Abstract
Objectives/Hypothesis
Studies on young volunteers have shown that aerodigestive reflexes are triggered before the maximum volume of fluid that can safely collect in the hypopharynx before spilling into the larynx is exceeded (hypopharyngeal safe volume [HPSV]). The objective of this study was to determine the influence of aging on HPSV and pharyngoglottal closure reflex (PGCR), pharyngo-UES contractile reflex (PUCR), and reflexive pharyngeal swallow (RPS).
Study Design
Comparison between two groups of different age ranges.
Methods
Ten young (25 ±3 standard deviation [SD] years) and 10 elderly (77 ±3 SD years) subjects were studied. PGCR, PUCR, and RPS were elicited by perfusing water into the pharynx rapidly and slowly. HPSV was determined by abolishing RPS with pharyngeal anesthesia.
Results
Frequency–elicitation of PGCR and PUCR were significantly lower in the elderly compared to the young during slow water perfusion (47% vs. 97% and 40% vs. 90%, respectively, P <.001). RPS was absent in five of the 30 (17%) slow injections in the elderly group. In these elderly subjects, HPSV was exceeded and laryngeal penetration of the water was seen. The threshold volume to elicit PGCR, PUCR, and RPS was significantly lower than the HPSV during rapid injections. Except for RPS, these volumes were also significantly lower than HPSV during slow injections.
Conclusions
PGCR, PUCR, and RPS reflexes are triggered at a threshold volume significantly lower than the HPSV in both young and elderly subjects. Lower frequency–elicitation of PGCR, PUCR, and RPS in the elderly can predispose them to the risks of aspiration.
Keywords: Laryngeal penetrations, aspiration, aging, elderly, aerodigestive reflexes, airway protection
INTRODUCTION
Anatomical contiguity between the digestive and the respiratory pathways in the pharynx can predispose the airways to the risks of aspiration during transit of food through the pharynx. Several aerodigestive reflexes have been proposed to protect the airways against aspiration. For example, augmentation of the upper esophageal sphincter (UES) pressure with distention of the esophagus (esophago-UES contractile reflex) may prevent entry of esophageal contents into the pharynx.1–3 Esophageal distention can also elicit adduction of the vocal cords.4,5 During esophagopharyngeal reflux, fluid in the pharynx can enhance UES pressure (pharyngo-UES contractile reflex [PUCR]).6–10 This may prevent additional material from entering the pharynx. Antegrade or retrograde entry of fluid into the pharynx can trigger an irrepressible swallow (reflexive pharyngeal swallow [RPS]).9–12 RPS not only closes the glottis but also clears pharyngeal contents.9–12 Fluid in the pharynx can also elicit vocal cord adduction (pharyngoglottal closure reflex [PGCR]).13–16 Compared to the laryngeal adductor reflex, which encompasses many reflexes with different afferent pathways, PGCR is a distinct reflex triggered by afferents from the glossopharyngeal nerve.13–15 Bilateral transection of the glossopharyngeal nerve in cats completely abolished this reflex but not swallows induced by pharyngeal water stimulation.16
We studied the correlation between the threshold volume required to elicit RPS, PUCR, and PGCR with the maximum volume that can safely collect in the hypopharynx before spilling into the larynx (hypopharyngeal safe volume [HPSV]).17,18 The above reflexes were triggered before HPSV was exceeded. In those with defective RPS,10,17 HPSV was exceeded resulting in laryngeal penetration. Therefore, conceptually speaking, the risks of laryngeal penetration/aspiration can increase either due to defective aerodigestive reflexes or due to decrease in the capacity of the pharynx to safely hold material (smaller HPSV).
Previous studies have shown that the aerodigestive reflexes may be impaired in the elderly.11,13,19–26 Whether this predisposes elderly subjects to risks of aspiration is not known. The aim of the present study was to determine the effect of aging on HPSV and the airway protective functions of the aerodigestive reflexes.
MATERIALS AND METHODS
This study was approved by the institutional review board. All subjects gave consent prior to the study. Healthy young (age ≤30 years) and healthy elderly (age ≥70 years) subjects were enrolled in the study.
Screening
Because previous studies have shown that smoking and alcohol can adversely affect the elicitation of aerodigestive reflexes,9,10,14 those who smoked or consumed alcohol were excluded from the study.
All subjects also underwent unsedated transnasal endoscopy.27–32 A previous study has shown that 18% of volunteers with no symptoms may have abnormal upper gastrointestinal findings when screened by transnasal upper gastrointestinal endoscopy using a thin gastroscope that can be advanced up to the second part of the duodenum (5.1-mm diameter, EG-1580K; Pentax Medical, Montvale, NJ.).33 Healthy young and elderly subjects with normal endoscopy were enrolled. Studies were performed within 1 week of the screening endoscopy. Except for aspirin in the elderly group, all subjects were on no medications.
Study Subjects and Study Protocol
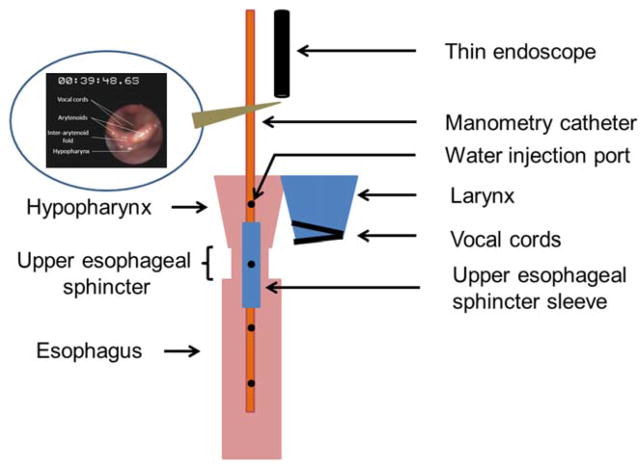
Ten healthy young (25 ±3 standard deviation [SD] years; 7 males and 3 females) and 10 healthy elderly (77 ±3 SD years; 6 males and 4 females) subjects were studied in the 45° inclined position. We used previously described techniques of concurrent transnasal unsedated videoendoscopy, UES manometry, and submental electromyography (Fig. 1). After lubricating a nostril with a nonanesthetic jelly using a cotton tipped applicator (Surgilube; E. Fougera & Co., Atlanta Inc., Melville, NY) a manometry catheter (Dentsleeve International Ltd., Mississauga, Ontario, Canada) was inserted through the nostril. Anesthetic jelly was not used as it could be carried with the catheter to the hypopharynx and therefore affect the elicitation of reflexes. The UES pressure and UES response to pharyngeal water stimulation was monitored with the UES sleeve (6 × 0.5 × 0.3 cm) that also had recording ports at the proximal and distal ends for manometric positioning across the UES (Fig. 1). The port immediately proximal to the sleeve was positioned 2 cm above the UES high-pressure zone and was oriented posteriorly. Aerodigestive reflexes (PGCR, PUCR, and RPS; Table I) were elicited by injecting water into the hypopharynx through a dedicated port in the manometry catheter.10,13,14,17,18 The posterior orientation prevented water from splashing toward the larynx (also visually monitored by an endoscope). The injection port, esophageal ports, and the sleeve sensor were connected to pressure transducers in line with a minimally compliant pneumohydraulic pump (Arndorfer Medical Specialties, Greendale, WI). MMS Motility System (Medical Measurement Systems, Enschede, the Netherlands) was used to record the onset and offset of water injection and UES pressure responses.
Fig. 1.
A diagram of the research setup. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
TABLE I.
Definitions.
| Name | Acronym | Definition |
|---|---|---|
| Reflexive pharyngeal swallow | RPS | An irresistible swallow triggered by stimulating the hypopharynx |
| Pharyngo-UES contractile reflex | PUCR | UES contractile response triggered by stimulation of the hypopharynx (unassociated with a swallow) |
| Pharyngoglottal closure reflex | PGCR | Adduction of the vocal cords in response to hypopharyngeal stimulation (unassociated with a swallow) |
| Hypopharyngeal safe volume | HPSV | Maximum amount of water that can safely collect in the hypopharynx before spilling into the larynx |
UES =upper esophageal sphincter.
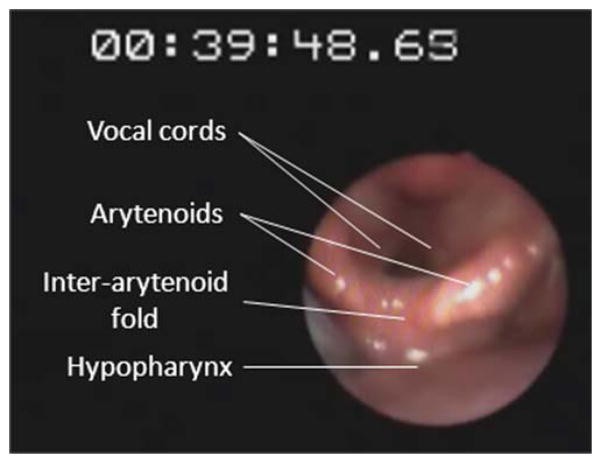
To monitor glottal response to pharyngeal water stimulation (PGCR), a laryngo-pharyngoscope (3.2-mm diameter, Pentax FNL-10AP; Pentax Medical) was passed through the other nostril and positioned within the pharynx such that the vocal cords were adequately visualized (Fig. 2). For better endoscopic visualization, green-colored water was used for perfusion (Figs. 3 and 4). Endoscopic images were recorded on a digital recorder for subsequent analysis in real time and slow motion. All modalities (onset–offset of water perfusion, laryngopharyngoscopy, manometric recording, and submental surface electromyography) were synchronized using a specially designed timer (Thalner Electronics Labs Inc., Ann Arbor, MI). All subjects were given 15 minutes to adapt before starting the study.
Fig. 2.
Pharyngeal water perfusion and glottal response to water stimulation was monitored by using an ultrathin laryngo-pharyngoscope (Pentax FNL-10AP; Pentax Medical, Montvale, NJ) passed through the nostril and positioned within the pharynx such that the vocal cords were adequately visualized. For better endoscopic visualization, green-colored water was used for perfusion. Endoscopic views were synchronized with the manometry and the electromyography tracing in real time using a timer. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
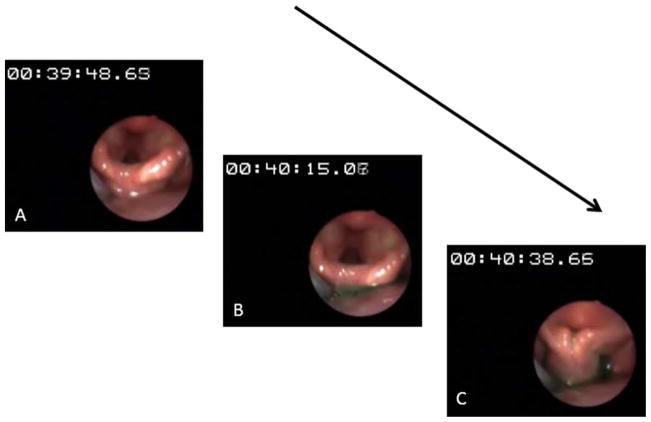
Fig. 3.
Infusion of green-colored water into the hypopharynx at 1 mL/ min in a young volunteer. (A) Onset of water perfusion (timer at 00:39: 48.63). (B) Colored water seen accumulating in the hypopharynx. (C) With ongoing perfusion, a reflexive pharyngeal swallow was triggered before the water could spill into the larynx. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
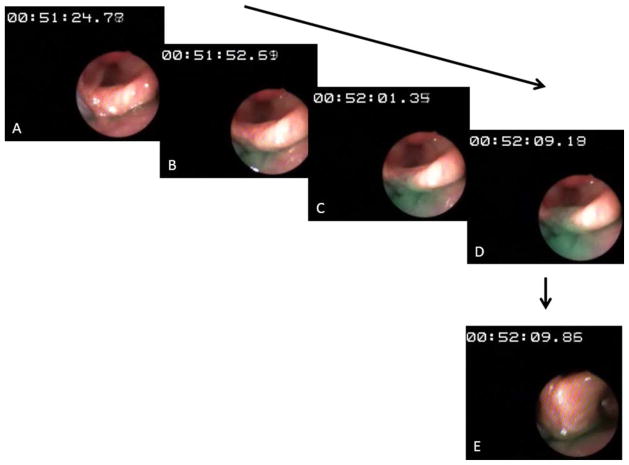
Fig. 4.
Infusion of green-colored water into the hypopharynx at 1 mL/ min in an elderly subject. (A) Onset of water perfusion (timer at 00:51: 24.77). (B) Colored water seen accumulating in the hypopharynx. (C) With ongoing perfusion, the colored water was seen rising up to the superior margin of the interarytenoid fold. (D) Further perfusion did not elicit reflexive pharyngeal swallow, which led to water spilling into the larynx. (E) To prevent aspiration, the subject was asked to swallow (command swallow). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
PGCR, PUCR, and RPS were elicited by pharyngeal stimulation with water injected at slow and rapid rates. For slow injections, water at room temperature was perfused into the hypopharynx at a rate of 1 mL/min using a Harvard infusion pump (model N0975; Harvard Apparatus Co., Inc., Dover, MA). Subjects were asked to avoid volitional swallow and perfusion was continued until an irrepressible swallow occurred (RPS). Each injection was performed three times, and subjects were asked to swallow between injections to clear the pharynx of any residual water. For rapid injections, water was rapidly injected using a handheld syringe. Rapid injections were started at 0.05 mL, then 1 mL, followed by 1-mL increments until an irrepressible swallow occurred at three of three injections (RPS). For both slow and rapid injections, pharyngeal perfusion was started after the UES pressure returned to baseline following a command swallow. After the onset of pharyngeal water injection, rise in UES pressure that was not associated with submental electromyography activity was considered as PUCR. The average end-expiratory UES pressure at and after the injection was recorded. The postinjection pressure was defined as the maximum UES pressure following injection, excluding the 3-second interval prior to deglutitive relaxation, if a swallow occurred. This 3-second interval was used to avoid counting the commonly seen pressure increase that is registered by the sleeve immediately before its swallow-induced relaxation. We determined in each subject the frequency–elicitation response and the smallest volume of injected water (threshold volume) that triggered the above reflexes.
After determining their frequency–elicitation and threshold volumes for elicitation of the above reflexes, HPSV (Table I) was determined by abolishing RPS. Although the sleeve catheter and the thin endoscope were still in place, 4% topical lidocaine (Roxane Laboratories, Columbus, OH) was sprayed into the hypopharynx with an atomizer nozzle (two puffs). Water was then injected into the hypopharynx. If the reflexive pharyngeal swallow was still elicitable, two further sprays of lidocaine were administered. Using this approach, we were able to abolish RPS in all subjects.
After abolishing RPS, while monitoring the larynx with the laryngo-pharyngoscope, colored water was perfused into the hypopharynx at 1 mL/min. Water perfusion was stopped when the water level rose up to the superior level of the interarytenoid folds (Fig. 3). This volume of water is the maximum volume that could safely collect in the hypopharynx before spilling into the larynx (HPSV; Table I). At this point perfusion was stopped and the volunteers were asked to swallow. Occurrence of laryngeal penetration was documented by observing colored water rise up to the interarytenoid fold and spill into the larynx (laryngeal penetration; Fig. 4).
Statistical Analysis
Comparison of frequency–elicitation response of PUCR, PGCR, RPS and aspiration between young and elderly subjects was done using the Fischer exact test. Comparison of threshold volumes between the two groups was done by unpaired t test. Within each group, the threshold volumes to elicit the reflexes were compared with HPSV using a t test. For data that did not pass normality test, the Mann-Whitney rank sum method was used. Values are presented as mean ±SD.
RESULTS
All subjects completed the study with no adverse events.
Pharyngo-glottal closure reflex
Frequency–elicitation
As shown in Table II, PGCR was triggered in all young volunteers during the 30 rapid water injections (100%). In the elderly group, frequency–elicitation was 80% (P =NS). During slow water infusion, PGCR was elicited in 97% of the young volunteers compared to only 47% in the elderly group (P <.001).
TABLE II.
Frequency–Elicitation of PGCR, PUCR, and RPS in Young and Elderly Subjects Using Slow and Rapid Pharyngeal Water Injections.
| Reflex | Injection | Young (%) | Elderly (%) | P Value |
|---|---|---|---|---|
| PGCR | Slow | 97 | 47 | <.001 |
| Rapid | 100 | 80 | NS | |
| PUCR | Slow | 97 | 40 | <.001 |
| Rapid | 100 | 90 | NS | |
| RPS | Slow | 100 | 83* | <.05 |
| Rapid | 100 | 100 | NS | |
| Laryngeal penetration* | No | Yes |
Of the 30 slow pharyngeal perfusions performed in 10 elderly subjects, RPS was absent in five perfusions without any pharyngeal anesthesia (in one subject it was absent in three of three perfusions). During these injections, colored water was seen to rise up to the superior margin of the interarytenoid fold and spill into the larynx (laryngeal penetration), at which point the perfusion was stopped.
NS =not significant; PGCR =pharyngoglottal closure reflex; PUCR =pharyngo-UES contractile reflex; RPS =reflexive pharyngeal swallow.
Threshold volume
The mean threshold volume to elicit PGCR during both slow and rapid pharyngeal water injections were similar between the young and the elderly groups (slow injections: elderly 0.18 ±0.13 mL, young 0.13±0.06 mL; rapid injections: elderly 0.08 ± 0.03, young 0.07 ±0.03; P =NS; Table III).
TABLE III.
Threshold Volume to Elicit PGCR, PUCR, and RPS in Young and Elderly Subjects Using Slow and Rapid Pharyngeal Water Injections.
| Injection | Young, mL (mean ±SD) | Elderly, mL (mean ±SD) | P Value | |
|---|---|---|---|---|
| PGCR | Slow | 0.13 ±0.06 | 0.18 ±0.13 | NS |
| Rapid | 0.07 ±0.03 | 0.08 ±0.03 | NS | |
| PUCR | Slow | 0.20 ±0.08 | 0.20 ±0.13 | NS |
| Rapid | 0.19 ±0.15 | 0.22 ±0.14 | NS | |
| RPS | Slow* | 0.59 ±0.27 | 0.63 ±0.47 | NS |
| Rapid | 0.38 ±0.19 | 0.40 ±0.23 | NS | |
| HPSV | 0.82 ±0.41 | 0.80 ±0.46 | NS |
By abolishing RPS with topical pharyngeal anesthesia, HPSV was measured.
During slow water perfusion, RPS was absent in five of 30 injections in the elderly subjects (excluded from analysis of threshold volume).
HPSV =hypopharyngeal safe volume; NS =not significant; PGCR = pharyngoglottal closure reflex; PUCR =pharyngo-UES contractile reflex; RPS =reflexive pharyngeal swallow; SD =standard deviation.
Pharyngo-UES Contractile Reflex
Frequency–elicitation
Similar to PGCR, PUCR was elicited in all young volunteers with rapid injection of water compared to 90% in the elderly subjects (P =NS). During slow infusion, the frequency–elicitation of PUCR was 97% in the young group compared to 40% in the elderly (P <.001) (Table II). This reflex was abolished after topical pharyngeal anesthesia in both the groups.
Threshold volume
The mean threshold volume of water required to trigger PUCR during rapid injections was 0.19 ±0.15 mL in the young volunteers. This was not significantly different from the elderly group (mean 0.22 ±0.14 mL). Similarly, the threshold volumes to elicit PUCR during slow water infusions were similar in the two groups (young: 0.20 ±0.08 mL, elderly: 0.20 ± 0.13 mL; P =NS) (Table III).
In both groups and for both rapid and slow injections, the threshold volume to elicit PUCR was significantly larger than the threshold volumes to elicit PGCR (P<.001).
UES pressure
Mean baseline UES pressure was 54 ±11 mm Hg in the young group compared to 28 ±8 mm Hg in the elderly subjects (P <.001). However, the percent rise in UES pressure following injection of water into the pharynx at 1 mL/min was similar in the two groups (young: 174% ±34%, elderly 183% ±26%; P=NS).
Reflexive Pharyngeal Swallow
Frequency–elicitation
Frequency–elicitation of RPS during rapid water injections was 100% in both groups. During slow water infusion, RPS was elicited in all young volunteers (100%) compare to 83% in the elderly group (P <.05) (Table II). Of the 30 slow water perfusions performed in 10 elderly subjects, RPS was absent in five perfusions (in one subject it was absent in three of three perfusions). After pharyngeal topical anesthesia, the reflex was absent in all young and elderly volunteers for both rapid and slow water injections.
Threshold volume
Mean threshold volume to trigger RPS during rapid water injections was 0.38 ±0.19 mL in the young and 0.40 ±0.23 mL in the elderly (P =NS). During slow water infusion, the mean threshold volumes were 0.59 ±0.27 mL and 0.63 ±0.47 mL in the young and the elderly subjects, respectively (P =NS) (Table III).
Similar to PUCR, in both groups and for both rapid and slow injections, the threshold volume to elicit RPS was significantly larger than the threshold volumes to elicit PGCR (P <.001).
Laryngeal Penetration/Hypopharyngeal Safe Volume
None of the young volunteers had laryngeal penetration of the perfused fluid prior to pharyngeal anesthesia (Fig. 2). In the elderly group, five of 30 infusions (three of three in one patient) were associated with absent RPS. In these subjects, during perfusion, colored water was seen rising up to the superior margin of the interarytenoid folds and spilling into the larynx (laryngeal penetration; Fig. 3).
After abolishing RPS by topical pharyngeal anesthesia, HPSV was determined. The mean HPSV was 0.82 ±0.41 mL in the young compared to 0.80 ±0.46 mL in the elderly (P =NS; Table III). In both young and the elderly subjects, HPSV was significantly larger than the threshold volumes to elicit PGCR, PUCR, and RPS during rapid water injection and was also significantly larger than the threshold volume to elicit PGCR and PUCR during slow water perfusion. This difference did not reach statistical significance for RPS during slow water perfusion in both the groups.
DISCUSSION
Anatomical contiguity of the digestive and the respiratory pathways in the pharynx can predispose the airways to the risks of aspiration. The capacity of the pharynx to safely accommodate material (HPSV) and the efficacy of the aerodigestive reflexes in clearing the material from the pharynx play an integral part in airway protection during swallowing and during retrograde transit of gastric contents. Laryngeal penetration or aspiration can occur when the material entering the pharynx exceeds the HPSV.17,18 However, before HPSV is exceeded, fluid in the pharynx can trigger an irrepressible swallow (RPS).9–12 This reflex protects the airways by not only clearing the pharynx of residual fluid but also by closing the glottis and elevating the larynx. By abolishing RPS with pharyngeal anesthesia and in cigarette smokers with defective RPS, HPSV can be exceeded, resulting in laryngeal penetration.17,18
Around 40% of healthy elderly subjects have dysphagia with risks of aspiration.34,35 Studies have shown that the aerodigestive reflexes deteriorate with aging.11,13,18–25 The current study was undertaken to address the dynamics of the interaction between the deteriorating aerodigestive reflexes and HPSV in the elderly. We found the frequency–elicitation of PGCR and PUCR was significantly lower in the elderly group compared to the young subjects. RPS was elicited in all young subjects but was absent in 17% of the slow pharyngeal water injections in the elderly. In these subjects, water was seen rising up to the superior margin of the interarytenoid fold and spill into the larynx. Lower frequency–elicitation of PGCR (47%) and PUCR (40%) may further predispose the elderly to risks of aspiration during slow entry of material into the pharynx. For example, during esophagopharyngeal reflux, absence of PUCR in the elderly will not raise the UES pressure when refluxate enters the hypopharynx, thereby allowing more esophageal contents to enter the pharynx. The pharynx may not be able to clear this material if RPS is absent as was observed only in the elderly group. Lack of reflexive glottal adduction may further increase the risks of aspiration. However, because water was perfused in an antegrade manner in this study, whether the same applies to retrograde entry of fluid into the hypopharynx is speculative. Our current ongoing studies do suggest that aerodigestive reflexes react similarly to retrograde entry of material into the hypopharynx as they do with antegrade entry.36
Despite these observations, aspiration does not commonly occur in healthy elderly subjects. This could be due to the presence of a multilayered airway protection mechanism from reflexes triggered at various levels. For example, retrograde transit of material into the esophagus can elicit vocal cord closure by triggering the esophagoglottal closure reflex.1–3,5 If material overwhelms the UES barrier and enters the pharynx (esophagopharyngeal reflux), pharyngoglottal closure reflex13,14,16 will result in reflexive vocal cord closure. Spillage of material into the larynx will trigger the laryngo-laryngeal closure reflex.37 This arrangement provides redundancy in airway protection that may have clinical significance. Another factor that could have increased the risks of aspiration, namely, diminished pharyngeal capacity (HPSV), was not observed in the elderly. In those in whom RPS, PGCR, and PUCR were elicited, the threshold volume to elicit these reflexes was similar between the two groups. This is contrary to what we had observed in our previous studies, where the threshold volume to elicit these reflexes was higher in the elderly subjects.11,13 The reason for this could be multifactorial. Slow perfusion in our previous studies was performed at 5.5 mL/min compared to 1 mL/ min in the present study. We have previously shown that elicitation of aerodigestive reflex can be affected by water perfusion rate.38 The body position in the previous studies was either upright or supine compared to the 45° semi-inclined position in the current study. Rapid pharyngeal injections were performed by using a handheld syringe. Differences in the force of injections between operators of the previous and the current study could have also influenced the threshold volume for elicitation of these reflexes. To avoid interoperator variability, in the current study, the same operator performed all the injections in both the young and elderly groups.
The exact mechanism/s for the absent/lower frequency–elicitation rates of studied aerodigestive reflexes in the elderly subjects is not known. Age related degenerative changes in the sensory-motor functions could be a contributory factor. Aviv studied laryngo-pharyngeal sensory discrimination in 80 young and elderly subjects and showed progressive decrease in sensory discrimination with advancing age.39 A higher air pulse pressure threshold was required for sensory discrimination with each advancing decade of life. Kidd et al. showed that diminished pharyngeal sensation is associated with higher incidence of aspiration pneumonia.40 This observation can be explained by our findings that showed laryngeal penetration of the fluid HPSV was exceeded in the elderly volunteers with absent RPS. Reduced frequency–elicitation of RPS in the elderly can be partially explained by the findings of Malandraki et al.41 They found that areas involved in sensory processing, sensori-motor integration and/or motor coordination, and control of swallowing showed reduced or limited activity in the elderly compared to the young. A previous study has also shown slow laryngeal elevation in the elderly resulting in more residue lying within the pharyngeal cavities.42
During rapid pharyngeal water injections, the threshold volumes of water required to trigger PGCR, PUCR, and RPS were significantly lower than HPSV in both the young and the elderly. Therefore, if material enters the pharynx abruptly, the aerodigestive reflexes appear to be effective in clearing the pharynx before HPSV is exceeded in both the young and the elderly. However, during slow water perfusion, the frequency–elicitation of PGCR, PUCR, and RPS was significantly lower in the elderly. During slow water perfusion, although RPS was triggered before HPSV was exceeded, the threshold volume to elicit RPS was not significantly different from the HPSV. This finding suggests that RPS is triggered by receptors located at the superior margin of the interarytenoid fold and that these receptors are not amenable to volitional suppression of swallowing, compared to those receptors located in the rest of pharyngeal swallow trigger zone such as posterior pharyngeal wall or tonsillar pillars. Therefore, RPS is triggered when the fluid accumulation reaches the superior margin of the interarytenoid folds, which is also the level beyond which water will spill into the larynx (HPSV). Thus, there was no significant difference between HPSV and the threshold volume required to trigger RPS during slow water injections.
Some of the limitations of this study are the small number of subjects studied, lack of data on the subjects’ body habitus that may have an influence on pharyngeal volumes, and that all subjects were studied only in one position (45° supine, neck neutral). HPSV can be affected by body position.43 Further studies will be required to address the above limitations. We also speculated that data obtained with antegrade perfusion of fluid into the hypopharynx can be extrapolated to retrograde transit of material. Our recent ongoing study supports this assumption.36
CONCLUSION
This study has demonstrated that the aerodigestive reflexes triggered with pharyngeal stimulation deteriorate with aging, and may not be as effective as in the young in clearing the pharynx, before the maximum volume of fluid that can safely accumulate in the pharynx is exceeded. This may result in spillage of the pharyngeal contents into the larynx. This deterioration is predominantly observed with material entering the pharynx slowly rather than abruptly. Despite these observations, aspiration does not commonly occur in healthy elderly subjects, probably due to the presence of a multilayered airway protection mechanism from reflexes triggered at various levels. This study again demonstrates the airway protective function of the reflexive pharyngeal swallow that is triggered before the hypopharyngeal safe volume is exceeded.
Acknowledgments
This work is supported in part by NIH grants 1P01DK068051-01A1 and 5RO1DK025731.
Footnotes
Level of Evidence: 4.
Author contributions: Kulwinder S. Dua: Study concept and design; acquisition, analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; obtained funding, technical, or material support; study supervision. Sri Naveen Surapaneni: Acquisition of data, analysis and interpretation of data, statistical analysis, technical support. Shiko Kuribayashi: Acquisition of data, analysis of data, statistical analysis, technical support. Mohammed Hafeezullah: Acquisition of data, technical support. Reza Shaker: Study concept and design; interpretation of data; critical revision of the manuscript for important intellectual content; obtained funding, technical, or material support; study supervision.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1.Enzmann DR, Harell GS, Zboralske FF. Upper esophageal responses to intraluminal distention in man. Gastroenterology. 1977;72:1292–1298. [PubMed] [Google Scholar]
- 2.Freiman JM, El-Sharkawy TY, Diamant NE. Effect of bilateral vagosym-pathetic nerve blockade on response of the dog upper esophageal sphincter (UES) to intraesophageal distention and acid. Gastroenterology. 1981;81:78–84. [PubMed] [Google Scholar]
- 3.Lang IM, Medda BK, Shaker R. Mechanisms of reflexes induced by esophageal distension. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1246–G1263. doi: 10.1152/ajpgi.2001.281.5.G1246. [DOI] [PubMed] [Google Scholar]
- 4.Shaker R, Ren J, Medda B, et al. Identification and characterization of the esophagoglottal closure reflex in a feline model. Am J Physiol. 1994;266:G147–G153. doi: 10.1152/ajpgi.1994.266.1.G147. [DOI] [PubMed] [Google Scholar]
- 5.Shaker R, Dodds WJ, Ren J, et al. Esophagoglottal closure reflex: a mechanism of airway protection. Gastroenterology. 1992;102:857–861. doi: 10.1016/0016-5085(92)90169-y. [DOI] [PubMed] [Google Scholar]
- 6.Shaker R, Ren J, Xie P, et al. Characterization of the pharyngo-UES contractile reflex in humans. Am J Physiol. 1997;273:G854–G858. doi: 10.1152/ajpgi.1997.273.4.G854. [DOI] [PubMed] [Google Scholar]
- 7.Ren J, Xie P, Lang IM, et al. Deterioration of the pharyngo-UES contractile reflex in the elderly. Laryngoscope. 2000;110:1563–1566. doi: 10.1097/00005537-200009000-00031. [DOI] [PubMed] [Google Scholar]
- 8.Medda BK, Lang IM, Layman R, et al. Characterization and quantification of a pharyngo-UES contractile reflex in cats. Am J Physiol. 1994;267:G972–G983. doi: 10.1152/ajpgi.1994.267.6.G972. [DOI] [PubMed] [Google Scholar]
- 9.Dua KS, Surapaneni SN, Santharam R, et al. Effect of systemic alcohol and nicotine on airway protective reflexes. Am J Gastroenterol. 2009;104:2431–2438. doi: 10.1038/ajg.2009.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dua K, Bardan E, Ren J, et al. Effect of chronic and acute cigarette smoking on the pharyngo-upper oesophageal sphincter contractile reflex and reflexive pharyngeal swallow. Gut. 1998;43:537–541. doi: 10.1136/gut.43.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaker R, Ren J, Zamir Z, et al. Effect of aging, position, and temperature on the threshold volume triggering pharyngeal swallows. Gastroenterology. 1994;107:396–402. doi: 10.1016/0016-5085(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 12.Nishino T. Swallowing as a protective reflex for the upper respiratory tract. Anesthesiology. 1993;79:588–601. doi: 10.1097/00000542-199309000-00024. [DOI] [PubMed] [Google Scholar]
- 13.Shaker R, Ren J, Bardan E, et al. Pharyngoglottal closure reflex: characterization in healthy young, elderly and dysphagic patients with prede-glutitive aspiration. Gerontology. 2003;49:12–20. doi: 10.1159/000066504. [DOI] [PubMed] [Google Scholar]
- 14.Dua K, Bardan E, Ren J, et al. Effect of chronic and acute cigarette smoking on the pharyngoglottal closure reflex. Gut. 2002;51:771–775. doi: 10.1136/gut.51.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jadcherla SR, Gupta A, Wang M, et al. Definition and implications of novel pharyngoglottal reflex in human infants using concurrent manometry ultrasonography. Am J Gastroenterol. 2009;104:2572–2582. doi: 10.1038/ajg.2009.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaker R, Medda BK, Ren J, et al. Pharyngoglottal closure reflex: identification and characterization in a feline model. Am J Physiol. 1998;275:G521–G525. doi: 10.1152/ajpgi.1998.275.3.G521. [DOI] [PubMed] [Google Scholar]
- 17.Dua K, Surapaneni SN, Kuribayashi S, et al. Protective role of aerodigestive reflexes against aspiration: study on subjects with impaired and preserved reflexes. Gastroenterology. 2011;140:1927–1933. doi: 10.1053/j.gastro.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dua KSS, Kuribayashi S, Hafeezullah M, Shaker R. Pharyngeal airway protective reflexes are triggered before the maximum volume of fluid that the hypopharynx can safely hold is exceeded. Am J Physiol. 2011;301:G197–G202. doi: 10.1152/ajpgi.00046.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zamir Z, Ren J, Hogan WJ, et al. Coordination of deglutitive vocal cord closure and oral-pharyngeal swallowing events in the elderly. Eur J Gastroenterol Hepatol. 1996;8:425–429. [PubMed] [Google Scholar]
- 20.Staff D, Shaker R. Aging in the gastrointestinal tract. Dis Mon. 2001;47:72–101. doi: 10.1067/mda.2000.114726. [DOI] [PubMed] [Google Scholar]
- 21.Shaker R, Staff D. Esophageal disorders in the elderly. Gastroenterol Clin North Am. 2001;30:335–361. vii–viii. doi: 10.1016/s0889-8553(05)70185-0. [DOI] [PubMed] [Google Scholar]
- 22.Shaker R, Ren J, Podvrsan B, et al. Effect of aging and bolus variables on pharyngeal and upper esophageal sphincter motor function. Am J Physiol. 1993;264:G427–G432. doi: 10.1152/ajpgi.1993.264.3.G427. [DOI] [PubMed] [Google Scholar]
- 23.Ren J, Shaker R, Zamir Z, et al. Effect of age and bolus variables on the coordination of the glottis and upper esophageal sphincter during swallowing. Am J Gastroenterol. 1993;88:665–669. [PubMed] [Google Scholar]
- 24.Ren J, Shaker R, Kusano M, et al. Effect of aging on the secondary esophageal peristalsis: presbyesophagus revisited. Am J Physiol. 1995;268:G772–G779. doi: 10.1152/ajpgi.1995.268.5.G772. [DOI] [PubMed] [Google Scholar]
- 25.Kawamura O, Easterling C, Aslam M, et al. Laryngo-upper esophageal sphincter contractile reflex in humans deteriorates with age. Gastroenterology. 2004;127:57–64. doi: 10.1053/j.gastro.2004.03.065. [DOI] [PubMed] [Google Scholar]
- 26.Bardan E, Xie P, Brasseur J, et al. Effect of ageing on the upper and lower oesophageal sphincters. Eur J Gastroenterol Hepatol. 2000;12:1221–1225. doi: 10.1097/00042737-200012110-00009. [DOI] [PubMed] [Google Scholar]
- 27.Dean R, Dua K, Massey B, et al. A comparative study of unsedated transnasal esophagogastroduodenoscopy and conventional EGD. Gastrointest Endosc. 1996;44:422–424. doi: 10.1016/s0016-5107(96)70092-5. [DOI] [PubMed] [Google Scholar]
- 28.Saeian K. Unsedated transnasal endoscopy (the Shaker technique): an alternative for assessment of supraesophageal complications of gastro-esophageal reflux. Am J Med. 2003;115(suppl 3A):144S–149S. doi: 10.1016/s0002-9343(03)00213-4. [DOI] [PubMed] [Google Scholar]
- 29.Saeian K, Staff D, Knox J, et al. Unsedated transnasal endoscopy: a new technique for accurately detecting and grading esophageal varices in cirrhotic patients. Am J Gastroenterol. 2002;97:2246–2249. doi: 10.1111/j.1572-0241.2002.05906.x. [DOI] [PubMed] [Google Scholar]
- 30.Saeian K, Staff DM, Vasilopoulos S, et al. Unsedated transnasal endoscopy accurately detects Barrett’s metaplasia and dysplasia. Gastrointest Endosc. 2002;56:472–478. doi: 10.1067/mge.2002.128131. [DOI] [PubMed] [Google Scholar]
- 31.Saeian K, Townsend WF, Rochling FA, et al. Unsedated transnasal EGD: an alternative approach to conventional esophagogastroduodenoscopy for documenting Helicobacter pylori eradication. Gastrointest Endosc. 1999;49:297–301. doi: 10.1016/s0016-5107(99)70004-0. [DOI] [PubMed] [Google Scholar]
- 32.Shaker R, Saeian K. Unsedated transnasal laryngo-esophagogastroduodenoscopy: an alternative to conventional endoscopy. Am J Med. 2001;111(Suppl 8A):153S–156S. doi: 10.1016/s0002-9343(01)00852-x. [DOI] [PubMed] [Google Scholar]
- 33.Siwiec RM, Dua K, Surapaneni SN, et al. Unsedated transnasal endoscopy with ultrathin endoscope as a screening tool for research studies. Laryngoscope. 2012;122:1719–1723. doi: 10.1002/lary.23304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feinberg MJ, Knebl J, Tully J, et al. Aspiration and the elderly. Dysphagia. 1990;5:61–71. doi: 10.1007/BF02412646. [DOI] [PubMed] [Google Scholar]
- 35.Roy N, Stemple J, Merrill RM, et al. Dysphagia in the elderly: preliminary evidence of prevalence, risk factors, and socioemotional effects. Ann Otol Rhinol Laryngol. 2007;116:858–865. doi: 10.1177/000348940711601112. [DOI] [PubMed] [Google Scholar]
- 36.Babaei A, Hogan W, Rahimi, Sohrab DM, Shaker R. Mechanism of esophagopharyngeal reflux: a concurrent videopharyngoscopy and high resolution manometry/impedance study. Gastroenterology. 2013;144:S50. [Google Scholar]
- 37.Aviv JE, Spitzer J, Cohen M, et al. Laryngeal adductor reflex and pharyngeal squeeze as predictors of laryngeal penetration and aspiration. Laryngoscope. 2002;112:338–341. doi: 10.1097/00005537-200202000-00025. [DOI] [PubMed] [Google Scholar]
- 38.Bajaj JS, Dua K, Bajaj S, Rittmann T, Shaker R. Effect of infusion rate on elicitation of pharyngeal reflexes and development of aspiration among smokers [abstract] Neurogastroenterol Motil. 2004;16:672. [Google Scholar]
- 39.Aviv JE. Effects of aging on sensitivity of the pharyngeal and supraglottic areas. Am J Med. 1997;103:74S–76S. doi: 10.1016/s0002-9343(97)00327-6. [DOI] [PubMed] [Google Scholar]
- 40.Kidd D, Lawson J, Nesbitt R, et al. Aspiration in acute stroke: a clinical study with videofluoroscopy. Q J Med. 1993;86:825–829. [PubMed] [Google Scholar]
- 41.Malandraki GA, Perlman AL, Karampinos DC, et al. Reduced somatosensory activations in swallowing with age. Hum Brain Mapp. 2011;32:730–743. doi: 10.1002/hbm.21062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robbins J, Hamilton JW, Lof GL, et al. Oropharyngeal swallowing in normal adults of different ages. Gastroenterology. 1992;103:823–829. doi: 10.1016/0016-5085(92)90013-o. [DOI] [PubMed] [Google Scholar]
- 43.Surapaneni SN, Dua KS, Kuribayashi S, Hafeezullah M, Tatro L, Shaker R. Influence of position on the maximum volume of fluid that can safely dwell in the hypopharynx; “Hypopharyngeal Safe Volume” (HPSV) [abstract] Gastroenterology. 2009;136:A734. [Google Scholar]