Abstract
Despite advances in the treatment of schizophrenia spectrum disorders with atypical antipsychotics (AAPs), there is still need for compounds with improved efficacy/side-effect ratios. Evidence from challenge studies suggests that the assessment of gating functions in humans and rodents with naturally low-gating levels might be a useful model to screen for novel compounds with antipsychotic properties. To further evaluate and extend this translational approach, three AAPs were examined. Compounds without antipsychotic properties served as negative control treatments. In a placebo-controlled, within-subject design, healthy males received either single doses of aripiprazole and risperidone (n=28), amisulpride and lorazepam (n=30), or modafinil and valproate (n=30), and placebo. Prepulse inhibiton (PPI) and P50 suppression were assessed. Clinically associated symptoms were evaluated using the SCL-90-R. Aripiprazole, risperidone, and amisulpride increased P50 suppression in low P50 gaters. Lorazepam, modafinil, and valproate did not influence P50 suppression in low gaters. Furthermore, low P50 gaters scored significantly higher on the SCL-90-R than high P50 gaters. Aripiprazole increased PPI in low PPI gaters, whereas modafinil and lorazepam attenuated PPI in both groups. Risperidone, amisulpride, and valproate did not influence PPI. P50 suppression in low gaters appears to be an antipsychotic-sensitive neurophysiologic marker. This conclusion is supported by the association of low P50 suppression and higher clinically associated scores. Furthermore, PPI might be sensitive for atypical mechanisms of antipsychotic medication. The translational model investigating differential effects of AAPs on gating in healthy subjects with naturally low gating can be beneficial for phase II/III development plans by providing additional information for critical decision making.
INTRODUCTION
Antipsychotic medication remains the first-line treatment of psychiatric disorders such as schizophrenia and bipolar mania (Moritz et al, 2013). Despite the reported advantage of second-generation (atypical) antipsychotics (AAPs) over the first-generation (typical) antipsychotics in terms of higher efficacy and better tolerability (Barnes and McPhillips, 1999; Breier et al, 1994; Kane et al, 1988), only moderate symptom reduction (Leucht et al, 2009) and distressing side effects (eg, neurologic, metabolic, emotional) may decrease the quality of life for patients and increase the problem of noncompliance (Moritz et al, 2013; Schimmelmann et al, 2005). Therefore, unmet medical needs for the treatment of schizophrenia spectrum disorders exist and novel neuropsychopharmacologic treatment strategies with improved efficacy/side-effect ratios are needed (Breier, 2005; Leucht et al, 2009). However, drug development has become more and more challenging. Although the number of new CNS drug applications has decreased, development cost for new molecular entities has increased dramatically. Less than 10% of the compounds that entered phase I studies were introduced to the market (Breier, 2005; Hurko and Ryan, 2005).
In recent years, considerable efforts have been made to develop translational strategies to bridge the gap between preclinical and clinical studies for the efficient development of novel antipsychotics (Geyer et al, 2001; Swerdlow et al, 2006b). Models based on neurophysiologic markers that can be translated to normal human populations would offer the potential to facilitate antipsychotic development at an early stage in the clinical development cycle and save resources and time (Swerdlow et al, 2006b). Prepulse inhibition (PPI) of the acoustic startle reflex (ASR) and the suppression of the P50 auditory evoked event-related potential in a condition test paradigm (P50 suppression) have been identified as such translational neurophysiologic markers associated with schizophrenia, and may be predictive for the antipsychotic effects of pharmacological treatments. It has repeatedly been shown that patients suffering from schizophrenia spectrum disorders show a deficiency in these measures of early information processing; that is, more precisely, the ability to gate extraneous stimuli (Braff et al, 1992; Clementz et al, 1998; Csomor et al, 2009).
Preclinical studies showed that AAPs like clozapine, risperidone, olanzapine, aripiprazole, or quietapine improved PPI in naturally low-gating (C57BL/6J or DBA/2) mice or rats, whereas a number of psychoactive compounds without antipsychotic properties (eg, buspirone, desipramine, morphine, and scopolamine) did not improve PPI, except diazepam (Flood et al, 2007; Ouagazzal et al, 2001; Swerdlow et al, 2006b). Furthermore, clozapine and olanzapine but not the typical antipsychotic haloperidol improved N40 suppression—the rodent analog to P50 suppression—in DBA/2 mice (Simosky et al, 2003, 2008).
Similarly, healthy human subjects with a ‘low-gating trait' may be viewed as a potential surrogate marker for impaired gating as reported in different clinical populations (Csomor et al, 2008a; Swerdlow et al, 2006b; Vollenweider et al, 2006). Therefore, to help bridge the gap between preclinical and clinical studies, we and others demonstrated that the atypical antipsychotics clozapine (Vollenweider et al, 2006), sertindole (Holstein et al, 2011), and quetiapine (Swerdlow et al, 2006b), but not the typical antipsychotic haloperidol (Csomor et al, 2008a), have the capacity to increase PPI in healthy subjects with low sensorimotor gating levels. These findings strongly suggest that antipsychotics modulate sensorimotor gating over a large (notably physiological) range in normal subjects and not simply the disruption by pathophysiologcal states (Swerdlow et al, 2006b).
In the present study, the putative progating effects of three AAPs, aripiprazole, risperidone, and amisulpride, on PPI and P50 suppression were tested in healthy subjects exhibiting either low- or high-gating traits. Please see Miyamoto et al (2005) for a detailed review of the pharmacology and mechanisms of action of these AAPs. Moreover, the anxiolytic lorazepam, the psychostimulant modafinil, and the anticonvulsant valproate, all of which fail to exhibit antipsychotic properties in the narrow sense, served as negative control treatments. For further information on these compounds, please refer to Ballon and Feifel (2006), Loscher (2002), and Altamura et al (2013). Furthermore, to investigate the potential relationship between clinically associated psychological traits and symptoms and differences in gating, the participants completed the Hopkins Symptom Checklist (SCL-90-R) (Franke, 1995).
We hypothesized that the administration of a single dose of the antipsychotics, but not the negative control treatments will improve sensory and/or sensorimotor gating in healthy subjects exhibiting naturally low PPI and/or P50-suppression levels. This study shall help to further elucidate whether antipsychotic effects on gating functions in low-gating normal humans might be a useful model to predict clinical efficacy of novel compounds for the treatment of schizophrenia spectrum disorders.
MATERIALS AND METHODS
Participants
A total of 88 healthy male volunteers were recruited by advertisement. As it has been shown that gating is influenced by the menstrual cycle in women (Swerdlow et al, 1997), only male participants were included. The study was approved by the Cantonal Ethics Committee of Zurich and Swissmedic (Swiss Agency for Therapeutic Products). All subjects provided written informed consent. All participants were without a history of mental (according to DSM-IV (American Psychiatric Association, 1994), axis I and II) or neurological disorders, and were free of any medication for at least 3 weeks before the experiment. For further details see Supplementary Methods 1.
Experimental Design
Three independent experiments adopting a double-blind, placebo-controlled, within-subject design were conducted. All participants completed a placebo testing (maltose), whereas 28 subjects participated in the experimental procedure receiving aripiprazole (15 mg) and risperidone (2 mg) (cohort 1), 30 subjects underwent treatment with amisulpride (400 mg) and lorazepam (2 mg) (cohort 2), and 30 volunteers participated in the experiment with modafinil (200 mg) and valproate (500 mg) (cohort 3). Participants received the substances orally in a balanced and random sequence on three experimental days 10 to 20 days apart. Subjects participating in one of the experimental series were not allowed in any of the two other series. Aripiprazole (Abilify), risperidone (Risperdal), amisulpride (Solian), lorazepam (Temesta), modafinil (Modasomil), and valproate (Convulex) were obtained from the respective marketing authorization holders in Switzerland. Selection criteria for negative control compounds were: (1) absence of an antipsychotic effect, (2) no application in schizophrenia treatment, (3) psychoactive effect, and (4) no previous knowledge of the influence on gating to not introduce a bias. On each of the three experimental days, participants received active drug or placebo after a short assessment of electrocardiogram, oxygen saturation, and blood pressure. Shortly before the onset of peak drug effect (aripiprazole: 115 min; risperidone: 70 min; amisulpride: 45 min; lorazepam: 100 min; modafinil: 120 min; and valproate 120 min) the subject was prepared for the electrophysiological recordings that took 45 min. It should be noted that the study nurse was not blind to the medication as different time until onset of peak drug effect for the different medications had to be preserved. However, the subject, the experimenter, and the data analyst were blind to the treatment condition. The assessment of P50 suppression and PPI were conducted in two experiments that were separated by a 5-min break. P50 suppression was assessed first.
P50 Suppression and PPI Session Definition
The P50 suppression test session was composed of 80 pairs of auditory clicks with a 500 ms interclick interval presented every 10 s (first click stimulus: S1; second click stimulus: S2). Stimuli consisted of 85 dBA white noise with a duration of 1 ms. The P50 suppression session lasted for ∼15 min.
The PPI test session was composed of a mixture of 40 pulse-alone, prepulse-pulse trials, and trials in which no discrete stimulus other than the constant background noise was presented (‘NS trials'). For details see Supplementary Methods 2.
Apparatus, Data Recording, and Data Processing
Electromyographic (EMG) (PPI paradigm) and electroencephalographic (EEG) (P50 suppression paradigm) data were recorded and processed as described in detail previously (Csomor et al, 2008a; Csomor et al, 2008b). Analyzer (Brainvision, Germany) was used to preprocess the recorded data. For the P50 suppression paradigm, the P50 component of the AEP was identified and scored as described by Nagamoto et al (1989). For further details see Supplementary Methods 3. For the PPI paradigm, the EMG record of each trial was separately scored using emgBLINK version 1.3 (CST, Switzerland) and further processed as described in Supplementary Methods 4.
Assessed Parameters
For the P50 suppression paradigm, the following ERP measures were examined: P50 amplitudes: P50 amplitude evoked by S1 and S2; and P50 suppression: percentage P50 suppression (%P50sup) was calculated by the formula: (1−(amplitudes2)/(amplitudes1)) × 100%.
For the PPI paradigm the following startle measures were analyzed. Startle reactivity: the mean magnitude of the startle reaction elicited by pulse-alone stimuli. Prepulse Inhibition: percentage PPI (%PPI) was calculated for each SOA according to the formula: (1−(amplitudeprepulse-pulse)/(amplitudepulse-alone (block2)) ) × 100%. Habituation: percentage habituation was calculated as the reduction in startle magnitude between the second block and following block of PA trials to avoid sensitization effects: %Habituation=100 × (block 2−block 3)/block 2.
Statistical Analysis
As a reliable startle reaction elicited by pulse-alone stimuli is a prerequisite for a meaningful calculation of PPI, only data sets in which a reliable startle reaction was elicited (mean pulse-alone reactivity >10 μV in the second block of the PPI session) were included in the analysis of PPI. Similarly, only P50 data sets showing a distinct AEP and a clearly identifiable P50 component elicited by S1 were included in the statistical analysis of P50 suppression. The number of valid data sets for each condition can be extracted from Table 1 and Supplementary Methods 5.
Table 1. Demographic Characteristics of Subjects Stratified into Low and High P50 and PPI Subgroups.
|
Study cohort 1 |
Study cohort 2 |
Study cohort 3 |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Mean |
SE |
Mean |
SE |
F/χ2a |
P-value |
Mean |
SE |
Mean |
SE |
F/χ2a |
P-value |
Mean |
SE |
Mean |
SE |
F/χ2a |
P-value |
|
| Low P50 subgroup (n=14) | High P50 group (n=14) | Main effect of group | Low P50 subgroup (n=15) | High P50 subgroup (n=15) | Main effect of group | Low P50 subgroup (n=15) | High P50 subgroup (n=15) | Main effect of group | ||||||||||
| Age (years) | 22.64 | 0.82 | 24.36 | 0.94 | 1.88 | 0.18 | 25.87 | 1.80 | 25.33 | 1.10 | 0.06 | 0.80 | 25.73 | 1.26 | 25.07 | 1.30 | 0.14 | 0.72 |
| BMI | 22.43 | 0.45 | 23.11 | 0.53 | 0.94 | 0.34 | 23.10 | 0.80 | 22.43 | 0.48 | 0.51 | 0.48 | 23.07 | 0.44 | 22.77 | 0.60 | 0.16 | 0.69 |
| IQ (MWT-B) | 110.93 | 3.09 | 117.36 | 2.99 | 2.24 | 0.15 | 111.00 | 2.43 | 107.80 | 1.32 | 1.34 | 0.26 | 112.27 | 3.03 | 111.13 | 2.62 | 0.08 | 0.78 |
| Smokers | 4 | 5 | 0.16 | 0.50 | 3 | 5 | 0.68 | 0.34 | 6 | 4 | 0.60 | 0.35 | ||||||
| Low PPI subgroup (n=14) | High PPI subgroup (n=14) | Main effect of group | Low PPI subgroup (n=15) | High PPI subgroup (n=15) | Main effect of group | Low PPI subgroup (n=15) | High PPI subgroup (n=15) | Main effect of group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 23.35 | 0.82 | 23.85 | 1.06 | 0.14 | 0.72 | 24.00 | 1.58 | 26.67 | 1.37 | 1.62 | 0.21 | 23.87 | 0.88 | 27.07 | 1.57 | 3.28 | 0.08 |
| BMI | 23.17 | 0.45 | 22.18 | 1.91 | 2.06 | 0.16 | 22.55 | 0.77 | 22.76 | 0.57 | 0.05 | 0.83 | 22.85 | 0.50 | 23.12 | 0.57 | 0.13 | 0.72 |
| IQ (MWT-B) | 115.71 | 3.38 | 111.69 | 2.94 | 0.79 | 0.38 | 112.87 | 1.85 | 107.45 | 1.80 | 4.36 | 0.04 | 110.87 | 2.49 | 111.71 | 3.24 | 0.04 | 0.84 |
| Smokers | 4 | 5 | 0.16 | 0.50 | 3 | 5 | 0.68 | 0.34 | 6 | 4 | 0.60 | 0.35 |
Significant p-values are shown in bold.
The χ2 test for smoking data.
All statistical analyses were conducted using the statistical software packages R (http://www.r-project.org/) and Statistica 7 (Statasoft, Tulsa, OK). To test whether the different medications exhibited differential effects on subjects with low- or high-gating measures after placebo, subjects were grouped by a median-split procedure into low- and high-gating subgroups. The splitting procedure was conducted separately for each of the three study cohorts. For the PPI paradigm, this median split was based on the results of %PPI of the SOA60ms lead interval in the placebo condition (median PPI cohort 1=54.46% median PPI cohort 2=58.34% median PPI cohort 3=62.90%) as a majority of studies show that schizophrenia patients exhibit a deficiency in PPI under this stimulus condition (Csomor et al, 2009; Swerdlow et al, 2006a; Wang et al, 2013). Similarly, for the P50 suppression paradigm, the median split was applied using the scores at %P50sup in the placebo condition (median P50 cohort 1=51.85% median P50 cohort 2=56.18%, median P50 cohort 3=63.60%). Assessed parameters were compared between the active drug and placebo for the six different treatment conditions (aripiprazole, risperidone, amisulpride, lorazepam, modafinil, and valproate). For details on data distribution and homogeneity see Supplementary Methods 6.
To investigate the influence of treatments on electrophysiological indices in the low- and high-gating cohorts, linear mixed-effects models were fitted for each of the six treatment conditions and for each dependent variable (%P50sup, P50 amplitudes, %PPI, and PPI amplitudes). In all models, repeated measurements were accounted for by including random intercepts for the subjects. In cases of heteroscedastic within-group errors, the models were allowed to estimate separate variances for each group. Akaike's information criterion (AIC) values were used to decide on appropriate variance models of the within-group errors and correlation structures of random effects in model specifications. Significant main effects and interactions were followed by Bonferroni-corrected pairwise comparisons. If data were not normally distributed (PShapiro–Wilk W<0.05), nonparametric Wilcoxon matched-pairs tests were calculated in addition to contrast tests. For all statistical tests, the significance level was set to p<0.05 (two tailed).
For the analysis of P50 suppression data, the within-subject factor ‘drug' (placebo vs active drug) and the between-subject factor ‘subgroup' (low vs high gating) as well as their interaction were included in the models as fixed effects factors. The models predicting P50 amplitudes additionally included the within-subject factors ‘stimulus' (S1 and S2) and all possible interactions between the factors as fixed effects terms. To test whether the divergence in %P50sup between the low and the high subgroups was based on differences in amplitudes elicited by S1 or S2, and to link changes in gating to modulation of a specific P50 amplitude (S1 or S2 elicited), pairwise comparisons were carried out.
Analysis of the %PPI values was performed with ‘SOA' (30, 60, and 120 ms) and ‘drug' as within-subject and ‘subgroup' as between-subject factor. Startle amplitudes were subjected to the linear mixed model with ‘block' (1 to 3) and ‘drug' as within-subject factors and ‘subgroup' as between-subject factor.
SCL-90-R data were analyzed by multivariate analyses of variance (MANOVA) with ‘subgroup' as between-subject factor and SCL-90-R scales as within-subject factor. Pearson's product-moment correlations were calculated to relate placebo gating measures to the SCL-90-R global severity index (GSI).
RESULTS
Demographic Characteristics
Demographic characteristics are summarized in Table 1 for low and high P50 and PPI gating subgroups in the three study cohorts. The low- and high-gating subgroups did not differ in age, body mass index (BMI), IQ, or smoking behavior, with the exception of the low PPI gating group having a higher mean verbal IQ than high PPI gating group in study cohort 2.
P50 Suppression Paradigm
Antipsychotics (aripiprazole, risperidone, amisulpride)
As forced by the splitting of the groups into low and high P50 gating subgroups, analysis of %P50sup revealed significant main effects of ‘subgroup' (treatments: aripiprazole vs placebo: F(1, 26)=21.41, p<0.0005; risperidone vs placebo: F(1, 26)=16.30, p<0.0005, amisulpride vs placebo: F(1, 27)=29.82, p<0.0001). Furthermore, the interaction between ‘drug' and ‘subgroup' attained significance in all three study cohorts (aripiprazole vs placebo F(1, 25)=9.08, p<0.01; risperidone vs placebo: F(1, 24)=13.47, p<0.005, amisulpride vs placebo: F(1, 26)=5.38, p<0.05)). Pairwise comparisons revealed that all AAPs significantly increased %P50sup in the low subgroups (all p<0.05), whereas no significant differences between active drug and placebo were found in the high subgroups (Figure 1a–c). These results indicate that antipsychotic medication can increase P50 gating in low-gating subjects, whereas it has no significant influence on the high-gating group.
Figure 1.
The influence of the AAPs aripiprazole (a), risperidone (b), and amisulpride (c) and negative control treatments lorazepam (d), modafinil (e), and valproate (f) on sensory gating, expressed as percent P50 suppression. All AAPs significantly increased P50 suppression in low-gating healthy volunteers. Lorazepam and modafinil reduced percent P50 suppression independently of low- and high-gating subgroups. Differences in placebo gating within one cohort originate from the exclusion of invalid data sets in a nonpairwise manner. *Significant difference between active drug and placebo. Error bars refer to SEM.
Negative control treatments (lorazepam, modafinil, valproate)
As expected, the low and high P50 subgroups differed significantly in %P50sup as indicated by significant main effects of ‘subgroup' (lorazepam vs placebo: F(1, 27)=17.01, p<0.0005; modafinil vs placebo: F(1, 28)=28.96, p<0.001; valproate vs placebo: F(1, 28)=23.03, p<0.001). Main effect for the factor ‘drug' was significant for treatment with lorazepam (F(1, 22)=4.74; p<0.05) and modafinil (F(1, 26)=5.32; p<0.03), but not for valproate, indicating a reduction of %P50sup with lorazpam and modafinil treatment independently of the subgroups. Furthermore, there was a significant interaction between the two factors (drug, subgroup) in the analysis with valproate (F(1, 26)=45.54; p<0.05). However, pairwise comparisons did not reveal significant differences between placebo and valproate in either subgroup (Figure 1d–f).).
Data were not normally distributed in the following subgroups and treatment conditions: modafinil, high-gating subgroup; valproate, high-gating subgroup. Wilcoxon matched-pairs tests revealed a significant reduction in P50 suppression in the modafinil condition compared with placebo in the high-gating subgroup (modafinil vs placebo: Z=2.23, p<0.03). The analysis for valproate did not reveal significant results.
Results for the analysis of P50 amplitudes are described in detail in the Supplementary Results 1 and Table 2. In short, the three AAPs, aripiprazole, risperidone, and amisulpride, increased %P50sup in the low-gating subgroup by an attenuation of S2-elicited P50 amplitude, rather than by changes in S1-elicited P50 amplitude. In contrast, the negative control treatments reduced P50 suppression by an enhancement of S2-elicited P50 amplitude (modafinil) or a reduction of S1-elicited amplitude (lorazepam).
Table 2. Startle and P50 Amplitudes, and Habituation of Low- and High-Gating Groups Receiving Treatment with Placebo and Active Drug.
|
Study cohort 1 |
Study cohort 2 |
Study cohort 3 |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Placebo |
Aripiprazole |
Risperidone |
Placebo |
Amisulpride |
Lorazepam |
Placebo |
Modafinil |
Valproate |
||||||||||
| Group | Low | High | Low | High | Low | High | Low | High | Low | High | Low | High | Low | High | Low | High | Low | High |
| n (PPI, P50) | 14, 14 | 14, 14 | 11, 13 | 13, 14 | 11, 12 | 13, 14 | 15, 15 | 15, 14 | 15, 14 | 15, 14 | 12, 13 | 13, 11 | 15, 15 | 14, 15 | 15, 14 | 13, 14 | 14, 14 | 14, 14 |
| Startle amplitude (μV)a | ||||||||||||||||||
| Block 1 | 4.45 (0.25) | 5.04 (0.14) | 4.69 (0.24) | 4.72 (0.29) | 4.27 (0.27) | 4.81 (0.15) | 4.85 (0.18) | 4.57 (0.18) | 4.86 (0.22) | 4.74 (0.14) | 4.73 (0.23) | 4.17 (0.23) | 4.82 (0.16) | 4.36 (0.19) | 4.96 (0.15) | 4.41 (0.20) | 4.94 (0.17) | 4.48 (0.26) |
| Block 2 | 3.79 (0.25) | 4.58 (0.18) | 4.27 (0.29) | 4.47 (0.24) | 3.52 (0.30) | 4.21 (0.21) | 4.52 (0.22) | 4.18 (0.15) | 4.36 (0.27) | 4.10 (0.13) | 4.05 (0.22) | 3.77 (0.20) | 4.44 (0.17) | 4.10 (0.21) | 4.64 (0.16) | 4.06 (0.21) | 4.57 (0.16) | 4.16 (0.23) |
| Block 3 | 3.57 (0.25) | 4.27 (0.20) | 3.51 (0.32) | 4.22 (0.17) | 3.19 (0.38) | 3.76 (0.27) | 4.21 (0.31) | 3.77 (0.20) | 4.24 (0.26) | 3.75 (0.22) | 3.63 (0.29) | 3.04 (0.35) | 4.27 (0.19) | 3.71 (0.20) | 4.46 (0.18) | 3.87 (0.23) | 4.45 (0.18) | 3.78 (0.18) |
| Habituation (%) | 19.78 (39.61) | 10.64 (57.19) | 19.22 (32.19) | 48.15 (17.55) | 22.01 (40.60) | 16.20 (42.87) | 22.65 (45.58) | 18.11 (35.71) | 13.46 (54.71) | 3.63 (33.03) | 37.14 (43.10) | 31.84 (36.39) | 29.87 (28.58) | 12.31 (27.97) | 12.44 (30.12) | 11.90 (27.90) | 28.41 (24.62) | 8.54 (23.89) |
| P50 amplitudes (μV)b | ||||||||||||||||||
| S1 | 1.25 (0.08) | 1.35 (0.09) | 1.34 (0.08) | 1.31 (0.08) | 1.20 (0.06) | 1.29 (0.08) | 1.89 (0.08) | 1.29 (0.09) | 1.27 (0.11) | 1.32 (0.08) | 1.05 (0.07) | 1.03 (0.10) | 1.31 (0.11) | 1.17 (0.07) | 1.25 (0.11) | 1.30 (0.11) | 1.30 (0.14) | 1.25 (0.08) |
| S2 | 1.10 (0.07) | 0.61 (0.08) | 0.86 (0.12) | 0.65 (0.12) | 0.82 (0.09) | 0.78 (0.10) | 1.00 (0.07) | 0.65 (0.07) | 0.89 (0.12) | 0.71 (0.09) | 0.88 (0.08) | 0.76 (0.06) | 0.98 (0.07) | 0.44 (0.06) | 0.96 (0.07) | 0.75 (0.07) | 0.80 (0.08) | 0.61 (0.09) |
ln-transformed.
Sqrt-transformed, SD values are shown in brackets.
Prepulse Inhibition Paradigm
Antipsychotics (aripiprazole, risperidone, amisulpride)
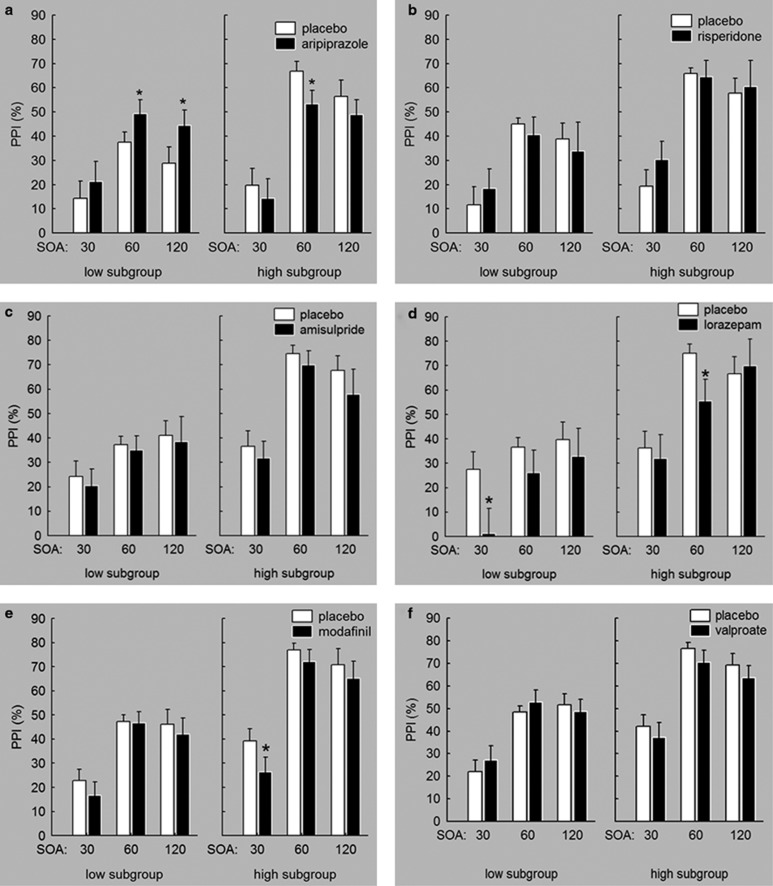
Within all three analyses, the main effect of factors ‘subgroup' (aripiprazole vs placebo: F(1, 26)=10.01, p<0.005; risperidone vs placebo: F(1, 26)=26.25, p<0.0001; amisulpride vs placebo: F(1, 28)=20.02, p<0.0001) and ‘SOA' (aripiprazole vs placebo: F(2, 118)=39.84, p<0.0001; risperidone vs placebo: F(2,118)=25.45, p<0.0001; amisulpride vs placebo: F(2, 140)=29.18, p<0.0001) attained statistical significance. Furthermore, the interaction between the factors ‘drug' and ‘subgroup' in the analysis with aripiprazole (F(1, 118)=12.98, p<0.0005) and between ‘subgroup' and ‘SOA' in the amisulpride analysis (F(2, 140)=6.44, p<0.005) were significant. Pairwise comparisons revealed that aripiprazole (p<0.01) increased %PPI in the low sensorimotor gating subgroup and reduced %PPI in the high sensorimotor gating subgroup (p<0.05; Figure 2a–c).
Figure 2.
The influence of the AAPs aripiprazole (a), risperidone (b), and amisulpride (c) and negative control treatments lorazepam (d), modafinil (e), and valproate (f) on sensorimotor gating expressed as percent PPI. Aripiprazole increased PPI in the low-gating subgroup and decreased PPI in the high-gating subgroup. Risperidone and amisulpride did not significantly influence sensorimotor gating. Whereas valproate did not significantly modulate PPI, lorazepam and modafinil attenuated sensorimotor gating independently of high- and low-gating group. Differences in placebo gating within one cohort originate from the exclusion of invalid data sets in a nonpairwise manner. *Significant difference between active drug and placebo. Error bars refer to SEM.
The analysis of startle habituation did not reveal significant main effects for the factors ‘drug' and ‘subgroup' or significant drug × subgroup interactions in any treatment group (Table 2; all p>0.05).
Negative control treatments (lorazepam, modafinil, valproate)
As expected, the main effects of the factors ‘subgroup' (lorazepam vs placebo: F(1, 28)=21.65, p<0.0001; modafinil vs placebo: F(1, 27)=28.42, p<0.0001; valproate vs placebo: F(1, 27)=23.4, p<0.0001) and ‘SOA' (lorazepam vs placebo: F(2, 125)=18.82, p<0.0001; modafinil vs placebo: F(2, 132)=59.76, p<0.0001; valproate vs placebo: F(2, 132)=40.66, p<0.0001) were significant in all three treatment conditions. There was a significant main effect of the factor ‘drug' for lorazepam (F(1, 125)=6.54, p<0.05) and modafinil (F(1, 132)=4.69, p<0.05) indicating a reduction of %PPI upon these treatments (Figure 2d and f). None of the possible interactions between the factors attained significance. No significant effects were obtained for startle habituation (Table 2; all p>0.05).
Taken together, the antipsychotic aripiprazole increased %PPI in subjects with low levels of sensorimotor gating and reduced %PPI in the high-gating subgroup. Furthermore, lorazepam and modafinil reduced %PPI independently of subgroups.
Data were not normally distributed in the following subgroups and treatment conditions: risperidone, SOA 60, low-gating subgroup; risperidone, SOA 120, low-gating subgroup; risperidone, SOA 120, high-gating subgroup; amisulpride, SOA 120, high-gating subgroup; lorazepam, SOA 120, low-gating subgroup; modafinil, SOA 120, high-gating subgroup; valproate, SOA 60, low-gating subgroup. Wilcoxon matched-pairs tests did not reveal any significant differences between active drug and placebo (all p>0.05).
Results for the analysis of startle amplitudes are described in detail in the Supplementary Results 2 and Table 2. In short, risperidone and lorazepam reduced startle reactivity, whereas valproate increased startle reactivity independently of the subgroup. Aripiprazole increased startle reactivity in the low-gating subgroup and decreased startle reactivity in the high-gating subgroup.
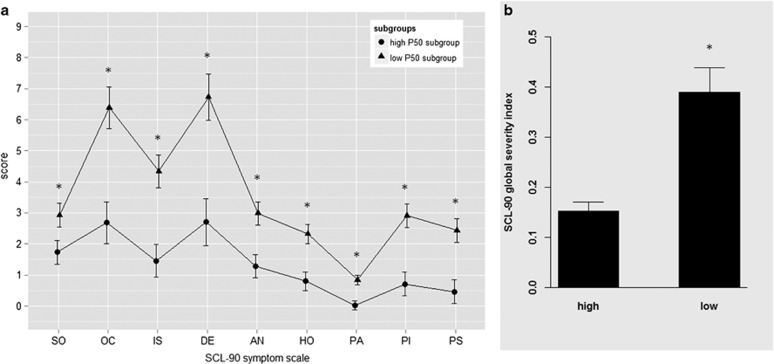
SCL-90-R
Low (n=44) and high (n=44) P50 gating subgroups differed significantly in the SCL-90-R GSI (F(1, 87)=21.16, p<0.001) and in each of the nine symptom scales (all p<0.03), with low P50 gaters reporting more pronounced clinically associated ratings than high P50 gaters (Figure 3). SCL-90-R GSI score also differed significantly between low- and high-gating subgroups when cohorts were analyzed separately (all p<0.03). SCL-90-R GSI was significantly correlated with placebo %P50sup (r=−0.45, p<0.001).
Figure 3.
SCL-90 symptom scales (a) and global severity index (b) in the low (n=44) and the high (n=44) P50 subgroups. SO, somatization; OC, obsessive–compulsive; IS, interpersonal sensitivity; DE, depression; AN, anxiety; HO, hostility; PA, phobic anxiety; PI, paranoid ideation; PS, psychoticism. *Significant difference between low- and high-gating subgroups. Error bars refer to SEM.
For low (n=44) and high (n=44) PPI gating subgroups, no differences were found in any of the SCL-90-R subscales or the GSI (all p>0.35). Furthermore, SCL-90-R GSI was not significantly correlated with placebo %meanPPI (r=0.12, p>0.28).
DISCUSSION
The present study demonstrates first that a single dose of the antipsychotic aripiprazole, risperidone, and amisulpride increased P50 gating in low-gating healthy volunteers, whereas the negative control treatments with lorazepam, modafinil, and valproate did not increase low levels of P50 suppression. Furthermore, low P50 gaters scored significantly higher than high P50 gaters on the SCL-90-R global and all subscale scores measuring psychological traits and symptoms of psychopathology. Second, the atypical antipsychotic aripiprazole significantly increased sensorimotor gating in low PPI gaters.
P50 Supression
The increase of P50 gating in low-gating healthy subjects by the three antipsychotics used in this study is in line with a previous study reporting that atypical sertindole increased P50 suppression in low-gating healthy human subjects (Holstein et al, 2011). Similarly, studies in rodents revealed increased N40 suppression in low-gating DBA/2 mice acutely treated with the atypical clozapine and olanzapine (Simosky et al, 2003, 2008). Also, in accordance with the present P50 increasing effects of the DA-2/3 antagonist amisulpride is the finding that the typical antipsychotic haloperidol acutely augmented P50 suppression in low-gating subjects (Csomor et al, 2008a). The increase of P50 suppression in the low-gating group was because of reduction in S2-elicited amplitudes in the antipsychotic treatment conditions, suggesting that the ability to inhibit the response to the second stimulus was improved and gating was therefore increased. A similar increase of P50 gating was repeatedly shown in drug-naive first-episode and chronically ill schizophrenia patients after long-term treatment with AAPs (Devrim-Ucok et al, 2008; Light et al, 2000; Nagamoto et al, 1999; Oranje et al, 2013). However, Hong et al (2009) did not find a normalization of P50 suppression after 6 weeks of treatment with risperidone or clozapine in first-episode schizophrenia patients. The discrepancy between these findings might be because of differences in symptomatology, course of the illness, or methodological differences in P50 recording. In contrast to the increase of sensory gating in the low P50 gating subgroups observed in this study, P50 suppression in high-gating healthy subjects was not significantly affected by any of the antipsychotic treatments. In line with Holstein et al (2011), we rather found a tendency for reduced sensory gating in subjects with high P50 gating during antipsychotic treatment (see Figure 1).
The three psychoactive control substances without antipsychotic properties used here did not increase P50 gating in either the high- or low-gating subgroup. However, although valproate had no influence on P50 suppression, both lorazepam and modafinil reduced P50 suppression independently of the subgroup. Taken together, the present results strongly suggest that compounds with antipsychotic properties can be distinguished from nonantipsychotics by their effect on P50 gating in naturally low-gating healthy subjects.
Prepulse Inhibition
Treatment with aripiprazole resulted in a PPI increase in subjects with low levels of sensorimotor gating, whereas risperidone and amisulpride did not show the expected PPI-increasing effect. The present results (with aripiprazole and amisulpiride) confirm and extend previous findings demonstrating that the atypical antipsychotics sertindole, clozapine, and quetiapine (Holstein et al, 2011; Swerdlow et al, 2006b; Vollenweider et al, 2006), but not the typical antipsychotic haloperidol (Csomor et al, 2008a), have the capacity to increase PPI in subjects with low gating. Considering the receptor profile of the antipsychotics tested in this study, the high and selective affinity of amisulpride for dopamine D2/3 receptors is unlike other atypicals such as aripiprazole and clozapine that are multireceptor acting agents (Natesan et al, 2008). The receptor profile of amisulpride is rather similar to typical antipsychotics such as haloperidol or chlorpromazine (D2 receptor antagonists) that were found to have no effect on PPI in healthy volunteers in a number of studies (Abduljawad et al, 1999; Barrett et al, 2004; Csomor et al, 2008a). Therefore, PPI in low gaters seems to be particularly sensitive for atypical multireceptor mechanisms of antipsychotic compounds. This assumption is supported by previous studies reporting the significance of multiple transmitter systems in the modulation of PPI (Quednow et al, 2008, 2009).
Based on its multireceptor profile, risperidone has been classified as an atypical antipsychotic. Therefore, it is somewhat surprising that risperidone did not increase PPI in subjects with low PPI traits. Nevertheless, the lack of PPI-increasing effects of risperidone and amisulpride in the present study accords with the finding of Barrett et al (2004) who reported that neither risperidone nor amisulpride influenced PPI in healthy subjects. However, as subjects in this latter study were not stratified for low vs high gating, the comparability of these results with the present findings is limited because potential differential effects on low and high gaters might have been masked. Our results are in line with a previous study reporting that PPI was increased in naturally low-gating Wistar rats by some antipsychotic drugs (clozapine, olanzapine, and sertindole) but not by others (risperidone, remoxipride, and haloperidol) (Depoortere et al, 1997). Taken together, although low P50 gating seems to represent a model sensitive for compounds with antipsychotic properties in general, low PPI gating might rather capture atypical mechanisms of antipsychotic medication. Future studies are necessary to obtain a clearcut picture, as atypical antipsychotics involve complex receptor profiles that might differentially influence PPI capacity.
The analysis of startle amplitude revealed that startle reactivity was influenced by aripiprazole. As startle reactivity serves as the denominator when calculating PPI, startle reactivity can influence PPI. However, as higher startle reactivity is mathematically associated with lower %PPI and vice versa (Csomor et al, 2008b), and startle reactivity was rather increased in the low-gating and decreased in the high-gating subgroup treated with aripiprazole, it is unlikely that the PPI increase in the low-gating subgroup can be attributed to changes in startle reactivity. This conclusion is supported by obtaining the same results when introducing the difference in startle amplitude between placebo and active drug as a covariate in the analysis. Furthermore, it has to be noted that %PPI of the low-gating subgroup in the placebo condition was substantially higher in the current study than in the previous investigations with clozapine and quetiapine (mean SOA 60=3.3% (Vollenweider et al, 2006); %PPI cutoff <16% (Swerdlow et al, 2006b)). More stringent inclusion criteria for the low-gating PPI subgroup might be necessary to evaluate the effects of antipsychotic treatment on PPI in healthy human subjects in future studies. Furthermore, PPI-increasing effects obtained with quetiapine were most prominent at very short SOA conditions (20 and 30 ms) (Swerdlow et al, 2006b) that were only partially assessed in this study. No significant effects were obtained for %habituation, indicating that acute treatment did not influence startle habituation.
In the high-gating subgroup, aripiprazole reduced PPI gating, whereas risperidone and amisulpride had no significant influence on PPI. The reduction of PPI in the high-gating subgroup treated with aripiprazole is in line with previous studies reporting at least a trend for a PPI reduction in relatively high gaters treated with AAPs, a finding that might be explained by an inverted U-shaped dose response (Csomor et al, 2008a; Holstein et al, 2011).
Negative control treatments, as hypothesized, did not increase PPI. Although valproate did not affect PPI, lorazepam and modafinil even reduced PPI independently of subgroups. The reduction of PPI caused by modafinil is in line with recent results obtained in mice (Kwek and van den Buuse, 2013). However, valproate has been shown to increase PPI at high doses in low-gating mice (Flood et al, 2009). In general, more research is necessary to be able to clearly differentiate the effects of antipsychotic and nonantipsychotic medication on PPI in low- and high-gating human subjects.
SCL-90-R
Low P50 gaters scored significantly higher on every scale of the SCL-90 than the high-gating subgroup. Importantly, in the present study all subjects were carefully screened to rule out clinically relevant psychopathology and a family history of an axis I or II psychiatric disorder. Furthermore, SCL-90-R GSI scores in the low and high P50 suppression subgroups were comparable to the healthy SCL-90-R standardization sample (GSI mean=0.33, SD=0.25) (Franke, 1995), confirming that our study sample does not represent a clinical population. Holstein et al (2011) also reported that low P50 (and PPI) gaters scored higher in most of the SCL-90 scales compared with high gaters, even though these differences did not reach significance in that previous study. In contrast to P50 suppression subgroups, SCL-90 scores did not differ between high and low PPI gating subgroups and were not correlated with PPI levels in the placebo condition. However, low PPI levels were associated with worse executive functioning in healthy subjects in previous studies (Csomor et al, 2008a; Holstein et al, 2011). The different relationship between these two gating measures and SCL-90 symptom scores might be reflected by the finding that P50 suppression and PPI are not correlated in healthy subjects (Csomor et al, 2008a), schizophrenia patients (Light and Braff, 2001), or rodents (de Bruin et al, 2001). Thus, the present results might indicate that low P50 gating and low PPI gating in healthy subjects might be linked to different psychological processes and traits with low PPI gating being associated with cognitive deficits and low P50 gating with general psychopathology.
Our present findings should be interpreted with the following limitations in mind. The generalizability may be constricted by the use of a single drug dose and the exclusion of women. Therefore, the effects may not be fully comparable to long-term treatment effects in the clinical setting. Furthermore, it has still to be assessed whether the improvement of P50 gating in the low-gating subgroup treated with AAPs is also related to improvements of clinically associated indices. In addition, it is possible that high gaters had such high suppression levels that further increases were not detectable because of ceiling effects. Moreover, drugs of interest and negative controls were not assigned equally to the three study cohorts (eg, cohort 1: 2 AAPs; cohort 3: 2 negative controls). Finally, more recently, genetic differences were reported to be related to low PPI gating levels in healthy subjects (Quednow et al, 2009) and P50 suppression changes induced by nicotine (Millar et al, 2011) and should therefore be considered in future studies.
CONCLUSIONS
In summary, AAPs seem to increase low P50 suppression in healthy human subjects in line with a number of studies on treatment with AAPs in schizophrenia patients. Effects of AAPs on low P50 gaters can be differentiated from the effect on high gaters, and from the effect of negative control treatments in this study. Although low P50 gating seems to be increased by antipsychotic medication in general, low PPI gating might rather capture complex atypical multireceptor mechanisms of antipsychotic compounds. The results regarding psychopathologically associated indices as indexed by the SCL-90-R are of great importance in the context of translational models, as they bridge basic laboratory measures and clinically relevant indices. In potential phase Ib trials, the low-gating subgroup may be considered as a ‘surrogate patient group', whose response can be differentiated from the high-gating group. The results might be beneficial for planning phase II/III development plans by providing additional information for critical decision-making processes (eg, in dose finding), while saving both resources and time, as healthy subjects are widely available and compliant. Furthermore, confounding effects of previous medication exposure and the generally nonrandom allocation of patients to treatment regimens are eliminated. Furthermore, low P50 gating and low PPI gating models might reflect differential psychological processes and traits.
FUNDING AND DISCLOSURE
Over the past 3 years, MAG has received consulting compensation from Abbott, Addex, Cerca, Dart, Lundbeck/Otsuka, Neurocrine, Omeros, Sunovion, Takeda, and Teva, and holds an equity interest in San Diego Instruments. MAG also has research grant support from Intracellular Therapeutics, Johnson & Johnson, NIDA, NIMH, and the U.S. Veteran's Administration VISN 22 Mental Illness Research, Education, and Clinical Center. PAC is currently employed by Roche Pharma, Switzerland. The authors declare no conflict of interest.
Acknowledgments
This work was supported by AstraZeneca, USA (IIT Grant to FXV) and Swiss Neuromatrix Foundation, Switzerland (Achievement Grant to FXV and KHP). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Abduljawad KA, Langley RW, Bradshaw CM, Szabadi E. Effects of bromocriptine and haloperidol on prepulse inhibition: comparison of the acoustic startle eyeblink response and the N1/P2 auditory-evoked response in man. J Psychopharmacol. 1999;13:3–9. doi: 10.1177/026988119901300101. [DOI] [PubMed] [Google Scholar]
- Altamura AC, Moliterno D, Paletta S, Maffini M, Mauri MC, Bareggi S. Understanding the pharmacokinetics of anxiolytic drugs. Expert Opin Drug Metab Toxicol. 2013;9:423–440. doi: 10.1517/17425255.2013.759209. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association 1994Diagnostic and Statistical Manual of Mental Disorders: DSM-IV4th ednAmerican Psychiatric Association (APA): Washington, DC [Google Scholar]
- Ballon JS, Feifel D. A systematic review of modafinil: Potential clinical uses and mechanisms of action. J Clin Psychiatry. 2006;67:554–566. doi: 10.4088/jcp.v67n0406. [DOI] [PubMed] [Google Scholar]
- Barnes TR, McPhillips MA. Critical analysis and comparison of the side-effect and safety profiles of the new antipsychotics. Br J Psychiatry Suppl. 1999;38:34–43. [PubMed] [Google Scholar]
- Barrett SL, Bell R, Watson D, King DJ. Effects of amisulpride, risperidone and chlorpromazine on auditory and visual latent inhibition, prepulse inhibition, executive function and eye movements in healthy volunteers. J Psychopharmacol. 2004;18:156–172. doi: 10.1177/0269881104042614. [DOI] [PubMed] [Google Scholar]
- Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry. 1992;49:206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- Breier A. Developing drugs for cognitive impairment in schizophrenia. Schizophr Bull. 2005;31:816–822. doi: 10.1093/schbul/sbi051. [DOI] [PubMed] [Google Scholar]
- Breier A, Buchanan RW, Kirkpatrick B, Davis OR, Irish D, Summerfelt A, et al. Effects of clozapine on positive and negative symptoms in outpatients with schizophrenia. Am J Psychiatry. 1994;151:20–26. doi: 10.1176/ajp.151.1.20. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Geyer MA, Braff DL. Multiple site evaluation of P50 suppression among schizophrenia and normal comparison subjects. Schizophr Res. 1998;30:71–80. doi: 10.1016/s0920-9964(97)00122-9. [DOI] [PubMed] [Google Scholar]
- Csomor PA, Stadler RR, Feldon J, Yee BK, Geyer MA, Vollenweider FX. Haloperidol differentially modulates prepulse inhibition and p50 suppression in healthy humans stratified for low and high gating levels. Neuropsychopharmacology. 2008;33:497–512. doi: 10.1038/sj.npp.1301421. [DOI] [PubMed] [Google Scholar]
- Csomor PA, Yee BK, Feldon J, Theodoridou A, Studerus E, Vollenweider FX. Impaired prepulse inhibition and prepulse-elicited reactivity but intact reflex circuit excitability in unmedicated schizophrenia patients: a comparison with healthy subjects and medicated schizophrenia patients. Schizophr Bull. 2009;35:244–255. doi: 10.1093/schbul/sbm146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csomor PA, Yee BK, Vollenweider FX, Feldon J, Nicolet T, Quednow BB. On the influence of baseline startle reactivity on the indexation of prepulse inhibition. Behav Neurosci. 2008;122:885–900. doi: 10.1037/0735-7044.122.4.885. [DOI] [PubMed] [Google Scholar]
- de Bruin NM, van Luijtelaar EL, Cools AR, Ellenbroek BA. Auditory information processing in rat genotypes with different dopaminergic properties. Psychopharmacology (Berl) 2001;156:352–359. doi: 10.1007/s002130100785. [DOI] [PubMed] [Google Scholar]
- Depoortere R, Perrault G, Sanger DJ. Some, but not all, antipsychotic drugs potentiate a low level of prepulse inhibition shown by rats of the Wistar strain. Behav Pharmacol. 1997;8:364–372. doi: 10.1097/00008877-199708000-00009. [DOI] [PubMed] [Google Scholar]
- Devrim-Ucok M, Keskin-Ergen HY, Ucok A. P50 gating at acute and post-acute phases of first-episode schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1952–1956. doi: 10.1016/j.pnpbp.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Flood DG, Choinski M, Marino MJ, Gasior M. Mood stabilizers increase prepulse inhibition in DBA/2NCrl mice. Psychopharmacology (Berl) 2009;205:369–377. doi: 10.1007/s00213-009-1547-y. [DOI] [PubMed] [Google Scholar]
- Flood DG, Gasior M, Marino MJ. Variables affecting prepulse inhibition of the startle reflex and the response to antipsychotics in DBA/2NCrl mice. Psychopharmacology (Berl) 2007;195:203–211. doi: 10.1007/s00213-007-0894-9. [DOI] [PubMed] [Google Scholar]
- Franke G. SCL-90-R: Die Symptom-Check-Liste von Derogatis—Deutsche Version. Beltz Test Gesellschaft: Göttingen; 1995. [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Holstein DH, Csomor PA, Geyer MA, Huber T, Brugger N, Studerus E, et al. The effects of sertindole on sensory gating, sensorimotor gating, and cognition in healthy volunteers. J Psychopharmacol. 2011;25:1600–1613. doi: 10.1177/0269881111415734. [DOI] [PubMed] [Google Scholar]
- Hong X, Chan RC, Zhuang X, Jiang T, Wan X, Wang J, et al. Neuroleptic effects on P50 sensory gating in patients with first-episode never-medicated schizophrenia. Schizophr Res. 2009;108:151–157. doi: 10.1016/j.schres.2008.11.016. [DOI] [PubMed] [Google Scholar]
- Hurko O, Ryan JL. Translational research in central nervous system drug discovery. NeuroRx. 2005;2:671–682. doi: 10.1602/neurorx.2.4.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45:789–796. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- Kwek P, van den Buuse M. Modafinil disrupts prepulse inhibition in mice: strain differences and involvement of dopaminergic and serotonergic activation. Eur J Pharmacol. 2013;699:132–140. doi: 10.1016/j.ejphar.2012.11.041. [DOI] [PubMed] [Google Scholar]
- Leucht S, Arbter D, Engel RR, Kissling W, Davis JM. How effective are second-generation antipsychotic drugs? A meta-analysis of placebo-controlled trials. Mol Psychiatry. 2009;14:429–447. doi: 10.1038/sj.mp.4002136. [DOI] [PubMed] [Google Scholar]
- Light GA, Braff DL. Measuring P50 suppression and prepulse inhibition in a single recording session. Am J Psychiatry. 2001;158:2066–2068. doi: 10.1176/appi.ajp.158.12.2066. [DOI] [PubMed] [Google Scholar]
- Light GA, Geyer MA, Clementz BA, Cadenhead KS, Braff DL. Normal P50 suppression in schizophrenia patients treated with atypical antipsychotic medications. Am J Psychiatry. 2000;157:767–771. doi: 10.1176/appi.ajp.157.5.767. [DOI] [PubMed] [Google Scholar]
- Loscher W. Basic pharmacology of valproate: a review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs. 2002;16:669–694. doi: 10.2165/00023210-200216100-00003. [DOI] [PubMed] [Google Scholar]
- Millar A, Smith D, Choueiry J, Fisher D, Albert P, Knott V. The moderating role of the dopamine transporter 1 gene on P50 sensory gating and its modulation by nicotine. Neuroscience. 2011;180:148–156. doi: 10.1016/j.neuroscience.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10:79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- Moritz S, Andreou C, Klingberg S, Thoring T, Peters MJ. Assessment of subjective cognitive and emotional effects of antipsychotic drugs. Effect by defect. Neuropharmacology. 2013;72:179–186. doi: 10.1016/j.neuropharm.2013.04.039. [DOI] [PubMed] [Google Scholar]
- Nagamoto HT, Adler LE, McRae KA, Huettl P, Cawthra E, Gerhardt G, et al. Auditory P50 in schizophrenics on clozapine: improved gating parallels clinical improvement and changes in plasma 3-methoxy-4-hydroxyphenylglycol. Neuropsychobiology. 1999;39:10–17. doi: 10.1159/000026553. [DOI] [PubMed] [Google Scholar]
- Nagamoto HT, Adler LE, Waldo MC, Freedman R. Sensory gating in schizophrenics and normal controls: effects of changing stimulation interval. Biol Psychiatry. 1989;25:549–561. doi: 10.1016/0006-3223(89)90215-1. [DOI] [PubMed] [Google Scholar]
- Natesan S, Reckless GE, Barlow KB, Nobrega JN, Kapur S. Amisulpride the ‘atypical' atypical antipsychotic–comparison to haloperidol, risperidone and clozapine. Schizophr Res. 2008;105:224–235. doi: 10.1016/j.schres.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Oranje B, Aggernaes B, Rasmussen H, Ebdrup BH, Glenthoj BY. P50 suppression and its neural generators in antipsychotic-naive first-episode schizophrenia before and after 6 months of quetiapine treatment. Schizophr Bull. 2013;39:472–480. doi: 10.1093/schbul/sbr183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouagazzal AM, Jenck F, Moreau JL. Drug-induced potentiation of prepulse inhibition of acoustic startle reflex in mice: a model for detecting antipsychotic activity. Psychopharmacology (Berl) 2001;156:273–283. doi: 10.1007/s002130100763. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Kuhn KU, Mossner R, Schwab SG, Schuhmacher A, Maier W, et al. Sensorimotor gating of schizophrenia patients is influenced by 5-HT2A receptor polymorphisms. Biol Psychiatry. 2008;64:434–437. doi: 10.1016/j.biopsych.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Schmechtig A, Ettinger U, Petrovsky N, Collier DA, Vollenweider FX, et al. Sensorimotor gating depends on polymorphisms of the serotonin-2A receptor and catechol-O-methyltransferase, but not on neuregulin-1 Arg38Gln genotype: a replication study. Biol Psychiatry. 2009;66:614–620. doi: 10.1016/j.biopsych.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmelmann BG, Paulus S, Schacht M, Tilgner C, Schulte-Markwort M, Lambert M. Subjective distress related to side effects and subjective well-being in first admitted adolescents with early-onset psychosis treated with atypical antipsychotics. J Child Adolesc Psychopharmacol. 2005;15:249–258. doi: 10.1089/cap.2005.15.249. [DOI] [PubMed] [Google Scholar]
- Simosky JK, Freedman R, Stevens KE. Olanzapine improves deficient sensory inhibition in DBA/2 mice. Brain Res. 2008;1233:129–136. doi: 10.1016/j.brainres.2008.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simosky JK, Stevens KE, Adler LE, Freedman R. Clozapine improves deficient inhibitory auditory processing in DBA/2 mice, via a nicotinic cholinergic mechanism. Psychopharmacology (Berl) 2003;165:386–396. doi: 10.1007/s00213-002-1285-x. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Hartman PL, Auerbach PP. Changes in sensorimotor inhibition across the menstrual cycle: implications for neuropsychiatric disorders. Biol Psychiatry. 1997;41:452–460. doi: 10.1016/S0006-3223(96)00065-0. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Light GA, Cadenhead KS, Sprock J, Hsieh MH, Braff DL. Startle gating deficits in a large cohort of patients with schizophrenia: relationship to medications, symptoms, neurocognition, and level of function. Arch Gen Psychiatry. 2006;63:1325–1335. doi: 10.1001/archpsyc.63.12.1325. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Talledo J, Sutherland AN, Nagy D, Shoemaker JM. Antipsychotic effects on prepulse inhibition in normal ‘low gating' humans and rats. Neuropsychopharmacology. 2006;31:2011–2021. doi: 10.1038/sj.npp.1301043. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Barro M, Csomor PA, Feldon J. Clozapine enhances prepulse inhibition in healthy humans with low but not with high prepulse inhibition levels. Biol Psychiatry. 2006;60:597–603. doi: 10.1016/j.biopsych.2006.03.058. [DOI] [PubMed] [Google Scholar]
- Wang ZR, Tan YL, Yang FD, Zhang WF, Zou YZ, Tan SP, et al. Impaired prepulse inhibition of acoustic startle in Chinese patients with first-episode, medication-naive schizophrenia. Chin Med J. 2013;126:526–531. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.