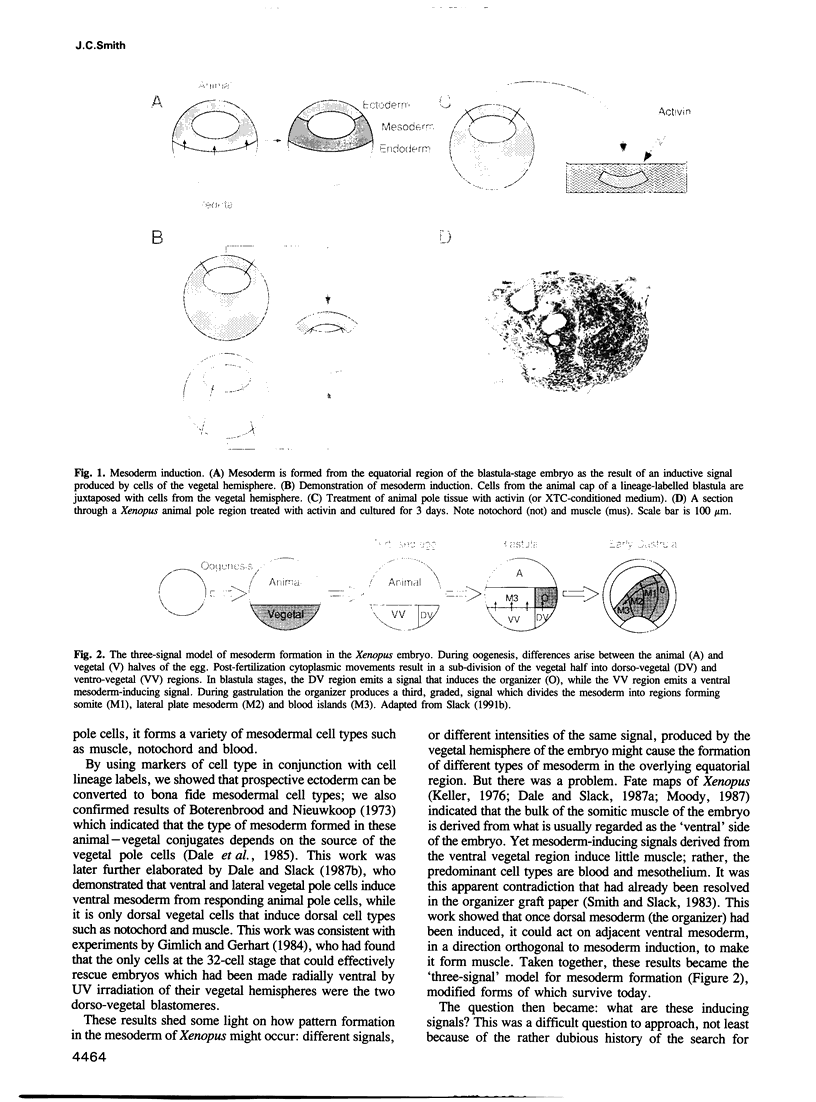
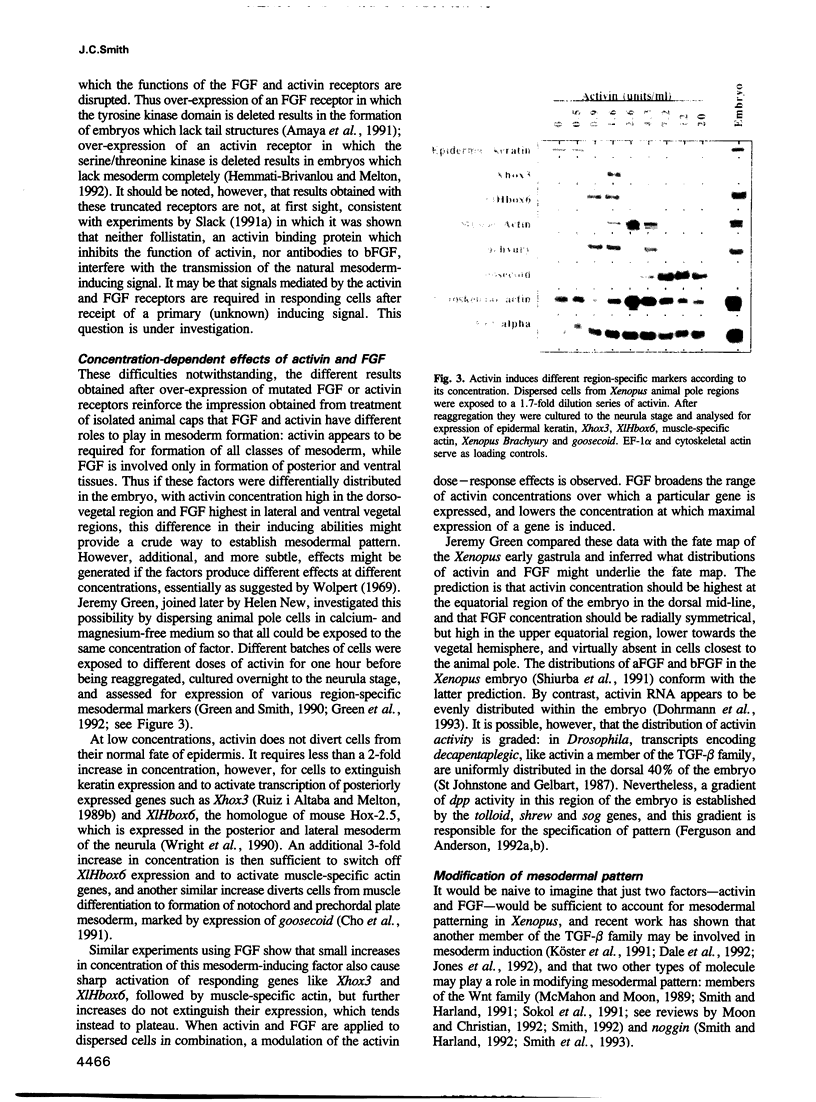
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano R. M., Godsave S. F., Huylebroeck D., Van Nimmen K., Isaacs H. V., Slack J. M., Smith J. C. A mesoderm-inducing factor produced by WEHI-3 murine myelomonocytic leukemia cells is activin A. Development. 1990 Oct;110(2):435–443. doi: 10.1242/dev.110.2.435. [DOI] [PubMed] [Google Scholar]
- Albano R. M., Groome N., Smith J. C. Activins are expressed in preimplantation mouse embryos and in ES and EC cells and are regulated on their differentiation. Development. 1993 Feb;117(2):711–723. doi: 10.1242/dev.117.2.711. [DOI] [PubMed] [Google Scholar]
- Amaya E., Musci T. J., Kirschner M. W. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991 Jul 26;66(2):257–270. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- Asashima M., Nakano H., Uchiyama H., Sugino H., Nakamura T., Eto Y., Ejima D., Nishimatsu S., Ueno N., Kinoshita K. Presence of activin (erythroid differentiation factor) in unfertilized eggs and blastulae of Xenopus laevis. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6511–6514. doi: 10.1073/pnas.88.15.6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum M., Gaunt S. J., Cho K. W., Steinbeisser H., Blumberg B., Bittner D., De Robertis E. M. Gastrulation in the mouse: the role of the homeobox gene goosecoid. Cell. 1992 Jun 26;69(7):1097–1106. doi: 10.1016/0092-8674(92)90632-m. [DOI] [PubMed] [Google Scholar]
- Born J., Geithe H. P., Tiedemann H., Kocher-Becker U. Isolation of a vegetalizing inducing factor. Hoppe Seylers Z Physiol Chem. 1972 Jul;353(7):1075–1084. doi: 10.1515/bchm2.1972.353.2.1075. [DOI] [PubMed] [Google Scholar]
- Chakrabarti A., Matthews G., Colman A., Dale L. Secretory and inductive properties of Drosophila wingless protein in Xenopus oocytes and embryos. Development. 1992 May;115(1):355–369. doi: 10.1242/dev.115.1.355. [DOI] [PubMed] [Google Scholar]
- Cho K. W., Blumberg B., Steinbeisser H., De Robertis E. M. Molecular nature of Spemann's organizer: the role of the Xenopus homeobox gene goosecoid. Cell. 1991 Dec 20;67(6):1111–1120. doi: 10.1016/0092-8674(91)90288-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian J. L., McMahon J. A., McMahon A. P., Moon R. T. Xwnt-8, a Xenopus Wnt-1/int-1-related gene responsive to mesoderm-inducing growth factors, may play a role in ventral mesodermal patterning during embryogenesis. Development. 1991 Apr;111(4):1045–1055. doi: 10.1242/dev.111.4.1045. [DOI] [PubMed] [Google Scholar]
- Christian J. L., Moon R. T. Interactions between Xwnt-8 and Spemann organizer signaling pathways generate dorsoventral pattern in the embryonic mesoderm of Xenopus. Genes Dev. 1993 Jan;7(1):13–28. doi: 10.1101/gad.7.1.13. [DOI] [PubMed] [Google Scholar]
- Christian J. L., Olson D. J., Moon R. T. Xwnt-8 modifies the character of mesoderm induced by bFGF in isolated Xenopus ectoderm. EMBO J. 1992 Jan;11(1):33–41. doi: 10.1002/j.1460-2075.1992.tb05024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. Mesoderm-inducing factors and Spemann's organiser phenomenon in amphibian development. Development. 1989 Oct;107(2):229–241. doi: 10.1242/dev.107.2.229. [DOI] [PubMed] [Google Scholar]
- Cooke J., Smith J. C. Gastrulation and larval pattern in Xenopus after blastocoelic injection of a Xenopus-derived inducing factor: experiments testing models for the normal organization of mesoderm. Dev Biol. 1989 Feb;131(2):383–400. doi: 10.1016/s0012-1606(89)80012-0. [DOI] [PubMed] [Google Scholar]
- Cooke J., Smith J. C., Smith E. J., Yaqoob M. The organization of mesodermal pattern in Xenopus laevis: experiments using a Xenopus mesoderm-inducing factor. Development. 1987 Dec;101(4):893–908. doi: 10.1242/dev.101.4.893. [DOI] [PubMed] [Google Scholar]
- Cooke J., Wong A. Growth-factor-related proteins that are inducers in early amphibian development may mediate similar steps in amniote (bird) embryogenesis. Development. 1991 Jan;111(1):197–212. doi: 10.1242/dev.111.1.197. [DOI] [PubMed] [Google Scholar]
- Cunliffe V., Smith J. C. Ectopic mesoderm formation in Xenopus embryos caused by widespread expression of a Brachyury homologue. Nature. 1992 Jul 30;358(6385):427–430. doi: 10.1038/358427a0. [DOI] [PubMed] [Google Scholar]
- Dale L., Howes G., Price B. M., Smith J. C. Bone morphogenetic protein 4: a ventralizing factor in early Xenopus development. Development. 1992 Jun;115(2):573–585. doi: 10.1242/dev.115.2.573. [DOI] [PubMed] [Google Scholar]
- Dale L., Slack J. M. Fate map for the 32-cell stage of Xenopus laevis. Development. 1987 Apr;99(4):527–551. doi: 10.1242/dev.99.4.527. [DOI] [PubMed] [Google Scholar]
- Dale L., Slack J. M. Regional specification within the mesoderm of early embryos of Xenopus laevis. Development. 1987 Jun;100(2):279–295. doi: 10.1242/dev.100.2.279. [DOI] [PubMed] [Google Scholar]
- Dale L., Smith J. C., Slack J. M. Mesoderm induction in Xenopus laevis: a quantitative study using a cell lineage label and tissue-specific antibodies. J Embryol Exp Morphol. 1985 Oct;89:289–312. [PubMed] [Google Scholar]
- Dohrmann C. E., Hemmati-Brivanlou A., Thomsen G. H., Fields A., Woolf T. M., Melton D. A. Expression of activin mRNA during early development in Xenopus laevis. Dev Biol. 1993 Jun;157(2):474–483. doi: 10.1006/dbio.1993.1150. [DOI] [PubMed] [Google Scholar]
- Driever W., Nüsslein-Volhard C. The bicoid protein is a positive regulator of hunchback transcription in the early Drosophila embryo. Nature. 1989 Jan 12;337(6203):138–143. doi: 10.1038/337138a0. [DOI] [PubMed] [Google Scholar]
- Ferguson E. L., Anderson K. V. Decapentaplegic acts as a morphogen to organize dorsal-ventral pattern in the Drosophila embryo. Cell. 1992 Oct 30;71(3):451–461. doi: 10.1016/0092-8674(92)90514-d. [DOI] [PubMed] [Google Scholar]
- Ferguson E. L., Anderson K. V. Localized enhancement and repression of the activity of the TGF-beta family member, decapentaplegic, is necessary for dorsal-ventral pattern formation in the Drosophila embryo. Development. 1992 Mar;114(3):583–597. doi: 10.1242/dev.114.3.583. [DOI] [PubMed] [Google Scholar]
- Gimlich R. L., Gerhart J. C. Early cellular interactions promote embryonic axis formation in Xenopus laevis. Dev Biol. 1984 Jul;104(1):117–130. doi: 10.1016/0012-1606(84)90042-3. [DOI] [PubMed] [Google Scholar]
- Green J. B., Howes G., Symes K., Cooke J., Smith J. C. The biological effects of XTC-MIF: quantitative comparison with Xenopus bFGF. Development. 1990 Jan;108(1):173–183. doi: 10.1242/dev.108.1.173. [DOI] [PubMed] [Google Scholar]
- Green J. B., New H. V., Smith J. C. Responses of embryonic Xenopus cells to activin and FGF are separated by multiple dose thresholds and correspond to distinct axes of the mesoderm. Cell. 1992 Nov 27;71(5):731–739. doi: 10.1016/0092-8674(92)90550-v. [DOI] [PubMed] [Google Scholar]
- Green J. B., Smith J. C. Graded changes in dose of a Xenopus activin A homologue elicit stepwise transitions in embryonic cell fate. Nature. 1990 Sep 27;347(6291):391–394. doi: 10.1038/347391a0. [DOI] [PubMed] [Google Scholar]
- Heasman J., Wylie C. C., Hausen P., Smith J. C. Fates and states of determination of single vegetal pole blastomeres of X. laevis. Cell. 1984 May;37(1):185–194. doi: 10.1016/0092-8674(84)90314-3. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A., Melton D. A. A truncated activin receptor inhibits mesoderm induction and formation of axial structures in Xenopus embryos. Nature. 1992 Oct 15;359(6396):609–614. doi: 10.1038/359609a0. [DOI] [PubMed] [Google Scholar]
- Herrmann B. G., Labeit S., Poustka A., King T. R., Lehrach H. Cloning of the T gene required in mesoderm formation in the mouse. Nature. 1990 Feb 15;343(6259):617–622. doi: 10.1038/343617a0. [DOI] [PubMed] [Google Scholar]
- Hirose G., Jacobson M. Clonal organization of the central nervous system of the frog. I. Clones stemming from individual blastomeres of the 16-cell and earlier stages. Dev Biol. 1979 Aug;71(2):191–202. doi: 10.1016/0012-1606(79)90163-5. [DOI] [PubMed] [Google Scholar]
- Howard J. E., Hirst E. M., Smith J. C. Are beta 1 integrins involved in Xenopus gastrulation? Mech Dev. 1992 Aug;38(2):109–119. doi: 10.1016/0925-4773(92)90003-3. [DOI] [PubMed] [Google Scholar]
- Isaacs H. V., Tannahill D., Slack J. M. Expression of a novel FGF in the Xenopus embryo. A new candidate inducing factor for mesoderm formation and anteroposterior specification. Development. 1992 Mar;114(3):711–720. doi: 10.1242/dev.114.3.711. [DOI] [PubMed] [Google Scholar]
- Jones C. M., Lyons K. M., Lapan P. M., Wright C. V., Hogan B. L. DVR-4 (bone morphogenetic protein-4) as a posterior-ventralizing factor in Xenopus mesoderm induction. Development. 1992 Jun;115(2):639–647. doi: 10.1242/dev.115.2.639. [DOI] [PubMed] [Google Scholar]
- Keller R. E. Vital dye mapping of the gastrula and neurula of Xenopus laevis. II. Prospective areas and morphogenetic movements of the deep layer. Dev Biol. 1976 Jul 1;51(1):118–137. doi: 10.1016/0012-1606(76)90127-5. [DOI] [PubMed] [Google Scholar]
- Keller R. Early embryonic development of Xenopus laevis. Methods Cell Biol. 1991;36:61–113. doi: 10.1016/s0091-679x(08)60273-3. [DOI] [PubMed] [Google Scholar]
- Kimelman D., Abraham J. A., Haaparanta T., Palisi T. M., Kirschner M. W. The presence of fibroblast growth factor in the frog egg: its role as a natural mesoderm inducer. Science. 1988 Nov 18;242(4881):1053–1056. doi: 10.1126/science.3194757. [DOI] [PubMed] [Google Scholar]
- Kimelman D., Christian J. L., Moon R. T. Synergistic principles of development: overlapping patterning systems in Xenopus mesoderm induction. Development. 1992 Sep;116(1):1–9. doi: 10.1242/dev.116.Supplement.1. [DOI] [PubMed] [Google Scholar]
- Kimelman D., Kirschner M. Synergistic induction of mesoderm by FGF and TGF-beta and the identification of an mRNA coding for FGF in the early Xenopus embryo. Cell. 1987 Dec 4;51(5):869–877. doi: 10.1016/0092-8674(87)90110-3. [DOI] [PubMed] [Google Scholar]
- Kispert A., Herrmann B. G. The Brachyury gene encodes a novel DNA binding protein. EMBO J. 1993 Aug;12(8):3211–3220. doi: 10.1002/j.1460-2075.1993.tb05990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köster M., Plessow S., Clement J. H., Lorenz A., Tiedemann H., Knöchel W. Bone morphogenetic protein 4 (BMP-4), a member of the TGF-beta family, in early embryos of Xenopus laevis: analysis of mesoderm inducing activity. Mech Dev. 1991 Mar;33(3):191–199. doi: 10.1016/0925-4773(91)90027-4. [DOI] [PubMed] [Google Scholar]
- Ling N., Ueno N., Ying S. Y., Esch F., Shimasaki S., Hotta M., Cuevas P., Guillemin R. Inhibins and activins. Vitam Horm. 1988;44:1–46. doi: 10.1016/s0083-6729(08)60692-5. [DOI] [PubMed] [Google Scholar]
- Lyons K., Graycar J. L., Lee A., Hashmi S., Lindquist P. B., Chen E. Y., Hogan B. L., Derynck R. Vgr-1, a mammalian gene related to Xenopus Vg-1, is a member of the transforming growth factor beta gene superfamily. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4554–4558. doi: 10.1073/pnas.86.12.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manova K., Paynton B. V., Bachvarova R. F. Expression of activins and TGF beta 1 and beta 2 RNAs in early postimplantation mouse embryos and uterine decidua. Mech Dev. 1992 Feb;36(3):141–152. doi: 10.1016/0925-4773(92)90065-r. [DOI] [PubMed] [Google Scholar]
- McMahon A. P., Moon R. T. Ectopic expression of the proto-oncogene int-1 in Xenopus embryos leads to duplication of the embryonic axis. Cell. 1989 Sep 22;58(6):1075–1084. doi: 10.1016/0092-8674(89)90506-0. [DOI] [PubMed] [Google Scholar]
- Mitrani E., Gruenbaum Y., Shohat H., Ziv T. Fibroblast growth factor during mesoderm induction in the early chick embryo. Development. 1990 Jun;109(2):387–393. doi: 10.1242/dev.109.2.387. [DOI] [PubMed] [Google Scholar]
- Mitrani E., Ziv T., Thomsen G., Shimoni Y., Melton D. A., Bril A. Activin can induce the formation of axial structures and is expressed in the hypoblast of the chick. Cell. 1990 Nov 2;63(3):495–501. doi: 10.1016/0092-8674(90)90446-l. [DOI] [PubMed] [Google Scholar]
- Moody S. A. Fates of the blastomeres of the 32-cell-stage Xenopus embryo. Dev Biol. 1987 Aug;122(2):300–319. doi: 10.1016/0012-1606(87)90296-x. [DOI] [PubMed] [Google Scholar]
- Moon R. T., Christian J. L. Competence modifiers synergize with growth factors during mesoderm induction and patterning in Xenopus. Cell. 1992 Nov 27;71(5):709–712. doi: 10.1016/0092-8674(92)90545-n. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Asashima M., Eto Y., Takio K., Uchiyama H., Moriya N., Ariizumi T., Yashiro T., Sugino K., Titani K. Isolation and characterization of native activin B. J Biol Chem. 1992 Aug 15;267(23):16385–16389. [PubMed] [Google Scholar]
- Niswander L., Martin G. R. Fgf-4 expression during gastrulation, myogenesis, limb and tooth development in the mouse. Development. 1992 Mar;114(3):755–768. doi: 10.1242/dev.114.3.755. [DOI] [PubMed] [Google Scholar]
- Padgett R. W., St Johnston R. D., Gelbart W. M. A transcript from a Drosophila pattern gene predicts a protein homologous to the transforming growth factor-beta family. Nature. 1987 Jan 1;325(6099):81–84. doi: 10.1038/325081a0. [DOI] [PubMed] [Google Scholar]
- Rebagliati M. R., Weeks D. L., Harvey R. P., Melton D. A. Identification and cloning of localized maternal RNAs from Xenopus eggs. Cell. 1985 Oct;42(3):769–777. doi: 10.1016/0092-8674(85)90273-9. [DOI] [PubMed] [Google Scholar]
- Rosa F. M. Mix.1, a homeobox mRNA inducible by mesoderm inducers, is expressed mostly in the presumptive endodermal cells of Xenopus embryos. Cell. 1989 Jun 16;57(6):965–974. doi: 10.1016/0092-8674(89)90335-8. [DOI] [PubMed] [Google Scholar]
- Rosa F., Roberts A. B., Danielpour D., Dart L. L., Sporn M. B., Dawid I. B. Mesoderm induction in amphibians: the role of TGF-beta 2-like factors. Science. 1988 Feb 12;239(4841 Pt 1):783–785. doi: 10.1126/science.3422517. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A., Melton D. A. Bimodal and graded expression of the Xenopus homeobox gene Xhox3 during embryonic development. Development. 1989 May;106(1):173–183. doi: 10.1242/dev.106.1.173. [DOI] [PubMed] [Google Scholar]
- Sargent M. G., Bennett M. F. Identification in Xenopus of a structural homologue of the Drosophila gene snail. Development. 1990 Aug;109(4):967–973. doi: 10.1242/dev.109.4.967. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S., Ho R. K., Herrmann B. G., Nüsslein-Volhard C. The protein product of the zebrafish homologue of the mouse T gene is expressed in nuclei of the germ ring and the notochord of the early embryo. Development. 1992 Dec;116(4):1021–1032. doi: 10.1242/dev.116.4.1021. [DOI] [PubMed] [Google Scholar]
- Shiurba R. A., Jing N., Sakakura T., Godsave S. F. Nuclear translocation of fibroblast growth factor during Xenopus mesoderm induction. Development. 1991 Oct;113(2):487–493. doi: 10.1242/dev.113.2.487. [DOI] [PubMed] [Google Scholar]
- Sive H. L. The frog prince-ss: a molecular formula for dorsoventral patterning in Xenopus. Genes Dev. 1993 Jan;7(1):1–12. doi: 10.1101/gad.7.1.1. [DOI] [PubMed] [Google Scholar]
- Slack J. M., Darlington B. G., Heath J. K., Godsave S. F. Mesoderm induction in early Xenopus embryos by heparin-binding growth factors. Nature. 1987 Mar 12;326(6109):197–200. doi: 10.1038/326197a0. [DOI] [PubMed] [Google Scholar]
- Slack J. M., Forman D. An interaction between dorsal and ventral regions of the marginal zone in early amphibian embryos. J Embryol Exp Morphol. 1980 Apr;56:283–299. [PubMed] [Google Scholar]
- Slack J. M. The nature of the mesoderm-inducing signal in Xenopus: a transfilter induction study. Development. 1991 Oct;113(2):661–669. doi: 10.1242/dev.113.2.661. [DOI] [PubMed] [Google Scholar]
- Smith J. C. A mesoderm-inducing factor is produced by Xenopus cell line. Development. 1987 Jan;99(1):3–14. doi: 10.1242/dev.99.1.3. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Dale L., Slack J. M. Cell lineage labels and region-specific markers in the analysis of inductive interactions. J Embryol Exp Morphol. 1985 Nov;89 (Suppl):317–331. [PubMed] [Google Scholar]
- Smith J. C., Howard J. E. Mesoderm-inducing factors and the control of gastrulation. Dev Suppl. 1992:127–136. [PubMed] [Google Scholar]
- Smith J. C., Malacinski G. M. The origin of the mesoderm in an anuran, Xenopus laevis, and a urodele, Ambystoma mexicanum. Dev Biol. 1983 Jul;98(1):250–254. doi: 10.1016/0012-1606(83)90354-8. [DOI] [PubMed] [Google Scholar]
- Smith J. C. Mesoderm induction and mesoderm-inducing factors in early amphibian development. Development. 1989 Apr;105(4):665–677. doi: 10.1242/dev.105.4.665. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Price B. M., Green J. B., Weigel D., Herrmann B. G. Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell. 1991 Oct 4;67(1):79–87. doi: 10.1016/0092-8674(91)90573-h. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Price B. M., Van Nimmen K., Huylebroeck D. Identification of a potent Xenopus mesoderm-inducing factor as a homologue of activin A. Nature. 1990 Jun 21;345(6277):729–731. doi: 10.1038/345729a0. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Singh J. P., Lillquist J. S., Goon D. S., Stiles C. D. Growth factors adherent to cell substrate are mitogenically active in situ. Nature. 1982 Mar 11;296(5853):154–156. doi: 10.1038/296154a0. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Slack J. M. Dorsalization and neural induction: properties of the organizer in Xenopus laevis. J Embryol Exp Morphol. 1983 Dec;78:299–317. [PubMed] [Google Scholar]
- Smith J. C., Stiles C. D. Cytoplasmic transfer of the mitogenic response to platelet-derived growth factor. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4363–4367. doi: 10.1073/pnas.78.7.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. C., Symes K., Hynes R. O., DeSimone D. Mesoderm induction and the control of gastrulation in Xenopus laevis: the roles of fibronectin and integrins. Development. 1990 Feb;108(2):229–238. doi: 10.1242/dev.108.2.229. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Tickle C., Wolpert L. Attenuation of positional signalling in the chick limb by high doses of gamma-radiation. Nature. 1978 Apr 13;272(5654):612–613. doi: 10.1038/272612a0. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Watt F. M. Biochemical specificity of Xenopus notochord. Differentiation. 1985;29(2):109–115. doi: 10.1111/j.1432-0436.1985.tb00302.x. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Yaqoob M., Symes K. Purification, partial characterization and biological effects of the XTC mesoderm-inducing factor. Development. 1988 Jul;103(3):591–600. doi: 10.1242/dev.103.3.591. [DOI] [PubMed] [Google Scholar]
- Smith W. C., Harland R. M. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992 Sep 4;70(5):829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- Smith W. C., Harland R. M. Injected Xwnt-8 RNA acts early in Xenopus embryos to promote formation of a vegetal dorsalizing center. Cell. 1991 Nov 15;67(4):753–765. doi: 10.1016/0092-8674(91)90070-f. [DOI] [PubMed] [Google Scholar]
- Smith W. C., Knecht A. K., Wu M., Harland R. M. Secreted noggin protein mimics the Spemann organizer in dorsalizing Xenopus mesoderm. Nature. 1993 Feb 11;361(6412):547–549. doi: 10.1038/361547a0. [DOI] [PubMed] [Google Scholar]
- Snape A., Wylie C. C., Smith J. C., Heasman J. Changes in states of commitment of single animal pole blastomeres of Xenopus laevis. Dev Biol. 1987 Feb;119(2):503–510. doi: 10.1016/0012-1606(87)90053-4. [DOI] [PubMed] [Google Scholar]
- Sokol S., Christian J. L., Moon R. T., Melton D. A. Injected Wnt RNA induces a complete body axis in Xenopus embryos. Cell. 1991 Nov 15;67(4):741–752. doi: 10.1016/0092-8674(91)90069-b. [DOI] [PubMed] [Google Scholar]
- Sokol S., Wong G. G., Melton D. A. A mouse macrophage factor induces head structures and organizes a body axis in Xenopus. Science. 1990 Aug 3;249(4968):561–564. doi: 10.1126/science.2382134. [DOI] [PubMed] [Google Scholar]
- St Johnston D., Nüsslein-Volhard C. The origin of pattern and polarity in the Drosophila embryo. Cell. 1992 Jan 24;68(2):201–219. doi: 10.1016/0092-8674(92)90466-p. [DOI] [PubMed] [Google Scholar]
- St Johnston R. D., Gelbart W. M. Decapentaplegic transcripts are localized along the dorsal-ventral axis of the Drosophila embryo. EMBO J. 1987 Sep;6(9):2785–2791. doi: 10.1002/j.1460-2075.1987.tb02574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles C. D., Capone G. T., Scher C. D., Antoniades H. N., Van Wyk J. J., Pledger W. J. Dual control of cell growth by somatomedins and platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1279–1283. doi: 10.1073/pnas.76.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G., Struhl K., Macdonald P. M. The gradient morphogen bicoid is a concentration-dependent transcriptional activator. Cell. 1989 Jun 30;57(7):1259–1273. doi: 10.1016/0092-8674(89)90062-7. [DOI] [PubMed] [Google Scholar]
- Strähle U., Blader P., Henrique D., Ingham P. W. Axial, a zebrafish gene expressed along the developing body axis, shows altered expression in cyclops mutant embryos. Genes Dev. 1993 Jul;7(7B):1436–1446. doi: 10.1101/gad.7.7b.1436. [DOI] [PubMed] [Google Scholar]
- Thomsen G., Woolf T., Whitman M., Sokol S., Vaughan J., Vale W., Melton D. A. Activins are expressed early in Xenopus embryogenesis and can induce axial mesoderm and anterior structures. Cell. 1990 Nov 2;63(3):485–493. doi: 10.1016/0092-8674(90)90445-k. [DOI] [PubMed] [Google Scholar]
- Tiedemann H., Lottspeich F., Davids M., Knöchel S., Hoppe P., Tiedemann H. The vegetalizing factor. A member of the evolutionarily highly conserved activin family. FEBS Lett. 1992 Mar 30;300(2):123–126. doi: 10.1016/0014-5793(92)80178-j. [DOI] [PubMed] [Google Scholar]
- Wagner M., Thaller C., Jessell T., Eichele G. Polarizing activity and retinoid synthesis in the floor plate of the neural tube. Nature. 1990 Jun 28;345(6278):819–822. doi: 10.1038/345819a0. [DOI] [PubMed] [Google Scholar]
- Weeks D. L., Melton D. A. A maternal mRNA localized to the vegetal hemisphere in Xenopus eggs codes for a growth factor related to TGF-beta. Cell. 1987 Dec 4;51(5):861–867. doi: 10.1016/0092-8674(87)90109-7. [DOI] [PubMed] [Google Scholar]
- Weisblat D. A., Sawyer R. T., Stent G. S. Cell lineage analysis by intracellular injection of a tracer enzyme. Science. 1978 Dec 22;202(4374):1295–1298. doi: 10.1126/science.725606. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Bhatt S., Herrmann B. G. Expression pattern of the mouse T gene and its role in mesoderm formation. Nature. 1990 Feb 15;343(6259):657–659. doi: 10.1038/343657a0. [DOI] [PubMed] [Google Scholar]
- Wolpert L. Positional information and the spatial pattern of cellular differentiation. J Theor Biol. 1969 Oct;25(1):1–47. doi: 10.1016/s0022-5193(69)80016-0. [DOI] [PubMed] [Google Scholar]
- Wozney J. M., Rosen V., Celeste A. J., Mitsock L. M., Whitters M. J., Kriz R. W., Hewick R. M., Wang E. A. Novel regulators of bone formation: molecular clones and activities. Science. 1988 Dec 16;242(4885):1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- Wright C. V., Morita E. A., Wilkin D. J., De Robertis E. M. The Xenopus XIHbox 6 homeo protein, a marker of posterior neural induction, is expressed in proliferating neurons. Development. 1990 May;109(1):225–234. doi: 10.1242/dev.109.1.225. [DOI] [PubMed] [Google Scholar]
- Wylie C. C., Snape A., Heasman J., Smith J. C. Vegetal pole cells and commitment to form endoderm in Xenopus laevis. Dev Biol. 1987 Feb;119(2):496–502. doi: 10.1016/0012-1606(87)90052-2. [DOI] [PubMed] [Google Scholar]