Abstract
The role of the hypothalamus in female reproductive senescence is unclear. Here we identified novel molecular neuroendocrine changes during the natural progression from regular reproductive cycles to acyclicity in middle-aged female rats, comparable with the perimenopausal progression in women. Expression of 48 neuroendocrine genes was quantified within three hypothalamic regions: the anteroventral periventricular nucleus, the site of steroid positive feedback onto GnRH neurons; the arcuate nucleus (ARC), the site of negative feedback and pulsatile GnRH release; and the median eminence (ME), the site of GnRH secretion. Surprisingly, the majority of changes occurred in the ARC and ME, with few effects in anteroventral periventricular nucleus. The overall pattern was increased mRNA levels with chronological age and decreases with reproductive cycle status in middle-aged rats. Affected genes included transcription factors (Stat5b, Arnt, Ahr), sex steroid hormone receptors (Esr1, Esr2, Pgr, Ar), steroidogenic enzymes (Sts, Hsd17b8), growth factors (Igf1, Tgfa), and neuropeptides (Kiss1, Tac2, Gnrh1). Bionetwork analysis revealed region-specific correlations between genes and hormones. Immunohistochemical analyses of kisspeptin and estrogen receptor-α in the ARC demonstrated age-related decreases in kisspeptin cell numbers as well as kisspeptin-estrogen receptor-α dual-labeled cells. Taken together, these results identify unexpectedly strong roles for the ME and ARC during reproductive decline and highlight fundamental differences between middle-aged rats with regular cycles and all other groups. Our data provide evidence of decreased excitatory stimulation and altered hormone feedback with aging and suggest novel neuroendocrine pathways that warrant future study. Furthermore, these changes may impact other neuroendocrine systems that undergo functional declines with age.
Reproductive aging in females is a complex physiological process involving gradual changes in hypothalamic-pituitary-ovarian (HPO) functions and ultimately culminating in reproductive senescence. Although each level of the HPO axis changes with age, evidence indicates a key involvement of the hypothalamus (1). In rodents, age-related changes to the hypothalamic GnRH neurons include diminished GnRH release, neural activation, and an attenuated preovulatory GnRH/LH surge (2–7). Many of these processes are observed in middle age prior to obvious age-related changes in the pituitary or ovary or a decline in fertility (2). However, hypothalamic senescence cannot be attributable solely to properties of the GnRH neurons because GnRH neuronal activity is determined by coordinated inputs from neurotransmitter systems, glia, and hormone signaling (8–12). In fact, it is likely that hypothalamic senescence is due to age-related changes in this broader network of neurotransmitters, neurotrophic factors, and steroid feedback in the brain.
Rats provide an excellent model for hypothalamic aging. At middle age, female rats progress from regular estrous cycles to irregular cycles and finally become acyclic (persistent estrus). This process is similar to the transitions in women from premenopausal, perimenopausal, and postmenopausal stages (13, 14). Despite these similarities between female rats and humans with reproductive aging, few studies have capitalized upon the natural transition to acyclicity in rodents to better understand hypothalamic changes. More commonly used ovariectomized models are useful in replicating rapid hormone depletion but may miss underlying neuroendocrine factors that initiate and perpetuate the dysregulation of reproductive physiology at middle age.
Here we used middle-aged Sprague Dawley females undergoing natural reproductive senescence to investigate hypothalamic molecular changes and their relationship to physiological aging processes. Work was focused on three regions within the hypothalamus: 1) the anteroventral periventricular nucleus (AVPV), involved in positive hormonal feedback during the GnRH/LH surge (15); 2) the arcuate nucleus (ARC), related to negative feedback and GnRH pulsatile release (15, 16); and 3) the median eminence (ME), in which GnRH neuroterminals release the decapeptide (17, 18). By designing a 48-gene low-density PCR array representing neuropeptides, neurotransmitters and receptors, neurotrophic factors, and steroid hormone receptors and steroidogenic enzymes, we were both able to test specific hypotheses as well as to identify novel candidates involved in reproductive senescence. Based on gene expression results and the importance of neurons in the ARC that coexpress kisspeptin, neurokinin B, and dynorphin [KNDy neurons (16, 19, 20)] we also performed immunohistochemistry on kisspeptin and estrogen receptor (ER)-α-labeled cells in the caudal ARC.
Materials and Methods
Animal care
All animal procedures were conducted in accordance to National Institutes of Health and US Department of Agriculture guidelines and were approved by the Institutional Animal Care and Use Committee at The University of Texas at Austin. Subjects were young (3–5 mo, sexually naïve) and middle-aged (10–14 mo, retired breeders) female Sprague Dawley rats (Harlan). Upon arrival, the rats were pair housed on a 12-hour light, 12-hour dark cycle (lights on at 7:00 am) and received food and water ad libitum. Rats were handled for 5 min/d for 1 week before monitoring estrous cyclicity by daily vaginal lavage of sterile saline for approximately 4 weeks. Animals were categorized as regular, irregular, and acyclic based on the last 2 weeks of their smears. Regular animals showed three consecutive 4- to 5-day cycles, irregular animals showed two consecutive cycles that were 6 days or longer, and acyclic (persistent estrus) animals showed 14 continuous days in estrus. Based on age and cycle status, the rats were assigned to one of the following four categories: young, regularly cycling (YgReg), middle-aged regularly cycling (MaReg), middle-aged irregularly cyling (MaIrreg), and middle-aged persistent estrus (MaPE).
Tissue processing
Rats were weighed and humanely euthanized 1–3 hours prior to the dark period on proestrus in cycling rats (YgReg, MaReg, MaIrreg) or estrus if acyclic (MaPE). One set of rats was perfused for immunohistochemistry experiments (YgReg, n = 10; MaReg, n = 14; MaIrreg, n = 10; MaPE, n = 11), as previously described (21). A second set of females was euthanized by rapid decapitation for gene expression experiments (YgReg, n = 9; MaReg, n = 10; MaIrreg, n = 11; MaPE, n = 11), and micropunches from each region of interest (AVPV, ARC, and ME) were dissected, as previously described (22). Photographs were taken before and after punching to confirm that the regions (AVPV, ARC, and ME) were consistently and accurately dissected. When removing the ME, we tried to avoid inclusion of any ARC tissue. However, we note that we cannot eliminate the possibility that a very small portion of the ventral ARC may have been captured in a subset of ME dissections.
Serum hormone assays
A terminal blood sample was obtained from the trunk in decapitated rats or by cardiac puncture immediately before perfusion in anesthetized rats and allowed to clot, and serum was separated using centrifugation at 2300 × g for 5 minutes and stored at −80°C. Serum levels of LH were measured in duplicate 50-μL samples in the laboratory of Dr Michael J. Woller (University of Wisconsin, Whitewater, Wisconsin) using a double-antibody competitive binding RIA in three assays over a period of 3 years. Intraassay and interassay variability was 24.6% and 4.3%, respectively. Using the Milliplex rat pituitary magnetic bead assay (catalog number RPTMAG-86K; EMD Millipore), serum FSH and LH concentrations were measured simultaneously as single replicates of 25-μL samples in the laboratory of Dr Andrew Wolfe (Johns Hopkins University, Baltimore, Maryland) in one run. The assay detection limits were 96 pg/mL (FSH) and 48 pg/mL (LH). Although the LH RIA had high interassay variability, all four animal groups were evenly distributed among the assays over the years, and both the RIA and Milliplex assays had the same LH pattern across the groups. We chose to present the RIA data here to include the entire sample set.
Concentrations of serum estradiol were detected in duplicate using the estradiol RIA kit, as recommended by the manufacturer (catalog number DSL-4800; Beckman Coulter). Samples were run across four assays, and assay sensitivity was 2.2 pg/mL. The interassay and interplate variability was 6.6% and 4.7%, respectively. Serum progesterone levels were measured in triplicate on four plates using the progesterone competitive enzyme immunoassay (catalog number 582601; Cayman Chemical), according to the manufacturer's protocol. Serum was diluted 1:200. The assay detection limit was 10 pg/mL. Intraassay and interplate variability was 5.7% and 10.9%, respectively.
Real-time PCR assays
RNA was extracted from frozen AVPV, ARC, and ME punches using an in-house double-detergent lysis buffer protocol (23). RNA (200 ng) was converted to single-stranded cDNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems), using the manufacturer's protocol, and stored at −20°C. Samples (n = 6 per group) from the AVPV, ARC, and ME were run on customized rat Taqman low-density array (TLDA) Microfluidic 48-gene real-time PCR cards (Applied Biosystems) with specific gene assays chosen based on a priori hypotheses and publications on their importance in neuroendocrine function and reproductive aging (46 genes of interest and two normalizing genes; Supplemental Table 1).
To confirm the TLDA findings in the ME, we performed an additional experiment with RNA samples from a subset of the above animals, supplemented by an additional three to five animals per group. We used conventional gene-by-gene PCR for 10 of the genes in the ME: Esr1, Esr2, Pgr, Kiss1, Kiss1r, Tgfa, Tgfb1, Grin1, Grin2b, and Gapdh. For Kiss1, Kiss1r, Tgfa, and Tgfb1 assays, Taqman primer and probe sets were used. For Esr1, Esr2, Pgr, Grin1, Grin2b, and Gapdh assays, we used predesigned primer (IDT) and probe (Eurogentec and MWG Biotech) sequences whose optimal concentrations have been previously published (24–26) and that show excellent replication with TLDA cards (24). Samples using conventional gene-by-gene PCR were run in triplicate and any of the triplicates with a value of 1.5 SD above the mean of that animal was removed. Additionally, we confirmed that animal triplicates did not have a coefficient of variation greater than 3%.
In both experiments, real-time PCR was performed using Taqman universal mastermix (Applied Biosystems) on an ABI ViiA7 machine with the following run parameters: 50°C for 2 minutes, 95°C for 10 minutes, 45 cycles of 95°C for 15 seconds, and 60°C for 1 minute. Relative expression of each gene was determined using the comparative cycle threshold method (27). Each sample was normalized to Gapdh expression and then calibrated to the median δ-cycle threshold of the lowest-expressing group within each gene.
Immunofluorescence histochemistry and confocal microscopy
Double-labeled immunofluorescence histochemistry was performed for ERα and kisspeptin. Three sections in a 1:7 series were used from the caudal half of the ARC from each animal, approximately bregma −2.60 through −3.60. All tissues were processed in a single run. First, antigen retrieval with 10 mM citrate buffer (pH 6.0) for 30 minutes at 75°C was performed, followed by incubation for 1 hour in 10% normal goat serum (NGS), 10% normal horse serum (NHS), and 2% BSA. Next, sections were incubated with rabbit antikisspeptin (1:10 000, number 566, a generous gift from Dr Alain Caraty, Université de Tours, Tours, France) and mouse anti-ERα (1:1000, ER6F11; Leica Biosystems) for 72 hours at 4°C in 2% NGS/2% NHS/0.4% BSA. Texas red-conjugated horse antimouse (1:400; Vector Laboratories) and fluorescein-conjugated goat antirabbit (1:400, Vector Laboratories) were used as secondary antibodies in 5% NGS/5% NHS/1% BSA for 2 hours. Finally, 0.1% Sudan black staining was performed (28). Sections were mounted and coverslipped with VectaShield with 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories). The specificity of both the anti-kisspeptin and anti-ERα primary antibodies has been extensively validated in neural tissue (29–32). Specificity was verified by omitting primary antibodies in control samples, and no staining was observed.
Stacks of fluorescent images spaced 2.7 μm apart (for a total of 11 μm) were obtained with a 1.25 NA ×40 oil immersion lens on a confocal laser-scanning microscope (TCS SP2; Leica Microsystems). We sampled within the caudal half of the ARC, chosen based on preliminary results showing a greater distribution of kisspeptin neurons in the caudal vs rostral regions. Sections were scanned sequentially between frames at excitation wavelengths of 350 nm, 488 nm, and 568 nm using line averaging of 4 after passing the guard zone.
Cell counting in the caudal ARC
Cells labeled for DAPI, kisspeptin, and ERα were quantified within the caudal ARC using the unbiased optical dissector method. Using the acquired confocal stacks, cells were counted when they disappeared in the next confocal slice. Due to poor penetration, DAPI-labeled cells were counted within the top 2.7 μm of the confocal stack and estimated for the total 11 μm. Kisspeptin, ERα, and double-labeled cells were quantified throughout the entire 11 μm. Cell numbers were then normalized to the estimated total number of cells (DAPI labeled) and expressed as a percentage of cells within the caudal ARC region. We also examined the proportion of kisspeptin and ERα cells that were colocalized as well as the kisspeptin to ERα cell ratio. Cells were manually counted by an experimenter blind to treatment with a within-counting variability of 8.5%.
Statistical analysis
SPSS (version 18.0) was used to analyze hormone, cell number percentages, and TLDA gene expression data. Effects of chronological age were ascertained through comparisons of young and middle-aged regularly cycling rats (YgReg vs MaReg) and effects of reproductive cycle status through comparisons of middle-aged rats (MaReg, MaIrreg vs MaPE). All end points were analyzed using one-way ANOVA or Kruskal-Wallis tests, except for gene expression data from the ME that were quantified by two different methods (see below). Appropriate post hoc comparisons (Tukey or Mann-Whitney), corrected for multiple comparisons, were used to determine group differences where there was a main effect of cycle status. For gene expression data, outliers were removed using the Grubbs test prior to statistical analysis, and P values were corrected for multiple comparisons using the Benjamini-Hochberg false discovery rate (33), but no genes survived. However, because each gene was chosen a priori based on specific hypotheses, group differences were considered statistically significant at the two-tailed P < .05 level, as described previously (22).
A linear mixed-model approach was used to analyze those genes in the ME that were quantified both by the TLDA array and confirmed using conventional gene-by-gene PCR: Esr1, Esr2, Pgr, Kiss1, Kiss1r, Tgfa, Tgfb1, Grin1, and Grin2b. The linear mixed-model approach is preferred over general linear models for data with missing values, correlated errors, and nonconstant variability (34). Data from both runs were combined and analyzed in one model with the diagonal repeated covariance type using fixed factors of chronological age or reproductive cycle status. Animal identification was included as a random factor and PCR run as a repeated variable.
To examine relationships among gene expression, circulating hormone concentrations, and body weight within each brain region, a correlation network was created based on a bootstrapping technique that our laboratory has used previously (22). Significant Pearson coefficients after the false discovery rate were uploaded into Cytoscape version 2.8.3 (35) to create a correlation network.
Results
Serum hormone levels
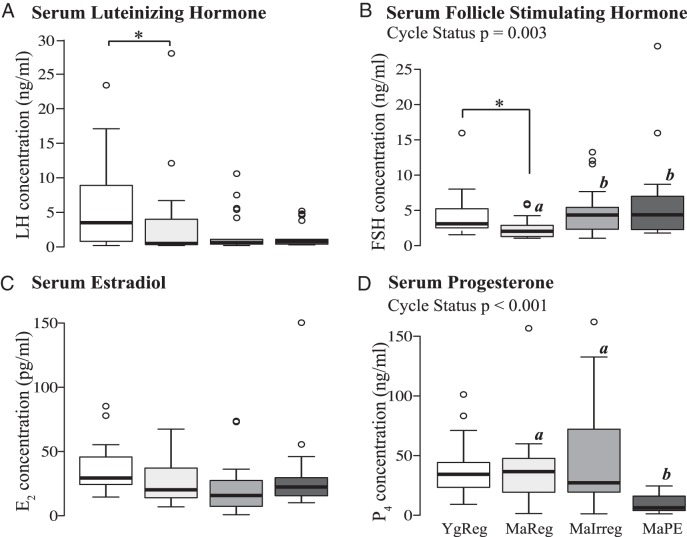
Both serum LH[(H (1) = 3.96, P = .047; Figure 1A] and FSH [H (1) = 6.99, P = .008; Figure 1B] concentrations were significantly lower on the afternoon of proestrus in the middle-aged than the young regularly cycling rats. In the middle-aged rats, a significant effect of reproductive cycle status was found for FSH [H (2) = 11.771, P = .003] and progesterone serum levels [H (2) = 20.54, P < .001; Figure 1, B and D, respectively]. Specifically, FSH levels increased as middle-aged females progressed to reproductive senescence, with both MaIrreg (P = .011) and MaPE (P = .009) females showing significantly greater levels than the MaReg females. On the other hand, progesterone concentrations were decreased in the MaPE females compared with the MaReg and the MaIrreg females (P < .001 for each). No effects were found for circulating estradiol concentrations (Figure 1C).
Figure 1.
Serum luteinizing hormone (A), follicle stimulating hormone (B), estradiol (C), and progesterone (D) levels during reproductive aging. Circulating serum hormone levels were measured in ovarian-intact Sprague Dawley females of the four experimental groups. Boxes show the 25th to 75th percentiles with the median value depicted as a black bar. Whiskers represent 1.5 times the interquartile range with extreme values shown as open circles. MaIrreg, middle-aged irregularly cycling; MaPE, middle-aged persistent estrus; MaReg, middle-aged regularly cycling; YgReg, young regularly cycling. *, P < .05 between YgReg and MaReg. Different letters denote significant (P < .05) differences between middle-aged groups.
Gene expression in the AVPV, ARC, and ME
Gene expression results are summarized for the AVPV, ARC, and ME in Supplemental Table 1.
Anteroventral periventricular nucleus
There were no gene differences with age between the YgReg and MaReg females. Only one gene, the transcription factor aryl hydrocarbon receptor nuclear translocator (Arnt), showed a significant effect of cycle status [F (2, 14) = 4.0, P = .041]. The Tukey pairwise comparisons revealed trends for increased Arnt expression in MaReg vs MaIrreg and MaPE (P = .069 for each).
Arcuate nucleus
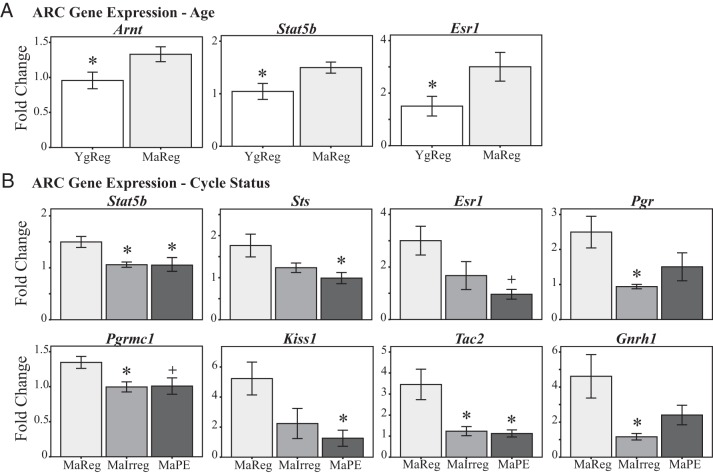
Three genes in the ARC showed significant increases in expression with age (MaReg vs YgReg; Figure 2A): Arnt [F (1, 10) = 5.5, P = .041], ERα [Esr1; F (1, 10) = 5.1, P = .047], and signal transducer and activator of transcription 5b [Stat5b; F (1, 10) = 6.0, P = .034].
Figure 2.
ARC gene expression during reproductive aging. Expression of genes with significant effects of chronological age (A) or reproductive cycle status (B) is shown. MaIrreg, middle-aged irregularly cycling; MaPE, middle-aged persistent estrus; MaReg, middle-aged regularly cycling; YgReg, young regularly cycling. +, P < .1 (trend) and *, P < .05 vs MaReg.
Furthermore, eight genes demonstrated changes in expression with cycle status among the middle-aged females (Figure 2B): Stat5b (F (2, 15) = 6.3, P = .010), Esr1 (H (2) = 6.2, P = .045), progesterone receptor (Pgr; H (2) = 7.4, P = .025), membrane progesterone receptor (Pgrmc1; F (2, 15) = 4.5, P = .029), steroid sulfatase (Sts; F (2, 15) = 4.5, P = .029), GnRH 1 (Gnrh1; H (2) = 7.3, P = .025), kisspeptin 1 (Kiss1; H (2) = 6.8, P = .034), and neurokinin B (Tac2; H (2) = 8.6, P = .014). In all cases, post hoc analysis showed that MaReg females had higher expression compared to MaIrreg and/or MaPE females (Figure 2B).
Median eminence
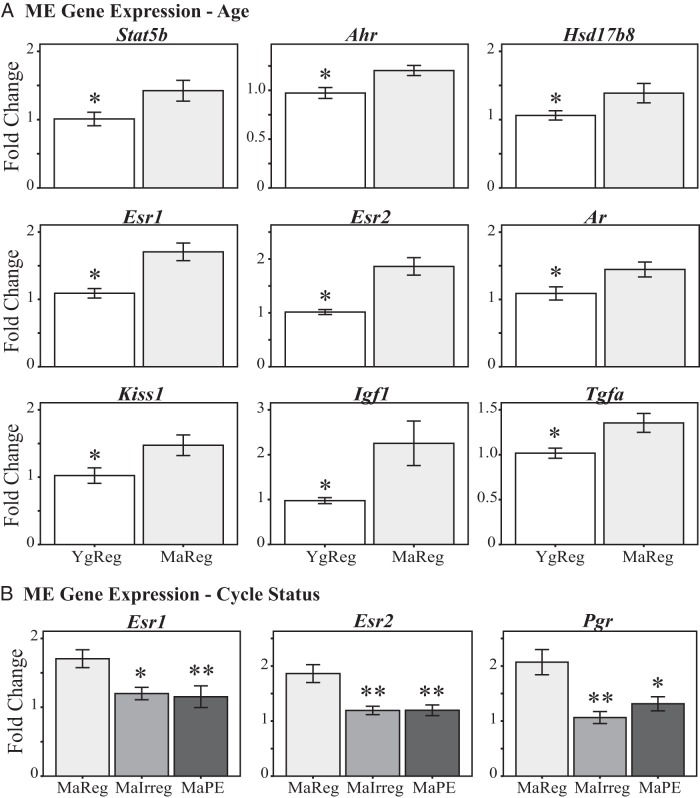
Within the ME, age-related increases (Figure 3A) were seen for the following genes: aryl hydrocarbon receptor [Ahr; F (1, 8) = 9.2, P = .016], Stat5b [F (1, 10) = 5.2, P = .045], androgen receptor [Ar; F (1, 10) = 5.8, P = .037], Esr1 (P = .005); ERβ (Esr2; P = .007), hydroxysteroid (17β) dehydrogenase 8 [Hsd17b8; H (1) = 4.3, P = .037], Kiss1 (P = .029), IGF-I [Igf1; H (1) = 4.0, P = .045], and TGFα (Tgfa; P = .020).
Figure 3.
ME gene expression during reproductive aging. Expression of genes with significant effects of chronological age (A) or reproductive cycle status (B) is shown. MaIrreg, middle-aged irregularly cycling; MaPE, middle-aged persistent estrus; MaReg, middle-aged regularly cycling; YgReg, young regularly cycling. *, P < .05 and **, P < .01 vs MaReg.
Three of the sex steroid hormone receptors were significantly affected by cycle status (Figure 3B): Esr1 (P = .011), Esr2 (P = .002), and Pgr (P = .007). All of these genes were higher in the MaReg than MaIrreg or MaPE females.
Bionetwork analysis
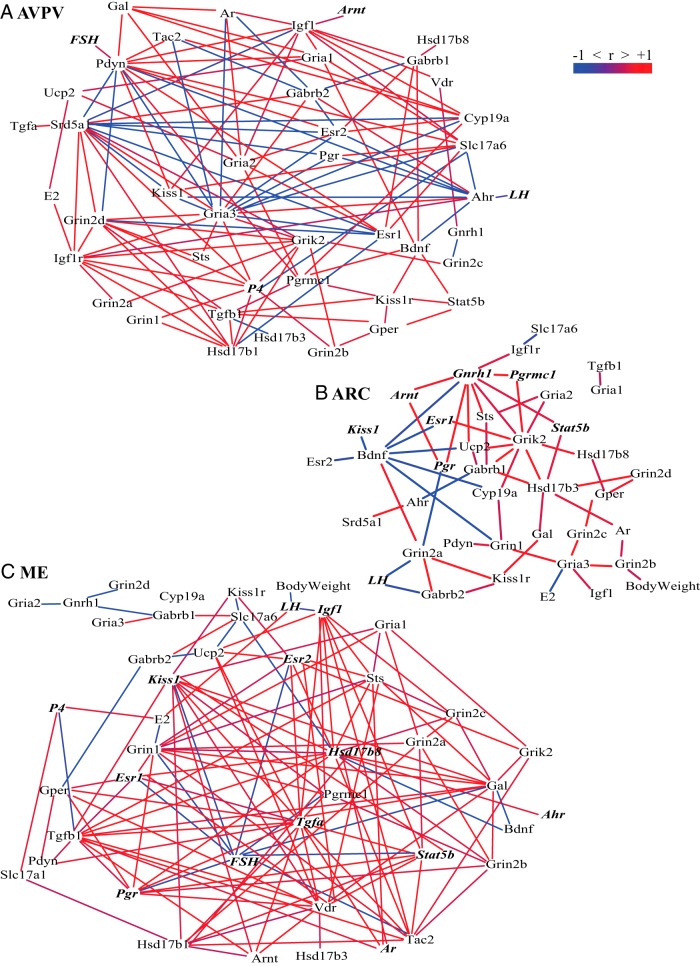
To determine the relationship between gene expression and physiological end points, data were collapsed across all females into one network for each brain region (Figure 4). Networks were subtracted from each other to reveal relationships unique to each region. Despite showing few overall changes in expression of individual genes, we found many significant interactions within the AVPV. Hubs of positive correlations were found for serum progesterone levels, sex steroid hormone enzyme expression (Hsd17b1, Cyp19a1), insulin-like GH signaling (Igf1, Igf1r), and the vesicular glutamate transporter 2 (Slc17a6). In the ARC, fewer correlations were detected. Positive hubs were found for Gnrh1, Grik2, and Hsd17b3 expression. Additionally, Bdnf served as a major negative hub. By contrast to the ARC, the ME displayed a large number of positive correlations in its network. In particular, gene expression of neuropeptides (Kiss1, Tac2), steroidogenic enzymes (Hsd17b8, Hsd17b1), growth factors (Tgfa, Igf1), and the progesterone receptor (Pgr) were positive hubs. FSH serum levels demonstrated a large number of negative correlations.
Figure 4.
Bionetwork analysis in the AVPV, ARC, and ME. Correlation networks of genes, serum hormones, and body weight of all females in the AVPV (A), the ARC (B), and the ME (C) are shown. Positive Pearson correlations are shown in red and negative in blue. Genes and hormones that displayed a significant main effect of age or cycle status are indicated by bold, italicized font (P < .05).
Percentage of kisspeptin, ERα, and colabeled cells in the caudal ARC
Immunofluorescence histochemistry was performed to follow up on gene expression results for Esr1 and Kiss1 in the caudal ARC (Figure 5A). The percentage of cells that colabeled with kisspeptin and ERα was significantly lower in MaReg females compared with YgReg [F (1, 17) = 4.49, P = .049; Figure 5B]. This effect may be driven by changes in kisspeptin neurons because further analysis revealed the proportion of kisspeptin cells that colabeled ERα also decreased with age [from 47% to 30%; F (1, 17) = 6.87, P = .018; Figure 5C], but there were no differences in the proportion of ERα cells colabeling kisspeptin (∼9%). Although the percentage of kisspeptin cells that were colocalized may be underestimates based on the literature (30, 36), we believe the results are biologically relevant because cell counting was performed using unbiased methods (37), and the same pattern was found, regardless of whether the overall density of colocalized cells, the proportion of total DAPI cells, or the proportion of kisspeptin cells were examined. Differences between our study and others may have arisen due to the use of different species and primary antibodies as well as the examination of mRNA (via in situ hybridization) vs protein expression. No effects of age were found for the total percentage of kisspeptin or ERα cells.
Figure 5.
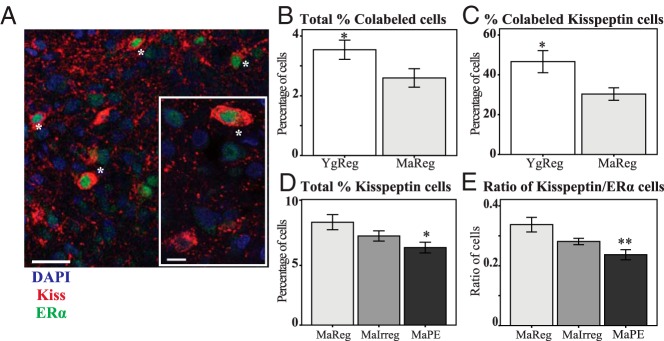
Immunofluorescence histochemistry in the ARC during reproductive aging. The percentage of kisspeptin, ERα, and colabeled cells were quantified in the caudal ARC of middle-aged female rats during the transition to acyclicity. A, Representative micrograph from one confocal slice showing cells colabeled for kisspeptin (red) and ERα (green), marked with an asterisk, with a DAPI counterstain (blue). A higher resolution image is shown in the inset of panel A. Single-labeled cells can also be seen for both proteins. The total percentage of colabeled cells (B) and the percentage of kisspeptin cells that colabeled ERα (C) decreased at middle age in regularly cycling females. The percentage of kisspeptin cells (D) and the ratio of kisspeptin to ERα cells (E) decreased with reproductive cycle status. Kiss, kisspeptin. *, P < .05 and **, P < .01 vs MaReg. Scale bar, 30 μm (20 μm for inset).
Within middle-aged rats, we found a significant effect of cycle status in the percentage of kisspeptin neurons [F (2, 27) = 4.45, P = .021; Figure 5D]. Pairwise comparisons revealed that MaPE rats had significantly fewer kisspeptin cells than MaReg rats (P = .016). The percentage of ERα and colabeled cells did not change with cycle status at middle age; however, the ratio of kisspeptin to ERα cells significantly decreased [F (2, 27) = 7.69, P = .002; Figure 5E]. Again, MaPE rats expressed fewer kisspeptin to ERα cells compared with MaReg (P = .002). There was no difference in the number of DAPI cells or DAPI cell density with age or cycle status.
Discussion
Using the ovarian-intact rat model of menopause, this study revealed novel alterations in gene expression within the ARC and ME during the natural transition to reproductive senescence. Furthermore, we demonstrated age and cycle status-related changes in two of the proteins expressed in KNDy neurons that are proposed to be involved in age-related changes in steroid feedback (19, 38): kisspeptin- and ERα-immunoreactive neurons of the caudal ARC. Finally, gene expression and physiological data from all females were integrated into correlation networks within each brain region to provide a holistic view of the relationships among the variables. The results identified region-specific, and sometimes unexpected, key pathways and players in reproductive aging that warrant future study.
Although the findings of this study provide great insight into possible mechanisms of reproductive aging, there are important caveats to the data. First, gene transcript levels do not necessarily correlate with protein levels, their posttranslational regulation, or the secretion of peptides. Second, the descriptive nature of this study precludes firm mechanistic interpretations. Nevertheless, this novel data set provides potential targets for future work involving up- or down-regulation of pathways identified here in age- and cycle status-dependent processed involved in the hypothalamic control of reproductive senescence.
Circulating hormone levels change during natural reproductive aging
Our measures of peripheral hormones confirm and extend past work through the inclusion of animals of different chronological ages and at different phases of reproductive aging. Estradiol concentrations did not vary, a result that is not surprising because ovarian follicles are maintained in the rat ovary until much later in life. Serum progesterone levels were substantially lower in the MaPE than other groups, as found previously (14, 39–41), probably due to a lack of ovulation and corpus luteum production. In addition, our middle-aged cycling females exhibited decreased LH and FSH levels, consistent with a delayed and attenuated gonadotropin surge reported in the aging rodent literature (1, 3, 4, 8–12). This may be translationally relevant: only half of perimenopausal women showed LH surges with estrogen peaks (42), suggesting a loss of responsiveness to steroid feedback in women under observational conditions. However, postmenopausal women who were supplied exogenous gonadal hormones did show LH surges (43). Thus, it is unclear the extent to which positive feedback in women is altered with reproductive aging.
Although LH levels remained low once rats entered middle age, FSH concentrations steadily rose as middle-aged rats transitioned to acyclicity, as reported previously (14, 44). In primates, including humans, FSH levels also increase with reproductive aging (13, 14, 45), but this is believed to be driven by a decline in peripheral inhibin levels (15, 46), which does not occur in the rodent (44). However, blunted inhibin responses to ovarian challenges have been shown in aging female rats (44, 47). Altered GnRH pulse frequency and amplitude parameters may explain the differential patterns of LH and FSH levels in our model (15, 16, 48). Finally, our bionetwork analysis revealed interesting negative correlations between serum FSH levels and gene expression in the ME, including with Esr1, Esr2, and Pgr, suggesting a potential link between FSH secretion and sex steroid hormone receptor expression in the ME.
Changes in the hypothalamic network governing GnRH during the transition to acyclicity
We selected the AVPV, ARC, and ME for our gene expression analyses because of their key role in the network driving HPO and other neuroendocrine functions. These regions encompass the site of GnRH cell bodies, axons, and terminals, respectively, as well as the AVPV and ARC populations of kisspeptin neurons that regulate GnRH function and mediate positive and negative feedback effects of steroids. Our expectation was that most changes would be identified in the AVPV based on its key role in mediating steroid inputs involved in the positive feedback of steroids to GnRH neurons, a function that declines with age (36, 49). Surprisingly, only one gene (Arnt) was identified by the 48-gene PCR array. Although we anticipated changes in Kiss1 expression (15), our results are consistent with two recent reports in intact aging female rats showing no change (22, 50).
Despite our finding few individual gene expression changes with reproductive aging in the AVPV, bionetwork analysis identified several hubs, particularly serum progesterone, steroidogenic enzymes, and IGF-I signaling. Progesterone is somewhat understudied in this context, but along with estradiol, it plays an important role in the disruption of the LH surge with aging (51). This aspect of hormonal regulation may be especially affected in the MaPE group, whose serum progesterone levels were significantly decreased. In addition, the finding that steroidogenic enzymes (eg, Srd5a, Hsd17b1, and Cyp19a1) were positively correlated with a large number of genes within the AVPV highlights the possible role of local hormone synthesis and metabolism in the regulation of reproductive function (52, 53).
The most surprising finding of this study was the large number of genes affected in the ME, the expression of which had not been previously reported. The ME is the site of GnRH and other hypothalamic-releasing hormone release and is not normally considered a neurobiological locus of gene regulation. Because neural cell bodies are sparse to absent in the ME, expression of genes in this region may occur in glia or may represent RNA transport along the axon for local translation in the terminals, as has been found for some neurosecretory cells [eg, oxytocin and vasopressin (54)]. For example, Kiss1 mRNA was detectable in the ME and increased with age; and Tac2 as well as Kiss1 was positively correlated with many genes in the ME bionetwork. Kisspeptin neurons project axons to the ME, especially the internal zone (55, 56), consistent with this possibility.
Steroid hormone gene expression in the ME was also affected in our model. During reproductive aging, the three genes that decreased expression in this region were Esr1, Esr2, and Pgr. These findings suggest the intriguing possibility that the ME may be a direct site of steroid hormone feedback and that gene transcription may be a heretofore unknown mechanism for ME regulation. There are also alternative explanations for our finding of Kiss1 and some other genes in the ME. It is possible that we have a small amount of ARC tissue included in the ME dissection. Photomicrographs were taken of all our brain dissections before and after the removal of the ARC; inspection confirmed that the dissections were extremely consistent and that we took a very conservative ME cut to avoid inclusion of any ARC. This, along with our findings of many gene expression pattern differences between the ARC and ME (see Figures 2 and 3) and the fact that some genes were detected in one region but not the other, suggests that any contamination of the ME dissections by ARC tissues would have been extremely small. Thus, we favor the possibility that Kiss1 mRNA is indeed expressed in the ME.
Gene expression of the trophic factors Tgfa and Igf1 increased with age in the ME, and these genes also served as positive correlation hubs in the ME bionetwork. Growth factor signaling via glia is an important mechanism in the regulation of neuropeptide secretion in the ME and is modulated by hormone signaling (57). Glia also undergo structural organizational changes with age in this region (58, 59), which may further disrupt neuropeptide release into the portal vasculature.
Results in the ARC were more in line with our expectations because three genes increased with age and eight genes (two in common with the former three) decreased in middle-aged rats undergoing reproductive senescence. The ARC mediates negative hormone feedback and pulsatile GnRH release via KNDy neurons, many of which are ERα positive (14, 16, 20, 39–41, 60). Here Kiss1 and Tac2 decreased with reproductive cycle status in the ARC, in parallel with decreased Gnrh1 and Esr1. Their decline supports our hypothesis that neuroendocrine changes contribute to decreased GnRH drive, possibly by modulating pulsatile GnRH release or the GnRH/LH surge (61). An alternative explanation is that lower expression of Kiss1, Tac2, and Gnrh1 expression in MaPE females may be attributed to their persistent estrous status. Unlike the other groups, which were euthanized on proestrus, the MaPE group's serum estradiol levels do not undergo cyclic change but remain constantly high. Future studies should include females at each stage in the estrous cycle to better ascertain how changing hormone and gene expression levels throughout the estrous cycle vary with reproductive aging and to include comparison estrous groups in the cycling females.
Interestingly, Tac2 but not Kiss1 levels were significantly reduced in MaIrreg vs MaReg females. Neurokinin B in the ARC is proposed to aid the coordination of pulsatile GnRH release (62), and a dysregulation of this peptide may contribute to the irregularity of ovulation in these females. Although GnRH cell bodies are mainly located in the preoptic area of the rat (63), we previously not only detected Gnrh1 in the ARC but also found it to be a major hub of positive correlations in the region (22), which was replicated in the bionetwork analysis of this study. Our measures may reflect a specific pool of GnRH that is locally translated in the axons. In fact, a decrease in Gnrh1 in the ARC may reflect diminished transport of GnRH to the terminals, consistent with attenuated GnRH release with aging (64).
Of the gene expression targets identified, steroid hormone receptors (Esr1, Esr2, Pgr, Pgrmc1, Ar) and steroidogenic enzymes (eg, Srd5a, Hsd17b1, Hsd17b8, Cyp19a1) were commonly identified both through individual gene profiling as well as through bionetwork analyses. These overall results of steroid receptor genes underscore steroid hormone responsiveness changes with age. The steroidogenic enzyme changes suggest the possible role of local hormone synthesis and metabolism in the regulation of reproductive function (52, 53). In addition, it is important to note that those genes that increased with age, and/or decreased with senescence, are involved in the stimulatory control of GnRH neurons (65). Although an increase in the inhibitory inputs to the GnRH neurons with aging has been proposed (66) and was expected here, we did not find any such genes.
The transcription factors Stat5b and Ahr/Arnt were identified as common targets, with Ahr and/or Arnt expression altered in all three regions. The Ahr system plays a physiological role in estrous cyclicity, fertility, and reproductive senescence (67). It is also important in mediating responses to exogenous toxins, such as dioxins and polychlorinated biphenyls, and modulates inflammation, immune, and metabolic functions (68), and thus may impact many different cellular pathways with reproductive aging. Additionally, knockout studies in mice demonstrate the importance of Stat5a/b expression for reproductive function (69), and Stat5b mediates the effects of prolactin in the hypothalamus (70). Although beyond the scope of this study, it would be beneficial to examine serum prolactin levels, as well as other pituitary hormones, in the context of this model.
Based on the gene expression results in the ARC, we focused the immunohistochemical work on kisspeptin and ERα colabeling. We replicated the finding that total kisspeptin-positive cell numbers do not change with age in regularly cycling females (42, 49). However, we discovered a decrease in the percentage of kisspeptin neurons that coexpressed ERα, consistent with a reduced capacity to respond to estradiol signaling on proestrus at middle age. This result raises the intriguing possibility that the ARC plays an underappreciated role in mediating estradiol-positive feedback on proestrus in rodents, as suggested by studies in sheep (14, 61), and that this feedback is disrupted with age. In addition, our data show that the percentage of kisspeptin cells decreased at middle age as females transitioned to acyclicity, mirroring the decline in Kiss1 gene expression. Projections from these neurons in rodents innervate a large number of hypothalamic and limbic regions, reaching GnRH cell bodies in the preoptic area (71, 72) and GnRH terminals (55). Thus, age-related changes in kisspeptin regulation of GnRH activity within the ARC may be central to GnRH dysregulation, as well as other neuroendocrine systems, with reproductive age.
Hypothalamic mechanisms of natural reproductive aging
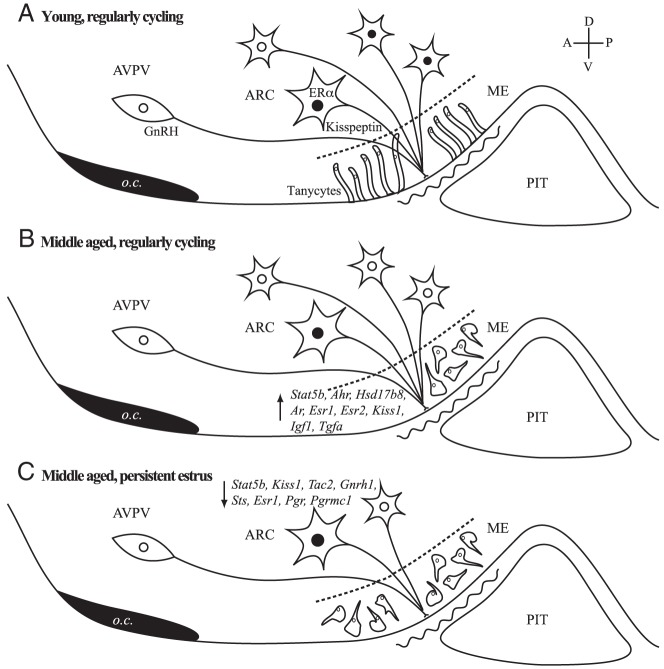
In the ARC and ME, all genes affected by aging were up-regulated in MaReg compared with YgReg, and all genes altered by cycle status were decreased in MaIrreg and/or MaPE compared with MaReg rats, with cycle status accounting for most effects in the ARC and chronological age more important in the ME. Increases in gene expression within the ME of MaReg vs YgReg females may reflect compensatory changes early in middle age to facilitate GnRH release at the site of the terminals, despite decreased GnRH drive in the anterior hypothalamus (66, 73). These compensatory mechanisms may then be lost, or cease to be effective, as middle-aged females progress into reproductive senescence. During this transition at middle age, decreases in expression dominate within the ARC and may underlie GnRH dysregulation, leading to acyclicity. These results are summarized in Figure 6, illustrating our working model for novel pathways that may underlie reproductive aging.
Figure 6.
Proposed model of neuroendocrine mechanisms of reproductive aging. As regularly cycling females age from young (A) to middle aged (B), expression of many genes are increased in the ARC and ME. This up-regulation (indicated by up arrow in panel B) is proposed to compensate for a diminishing drive to GnRH neurons with age. Additionally, the percentage of kisspeptin cells in the ARC that express ERα is decreased. During aging, there are also structural changes to tanycytes in the ME, shown in young rats with a linear organization, and becoming progressively disorganized in middle-age, possibly reflecting changes in access of hypothalamic nerve terminals to the portal capillaries (58, 59). As middle-aged females transition from regular cycles (B) through acyclicity (C), they show decreased kisspeptin cell number together with decreases in neuroendocrine gene expression (indicated by down arrow in panel C). A, anterior; D, dorsal; o.c., optic chiasm; P, posterior; PIT, pituitary; V, ventral.
It is important to note that although our current work enabled us to test hypotheses about the HPO axis, the regions and genes studied here are involved in many other neurobiological processes. Thus, the gene expression and protein changes described here may have broader impacts and even contribute to understanding changes that occur in other nonreproductive neuroendocrine systems in aging animals.
Acknowledgments
Dr Alain Caraty made the kind gift of the kisspeptin antibody. Dr A. M. Parlow (National Hormone and Peptide Program, National Institute of Diabetes and Digestive and Kidney Diseases) provided reagents for the LH RIA. We thank Dr Deena Walker and Christine Dinh for help with the tissue processing, Dr. Weiling Yin for the confocal and cell counting advice, Guillermo Olmedo for help with the cell counting, and Dean Kirson and Dr M. Shel Swenson for the matlab and python scripts for the network analysis, respectively.
This work was supported by National Institutes of Health Grants AG16765 and AG028051 (to A.C.G.) and Grant P60DK079637 from the Baltimore Diabetes Research and Training Center (to A.W.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ARC
- arcuate nucleus
- AVPV
- anteroventral periventricular nucleus
- DAPI
- 4′,6-diamidino-2-phenylindole
- ER
- estrogen receptor
- HPO
- hypothalamic-pituitary-ovarian
- KNDy
- kisspeptin, neurokinin B, and dynorphin
- ME
- median eminence
- NGS
- normal goat serum
- NHS
- normal horse serum
- TLDA
- Taqman low-density array.
References
- 1. Kermath BA, Gore AC. Neuroendocrine control of the transition to reproductive senescence: lessons learned from the female rodent model. Neuroendocrinology. 2012;96:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Scarbrough K, Wise PM. Age-related changes in pulsatile luteinizing hormone release precede the transition to estrous acyclicity and depend upon estrous cycle history. Endocrinology. 1990;126:884–890 [DOI] [PubMed] [Google Scholar]
- 3. Matt DW, Gilson MP, Sales TE, et al. Characterization of attenuated proestrous luteinizing hormone surges in middle-aged rats by deconvolution analysis. Biol Reprod. 1998;59:1477–1482 [DOI] [PubMed] [Google Scholar]
- 4. Zuo Z, Mahesh VB, Zamorano PL, Brann DW. Decreased gonadotropin-releasing hormone neurosecretory response to glutamate agonists in middle-aged female rats on proestrus afternoon: a possible role in reproductive aging? Endocrinology. 1996;137:2334–2338 [DOI] [PubMed] [Google Scholar]
- 5. Gore AC, Oung T, Yung S, Flagg RA, Woller MJ. Neuroendocrine mechanisms for reproductive senescence in the female rat: gonadotropin-releasing hormone neurons. Endocrine. 2000;13:315–323 [DOI] [PubMed] [Google Scholar]
- 6. Rubin BS, Lee CE, King JC. A reduced proportion of luteinizing hormone (LH)-releasing hormone neurons express Fos protein during the preovulatory or steroid-induced LH surge in middle-aged rats. Biol Reprod. 1994;51:1264–1272 [DOI] [PubMed] [Google Scholar]
- 7. Le W, Wise P, Murphy A, Coolen L, Hoffman G. Parallel declines in Fos activation of the medial anteroventral periventricular nucleus and LHRH neurons in middle-aged rats. Endocrinology. 2001;142:4976–4982 [DOI] [PubMed] [Google Scholar]
- 8. Brann D, Mahesh V. Excitatory amino acids: function and significance in reproduction and neuroendocrine regulation. Front Neuroendocrinol. 1994;15:3–49 [DOI] [PubMed] [Google Scholar]
- 9. Neal-Perry G, Lebesgue D, Lederman M, Shu J, Zeevalk GD, Etgen AM. The excitatory peptide kisspeptin restores the luteinizing hormone surge and modulates amino acid neurotransmission in the medial preoptic area of middle-aged rats. Endocrinology. 2009;150:3699–3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Daftary SS, Gore AC. IGF-1 in the brain as a regulator of reproductive neuroendocrine function. Exp Biol Med (Maywood). 2005;230:292–306 [DOI] [PubMed] [Google Scholar]
- 11. Sahu A. Alteration in hypothalamic neuropeptide Y (NPY) secretion may underlie female reproductive ageing: induction of steroid-induced luteinising hormone surge by NPY in ovariectomised aged rats. J Neuroendocrinol. 2006;18:584–593 [DOI] [PubMed] [Google Scholar]
- 12. Prevot V. Glial-neuronal-endothelial interactions are involved in the control of GnRH secretion. J Neuroendocrinol. 2002;14:247–255 [DOI] [PubMed] [Google Scholar]
- 13. LeFevre J, McClintock MK. Reproductive senescence in female rats: a longitudinal study of individual differences in estrous cycles and behavior. Biol Reprod. 2004;38:780–789 [DOI] [PubMed] [Google Scholar]
- 14. Lu KH, Hopper BR, Vargo TM, Yen SS. Chronological changes in sex steroid, gonadotropin and prolactin secretions in aging female rats displaying different reproductive states. Biol Reprod. 1979;21:193–203 [DOI] [PubMed] [Google Scholar]
- 15. Maeda K-I, Adachi S, Inoue K, Ohkura S, Tsukamura H. Metastin/kisspeptin and control of estrous cycle in rats. Rev Endocr Metab Disord. 2007;8:21–29 [DOI] [PubMed] [Google Scholar]
- 16. Goodman RL, Hileman SM, Nestor CC, et al. Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology. 2013;154:4259–4269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. d'Anglemont de Tassigny X, Fagg L, Carlton M, Colledge W. Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology. 2008;149:3926–3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kawakami S, Ichikawa M, Murahashi K, Hirunagi K, Tsukamura H, Maeda K. Excitatory amino acids act on the median eminence nerve terminals to induce gonadotropin-releasing hormone release in female rats. Gen Comp Endocrinol. 1998;112:372–382 [DOI] [PubMed] [Google Scholar]
- 19. Rance NE, Dacks PA, Mittelman-Smith MA, Romanovsky AA, Krajewski-Hall SJ. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34:211–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151:3479–3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kermath BA, Riha PD, Sajjad A, Gore AC. Effects of chronic NMDA-NR2b inhibition in the median eminence of the reproductive senescent female rat. J Neuroendocrinol. 2013;25:887–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Walker DM, Kermath BA, Woller MJ, Gore AC. Disruption of reproductive aging in female and male rats by gestational exposure to estrogenic endocrine disruptors. Endocrinology. 2013;154:2129–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jakubowski M, Blum M, Roberts JL. Postnatal development of gonadotropin-releasing hormone and cyclophilin gene expression in the female and male rat brain. Endocrinology. 1991;128:2702–2708 [DOI] [PubMed] [Google Scholar]
- 24. Walker D, Juenger T, Gore A. Developmental profiles of neuroendocrine gene expression in the preoptic area of male rats. Endocrinology. 2009;150:2308–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Durrer S, Maerkel K, Schlumpf M, Lichtensteiger W. Estrogen target gene regulation and coactivator expression in rat uterus after developmental exposure to the ultraviolet filter 4-methylbenzylidene camphor. Endocrinology. 2005;146:2130–2139 [DOI] [PubMed] [Google Scholar]
- 26. Medhurst AD, Harrison DC, Read SJ, Campbell CA, Robbins MJ, Pangalos MN. The use of TaqMan RT-PCR assays for semiquantitative analysis of gene expression in CNS tissues and disease models. J Neurosci Methods. 2000;98:9–20 [DOI] [PubMed] [Google Scholar]
- 27. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schnell SA, Staines WA, Wessendorf MW. Reduction of lipofuscin-like autofluorescence in fluorescently labeled tissue. J Histochem Cytochem. 1999;47:719–730 [DOI] [PubMed] [Google Scholar]
- 29. Clarkson J, d'Anglemont de Tassigny X, Colledge WH, Caraty A, Herbison AE. Distribution of kisspeptin neurones in the adult female mouse brain. J Neuroendocrinol. 2009;21:673–682 [DOI] [PubMed] [Google Scholar]
- 30. Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor α. Neurosci Lett. 2006;401:225–230 [DOI] [PubMed] [Google Scholar]
- 31. Pérez SE, Chen EY, Mufson EJ. Distribution of estrogen receptor α and β immunoreactive profiles in the postnatal rat brain. Dev Brain Res. 2003;145:117–139 [DOI] [PubMed] [Google Scholar]
- 32. Blurton-Jones MM, Roberts JA, Tuszynski MH. Estrogen receptor immunoreactivity in the adult primate brain: neuronal distribution and association with p75, trkA, and choline acetyltransferase. J Comp Neurol. 1999;405:529–542 [PubMed] [Google Scholar]
- 33. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc B Met. 1995;57:289–300 [Google Scholar]
- 34. Breslow NE, Clayton DG. Approximate inference in generalized linear mixed models. J Am Stat Assoc. 1993;88:9–25 [Google Scholar]
- 35. Smoot ME, Ono K, Ruscheinski J, Wang P-L, Ideker T. Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics. 2011;27:431–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26:6687–6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. West MJ. Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends Neurosci. 1999;22:51–61 [DOI] [PubMed] [Google Scholar]
- 38. Eghlidi D, Haley G, Noriega N, Kohama S, Urbanski H. Influence of age and 17β-estradiol on kisspeptin, neurokinin B, and prodynorphin gene expression in the arcuate-median eminence of female rhesus macaques. Endocrinology. 2010;151:3783–3794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wise PM, Ratner A. Effect of ovariectomy on plasma LH, FSH, estradiol, and progesterone and medial basal hypothalamic LHRH concentrations old and young rats. Neuroendocrinology. 1980;30:15–19 [DOI] [PubMed] [Google Scholar]
- 40. Steger RW, Huang HH, Meites J. Relation of aging to hypothalamic LHRH content and serum gonadal steroids in female rats. Proc Soc Exp Biol Med. 1979;161:251–254 [DOI] [PubMed] [Google Scholar]
- 41. Wise PM. Alterations in the proestrous pattern of median eminence LHRH, serum LH, FSH, estradiol and progesterone concentrations in middle-aged rats. Life Sci. 1982;31:165–173 [DOI] [PubMed] [Google Scholar]
- 42. Weiss G, Skurnick JH, Goldsmith LT, Santoro NF, Park SJ. Menopause and hypothalamic-pituitary sensitivity to estrogen. JAMA. 2004;292:2991–2996 [DOI] [PubMed] [Google Scholar]
- 43. Shaw ND, Srouji SS, Histed SN, Hall JE. Differential effects of aging on estrogen negative and positive feedback. Am J Physiol Endocrinol Metab. 2011;301:E351–E355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Matt DW, Dahl KD, Sarkissian A, Sayles TE. Apparent absence of negative feedback in middle-aged persistent-estrous rats following luteinizing hormone-releasing hormone agonist treatment: relation to plasma inhibin and 17β-estradiol. Biol Reprod. 1993;48:333–339 [DOI] [PubMed] [Google Scholar]
- 45. Gore AC, Windsor-Engnell BM, Terasawa E. Menopausal increases in pulsatile gonadotropin-releasing hormone release in a nonhuman primate (Macaca mulatta). Endocrinology. 2004;145:4653–4659 [DOI] [PubMed] [Google Scholar]
- 46. Reame NE, Wyman TL, Phillips DJ, de Kretser DM, Padmanabhan V. Net increase in stimulatory input resulting from a decrease in inhibin B and an increase in activin A may contribute in part to the rise in follicular phase follicle-stimulating hormone of aging cycling women. J Clin Endocrinol Metab. 1998;83:3302–3307 [DOI] [PubMed] [Google Scholar]
- 47. Yeh J, Kim B. Increasing blunting of inhibin responses todynamic ovarian challenge is associated with reproductive aging in the rat. Reprod Sci. 2007;14:10–19 [DOI] [PubMed] [Google Scholar]
- 48. Thompson IR, Kaiser UB. GnRH pulse frequency-dependent differential regulation of LH and FSH gene expression. Mol Cell Endocrinol. 2013;385:28–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lederman MA, Lebesgue D, Gonzalez VV, et al. Age-related LH surge dysfunction correlates with reduced responsiveness of hypothalamic anteroventral periventricular nucleus kisspeptin neurons to estradiol positive feedback in middle-aged rats. Neuropharmacology. 2010;58:314–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ishii MN, Matsumoto K, Matsui H, et al. Reduced responsiveness of kisspeptin neurons to estrogenic positive feedback associated with age-related disappearance of LH surge in middle-age female rats. Gen Comp Endocrinol. 2013;193:121–129 [DOI] [PubMed] [Google Scholar]
- 51. Tsai H-W, LaPolt PS, Olcott AP, Lu JKH. Temporal changes occur in the neuroendocrine control of gonadotropin secretion in aging female rats: role of progesterone. Biol Reprod. 2004;71:845–852 [DOI] [PubMed] [Google Scholar]
- 52. Kuo J, Micevych P. Neurosteroids, trigger of the LH surge. J Steroid Biochem Mol Biol. 2012;131:57–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Terasawa E, Kenealy BP. Neuroestrogen, rapid action of estradiol, and GnRH neurons. Front Neuroendocrinol. 2012;33:364–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jung H, Yoon BC, Holt CE. Axonal mRNA localization and local protein synthesis in nervous system assembly, maintenance and repair. Nat Rev Neurosci. 2012;13:308–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Desroziers E, Mikkelsen J, Simonneaux V, et al. Mapping of kisspeptin fibres in the brain of the pro-oestrous rat. J Neuroendocrinol. 2010;22:1101–1112 [DOI] [PubMed] [Google Scholar]
- 56. Uenoyama Y, Inoue N, Pheng V, et al. Ultrastructural evidence of kisspeptin-gonadotrophin-releasing hormone interaction in the median eminence of female rats: implication of axo-axonal regulation of GnRH release. J Neuroendocrinol. 2011;23:863–870 [DOI] [PubMed] [Google Scholar]
- 57. Prevot V, Bellefontaine N, Baroncini M, et al. Gonadotrophin-releasing hormone nerve terminals, tanycytes and neurohaemal junction remodelling in the adult median eminence: functional consequences for reproduction and dynamic role of vascular endothelial cells. J Neuroendocrinol. 2010;22:639–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yin W, Wu D, Noel M, Gore A. Gonadotropin-releasing hormone neuroterminals and their microenvironment in the median eminence: effects of aging and estradiol treatment. Endocrinology. 2009;150:5498–5508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yin W, Mendenhall JM, Monita M, Gore AC. Three-dimensional properties of GnRH neuroterminals in the median eminence of young and old rats. J Comp Neurol. 2009;517:284–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rance N. Menopause and the human hypothalamus: evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides. 2009;30:111–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Merkley CM, Porter KL, Coolen LM, et al. KNDy (kisspeptin/neurokinin B/dynorphin) neurons are activated during both pulsatile and surge secretion of LH in the ewe. Endocrinology. 2012;153:5406–5414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Navarro VM. New insights into the control of pulsatile GnRH release: the role of Kiss1/neurokinin B neurons. Front Endocrinol (Lausanne). 2012;3:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. King JC, Tobet SA, Snavely FL, Arimura AA. LHRH immunopositive cells and their projections to the median eminence and organum vasculosum of the lamina terminalis. J Comp Neurol. 1982;209:287–300 [DOI] [PubMed] [Google Scholar]
- 64. Rubin BS, Bridges RS. Alterations in luteinizing hormone-releasing hormone release from the mediobasal hypothalamus of ovariectomized, steroid-primed middle-aged rats as measured by push-pull perfusion. Neuroendocrinology. 1989;49:225–232 [DOI] [PubMed] [Google Scholar]
- 65. Gore AC. GnRH: The Master Molecule of Reproduction. Boston: Kluwer Academic Publishers; 2002 [Google Scholar]
- 66. Neal-Perry GS, Zeevalk GD, Shu J, Etgen AM. Restoration of the luteinizing hormone surge in middle-aged female rats by altering the balance of GABA and glutamate transmission in the medial preoptic area. Biol Reprod. 2008;79:878–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hernández-Ochoa I, Karman B, Flaws J. The role of the aryl hydrocarbon receptor in the female reproductive system. Biochem Pharmacol. 2009;77:547–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Barouki R, Aggerbeck M, Aggerbeck L, Coumoul X. The aryl hydrocarbon receptor system. Drug Metab Drug Interact. 2012;27:3–8 [DOI] [PubMed] [Google Scholar]
- 69. Teglund S, McKay C, Schuetz E, et al. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93:841–850 [DOI] [PubMed] [Google Scholar]
- 70. Yip SH, Eguchi R, Grattan DR, Bunn SJ. Prolactin signalling in the mouse hypothalamus is primarily mediated by signal transducer and activator of transcription factor 5b but not 5a. J Neuroendocrinol. 2012;24:1484–1491 [DOI] [PubMed] [Google Scholar]
- 71. Yeo SH. Neuronal circuits in the hypothalamus-controlling GnRH release: the neuroanatomical projections of kisspeptin neurons. Exp Physiol. 2013;11:1544–1159 [DOI] [PubMed] [Google Scholar]
- 72. Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience. 2010;166:680–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Neal-Perry GS, Zeevalk GD, Santoro NF, Etgen AM. Attenuation of preoptic area glutamate release correlates with reduced luteinizing hormone secretion in middle-aged female rats. Endocrinology. 2005;146:4331–4339 [DOI] [PubMed] [Google Scholar]