Abstract
Sedatives alter the metrics of saccadic eye movements. If these effects are nonspecific consequences of sedation, like drowsiness and loss of attention to the task, or differ between sedatives is still unresolved. A placebo-controlled multi-step infusion of one of three sedatives, propofol or midazolam, both GABA-A agonists, or dexmedetedomidine, an α2-adrenergic agonist, was adopted to compare the effects of these three drugs in exactly the same experimental conditions. 60 healthy human volunteers, randomly divided in 4 groups, participated in the study. Each infusion step, delivered by a computer-controlled infusion pump, lasted 20 min. During the last 10 min of each step, the subject executed a saccadic task. Target concentration was doubled at each step. This block was repeated until the subject was too sedated to continue or for a maximum of 6 blocks. Subjects were unaware which infusion they were receiving. A video eye tracker was used to record the movements of the right eye. Saccadic parameters were modeled as a function of block number, estimated sedative plasma concentration, and subjective evaluation of sedation. Propofol and midazolam had strong effects on the dynamics and latency of the saccades. Midazolam, and to a less extent, propofol, caused saccades to become increasingly hypometric. Dexmedetedomidine had less impact on saccadic metrics and presented no changes in saccadic gain. Suppression of the sympathetic system associated with dexmedetomidine has different effects on eye movements from the increased activity of the inhibitory GABA-A receptors by propofol and midazolam even when the subjects reported similar sedation level.
Keywords: sedative, saccade, main sequence, oculomotor, human
Sedatives alter the dynamics of visually-driven saccadic eye movements between stationary targets. It is not known if these changes are nonspecifically linked to the sedated state of the subject, like drowsiness and loss of attention to the task, or if they also depend on the type of sedative. We measured the effects of three commonly used sedatives, propofol, midazolam, and dexmedetomidine, and of saline control on saccadic responses. Dexmedetomidine has a different pharmacological mechanism of sedation than propofol and midazolam, and therefore it is a potential candidate to verify if the effects on saccades are drug-specific. The intensity of “placebo effects” on saccadic eye movements inside a sedation study, where the subject does not know if receiving a sedative or saline, is not well quantified in the literature. Using the placebo group, we were also able to quantify how much of the observed changes in saccadic behavior during the session were associated with the experimental paradigm per se, most likely fatigue, boredom, and on-the-task learning, all being naïve subjects to oculomotor tasks inside a controlled laboratory setting. In some subjects, physical and/or psychological effects associated with the two IV needles might have also influenced the execution of the task and the overall number of blocks obtained from the subject. The finding of an irregular, but still very significant on average, increase in the self-reported sedation level inside the placebo group as the session progressed was also used as an additional tool in determining the importance of the subjective state of sedation on eye movements.
Propofol is widely used perioperatively to induce and maintain anesthesia and for procedural sedation. Midazolam is used to induce sedation and amnesia before medical procedures, for prolonged sedation in individuals receiving mechanical ventilation, and as anxiolytic. Both are agonists of the gamma-aminobutyric acid type A (GABA-A) benzodiazepine receptors. These receptors are present in several brain areas and have an inhibitory action on their target neurons. Ibotenic acid and its derivative muscimol, also GABA-A agonists, are commonly used for microinjections in experimental animals to induce a reversible inhibitory action on the targeted brain location. It is not surprising, therefore, that significant effects on the peak velocity of saccades were reported for propofol [1], midazolam [2,3], and diazepam [4]. Propofol was also found to reduce ocular microtremor [5,6], which is a small high frequency random tremor of the eyes linked to neural activity in the brainstem and midbrain reticular formation [7].
As third sedative we used dexmedetomidine, which is a selective α2-adrenoceptor agonist [8]. Virtanen et al. [9] found that medetomidine – dexmedetomidine is the pharmacologically active d-isomer of medetomidine – has no binding activity with benzodiazepine receptors. By activating the inhibitory α2-adrenoceptors both at the central level and at the peripheral sympathetic nerve endings, it inhibits, in a dose-dependent function, the release of noradrenaline, with a corresponding reduction in the sympathetic neural activity. It is commonly used as short-term sedative in mechanically ventilated critically ill patients, as adjunct to anaesthesia, and as sedative for invasive procedures. This drug has sedative, analgesic, and antishivering properties [10] without causing respiratory depression. The sedated patient remains cooperative [11], which is a critical factor in many procedures and makes it a highly desirable alternative, in several applications, to benzodiazepines. The brain area presenting the strongest attenuation of activity during dexmedetomidine sedation in rats is the locus coeruleus [12], the principal site in the brain for the synthesis of noradrenaline. Located in the rostral pons, it projects to several areas, including spinal cord, brainstem, cerebellum, hypothalamus, thalamic relay nuclei, amygdala, basal telencephalon, and cortex [13–16]. The main afferents to the locus coeruleus are from the paragigantocellularis and the prepositus hypoglossi nuclei in the rostral medulla [17,18]. The prepositus hypoglossi is part of the oculomotor neural integrator responsible for maintaining horizontal gaze [19] and its action on the locus coeruleus seems to regulate REM sleep [20]. Saccadic peak velocity is affected by dexmedetomidine [21]. Our study is the first to compare these three sedatives and saline in exactly the same paradigm configuration. We also determined the optimal concentration of each drug for single-dosage studies, i.e., the value that produced the strongest oculomotor effects at the group level with the majority of the subjects still able to perform the saccadic task.
1. Methods and procedures
1.1 Subjects
Sixty healthy volunteers (25 males, 35 females, age 19 to 56) were randomly assigned to one of four groups (placebo, propofol, midazolam, dexmedetomidine) of 15 subjects each. All subjects had a preliminary physical examination prior to the day of the test, and at the day of the test females were tested for pregnancy. Sedation monitoring followed the guidelines of the American Society of Anesthesiology, which include continuous evaluation of respiration and circulation using pulse oximetry, non-invasive blood pressure monitoring, and ECG. At the end of the session the subject rested for as long as necessary, and was released only when the accompanying person, identified by the subject at the beginning of the session, arrived at the clinic. Written instructions were given to the subject not to drive or do other potentially dangerous tasks for the remainder of the day. The study was approved by the University of Alabama at Birmingham Institutional Review Board (IRB) and it adhered to the tenets of the Declaration of Helsinki for clinical research. Subjects were previously informed about the experimental protocol and the possible effects of the sedatives and had signed an IRB-approved informed consent.
1.2 Protocols and data acquisition
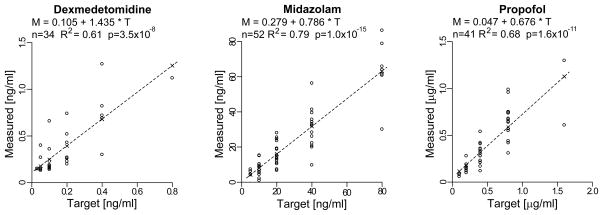
All subjects were naïve to oculomotor tasks performed in a controlled laboratory setting and to the purpose of the experiment. After some saccadic training trials and a brief analog calibration of the eye signals, the subjects received computer-assisted infusions with a Graseby® 3400 infusion pump. The profile of the infusion rate was designed to stepwise increase the plasma drug concentration [22], with each step lasting approximately 20 min. The subject rested for the first 10 min to give time to the blood concentration to stabilize, and saccadic testing was carried out during the last 10 min of each step. At the end of the saccadic task, the subjects were asked to self-evaluate their level of sedation (SLS) by using a visual analogue scale of sedation ranging from fully awake (perceived sedation level 0) to very sedated (perceived sedation level 10), and a venous blood sample for verification of actual plasma concentration was obtained from an intravenous cannula on the arm opposite to the side of the infusion. The infusion was then stepped to the next target concentration. The sequences of rest/testing/SLS/blood-sampling (blocks) were repeated until the subject was too sedated to continue or for a maximum of 6 blocks. An average of 150–250 trials was acquired in each block. Subjects were unaware of what they were given and, during the initial block (BLOCK=0), all subjects received a saline infusion in order to obtain the subject’s baseline saccadic metrics. For the placebo group, the subsequent blocks continued to be saline. For the other three groups, the saline was replaced by the sedative, and the target blood concentration was set to double at each subsequent block. For dexmedetomidine, the set of target concentrations was 0.0125, 0.025, 0.05, 0.10, 0.20, 0.40, and 0.80 ng/ml. For midazolam it was 5, 10, 20, 40, and 80 ng/ml. For propofol it was 0.05, 0.10, 0.20, 0.40, 0.80, and 1.60 μg/ml. In order to take into account differences in individual sensitivity to the sedative, we varied the starting value of the target concentration between subjects. This assured that a sufficient number of subjects received their highest tolerable drug concentration, in terms of still being able to perform the saccadic task, between blocks #3 and #5. For example, some subjects in the propofol group had assigned target concentrations of 0, 0.05, 0.10, 0.20, 0.40 μg/ml, while others covered the 0, 0.20, 0.40, 0.80, 1.60 μg/ml values. The measured concentrations from the blood samples, using a gas chromatographic--mass spectrometric procedure, are illustrated in Fig. 1, together with the linear regressions that were used to compute the group-wide estimated blood concentration values (indicated in the plots by ×) at each target value, which were used in the subsequent statistical and model analyses (CONC values).
Fig. 1.
Comparison between target (x-axis) and measured (y-axis) blood concentrations for the three sedatives. A linear model (top of graphs and dashed lines) was used to determine the group-wide estimated blood concentrations (×) at each target value utilized in the saccadic analysis (CONC values). For each linear model we also report the number of available samples n, the adjusted R2, and the p value of the slope of the linear regression.
The subjects were seated in a hospital sleeper chair that was modified to carry a chin rest and two temporal pads to minimize head movements. The room was dimly illuminated by the room window and/or indirect light. A board with LEDs was placed in front of the subject at a distance of 80 cm. The board had custom-made attachments for the 240 frame/s infrared video camera and the infrared illuminator of the ISCAN® video eye tracker that was used to follow the horizontal and vertical positions of the right eye. Its analog outputs were acquired by a real-time Ubuntu Linux system at 1000 Hz, which also controlled the stimulus presentation. Each saccadic trial started with a brief sound, after which the center target was turned on and the subject had to acquire and fixate the center target for 500 ms. Proper fixation was determined by software windows on the eye position signals. The center target was then extinguished, and, at the same time, a second target randomly appeared at one of 12 possible locations: 5°, 10°, or 15° right, left, up or down. The subject had to acquire and fixate the new target for 500 ms before it was turned off. The computer started a new trial after the subject had pressed a button on a keypad after the end of the second fixation. As control of the subject’s alertness, the subject had 2000 ms to acquire each target from the time when it was turned on. If he/she failed to do so, the trial was aborted, the target turned off, and the computer waited for the pressing of the push button to start a new trial.
1.3 Data analysis
The data were transferred to Red-Hat Linux systems, where each trial was visually inspected for blinks and artifacts. Eye traces were calibrated with a third-order polynomial correction using the initial saline set as calibration data. The position data were filtered with a cubic spline and velocity traces were obtained by a 2-point backward differentiation of the splined position data. The analysis was restricted to the primary saccade. Saccadic onset and offset were automatically determined by a fitting algorithm [23], and using these two points in time as anchors, we determined saccadic latency (LAT), size (SIZE), duration (DUR), peak velocity (PKV), and gain (GAIN), the latter defined as the ratio between the size of the primary saccade and the size of the target step from the center. From the observation that saccadic latency follows a reciprobit distribution [24–26] and therefore the reciprocal of the latency has a normal distribution, the latency analysis was done on the reciprocal of saccadic latency (RLAT). The latency, from the offset of the second target, of the pressing of the push-button needed to start a new trial was also extracted. This latency presented a very strong learning component, with the subject pressing the button closer to the end of the saccade as the session progressed in order to move more rapidly to the next trial. Pressing of the button before the turning off of the second target was ignored and the subject had to press it again. Although there was often an increase in this latency with the highest levels of sedation with, as a result, a U-shape function, the strong learning component made this measure of little value and was abandoned.
We first determined the drug dosages that gave the most significant effects in the majority of the subjects. These dosages are not necessarily the highest target concentrations we used, because some subjects stopped working at those values or even earlier. They can be seen as the optimal values for single-dosage studies because maximizing the oculomotor effects while still having the highest number of subjects able to perform the required behavioral task. The analysis adopted for this determination is described below.
The saccadic metrics of the initial saline sets varied between subjects and, within each subject, not only with the size of the target step, as expected by the saccadic main sequences, but often also with saccadic direction. With our focus here on the relative changes in saccadic metrics with respect to the initial saline set of the subject, for each subject we first normalized all measures with respect to the corresponding average of the values obtained during the initial saline step for the same target step and direction. From the models, which we will describe later, we verified that all saccadic directions were, on average, equally affected by the drugs. Thus, the normalization with the initial saline set allowed, for each BLOCK (block number) or CONC (group-wide estimated blood concentration of the sedative, see also Fig. 1), the pooling together, for each subject, of all the values for all saccadic directions and target steps. Note that, for the peak velocity and duration measures, these normalized values combine both drug-related changes in the peak-velocity and duration main sequences [27,28] and changes in the gain of the saccadic responses, the main sequences being related to the size of the saccade, not the size of the target step (see also our model analysis later on). As intra-subject analysis, we used non-paired t-tests (separate variances) between the normalized saline values (CONC = 0, BLOCK = 0) and the normalized values obtained at each BLOCK > 0 or CONC > 0. At the group level, we pooled together the averages of the normalized measures from each subject for the same BLOCK or CONC values and used a single-value t-test with H0: mean=1 on these subsets.
Our second analysis was designed to use the natural properties of the saccadic system to better understand the effects of the sedative and of the placebo on saccadic metrics. For example, we wanted to verify if a decrease in peak velocity was due to a true slowing of the saccades and/or because, for the same target step, the primary saccades became smaller as the session progressed. Saccades present a characteristic soft-saturated relationship between the size of the movement (SIZE) and its peak velocity (peak velocity main sequence) and a linear dependence between size and duration (duration main sequence) [27]. The most traditional fit of the peak velocity main sequence, introduced by Carpenter [28], is PKV = Vmax × (1 − e−SIZE/SAT), with Vmax the asymptotic value and SAT the exponential saturation coefficient. Although weaker and somewhat irregular, the retinal distance (eccentricity) of the new target from the fovea affects the latency of the saccade [29,30], and we adopted a linear model of the reciprocal of the latency (RLAT) as a function of size of the target step (TSTEP) to estimate this effect. We did not expect significant changes in primary saccadic accuracy (GAIN) as a function of TSTEP within our limited range of target steps. Nonetheless, we adopted a linear fit of GAIN as a function of TSTEP. We modeled the effects of the block number (BLOCK), the group-wide estimated blood concentration of the sedative (CONC), and the subjective level of sedation (SLS) as linear modulations of the oculomotor relationships defined above, which took into account the natural contributions of saccadic size or target step to the metrics of the oculomotor response. The equations that we used in our study were therefore:
| (1) |
| (2) |
| (3) |
| (4) |
where BLOCK can be replaced by CONC or SLS depending on the analysis. With the first block (BLOCK=0) also having CONC=0, the infusion being saline for all subjects during the initial block, the sections in italics in equations 1–4 represent, excluding placebo effects, the natural (saline) saccadic behavior of the subject. Our results will strongly suggest that placebo effects have no impact on saccadic metrics, only on SLS. We computed the same models as a function of the subject’s self-evaluation of the level of sedation (SLS) to directly estimate the linkage between (subjective) level of sedation and oculomotor effects. Being SLS a shared variable with the placebo group and more biologically relevant than BLOCK at the single subject level, we were able to perform a direct statistical cross-comparison of the oculomotor effects between drugs and placebo and between drugs as a function of SLS. With different concentration ranges between the drugs and no placebo equivalent to CONC, this crossed comparison could not be done using estimated concentrations (CONC) or, for the same reason, target or measured concentration values. Note that SLS could be ≠ 0 already at BLOCK=0. Our focus will be exclusively on the modulatory parameter C (sections in bold) as a function of BLOCK, CONC, or SLS, and its significance. The non-linear regression model menu in Systat® was used to determine the model parameters. For this analysis we kept the 4 saccadic directions separate, with no need therefore for the data to be normalized. This allowed us to see if there was any modulation pattern with saccadic direction. Thus, for each group of 15 subjects we had 60 estimates for each equation.
All reported statistical significances are with p < 0.05 and the R2 are adjusted R-square observed vs. predicted values. For the C parameter in the models, significance was defined as its 95% confidence interval not containing zero.
2. Results
2.1 Subjective evaluation of sedation
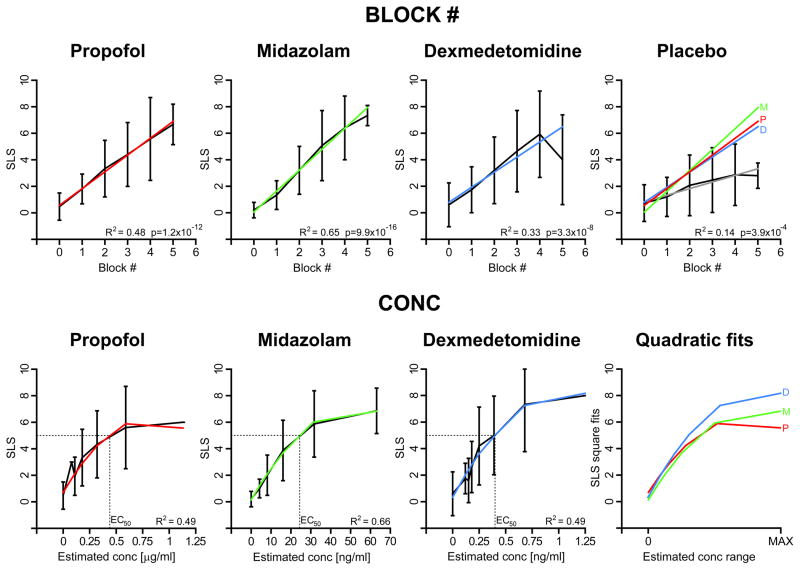
The ranges of target concentrations for the three drugs were selected with the goal of achieving similar subjective levels of sedation. To verify if this goal were achieved, at the end of each saccadic block the subject reported his/her self-evaluation of the level of sedation (SLS) on a scale from 0 to 10. As a first step, for each group we pooled the reported SLS at each block number and fitted the results with a linear regression. The results are graphically illustrated in the top four panels in Fig. 2, with, on the bottom right corner, the R2 and the slope p-values of the linear regression. The subjects in the placebo group also reported some sedation, with a significant monotonic increase of SLS with the block number. Although, as indicated by the large standard deviation bars, there was a pronounced variability between subjects, the average SLS as a function of BLOCK was, with the exclusion of the BLOCK=5 value for dexmedetomidine, remarkably linear in all four cases. Most important, when the drug regressions lines were superimposed (color lines in “Placebo” upper panel) they were practically on top of each other while, at the same time, well separated from the placebo regression (grey line). A 6-pair cross-comparison between drugs and between drugs and placebo as a function of BLOCK using a linear regression with the omitted group technique confirmed what is qualitatively illustrated in the “Placebo” panel. All drug slopes were significantly different from the placebo slope (propofol: t=3.8 p<0.00019; midazolam: t=5.6 p<1.1E-7; dexmedetedomidine: t=2.8 p<0.0062), and there were no significant differences between the drug slopes. When SLS was pooled as a function of CONC, a clear non-linear trend was apparent (first three lower panels of Fig. 2). Note also as the drop from linearity of the average at BLOCK=5 for dexmedetomidine was actually an artifact due to the variation in target concentration, and therefore CONC values, between subjects for the same BLOCK. To determine the EC50 value, defined as the estimated blood concentration of sedative where SLS has a value of 5, we used therefore a quadratic fit. For propofol, the quadratic fit gave a R2=0.49 and the EC50 value for the propofol group was 0.42 μg/ml. For midazolam the R2 was a relatively good value of 0.66, with the EC50 at 24 ng/ml. For dexmedetomidine, the R2 was 0.49, with the EC50 at 0.40 ng/ml. The “Quadratic fits” panel illustrates the three SLS quadratic models of the drugs with the horizontal axis magnification adjusted in such a way to have the three drug CONC ranges fitting inside the x-axis segment “0 to MAX”. Again, the similarity is quite striking and dexmedetomidine giving, qualitatively, slightly stronger SLS values inside the scaled CONC range. As we will illustrate later, dexmedetomidine was associated with the weaker oculomotor effects. From the results of this section it is evident that we achieved our goal of similar subjective levels of sedation for the three drugs. Therefore, any finding showing differences in the oculomotor effects between the three drugs has to be, at least in part, drug-specific.
Fig. 2.
SLS analysis. Top four panels: SLS as a function of BLOCK # in each group as average and ±SD. The linear regression is reported in color with, on the bottom right corner, the R2 and p-value of the slope of the linear regression. In the “Placebo” panel, the linear regressions of the three drugs are superimposed on the placebo results for comparison. First three lower panels: SLS as a function of CONC for the three drugs, unmasking a non-linear relationship. The plots also show the associated EC50 values, defined as the estimated blood concentration of sedative where SLS has a value of 5, and the R2 of the quadratic fits. “Quadratic fits” panel: superimposition of the SLS quadratic models of the drugs with the horizontal axis magnification adjusted in such a way to have the three drug CONC ranges fitting inside the x-axis segment “0 to MAX”.
2.2 Normalized analysis and search for the sedative optimal single-dosage concentration
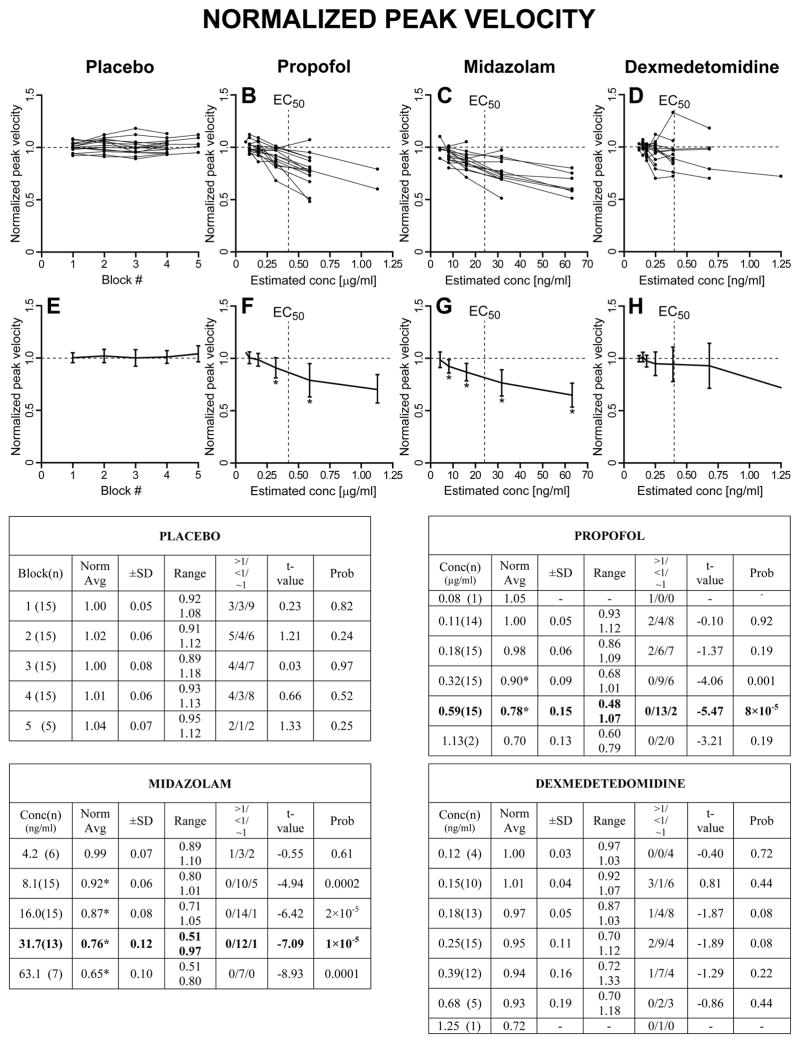
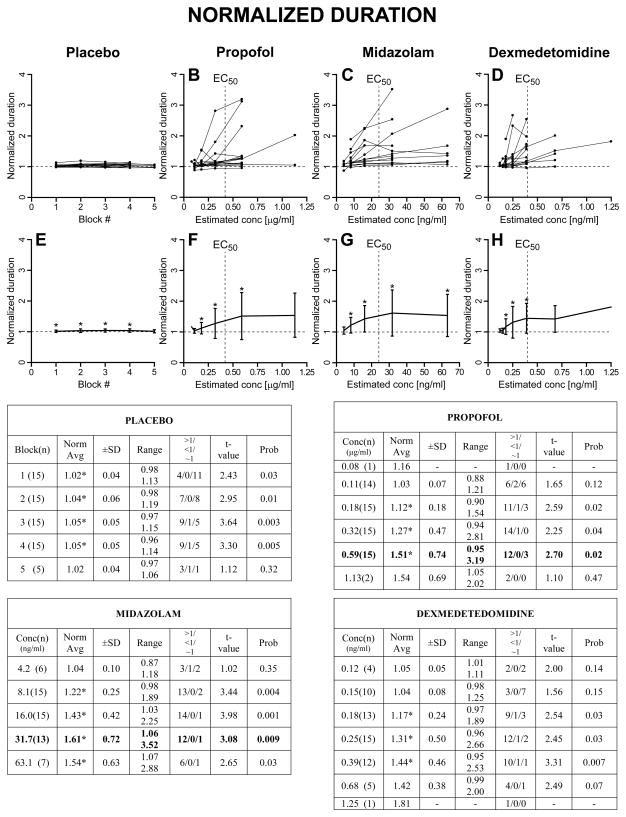
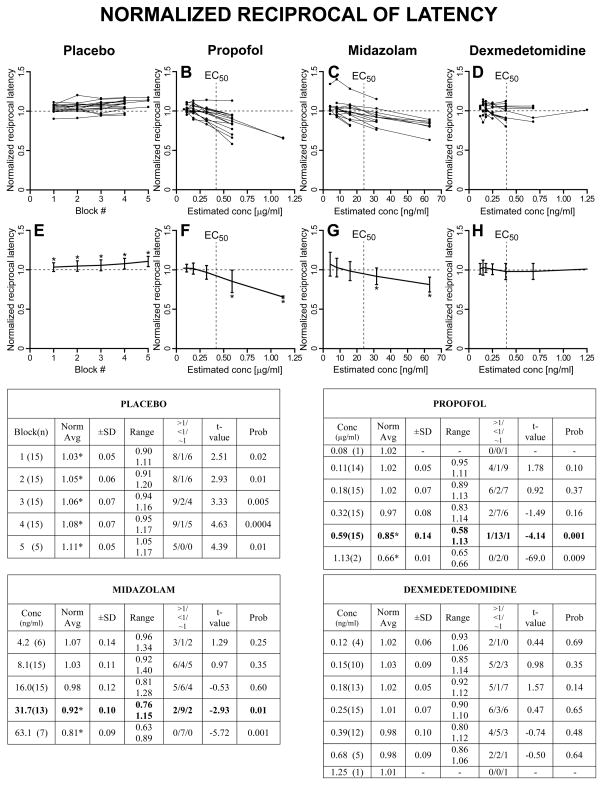
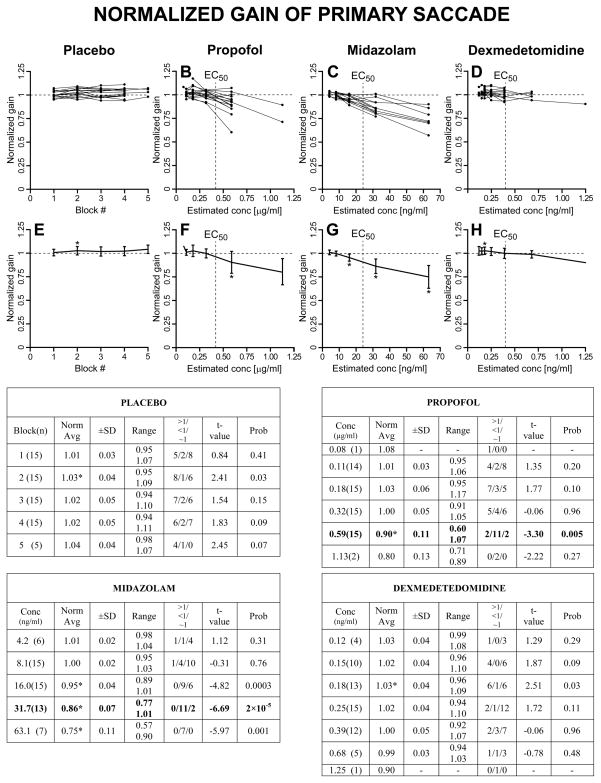
As described in Methods, we first computed, for each trial, the normalized saccadic measures with respect to the subject’s averages from BLOCK=0 (saline for all subjects) for the same target step and direction. Then, for each subject, the normalized values for all target steps and directions in each BLOCK (placebo) or CONC (for the drugs) were averaged together. A graphical representation, together with summary tables, of these results is reported in Fig. 3 for the normalized peak velocity, in Fig. 4 for the normalized duration, in Fig. 5 for the normalized reciprocal of latency, and in Fig. 6 for the normalized gain of the primary saccade. The upper panels show the superimposed normalized averages of each subject as a function of BLOCK for the placebo set and CONC for the sedative sets. The lower panels show the population estimates (±SD) as averages of the subject averages from the upper panels. The asterisks in the lower panels indicate that the associated value is significantly different from 1, >1 if the asterisk is at the top of the SD bar, <1 if the asterisk is at the bottom of the SD bar, using a one-sample t-test with H0=1 (p<0.05). Each summary table reports the averages (±SD and range) of the average normalized measures from each subject at each BLOCK or CONC. An asterisk near the average indicates that the value is significantly different from 1. The values in parenthesis in the first column are the number of subjects from which we have data. The t-value and probability of these one-sample t-tests are reported in columns “t-value” and “Prob”. The “>1/<1/~1” columns report the number of subjects with an average normalized value significantly higher than 1 (>1), significantly lower than 1 (<1) and not significantly different from 1 (~1), using, within each subject, unpaired two-sample t-tests between the normalized measures at each BLOCK or CONC >0 and the normalized (saline) measures at BLOCK = 0.
Fig. 3.
Normalized peak velocity. Upper panels: superimposed normalized averages from each subject as a function of BLOCK # (placebo group) or CONC (sedative groups). Lower panels: population averages (±SD). Asterisk near top of SD bar: population average value significantly >1. Asterisk near bottom of SD bar: average value significantly <1. Summary table for the placebo group: “Block(n)”: Block # and number of subjects (n) from which we have data; “Norm avg”: population average (±SD and range) of the average normalized measures from each subject at each BLOCK. An asterisk near the average indicates (as the asterisks in the lower panels) value significantly different from 1. The t-value and probability are reported in columns “t-value” and “Prob”. The “>1/<1/~1” columns report the number of subjects with an average normalized value significantly higher than 1 (>1), significantly lower than 1 (<1) or not significantly different from 1 (~1). Identical layout for the three drugs, with BLOCK substituted by CONC.
Fig. 4.
Normalized saccadic duration. Same layout of Fig. 3.
Fig. 5.
Normalized reciprocal of latency. Same layout of Fig. 3.
Fig. 6.
Normalized saccadic gain. Same layout of Fig. 3.
2.2.1 Normalized peak velocity
For the placebo group there were no significant relative changes in peak velocity with respect to the initial block as the session progressed. The monotonic increase in the subjective level of sedation did not correspond, on average, to a decrease in saccadic peak velocity. For propofol, there was a clear decrease in the peak velocity of the primary saccade starting at CONC=0.32 μg/ml. Only two subjects managed to perform the task at CONC=1.13 μg/ml, and the lack of significance of some measures at this level is likely due to this fact. Midazolam generated the most reliable oculomotor alterations. The decrease in peak velocity was already significant at CONC=8.1 ng/ml. Propofol and midazolam are both GABA-A agonists, and, using the EC50 as reference, the oculomotor effects in terms of peak velocity were highly comparable. As we will observe later, this was true also for the other saccadic parameters. If these effects are mostly related to the level of sedation, for which we have the subjective estimates, and not also to the specific drug mechanism, we expect with dexmedetedomidine, an α2-adrenergic agonist, to see similar normalized values around the EC50 CONC level. The slowing of peak velocity did not reach significance for any of the CONC used, even for the value (CONC=0.39 ng/ml) closest to EC50 (0.40 ng/ml). The normalized results for dexmedetomidine do not support this hypothesis, indicating that the oculomotor effects are, at least in part, drug-specific.
2.2.2 Normalized duration
Inside the placebo group there were very small, but often significant, increases in saccadic duration. As population, the duration increase was already significant at BLOCK=1, indicating it was not fatigue. This was also confirmed by the fact that it was not significant, as average, on the 5 subjects that performed the maximum number of blocks. Consistent with the decrease in peak velocity for increasing CONC values, both propofol and midazolam presented an increase in saccadic duration, although the scatter for this parameter was quite pronounced. This very large scatter was associated with the presence, in some subjects, of several saccades with very long deceleration periods but with only a partial decrease in their peak velocity and lengthening of their acceleration period, making them very asymmetric. Their frequency increased with increasing CONC values, and they were intermixed, in a bistable-like pattern, with saccades of only slightly longer duration than the initial saline ones and much more symmetric temporal profiles. From the top panels it is noticeable that, for these subjects, the increase in saccadic duration was often present already at low CONC values and they could not perform the saccadic task at the highest sedative dosages we tested. This was true also for dexmedetedomidine.
2.2.3 Normalized reciprocal of latency
The placebo group presented a quite robust increase in the reciprocal of saccadic latency, i.e., a decrease in saccadic latency, as the session progressed, which was likely a short-term task optimization by the subject. For propofol, significant reductions in the reciprocal of latency, i.e., longer saccadic latencies, were observed starting at CONC=0.59 μg/ml. For midazolam, similar reductions in the reciprocal of latency were detected at CONC=31.7 ng/ml. Most strikingly, for dexmedetomidine there were no net effects on the reciprocal of latency.
2.2.4 Normalized gain of primary saccade
Probably another form of task optimization, in several subjects in the placebo group there was a small increase in the gain of the primary saccade relatively to the saline set. Some subjects had a tendency to generate more frequent hypometric saccades very early in the session. Later they became less frequent, and, by landing closer to the target, the subjects reduced the need for corrective saccades. At the population level, this was significant at BLOCK=2. Perhaps the increase in saccadic duration reported earlier is an secondary consequence of this increase in saccadic gain, i.e., size, although we cannot rule out that the much more robust reduction in latency might have influenced saccadic dynamics through some unknown mechanism. A pronounced decrease in saccadic gain was observed for both propofol and midazolam starting around the EC50 values. As the session progressed, more and more often transfers of gaze were achieved with series of smaller saccades. On the contrary, no changes in saccadic gain were observed for dexmedetomidine.
2.2.5 Optimal concentration values
The normalized analysis reported above offers the opportunity to determine the optimal concentrations of the three sedatives for single-dosage studies that maximize the oculomotor effects while still having the highest number of subjects able to perform the required behavioral task. There are several experimental conditions, like fMRI imaging or eye movement recordings using search coils, where protocol or time constrains do not allow a stepwise infusion. In performing this estimate we are nonetheless aware that reaching the optimal concentration value using a stepwise infusion may give sedative and oculomotor effects that are different from a single-step infusion. From Figs. 3–6 it is evident that, for propofol, CONC=0.59 μg/ml, corresponding to a target concentration of 0.80 μg/ml, gave the most significant oculomotor effects with all 15 subjects still being able to perform the task. The >1/<1/~1 columns also show that the majority of the subjects presented significant effects. The EC50 of the self-evaluation of sedation for propofol was estimated to be between the 0.32 and the 0.59 μg/ml CONC values. Although the strength of the effects for midazolam was comparable to propofol, the consistency of the results made them statistically more robust. As optimal CONC value, all subjects were able to perform the task at CONC=16.0 ng/ml, corresponding to a target concentration of 20 ng/ml. For CONC=31.7 ng/ml, corresponding to a target concentration of 40 ng/ml, we had data only from 13 subjects, but with remarkably stronger effects on all four measures, making this concentration the preferred value for single-dosage studies. As for propofol, the EC50 was just before the optimal CONC value. For dexmedetomidine, only 5 subjects performed the task at CONC=0.68 ng/ml, corresponding to a target concentration of 0.40 ng/ml. For CONC=0.39 ng/ml, corresponding to a target concentration of 0.20 ng/ml, and with 12 subjects still able to perform the saccadic task, the increase in saccadic duration was significant in 10 subjects, but none of the other three normalized measures reached significance. For dexmedetomidine, there was no identifiable optimal dosage in terms of oculomotor effects because, with the exclusion of saccadic duration, effects on peak velocity and gain started to appear at the same dosage several subjects could not anymore perform the task, and no effects on latency were detected at any of tested concentrations.
2.3 Equations 1–4 as a function of BLOCK (placebo) and CONC (drugs)
The model analysis (eqs. 1–4) has two desirable features, not offered by the normalized measures. The first is that, by exploiting the known dependences of the saccadic parameters with saccadic size (peak velocity and duration) or the size of the target step (latency and gain), we were able to quantify the oculomotor effects for each saccadic direction with a single number (the modulation coefficient C). For this analysis we maintained the directions separate to take into account that the initial saline set (CONC=0) often presented different saccadic metrics with saccadic direction and, at the same time, allowing us to search for direction-specific modulatory patterns. The normalization process took care of this variability using the initial saline data as reference. The second was that we could test if the peak velocity and duration changes were not solely a secondary consequence of changes in saccadic gain but true alterations in the main sequences. This is an important factor for propofol and midazolam, which presented significant gain changes.
We first tested equations 1–4 on the placebo group as a function of BLOCK. The results are illustrated in the Placebo rows in Tab. 1. This table reports, for the four measures, the average of the parameter C, its ±SD, and range (n=60). An asterisk near the average indicates that the average value of C was significantly different from 0, using a one-sample t-test with H0=0 (p<0.05). The t-value and probability of these tests are reported in columns “t-value” and “Prob”. Column “>0/<0/~0” illustrates the number of >0 significances, the number of <0 significances, and the number of no significances (~0) of the C values among the 60 sets. We considered C significantly different from zero if its 95% confidence interval was entirely positive (>0) or entirely negative (<0). It was considered not significantly different from zero if its 95% confidence interval contained zero. The average, ±SD and range of the R2 of the 60 model fits are also reported. Equation 1 well represented the relationship between peak velocity and size of the saccade, with the average R2 for the placebo group of 0.76. The average value of CPKV was −0.002 1/block and not significantly different from zero. The relationship between duration and saccadic size (eq. 2) was slightly less robust, with the average R2 for the duration main sequence equal to 0.62. The average value of CDUR was 0.004 1/block and not significantly different from zero. The ability of the model to predict the reciprocal of the latency (RLAT) and the gain of the primary saccade (GAIN) were much poorer. The reciprocal of saccadic latency model had an average R2 of 0.14. The average value of CRLAT was 0.015 1/block and was strongly different from zero (t=7.9, p<8×10−11). Several subjects reduced the latency of the saccades as the session progressed, showing therefore some task optimization, with, as a result, a highly significant overall increase of the reciprocal of the latency as a function of BLOCK. Notice as this effect compensated any increase in saccadic latency due to fatigue or loss of attention in the task. The gain model had an average R2 of 0.21. The average value of CGAIN was 0.006 1/block and weakly different from zero (t=3.1, p<0.003). As reported earlier, some subjects tended to undershoot during the initial blocks, requiring more frequent corrective saccades, and gradually learned to land on target with higher precision. Both the normalization and the model analyses of the placebo sets show two important elements for the interpretation of the sedative data. The block structure of the task, with 10 min of rest followed by 10 min of saccadic testing, likely combined with some on-the-task learning/optimization, practically eliminated the effects of fatigue or loss of attention in the task on the peak velocity, and the effects on duration were very small and not significant using the model analysis. Significant effects were present on the reciprocal of saccadic latency and, albeit weaker, on gain, but with signs opposite to the ones expected with fatigue. More important, the “placebo effects” unmasked by the increasing SLS values as the session progressed, had no impact on saccadic metrics.
Tab. 1.
BLOCK models for the placebo and CONC models for the sedative groups.
| BLOCK/CONC MODEL PEAK VELOCITY [eq. 1] (n=60) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | CPKV Average 1/unit | CPKV ±SD 1/unit | CPKV Range 1/unit | CPKV >0/<0/~0 # | t-value | Prob | R2 Average | R2 ±SD | R2 Range |
| Placebo (Blocks) | −0.002 | 0.015 | −0.047 0.028 |
14/19/27 | −1.2 | 0.2 | 0.76 | 0.13 | 0.36 0.97 |
| Propof. (μg/ml) | −0.33* | 0.24 | −0.91 0.22 |
1/56/3 | −10.3 | 1×10−14 | 0.72 | 0.18 | 0.27 0.97 |
| Midaz. (ng/ml) | −0.0050* | 0.0048 | −0.0199 0.0060 |
0/49/11 | −8.0 | 6×10−11 | 0.65 | 0.18 | 0.27 0.93 |
| Dexmed. (ng/ml) | −0.21* | 0.33 | −0.96 0.79 |
6/39/15 | −4.9 | 8×10−6 | 0.68 | 0.16 | 0.38 0.94 |
| BLOCK/CONC MODEL DURATION [eq. 2] (n=60) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | CDUR Average 1/unit | CDUR ±SD 1/unit | CDUR Range 1/unit | CDUR >0/<0/~0 # | t-value | Prob | R2 Average | R2 ±SD | R2 Range |
| Placebo (Blocks) | 0.004 | 0.020 | −0.050 0.048 |
25/8/27 | 1.7 | 0.09 | 0.62 | 0.28 | 0.04 0.98 |
| Propof. (μg/ml) | 1.82* | 4.10 | −0.44 25.71 |
49/2/9 | 3.4 | 1×10−3 | 0.50 | 0.25 | 0.05 0.89 |
| Midaz. (ng/ml) | 0.029* | 0.041 | −0.005 0.186 |
54/0/6 | 5.4 | 1×10−6 | 0.42 | 0.20 | 0.09 0.86 |
| Dexmed. (ng/ml) | 1.08* | 1.27 | −0.17 6.48 |
45/2/13 | 6.6 | 1×10−8 | 0.44 | 0.21 | 0.07 0.86 |
| BLOCK/CONC MODEL RECIPROCAL OF LATENCY [eq. 3] (n=60) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | CRLAT Average 1/unit | CRLAT ±SD 1/unit | CRLAT Range 1/unit | CRLAT >0/<0/~0 # | t-value | Prob | R2 Average | R2 ±SD | R2 Range |
| Placebo (Blocks) | 0.015* | 0.015 | −0.020 0.054 |
41/3/16 | 7.9 | 8×10−11 | 0.14 | 0.10 | 0.00 0.41 |
| Propof. (μg/ml) | −0.27* | 0.21 | −0.63 0.21 |
4/46/10 | −9.8 | 6×10−14 | 0.23 | 0.16 | 0.00 0.63 |
| Midaz. (ng/ml) | −0.0041* | 0.0040 | −0.0170 0.0038 |
0/46/14 | −8.0 | 5×10−11 | 0.16 | 0.11 | 0.00 0.45 |
| Dexmed. (ng/ml) | −0.03 | 0.27 | −0.86 0.78 |
12/21/27 | −0.9 | 0.3 | 0.12 | 0.09 | 0.00 0.45 |
| BLOCK/CONC MODEL GAIN PRIMARY SACCADE [eq. 4] (n=60) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | CGAIN Average 1/unit | CGAIN ±SD 1/unit | CGAIN Range 1/unit | CGAIN >0/<0/~0 # | t-value | Prob | R2 Average | R2 ±SD | R2 Range |
| Placebo (Blocks) | 0.006* | 0.015 | −0.026 0.049 |
25/8/27 | 3.1 | 0.003 | 0.21 | 0.15 | 0.00 0.62 |
| Propof. (μg/ml) | −0.18* | 0.20 | −0.79 0.18 |
4/40/16 | −6.9 | 4×10−9 | 0.23 | 0.17 | 0.00 0.76 |
| Midaz. (ng/ml) | −0.0045* | 0.0036 | −0.0124 0.0086 |
1/50/9 | −9.7 | 8×10−14 | 0.27 | 0.16 | 0.00 0.70 |
| Dexmed. (ng/ml) | 0.04 | 0.25 | −0.43 0.62 |
14/14/32 | 1.1 | 0.3 | 0.18 | 0.14 | 0.01 0.55 |
“C[] Average”: average of the parameter C from eqs. [1–4] (±SD and range). The unit is reported in parenthesis in the first column below the group label. An asterisk near the average indicates that the value was significantly different from 0, using a one-sample t-test with H0=0 (p<0.05, n=60). The t-value and probability of these tests are reported in columns “t-value” and “Prob”. The “>0/<0/~0” columns report the number of >0 significances, the number of <0 significances, and the number of no significances (~0) of the C values at the subject level. Last three columns: average, ±SD and range of the R2 of the model fits.
For the three sedatives we tested both BLOCK and CONC models, which gave only minor differences in the overall results. For brevity, Tab.1 reports only the CONC results. For the peak velocity main sequence, the average R2 of eq. 1 for the sedatives was comparable with the placebo. The lowest average value of the R2 was for the midazolam group, with mean 0.65. For the duration main sequence, again the midazolam group had the lowest R2 on average, with mean 0.42. The lower values of the duration R2 for the sedative groups with respect to the placebo group were due, as reported earlier, by the presence of very long-duration saccades in some of the subjects. For all drug groups, the majority of the subjects showed, for all saccadic directions, a significant slowing of the peak velocity with increasing CONC (negative CPKV), mirrored by an equivalent increase in saccadic duration (positive CDUR). The averages of the parameters CPKV and CDUR were all strongly different from zero. The lowest t-value was 3.4 for propofol CDUR (p=1×10−3) and the highest, as absolute value, was t= −10.3 for propofol CPKV (p=1×10−14). Clearly, as their blood concentration increased, the three drugs caused pronounced slowing and lengthening of the saccades for the same saccadic size. This indicates that, independently of the changes in saccadic gain, there was a true alteration in the peak and duration main sequences. The average and ranges of the R2 for RLAT and GAIN for the three drugs were again low, but also similar to the placebo values, indicating that the data scatter was not affected by the sedatives. The effects of propofol and midazolam were quite pronounced for both RLAT and GAIN when compared to placebo. This is readily evident from the C averages and the signs and significances of the slopes. The lowest t-value, as absolute value, was −6.9 for propofol CGAIN (p=4×10−9) and the highest, as absolute value, was −9.8 for propofol CRLAT (p=6×10−14). Very different results were found for dexmedetomidine, with no significant changes in gain or the reciprocal of latency as a function of CONC. For the latter, its effects mostly eliminated the optimization in latency observed in the placebo group.
For the sedative groups, BLOCK as parameter was used to address two specific questions. For each group, we tested if the number of blocks that the subject was able to execute correlated with the C values. For CPKV, only propofol showed a significant dependence (t=5.0; p=1×10−5), matched with a slightly weaker correlation (t=−3.6; p=6×10−4) for CDUR. In both cases, the modulation coefficient C was smaller, in absolute terms, the higher the number of blocks executed by the subject. Thus, subjects more sensitive to the drug propofol, i.e., with the steepest negative CPKV slopes and the steepest positive CDUR slopes, also quit earlier. This was not detected for the other two drugs. The trend for the subjects that presented a higher number of longer saccades also to quit earlier (Fig. 4) was not significant for midazolam or dexmedetomidine. No correlations were found for CRLAT or CGAIN. Similar results were obtained using CONC.
The neuronal circuitry downstream of the superior colliculus, including the neural integrators, is segregated between horizontal and vertical saccades [31]. Furthermore, the major area in the rat affected by dexmedetomidine receive afferents from the prepositus hypoglossi [18], involved in horizontal oculomotor integration [19]. To test if the changes in peak velocity and duration main sequences with the BLOCK # differed for the 4 saccadic directions, in each group we performed a paired t-test (matched for subject and direction) of the parameter C for each of the 6 possible pairs of directions. Seven of the 24 comparisons were significant for peak velocity and 2 of the 24 for duration, but there were no evident patterns, in the placebo or in the drug groups, in terms of horizontal vs. vertical saccadic directions. Similar results were obtained using CONC (drug sets only). Thus, for the normalized data, which normalization with the initial saline averages took into account the natural variability of the metrics with saccadic direction and target step, all the normalized data inside each BLOCK (or CONC), were pooled together.
2.4 Equations 1–4 as a function of SLS
As expected from the quite scattered, but on average relatively consistent relationship between BLOCK and SLS and between CONC and SLS illustrated in Fig. 2, the results, in terms of significances and quality of the Eqs. 1–4 models using SLS as parameter were similar, albeit slightly weaker and with worse R2 values, to the results described in Tab. 1 using BLOCK (placebo) and CONC (sedatives). The main purpose of determining the C values using SLS was to directly test, using a subjective measure of sedation shared by all groups, if subjects presented different sensitivities to SLS depending on the assigned group. For each saccadic parameter, we used unpaired two-sample t-tests (separate variances) to cross-compare sedatives and sedatives to placebo. The results are illustrated in Tab. 3. For peak velocity, there was a strong difference in SLS sensitivity between propofol and placebo and between midazolam and placebo. This difference was much weaker between dexmedetomidine and placebo. The distributions of C values as a function of SLS for midazolam and propofol were remarkably similar, with no significant difference in the means. As expected, the sensitivity to SLS was also stronger for propofol and midazolam when directly compared to dexmedetomidine. For duration, the positive skew of the distributions of the C values in the sedative groups caused by the presence of subjects with very long saccades was such that while the drug groups presented very different SLS sensitivities with respect to the placebo group, no significant differences were found between the drug groups. We also confirmed these findings using non-parametric two-sample Mann-Whitney tests. As expected from the previous analyses, the most striking differences were found for the reciprocal of latency and the gain of the primary saccade. For the reciprocal of latency, all three drug groups presented a significantly stronger sensitivity to SLS with respect to the placebo group, but it was weaker for dexmedetomidine. As for peak velocity, there was no significant difference between the two GABA-A agonists, and both presented a much stronger SLS sensitivity than dexmedetomidine. In terms of gain, again the two GABA-A agonists had similarly strong sensitivities when compared to placebo and together. Dexmedetomidine, on the contrary, was not statistically different from the placebo set. In summary, the results from Tab. 3 show that the sensitivity to SLS in the placebo group was much smaller than the one presented by propofol and midazolam for all four saccadic parameters. With the exclusion of duration, dexmedetomidine had sensitivity to SLS that was significantly different from the two GABA-A agonists, and for the gain, much more similar to the placebo group. It is clear that SLS, i.e., the subjective sedation level of the subject, is not the only, if any, factor determining the depth of alteration in saccadic dynamics.
Tab. 3.
Cross-comparisons of the C values from the SLS models, using unpaired two-sample t-tests (separate variances). For duration, the distributions were strongly skewed by the subjects presenting very long-duration saccades at the highest drug concentrations. Thus, together with the t-test, it is reported the p-value of a Mann-Whitney 2-sample test.
| CROSS-COMPARISONS PEAK VELOCITY [eq. 1] (n=60) | ||||||
|---|---|---|---|---|---|---|
| Groups | Propof. Vs Placebo | Midaz. Vs Placebo | Dexmed. Vs Placebo | Midaz. Vs Propof. | Dexmed. Vs Propof. | Dexmed. Vs Midaz. |
| Difference | −0.035* | −0.032* | −0.017* | 0.003 | 0.018* | 0.015* |
| t-value | −6.2 | −5.5 | −3.4 | 0.4 | 3.2 | 2.6 |
| Prob | 1×10−8 | 3×10−7 | 0.001 | 0.7 | 0.001 | 0.009 |
| CROSS-COMPARISONS DURATION [eq. 2] (n=60) | ||||||
|---|---|---|---|---|---|---|
| Groups | Propof. Vs Placebo | Midaz. Vs Placebo | Dexmed. Vs Placebo | Midaz. Vs Propof. | Dexmed. Vs Propof. | Dexmed. Vs Midaz. |
| Difference | 0.149* | 0.135* | 0.100* | −0.014 | −0.049 | −0.035 |
| t-value | 3.8 | 6.4 | 4.9 | −0.4 | −1.1 | −1.2 |
| Prob | 3×10−4 | 4×10−9 | 3×10−6 | 0.7 | 0.3 | 0.2 |
| Mann- Whitney | 3×10−9 | 9×10−14 | 5×10−9 | 0.04 | 0.97 | 0.04 |
| CROSS-COMPARISONS RECIPROCAL OF LATENCY [eq. 3] (n=60) | ||||||
|---|---|---|---|---|---|---|
| Groups | Propof. Vs Placebo | Midaz. Vs Placebo | Dexmed. Vs Placebo | Midaz. Vs Propof. | Dexmed. Vs Propof. | Dexmed. Vs Midaz. |
| Difference | −0.044* | −0.041* | −0.027* | 0.003 | 0.017* | 0.014* |
| t-value | −6.9 | −6.9 | −4.4 | 1.0 | 4.2 | 4.0 |
| Prob | 6×10−10 | 2×10−9 | 4×10−5 | 0.3 | 6×10−5 | 1×10−4 |
| CROSS-COMPARISONS GAIN PRIMARY SACCADE [eq. 4] (n=60) | ||||||
|---|---|---|---|---|---|---|
| Groups | Propof. Vs Placebo | Midaz. Vs Placebo | Dexmed. Vs Placebo | Midaz. Vs Propof. | Dexmed. Vs Propof. | Dexmed. Vs Midaz. |
| Difference | −0.018* | −0.029* | −0.003 | −0.011* | 0.015* | 0.026* |
| t-value | −5.9 | −8.1 | −0.9 | −2.6 | 4.1 | 6.4 |
| Prob | 4×10−8 | 2×10−12 | 0.4 | 0.01 | 6×10−5 | 4×10−9 |
2.5 Sedative effects: model outliers
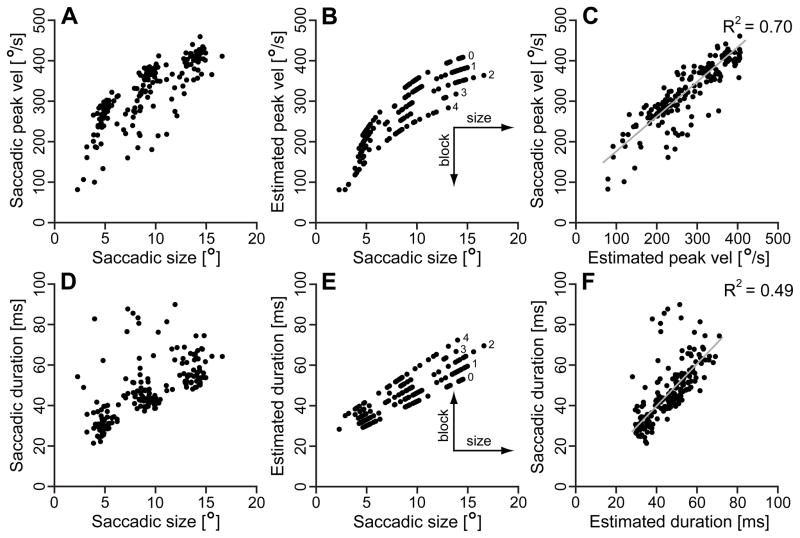
Figure 7 reports an example of the peak velocity and duration main sequences and associated models as a function of BLOCK for the data set with the R2 closest to the average value (subject 009, propofol group). Panel A shows the measured peak velocity vs. saccadic size main sequence, and panel B the estimated values using eq. 1. The numbers near the model data points are the block #. Panel C illustrates the direct comparison between measured and estimated values, with linear regression and R2. With the same layout, panels D, E, and F show the duration vs. saccadic size main sequence, using eq. 2 as a function of BLOCK. As expected by the decrease in peak velocity, duration increased with the block number and, therefore, with the sedative concentration. Evident in panel C, and even more in panel F, there were trials with saccades slower and longer than what the model predicted. These relatively slower and longer (duration < 200 ms) saccades equally affected the R2 of both placebo and sedative sets, and occurred randomly during the session, although, in the sedative groups, their number increased with the level of sedative concentration. Less common, and only in some subjects, we observed very slow and long saccadic outliers (duration > 200 ms, up to 1–2 s). These occurred most frequently at the highest drug concentrations, and, interestingly, also in two placebo subjects. Although statistically identified as “strong outliers” by the fitting program, they were true biological responses and were not removed. These were the main reason for the drop in average R2 for the duration main sequences in the sedative groups. They were very asymmetric, with the lengthening of the duration mostly affecting the deceleration phases. Thus, their impact on the peak velocity main sequences was relatively minor and this was particularly evident for dexmedetomidine.
Fig. 7.
Examples of measured main sequences and estimated values using eq. 1 (peak velocity) and eq. 2 (duration) as a function of BLOCK. This set, rightward saccades, subject 009CB, propofol group, was selected as an illustrative example for having the R2 closest to the average R2 values. Panel A: measured saccadic peak velocity vs. saccadic size main sequence; Panel B: model, using eq. 1; Panel C: direct comparison between measured peak velocities and estimates; Panel D: measured saccadic duration vs. saccadic size main sequence; Panel E: model, using eq. 2; Panel F: direct comparison between measured durations and estimates. The grey lines in panels C and F are linear regressions, with the associated R2 top right of panels. The numbers in the plots are the block #.
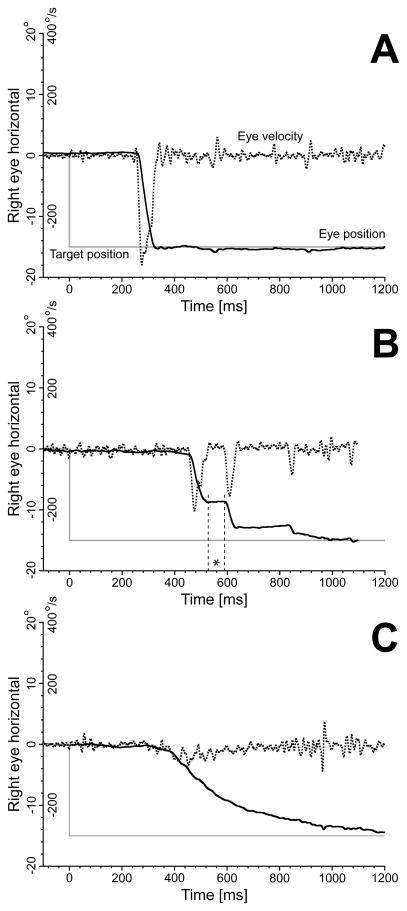
The trial-by-trial scatter in saccadic metrics and temporal profiles for the highest sedative blood concentrations was often quite large. Figure 8 illustrates some 15° leftward trials from 029JG acquired at the highest propofol dosage this subject was still able to perform the saccadic task (BLOCK=4; target concentration 0.80 ng/ml; estimated concentration 0.59 ng/ml). The trial in panel A was similar to the subject’s placebo responses, with a single saccade reaching the desired target. These saccades were only slightly slower and longer than the initial placebo saccades of similar size. In panel B, the saccade was broken into a sequence of smaller saccades, with an evident reduction in gain of the primary saccade. In panel C, the saccade was extremely slow. We often looked at the real-time eye images acquired by the eye tracker during the saccadic task and we can confirm that most of these responses were not contaminations caused by head movements, but true very slow eye saccades. It is possible that subjects were fighting the effects of the sedative with rapid transitions in behavior between quick waking up periods and drifting off periods. There was no evidence for these very long saccades to be concentrated around the end of the 10 min period of recordings, with all three patterns usually intermixed in an apparent random fashion inside the block.
Fig. 8.
Sample 15° leftward trials from subject 029JG acquired at the highest propofol dosage for this subject (BLOCK=4; target concentration 0.80 ng/ml; estimated concentration 0.59 ng/ml). Panel A: trial similar to the subject’s placebo responses; Panel B: response broken into a sequence of smaller saccades, with an evident reduction in gain of the primary saccade. The asterisk indicates the first intrasaccadic interval, which duration was only 62 ms; Panel C: saccade with an extremely slow dynamics. Vertical axis: target position (grey traces); horizontal right eye position (continuous black traces); horizontal right eye velocity (dotted black traces). Position and velocity scales reported on the y-axis. Horizontal axis: time in ms.
As illustrated in the example in Fig. 6, there were often longer and slower saccades than predicted by the models, but their duration was usually less than 200 ms. Saccades longer than 200 ms, like the one in Fig. 8C, are clearly well outside the duration vs. size main sequence. In the placebo group, 2 subjects had more than 1% (1.2% and 6.0%) of the total number of saccades with duration longer than 200 ms. The other 13 placebo subjects had a percentage of very long saccades of less than 0.2%, i.e., 0, 1, or 2 in the entire session. In each of the sedation groups, 7 subjects had more than 1% of the saccades longer than 200 ms, and one subject in each of the three groups had a percentage higher than 10%. Eight of the subjects in each of the sedation groups did not reach the 1% value.
2.6 Sedative effects: alterations in post-saccadic profiles
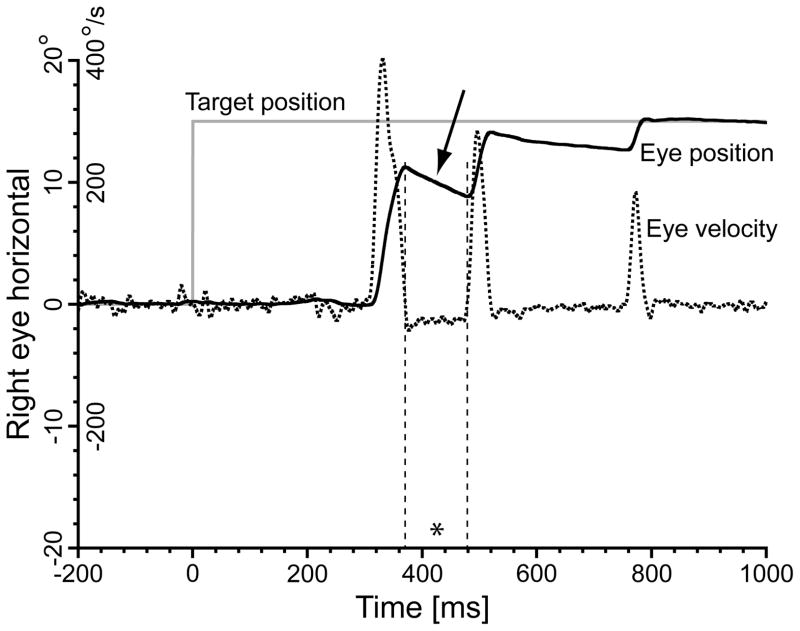
Visual inspection of the saccadic profiles from the subjects in the placebo group did not show any consistent post-saccadic drift changes as the session progressed. In the sedative groups, we observed several trials like the one illustrated in Fig. 9. An evident post-saccadic backward drift, indicated by the arrow, was present after the primary saccade. The leakage was usually smaller after the first corrective, and undetectable after later correctives. The primary saccade was also the largest saccade in the sequence, suggesting that the amplitude of the saccade, i.e., the velocity pulse, determined the amplitude of the post-saccadic drift. In these trials the primary saccade was quite hypometric, with two or more correctives afterward. Although this pattern was more common for propofol and midazolam, several cases were observed also for dexmedetomidine. No clear pattern was found regarding their occurrence, albeit most of them appeared at the highest sedative concentrations, or saccadic direction. Not all subjects presented these profiles.
Fig. 9.
Some trials in the sedative groups showed alterations in the post-saccadic profiles, with clear post-saccadic backward drifts, the largest affecting the primary saccade (arrow). In these trials, the primary saccade was always hypometric and followed by two or more corrective saccades. Smaller backward drifts were often present also after the corrective saccades, but the subject was able to hold the eye position at the end of the sequence indefinitely. The asterisk indicates the first intrasaccadic interval, which duration was 108 ms. 15° rightward trial, propofol group, subject 060MB, trial 1447, target concentration 1.60 μg/ml; estimated concentration 1.13 μg/ml. Vertical axis: target position (grey trace); horizontal right eye position (continuous black trace); horizontal right eye velocity (dotted black trace). Position and velocity scales reported on the y-axis. Horizontal axis: time in ms.
3. Discussion
3.1 Comparisons with previous studies
The relative decrease in saccadic peak velocity in our propofol group was similar to the amount observed by Gao et al. [1], although a comparison of the estimated blood concentrations can only be approximate. Our blood samples were venous samples, while Gao et al. used arterialized venous samples as better estimates of arterial concentration [32,33]. The protocol was similar, with a stepwise continuous infusion with increasing levels every 25 min. The 24% average decrease in peak velocity at their highest mean arterialized venous concentration of 0.80 μg/ml, estimated from their Fig. 3, is similar to our 22% decrease at the estimated venous concentration of 0.59 μg/ml (Fig. 3). They found that peak saccadic velocity decreased linearly with increasing log10 of propofol concentrations, but SLS correlated with log10 propofol or the % reduction in peak velocity in only three of their six subjects. Our SLS data were also not reliable at the single subject level, and only as group average we found a robust trend, with the quadratic fit for propofol having a R2 of 0.49 (Fig. 2). No other saccadic measures were reported in their study.
Ball et al. [2], in their midazolam and flumazenil study, used three repeated bolus injections separated in time by 15 min, with a venous sampling. Their 20% decrease in peak velocity at the average venous concentration of 50.8 ng/ml, estimated from their Fig. 2, well compares with our 24% at 31.7 ng/ml (Fig. 3). Several other measures were reported in their study, and two, latency and gain, did not match our results. They found no changes in saccadic latency and reported a decrease in saccadic gain only briefly after the bolus infusion, with the gain returning near placebo values during the 15 min periods before the next midazolam injection. We did not observe any recovery of gain during our 10 min saccadic tasks, likely due to our continuous infusion. Paut et al. [3], using a single bolus injection of midazolam, found a significant increase in latency soon after the infusion. At 120 min after the infusion, where the plasma concentration was estimated to be 90 ng/ml, the increase in latency was still around 150% of the placebo values (estimated from their Figs. 1 and 4). At the estimated concentration of 63.1 ng/ml, our decrease in reciprocal of latency was 19%, corresponding to an increase in latency of 123% (Fig. 5).
Aantaa [21] used a single bolus infusion of dexmedetomidine lasting approximately 60 s. For their largest bolus of 1.0 μg/kg, the highest average decrease in peak velocity was obtained after 30 min, with a reduction of 32%. Although no blood concentration measures are available for a direct comparison, it is important to note that even at their highest level of reduction in peak velocity the authors reported no consistent changes in latency. Our changes in dynamics were not significant using the normalized values while they were significant using the models, but still smaller than for propofol and midazolam. Only one of our subjects managed to perform the saccadic task at CONC=1.25 ng/ml, where we observed a normalized peak velocity of 0.72, equivalent to a 28% reduction (Fig. 3). It is possible that, for similar blood concentrations, single bolus infusions do not impair the subject’s ability to perform a saccadic task as much as multistep infusions.
3.2 Pharmacological considerations
The changes in saccadic metrics caused by sedatives are often attributed to their suppression of the brainstem reticular formation, which is supported by the alterations in the ocular microtremor observed in anesthetized patients [5,6]. Although Spauschus et al. [7] presented evidence for a brainstem link to microtremor, other sources of this neural noise might be further upstream, such as the cerebellum, superior colliculus, or the vestibular nuclei. Using an autoradiographic technique in rats, Freo et al. [34] found that midazolam caused a decrease in glucose intake in a large number of cortical and subcortical areas, and that the progression in the recovery of the glucose intake closely followed the behavioral recovery of the rat from the anesthesia. Of our direct interest, the recovery of motor function well correlated with the return to normal glucose uptake by the frontal motor, limbic, and thalamic areas. The finding of a decreased glucose uptake in cerebellar vermis is of particular significance regarding the slowing of saccadic dynamics and the hypometria observed with midazolam and propofol. Kojima et al. [35] reported that injection of muscimol, a GABA agonist, in cerebellar vermis caused hypometric saccades with lower peak velocities and longer durations. In monkeys, GABA-A/benzodiazepine receptors are present in all five types of cells present in cerebellar cortex [36]. Significant alterations in glucose uptake with midazolam were also reported in the basal ganglia, including the substantia nigra pars reticulata, which could affect saccadic latency [37,38], and both superficial and deep layers of the superior colliculus. Injections of muscimol in monkey superior colliculus are known to cause hypometric saccades with longer latencies as an indirect consequence of the increase in tonic inhibition originating from the substantia nigra efferents [39]. For the two benzodiazepines, cortical suppression, particularly at the level of pyramidal cells, might have also altered saccadic dynamics. Schönle et al. [40] have reported that the evoked motor responses elicited by magnetic brain stimulation were suppressed by a midazolam infusion. At the peripheral level, Dueck et al. [41] have described depression at the level of the α-motoneurons in the spinal cord by propofol.
Our knowledge of specific effects of α2-adrenergic agonists on the saccadic system is much more fragmentary. There is evidence that noradrenaline (norepinephrine) is involved in neuroplasticity and adaptation. For example, depletion of noradrenaline affected the ability of the vestibular ocular reflex to adapt [42]. α2-adrenergic receptors in dorsolateral prefrontal cortex modulated the performance of monkeys in an oculomotor delayed-response task [43]. A pharmacological depletion of dopamine and noradrenaline in normal volunteers using metyrosine caused an increase in saccadic intrusions during fixation and during smooth pursuit tracking [44] and similar behavioral effects were reported when the inhibitory action of the substantia nigra pars reticulata on the superior colliculus was reduced using bicuculline [39], a GABA antagonist. No saccadic intrusions or nystagmus were observed in any of our groups, although the resolution of our recordings was too coarse to detect changes in the “fixational” eye movements (microsaccades, slow drifts, and microtremor) during the periods of target fixation.
3.3 Possible effects on saccadic mechanisms
In alert monkeys, omnipause neurons (OPNs) have a high baseline firing rate and briefly stop during saccades [45] and blinks [46]. This cell group, which exerts a powerful inhibitory action on the saccadic burst neurons [47], has glycine as neurotransmitter [48,49] but it receives GABAergic, glycinergic, as well as glutaminergic afferents [48]. Sedation directly affects alertness, which is known to also alter the tonic firing of the saccadic omnipause neurons [50,51]. Shaikh et al. [52] have reported extremely long saccades with sustained voluntary eyelid closures, which are believed to cause OPNs to stop firing. Particularly puzzling was therefore the quite inconsistent presence of saccades with very long durations (Fig. 8C) in our sedated subjects. Eight of the subjects in each of the sedative groups did not reach the 1% threshold, indicating that they are not necessarily present even when the subjects were moderately sedated and had hard times in executing the task or even had be repeatedly awakened to continue. It is important to note that our video eye tracker could not record with the eyelids closed or partially closed, and saccades not reaching the acceptance angular window around the second target inside 2000 ms from target onset were rejected. In other words, the subject had to be sufficiently alert to still roughly perform the task and with the eyes open for the data to be accepted. We hypothesize that fluctuating alertness of the subject, and not the sedative per se, was responsible for the very long saccades, as evident from the fact that two sleepy placebo subjects also presented very long saccades.
The first intrasaccadic intervals in Fig. 8B and 9, indicated with an asterisk, are too short (62 ms and 108 ms, respectively) for the first secondary saccade to be a visually-driven and independently-generated corrective eye movement. This was a common feature in several of these trials, usually between the primary and the first secondary saccade. There is neural evidence that short-latency corrective saccades could be elicited by internal non-visual feedback [53]. The large decrease in gain, with the need of several saccades to reach the target, might have facilitated the deployment of this internal corrective mechanism. A few trials presented rapid staircases of several small saccades that resembled what is observed during prolonged electrical stimulation of the motor layers of the superior colliculus [54–56]. The staircase pattern in these stimulation experiments is associated with an on-off cycling of the OPNs [57]. Is it possible that some of our staircases, perhaps including the two examples in Fig. 8B and 9, were originally programmed as a larger (and faster) single saccade but that instability of the triggering/OPN circuitry caused them to be split into staircases? If so, the peak velocity of the primary saccade of the staircase would be closer to the peak velocity of the original single saccade than to the peak velocity of normal saccades of its size. In other words, the curtailed primary saccade would appear faster than what expected by the peak velocity/size main sequence. We analyzed all staircases in our data sets and we found no evidence for this, but we consider this observation inconclusive. With these saccadic sequences mostly occurring at high CONC values and therefore where saccades are slower with respect to the associated saline main sequence and presenting large between-trials dynamic scatter, it is impossible to confidently estimate the peak velocity of the hypothetical single saccade.
We further explored possible mechanisms for the longer latencies. One of the most popular models of saccadic reaction times is the Linear Approach to Threshold with Ergodic Rate (LATER) proposed by Carpenter et al. [58,59]. Sedation may affect the velocity of the reaching of the “go” threshold, add internal noise to the ergodic process, and/or interfere with the detection of the target transition. The R2 of the RLAT models for the sedatives were poor, but also similar to the R2 of the placebo, suggesting that the internal noise was not significantly affected. Unwanted eye movements like drifts, nystagmus, or saccadic intrusions during the period of fixation of the central target could have delayed the detection of the target transition and/or delayed the saccadic response. We visually inspected all traces during the time interval between −500 ms from the target transition from the center to the eccentric position up to the onset of the primary saccade. Although the eye traces were noisier, we did not see evidence for abnormal eye movements with any of the three sedatives. Even when the latencies were longer than 1000 ms, many trials did not show any small spurious saccade during this period, which, due to the saccadic refractory period, could have delayed the subject’s response. Some of the noise increase in the traces was likely due to the reduction of the diameter of the pupil in several subjects caused by the sedative [60], the drooping eyelids interfering with the view of the pupil, and the subject fighting sleepiness and putting mechanical pressure on the head holder causing small head movements. These are all factors that would have degraded the performance of the video eye tracker with the increasing sedation of the subject. No subject complained about poor vision, loss of focus or loss of contrast. The fact that the effects on latency by dexmedetomidine were significantly weaker than for the two GABA-A agonists suggests a drug-specific effect on latency unrelated to an hypothetical degradation in the visual detection of the target transition, which would have likely affected, on average, all sedative groups equally.
The postsaccadic drift in Fig. 9 (arrow), which was present in several “staircase” trials in the sedative groups, but never in the placebo group, might indicate that sedation affects the saccadic pulse-step matching. When the tonic step needed to hold the eye in the new position, obtained by integration of the saccadic velocity command (pulse) [61], has a gain that is too low, a backward drift follows after the end of the movement. The corrective saccades, which have smaller pulses, show smaller or no backward drifts. Once the goal is reached, the subject could hold the eye in the final position indefinitely, which is not consistent with a leaking oculomotor integrator. Sedation causes an increase in exophoria [62], clearly seen by Aantaa [21] for dexmedetedomidine using a standard Maddox wing test. This effect is traditionally seen as an impairment of extraocular muscle balance by the sedative, although no binocular recordings are available. The alternative hypothesis of an alteration in tonic accommodation, with the exophoria elicited by the abnormal accommodation level through the accommodation/vergence cross-links [63] seems, in our view, more likely. Accommodation is a balance of actions by the sympathetic and the parasympathetic systems, and GABAergic effects are likely as well. It is therefore possible that some of the smooth drifts observed in our traces were corrective vergence responses after a transient loss of binocular alignment. Our monocular recordings cannot discriminate between conjugate and vergence smooth responses, but patterns similar to Fig. 9 were also observed for vertical eye movements. This makes it unlikely that the between-saccades drifts described in Fig. 9 are vergence responses. Vertical binocular alignment is much more robust, and its disruption would have been immediately detected by the subject as vertical diplopia. Furthermore, vertical vergence has a much smaller functional range.
3.4 Conclusions
As reported by earlier studies, the intensity of the effects of sedatives on saccadic metrics presented large between-subjects variability, quite evident in Figs. 3–6. The effects became more consistent at the single-subject level around the EC50 value for propofol and midazolam. For dexmedetomidine, the effects were much more irregular at all the concentrations we tested, including around the EC50 value. The major finding of our study is that while propofol and midazolam had comparable effects on saccadic dynamics, gain and latency, dexmedetomidine caused smaller dynamical changes and weak or no changes were observed on gain and latency, even if the subjects reported similar SLS values (Fig. 2 and Tab. 3). Placebo effects, in terms of increasing SLS as the session progressed, had no impact on saccadic metrics. Our results show clear evidence that the oculomotor effects of sedation are, for the most part, drug-specific and not linked to the subjective level of sedation.
Tab. 2.
SLS models for all 4 groups. Layout as Tab. 1.
| SLS MODEL PEAK VELOCITY [eq. 1] (n=60) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | CPKV Average | CPKV ±SD | CPKV Range | CPKV >0/<0/~0 | t-value | Prob | R2 Average | R2 ±SD | R2 Range |
| Placebo | −0.003 | 0.027 | −0.094 0.054 |
13/16/31 | −0.9 | 0.4 | 0.76 | 0.13 | 0.33 0.97 |
| Propof. | −0.038* | 0.033 | −0.117 0.018 |
1/50/9 | −8.7 | 3×10−12 | 0.69 | 0.19 | 0.23 0.94 |
| Midaz. | −0.035* | 0.036 | −0.191 0.073 |
0/51/9 | −7.5 | 4×10−10 | 0.66 | 0.18 | 0.25 0.92 |
| Dexmed. | −0.020* | 0.027 | −0.095 0.024 |
4/35/21 | −5.7 | 4×10−7 | 0.69 | 0.16 | 0.19 0.95 |
| SLS MODEL DURATION [eq. 2] (n=60) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | CDUR Average | CDUR ±SD | CDUR Range | CDUR >0/<0/~0 | t-value | Prob | R2 Average | R2 ±SD | R2 Range |
| Placebo | 0.008 | 0.037 | −0.047 0.150 |
22/14/24 | 1.7 | 0.09 | 0.63 | 0.27 | 0.05 0.98 |
| Propof. | 0.157* | 0.298 | −0.037 1.741 |
44/2/14 | 4.1 | 1×10−4 | 0.43 | 0.27 | 0.03 0.89 |
| Midaz. | 0.143* | 0.159 | −0.017 0.887 |
55/0/5 | 7.0 | 3×10−9 | 0.39 | 0.20 | 0.07 0.82 |
| Dexmed. | 0.108* | 0.153 | −0.062 0.752 |
42/1/17 | 5.5 | 1×10−6 | 0.44 | 0.20 | 0.04 0.86 |
| SLS MODEL RECIPROCAL OF LATENCY [eq. 3] (n=60) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | CRLAT Average | CRLAT ±SD | CRLAT Range | CRLAT >0/<0/~0 | t-value | Prob | R2 Average | R2 ±SD | R2 Range |
| Placebo | 0.019* | 0.043 | −0.123 0.197 |
32/5/23 | 3.5 | 0.001 | 0.13 | 0.10 | 0.01 0.39 |
| Propof. | −0.025* | 0.025 | −0.086 0.022 |
4/40/16 | −7.8 | 1×10−10 | 0.17 | 0.12 | 0.01 0.57 |
| Midaz. | −0.022* | 0.016 | −0.070 0.026 |
1/47/12 | −10.3 | 9×10−15 | 0.15 | 0.10 | 0.00 0.39 |
| Dexmed. | −0.008* | 0.021 | −0.071 0.032 |
12/23/25 | −2.9 | 0.006 | 0.12 | 0.09 | 0.01 0.39 |
| SLS MODEL GAIN PRIMARY SACCADE [eq. 4] (n=60) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | CGAIN Average | CGAIN ±SD | CGAIN Range | CGAIN >0/<0/~0 | t-value | Prob | R2 Average | R2 ±SD | R2 Range |
| Placebo | 0.004* | 0.013 | −0.026 0.031 |
24/11/25 | 2.5 | 0.014 | 0.21 | 0.15 | 0.00 0.61 |
| Propof. | −0.014* | 0.021 | −0.096 0.029 |
5/34/21 | −5.3 | 2×10−6 | 0.17 | 0.13 | 0.00 0.49 |
| Midaz. | −0.025* | 0.025 | −0.153 0.015 |
2/49/9 | −7.9 | 9×10−11 | 0.25 | 0.15 | 0.01 0.63 |
| Dexmed. | 0.001 | 0.021 | −0.075 0.073 |
14/17/29 | 0.5 | 0.6 | 0.17 | 0.13 | 0.01 0.54 |
Research Highlights.
We tested if the effects of sedatives on saccades are nonspecific or differ between sedatives.
The dosages were selected for the subjects to report similar subjective levels of sedation (SLS).
Propofol and midazolam had strong effects on saccadic dynamics, latency, and gain.
Dexmedetedomidine had less impact on saccadic metrics and presented no changes in saccadic gain.
Sympathetic system suppression differs from inhibitory GABA-A receptors activation at same SLS.
Acknowledgments
Grant information:
The effect of sedation on pain perception NIH-NCRR K23 RR021874 and UAB GCRC M01 RR-00032 to M. A. Frölich
NIH-NEI P30 EY-03039 Vision Science Research Center Core Grant
The authors thank the UAB General Clinical Research Center for their clinical and organizational support, the UAB Vision Science Research Center for helping in the development of the recording equipment, Drs. A. Arabshahi, S. Barnes, and J. Prasain for the gas chromatography--mass spectrometry measurements, and Christopher Williams for helping in the data acquisition and analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gao F, Mapleson WW, Vickers MD. Effect of sub-anaesthetic infusions of propofol on peak velocity of saccadic eye movements. Eur J Anaesthesiol. 1991;8:267–76. [PubMed] [Google Scholar]
- 2.Ball DM, Glue P, Wilson S, Nutt DJ. Pharmacology of saccadic eye movements in man. 1. Effects of the benzodiazepine receptor ligands midazolam and flumazenil. Psychopharmacology (Berl) 1991;105:361–7. doi: 10.1007/BF02244431. [DOI] [PubMed] [Google Scholar]
- 3.Paut O, Vercher J-L, Blin O, Lacarelle B, Mestre D, Durand A, et al. Evaluation of saccadic eye movements as an objective test of recovery from anaesthesia. Acta Anaesthesiol Scand. 1995;39:1117–24. doi: 10.1111/j.1399-6576.1995.tb04241.x. [DOI] [PubMed] [Google Scholar]
- 4.Hommer DW, Matsuo V, Wolkowitz O, Chrousos G, Greenblatt DJ, Weingartner H, et al. Benzodiazepine sensitivity in normal human subjects. Arch Gen Psychiatry. 1986;43:542–51. doi: 10.1001/archpsyc.1986.01800060032005. [DOI] [PubMed] [Google Scholar]
- 5.Bojanic S, Simpson T, Bolger C. Ocular microtremor: a tool for measuring depth of anaesthesia? Br J Anaesth. 2001;86:519–22. doi: 10.1093/bja/86.4.519. [DOI] [PubMed] [Google Scholar]
- 6.Heaney M, Kevin LG, Manara AR, Clayton TJ, Timmons SD, Angel JJ, et al. Ocular microtremor during general anesthesia: Results of a multicenter trial using automated signal analysis. Anesth Analg. 2004;99:775–80. doi: 10.1213/01.ANE.0000133145.98702.C0. [DOI] [PubMed] [Google Scholar]
- 7.Spauschus A, Marsden J, Halliday DM, Rosenberg JR, Brown P. The origin of ocular microtremor in man. Exp Brain Res. 1999;126:556–62. doi: 10.1007/s002210050764. [DOI] [PubMed] [Google Scholar]
- 8.Savola JM, Ruskoaho J, Puurunen J, Salonen JS, Kärki NT. Evidence for medetomidine as a selective and potent agonist of alpha2-adrenoceptors. J Auton Pharmacol. 1986;5:275–84. doi: 10.1111/j.1474-8673.1986.tb00654.x. [DOI] [PubMed] [Google Scholar]
- 9.Virtanen R, Savola JM, Saano V, Nyman L. Characterization of the selectivity, specificity, and potency of medetomidine as an alpha2-adrenoceptor agonist. Eur J Pharmacol. 1988;150:9–14. doi: 10.1016/0014-2999(88)90744-3. [DOI] [PubMed] [Google Scholar]
- 10.Bhana N, Goa KL, McClellan K. Dexmedetomidine. Drugs. 2000;59:263–8. doi: 10.2165/00003495-200059020-00012. [DOI] [PubMed] [Google Scholar]
- 11.Unger RJ, Gallagher CJ. Dexmedetomidine sedation for awake fiberoptic intubation. Semin Anest Periop Med Pain. 2006;25:65–70. [Google Scholar]
- 12.Chiu TH, Chen MJ, Yang YR, Yang JJ, Tang FL. Action of dexmedetomidine on rat locus coeruleus neurons: intracellular recording in vitro. Eur J Pharmacol. 1995;285:261–8. doi: 10.1016/0014-2999(95)00417-j. [DOI] [PubMed] [Google Scholar]
- 13.Benarroch EE. The locus coeruleus norepinephrine system: functional organization and potential clinical significance. Neurology. 2009;73:1699–704. doi: 10.1212/WNL.0b013e3181c2937c. [DOI] [PubMed] [Google Scholar]
- 14.Dietrichs E. Cerebellar cortical and nuclear afferents from the feline locus coeruleus complex. Neurosci. 1988;27:77–91. doi: 10.1016/0306-4522(88)90220-5. [DOI] [PubMed] [Google Scholar]
- 15.Clark FM, Proudfit HK. The projection of locus coeruleus neurons to the spinal cord in the rat determined by anterograde tracing combined with immunocytochemistry. Brain Res. 1991;538:231–45. doi: 10.1016/0006-8993(91)90435-x. [DOI] [PubMed] [Google Scholar]
- 16.Simpson KL, Altman DW, Wang L, Kirifides ML, Lin RC, Waterhouse BD. Lateralization and functional organization of the locus coeruleus projection to the trigeminal somatosensory pathway in rat. J Comp Neurol. 1997;385:135–47. [PubMed] [Google Scholar]
- 17.Aston-Jones G, Ennis M, Pieribone VA, Nickell WT, Shipley MT. The brain nucleus locus coeruleus: restricted afferent control of a broad efferent network. Science. 1986;234:734–7. doi: 10.1126/science.3775363. [DOI] [PubMed] [Google Scholar]
- 18.Ennis M, Aston-Jones G. Potent inhibitory input to locus coeruleus from the nucleus prepositus hypoglossi. Brain Res Bull. 1989;22:793–803. doi: 10.1016/0361-9230(89)90022-1. [DOI] [PubMed] [Google Scholar]
- 19.McFarland JL, Fuchs AF. Discharge patterns in nucleus prepositus hypoglossi and adjacent medial vestibular nucleus during horizontal eye movements in behaving macaques. J Neurophysiol. 1992;68:319–32. doi: 10.1152/jn.1992.68.1.319. [DOI] [PubMed] [Google Scholar]
- 20.Kaur S, Saxena RN, Mallick BN. GABAergic neurons in prepositus hypoglossi regulate REM sleep by its action on locus coeruleus in freely moving rats. Synapse. 2001;42:141–50. doi: 10.1002/syn.1109. [DOI] [PubMed] [Google Scholar]
- 21.Aantaa R. Assessment of the sedative effects of dexmedetomidine, an α2-adrenoceptor agonist, with analysis of saccadic eye movements. Pharmacol Toxicol. 1991;68:394–8. doi: 10.1111/j.1600-0773.1991.tb01259.x. [DOI] [PubMed] [Google Scholar]
- 22.Frölich MA, Dennis DM, Shuster JA, Melker RJ. Precision and bias of target controlled propofol infusion for sedation. Br J Anaesth. 2005;94:434–7. doi: 10.1093/bja/aei081. [DOI] [PubMed] [Google Scholar]
- 23.Busettini C, Mays LE. Saccade-vergence interactions in macaques. I. Test of the omnipause multiply model. J Neurophysiol. 2005;94:2295–311. doi: 10.1152/jn.01336.2004. [DOI] [PubMed] [Google Scholar]
- 24.Carpenter RHS. Oculomotor procrastination. In: Fisher DF, Monty RA, Senders JW, editors. Eye movements: cognition and visual perception. New Jersey: Lawrence Erlbaum; 1981. pp. 237–46. [Google Scholar]
- 25.Reddi BA, Asrress KN, Carpenter RHS. Accuracy, information, and response time in a saccadic decision task. J Neurophysiol. 2003;90:3538–46. doi: 10.1152/jn.00689.2002. [DOI] [PubMed] [Google Scholar]
- 26.Michell AW, Xu Z, Fritz D, Lewis SJG, Foltynie T, Williams-Gray CH, et al. Saccadic latency distributions in Parkinson’s disease and the effect of L-dopa. Exp Brain Res. 2006;174:7–18. doi: 10.1007/s00221-006-0412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bahill AT, Clark MR, Stark L. The main sequence, a tool for studying human eye movements. Math Biosci. 1975;24:191–204. [Google Scholar]
- 28.Carpenter RHS. Movements of the eyes. London: Pion Ltd; 1988. [Google Scholar]
- 29.Kalesnykas RP, Hallett PE. Retinal eccentricity and the latency of eye saccades. Vision Res. 1994;34:517–31. doi: 10.1016/0042-6989(94)90165-1. [DOI] [PubMed] [Google Scholar]
- 30.Hodgson TL. The location marker effect. Saccadic latency increases with target eccentricity. Exp Brain Res. 2002;145:539–42. doi: 10.1007/s00221-002-1162-1. [DOI] [PubMed] [Google Scholar]
- 31.Moschovakis AK, Scudder CA, Highstein SM. The microscopic anatomy and physiology of the mammalian saccadic system. Prog Neurobiol. 1996;50:133–254. doi: 10.1016/s0301-0082(96)00034-2. [DOI] [PubMed] [Google Scholar]
- 32.Davies DN, Steward A, Allott PR, Mapleson WW. A comparison of arterial and arterialized venous concentrations of halothane. Br J Anaesth. 1972;44:548–50. [PubMed] [Google Scholar]
- 33.Johnston KR, Vickers MD, Mapleson WW. Comparison of arterialized venous with arterial blood propofol concentrations during sub-anaesthetic infusion in volunteers. Br J Anaesth. 1996;76:401–4. doi: 10.1093/bja/76.3.401. [DOI] [PubMed] [Google Scholar]
- 34.Freo U, Dam M, Ori C. The time-dependent effects of midazolam on regional cerebral glucose metabolism in rats. Anesth Analg. 2008;106:1516–23. doi: 10.1213/ane.0b013e31816a64a8. [DOI] [PubMed] [Google Scholar]
- 35.Kojima Y, Soetedjo F, Fuchs AF. Effects of GABA agonist and antagonist injections into the oculomotor vermis on horizontal saccades. Brain Res. 2010;1366:93–100. doi: 10.1016/j.brainres.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meinecke DL, Tallman J, Rakic P. GABAA/benzodiazepine receptor-like immunoreactivity in rat and monkey cerebellum. Brain Res. 1989;493:303–19. doi: 10.1016/0006-8993(89)91165-7. [DOI] [PubMed] [Google Scholar]
- 37.Temel Y, Visser-Vandewalle V, Carpenter RHS. Saccadic latency during electrical stimulation of the human subthalamic nucleus. Curr Biol. 2008;18:R412–4. doi: 10.1016/j.cub.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 38.Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev. 2000;80:953–78. doi: 10.1152/physrev.2000.80.3.953. [DOI] [PubMed] [Google Scholar]
- 39.Hikosaka O, Wurtz RH. Effects on eye movements of a GABA agonist and antagonist injected into monkey superior colliculus. Brain Res. 1983;272:368–72. doi: 10.1016/0006-8993(83)90586-3. [DOI] [PubMed] [Google Scholar]
- 40.Schönle PW, Isenberg C, Crozier TA, Dressler D, Machetanz J, Conrad B. Changes of transcranially evoked motor responses in man by midazolam, a short acting benzodiazepine. Neurosci Lett. 1989;101:321–4. doi: 10.1016/0304-3940(89)90553-3. [DOI] [PubMed] [Google Scholar]
- 41.Dueck MH, Oberthuer A, Wedekind C, Paul M, Boerner U. Propofol impairs the central but not the peripheral part of the motor system. Anesth Analg. 2003;96:449–55. doi: 10.1097/00000539-200302000-00029. [DOI] [PubMed] [Google Scholar]
- 42.McElligott JG, Freedman W. Vestibulo-ocular reflex adaptation in cats before and after depletion of norepinephrine. Exp Brain Res. 1988;69:509–21. doi: 10.1007/BF00247305. [DOI] [PubMed] [Google Scholar]
- 43.Sawaguchi T. Attenuation of delay-period activity of monkey prefrontal neurons by an alpha2-adrenergic antagonist during an oculomotor delayed-response task. J Neurophysiol. 1988;80:2200–5. doi: 10.1152/jn.1998.80.4.2200. [DOI] [PubMed] [Google Scholar]
- 44.Tychsen L, Sitaram N. Catecholamine depletion produces irrepressible saccadic eye movements in normal humans. Ann Neurol. 1989;25:444–9. doi: 10.1002/ana.410250505. [DOI] [PubMed] [Google Scholar]
- 45.Busettini C, Mays LE. Pontine omnipause activity during conjugate and disconjugate eye movements in macaques. J Neurophysiol. 2003;90:3838–53. doi: 10.1152/jn.00858.2002. [DOI] [PubMed] [Google Scholar]
- 46.Schultz KP, Williams CR, Busettini C. Macaque pontine omnipause neurons play no direct role in the generation of eye blinks. J Neurophysiol. 2010;103:2255–74. doi: 10.1152/jn.01150.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strassman A, Evinger C, McCrea RA, Baker RG, Highstein SM. Anatomy and physiology of intracellularly labelled omnipause neurons in the cat and squirrel monkey. Exp Brain Res. 1987;67:436–40. doi: 10.1007/BF00248565. [DOI] [PubMed] [Google Scholar]
- 48.Horn AK, Büttner-Ennever JA, Wahle P, Reichenberger I. Neurotransmitter profile of saccadic omnipause neurons in nucleus raphe interpositus. J Neurosci. 1994:2032–46. doi: 10.1523/JNEUROSCI.14-04-02032.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Optican LM. The role of omnipause neurons: why glycine? Prog Brain Res. 2008;171:115–21. doi: 10.1016/S0079-6123(08)00615-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henn V, Baloh RW, Hepp K. The sleep-wake transition in the oculomotor system. Exp Brain Res. 1984;54:166–76. doi: 10.1007/BF00235828. [DOI] [PubMed] [Google Scholar]
- 51.Keller EL. Control of saccadic eye movements by midline brain stem neurons. In: Baker R, Berthoz A, editors. Control of Gaze by Brain Stem Neurons. Amsterdam: Elsevier; 1977. pp. 327–36. [Google Scholar]
- 52.Shaikh AG, Wong AL, Optican LM, Miura K, Solomon D, Zee DS. Sustained eye closure slows saccades. Vision Res. 2010;50:1665–75. doi: 10.1016/j.visres.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murthy A, Ray S, Shorter SM, Priddy EG, Schall JD, Thompson KG. Frontal eye field contributions to rapid corrective saccades. J Neurophysiol. 2007;97:1457–69. doi: 10.1152/jn.00433.2006. [DOI] [PubMed] [Google Scholar]
- 54.Robinson DA. Eye movements evoked by collicular stimulation in the alert monkey. Vision Res. 1972;12:1795–808. doi: 10.1016/0042-6989(72)90070-3. [DOI] [PubMed] [Google Scholar]
- 55.Schiller PH, Stryker M. Single-unit recording and stimulation in the superior colliculus of the alert rhesus monkey. J Neurophysiol. 1972;35:915–24. doi: 10.1152/jn.1972.35.6.915. [DOI] [PubMed] [Google Scholar]
- 56.Breznen B, Lu S-M, Gnadt JW. Analysis of the step response of the saccadic feedback: system behavior. Exp Brain Res. 1997;117:181–91. doi: 10.1007/s002210050214. [DOI] [PubMed] [Google Scholar]
- 57.Paul K, Gnadt JW. Activity of omnipause neurons during “staircase saccades” elicited by persistent microstimulation of the superior colliculus. Vision Res. 2006;46:3430–42. doi: 10.1016/j.visres.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 58.Carpenter RHS. A neural mechanism that randomizes behavior. J Consciousness Stud. 1999;6:13–22. [Google Scholar]
- 59.Ratcliff R, Carpenter RHS, Reddi BAJ. Putting noise into neurophysiological models of simple decision making. Nat Neurosci. 2001;4:336–7. doi: 10.1038/85956. [DOI] [PubMed] [Google Scholar]
- 60.Loewenfeld IE. The Pupil: Anatomy, Physiology, and Clinical Applications. 2. Oxford: Butterworth-Heinemann; 1999. [Google Scholar]
- 61.Robinson DA. Oculomotor control signals. In: Lennerstrand G, Bach-Y-Rita P, editors. Basic Mechanisms of Ocular Motility and Their Clinical Implications. Oxford: Pergamon Press; 1975. pp. 337–74. [Google Scholar]
- 62.Hannington-Kiff JG. Measurement of recovery from outpatient general anaesthesia with a simple ocular test. Brit Med J. 1970;3:132–5. doi: 10.1136/bmj.3.5715.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Semmlow JJ, Heerema D. The synkinetic interaction of convergence accommodation and accommodative convergence. Vision Res. 1979;19:1237–42. doi: 10.1016/0042-6989(79)90189-5. [DOI] [PubMed] [Google Scholar]