Abstract
The cAMP-dependent protein kinase A (PKA) signaling system is widely expressed and has a central role in regulating cellular metabolism in all organ systems affected by obesity. PKA has four regulatory (RIα, RIIα, RIβ, RIIβ) and four catalytic (Cα, Cβ, Cγ, Prkx) subunit isoforms that have tissue-specific expression profiles. In mice, knockout (KO) of RIIβ, the primary PKA regulatory subunit in adipose tissue or knockout of the catalytic subunit Cβ resulted in a lean phenotype that resists diet-induced obesity and associated metabolic complications. Here we report that the disruption of the ubiquitously expressed PKA RIIα subunit in mice (RIIαKO) confers resistance to diet-induced obesity, glucose intolerance, and hepatic steatosis. After 2-week high-fat diet exposure, RIIαKO mice weighed less than wild-type littermates. Over time this effect was more pronounced in female mice that were also leaner than their wild-type counterparts, regardless of the diet. Decreased intake of a high-fat diet contributed to the attenuated weight gain in RIIαKO mice. Additionally, RIIα deficiency caused differential regulation of PKA in key metabolic organs: cAMP-stimulated PKA activity was decreased in liver and increased in gonadal adipose tissue. We conclude that RIIα represents a potential target for therapeutic interventions in obesity, glucose intolerance, and nonalcoholic fatty liver disease.
In the United States, an estimated 35.7% of adults and nearly 17% of children and adolescents were obese in 2009–2010 (1). Because obesity contributes to several of the leading causes of death, the high obesity rate poses a significant health threat to the general public. Genetic differences in signaling systems clearly contribute to the high interindividual variability observed in susceptibility or resistance to obesity. cAMP-dependent protein kinase (PKA) signaling represents one of the primary pathways that mediates the effects of sympathetic signaling molecules and hormones that regulate energy metabolism. PKA is widely involved in energy homeostasis in humans and other species and therefore represents a good target for therapeutic intervention. PKA comprises four regulatory subunit isoforms (RIα, RIIα, RIβ, RIIβ) and four catalytic subunit isoforms (Cα, Cβ, Cγ, Prkx). Two regulatory and two catalytic subunits form the tetrameric PKA holoenzyme. The PKA subunits have distinct expression patterns: α-subunits are expressed ubiquitously, whereas β-subunits are expressed in a more tissue-specific fashion. The PKA holoenzyme can confer type I or type II kinase activity, depending on regulatory subunit identity. Local cAMP levels regulate intracellular PKA activity because cAMP binds to PKA regulatory subunit dimers (four cAMP molecules per dimer), thereby activating catalytic subunits that in turn phosphorylate numerous targets. RIα has the highest affinity for cAMP of the regulatory subunits and is therefore more sensitive to cAMP, which makes RIα essential for maintaining regulated PKA activity (2).
RIIβ is highly and specifically expressed in brain and white and brown adipose tissue (AT). Global knockout (KO) of RIIβ produced a genetically lean mouse (RIIβKO) that resisted obesity and had improved insulin resistance when challenged with a high-fat diet (HFD) (3, 4). An isoform shift to the more cAMP-sensitive type I PKA led to enhanced PKA activity (5), increased locomotor activity, and brown AT activation in RIIβKO mice (3, 6). Recently the lean phenotype of the RIIβKO mouse was attributed to altered γ-aminobutyric acid (GABA)ergic signaling in hypothalamic neurons despite the numerous changes observed in AT (7). Deletion of all isoforms of PKA catalytic subunit β (Cβ) (8) also led to a lean phenotype in mice (CβKO) and significant sex differences (9). Altered energy intake or locomotor activity did not fully explain the metabolic phenotype of the CβKO mouse. Although the RIIβ KO mouse had increased basal PKA activity in the brain, CβKO mice had decreased basal activity in the brain (10). Unlike RIIβ, the Cβ subunit is not highly expressed in AT (4). RIIβKO and CβKO mouse studies in conjunction with evidence that melanocortin receptors and other hypothalamic molecular systems regulate metabolic rate through cAMP/PKA signaling (5) suggest that although the mechanisms likely differ between RIIβ and Cβ mutants, PKA signaling in brain is critical to the observed resistance to diet-induced obesity.
The metabolic consequences of disrupting other PKA subunits have not been reported. Here we provide evidence for a role for RIIα in regulation of energy balance. The RIIαKO mouse was initially studied for what was thought to be an essential role of RIIα in localizing PKA to facilitate PKA-dependent modulation of Ca2+ channel activity via specific associations with A kinase anchor proteins. Instead of identifying tissues such as skeletal muscle that lacked these critical interactions, RIα was found to bind A kinase anchor proteins in the absence of RIIα, and the RIIαKO mouse was reportedly normal and healthy (11). RIIα was shown to localize PKA in late spermatogenesis to enhance motility, yet RIIαKO mice maintain fertility and sperm motility despite an isoform shift and relocalization of PKA (12).
In the present study, we found that RIIαKO mice resisted diet-induced obesity, glucose intolerance, and hepatic steatosis in a sex-dependent manner. Additionally, there were substantial phenotypic differences from RIIβKO and CβKO mice that provide new insights into the role of PKA signaling in the regulation of obesity, liver function, and adiposity.
Materials and Methods
RIIαKO mice
The RIIαKO mouse line was obtained from the Mutant Mouse Regional Resource Center (http://www.mmrrc.org) and has been described (11). RIIα heterozygote breeding pairs with a C57/BL6, 129 Sv/J genetic background were backcrossed to C57/BL6 for at least five generations. Use of heterozygote breeding pairs enabled the generation of RIIαKO and wild-type (WT) littermates for the studies. PCR genotyping was performed as described (11). Both male and female mice were used for the described feeding studies, body composition, glucose tolerance tests, and mRNA experiments. Follow-up studies including indirect calorimetry, protein expression, and PKA assays were conducted in female mice. Procedures were approved by and conducted in accordance with the Eunice Kennedy Shriver National Institute for Child Health and Human Development Institutional Animal Care and Use Committee.
Specialized diet cohorts
Chow and water were provided ad libitum during the 5- to 7-day acclimation period. The diets used for the feeding studies were as follows: 1) control diet (CD) (NIH-31 Open Formula; Harlan Teklad) with an energy density of 12.552 kJ/g with 24%, 14%, and 62% of energy derived from protein, fat, and carbohydrate, respectively; and 2) high-fat/high-sucrose diet (HFD) (F3282; Bio-Serv) with an energy density of 23.01 kJ/g and 21%, 36%, and 36% of energy derived from protein, fat, and carbohydrate, respectively. Mice were maintained on the CD until 12 weeks of age at which time they were assigned to either the HFD or CD for 14 weeks. Four groups of mice included for each sex were WT CD, WT HFD, RIIαKO CD, and RIIαKO HFD (n = 8/group; total mice, n = 64). Mice were housed two to four per cage and maintained on a 12-hour light, 12-hour dark cycle at 22–23°C.
Body weight and adiposity
Mice were weighed weekly at 9:00 am during the 14-week feeding study. Body composition was measured by EchoMRI-100H body composition analyzer for mice after 14 weeks (EchoMRI). Inguinal, gonadal, mesenteric, perirenal, retroperitoneal, and intrascapular brown fat pads and liver were weighed at the time of euthanasia.
Tissue and serum collection
Mice were killed by slow replacement of air with CO2 followed by cervical dislocation and exsanguination. Collected tissues were snap frozen in liquid nitrogen and stored at −80°C. Tissues for RNA extraction were stored in RNAlater (QIAGEN). Trunk blood was collected in serum separator tubes and serum was stored at −20°C.
Intraperitoneal glucose tolerance test (GTT) and insulin tolerance test (ITT)
On day 3 of week 14, mice were fasted overnight for 16 hours (n = 8/group) for GTT. An ip glucose injection (2 g/kg) (Sigma-Aldrich) was administered and blood glucose measured in 3 μL of tail blood at baseline and 15, 30, 60, and 120 minutes with a Contour glucometer and test strips (Bayer Healthcare LLC). ITT was performed on nonfasted mice (n = 5/group) after 12 weeks on the HFD or CD. An ip injection of human insulin (Humulin R, 0.75 U/kg) was administered and glucose measured in 3 μL of blood obtained from the tail vein at baseline and 15, 30, 45, and 60 minutes.
Energy intake and expenditure and total activity
Indirect calorimetry was performed in a 12-chamber Oxymax/CLAMS (Columbus Instruments, Inc) on 20- to 22-week-old female mice fed either a CD or HFD for 10 weeks prior to testing (n = 4–5/group, one mouse per chamber, each mouse tested every 13 min). Food and water were provided ad libitum. Mice were first acclimated to the metabolic chambers for 2 days at 23°C. The respiratory exchange ratio, resting and total energy expenditure, energy intake, and locomotor activity were measured for 24 hours at 23°C and then for 24 hours at 30°C. Total activity was determined by infrared beam interruption using an Opto-Varimex mini (Columbus Instruments, Inc).
Biochemical serum measurements
Colorimetric assays were used to measure serum cholesterol (Thermo Scientific), triacylglycerols (Pointe Scientific Inc), and free fatty acids (Roche Diagnostics GmBH). Insulin was measured by a RIA (Millipore). Serum leptin was measured by an ELISA (R&D Systems). Blood glucose was measured with a Contour glucomoter and test strips (Bayer Healthcare).
Immunohistochemistry
Fresh liver tissue (left lobe) was fixed in 4% paraformaldehyde for 48 hours, rinsed in 1× PBS for 5 minutes (three times), transferred to 30% sucrose to equilibriate for 24 hours, rinsed again with 1× PBS, and then set in optimum cutting temperature compound (Tissue-Tek) using dry ice-chilled isopentane. Slides were prepared from 12-μM-thick sections and then stained with Oil Red O (Sigma-Aldrich) and counterstained with hematoxylin and eosin.
mRNA and protein expression
RNA was extracted using RNEasy Lipid Mini (AT) and Mini (liver) kits (QIAGEN). cDNA was synthesized from 250 ng (AT) and 500 ng (liver) total RNA with SuperScript III first-strand synthesis supermix for quantitative RT-PCR (Invitrogen). Relative mRNA expression was determined by quantitative RT-PCR (ViAA7; Applied Bioscience) using the 2−ΔΔCT method (13). β-Actin served as the housekeeper gene for liver and averaged values for β-actin, Rblpo, B2m, and Hprt were used for AT. All quantitative PCR primers were tested and optimized previously (Supplemental Table 1). Gonadal AT and liver samples were homogenized in ice-cold TPER lysis buffer with 1:100 protease and phosphatase inhibitor cocktails (EMD Biosciences) using a BulletBlender (Next Advance). Total protein was measured by a BCA assay (Pierce). PKA subunit protein expression was quantified by Western blot with commercially available antibodies (Supplemental Table 1) and analyzed by ChemiDoc using Image Lab software (BioRad Laboratories).
PKA activity
Gonadal AT and liver samples were homogenized in ice-cold buffer [10 mM Tris-HCl (pH 7.5), 1 mM EDTA, 1 mM dithiothreitol, and protease inhibitor cocktail I (EMD Biosciences)]. Lysates were centrifuged at 10 000 × g for 10 minutes at 4°C. The total protein concentration of the supernatant was determined by a BCA assay (Pierce). The PKA enzymatic activity was measured using a previously described method (14).
Diethylaminoethyl (DEAE)-cellulose chromatography
All procedures were performed at 0–4°C as described by Nesterova et al (14). Briefly, after two washes with ice-cold 1× PBS, AT and liver samples were suspended in 10 mL of 10 mM Tris/HCI, 1 mM EDTA, 1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 1 homogenized, and centrifuged at 10 000 × g for 10 minutes. The resulting supernatants were subjected to chromatography on an equilibriated DEAE column developed with a 0- to 350-mM NaCl gradient in 10 mM Tris/HCI (pH 7.1), 1 mM EDTA, and 1 mM phenylmethylsulfonyl fluoride at a flow rate of 15 mL/h. Two-milliliter fractions were collected on ice and assayed for protein kinase activity.
Statistical analysis
Data were described using simple descriptive statistics, which are reported as mean ± SEM; relative changes are described as a percentage of each respective comparison. All data distributions were assessed for approximate normality. Continuous data were compared between two independent groups using t tests. Analysis of covariance (ANCOVA) considered the roles of sex, diet, body weight, and temperature, as appropriate, when comparing genotype for the various outcomes of AT depot and liver weights, serum parameters, energy expenditure and locomotor activity, mRNA and protein expression, and PKA activity. As appropriate, certain results were stratified by sex or temperature; additional stratification by diet was carried out when statistically significant interactions were observed. A value of P ≤ .05 was considered statistically significant. Data were analyzed using SAS version 9.2 (SAS Institute, Inc).
Results
RIIαKO mice were leaner and gained less weight than WT littermates during HFD feeding
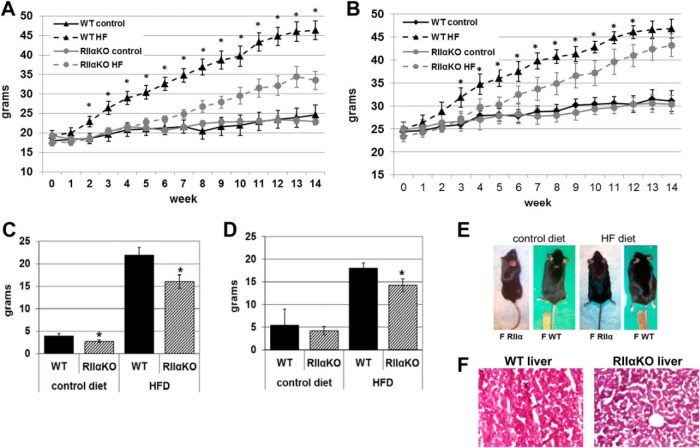
RIIαKO and WT mice fed the CD had similar body weights when compared within same-sex groups. Female and male RIIαKO mice weighed less than their sex-matched WT littermates within 2 and 3 weeks, respectively, after the introduction of the HFD (Figure 1, A and B). This increase in body weight was maintained throughout the 14-week HFD feeding study in female mice and remained significant in males until week 12 of the study. RIIαKO females were significantly leaner than WT females (Figure 1, C and D). At week 14, the difference in body weight was no longer significant between HFD-fed WT and mutant males, yet male mutants had decreased absolute fat mass (Figure 1D). Female mutants weighed approximately 21.3% less and male mutants 7.6% than their respective WT counterparts after 14 weeks of HFD exposure. No differences in lean mass were observed in RIIαKO and WT mice between genotypes or diet groups (data not shown).
Figure 1.
Mean weekly body weights of female (A) and male (B) mice during 14 weeks of ad libitum feeding of either chow (control diet) or high-fat/high-sucrose (HF) diet. Absolute fat mass in female (C) and male (D) WT and RIIαKO mice on control and HF diets as determined by magnetic resonance imaging. Female RIIαKO and WT mice (E) and livers counterstained with Oil Red O and hematoxylin and eosin (F) after 14 weeks of HF diet feeding Oil Red O. Values are mean ± SEM. *, P < .05.
Fat distribution, liver weight, and lipid accumulation in liver were altered in RIIαKO mice
RIIαKO females had significantly smaller mesenteric, perirenal, and retroperitoneal adipose depots, which were approximately 56%, 53%, and 31% that of WT mice, respectively, after 14 weeks on the CD and 44%, 39%, and 42% that of WT mice, respectively after 14 weeks of the HFD feeding (Table 1). Among female CD-fed mice, mutants had smaller gonadal, mesenteric, and retroperitoneal depots than WTs (Table 1).
Table 1.
RIIαKO Mice Had Reduced AT Depot and Liver Weights
| BW, g | Gonadal AT, g | Inguinal AT, g | Mesenteric AT, g | Perirenal AT, g | RP AT, g | IBAT, g | Liver, g | |
|---|---|---|---|---|---|---|---|---|
| Females | ||||||||
| CD | ||||||||
| WT (n = 8) | 24.35 ± 1.41 | 0.24 ± 0.04 | 0.39 ± 0.09 | 0.25 ± 0.04 | 0.13 ± 0.03 | 0.16 ± 0.03 | 0.25 ± 0.04 | 1.12 ± 0.07 |
| Mutant (n = 8) | 23.38 ± 0.70 | 0.14 ± 0.02 | 0.23 ± 0.02 | 0.15 ± 0.02 | 0.06 ± 0.02 | 0.05 ± 0.02 | 0.19 ± 0.02 | 0.96 ± 0.10 |
| HFD | ||||||||
| WT (n = 8) | 45.4 ± 2.51 | 1.64 ± 0.12 | 2.16 ± 0.19 | 1.75 ± 0.31 | 0.68 ± 0.13 | 1.24 ± 0.28 | 0.58 ± 0.08 | 1.62 ± 0.18 |
| Mutant (n = 7) | 35.73 ± 3.25 | 1.53 ± 0.37 | 1.59 ± 0.40 | 0.86 ± 0.27 | 0.30 ± 0.08 | 0.59 ± 0.18 | 0.66 ± 0.14 | 1.39 ± 0.19 |
| HFD, P = .011; CD, P = .76a | P = .55 | P = .097 | P = .029 | P = .008 | P = .040 | P = .98 | P = .17 | |
| Males | ||||||||
| CD | ||||||||
| WT (n = 7) | 31.94 ± 1.94 | 0.29 ± 0.09 | 0.40 ± 0.12 | 0.31 ± 0.11 | 0.15 ± 0.05 | 0.24 ± 0.09 | 0.19 ± 0.02 | 1.39 ± 0.16 |
| Mutant (n = 7) | 29.49 ± 0.66 | 0.22 ± 0.04 | 0.34 ± 0.07 | 0.19 ± 0.03 | 0.06 ± 0.03 | 0.19 ± 0.02 | 0.25 ± 0.05 | 1.37 ± 0.03 |
| HFD | ||||||||
| WT (n = 7) | 46.69 ± 1.21 | 0.71 ± 0.09 | 1.26 ± 0.11 | 0.98 ± 0.11 | 0.49 ± 0.06 | 1.14 ± 0.16 | 0.58 ± 0.07 | 2.20 ± 0.16 |
| Mutant (n = 7) | 43.14 ± 1.94 | 0.75 ± 0.09 | 1.50 ± 0.17 | 0.86 ± 0.12 | 0.56 ± 0.13 | 0.81 ± 0.19 | 0.54 ± 0.10 | 1.61 ± 0.09 |
| P = .058 | P = .84 | P = .47 | P = .25 | P = .86 | P = .16 | P = .82 | P = .024 |
Abbreviations: BW, body weight; IBAT, intrascapular brown adipose tissue; RP, retroperitoneal. Body weight and AT depot weights were measured after 14 weeks of dietary treatment. Values are mean ± SEM. P value was from multivariable ANOVA comparing genotype and adjusting for diet.
Reported separately for each diet due to statistically significant interaction of diet group with genotype.
Although RIIαKO males had decreased absolute fat mass after chronic HFD feeding, individual fat depot weights did not differ from those of WT mice (Figure 1D and Table 1). Total fat mass in female and male RIIαKOs was decreased by 27% and 21%, respectively, compared with sex-matched WT littermates after HFD feeding. Liver weight was significantly lower in male mutants compared with WTs; liver weight in HFD-fed mutants was approximately 27% less than that of their WT counterparts (Table 1). Oil Red O staining confirmed decreased lipid accumulation in liver from RIIαKO mice (Figure 1F).
Serum parameters of glucose and lipid metabolism
Apart from the expected increases in nonfasted serum glucose, triacylglycerols, cholesterol, insulin, and leptin between the CD- and HFD-fed mice (Table 2), there were several interesting differences in the serum parameters examined. Adjusting for body weight eliminated the significant differences between diet groups for these parameters; however, male RIIαKO mice had lower nonfasted serum insulin than that of WT males after correcting for body weight (Table 2). Nonfasted glucose was significantly lower in HFD-fed RIIαKO females compared with WTs, and this significant difference persisted after adjusting for body weight. Serum cholesterol was significantly decreased in the HFD-fed RIIαKO females compared with their WT counterparts, but the statistical significance was lost after adjusting for body weight (Table 2).
Table 2.
Serum Parameters in RIIαKO and WT Mice After 14 Weeks of CD or HFD Feeding Period in Nonfasted Mice
| BW, g | FFA, mM | TG, mg/dL | Glucose, mg/dL | Cholesterol, mg/dL | Insulin, ng/mL | Leptin, ng/mL | |
|---|---|---|---|---|---|---|---|
| Females | |||||||
| CD | |||||||
| Wild type (n = 7) | 24.35 ± 1.41 | 0.71 ± 0.23 | 119.09 ± 30.57 | 140.00 ± 16.23 | 87.25 ± 9.74 | 0.86 ± 0.38 | 4.64 ± 1.08 |
| Mutant (n = 8) | 23.38 ± 0.70 | 0.50 ± 0.09 | 119.14 ± 11.75 | 154.50 ± 37.98 | 108.29 ± 6.91 | 0.99 ± 0.29 | 5.03 ± 0.52 |
| HFD | |||||||
| Wild type (n = 10) | 44.94 ± 2.10 | 0.88 ± 0.2 | 153.82 ± 31.53 | 306.14 ± 48.84 | 204.89 ± 25.71 | 3.77 ± 0.64 | 131.28 ± 30.45 |
| Mutant (n = 8) | 34.88 ± 2.86 | 0.73 ± 0.09 | 133.25 ± 8.53 | 248.63 ± 44.66 | 172.44 ± 8.70 | 2.84 ± 0.87 | 75.42 ± 15.46 |
| HFD, P = .011; CD, P = .76a | P = .26 | P = .91 | HFD, P = .022; CD, P = .93a | P = 1.0 | P = .70 | P = .70 | |
| Males | |||||||
| CD | |||||||
| Wild type (n = 4) | 31.48 ± 2.14 | 0.70 ± 0.19 | 142.68 ± 27.99 | 131.50 ± 27.08 | 125.87 ± 12.88 | 1.33 ± 0.49 | 15.63 ± 3.62 |
| Mutant (n = 9) | 29.25 ± 1.20 | 0.54 ± 0.08 | 122.45 ± 26.80 | 158.50 ± 26.58 | 122.75 ± 3.72 | 0.77 ± 0.06 | 5.53 ± 2.35 |
| HFD | |||||||
| Wild type (n = 7) | 47273 ± 1.01 | 0.64 ± 0.14 | 230.48 ± 46.13 | 273.17 ± 81.78 | 209.00 ± 14.08 | 9.03 ± 2.64 | 87.75 ± 18.35 |
| Mutant (n = 6) | 44.41 ± 2.20 | 0.52 ± 0.06 | 164.34 ± 22.15 | 273.43 ± 40.36 | 202.75 ± 22.49 | 2.25 ± 0.32 | 63.65 ± 10.23 |
| P = .21 | P = .35 | P = .32 | P = 1.0 | P = .41 | P = .023 | P = .34 |
Abbreviations: BW, body weight; FFA, free fatty acids; TG, triacylglycerols. Values are mean ± SEM. P values are from multivariable ANCOVA comparing genotype adjusted for covariates (BW, diet).
Reported separately for each diet due to statistically significant interaction with genotype.
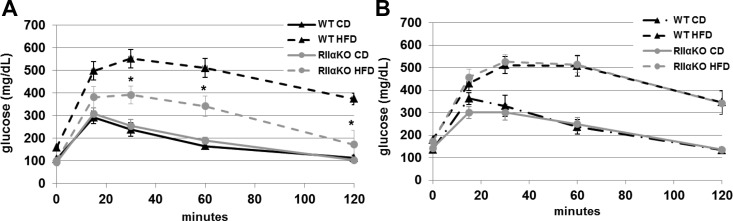
Female RIIαKO mice had improved glucose and insulin tolerance after chronic HFD feeding
After an overnight 16-hour fast and ip glucose administration, blood glucose was lower in HFD-fed female RIIαKO compared with WT mice at all time points measured (Figure 2A). This difference was absent in male mice (data not shown). Initial glucose clearance (15 min) after ip insulin administration was similar in nonfasted CD-fed and HFD-fed, regardless of genotype, and 1-hour blood glucose levels were the same between mutant mice, regardless of diet and CD-fed WT, unlike that of HFD-fed WT mice, which was significantly lower (Figure 2B).
Figure 2.
Glucose tolerance was attenuated and response to insulin tolerance test differed after chronic feeding in female RIIαKO mice HFD feeding. GTT (A) and ITT (B) in female WT and RIIαKO after CD and HFD feeding. Intraperitoneal glucose injection (2 g/kg) and insulin (HumulinR) (0.75 U/kg) was administered, respectively, for GTT and ITT. Blood glucose was measured at baseline and 15, 30, 60, and 120 minutes after glucose injection and at baseline and 15, 30, 45, and 60 minutes after insulin injection. Values are mean ± SEM. *, P < .05.
Indirect calorimetry
Indirect calorimetry data were collected at ambient (23°C) and then at thermoneutral (30°C) conditions. We were unable to detect differences in energy intake between mutant and WT mice with stratified comparisons for each temperature and diet group or by multivariable ANCOVA adjusting for temperature and diet (Table 3).
Table 3.
Energy Expenditure and Locomotor Activity in RIIαKO and WT Mice Were Determined by Indirect Calorimetry
| TEE, kJ/h | Energy Intake, kJ/h | RER | Total Activity, Beam Break/h) (×104) | Resting VO2, mL/g0.75 · h | Total VO2, mL/g0.75 · h | |
|---|---|---|---|---|---|---|
| 23 C | ||||||
| CD | ||||||
| WT (n = 6) | 48.90 ± 1.55 | 54.49 ± 6.16 | 0.91 ± 0.01 | 486.79 ± 72.47 | 7.95 ± 0.36 | 9.62 ± 0.48 |
| RIIαKO (n = 6) | 46.61 ± 1.05 | 47.67 ± 2.36 | 0.88 ± 0.01 | 606.91 ± 66.6 | 7.51 ± 0.18 | 9.13 ± 0.18 |
| HFD | ||||||
| WT (n = 3) | 53.18 ± 0.72 | 49.96 ± 7.00 | 0.73 ± 0.01 | 443.80 ± 50.33 | 6.46 ± 0.24 | 7.54 ± 0.25 |
| RIIαKO (n = 4) | 57.57 ± 1.14 | 49.19 ± 3.81 | 0.74 ± 0.01 | 453.14 ± 28.17 | 6.82 ± 0.17 | 7.87 ± 0.24 |
| HFD, P = .031; CD, P = .256a | P = .62 | P = .12 | P = .20 | P = .61 | P = .597 | |
| 30 C | ||||||
| CD | ||||||
| WT (n = 6) | 32.17 ± 0.81 | 40.53 ± 4.50 | 0.97 ± 0.02 | 437.04 ± 64.41 | 4.33 ± 0.19 | 6.25 ± 0.24 |
| RIIαKO (n = 6) | 31.37 ± 0.47 | 32.18 ± 1.74 | 0.94 ± 0.01 | 552.98 ± 54.12 | 4.37 ± 0.17 | 6.12 ± 0.14 |
| HFD | ||||||
| WT (n = 3) | 32.60 ± 0.34 | 52.56 ± 15.25 | 0.78 ± 0.01 | 394.18 ± 35.78 | 3.40 ± 0.16 | 4.60 ± 0.21 |
| RIIαKO (n = 4) | 35.85 ± 0.53 | 44.25 ± 1.74 | 0.77 ± 0.01 | 368.16 ± 38.83 | 4.23 ± 0.13 | 4.88 ± 0.23 |
| HFD, P = .005; CD, P = .416a | P = .058 | P = .28 | P = .12 | HFD, P = .010; CD, P = .89a | P = .92 |
Abbreviations: RER, respiratory exchange ratio; TEE, total energy expenditure. Energy expenditure and locomotor activity in RIIαKO and WT mice were determined by indirect calorimetry. Total energy expenditure and energy intake, respiratory exchange ratio, total locomotor activity, and VO2 in CD and HFD-fed mice were measured for 24 hours at 23°C and 30°C; rates are presented per hour. Groups consisted of CD (n = 5/group for each genotype) and HFD (n = 4/group for each genotype); P values are from multivariable ANCOVA comparing genotype, including temperature and diet as covariates; values are mean ± SEM. *, P < .05 for comparisons within diet group.
Reported separately for each diet due to statistically significant interaction of diet group with genotype.
Multivariable ANCOVA adjusting for diet and temperature revealed a significant interaction between diet group and genotype (Table 3), so we performed stratified comparisons for each diet group, which revealed higher total energy expenditure in RIIαKO compared with the WT mice after chronic HFD feeding (Table 3). The significant interaction between diet group and genotype for resting oxygen consumption (VO2) also led to pairwise comparisons for this variable, which indicated higher resting VO2 at 30°C in HFD- but not CD-fed RIIαKO compared with the WT mice. Differences in the respiratory exchange ratio between genotypes approached but did not achieve statistical significance after adjusting for diet and temperature (Table 3). No differences were detected in locomotor activity or in total VO2 at either temperature (Table 3). Because of the absence of differences in energy intake and activity, we focused on liver and AT in our initial PKA expression and activity studies.
PKA expression and activity were differentially regulated in AT and liver of RIIαKO mice
Gonadal AT mRNA and protein expression
Regulatory subunit RIIβ and catalytic subunit Cα mRNA expressions were higher in RIIαKO compared with WT mice compared within the same diet groups as well as when analyzed using a multivariable model that adjusted for diet. RIα mRNA was significantly decreased in male mutants compared with WT mice (data not shown). Differences in in RIIβ and Cα were not detected at the protein level (Table 4). Overall, HFD feeding elicited a tendency toward a decreased expression of the PKA subunit protein in RIIαKO mice, whereas only RIIβ protein decreased in WT mice after chronic HFD exposure (Table 4).
Table 4.
PKA Subunit mRNA and Protein Expression in Gonadal AT of WT and RIIαKO Mice After 14 Weeks of ad Libitum Feeding With CD or HFD
| RIα | RIIα | RIIβ | Cα | Cβ | |
|---|---|---|---|---|---|
| mRNA expression of PKA subunits (qPCR) | |||||
| CD | |||||
| WT (n = 8) | 1.07 ± 0.14 | 0.97 ± 0.15 | 1.40 ± 0.37 | 1.21 ± 0.27 | 1.06 ± 0.14 |
| Mutant (n = 7) | 1.22 ± 0.12 | 0.30 ± 0.22 | 2.73 ± 0.51 | 2.18 ± 0.29 | 1.27 ± 0.09 |
| HFD | |||||
| WT (n = 8) | 1.26 ± 0.08 | 1.70 ± 0.27 | 2.60 ± 0.18 | 1.67 ± 0.13 | 1.49 ± 0.12 |
| Mutant (n = 8) | 1.34 ± 0.07 | 0.24 ± 0.04 | 3.19 ± 0.36 | 1.72 ± 0.10 | 1.52 ± 0.12 |
| P = .30 | P < .001 | P = .017 | CD, P = .031; HFD, P = .80a | P = .32 | |
| Protein expression of PKA subunits (Western blot) | |||||
| CD | |||||
| WT (n = 5) | 1.47 ± 0.48 | 1.56 ± 0.41 | 1.22 ± 0.34 | 0.29 ± 0.09 | 0.13 ± 0.04 |
| Mutant (n = 2) | 1.36 ± 0.10 | 0.13 ± 0.10 | 1.89 ± 1.05 | 0.96 ± 0.07 | 0.08 ± 0.01 |
| HFD | |||||
| WT (n = 5) | 0.32 ± 0.08 | 1.17 ± 0.27 | 0.30 ± 0.21 | 1.65 ± 0.33 | 0.19 ± 0.03 |
| Mutant (n = 2) | 0.50 ± 0.30 | 0.22 ± 0.05 | 0.46 ± 0.37 | 0.40 ± 0.30 | 0.08 ± 0.00 |
| P = .94 | P = .012 | P = .45 | CD, P = .030; HFD, P = .083a | P = .054 |
Abbreviation: qPCR, quantitative PCR. mRNA expression was represented as fold change (2−ΔΔCT) based on WT mice on CD. Protein expression was calculated as the ratio of protein of interest to housekeeper. Data for female mice are shown. P values derive from multivariable ANCOVA adjusting for diet; values are mean ± SEM.
Reported separately for each diet due to statistically significant interaction with genotype.
We also investigated mRNA expression of the two rate-limiting lipolytic enzymes in gonadal AT. Adipose triglyceride lipase (ATGL) mRNA was significantly higher in RIIαKO compared with WT mice (Supplemental Figure 1A), and the increase in hormone-sensitive lipase (HSL) fell just outside the range of statstical significance (Supplemental Figure 1B).
Gonadal AT PKA activity
Basal PKA activity was significantly higher in gonadal AT of RIIαKO compared with WT mice after adjusting for diet (Table 5). Although total PKA activity did not differ between mutant and WT mice, HFD feeding led to a nearly 2-fold increase in total PKA activity in RIIαKO mice (P < .05), a response not observed in the WT mice (Table 5). The ratio of basal to total PKA activity was significantly different between mutant and WT AT, indicating differences in the regulation of the PKA system between genotypes (Table 5).
Table 5.
PKA Activity in Gonadal AT of WT and RIIαKO Mice After 14 Weeks of ad Libitum Feeding With CD or HFD
| PKA Activity |
|||
|---|---|---|---|
| −cAMP | +cAMP | Ratio | |
| CD | |||
| WT (n = 7) | 39.94 ± 8.55 | 358.36 ± 67.65 | 0.11 ± 0.01 |
| Mutant (n = 7) | 42.87 ± 3.00 | 296.69 ± 23.86 | 0.15 ± 0.01 |
| HFD | |||
| WT (n = 8) | 31.24 ± 5.03 | 414.45 ± 41.42 | 0.08 ± 0.01 |
| Mutant (n = 7) | 62.31 ± 12.82 | 539.16 ± 90.31 | 0.11 ± 0.01 |
| P = .047a | P = .58 | P = .001 | |
−cAMP activity (basal) was without and +cAMP activity was activity with 5 μmol added cAMP. Data for female mice are shown. P values derive from multivariable ANCOVA adjusting for diet; values are mean ± SEM.
Overall model was not statistically significant.
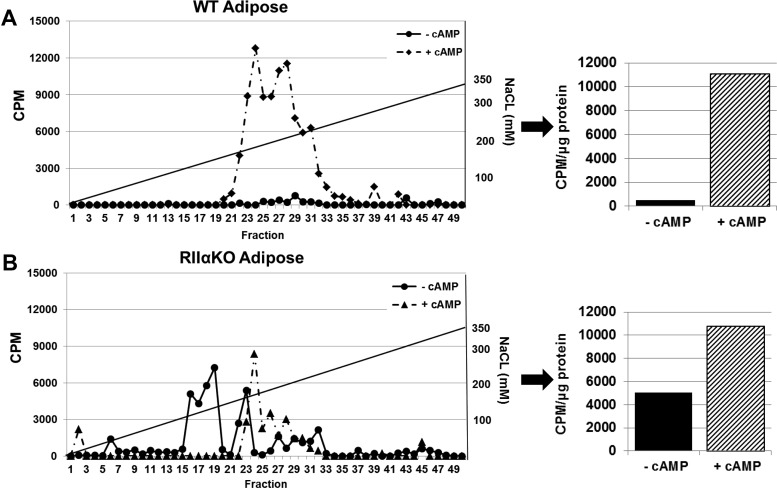
To further investigate the increased PKA activity we observed in AT of mutant mice, we subjected AT cell lysates to DEAE column chromatography and subsequent PKA activity assay to assess type I and type II kinase activity profiles in mutants. We found a distinctly different pattern of kinase activity in mutant AT compared with that of WT (Figure 3, A and B). In line with previous reports, the WT mice had exclusively type II kinase activity, and basal activity was barely detectable. Cumulative basal kinase activity that was assessed as the total amount of activity prior to HPLC column separation was approximately 10-fold higher in the RIIαKO than in the WT mice (Figure 3B, right panel). The peak in fractions 15–19 indicated free catalytic subunit activity (Figure 3B, left panel).
Figure 3.
DEAE-cellulose column separation and PKA activity assay of inguinal adipose from HFD-fed WT (A) and RIIαKO (B) mice showed substantially increased basal kinase activity in the mutant mice. Line graphs show kinase activity in HPLC-separated fractions with and without added cAMP (5 μM). Type I elutes between 40 and 80 mM (fractions 20–25), type II elutes between 120 and 200 mM (fractions 31–35), and free C subunit elutes just before the type I holoenzyme. Bar graphs show kinase activity (per microgram of protein) in lysate prior to HPLC separation (cumulative kinase activity with and without added cAMP).
Liver mRNA and protein expression
After adjusting for diet and sex, there were no differences in mRNA levels of PKA subunits in liver between genotypes except for RIIα, which was not detectable (Table 4). A multivariable analysis of PKA subunit protein expression adjusted for diet confirmed the significant increase in RIα protein among mutants, which was greater than 5-fold that of WT mice (Table 6). Mean protein levels of catalytic subunits Cα and Cβ were both decreased in RIIαKO mice after adjusting for diet (Table 6), and Cβ protein was decreased in the mutant mice after 14 weeks of the HFD compared with the CD-feeding (P = .002).
Table 6.
PKA Subunit mRNA and Protein Expression in Liver of WT and RIIαKO Mice After 14 Weeks of ad Libitum Feeding With CD or HFD
| RIα | RIIα | RIIβ | Cα | Cβ | |
|---|---|---|---|---|---|
| mRNA expression (qPCR) | |||||
| CD | |||||
| WT (n = 7) | 0.99 ± 0.12 | 1.10 ± 0.13 | 1.01 ± 0.18 | 0.98 ± 0.09 | 1.02 ± 0.13 |
| Mutant (n = 7) | 0.98 ± 0.05 | 0.19 ± 0.18 | 0.81 ± 0.15 | 0.72 ± 0.08 | 1.02 ± 0.12 |
| HFD | |||||
| WT (n = 7) | 0.65 ± 0.04 | 0.55 ± 0.12 | 1.19 ± 0.27 | 0.60 ± 0.05 | 0.49 ± 0.07 |
| Mutant (n = 6) | 0.91 ± 0.06 | 0.02 ± 0.02 | 0.80 ± 0.20 | 0.74 ± 0.06 | 1.05 ± 0.26 |
| P = .14 | P < .001 | P = .17 | HFD, P = .086; CD, P = .059a | P = .10 | |
| Protein expression (Western blot) | |||||
| CD | |||||
| WT (n = 3) | 3.30 ± 0.55 | 2.91 ± 0.58 | 13.77 ± 5.35 | 6.39 ± 1.59 | 6.94 ± 0.69 |
| Mutant (n = 3) | 18.52 ± 3.89 | 0.28 ± 0.15 | 5.95 ± 2.68 | 1.33 ± 0.34 | 3.19 ± 0.30 |
| HFD | |||||
| WT (n = 3) | 3.20 ± 2.55 | 1.71 ± 0.12 | 8.97 ± 4.30 | 5.07 ± 1.12 | 2.28 ± 0.44 |
| Mutant (n = 3) | 16.70 ± 6.60 | 0.17 ± 0.10 | 3.90 ± 1.89 | 0.87 ± 0.27 | 0.79 ± 0.12 |
| P = .005 | P < .001 | P = .11 | P < .001 | HFD, P = .032; CD, P = .007a |
Abbreviation: qPCR, quantitative PCR. mRNA expression was presented as fold-change (2−ΔΔCT); WT mice on CD served as the control. Protein expression was calculated as the ratio of protein of interest to housekeeper. Data shown are for female mice. P values derive from multivariable ANCOVA adjusting for diet; values are mean ± SEM.
Reported separately for each diet due to statistically significant interaction with genotype.
PKA activity in liver
In liver, chronic HFD feeding led to a nearly 4-fold suppression of cAMP-stimulated (total) PKA activity in RIIαKO compared with WT mice (Table 7). Basal PKA did not differ between genotypes, but the ratio of basal to total PKA activity was significantly different between genotypes after adjusting for diet, suggesting an altered regulation of the PKA system in mutants (Table 7).
Table 7.
PKA Activity in Liver of WT and RIIαKO Mice After 14 Weeks of ad Libitum Feeding With CD or HFD
| PKA Activity |
|||
|---|---|---|---|
| −cAMP | +cAMP | Ratio | |
| CS | |||
| WT (n = 8) | 860.09 ± 140.47 | 2803.01 ± 372.60 | 0.37 ± 0.08 |
| Mutant (n = 8) | 694.53 ± 261.32 | 1752.96 ± 724.79 | 0.77 ± 0.21 |
| HFD | |||
| WT (n = 8) | 1221.54 ± 412.92 | 2853.38 ± 441.15 | 0.43 ± 0.14 |
| Mutant (n = 7) | 483.35 ± 188.37 | 715.17 ± 306.70 | 1.17 ± 0.49 |
| P = .12 | P = .003 | P = .030a | |
−cAMP activity (basal) was activity without and +cAMP activity (total) was activity with 5 μmol added cAMP; ratio was the ratio of basal to total PKA activity. Data shown are for female mice. P values derive from multivariable ANCOVA adjusting for diet; values are mean ± SEM.
Overall model was not statistically significant.
Discussion
We have shown that the disruption of PKA regulatory subunit RIIα protects mice from diet-induced obesity, fatty liver, and glucose intolerance. These effects were more pronounced in female mice, suggesting sex-specific differences in the regulation of energy and glucose homeostasis by PKA. Female RIIαKO mice were leaner than their WT littermates when given ad libitum access to standard chow (CD), an effect that became even more profound when the HFD was provided, and the attenuated weight gain was maintained throughout the 14-week study. Despite the diminished difference in body weight between the male WT and RIIαKO mice by week 14, RIIαKO males had decreased mean fat mass and liver weight yet did not have improved glucose tolerance compared with their WT littermates. When challenged with an obesogenic diet, the female RIIαKO mice had improved glucose tolerance that was likely the result of their decreased fat mass. It is tempting to speculate that RIIαKO males would have displayed improved glucose tolerance compared with the WT males if a GTT was performed earlier when differences in body weight (BW) were greater. Mutant males had lower nonfasted insulin levels and were able to achieve similar glucose control as WT mice, which was suggestive of improved insulin sensitivity. Intrahepatic lipid accumulation that is associated with increased insulin secretion (15) is likely a contributor to this effect in RIIαKO males.
Thus, in parallel to what has been observed in mice with disruption of PKA catalytic subunit Cβ (9), there was sexual dimorphism in the obesity-resistant phenotype of RIIαKO mice in that females exhibited greater metabolic effects from the deletion of the Prkar2a gene. This leads to the question of possible involvement of estrogen signaling in the RIIαKO mice. Ovariectomy has been shown to increase adiposity in rats (16), and replacement of estrogens in overweight postmenopausal women can reduce central adiposity (17). Estradiol treatment of ovariectomized female as well as male mice can increase central leptin sensitivity (18). Further study of sex-specific effects of PKA signaling on adiposity may be useful in developing therapeutic obesity interventions aimed at women.
Comparing mouse models of PKA deficiency provides new insight on the role of PKA in energy and glucose homeostasis regulation. One distinct difference between RIIαKO and CβKO mice is that the energy intake of RIIαKO mice was similar to WT and may even have been slightly decreased when ad libitum access to the HFD was provided, whereas CβKO as well as RIIβ mice were hyperphagic. Longer-term intake studies may be required to detect potential differences in intake by mutants. Multivariable analysis identified significantly increased total energy expenditure in mutants during HFD feeding but not CD feeding. Conversely, in RIIβKO and CβKO mice, energy expenditure was increased quite dramatically, and the phenotypes included increased locomotor activity and brown fat activation in RIIβKO mice. This suggests that the mechanisms for the observed leanness in PKA KO models (RIIβ, Cβ, and RIIα) are likely different. RIIβ, Cβ, and RIIα are all expressed in the brain, although neither Cβ nor RIIα is highly expressed in AT as is RIIβ. Reexpression of RIIβ in GABAergic neurons of hypothalamus rescued the lean phenotype of the RIIβKO mouse, whereas the reexpression in AT did not (7). Further investigation into the role of RIIα in brain regions involved in energy and glucose homeostasis, which is currently underway in our laboratory, will aid in understanding the specificity of RIIα.
The changes in PKA expression and increased PKA activity in AT were unexpected in the RIIαKO mouse because the low RIIα expression we found in AT from WT mice confirmed previous reports of low RIIα expression in fat (19). We found significant changes in AT PKA activity despite the lack of increased RIα or catalytic subunits; Cα protein was increased but only in CD-fed mutants. There was an overall decrease in PKA subunit protein expression in gonadal AT from mutant mice. Following HFD challenge, Cα protein decreased in AT of RIIαKO mice and the relative importance of Cβ increased as evidenced by the increased ratio of Cβ to Cα protein expression in CD-fed mutants (0.08/0.96) compared to HFD-fed mutants (0.08/0.40) (Table 4). Compensatory PKA regulation does not involve transcriptional or translational control but instead is thought to result from stabilization of the protein through formation of type I holoenzyme (20), which does not appear to be the sole cause for increased PKA activity in RIIαKO mice. Low expression of type II regulatory subunits in AT of RIIαKO mice, absent RIIα, and low RIIβ compared with WTs was enhanced by chronic HFD.
Under normal physiological conditions, AT has almost exclusively type II kinase activity, unlike some tissues that display combined type I and II kinase activities (21). The type I holoenzyme has consistently been shown to be more sensitive to cAMP activation than the type II holoenzyme (3, 20, 22, 23). The DEAE assay confirmed the profound increase in basal PKA activity in AT that we found to be enhanced by HFD exposure and indicated that increased PKA activity was not solely the result of an isoform switch from type II to type I activity but also resulted from free catalytic subunit activity. We hypothesize that this unexpected, but robust increase in PKA activation in response to chronic HFD stimulated lipolysis and exerted a protective effect against excess fat accumulation. Determining what changes in the HFD-fed mutant mice can cause the observed changes in PKA regulation will help understand how the blockade of RIIα may provide protection from some of the metabolic complications associated with diet-induced obesity.
By virtue of its essential regulatory roles in lipolysis and adipogenesis, changes in PKA activity in AT would be expected to impact mobilization and storage of lipids. In fat cells, the actions of PKA oppose those of insulin. Catecholamines and glucagon, key regulators of triglyceride catabolism, act by raising cAMP levels and stimulating PKA-dependent phosphorylation. Two phosphorylation targets are the two primary lipolytic enzymes: HSL (24) and ATGL (25). We found increased HSL mRNA in the AT of RIIαKO mice and a similar trend in the ATGL mRNA expression compared with WT mice (Supplemental Figure 1, A and B) that support enhanced lipolysis as a contributing factor for the decreased fat mass of RIIα mutants.
Conversely, decreased hepatic PKA activity in RIIαKO mice appeared to protect from glucose intolerance and steatosis. Biguanides, the potent antihyperglycemic agents that have become the most commonly prescribed class of drug for type 2 diabetes (26), have recently been determined to function mechanistically by inhibiting production of cAMP and reducing PKA activity (27). Additionally, targeted expression of a dominant-negative form of RIα in liver caused decreased PKA activity and enhanced glucose clearance (28). It should be noted that RIIαKO mice did not exhibit improved glucose clearance compared with WTs on a CD: improved glucose tolerance became evident only after chronic HFD feeding. Although male RIIαKOs did not have improved glucose tolerance, we saw attenuated paleness of liver and decreased accumulation of triacylglycerols compared with WT mice. Fatty liver in nonalcoholic fatty liver disease and nonalchoholic steatohepatosis is associated with glucose intolerance (29) and insulin resistance (30), and hypertriglyceridemia plays a central role in its pathogenesis (31). The tendency toward decreased triacylglycerols in RIIαKO mice compared with WT counterparts was not significant but may be physiologically relevant to the improved metabolic phenotype.
The PKA pathway also regulates PPARα in hepatocytes that directly impacts genes involved in fatty acid β-oxidation and insulin sensitivity (32). It should be noted that skeletal muscle of RIIαKO mice showed a compensatory increase in RIα with concomitant decreases in catalytic subunits as well as PKA activity (11), similar to what we report here in liver. We propose that the suppression of PKA signaling in these key glucoregulatory tissues plays a role in the improved glucose metabolism and decreased insulin secretion observed in mutant female and male mice, respectively. Current studies are investigating gluconeogenic response in RIIαKO mice to gain insight into PKA regulation of glucose homeostasis.
In summary, previous studies of dysregulation of PKA activity by global KO of RIIα showed a fertile, healthy mouse (11, 12). The RIIαKO mouse, however, had an improved metabolic profile when challenged with a chronic obesogenic diet, a phenotype not recognized earlier. Disruption of RIα has dramatic physiological impacts including embryonic lethality in the RIαKO mouse (33) and multiple tumors in humans that have RIα haploinsufficiency caused by inactivating mutations of the PRKAR1A gene (34, 35). Mice heterozygous for inactivating RIα mutations have a less severe phenotype than humans but still develop a variety of tumors (36). The role of RIα in the regulation of insulin secretion was only recently recognized (37). Humans with inactivating RIα mutations have differences in their adiposity and insulin secretion when exposed to glucocorticoids (38) and during an oral GTT (37, 39), respectively. RIIα is ubiquitously expressed like RIα but is not thought to be the primary PKA regulatory subunit in either liver or AT; nevertheless, we found impressive effects of RIIα's loss in these tissues in RIIαKO mice. Thus, differential regulation of PKA activity in organs important to energy and glucose homeostasis is likely to be central in regulating the responses to diet, metabolic function, and adiposity.
Acknowledgments
We thank G. Stanley McKnight for his thoughtful input regarding our experiments in PKA RIIαKO mice. We also thank Marc L. Reitman for his advice regarding the data and for reviewing the manuscript. We also thank Nina Tseridiani and Vincent Huang, two students who participated in the Eunice Kennedy Shriver National Institute of Child Health and Human Development intramural summer student program and assisted with genotyping and tissue collection.
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ANCOVA
- analysis of covariance
- AT
- adipose tissue
- ATGL
- adipose triglyceride lipase
- BW
- body weight
- DEAE
- diethylaminoethyl
- GTT
- glucose tolerance test
- HFD
- high-fat diet
- HSL
- hormone-sensitive lipase
- ITT
- insulin tolerance test
- KO
- knockout
- PKA
- protein kinase A
- VO2
- oxygen consumption
- WT
- wild type.
References
- 1. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States. NCHS Data Brief. 2012;(82):1–8 [PubMed] [Google Scholar]
- 2. Amieux PS, McKnight GS. The essential role of RI α in the maintenance of regulated PKA activity. Ann NY Acad Sci. 2002;968:75–95 [DOI] [PubMed] [Google Scholar]
- 3. Cummings DE, Brandon EP, Planas JV, Motamed K, Idzerda RL, McKnight GS. Genetically lean mice result from targeted disruption of the RII β subunit of protein kinase A. Nature. 1996;382:622–626 [DOI] [PubMed] [Google Scholar]
- 4. Schreyer SA, Cummings DE, McKnight GS, LeBoeuf RC. Mutation of the RIIβ subunit of protein kinase A prevents diet-induced insulin resistance and dyslipidemia in mice. Diabetes. 2001;50:2555–2562 [DOI] [PubMed] [Google Scholar]
- 5. Czyzyk TA, Sikorski MA, Yang L, McKnight GS. Disruption of the RIIβ subunit of PKA reverses the obesity syndrome of Agouti lethal yellow mice. Proc Natl Acad Sci USA. 2008;105:276–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nolan MA, Sikorski MA, McKnight GS. The role of uncoupling protein 1 in the metabolism and adiposity of RII β-protein kinase A-deficient mice. Mol Endocrinol. 2004;18:2302–2311 [DOI] [PubMed] [Google Scholar]
- 7. Zheng R, Yang L, Sikorski MA, et al. Deficiency of the RIIβ subunit of PKA affects locomotor activity and energy homeostasis in distinct neuronal populations. Proc Natl Acad Sci USA. 2013;110:E1631–E1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guthrie CR, Skalhegg BS, McKnight GS. Two novel brain-specific splice variants of the murine Cβ gene of cAMP-dependent protein kinase. J Biol Chem. 1997;272:29560–29565 [DOI] [PubMed] [Google Scholar]
- 9. Enns LC, Morton JF, Mangalindan RS, et al. Attenuation of age-related metabolic dysfunction in mice with a targeted disruption of the Cβ subunit of protein kinase A. J Gerontol A Biol Sci Med Sci. 2009;64:1221–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Howe DG, Wiley JC, McKnight GS. Molecular and behavioral effects of a null mutation in all PKA Cβ isoforms. Mol Cell Neurosci. 2002;20:515–524 [DOI] [PubMed] [Google Scholar]
- 11. Burton KA, Johnson BD, Hausken ZE, et al. Type II regulatory subunits are not required for the anchoring-dependent modulation of Ca2+ channel activity by cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 1997;94:11067–11072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burton KA, Treash-Osio B, Muller CH, Dunphy EL, McKnight GS. Deletion of type IIα regulatory subunit delocalizes protein kinase A in mouse sperm without affecting motility or fertilization. J Biol Chem. 1999;274:24131–24136 [DOI] [PubMed] [Google Scholar]
- 13. Stratakis CA. Cushing syndrome in pediatrics. Endocrinol Metab Clin North Am. 2012;41:793–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nesterova M, Yokozaki H, McDuffie E, Cho-Chung YS. Overexpression of RII β regulatory subunit of protein kinase A in human colon carcinoma cell induces growth arrest and phenotypic changes that are abolished by site-directed mutation of RII β. Eur J Biochem. 1996;235:486–494 [DOI] [PubMed] [Google Scholar]
- 15. Finucane FM, Sharp SJ, Hatunic M, et al. Liver fat accumulation is associated with reduced hepatic insulin extraction and β cell dysfunction in healthy older individuals. Diabetol Metab Syndr. 2014;6:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sharp JC, Copps JC, Liu Q, et al. Analysis of ovariectomy and estrogen effects on body composition in rats by X-ray and magnetic resonance imaging techniques. J Bone Miner Res. 2000;15:138–146 [DOI] [PubMed] [Google Scholar]
- 17. Perry AC, Allison M, Applegate EB, Jackson ML, Miller PC. The relationship between fat distribution and coronary risk factors in sedentary postmenopausal women on and off hormone replacement therapy. Obes Res. 1998;6:40–46 [DOI] [PubMed] [Google Scholar]
- 18. Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978–987 [DOI] [PubMed] [Google Scholar]
- 19. McKnight GS, Cummings DE, Amieux PS, et al. Cyclic AMP, PKA, and the physiological regulation of adiposity. Recent Prog Horm Res. 1998;53:139–159; discussion 160–131 [PubMed] [Google Scholar]
- 20. Amieux PS, Cummings DE, Motamed K, et al. Compensatory regulation of RIα protein levels in protein kinase A mutant mice. J Biol Chem. 1997;272:3993–3998 [DOI] [PubMed] [Google Scholar]
- 21. Corbin JD, Keely SL, Park CR. The distribution and dissociation of cyclic adenosine 3′:5′-monophosphate-dependent protein kinases in adipose, cardiac, and other tissues. J Biol Chem. 1975;250:218–225 [PubMed] [Google Scholar]
- 22. Brandon EP, Logue SF, Adams MR, et al. Defective motor behavior and neural gene expression in RIIβ-protein kinase A mutant mice. J Neurosci. 1998;18:3639–3649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Planas JV, Cummings DE, Idzerda RL, McKnight GS. Mutation of the RIIβ subunit of protein kinase A differentially affects lipolysis but not gene induction in white adipose tissue. J Biol Chem. 1999;274:36281–36287 [DOI] [PubMed] [Google Scholar]
- 24. Hsiao HP, Kirschner LS, Bourdeau I, et al. Clinical and genetic heterogeneity, overlap with other tumor syndromes, and atypical glucocorticoid hormone secretion in adrenocorticotropin-independent macronodular adrenal hyperplasia compared with other adrenocortical tumors. J Clin Endocrinol Metab. 2009;94:2930–2937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pagnon J, Matzaris M, Stark R, et al. Identification and functional characterization of protein kinase A phosphorylation sites in the major lipolytic protein, adipose triglyceride lipase. Endocrinology. 2012;153:4278–4289 [DOI] [PubMed] [Google Scholar]
- 26. Bertherat J, Horvath A, Groussin L, et al. Mutations in regulatory subunit type 1A of cyclic adenosine 5′-monophosphate-dependent protein kinase (PRKAR1A): phenotype analysis in 353 patients and 80 different genotypes. J Clin Endocrinol Metab. 2009;94:2085–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Powell AC, Stratakis CA, Patronas NJ, et al. Operative management of Cushing syndrome secondary to micronodular adrenal hyperplasia. Surgery. 2008;143:750–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gunther DF, Bourdeau I, Matyakhina L, et al. Cyclical Cushing syndrome presenting in infancy: an early form of primary pigmented nodular adrenocortical disease, or a new entity? J Clin Endocrinol Metab. 2004;89:3173–3182 [DOI] [PubMed] [Google Scholar]
- 29. Umeki S, Hisamoto N, Hara Y. Study on background factors associated with impaired glucose tolerance and/or diabetes mellitus. Acta Endocrinol. 1989;120:729–734 [DOI] [PubMed] [Google Scholar]
- 30. Marchesini G, Brizi M, Bianchi G, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850 [DOI] [PubMed] [Google Scholar]
- 31. Fiatarone JR, Coverdale SA, Batey RG, Farrell GC. Non-alcoholic steatohepatitis: impaired antipyrine metabolism and hypertriglyceridaemia may be clues to its pathogenesis. J Gastroenterol Hepatol. 1991;6:585–590 [DOI] [PubMed] [Google Scholar]
- 32. Svegliati-Baroni G, Saccomanno S, Rychlicki C, et al. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int. 2011;31:1285–1297 [DOI] [PubMed] [Google Scholar]
- 33. Amieux PS, Howe DG, Knickerbocker H, et al. Increased basal cAMP-dependent protein kinase activity inhibits the formation of mesoderm-derived structures in the developing mouse embryo. J Biol Chem. 2002;277:27294–27304 [DOI] [PubMed] [Google Scholar]
- 34. Kirschner LS, Carney JA, Pack SD, et al. Mutations of the gene encoding the protein kinase A type I-α regulatory subunit in patients with the Carney complex. Nat Genet. 2000;26:89–92 [DOI] [PubMed] [Google Scholar]
- 35. Keil MF, Graf J, Gokarn N, Stratakis CA. Anthropometric measures and fasting insulin levels in children before and after cure of Cushing syndrome. Clin Nutr. 2012;31:359–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Griffin KJ, Kirschner LS, Matyakhina L, et al. A mouse model for Carney complex. Endocr Res. 2004;30:903–911 [DOI] [PubMed] [Google Scholar]
- 37. Hussain MA, Stratakis C, Kirschner L. Prkar1a in the regulation of insulin secretion. Horm Metab Res. 2012;44:759–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. London E, Rothenbuhler A, Lodish M, et al. Differences in adiposity in Cushing syndrome caused by PRKAR1A mutations: clues for the role of cyclic AMP signaling in obesity and diagnostic implications. J Clin Endocrinol Metab. 2014;99:E303–E310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Song WJ, Seshadri M, Ashraf U, et al. Snapin mediates incretin action and augments glucose-dependent insulin secretion. Cell Metab. 2011;13:308–319 [DOI] [PMC free article] [PubMed] [Google Scholar]