detected in brain extracts of mice (44), in vivo microdialysis found no such differences between the HP of rodents and the caudate putamen (35) or prefrontal cortex (45) or within subregions of the HP itself (28). In addition, in mammals 11βHSD2 in the brain is elevated early in life (eg, in the fetus), but levels appear to fall in adulthood (46), whereas songbird 11βHSD2 levels do not experience such a decline (19), suggesting a difference in the possible function and activity of this enzyme between songbirds and mammals. Taken together, these data suggest the possibility that songbirds use 11βHSD2 to regulate access of CORT to the brain in a manner fundamentally different from that of rodents.
This is also the first study to assess expression of the transporter p-glycoprotein (ABCB1) in a songbird. This protein is a member of the ATP-binding cassette transporter family and is localized to the blood-brain barrier (13). Work in a knockout rodent model suggests that p-glycoprotein may play a role in transporting steroid hormones such as CORT out of the brain, providing a potential mechanism for regional CORT regulation (47), although more recent studies suggest that CORT entry in rodents may not be regulated by ABCB1 (48, 49). We found no difference in p-glycoprotein expression between the HP and cNp, suggesting that this transporter is not responsible for the region-specific CORT differences we observed.
In addition to observing regional CORT differences within the brain, we documented differences between CORT levels in the brain vs those in the periphery. In the periphery, we detected a strong diel rhythm that, to our knowledge, is the first to be reported in zebra finches. Plasma CORT levels were lowest during midday and peaked in the evening and early morning hours (Figure 3). These results concur with those from other birds, such as Gambel's white-crowned sparrow (Zonotrichia leucophrys gambelii) (37), the European stonechat (fecal CORT) (Saxicola torquata rubicola) (50), blue tit (Parus caeruleus) (51), and house sparrow (Passer domesticus) (52). Interestingly, although the plasma CORT rhythm was robust, a diel rhythm in brain CORT levels was not observed when successive 30-minute bins were averaged at each time point. However, when brain CORT values were integrated over 4-hour time intervals, levels were found to be lowest in the evening-night hours, approximately 8 hours after the nadir in circulating CORT. A direct comparison between plasma and integrated brain levels is difficult to make, though, given that plasma CORT levels were not also continuously monitored and integrated over time (as in Reference 35). Regardless of the means of analysis, however, these results suggest that plasma CORT does not simply enter the brain unimpeded. If this were the case, one would expect direct mirroring of CORT patterns between the brain and the circulation. Unlike our results in the zebra finch, diel rhythms of CORT in rodents are present in the HP and other brain regions and directly parallel the blood rhythm (at least when free plasma CORT is assessed) (53). The lack of parallel responses in the blood and brain of male zebra finches observed in this study provides additional support for the hypothesis of CORT regulation in the HP and possibly the whole brain.
We also assessed circulating and brain CORT in response to a standard stressor. Our results indicate that zebra finches responded to the stress of being captured, bled, and restrained for 15 minutes with a significant elevation in plasma CORT of approximately 260% over baseline levels (Figure 4). It appears, however, that the stress-induced rise in plasma CORT (to ∼11 ng/mL) is insufficient to significantly elevate neural levels of CORT because we found no evidence of a stress response in the HP when using an integrated CORT measure or when assessing raw CORT levels. By contrast, im injections that elevate plasma CORT above approximately 20 ng/mL did elevate HP CORT levels. Such levels are typically seen in other songbird species subjected to a stressor (37–41) in which levels can reach as much as 200 ng/mL (song sparrow) (40) and can rise to approximately 750% above baseline (Smith's longspur, Calcarius pictus) (39). Our results therefore suggest that the magnitude of natural stress-induced CORT secretion may differentially impact the brain such that it is protected from exposure to lower levels, whereas a more severe stressor may induce higher CORT secretion that could reach the brain. It is important to note that although CORT itself may not reach the brain under conditions of minor stress, elevated plasma CORT presumably acts peripherally, such as through stimulation of hepatic gluconeogenesis, which may in turn impact the brain (2).
Studies using microdialysis in rodents suggest that they differ from birds in the availability of circulating CORT to the brain. Application of a series of foot shocks led to an approximately 275% increase in circulating CORT that was paralleled by an approximately 250% increase in the dorsal HP of young mice (54). In this same study, middle-aged mice that were stressed in the same manner achieved an approximately 430% increase in plasma CORT, whereas HP CORT increased by approximately 250%. Nevertheless, changes in neural CORT do not always parallel those in plasma. For example, HP CORT in Wistar rats rose after a forced swimming stressor but was delayed at the onset in the brain relative to plasma (35). Corticosterone binding globulins (CBGs) were responsible for producing this delay, which limited exposure of the HP to the full CORT response (55).
The lack of response to a stressor and the differential diel rhythms observed between the brain and periphery provide additional support for the idea of local regulation within the brain by a mediator such as 11βHSD2. However, there are other regulatory mechanisms that may have contributed to our results. CBGs are a likely regulator of CORT reaching the brain in our study system; they are present in the bloodstream and bind a large portion of circulating CORT. According to the free hormone hypothesis (56), only the unbound, free portion of CORT in circulation is available for binding to receptors in target tissues. Attenuated brain CORT in response to diel variation or stressors could thus be due to circulating CBGs or diel variation in CBG abundance or in 11βHSD2 activity. We measured total but not free plasma CORT in our study; thus, we are not able to determine whether free CORT rose in the plasma in response to stress, although Wada et al (57) documented an increase in both total and free CORT after 15 minutes of restraint stress in zebra finches. It is possible that a more severe or prolonged stressor could increase plasma levels to such a degree that the capabilities of the CBG pool or metabolic enzymes would be overwhelmed, leading to an elevation in brain CORT (58). This is a likely explanation for our result showing that exogenous CORT injections produced an elevation in the HP. Finally, because progesterone binds CBGs with equal affinity to CORT in birds (59), the availability of free CORT to the brain could potentially be regulated by factors such as reproductive stage (but see 60).
In addition, one cannot rule out neural CORT synthesis as a contributor to our results. The mammalian brain possesses all of the enzymes necessary for de novo CORT synthesis (61), although there is no concrete evidence for CORT synthesis in the songbird brain. The lack of concordance between brain and peripheral CORT in this study could therefore be the product of neural CORT production.
The results of the current study highlight the importance of assessing patterns of CORT fluctuations within the brain using sensitive techniques such as in vivo microdialysis to investigate neural hormone profiles. In contrast to some studies in rodents, we find that patterns of circulating CORT are not mirrored in the brain. Numerous studies have documented behavioral effects of exogenously manipulated CORT; however, the results of these studies are often contradictory and difficult to interpret. Indeed, our results suggest that the songbird brain is buffered from the effects of CORT fluctuations within the physiological range, and we provide evidence for a role of 11βHSD2 in regulating local, region-specific levels. Such regulation could prevent undesirable consequences, such as neurodegeneration, and ensure consistent neurogenesis required for spatial memory function within the HP as well as the maintenance of other CORT-sensitive circuits throughout the brain. The source and function of neural CORT in the songbird brain will therefore provide an exciting avenue for future research.
Acknowledgments
We thank Nicole Gomez and Brigit Harvey for assistance in collecting samples. In addition, we thank Drs Matthew Fuxjager, Anya Illes, and Jesse Ellis for providing valuable input and advice and Dr Luke Remage-Healey for reviewing this manuscript.
This work was supported by National Institutes of Health Grant NIMH061994 (to B.A.S.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CBG
- corticosterone binding globulin
- cNp
- caudal nidopallium
- CORT
- corticosterone
- CT
- cycle threshold
- CV
- coefficient of variation
- dPBS
- Dulbecco's PBS
- GAPdH
- glyceraldehyde-3-phosphate dehydrogenase
- HP
- hippocampus
- 11βHSD2
- 11β-hydroxysteroid dehydrogenase type 2.
References
- 1. Romero LM. Physiological stress in ecology: lessons from biomedical research. Trends Ecol Evol. 2004;19:249–255 [DOI] [PubMed] [Google Scholar]
- 2. Sapolsky R, Romero L, Munck A. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89 [DOI] [PubMed] [Google Scholar]
- 3. Young EA, Abelson J, Lightman SL. Cortisol pulsatility and its role in stress regulation and health. Front Neuroendocrinol. 2004;25:69–76 [DOI] [PubMed] [Google Scholar]
- 4. Landys MM, Ramenofsky M, Wingfield JC. Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. Gen Comp Endocrinol. 2006;148:132–149 [DOI] [PubMed] [Google Scholar]
- 5. Breuner CW, Greenberg AL, Wingfield JC. Noninvasive corticosterone treatment rapidly increases activity in Gambel's white-crowned sparrows (Zonotrichia leucophrys gambelii). Gen Comp Endocrinol. 1998;111:386–394 [DOI] [PubMed] [Google Scholar]
- 6. Loiseau C, Sorci G, Dano S, Chastel O. Effects of experimental increase of corticosterone levels on begging behavior, immunity and parental provisioning rate in house sparrows. Gen Comp Endocrinol. 2008;155:101–108 [DOI] [PubMed] [Google Scholar]
- 7. Pravosudov VV. Long-term moderate elevation of corticosterone facilitates avian food-caching behaviour and enhances spatial memory. Proc Roy Soc B. 2003;270:2599–2604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kitaysky AS. Corticosterone facilitates begging and affects resource allocation in the black-legged kittiwake. Behav Ecol. 2001;12:619–625 [Google Scholar]
- 9. Wada H, Breuner CW. Transient elevation of corticosterone alters begging behavior and growth of white-crowned sparrow nestlings. J Exp Biol. 2008;211:1696–1703 [DOI] [PubMed] [Google Scholar]
- 10. Vallarino A, Wingfield JC, Drummond H. Does extra corticosterone elicit increased begging and submissiveness in subordinate booby (Sula nebouxii) chicks? Gen Comp Endocrinol. 2006;147:297–303 [DOI] [PubMed] [Google Scholar]
- 11. Breuner CW, Wingfield JC. Rapid behavioral response to corticosterone varies with photoperiod and dose. Horm Behav. 2000;37:23–30 [DOI] [PubMed] [Google Scholar]
- 12. Busch DS, Sperry TS, Peterson E, Do CT, Wingfield JC, Boyd EH. Impacts of frequent, acute pulses of corticosterone on condition and behavior of Gambel's white-crowned sparrow (Zonotrichia leucophrys gambelii). Gen Comp Endocrinol. 2008;158:224–233 [DOI] [PubMed] [Google Scholar]
- 13. Pardridge W. Transport of nutrients and hormones through the blood-brain barrier. Diabetologia. 1981;20:246–254 [DOI] [PubMed] [Google Scholar]
- 14. McEwen B, Davis P, Parsons B, Pfaff D. The brain as a target for steroid hormone action. Ann Rev Neurosci. 1979;2:65–112 [DOI] [PubMed] [Google Scholar]
- 15. Moore FL, Evans SJ. Steroid hormones use non-genomic mechanisms to control brain functions and behaviors: a review of evidence. Brain Behav Evol. 1999;54:41–50 [DOI] [PubMed] [Google Scholar]
- 16. Celotti F, Negri-Cesi P, Poletti A. Steroid metabolism in the mammalian brain: 5α-reduction and aromatization. Brain Res Bull. 1997;44:365–375 [DOI] [PubMed] [Google Scholar]
- 17. Schlinger BA. Sex steroids and their actions on the birdsong system. J Neurobiol. 1997;33:619–631 [PubMed] [Google Scholar]
- 18. Newman AEM, Soma KK. Corticosterone and dehydroepiandrosterone in songbird plasma and brain: effects of season and acute stress. Eur J. Neurosci. 2009;29:1905–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Katz A, Oyama RK, Feng N, Chen X, Schlinger BA. 11β-Hydroxysteroid dehydrogenase type 2 in zebra finch brain and peripheral tissues. Gen Comp Endocrinol. 2010;166:600–605 [DOI] [PubMed] [Google Scholar]
- 20. Linthorst AC, Flachskamm C, Reul JM. Local administration of recombinant human interleukin-1 beta in the rat hippocampus increases serotonergic neurotransmission, hypothalamic-pituitary-adrenal axis activity, and body temperature. Endocrinol. 1994;135:520–532 [DOI] [PubMed] [Google Scholar]
- 21. Remage-Healey L, Maidment N, Schlinger B. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008;11:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Remage-Healey L, Oyama RK, Schlinger BA. Elevated aromatase activity in forebrain synaptic terminals during song. J Neuroendocrinol. 2009;21:191–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Remage-Healey L, Dong S, Maidment NT, Schlinger BA. Presynaptic control of rapid estrogen fluctuations in the songbird auditory forebrain. J Neurosci. 2011;31:10034–10038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sapolsky RM, Meaney MJ, McEwen BS. The development of the glucocorticoid receptor system in the rat limbic brain. III. Negative-feedback regulation. Brain Res. 1985;350:169–173 [DOI] [PubMed] [Google Scholar]
- 25. Dickens M, Romero LM, Cyr NE, Dunn IC, Meddle SL. Chronic stress alters glucocorticoid receptor and mineralocorticoid receptor mRNA expression in the European starling (Sturnus vulgaris) brain. J Neuroendocrinol. 2009;21:832–840 [DOI] [PubMed] [Google Scholar]
- 26. Patel SN, Clayton NS, Krebs JR. Hippocampal tissue transplants reverse lesion-induced spatial memory deficits in zebra finches (Taeniopygia guttata). J Neurosci. 1997;17:3861–3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Watanabe S, Bischof H. Effects of hippocampal lesions on acquisition and retention of spatial learning in zebra finches. Behav Brain Res. 2004;155:147–152 [DOI] [PubMed] [Google Scholar]
- 28. Dorey R, Piérard C, Chauveau F, David V, Béracochéa D. Stress-induced memory retrieval impairments: different time-course involvement of corticosterone and glucocorticoid receptors in dorsal and ventral hippocampus. Neuropsychopharmacol. 2012;37:2870–2880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roozendaal B, Griffith QK, Buranday J, de Quervain DJF, McGaugh JL. The hippocampus mediates glucocorticoid-induced impairment of spatial memory retrieval: dependence on the basolateral amygdala. Proc Nat Acad Sci USA. 2003;100:1328–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shahbazi M, Schmidt M, Carruth LL. Distribution and subcellular localization of glucocorticoid receptor-immunoreactive neurons in the developing and adult male zebra finch brain. Gen Comp Endocrinol. 2011;174:354–361 [DOI] [PubMed] [Google Scholar]
- 31. Amin N, Grace JA, Theunissen FE. Neural response to bird's own song and tutor song in the zebra finch field L and caudal mesopallium. J Comp Phys A. 2004;190:469–489 [DOI] [PubMed] [Google Scholar]
- 32. Spencer KA, Evans NP, Monaghan P. Postnatal stress in birds: a novel model of glucocorticoid programming of the hypothalamic-pituitary-adrenal axis. Endocrinol. 2009;150:1931–1934 [DOI] [PubMed] [Google Scholar]
- 33. Saldanha CJ, Popper P, Micevych PE, Schlinger BA. The passerine hippocampus is a site of high aromatase: inter- and intraspecies comparisons. Horm Behav. 1998;34:85–97 [DOI] [PubMed] [Google Scholar]
- 34. Fuxjager MJ, Schultz JD, Barske J, et al. Spinal motor and sensory neurons are androgen targets in an acrobatic bird. Endocrinology. 2012;153:3780–3791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Droste S, de Groote L, Atkinson HC, Lightman SL, Reul JMHM, Linthorst ACE. Corticosterone levels in the brain show a distinct ultradian rhythm but a delayed response to forced swim stress. Endocrinology. 2008;149:3244–3253 [DOI] [PubMed] [Google Scholar]
- 36. Woodroofe MN, Sarna GS, Wadhwa M, et al. Detection of interleukin-1 and interleukin-6 in adult rat brain, following mechanical injury, by in vivo microdialysis: evidence of a role for microglia in cytokine production. J Neuroimmunol. 1991;33:227–236 [DOI] [PubMed] [Google Scholar]
- 37. Breuner CW, Wingfield JC, Romero LM. Diel rhythms of basal and stress-induced corticosterone in a wild, seasonal vertebrate, Gambel's white-crowned sparrow. J Exp Zool. 1999;284:334–342 [PubMed] [Google Scholar]
- 38. Lendvai AZ, Chastel O. Experimental mate-removal increases the stress response of female house sparrows: the effects of offspring value? Horm Behav. 2008;53:395–401 [DOI] [PubMed] [Google Scholar]
- 39. Meddle SL, Owen-Ashley NT, Richardson MI, Wingfield JC. Modulation of the hypothalamic-pituitary-adrenal axis of an Arctic-breeding polygynandrous songbird, the Smith's longspur, Calcarius pictus. Proc Roy Soc B. 2003;270:1849–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Newman A, Pradhan D, Soma K. Dehydroepiandrosterone and corticosterone are regulated by season and acute stress in a wild songbird: jugular versus brachial plasma. Endocrinology. 2008;149:2537–2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Romero LM, Remage-Healey L. Daily and seasonal variation in response to stress in captive starlings (Sturnus vulgaris): corticosterone. Gen Comp Endocrinol. 2000;119:52–59 [DOI] [PubMed] [Google Scholar]
- 42. Remage-Healey L, Adkins-Regan E, Romero LM. Behavioral and adrenocortical responses to mate separation and reunion in the zebra finch. Horm Behav. 2003;43:108–114 [DOI] [PubMed] [Google Scholar]
- 43. Breuner CW, Orchinik M. Pharmacological characterization of intracellular, membrane, and plasma binding sites for corticosterone in house sparrows. Gen Comp Endocrinol. 2009;163:214–224 [DOI] [PubMed] [Google Scholar]
- 44. Croft AP, O'Callaghan MJ, Shaw SG, Connolly G, Jacquot C, Little HJ. Effects of minor laboratory procedures, adrenalectomy, social defeat or acute alcohol on regional brain concentrations of corticosterone. Brain Res. 2008;1238:12–22 [DOI] [PubMed] [Google Scholar]
- 45. Kitchener P, Di Blasi F, Borrelli E, Piazza PV. Differences between brain structures in nuclear translocation and DNA binding of the glucocorticoid receptor during stress and the circadian cycle. Eur J Neurosci. 2004;19:1837–1846 [DOI] [PubMed] [Google Scholar]
- 46. Brown RW, Diaz R, Robson AC, et al. The ontogeny of 11β-hydroxysteroid dehydrogenase type 2 and mineralocorticoid receptor gene expression reveal intricate control of glucocorticoid action in development. Endocrinol. 1996;137:794–797 [DOI] [PubMed] [Google Scholar]
- 47. Uhr M, Holsboer F, Müller MB. Penetration of endogenous steroid hormones corticosterone, cortisol, aldosterone and progesterone into the brain is enhanced in mice deficient for both mdr1a and mdr1b P-glycoproteins. J Neuroendocrinol. 2002;14:753–759 [DOI] [PubMed] [Google Scholar]
- 48. Karssen AM, Meijer OC, Van der Sandt IC, et al. Multidrug resistance P-glycoprotein hampers the access of cortisol but not of corticosterone to mouse and human brain. Endocrinology. 2001;142:2686–2694 [DOI] [PubMed] [Google Scholar]
- 49. Mason BL, Pariante CM, Thomas SA. A revised role for P-glycoprotein in the brain distribution of dexamethasone, cortisol, and corticosterone in wild-type and ABCB1A/B-deficient mice. Endocrinology. 2008;149:5244–5253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Goymann W, Trappschuh M. Seasonal and diel variation of hormone metabolites in European stonechats: on the importance of high signal-to-noise ratios in noninvasive hormone studies. J Biol Rhythms. 2011;26:44–54 [DOI] [PubMed] [Google Scholar]
- 51. Müller C, Jenni-Eiermann S, Blondel J, et al. Effect of human presence and handling on circulating corticosterone levels in breeding blue tits (Parus caeruleus). Gen Comp Endocrinol. 2006;148:163–171 [DOI] [PubMed] [Google Scholar]
- 52. Rich E, Romero LM. Daily and photoperiod variations of basal and stress-induced corticosterone concentrations in house sparrows (Passer domesticus). J Comp Phys B. 2001;171:543–547 [DOI] [PubMed] [Google Scholar]
- 53. Qian X, Droste SK, Lightman SL, Reul JMHM, Linthorst ACE. Circadian and ultradian rhythms of free glucocorticoid hormone are highly synchronized between the blood, the subcutaneous tissue, and the brain. Endocrinology. 2012;153:4346–4353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tronche C, Piérard C, Coutan M, Chauveau F, Liscia P, Béracochéa D. Increased stress-induced intra-hippocampus corticosterone rise associated with memory impairments in middle-aged mice. Neurobiol Learn Mem. 2010;93:343–351 [DOI] [PubMed] [Google Scholar]
- 55. Qian X, Droste SK, Gutièrrez-Mecinas M, et al. A rapid release of corticosteroid-binding globulin from the liver restrains the glucocorticoid hormone response to acute stress. Endocrinology. 2011;152:3738–3748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mendel CM. The free hormone hypothesis: a physiologically based mathematical model. Endocr Rev. 1989;10:232–274 [DOI] [PubMed] [Google Scholar]
- 57. Wada H, Salvante KG, Stables C, Wagner E, Williams TD, Breuner CW. Adrenocortical responses in zebra finches (Taeniopygia guttata): individual variation, repeatability, and relationship to phenotypic quality. Horm Behav. 2008;53:472–480 [DOI] [PubMed] [Google Scholar]
- 58. Breuner CW, Lynn SE, Julian GE, et al. Plasma-binding globulins and acute stress response. Horm Metab Res. 2006;38:260–268 [DOI] [PubMed] [Google Scholar]
- 59. Deviche P, Breuner C, Orchinik M. Testosterone, corticosterone, and photoperiod interact to regulate plasma levels of binding globulin and free steroid hormone in dark-eyed juncos, Junco hyemalis. Gen Comp Endocrinol. 2001;122:67–77 [DOI] [PubMed] [Google Scholar]
- 60. Love OP, Breuner CW, Vézina F, Williams TD. Mediation of a corticosterone-induced reproductive conflict. Horm Behav. 2004;46:59–65 [DOI] [PubMed] [Google Scholar]
- 61. Taves MD, Gomez-Sanchez CE, Soma KK. Extra-adrenal glucocorticoids and mineralocorticoids: evidence for local synthesis, regulation, and function. Am J Physiol Endocrinol Metab. 2011;301:E11–E24 [DOI] [PMC free article] [PubMed] [Google Scholar]