Abstract
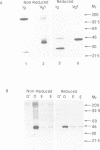
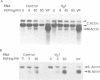
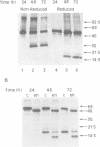
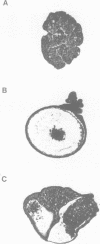
Vg1 is a maternal mRNA localized to the vegetal hemisphere of Xenopus embryos during blastula stages, a region responsible for the induction of mesoderm in the adjacent marginal zone. Its homology to the transforming growth factor-beta family, which includes several proteins with mesoderm-inducing activity, suggests a role for Vg1 as an endogenous mesoderm-inducing factor. However, expression of Vg1 protein in the animal hemisphere, following injection of synthetic mRNA, has no effect on development, and isolated animal caps are not mesodermalized. It is shown that Vg1 protein fails to form dimers and is not processed to release the putative bioactive domain. Furthermore it is shown that the N-terminal signal peptide of Vg1 is not cleaved following translocation into the ER, which may explain the failure of this protein to dimerize. To explore the role of Vg1 in amphibian development, a fusion protein has been made of the preproregion of Xenopus bone morphogenetic protein-4 and the putative bioactive C-terminal domain of Vg1. This fusion protein forms dimers and the C-terminal domain of Vg1 is secreted. Injection of this construct into Xenopus embryos induces the formation of a second dorsal axis and isolated animal caps are mesodermalized. The results are consistent with a role for Vg1 in mesoderm induction during Xenopus development.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaya E., Musci T. J., Kirschner M. W. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991 Jul 26;66(2):257–270. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- Asashima M., Nakano H., Uchiyama H., Sugino H., Nakamura T., Eto Y., Ejima D., Nishimatsu S., Ueno N., Kinoshita K. Presence of activin (erythroid differentiation factor) in unfertilized eggs and blastulae of Xenopus laevis. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6511–6514. doi: 10.1073/pnas.88.15.6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley R. S., Brown A. M. The proto-oncogene int-1 encodes a secreted protein associated with the extracellular matrix. EMBO J. 1990 May;9(5):1569–1575. doi: 10.1002/j.1460-2075.1990.tb08276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnahan P. A., Leduc R., Thomas L., Thorner J., Gibson H. L., Brake A. J., Barr P. J., Thomas G. Human fur gene encodes a yeast KEX2-like endoprotease that cleaves pro-beta-NGF in vivo. J Cell Biol. 1990 Dec;111(6 Pt 2):2851–2859. doi: 10.1083/jcb.111.6.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceriotti A., Colman A. Binding to membrane proteins within the endoplasmic reticulum cannot explain the retention of the glucose-regulated protein GRP78 in Xenopus oocytes. EMBO J. 1988 Mar;7(3):633–638. doi: 10.1002/j.1460-2075.1988.tb02857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceriotti A., Colman A. Protein transport from endoplasmic reticulum to the Golgi complex can occur during meiotic metaphase in Xenopus oocytes. J Cell Biol. 1989 Oct;109(4 Pt 1):1439–1444. doi: 10.1083/jcb.109.4.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti A., Matthews G., Colman A., Dale L. Secretory and inductive properties of Drosophila wingless protein in Xenopus oocytes and embryos. Development. 1992 May;115(1):355–369. doi: 10.1242/dev.115.1.355. [DOI] [PubMed] [Google Scholar]
- Christian J. L., Olson D. J., Moon R. T. Xwnt-8 modifies the character of mesoderm induced by bFGF in isolated Xenopus ectoderm. EMBO J. 1992 Jan;11(1):33–41. doi: 10.1002/j.1460-2075.1992.tb05024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale L., Howes G., Price B. M., Smith J. C. Bone morphogenetic protein 4: a ventralizing factor in early Xenopus development. Development. 1992 Jun;115(2):573–585. doi: 10.1242/dev.115.2.573. [DOI] [PubMed] [Google Scholar]
- Dale L., Matthews G., Tabe L., Colman A. Developmental expression of the protein product of Vg1, a localized maternal mRNA in the frog Xenopus laevis. EMBO J. 1989 Apr;8(4):1057–1065. doi: 10.1002/j.1460-2075.1989.tb03473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale L., Slack J. M. Fate map for the 32-cell stage of Xenopus laevis. Development. 1987 Apr;99(4):527–551. doi: 10.1242/dev.99.4.527. [DOI] [PubMed] [Google Scholar]
- Garbers D. L. Guanylyl cyclase receptors and their endocrine, paracrine, and autocrine ligands. Cell. 1992 Oct 2;71(1):1–4. doi: 10.1016/0092-8674(92)90258-e. [DOI] [PubMed] [Google Scholar]
- Gray A. M., Mason A. J. Requirement for activin A and transforming growth factor--beta 1 pro-regions in homodimer assembly. Science. 1990 Mar 16;247(4948):1328–1330. doi: 10.1126/science.2315700. [DOI] [PubMed] [Google Scholar]
- Hammonds R. G., Jr, Schwall R., Dudley A., Berkemeier L., Lai C., Lee J., Cunningham N., Reddi A. H., Wood W. I., Mason A. J. Bone-inducing activity of mature BMP-2b produced from a hybrid BMP-2a/2b precursor. Mol Endocrinol. 1991 Jan;5(1):149–155. doi: 10.1210/mend-5-1-149. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A., Melton D. A. A truncated activin receptor inhibits mesoderm induction and formation of axial structures in Xenopus embryos. Nature. 1992 Oct 15;359(6396):609–614. doi: 10.1038/359609a0. [DOI] [PubMed] [Google Scholar]
- Hurtley S. M., Helenius A. Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- Jones C. M., Lyons K. M., Lapan P. M., Wright C. V., Hogan B. L. DVR-4 (bone morphogenetic protein-4) as a posterior-ventralizing factor in Xenopus mesoderm induction. Development. 1992 Jun;115(2):639–647. doi: 10.1242/dev.115.2.639. [DOI] [PubMed] [Google Scholar]
- Kimelman D., Christian J. L., Moon R. T. Synergistic principles of development: overlapping patterning systems in Xenopus mesoderm induction. Development. 1992 Sep;116(1):1–9. doi: 10.1242/dev.116.Supplement.1. [DOI] [PubMed] [Google Scholar]
- Kimelman D., Kirschner M. Synergistic induction of mesoderm by FGF and TGF-beta and the identification of an mRNA coding for FGF in the early Xenopus embryo. Cell. 1987 Dec 4;51(5):869–877. doi: 10.1016/0092-8674(87)90110-3. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. In vitro RNA synthesis with SP6 RNA polymerase. Methods Enzymol. 1987;155:397–415. doi: 10.1016/0076-6879(87)55027-3. [DOI] [PubMed] [Google Scholar]
- Köster M., Plessow S., Clement J. H., Lorenz A., Tiedemann H., Knöchel W. Bone morphogenetic protein 4 (BMP-4), a member of the TGF-beta family, in early embryos of Xenopus laevis: analysis of mesoderm inducing activity. Mech Dev. 1991 Mar;33(3):191–199. doi: 10.1016/0925-4773(91)90027-4. [DOI] [PubMed] [Google Scholar]
- Lyons K. M., Jones C. M., Hogan B. L. The DVR gene family in embryonic development. Trends Genet. 1991 Nov-Dec;7(11-12):408–412. doi: 10.1016/0168-9525(91)90265-r. [DOI] [PubMed] [Google Scholar]
- Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- Matthews G., Colman A. A highly efficient, cell-free translation/translocation system prepared from Xenopus eggs. Nucleic Acids Res. 1991 Dec 11;19(23):6405–6412. doi: 10.1093/nar/19.23.6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohun T. J., Brennan S., Dathan N., Fairman S., Gurdon J. B. Cell type-specific activation of actin genes in the early amphibian embryo. Nature. 1984 Oct 25;311(5988):716–721. doi: 10.1038/311716a0. [DOI] [PubMed] [Google Scholar]
- Nishimatsu S., Suzuki A., Shoda A., Murakami K., Ueno N. Genes for bone morphogenetic proteins are differentially transcribed in early amphibian embryos. Biochem Biophys Res Commun. 1992 Aug 14;186(3):1487–1495. doi: 10.1016/s0006-291x(05)81574-8. [DOI] [PubMed] [Google Scholar]
- Papkoff J., Schryver B. Secreted int-1 protein is associated with the cell surface. Mol Cell Biol. 1990 Jun;10(6):2723–2730. doi: 10.1128/mcb.10.6.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters B. P., Krzesicki R. F., Hartle R. J., Perini F., Ruddon R. W. A kinetic comparison of the processing and secretion of the alpha beta dimer and the uncombined alpha and beta subunits of chorionic gonadotropin synthesized by human choriocarcinoma cells. J Biol Chem. 1984 Dec 25;259(24):15123–15130. [PubMed] [Google Scholar]
- Sive H. L. The frog prince-ss: a molecular formula for dorsoventral patterning in Xenopus. Genes Dev. 1993 Jan;7(1):1–12. doi: 10.1101/gad.7.1.1. [DOI] [PubMed] [Google Scholar]
- Slack J. M., Darlington B. G., Heath J. K., Godsave S. F. Mesoderm induction in early Xenopus embryos by heparin-binding growth factors. Nature. 1987 Mar 12;326(6109):197–200. doi: 10.1038/326197a0. [DOI] [PubMed] [Google Scholar]
- Smith W. C., Harland R. M. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992 Sep 4;70(5):829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- Smith W. C., Harland R. M. Injected Xwnt-8 RNA acts early in Xenopus embryos to promote formation of a vegetal dorsalizing center. Cell. 1991 Nov 15;67(4):753–765. doi: 10.1016/0092-8674(91)90070-f. [DOI] [PubMed] [Google Scholar]
- Sokol S., Christian J. L., Moon R. T., Melton D. A. Injected Wnt RNA induces a complete body axis in Xenopus embryos. Cell. 1991 Nov 15;67(4):741–752. doi: 10.1016/0092-8674(91)90069-b. [DOI] [PubMed] [Google Scholar]
- Tabe L., Krieg P., Strachan R., Jackson D., Wallis E., Colman A. Segregation of mutant ovalbumins and ovalbumin-globin fusion proteins in Xenopus oocytes. Identification of an ovalbumin signal sequence. J Mol Biol. 1984 Dec 15;180(3):645–666. doi: 10.1016/0022-2836(84)90031-7. [DOI] [PubMed] [Google Scholar]
- Tannahill D., Melton D. A. Localized synthesis of the Vg1 protein during early Xenopus development. Development. 1989 Aug;106(4):775–785. doi: 10.1242/dev.106.4.775. [DOI] [PubMed] [Google Scholar]
- Weeks D. L., Melton D. A. A maternal mRNA localized to the vegetal hemisphere in Xenopus eggs codes for a growth factor related to TGF-beta. Cell. 1987 Dec 4;51(5):861–867. doi: 10.1016/0092-8674(87)90109-7. [DOI] [PubMed] [Google Scholar]
- Wiren K. M., Ivashkiv L., Ma P., Freeman M. W., Potts J. T., Jr, Kronenberg H. M. Mutations in signal sequence cleavage domain of preproparathyroid hormone alter protein translocation, signal sequence cleavage, and membrane-binding properties. Mol Endocrinol. 1989 Feb;3(2):240–250. doi: 10.1210/mend-3-2-240. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. The signal peptide. J Membr Biol. 1990 May;115(3):195–201. doi: 10.1007/BF01868635. [DOI] [PubMed] [Google Scholar]