Abstract
Cardiac toxicity is a leading contributor to late-stage attrition in the drug discovery process and to withdrawal of approved from the market. In vitro assays that enable earlier and more accurate testing for cardiac risk provide early stage predictive indicators that aid in mitigating risk. Human cardiomyocytes, the most relevant subjects for early stage testing, are severely limited in supply. But human stem cell-derived cardiomyocytes (SC-hCM) are readily available from commercial sources and are increasingly used in academic research, drug discovery and safety pharmacology. As a result, SC-hCM electrophysiology has become a valuable tool to assess cardiac risk associated with drugs. This unit describes techniques for recording individual currents carried by sodium, calcium and potassium ions, as well as single cell action potentials, and impedance recordings from contracting syncytia of thousands of interconnected cells.
Keywords: Stem cell-derived cardiomyocytes, electrophysiology, impedance
INTRODUCTION
Increased use of in vitro safety assessment during preclinical drug discovery and development is an important trend fueled by rapid advances in cell biology coupled to development of powerful cell-based functional assays. Cardiac hazard identification in particular has benefitted from this trend, with safety assays moving to earlier stages of drug discovery to facilitate decision-making that helps avoid costly progression of compounds that ultimately prove to be cardiotoxic. The problem of drug-induced delayed cardiac repolarization is a case in point, non-cardiac drugs have been found to cause life-threatening arrhythmia primarily through off-target inhibition of the hERG potassium channel. This safety issue has led to the removal of some drugs from the market and restrictive labeling of other approved drugs, as well as the termination of otherwise promising research programs. The concerns raised by delayed repolarization have spurred interest in developing more predictive preclinical safety pharmacology assays that can be conducted in early drug development when the risk is more easily mitigated by medicinal chemistry or when the financial consequences of program termination are minimized.
The traditional approach to preclinical cardiac safety pharmacology focuses on animal testing – including in vivo models such as telemetered dog or monkey, and various in vitro or ex vivo models such as rabbit Purkinje fibers, guinea pig isolated or open chest heart, canine ventricular wedge, guinea pig papillary muscle or isolated myocytes. Until recently, the only extensively used human-based in vitro test system has been human cardiac ion channels expressed in recombinant non-cardiac cell lines.
Ideally, testing would be conducted in cardiac myocytes from human adult hearts. Although myocytes isolated from adult human atrial appendages obtained during cardiac surgery have been used, they are of atrial rather than ventricular origin, infrequently available; and because they come from heart disease patients, may have abnormal pathologic and pharmacologic characteristics. The situation has changed dramatically with the introduction of commercially available, stem cell-derived human cardiomyocytes (SC-hCM) originating either from embryonic stem cells (hESC-CM) or induced pluripotent stem cells reprogrammed from adult somatic cells (hiPSC-CM). The techniques for producing SC-hCM in large quantities have been industrialized by several companies enabling the adoption of both current testing techniques, such as manual patch clamp (MPC), as well as new, more efficient techniques, such as cellular impedance analysis (CIA), multi-electrode array (MEA) analysis of field potentials, fluorescent plate-based Calcium flux assays, and automated patch clamp. Furthermore, hiPS-CMs obtained from patients with congenital cardiac disorders, in particular, promise to open a new approach to testing drugs in at-risk patient populations, if it can be shown that the hiPSC-CM recapitulates the disease phenotype.
Assays using SC-hCM as single isolated cells in manual patch clamp in the current clamp mode have been developed to investigate compound -induced changes in action potential parameters, and as spontaneously contracting monolayers in impedance assays for contractility and cytotoxicity evaluations, and in multi-electrode array experiments to detect effects on conducted electrical activity.
This unit will describe protocols designed to characterize theaction potential and the major currents present in SC-hCM. We will focus on ionic currents with significant current density including thesodium current (INa), the L-type calcium current (ICa)and the transient activated potassium current (Ito). These currents are relatively easy to record, making them good subjects when learning topatch clamp SC-hCMs. Other relevant currents in SC-hCMs include the rapidly activated delayed rectifier potassium (hERG) current (IKr). However, the contribution of IKrto total outward current in SC-hCMs is very small and difficult to record. But the effect of hERG channel blockers is readily observed in action potential experiments (Peng et al. 2010) where changes membrane potential serve to amplify the effects of hERG blockers.
Finally, we will describe a new electrophysiological approach to characterize the contractile behavior of SC-hCMs populations using cellular impedance recordings. This new approach to SC-hCM function allows assessment ofdrug-induced effects on excitation-contraction coupling.
BASIC PROTOCOL 1
MEASUREMENT OF ACTION POTENTIALS INSTEM CELL-DERIVED HUMAN CARDIOMYOCYTES
Action potential recordings can be made from single stem cell-derived cardiac cells (Peng et al, 2010; Ma et al, 2011). The cells are commercially available from several sources (e.g., Cellular Dynamics International, Axiogenesis, Cellectis), and are generated in-houseby academic and industrial labs. This protocol describes the procedures used to record and analyze action potentials from SC-CMs in the whole cell perforated-patch clamp recording method. Fundamental action potential parameters are recorded and analyzed using appropriately configured hardware and software. Action potential changes in response to treatment with cardioactive compounds can be analyzed using real-time data acquisition and off-line software packages.
Solutions
Extracellular solution (in mM): 137 NaCl, 4 KCl, 1.8 CaCl2, 1.0 MgCl2, 10 HEPES, 10 Glucose; pH 7.4 with NaOH. Osmolality, 290 mMol/kg. Store refrigerated (2–8°C) up to one month.
Intracellular (micropipette) solution (in mM): 130 K-aspartate, 5 MgCl2, 5 EGTA, 10 HEPES; pH to 7.2 with KOH. Osmolality 275 mMol/kg. Store refrigerated (4 °C) up to 6 months. On each day of testing, add 1 ml of 130 mM KCl solution to an Amphotericin B aliquot and vortex for approximately one minute. Store on wet ice, protected from light, for no more than 4 hours.
Amphotericin B stock solution: 40 mg/ml in DMSO, amber vial. Vortex for approximately 3 minutes, then degas for approximately 1 minute. Amphotericin B will dissolve over the course of the next 5 minutes. Once the stock solution appears homogeneous, aliquot 6 µl per microcentrifuge tube. Store frozen (−80 °C), protected from light, up to one year.
Equipment
Air Table (Newport LW3036B-OPT)
Faraday Cage (Various)
Data Acquisition System – Digidata (Axon Instruments 1320A)
Laboratory DC Power Supply (Tenma 72-2005)
Micromanipulator Assembly (Burleigh, PCS-6000 (EXPO) Series)
Micromanipulator Axis Control Unit (Burleigh, PCS-6000 (EXPO) Series)
Micromanipulator Power Supply (Burleigh, PCS-6000 (EXPO) Series)
Micromanipulator Assembly Base (Burleigh,PCS-6000 (EXPO) Series)
Inverted Microscope (Zeiss Axiovert 25 CFL)
Patch Clamp Amplifier (Axon Instruments 200B)
Dual Channel Heater Controller (Warner Instruments, TC-344B)
Headstage (Axon Instruments)
Culture Dish Heater (Warner Instruments, DH-35)
Perfusion Chamber (Warner Instruments, RC-25F)
Heating Platform (Warner Instruments, PH4)
Cable Assembly (Warner Instruments, CC-28)
Solution Heater (Warner Instruments, SH-27B)
Manipulator for Perfusion (Fine Science Tools)
Magnetic Stand (Fine Science Tools)
Manipulator Stand (Sutter Instruments MT-75)
Manipulator for Solution Heater (World Precision Instruments KITE-L)
Pipet puller (Model P-97, Sutter Instruments)
Borosilicate glass tubing (World Precision Instruments TW150F-3)
Sonicator (Branson Ultrasonics)
Silicone tubing, size 24 (Cole Palmer Instrument)
Tygon tubing (Cole Palmer Instrument, cat. nos. AAQ-0127 and AAC02529-CP)
T-fittings (Cole Palmer Instrument, cat. no. 06365-77)
16 or 24-gauge needle
Vacuum source
35 mm dish containing cardiomyocytes (Support Protocol 1)
Positive Control Compounds (e.g., quinidine)
Protocol steps
Set-up the Electrophysiology Workstation
-
1
The workstation consists of 1) a computer running pClamp software, 2) a patch clamp amplifier and pre-amplifier, 3) an analog to digital converter, 4) an anti-vibration table, 5) an inverted microscope, and 6) a Faraday cage. Refer to Figure 1 for a schematic diagram of a typical workstation. Details for standard workstation installation can be found in the Axon Guide (Molecular Devices, Inc.).
-
2
Prepare the perfusion system. The perfusion system consists of a) a chamber heater placed in the stage of the microscope, b) 10 ml syringes connected to a stopcock, female luer and silicone tubing, c) a series of T-fittings connecting the silicone tubing to a common Tygon tubing (through T-fittings) that is connected to the in-line solution pre-heater, d) a Tygon tubing connected to solution outlet of the pre-heater and e) a suction device made of a needle connected to vacuum source. The perfusion rate is adjusted to maintain a stable solution level of ~ 2 mm, 1–2 mL/min. The Tygon tubing connected to the outlet of the pre-heater is placed close to the cell. The thermistor probe to measure and control the temperature is also placed close to the cell.
-
3
Prepare Microelectrodes. Use borosilicate glass tubing. Use the ramp test protocol of the model P-97 puller according to the manufacturer’s instruction manual to define the heat setting. Pull recording microelectrodes to the desired shape and tip resistance by adjusting the Heat, Pull, Velocity and Time parameters of the model P-97 puller in a 3 or 4 step protocol, according to the manufacturer’s instruction manual. Use the last step to adjust the point. A good electrode will have a tapered shape and resistance in the range of 1.5 – 8.0 MΩ when immersed in recording solution.
Fine tuning can be achieved changing the velocity parameter in 2–3 unit steps.
Figure 1.
Schematic diagram of a typical manual patch clamp electrophysiology workstation. The inverted microscope, perfusion system including the solution in-line heater, the recording chamber and the headstage are enclosed in a Faraday cage (not shown) for shielding from external electrical interference. The dual temperature controller, amplifier, digital converter and computer are located outside the cage. The complete equipment is listed in Basic Protocol 1.
Prepare for Recording
-
4
Set a 35 mm dish containing cardiomyocytes (Support Protocol 1) into the recording chamber.
-
5
Perfuse the chamber with recording solution at a rate of 1–2 ml/min and heated to 35 ± 2°C using a combination of in-line solution pre-heater and chamber heater. Aspirate the chamber solution using a suction pipet connected to a vacuum source. Position the outflow tubing close to the cell to be used.
-
6
Back-fill the recording electrode with the micropipette solution. With the pipette suction closed (no suction), gently fit the microelectrode into the electrode holder, and turn the pipette cap of the electrode holder to tighten the holder around the microelectrode. This will result in a small positive pressure inside the electrode that will prevent the influx of extracellular solution when the pipette is in the bath.
-
7
Immerse the electrode tip into the recording chamber containing recording solution and monitor the electrode resistance using the Seal Test protocol.
Achieve Perforated Patch Clamp
-
8
Carefully advance the recording electrode towards a single cardiomyocyte using the micromanipulator until sufficiently close to use the micromanipulator.
The SC-hCMs should be visibly beating when examined under a microscope. Choose a cell for patch clamping that is beating strongly. The cells will not resemble mature adult cardiomyocytes; they will be round or amorphous with disarrayed sarcomeric structures.
-
9
While continuously monitoring the pipette resistance, position the electrode close to the cell membrane and zero the pipette offset. Advance the electrode onto the cell very gently; the electrode resistance will suddenly increase when it touches the cell surface.
-
10
Using either mouth suction or syringe suction, gently apply negative pressure through the electrode to the cell membrane to form a seal. Measure the pipette resistance (≥ 0.8 GΩ) using the Seal Test protocol.
-
11
Switch the amplifier settings to the current-clamp mode with null current (I = 0). Monitor the baseline cell for 3 – 7 minutes. An action potential should become visible as the amphotericin slowly perforates the cell membrane. When action potentials are registered, switch the amplifier to current clamp fast mode and monitor until recordings are stable.
The action potential height (amplitude), shape and maximum diastolic potential should be stable before proceeding.
-
12
Switch the amplifier to voltage-clamp mode and measure the access resistance and cell capacitance using the Membrane Test protocol.
The access resistance of the electrode should be ≤ 30.0 MΩ. Series resistance can be compensated as described in Basic Protocol 2.
Monitor and Pace Action Potentials
-
13
Switch the amplifier settings to current-clamp fast mode.
-
14Monitor the action potential and proceed with the experiment only if the SC-hCM displays baseline values appropriate for the cell type you wish to record. For example, acceptance criteria for ventricular-like cells are shown below:
Measurement Acceptance Criteria Resting membrane potential (RMP) ≤ −60 mV Action potential duration at 90% of the initial resting potential (APD90) 150 ms Maximum rate of depolarization (Vmax) 20 V/s Action potential amplitude (APA) ≥ 70 mV -
15
Use episodic stimulation in the acquisition mode of pClamp to apply a 50 pA current pulse (up to 3 ms duration), then adjust the duration and magnitude of the stimulus to maintain pacing at a fixed rate. For example, the magnitude can be increased in 20 pA steps until the action potential period is maintained at a fixed rate. Most ventricular-like cardiomyocytes can be captured at a rate between 1 to 2 Hz.
Note that continuous recording using the gap-free function of pClamp will generate very large data files. Alternative software (e.g. Notocord-Hem software, Notocord Systems SA, Croissy, France) allows real-time display of action potential parameters as well as continuous recording at manageable data file sizes.
-
16
For pharmacological experiments, continuously pace the SC-hCM at a constant stimulation rate for at least one minute. This provides stabilization before baseline values of AP responses are obtained.
-
17
Apply solution containing test compound. Maintain a constant flow of solution for approximately 3 – 5 minutes or until measurable action potential parameters have stabilized.
Quinidine is a good positive control article. Quinidine at 3 µM blocks hERG ion channels (responsible for the rapid delayed rectifier potassium current, IKr) and will increase the duration of the action potential at 90% repolarization (APD90) by ~30–50% within 1- 3 minutes of application (Fig. 2).
-
18
Repeat Step 17 as necessary until all concentrations of test compounds have been tested or recording becomes unstable. Multiple test concentrations can be applied sequentially in increasing concentration.
-
19
Cease perfusion and end recording. Once the experiment is complete switch to voltage-clamp mode and measure the access resistance using the membrane test protocol.
Figure 2.
(A) Single action potentials recorded before and after equilibration with quinidine are superimposed. Note the marked prolongation of the action potential duration due the block of IKr by quinidine.(B) Representative action potential recordings made before (vehicle control) and after (quinidine) equilibration with 3 µM quinidine. Recordings were made continuously and the cell was paced at a frequency of 1 Hz. The action potential is a typical ventricular-like signal. Note that the upstroke velocity is relatively slow and the “spike and dome” morphology is absent, as has been reported (Peng et al., 2010).
The access resistance of the electrode should measure within ~ 20% of the initial measurement
SUPPORT PROTOCOL 1
PREPARATION OF STEM CELL-DERIVED HUMAN CARDIOMYOCYTES FOR RECORDING ACTION POTENTIALS OR ION CHANNEL CURRENTS
In this protocol, steps taken to thaw and plate Cytiva (GE Healthcare) SC-hCMs in 35 mm culture dishes for manual patch clamp recording are described.
Materials
Matrigel (BD Biosciences #354235)
RPMI 1640 (Invitrogen #22400-089)
B27 medium (Invitrogen #17504-044)
Cytiva Cardiomyocytes (1e5m, GE Healthcare 28-9774-35)
50 mL conical vials
35 mm culture plates (CELLSTAR Cat.-No. 627 170)
Water bath
Protocol steps
Thaw in refrigerator an appropriate number (2 – 3 mL) of aliquots of frozen Matrigel (~ 2 hours).
Place 14 mL of RPMI per 1 mL aliquot of Matrigel in a 50 mL conical vial and keep on ice.
Resuspend Matrigel in cold RPMI media by mixing 14 mL of RPMI per 1 mL aliquot of Matrigel (1:15 dilution, 15 mL RPMI total).
Add 0.75 mL of solution to 40–60, 35 mm culture plates (CELLSTAR Cat.-No. 627 170) and swirl each dish to coat the bottom with liquid.
Keep RPMI/Matrigel solution on plates for 3 hours or more.
Remove RPMI/Matrigel solution (plates are now coated with Matrigel) and replace with 2 mL culture media (RPMI + B27) before plating cardiomyocytes.
Remove a Cytiva cryovial from liquid nitrogen tank and let sit at room temperature for 1 – 2 minutes.
Thaw completely in a 37°C water bath with gentle agitation.
Transfer the contents to a 50 ml conical tube.
Rinse the cryovial with 2 ml of pre-warmed RPMI/B27 and add it to the cell suspension, drop wise with gentle swirling of the tube.
Add 16.5 ml more pre-warmed RPMI/B27 slowly (over the course of 2 – 3 minutes) until the total volume of cell suspension is 20 ml.
Centrifuge the 50 mL conical tube at 450 g for 10 min.
Remove supernatant from the50 mL conical tube and resuspendthecellsin5ml RPMI/B27.
Take cell counts and viability measurements using trypan blue solution on a hemocytometer.
Resuspend the cells in RPMI/B27 at final concentration of 1 × 105 viable cells/ml.
Plate cells onto the Matrigel-coated 35 mm culture dish (100 microliters/dish) that contains 2 mL of pre-warmed RPMI/B27.
Maintain cells by refreshing media every other day. Cardiomyocytes will recover and be optimal for manual patch clamp experiments after 2 weeks.
BASIC PROTOCOL 2
VOLTAGE-CLAMP STUDIES OF IONIC CURRENTS IN STEM CELL-DERIVED HUMAN CARDIOMYOCYTES
Three currents are considered in this basic protocol: the voltage-dependent L-type calcium current (ICa), the voltage-dependent sodium current (INa), and the transient outward potassium current (Ito).
ICa is mediated by L-type calcium channels which are key players in excitation-contraction coupling in the heart (Bers, 2002). ICa activates at membrane potentials more positive than −40 mV in physiological extracellular Ca2+ and inactivates following a bi-exponential time course that reflects both calcium- and voltage-dependent inactivation. To study ICa in isolation, both sodium and potassium currents must be inhibited. Voltage-dependent sodium currents are suppressed by holding the membrane potential at −40 mV. At this membrane potential INa is inactivated. Potassium currents are blocked by including tetraethylammonium (TEA) in the intracellular pipette solution, and Cs+ in the extracellular solution. Calcium currents decrease (“rundown”) after the whole-cell configuration is obtained, a process that is accelerated at physiological temperature.
INa is the fast depolarizing current underlying the upstroke of the cardiac action potential (Schram et al., 2002). In normal physiological conditions INa is fast and large making voltage control difficult to establish. INa shows fast and slow inactivation processes requiring hyperpolarizing potentials to reach maximal availability. It is common to reduce the extracellular Na+ concentration to reduce current amplitudes for better voltage control. Sodium currents are recorded in isolation by blocking potassium currents with Cs+ and TEA and calcium currents with 10 µM nifedipine.
The transient outward K+ current (Ito) mediates an early phase of repolarization of the cardiac action potential (Schram et al., 2002). The current is identified by its transient kinetics and by its sensitivity to flecainide. To study Ito in isolation, ICais blocked using Cd2+, and INa inactivated using a depolarizing pre-pulse to −40 mV.
Solutions to record ICa
Extracellular ICa recording solution (in mM): 137 NaCl, 5.4 CsCl, 1.8 CaCl2, 1.0 MgCl2, 10 HEPES, 10 Glucose; pH 7.4 with NaOH. Osmolality 290 mOsm.
Intracellular ICa recording solution (in mM): 130 K methanesulfonate, 20 TEA chloride, 1 MgCl2, 10 EGTA, 10 HEPES. 4 Mg-ATP, 14 Tris-phosphocreatine, 0.3 Tris-GTP, 50 creatine phosphokinase. pH 7.20 with N-methyl-D-glucamine. Osmolality 360 mOsm.
Solutions to record INa
Extracellular INa recording solution (in mM): 40 NaCl, 4 KCl, 1 MgCl2, 1.8 CaCl2, 10 glucose, 97 L-aspartic acid, 10 HEPES. pH 7.20 with N-methyl-D-glucamine. Supplement with 10 µM nifedipine on day of use. Osmolality 287 mOsm.
Intracellular INa recording solution (in mM): 130 CsOH, 130 L-aspartic acid, 5 MgCl2, 5 EGTA, 10 HEPES, 4 Na2-ATP, 0.1 Tris-GTP; pH 7.20 with L-aspartic or CsOH. Osmolality 281 mOsm.
Solutions to record Ito
Extracellular Ito recording solution (in mM): 137 NaCl, 0.5 CdCl2, 4 KCl, 1 MgCl2, 1.8 CaCl2, 10 glucose, 10 HEPES; pH 7.4 with NaOH. Osmolality 290 mOsm.
Intracellular Ito recording solution (in mM): 130 K-aspartate, 5 MgCl2, 5 EGTA, 4 Tris-ATP, 10 HEPES; pH 7.20 with KOH. Osmolality 274 mOsm.
Equipment
Refer to Basic Protocol 1
Protocol steps
-
1
Set-up the Electrophysiology Workstation and Prepare for Recording following the steps described in Basic Protocol 1.
-
2
Begin the experiment with the amplifier in voltage-clamp mode. Start the experiment with the Pipette Capacitance Compensation control and the Whole-Cell and Series Resistance potentiometers off.
-
3
Load the Seal Test Protocol from pClamp and monitor the tip resistance on the amplifier. It should read between 2 and 4 MΩ for ICa and Ito and less than 2 MΩ for INa. Compensate for pipette capacitance using the Fast Pipette Capacitance compensation control until a square waveform is obtained.
-
4
Approach the cell using the macro manipulator. Position the tip of the pipette on top of the cell and establish gentle contact with the cell membrane as evidenced by an increase in the pipette resistance. Open the pipette suction to release the positive pressure. A further increase in the pipette resistance should be apparent. Apply negative pressure using mouth suction or syringe suction to obtain a giga-ohm seal (i.e., the seal resistance measured using the Seal Test Protocol will be greater than 1 GΩ).
-
5
Switch to the Membrane Test Protocol, set the membrane potential to −40 mV for ICa and Itoor −60 mV for INa. Break the membrane by applying a mild negative pressure. The establishment of the whole cell configuration is noted by the appearance of a capacitive transient that decays mono-exponentially and an increase in signal noise. A stable whole-cell configuration may require applying a small negative pressure by closing the suction pipette.
If necessary, a 0.5 ms zap pulse can be used to rupture the membrane
-
6
Compensate for whole-cell capacitance and series resistance. Turn on the whole cell parameter. Use the Whole Cell Cap and Series Resistance potentiometers to remove the transient component. The values indicated in the controls after complete transient compensation are the values of the membrane cell capacitance and access resistance. Set the LAG to 10 µs and increase the % Compensation. The maximal compensation possible is the one below the threshold for oscillations.
Complete series resistance compensation may require the use of the Prediction protocol in the Series resistance ComP. function. Use this approach with caution since it also may induce current oscillations.
The cell capacitance, access resistance, and membrane resistance can also be read using the Membrane Test Protocol.The cell capacitance can be used to quantify the membrane surface area assuming 1µF/cm2.
-
7
Set the holding potential to −40 mV for ICa or −80 mV for INa and Ito.
-
8
Obtain a steady-state using the following voltage protocols:
ICa: Record ICa at a test potential level of 10 mV, 150 ms duration, repeated at 10 s intervals.ICamay decrease monotonically but the decrease should not exceed 10% within the first 3 min of recording.
INa:Record INa at a test potential of −10 mV, 25 ms duration, repeated at 10 s intervals.
Currents will increase slowly as Na channels recover from the slow inactivated state.
Ito: Record Ito current at a test potential of 0 mV, 300 ms duration, repeated at 10 s intervals.
-
9
Evaluate voltage-dependent gating using the procedures described in Support Protocols 2, 3, and 4.
-
10
Analyze the currents using Clampfit. The currents under consideration are transient in nature so they can be described by a rate of activation, a rate of inactivation, and peak current. The rates can be parameterized by time constants of exponential functions fitted to the rising and inactivating phases of the currents. The Clampfit help menu describes the different fitting algorithms available. Positive and negative peak currents are measured by defining an area with cursors that includes the peak, and indicating the expected polarity (positive or negative).
SUPPORT PROTOCOL 2
VOLTAGE PROTOCOL TO RECORD CALCIUM CURRENTS
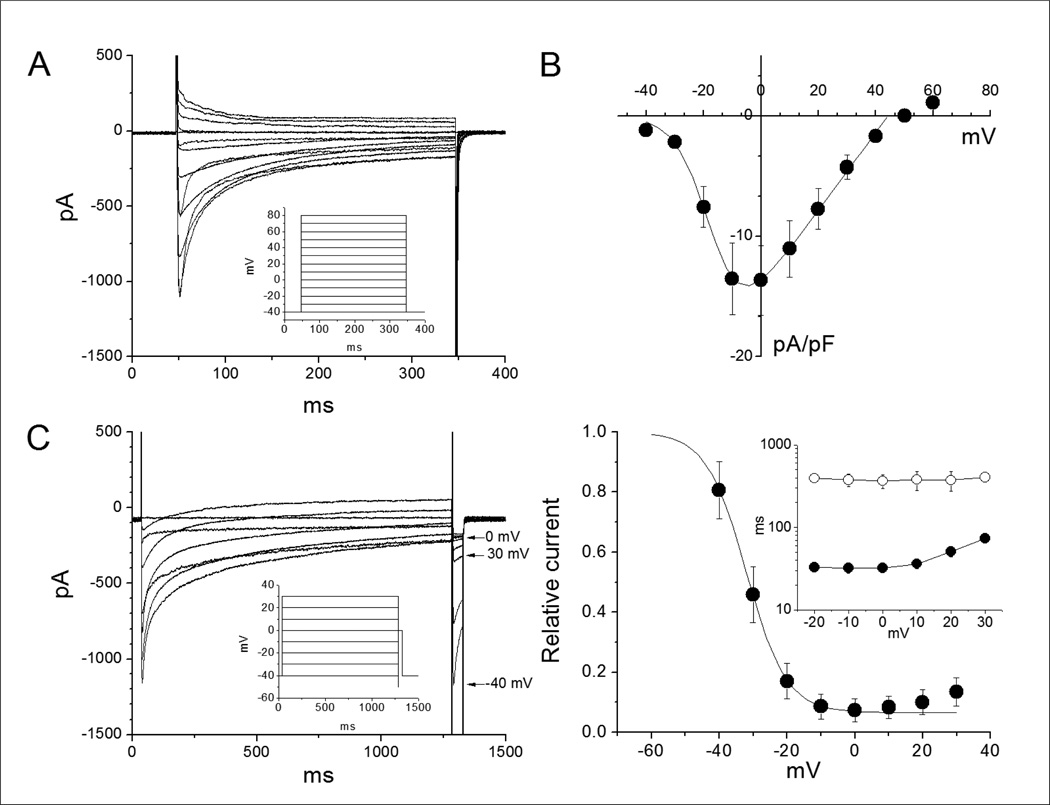
ICa current-voltage protocol (ICa I-V protocol): holding membrane potential: −40 mV. Pulse 1 duration: 150 ms. Pulse 1 level: 10 mV. Increase Pulse 1 level in 10 mV steps up to + 60 mV. Currents should be elicited at 0.1 Hz. See inset in Figure 3A.
Figure 3.
(A) Calcium currents elicited by 300 ms pulses as indicated in the inset. The pulse interval was 5 s. (B) Current-voltage relationship of peak currents normalized by cell capacitance. Data are mean ±sem of6 cells. (C) Calcium channels inactivation protocol (left) and inactivation curve (right). Inactivation was measured using a 1.25 s preconditioning pulse potentials ranging from –40 mV to 30 mV at 10 mV increments followed by a 1.25 ms conditioning pulse to –50 mV before applying a 42.5 ms test pulse to 0 mV. The inset shows the voltage-dependence of time constants from two exponential fits. The data is the average from 5 cells. The long time constants (empty symbols) accounts for the voltage-dependent inactivation component. The short time constants (filled symbols) are due to Ca2+-dependent inactivation. Note the increase of the fast time constants at potentials positive to 0 mV due to a decrease in driven force for Ca2+.
ICa inactivation protocol (ICa I protocol): holding membrane potential: −40 mV. Pulse 1 duration: 1 s. Pulse 1 level: −40 mV. Increase Pulse 1 level in 10 mV steps up to +30 mV. Pulse 2 duration: 5 ms. Pulse 2 level: −40 mV. Pulse 3 duration: 40 ms. Pulse 3 level: + 10 mV. Currents should be elicited at 0.1 Hz. See inset in Figure 3C.
ICa monitor protocol (ICa M protocol): holding membrane potential: −40 mV. Pulse 1 duration: 150 ms. Pulse 1 level: +10 mV. Currents should be elicited at 0.1 Hz.
SUPPORT PROTOCOL 3
VOLTAGE PROTOCOL TO RECORD SODIUM CURRENTS
INa current-voltage protocol (INa IV protocol): holding membrane potential: −80 mV. Pulse 1 duration: 200 ms. Pulse 1 level: −120 mV. Pulse 2 duration: 40 ms. Pulse 2 level: −80 mV. Increase Pulse 2level in 10 mV steps up to + 60 mV. Currents should be elicited at 0.1 Hz. See inset in Figure 4A.
Figure 4.
(A) Sodium currents activated by 45 ms step pulses from −80 in 10 mV increments that were preceded by 50 ms pre-pulses to −120 mV to recover from fast inactivation (see inset in right panel). Currents were recorded before (left panel) and after application of 3 mM lidocaine. (B) Normalized current-voltage relationship of 14 SC-hCMs. (C) Inactivation curve obtained using the protocol shown in the inset consisting of 1 s pulses between −140 and +40 mV in 20 mV increments followed by a 2 ms prepulse to −140 mV and a test pulse to −40 mV. The data are mean ± sem of five independent experiments.
INa inactivation protocol (INa I protocol): holding membrane potential: −100 mV. Pulse 1 duration: 1 s. Pulse 1 level: - 140 mV. Increase Pulse 1 level in 10 mV steps up to + 60 mV. Pulse 2 duration: 2 ms. Pulse 2 level: −140 mV. Currents should be elicited at 0.1 Hz. See inset in Figure 4C.
INa monitor protocol (INa M protocol): holding membrane potential: −80 mV. Pulse 1 duration: 200 ms. Pulse 1 level: −120 mV. Pulse 2 duration: 40 ms. Pulse 2 level: −10 mV.
SUPPORT PROTOCOL 4
VOLTAGE PROTOCOLS TO RECORD TRANSIENT OUTWARD CURRENTS
Ito current-voltage protocol (Ito IV protocol): holding membrane potential: −80 mV. Pulse 1 duration: 100 ms. Pulse 1 level: −30 mV. Pulse 2 duration: 500 ms. Pulse 2 level: −30 mV. Increase Pulse 2 level in 10 mV steps up to + 70 mV. Currents should be elicited at 0.1 Hz. See inset in Figure 5A.
Figure 5.
(A)Ito activated from a holding potential of −80 mV after a 100 ms prepulse to −30 mV to inactivate sodium currents (left panel) and the effect of 100µM flecainide (right panel). Calcium currents were blocked with 500 µM Cd2+. (B) Averaged current voltage relationship from eleven cells normalized for cell capacitance. (C) Relative inactivation from seven cells. The inset shows the protocol that consist of 500 ms pulses from −80 mV to +20 mV in 10 mV increments followed by a 600 ms test pulse to 50 mV. Symbols in B and C are mean ±sem.
Ito inactivation protocol (Ito I protocol): holding membrane potential: −80 mV. Pulse 1 duration: 500 ms. Pulse 1 level: −80 mV. Increase Pulse 1 level in 10 mV steps up to +20 mV. Pulse 2 duration: 600 ms. Pulse 2 level: 50 mV. Currents should be elicited at 0.1 Hz. See inset in Figure 5B.
Ito monitor protocol (Ito M protocol): holding membrane potential: −80 mV. Pulse 1 duration: 100 ms. Pulse 1 voltage: −30 mV. Pulse 2 duration: 600 ms. Pulse 2 voltage: 50 mV. Currents should be elicited at 0.1 Hz.
BASIC PROTOCOL 3
MEASUREMENT OF CONTRACTILE ACTIVITY OF iCELL CARDIOMYOCYTE MONOLAYERS IN THE xCELLigence RTCA CARDIO SYSTEM
The xCELLigence RTCA Cardio System (RTCA Cardio system, ACEA Biosciences, Inc.) is a label-free platform that measures impedance across asyncytiumof SC-hCMs. The system provides an indirect measure of cardiomyocyte viability and contractility in a 96-well plate format (e-plate).
The protocols and procedures described below are adapted from, and largely similar to, protocols provided by the cell supplier (Cellular Dynamics International, Inc.). We have optimized protocols for use with iCELL Cardiomyocytes, however our internal data and reports from other laboratories indicate that cells from other suppliers will provide similar results. SC-hCMs can be cultured and maintained on e-plates for extended durations, thus enabling measurement of acute and sub-acute drug-induced effects.
Materials
Maintenance Media (Cellular Dynamics International)
xCELLigence RTCA Cardio System (ACEA Biosciences)
iCell Cardiomyocytes Kit (Cellular Dynamics International)
E-Plate Cardio 96 (E-Plate) (ACEA Biosciences)
12-channel or 8-channel Multichannel Pipettor (20 and 200 µl)
Protocol steps
The impedance waveform stabilizes approximately 48 – 72 hours after transfer of iCell Cardiomyocytes to the E-Plate (Support Protocol 5). The optimal time window to perform an assay is between approximately 48 and 88 hours (2 and 4 days) post-plating.
-
1
Record Cell Index (CI) before addition of cells to the E-plate to measure the background electrode impedance. CI is the raw data output of the instrument and is a measure of overall impedance in each well. This background measurement is made with media in each well, but no cells. The manufacturer instructions detail how to set up the software to perform recordings. The background impedance will be very low and set to zero.
-
2
Add cardiomyocytes as described in Support Protocol 5. Measure the CI every 4 hours using 60s recordings.
The CI will increase to a range of 4 – 10 units over a time window of ~24–48 hours. Contractile activity (transient impedance changes) will appear within ~24 hours.
-
3
On the day of pharmacological experiments replace the Maintenance Medium 2 – 4 hours before recording. Tilt the E- Plate, remove the Maintenance Medium using a multichannel pipettor, and gently add 90 µl of Maintenance Medium to the side of each well to avoid disturbing the cardiomyocyte monolayer.
Evaporation rates can vary across the E-Plate. Changing the Maintenance Medium before compound treatment is required to ensure uniform medium volumes across the E-Plate.
-
4
Increase the sampling frequency to a 10 minute interval. Monitor the activity of the cardiomyocytes on the E-Plate to ensure regular beating rate and stable whole-peak amplitude values are reached.
-
5
Prepare test compounds in Maintenance Medium at 2X the final concentration in a regular 96-well cell culture plate.
Concentrations are selected based on known activity levels in other in vitro or in vivo settings. Not that the maintenance medium contains serum, and for highly protein-bound compounds this should be accounted for. Four concentrations applied to 4 wells each (n = 4) generally will be sufficient to define the concentration-response relation.
-
6
Place the formulated test compounds in a cell culture incubator at 37°C, 5% CO2 for 30 minutes to allow the compound solutions to equilibrate to the culture conditions.
-
7
Quickly transfer 90ul/well of the 2X solutions from the 96-well plate to the E-Plate.
Beating rate and amplitude are highly temperature dependent. The E-Plate should not be kept outside the incubator for more than 5 minutes while compounds are added. The RTCA Cardio Temperature Tool can also be used to minimize temperature changes while compounds are added.
-
8
Resume recording. Recording for compounds with expected acute effects (minutes to hours) should be made every 10 minutes. For compounds with longer-term effects, the interval can be increased to minimize file size (e.g., record every 4 hours).
-
9
Analyze Data. Refer to the RTCA Cardio Instrument Software Guide for specific instructions on using the RTCA software for data acquisition and analysis. The two basic measurements are the overall CI and the transient twitch activity (characterized by the beating rate, amplitude, and beating rhythm irregularity). Various classes of drugs induce signature changes in the measured parameters. For example, adrenergic agonists immediately increase in beat rate, whereas L-type calcium channel agonists immediately decrease the beat rate. Long-term cardiotoxic effects such as those induced by doxorubicin are observed as a decrease in the overall CI over the course of hours.
RTCA Cardio Settings for Analysis
| Method | PPB (Positive Peak Period Based) if analyzing beating rate or beating rhythm irregularity |
| WP (Whole Peak) | if analyzing amplitude |
| Threshold | 9 |
| Peak Adjustment | Square (0.02), Triangle (0.02), Diamond (0.02) |
| Noise Filtering | 0.005 |
SUPPORT PROTOCOL 5
PREPARATION OF iCELL CARDIOMYOCYTE MONOLAYERS FOR IMPEDANCE RECORDINGSUSING THE XCELLIGENCE RTCA CARDIO SYSTEM
Materials
Dulbecco’s Phosphate Buffered Saline without Ca2+ and Mg2+ (D-PBS) (Invitrogen)
Fibronectin (ACEABiosciences)
Trypsin 0.5%-EDTA (5X), no phenol red (Invitrogen), aliquoted for single use
12-channel or 8-channel Multichannel Pipettor (20 and 200 µl)
6-well Cell Culture Plates
Biological Safety Cabinet with UV Lamp
Sterile 15 and 50 ml Conical tubes
Sterile Reagent Reservoirs
iCell Cardiomyocytes Kit (Cellular Dynamics International (CDI))
E-Plate Cardio 96 (E-Plate) (ACEA Biosciences)
Protocol steps
The following steps describe the thawing, plating and maintenance of iCELL cardiomyocytes on 6 well plates, and the transfer and maintenance on 96 well e-plates. Note that the procedures described below are adapted from procedures provided by the cell supplier (Cellular Dynamics International, Inc.).
The following important points should be kept in mind:
iCell Cardiomyocytes are sensitive to over-digestion by trypsin and to excessive mechanical trituration.
When pipetting, do not allow the pipettor tips to touch the bottom of the wells during medium removal or addition. Pipette tips can damage cardiomyocytes and the gold electrodes on the bottom of the E-plate.
Thawing, Plating and Maintaining iCell Cardiomyocytes on 6 well plates
-
1
Dilute 1 mg/ml fibronectin solution in sterile D-PBS to a final concentration of 10 µg/ml immediately before use.
Reconstitute fibronectin in sterile water at 1 mg/ml according to the manufacturer’s instructions. Aliquot and store at −20°C.
-
2
Prepare two 6 well plates by adding 1 ml of the 10 µg/ml fibronectin solution to each well. Place at 4°C overnight.
-
3
Place the pre-coated 6-well cell culture plates in a biological safety cabinet to equilibrate to room temperature for at least 30 minutes.
-
4
Thaw 3 units of iCell Cardiomyocytes according to the iCell Cardiomyocytes User’s Guide.
-
5
Aspirate the fibronectin solution. Immediately add the cardiomyocyte suspension at a density of 750,000 plated cells per well. The plating volume should be 2 – 5 ml per well. Refer to the iCell Cardiomyocytes User’s Guide for instructions to calculate the Target Plating Density based on Plating Efficiency.
Each well of the 6-well cell culture plate must contain 750,000 plated cardiomyocytes. Any remaining cardiomyocytes can be distributed evenly between the wells to a maximum density of 1,000,000 plated cardiomyocytes/well. Cardiomyocytes remaining after this step should be discarded or plated for other purposes in accordance with the specific end-use protocol included in the iCell Cardiomyocytes Kit.
-
6
Incubate in a cell culture incubator at 37°C, 5% CO2.
-
7
Two days (48 hours) after plating, gently wash off the non-adherent cells and debris by pipetting the media up and down approximately 5 times using a serological pipette, or single- or multi-channel pipette. Remove 2 ml (of 3 ml) of the media, and replace with 2 ml pre-warmed Maintenance Medium.
-
8
Repeat step (7) two times.
-
9
Exchange media on days 4 and 6 post-thaw by removing 2 ml media (of 3 ml) and replacing with 2 ml pre-warmed Maintainence Medium.
Preparing the E-Plate for experiments
The E-Plate must be prepared the day before collecting iCell Cardiomyocytes from the 6-well cell culture plate(s) (day 6 post-thaw).
-
10
Dilute 1 mg/ml fibronectin in sterile D-PBS to a final concentration of 10 µg/ml immediately before use.
-
11
Add 50 µl of the 10 µg/ml fibronectin solution to each well of the E-Plate to evenly coat the bottom of the well. Place at 4°C overnight.
Detaching iCell Cardiomyocytes from the 6-well Cell Culture Plates
Dissociate a single 6-well cell culture plate at a time to avoid over-digesting the cardiomyocytes.
-
12
Warm 30 ml of Maintenance Medium and 18 ml of D-PBS per 6-well plate in a 37°C water bath for 30 minutes.
-
13
Dilute 0.5% trypsin solution in D-PBS to a final concentration of 0.1%. Warm the 0.1% trypsin solution in a 37°C water bath 10 minutes before use.
Thaw 0.5% trypsin aliquot(s) at 4°C overnight. Always use freshly prepared 0.1% trypsin and do not warm trypsin in a 37°C water bath for prolonged periods of time.
-
14
Aspirate the Maintenance Medium from each well containing iCell Cardiomyocytes.
-
15
Rinse cardiomyocytes twice with 2 ml/well of 37°C D-PBS.
-
16
Add 1 ml of 37°C 0.1% trypsin solution to each well. Incubate in a cell culture incubator at 37°C, 5% CO2 for 2 minutes. iCell Cardiomyocytes may not appear to be lifting from the bottom of the 6-well cell culture plate at the end of the 2-minute trypsin incubation. Proceed with step 17 to wash the cardiomyocytes from the plate to avoid over-digestion with trypsin.
-
17
Quickly wash the cardiomyocytes from the plate surface using a 1 ml pipettor by tilting the plate and repeatedly aspirating and dispensing the trypsin solution over the plate surface 2 – 3 times.
-
18
Rotate the plate 180° and repeat the previous step.
-
19
Add 3 ml of 37°C Maintenance Medium to each well to quench the trypsin.
-
20
Tilt the plate and triturate the cardiomyocytes 3 – 4 times using a 5 ml serological pipette. Avoid excessive trituration and introduction of air bubbles.
-
21
Transfer the cardiomyocyte suspension to a 50 ml tube. Rinse through each well with an additional 1 ml of 37°C Maintenance Medium, and transfer to the 50 ml tube.
Use a 1 ml pipettor to collect any remaining cardiomyocyte suspension from the wells after the cell transfer using the 5 ml serological pipette.
-
22
Centrifuge the cardiomyocyte suspension at 200 × g for 4 minutes.
-
23
Aspirate the supernatant, being careful not to disturb the cardiomyocyte pellet.
-
24
Add 4 ml of 37°C Maintenance Medium and mix gently to resuspend the cardiomyocyte pellet.
The resuspension volume depends on how many viable cells were harvested from the 6-well plate; ensure that the concentration at this step will be ≥800,000 viable cardiomyocytes/ml.
-
25
Remove an aliquot to count and determine the fraction of viable cardiomyocytes (do not use more than 500 µl for counting). [*Gwen: add cross-ref for cell counting protocol]
Ensure the cardiomyocytes are evenly suspended before removing an aliquot to count.
-
26
Dilute the cardiomyocyte suspension in Maintenance Medium to a final concentration of800,000 viable cardiomyocytes/ml.
Plating efficiency values are not used when transferring pre-plated cardiomyocytes to the E-Plate.
Transferring iCell Cardiomyocytes onto the E-Plate
-
27
Aspirate the fibronectin solution. Immediately add 50 µl/well of 37°C Maintenance Medium. Incubate in a cell culture incubator at 37°C, 5% CO2 for 5 minutes.
-
28
Record a background measurement according to the RTCA Cardio Instrument Operator’s Guide.
-
29
Gently pipette up and down the cardiomyocyte suspension 5 times using a serological pipette. Transfer to a reagent reservoir.
-
30
Mix the cardiomyocyte suspension by moving the reagent reservoir right to left 5 times. Pipette the cardiomyocyte suspension up and down 3 – 5 times before transferring to the E-Plate.
-
31
Add 50 µl/well of the cardiomyocyte suspension to each row of the E-Plate using a multichannel pipettor. Each well, already containing 50 µl of Maintenance Medium, will have a final volume of 100 µl and 40,000 viable cells.
Thoroughly mix the cardiomyocyte suspension between each addition to a row of the E-Plate as in steps 28 and 29 to ensure uniform seeding.
-
32
Place the E-Plate in the biological safety cabinet at room temperature for 30 minutes to allow the cardiomyocytes to settle and ensure an even distribution.
-
33
Incubate in a cell culture incubator at 37°C, 5% CO2.
Place the E-Plate in a low traffic incubator and away from the door to minimize fluctuations in temperature and air movement. Minimize opening the incubator’s door during the first 24 hours.
-
34
Replace the Maintenance Medium every 48 hours after transferring the cardiomyocytes to the E-Plate. Tilt the E-Plate, remove the Maintenance Medium using a multichannel pipettor, and gently add 100 µl of 37°C Maintenance Medium to the side of each well to avoid disturbing the cardiomyocyte monolayer. Perform additional medium replacements 2 – 4 hours before compound treatment.
COMMENTARY
Background Information
Investigation of cardiac myocyte electrophysiology for the purpose of understanding basic physiologic mechanisms of excitability and drug action has a long history extending back to the early development of techniques for intracellular membrane potential recording (reviewed by Cranefield and Hoffman, 1958). Moreover, action potential recording in isolated cardiac tissue from a variety of animal species is a well-established method of evaluating drugs for cardiac risk (Gintant 2001; Lu et al., 2001). The development of stem cell technology for producing SC-hCMs and their commercial availability provide expanding opportunities to assess the cardiac risk of drug candidates in a human in vitro test system, using both conventional patch-clamp methods (Braam et al., 2010; Peng et al., 2010; Ma et al., 2011) as well as novel techniques such as impedance measurement (Jonsson et al., 2011; Nguemo et al. 2012; Peters et al., 2012; Doherty et al., 2013).
Compared to traditional cardiac repolarization assays such as action potential recording in canine or rabbit Purkinje fibers, SC-hCM patch clamp assays have additional benefits beyond the species factor. As discussed by Peng et al., (2010) the benefits include: (a) higher pharmacological sensitivity to potassium channel blockers (i.e. fewer false negatives) while maintaining a high level of selectivity (i.e. few false positives), (b) reduced consumption of expensive test compounds by 10 to 15-fold, (c) avoidance of costs associated with animal use, and (d) faster throughput (i.e. patch clamp experiments lasting 30 minutes versus Purkinje fiber experiments of 2 – 4 hours).
The main disadvantages of the SC-hCM patch clamp assay are: (a) necessity of cell culture support facility, (b) low throughput compared to plate-based assays, (c) phenotypic heterogeneity in SC-hCMs (not 100% ventricular-like), and (d) incomplete differentiation into adult-like phenotype. The latter feature manifests itself as a relatively depolarized resting potential compared with adult ventricular cells. Consequently, sodium channels in SC-hCM are partially inactivated at normal resting potential and the action potential maximum rate of rise is reduced compared to adult cells.
Impedance-based measurements in SC-hCM monolayers offer the possibility of extending the evaluation of drug action on parameters such as contractility and cytotoxicity, which is not possible in patch clamp recordings from single isolated cells. This technique also offers the advantage of long-term application to test compounds to reveal potential effects of sub-acute or chronic exposure. Drawbacks to this technique include: (a) high cost of SC-hCM cells relative to native myocytes prepared from dissociated rodent hearts, (b) limited choice of instruments capable of resolving contractile beating, (c) necessity to include serum protein, that can reduce the amount of free test compound, in the culture medium, and (c) low throughput compared with other screening techniques.
Critical Parameters
Current-clamp studies
Current-clamp studies require a complete amphotericin B membrane perforation. If necessary follow the perforation process using the Membrane Test Protocol in Voltage Camp. A complete perforation will yield access resistance values of ~ 10 MΩ. The cardiac action potential upstrokein SC-hCMis slow enough that the maximal rate of depolarization will not be significantly affected by the filter time constant determined by Access Resistance (MΩ)×Cell capacitance (pF). For a typical cell with membrane capacitance 45 pF the time constant determined by a 10 MΩ access resistance is 0.45 ms.This time constant determines a rise time between 10–90% of the signal of 2.2 × 0.45 ms ~ 1 ms. Thus, typical action potentials with maximum rates of depolarization of 10 – 20 mV/ms will not be affected substantially. However, a decrease in the access resistance can be achieved using series resistance compensation. Remove the compensation when shifting to the voltage clamp mode; otherwise unwanted oscillations may damage the cell.
Voltage-clamp studies
Accurate ionic current recording requires minimal voltage error arising from series resistance, where Voltage Error (mV) =Series Resistance (MΩ) × Current (nA). Thus, there will be a 5 mV error when 1 nA current goes through a 5 MΩ access resistance. Voltage errors become obvious when plotting the current-voltage relationship. For instance, inward current through sodium channels increase monotonically with voltage in the range −60 to −20 mV as shown in Figure 4. However, when significant access resistance is present, inward current in particular will introduce a voltage clamp error that manifests as a disproportionate current jump and negative shift in the current-voltage relationship. Series resistance compensation and low pipette resistance (≤2 MΩ) are keys to measuring the INacurrent-voltage relationship in view of the large current densities observed in SC-hCMs. By contrast, ICais a relatively low amplitude inward current (compare Figure 3 and 4) that may be recorded without series resistance compensation. To record INaaccurately use pipettes less than.
Impedance studies
The impedance recordings should meet the following criteria: Overall impedance (Cell Index) > 4 Units (typical range 4 – 8), ΔCI amplitude >0.04 units (typical range 0.04 to 0.1), beat rate >30 bpm (typical range 30 – 50 bpm).
Troubleshooting
Current clamp experiments
Action potentials recorded from SC-hCMs have many shapes. The cells are a mixed population of ventricular-like, atrial-like and nodal-like cells. While recorded action potentials can often be used to classify individual cells into these groups, the action potentials can also be of intermediate morphology and not easily classified. Allowing the SC-hCMs to remain in culture for long durations (~ 3 - 4 weeks) can help with the success rate, particularly if targeting ventricular-like cells for safety pharmacology studies. Ventricular-like cells tend to be larger, of amorphous shape, and visibly contracting when examined under a microscope. Use of the perforated patch technique requires patience, as it may take ~20 minutes to perforate the cell membrane. Amphotericin B-containing micropipette solution will lose efficacy over time, and therefore should be stored on ice and protected from light during use, and should be used within 4 hours of addition of the Amphotericin B to the micropipette solution.
Voltage clamp experiments
Unusual current kinetics
As shown in the figures, voltage-dependent currents are transient in nature. A departure of this pattern may be due to several reasons including: a) coupling of the patched cell to another cell, b) large voltage error, c) incomplete isolation of the current under study, and d) low membrane resistance.
The membrane test protocol in Clampex uses the transient current elicited by a step pulse to measure the cell membrane capacitance, access resistance, membrane resistance, time constant of the transient relaxation and holding current. This is accomplished by fitting a single exponential to the current relaxation. Transient components in single cells are approximated by a single exponential function. Thus, the exponential fit will superpose the current exactly. When cells are coupled, current relaxation is a multi-exponential process. Solution: patch another cell.
Voltage-dependent currents should increase monotonically with voltage and currents should have comparable kinetics. However, large access resistances and/or large currents introduce an additional voltage that distorts the time course and magnitude of the currents. Solution: reduce the current density by reducing the concentration of the current carrier (e.g., reduce sodium when measuring sodium currents), use larger (lower resistance) pipettes, and apply series resistance compensation as described in
Incomplete current inhibition of outward currents would decrease the peak and exaggerate inactivation of calcium currents. Similarly, incomplete block of calcium currents would result in a persistent inward current that may be mistaken for a late sodium component. Solution: specific block of the current under inactivation will reveal the presence on contaminating currents. Use 10 µM tetrodotoxin to block INa, 10 µM nifedipine to block ICa, 10 µM flecainide to block Ito.
Leak currents due to low membrane resistance may add significant uncertainty to the recordings when they show some time dependent characteristics. Leak currents are indicative of cell viability. Solution: Pay attention to culture conditions and solution quality.
Current rundown
Decrease current amplitude over time (current rundown) is a serious problem in particular with calcium currents. It is due to disruption on the intracellular milieu and the metabolic support of the channel activity. Solution: solutions with regenerative ATP systems as the one shown in Basic Protocol 2 can help. Use pipettes with relatively high resistances (~ 4 MΩ). The perforated patch clamp configuration will help reduce rundown.
Giga-ohm seals are difficult to obtain
Immediately after releasing the positive pressure seal formation starts. You can help seal formation by applying negative gentle suction. Holding the membrane potential to negative values (between −30 and −70 mV) also may help seal formation.
Impedance experiments
Obtaining robust transient signals across plates is critical. Since the cells are transferred from 6 well “pre-plates” to 96 well E-plates, there is necessarily an enzymatic digestion step. Trypsin must be fresh and properly stored or variable results will be obtained. Alternatives such as TrypLE may also be used. Compared to commonly used cell lines (human embryonic kidney, Chinese hamster ovary, etc.) cardiomyocytes are very sensitive to stresses induced by handling. Pipetting should be gentle and contact with air should be avoided. Finally, methods that use direct plating are under development and may offer advantages including less handling after thawing the cardiomyocytes.
Anticipated Results
Action potentials
Figure 2 shows 10s recordings of action potentials recorded before (control) and after application of 3 µM quinidine. For display, representative action potentials from before and after application are superimposed by alignment along the x-axis at the point of maximal upstroke velocity. Note the large increase in action potential duration due to block of IKr.
L-type calcium current
Fig. 3A shows calcium currents elicited using the protocol shown in the inset (support protocol 2). Note that inward calcium currents inactivate over the pulse following a two exponential time course reflecting both calcium and voltage dependent inactivation. Fig. 3B shows the current-voltage relationship from 6 cells normalized by cell capacitance. Note that the currents show a maximum between −10 and 0 mV and then decrease at more positive potentials due to a decrease in the driving force for Ca2+. The current-voltage relationship was parameterized using a Boltzmann function with voltage for half activation (Vact) and slope factor (kact) of −17 mV and 5.8 mV, respectively. The calculatedmaximal conductance(Gmax) was 0.32 nS/pF. Fig 3C shows currents elicited by the voltage protocol (inset) designed to assess channel inactivation. Data are presented as mean ± sem (3 cells) Note that the more positive the pre-pulse potential the smaller the current elicited by the test pulse. The relative current reaches a minimum level at 0 mV prepulse. The test current elicited after a prepulse to 30 mV was larger than that elicited by a prepulse to 0 mV, reflecting a decrease in calcium dependent inactivation with strong membrane depolarization. To parameterize the voltage-dependence of inactivation we fitted the peak test currents between −40 and 0 mV to a single Boltzmann function plus a constant component. The maximum current was a free parameter used to normalize the test currents. Calcium currents inactivated with a half potential value (Vinact) of −32 mV and a slope factor (kinact) of 5.9 mV. However inactivation was not complete reaching a minimum between −20 and 0 mV where Ca2+ entry and Ca2+-dependent inactivation are maximal. As expected, inactivation decreases with the decrease in the driving force for Ca2+ entry. This is evidenced in 1) the increased availability (current) at more positive potentials (Panel 3C, right), and 2) the prolongation of the time constant of fast inactivation (Panel 3C, right inset).
Meaningful comparison of L-type calcium currents expressed in SC-hCMs with published data from adult cardiomyocytes (including Peltzman et al, 1998 and Magyar et al., 2000) is difficult to establish given the different ionic conditions, temperature voltage protocols and disease conditions. However, ICa in SC-hCMs shows qualitative similarities with ICa in adult cardiomyocytes in terms of voltage dependence, the presence of bothCa2+ and voltage-dependent inactivation, as well as sensitivity to nifedipine (not shown).
Sodium current
Fig. 4A shows an example of sodium currents activated using the protocol depicted in the inset (support protocol 3) and the complete block by 3000 µM lidocaine suggesting that the inward INa was well isolated. Fig.4B shows the averaged current-voltage relationship normalized by cell membrane capacitance. Inward sodium currents were activated at potentials positive to −60 mV, were maximal at −20 mV and decreased at more positive potentials due to the decrease in the driving force for sodium. The current-voltage relationship was parameterized using a Boltzmann function times a linear component. The free parameters included in the Boltzmann function were Vact(−37.2 ± 1.6 mV) and kact (5.8 ± 0.2 mV). The parameters determining the linear component according to the equation I = Gmax × (V – Er) were the reversal potential (Er = 34.1 ± 3.3 mV) and Gmax (2.48 ± 0.35 nS/pF). The potential for half activation agrees with published data from isolated human ventricular and atrial cardiomyocytes (Sakakibara et al., 1993, Furukawa et al., 1995, Furukawa et al., 1995, Sakakibara et al., 1992).
Fig. 4C shows the inactivation curve determined using the protocol shown in the inset (support protocol 3). Under this paradigm current inactivation was approximated by a Boltzmann function with a half inactivation parameter Vinact of −104.4 mV and a slope factor kinact of 7.5 mV. However inactivation was not complete as determined by a 5.6% plateau. The potential for half inactivation agrees with published data from isolated human ventricular (Sakakibara et al., 1993) and atrial (Sakakibara et al., 1992) cardiomyocytes. Note that the published data was obtained at 17 ◦C and low Na+ concentrations. Because warmer temperatures shift activation and inactivation towards more positive potentials (about 6 mV, Irvine et al., 1999) differences in Vinact between SC-hCM and isolated human cardiomyocytes may be larger.
Transient outward current
Fig. 5A shows an example of Ito elicited with the current I-V protocol (support protocol 4). The identity of the transient current was further confirmed by the sensitivity to 100 µM flecainide (Fig. 5A, right panel). Flecainide blocked Ito with an IC50 of 17 µM, a value similar to the one reported for the block of transient outward currents in rabbit atrial myocytes (Yamashita et al., 1995) and comparable to the values observed in human atrial myocytes (Wang et al., 1995). Also these values agree with those obtained in Kv 4.3 channels (Radicke et al., 2008).
The Ito current voltage-relationship normalized by cell capacitance is shown in Fig. 5B. Currents activated at membrane potentials positive to −20 mV and increases due to an increase in open probability and driving force. To calculate the activation parameters we assumed a linear open channel conductance and a reversal potential of −93 mV (theoretical K+ reversal potential). The fit of the individual current-voltage relationships to a Boltzmann function times a linear component gave a maximal slope conductance of 0.09 nS/pF with a Vact of 23 ± 2 mV and kact of 12 ± 1 mV (n = 11), values comparable to those observed in isolated human cardiomyocytes (Näbauer et al., 1996).
Fig. 5C shows the voltage dependence of Ito inactivation induced by 500 ms prepulses from −80 to +20 mV (support protocol 4). Current inactivation was approximated by a single Boltzmann function with Vinact of −15.5 mV and kinact of 8.9 mV. Whereas the nature of the sustained component remains to be investigated we anticipate that may be of the same nature as the sustained current described in human atrial myocytes (Wang et al., 1993).
xCELLigence RTCA Cardio
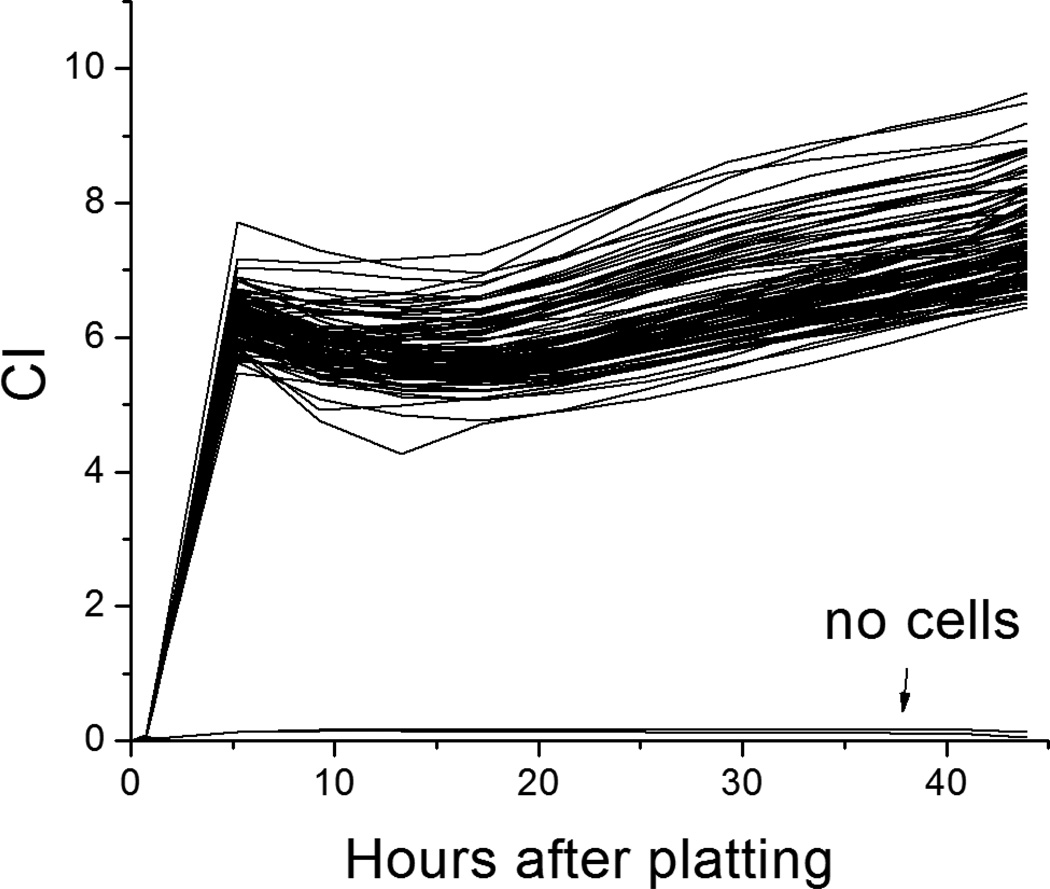
Figure 6 shows representative recordings of the overall Cell Index (CI) from a 96-well plate from time zero (pre-plating) to 48 hours after plating. The two lines at the bottom are data from wells to which no cells were added. Figure 7 shows the response to blebbistatin, a compound that disrupts actin-myosin interactions. While no effect is observed on the action potential recorded from a single SC-hCM, the transient impedance twitch is largely eliminated, indicating that the majority of the transient change in impedance (twitch) is due to contraction of the SC-hCMs.
Figure 6.
Overall CI recorded from a 96 well e-plate under no cell (media only) conditions (time zero), then post-plating up to 48 hours. The CI increases as cells attach and spread over the electrodes. The two lines with little increase in CI were recorded from wells to which no cells were added.
Figure 7.
Recordings were made in manual patch clamp (intracellular action potentials, left panel) or on the xCELLigence RTCA Cardio instrument (right panel), before and after application of 1 µM blebbistatin, which disrupts actin-myosin interaction. Note the large decrease in the impedance transient (contraction, right panel) in the absence of a change in the electrical signal (action potential, left panel).
Time Considerations
For all protocols described, SC-hCMs require time to recover after thawing from the frozen state. Performing experiments before the cells are allowed to recover will lead to a high degree of variability, inefficiency, and poor outcomes. Typically, experiments need to be planned at least 2 weeks in advance of actual data collection. The time requirements are detailed in the individual protocols.
Acknowledgments
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number R44HL104948.
LITERATURE CITED
- Amos GJ, Wettwer E, Metzger F, Li Q, Himmel HM, Ravens U. Differences between outward currents of human atrial and subepicardial ventricular myocytes. J Physiol. 1996;491:31–50. doi: 10.1113/jphysiol.1996.sp021194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- Cranefield PF, Hoffmann BF. Electrophysiology of single cardiac cells. Physiol Rev. 1958;38:41–76. doi: 10.1152/physrev.1958.38.1.41. [DOI] [PubMed] [Google Scholar]
- Doherty KR, Wappel RL, Talbert DR, Trusk PB, Moran DM, Kramer JW, Brown AM, Shell SA, Bacus S. Multi-parameter in vitro toxicity testing of crizotinib, sunitinib, erlotinib, and nilotinib in human cardiomyocytes. Toxicol Appl Pharmacol. 2013;272:245–255. doi: 10.1016/j.taap.2013.04.027. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Koumi S, Sakakibara Y, Singer DH, Jia H, Arentzen CE, Backer CL, Wasserstrom JA. An analysis of lidocaine block of sodium current in isolated human atrial and ventricular myocytes. J Mol Cell Cardiol. 1995;27:831–846. doi: 10.1016/0022-2828(95)90090-x. [DOI] [PubMed] [Google Scholar]
- Jonsson MK, Wang QD, Becker B. Impedance-based detection of beating rhythm and proarrhythmic effects of compounds on stem cell-derived cardiomyocytes. Assay Drug Dev Technol. 2011;9:589–599. doi: 10.1089/adt.2011.0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine LA, Jafri MS, Winslow RL. Cardiac sodium channel Markov model with temperature dependence and recovery from inactivation. Biophys J. 1999;76:1868–1885. doi: 10.1016/s0006-3495(99)77346-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HR, Mariën R, Saels A, De Clerck F. Species plays an important role in drug-induced prolongation of action potential duration and early afterdepolarizations in isolated Purkinje fibers. J Cardiovasc Electrophysiol. 2001;12:93–102. doi: 10.1046/j.1540-8167.2001.00093.x. [DOI] [PubMed] [Google Scholar]
- Magyar J, Iost N, Körtvély A, Bányász T, Virág L, Szigligeti P, Varró A, Opincariu M, Szécsi J, Papp JG, Nánási PP. Effects of endothelin-1 on calcium and potassium currents in undiseased human ventricular myocytes. Pflugers Arch. 2000;441:144–149. doi: 10.1007/s004240000400. [DOI] [PubMed] [Google Scholar]
- Näbauer M, Beuckelmann DJ, Uberfuhr P, Steinbeck G. Regional differences in current density and rate-dependent properties of the transient outward current in subepicardial and subendocardial myocytes of human left ventricle. Circulation. 1996;93:168–177. doi: 10.1161/01.cir.93.1.168. [DOI] [PubMed] [Google Scholar]
- Nguemo F, Šarić T, Pfannkuche K, Watzele M, Reppel M, Hescheler J. In vitro model for assessing arrhythmogenic properties of drugs based on high-resolution impedance measurements. Cell Physiol Biochem. 2012;29:819–832. doi: 10.1159/000188069. [DOI] [PubMed] [Google Scholar]
- Pelzmann B, Schaffer P, Bernhart E, Lang P, Mächler H, Rigler B, Koidl B. L-type calcium current in human ventricular myocytes at a physiological temperature from children with tetralogy of Fallot. Cardiovasc Res. 1998;38:424–432. doi: 10.1016/s0008-6363(98)00002-9. [DOI] [PubMed] [Google Scholar]
- Peng S, Lacerda AE, Kirsch GE, Brown AM, Bruening-Wright A. The action potential and comparative pharmacology of stem cell-derived human cardiomyocytes. J Pharmacol Toxicol Methods. 2010;61:277–286. doi: 10.1016/j.vascn.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Peters MF, Scott CW, Ochalski R, Dragan YP. Evaluation of cellular impedance measures of cardiomyocyte cultures for drug screening applications. Assay Drug Dev Technol. 2012;10:525–532. doi: 10.1089/adt.2011.442. [DOI] [PubMed] [Google Scholar]
- Radicke S, Vaquero M, Caballero R, Gómez R, Núñez L, Tamargo J, Ravens U, Wettwer E, Delpón E. Effects of MiRP1 and DPP6 beta-subunits on the blockade induced by flecainide of Kv4.3/KChIP2 channels. Br J Pharmacol. 2008;154:774–786. doi: 10.1038/bjp.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara Y, Furukawa T, Singer DH, Jia H, Backer CL, Arentzen CE, Wasserstrom JA. Sodium current in isolated human ventricular myocytes. Am J Physiol. 1993;265:H1301–H1309. doi: 10.1152/ajpheart.1993.265.4.H1301. [DOI] [PubMed] [Google Scholar]
- Sakakibara Y, Wasserstrom JA, Furukawa T, Jia H, Arentzen CE, Hartz RS, Singer DH. Characterization of the sodium current in single human atrial myocytes. Circ Res. 1992;71:535–546. doi: 10.1161/01.res.71.3.535. [DOI] [PubMed] [Google Scholar]
- Schram G, Pourrier M, Melnyk P, Nattel S. Differential distribution of cardiac ion channel expression as a basis for regional specialization in electrical function. Circ Res. 2002;90:939–950. doi: 10.1161/01.res.0000018627.89528.6f. [DOI] [PubMed] [Google Scholar]
- Wang Z, Fermini B, Nattel S. Sustained depolarization-induced outward current in human atrial myocytes. Evidence for a novel delayed rectifier K+ current similar to Kv1.5 cloned channel currents. Circ Res. 1993;73:1061–1076. doi: 10.1161/01.res.73.6.1061. [DOI] [PubMed] [Google Scholar]
- Wang Z, Fermini B, Nattel S. Effects of flecainide, quinidine, and 4-aminopyridine on transient outward and ultrarapid delayed rectifier currents in human atrial myocytes. J Pharmacol Exp Ther. 1995;272:184–196. [PubMed] [Google Scholar]
- Yamashita T, Nakajima T, Hamada E, Hazama H, Omata M, Kurachi Y. Flecainide inhibits the transient outward current in atrial myocytes isolated from the rabbit heart. J Pharmacol Exp Ther. 1995;274:315–321. [PubMed] [Google Scholar]