Abstract
Drug addiction is marked by pathological drug seeking and intense drug craving, particularly in response to drug-related stimuli. Repeated psychostimulant administration is known to induce long-term alterations in mesolimbic dopamine (DA) signaling that are hypothesized to mediate this heightened sensitivity to environmental stimuli. However, there is little direct evidence that drug-induced alteration in mesolimbic DA function underlies this hypersensitivity to motivational cues. In the current study, we tested this hypothesis using fast-scan cyclic voltammetry to monitor phasic DA signaling in the nucleus accumbens core of cocaine-pretreated (6 once-daily injections of 15 mg/kg, i.p.) and drug-naive rats during a test of cue-evoked incentive motivation for food—the Pavlovian-to-instrumental transfer task. We found that prior cocaine exposure augmented both reward seeking and DA release triggered by the presentation of a reward-paired cue. Furthermore, cue-evoked DA signaling positively correlated with cue-evoked food seeking and was found to be a statistical mediator of this behavioral effect of cocaine. Taken together, these findings provide support for the hypothesis that repeated cocaine exposure enhances cue-evoked incentive motivation through augmented phasic mesolimbic DA signaling. This work sheds new light on a fundamental neurobiological mechanism underlying motivated behavior and its role in the expression of compulsive reward seeking.
INTRODUCTION
One of the major goals of addiction research is to determine how environmental stimuli acquire the ability to elicit the exaggerated levels of drug craving and drug-seeking behavior exhibited by individuals with a history of drug abuse. Pavlovian (stimulus-reward) conditioning is likely to play an important role in this process (Jentsch and Taylor, 1999; O'Brien et al, 1998; Ostlund and Balleine, 2008a; Robinson and Berridge, 1993; Stewart et al, 1984). For instance, cues associated with passive cocaine delivery have been shown to develop incentive motivational properties that allow them to provoke cocaine seeking (LeBlanc et al, 2012). The mesolimbic dopamine (DA) system is likely to contribute to such behavior (Saunders et al, 2013). This system is implicated in various aspects of motivated behavior (Flagel et al, 2011; Nicola, 2010), including the excitatory influence that Pavlovian reward-paired cues have on instrumental reward-seeking actions (Dickinson et al, 2000; Lex and Hauber, 2008; Ostlund and Maidment, 2012; Wassum et al, 2011; Wassum et al, 2013). Furthermore, repeated drug exposure is known to induce long-lasting augmentation of mesolimbic DA signaling (Cadoni et al, 2000; Kalivas and Duffy, 1990; Pettit et al, 1990). Drug-induced augmentation of mesolimbic DA system function may therefore synergize with normal incentive processes to produce the exaggerated cue-evoked motivation, or craving, that characterizes the addicted state (Robinson and Berridge, 1993). However, more research is needed to determine and clarify the link between these neurochemical and behavioral effects of repeated drug administration.
Interestingly, the facilitatory effects of repeated drug exposure on DA signaling and motivated behavior are wide ranging, extending to situations involving food and other natural rewards (LeBlanc et al, 2013a, 2013b; Mendez et al, 2009; Nocjar and Panksepp, 2002; Nordquist et al, 2007; Saddoris et al, 2011; Taylor and Jentsch, 2001; Wyvell and Berridge, 2001). Such phenomena not only reveal the fundamental and pervasive impact of drug abuse on motivated behavior and its underlying neurochemistry, but also provide an important tool with which to test this impact in a drug-free state and in the absence of other behavioral confounds (discussed below). Importantly, however, it remains largely unknown whether these generalized effects of repeated drug exposure on reward-motivated behavior are related to changes in mesolimbic DA signaling.
To address this question, we compared the performance of rats with or without a history of repeated cocaine exposure on a test of cue-evoked incentive motivation for food—the Pavlovian-to-instrumental transfer (PIT) task (see Figure 1 for experimental design). At test, we applied in vivo fast-scan cyclic voltammetry (FSCV) to concurrently monitor phasic DA signaling in the nucleus accumbens core—an area in which DA signaling is strongly associated with expression of cue-evoked motivation for natural and cocaine reward (Lex and Hauber, 2008; Saunders et al, 2013; Wassum et al, 2013)—to determine whether the impact of cocaine exposure on incentive motivation was encoded by mesolimbic DA release.
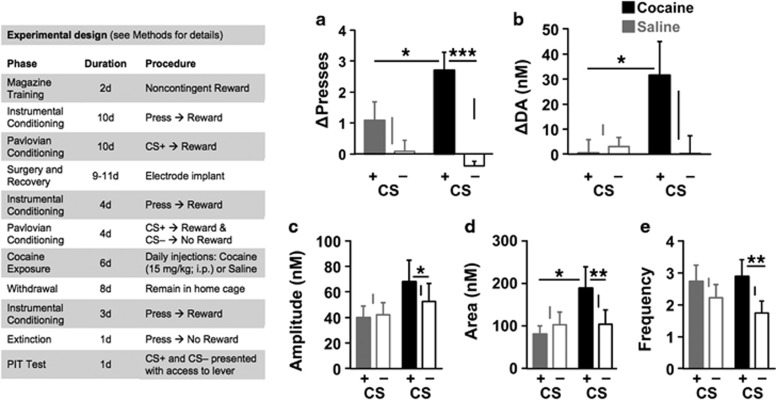
Figure 1.
Effect of cocaine preexposure on phasic mesolimbic DA signaling during PIT testing (see left panel for experimental design). (a) Mean change in lever pressing (CS−pre-CS; +SEM) during cue presentations for rats in groups saline and cocaine, plotted separately for CS+ (filled) and CS− (open) trials. (b) Mean change in DA concentration over 30-s CS periods, relative to last second of pre-CS period (background). See Figure 2 for representative and average DA by time traces. (c–e) Results of DA transient analysis. Mean (+SEM) DA transient amplitude (c), area (d), and frequency (e). Floating vertical lines show standard error of the mean difference (SED) for the simple effect of cue for each group and asterisks indicate significance at *p<0.05, **p<0.01, or ***p<0.001 for planned contrasts (two tailed). Results of linear mixed-model analyses are summarized in Table 1.
MATERIALS AND METHODS
See Supplementary Information for detailed Materials and Methods.
Subjects and Apparatus
Adult male Sprague-Dawley rats (n=19) were maintained at ∼85% of their free-feeding body weight during behavioral procedures that took place in sound- and light-attenuated operant chambers. Importantly, rats were given initial training, postsurgical retraining, and cocaine administration in a set of eight chambers but they underwent postcocaine retraining and PIT/FSCV testing in a distinctive chamber (see Supplementary Information for further details). All experimental procedures involving rats were approved by the UCLA Institutional Animal Care and Use Committee and were in accord with the National Research Council Guide for the Care and Use of Laboratory Animals.
Behavioral Training
After food cup training (2 days), rats were given 10 days of instrumental training, with each session consisting of 30 min of continuous access to a lever that rats could press to produce food pellets (reinforcement shifted over days from random interval (RI) 5 s to RI 45 s. Rats were then given 10 days of Pavlovian training in the same chambers, but with the levers withdrawn, during which they received pairings between one of the two auditory stimuli (CS+ either 3 kHz tone or 2 Hz clicker, ∼75 dB, 30 s duration; 10 presentations per session, separated by variable 150 s intervals) and reward (3 pellets per trial, delivered at cue offset).
Surgery and Retraining
Rats were anesthetized with isoflurane and prepared for aseptic surgery, during which they were implanted with a precalibrated carbon fiber microelectrode aimed at the core of the nucleus accumbens (AP: +1.3 mm, ML: +1.3 mm, relative to bregma, and 7 mm below dura surface) and an Ag/AgCl reference electrode in the contralateral cortex. Rats were allowed to recover for 8–10 days before undergoing 4 days of instrumental retraining (RI 45 s), followed by 4 days of Pavlovian retraining, with two nonreinforced trials with the other cue (CS− either tone or clicker) in the last session (see Supplementary Figure S1A for results). These postsurgical retraining sessions took place in the same chambers used for initial testing.
Cocaine Administration
Rats were then given 6 once-daily injections of either cocaine (15 mg/kg in sterile saline; n=9) or sterile saline (1 ml/kg; i.p.; n=10). Immediately after each injection the rats were placed in the behavioral chamber used for initial training for 45 min. This cocaine administration protocol was previously shown to support locomotor sensitization and facilitate the expression of the PIT effect (LeBlanc et al, 2013b). Rats remained undisturbed in their home cages for 8 days before further behavioral testing.
Pavlovian-to-Instrumental Transfer
The PIT paradigm was used to selectively assess the impact of repeated cocaine exposure on Pavlovian incentive motivation in the absence of other processes through which cues trigger such actions, including stimulus–response learning (Dickinson and Balleine, 1994; Ostlund and Balleine, 2008a; Rescorla and Solomon, 1967). Here, we used a suboptimal PIT protocol known to support minimal cue-evoked reward seeking in normal rats in order to facilitate detection of psychostimulant-induced potentiation of such behavior (LeBlanc et al, 2013a, 2013b; Wyvell and Berridge, 2001). After the cocaine withdrawal period, rats were given another 3 days of instrumental retraining (Supplementary Figure S1B), followed by a 30-min session of extinction (Supplementary Figure S2) in a distinct behavioral chamber that would also be used for PIT testing (see Supplementary Information). On the next day (13 days after cocaine), rats were given a PIT test, during which the lever was continuously available but produced no rewards. The CS+ and CS− were each presented 4 times (30 s per trial) in pseudorandom order and separated by a 3.5-min fixed interval. Histological verification of electrode placements was carried out after each experiment using standard procedures (see Supplementary Figure S3 for placements).
Voltammetric Recording and Dopamine Detection
A voltammetric potentiostat was used to apply a triangular waveform (−0.4 V to +1.3 V at 400 V/s; scan rate of 10 Hz) to the carbon fiber microelectrode through a head-mounted amplifier. DA at the electrode surface undergoes oxidation and reduction reactions at approximately +0.64 V and −0.2 V, respectively, generating distinct currents that are recorded as cyclic voltammograms. Background subtraction was used to quantify changes in the oxidation current over time. Principal component regression analysis (Tar Heel CV software) of the voltammetric data was used to distinguish DA currents from those arising from other electroactive species. Individual preimplant calibration factors were used to convert DA currents to concentration estimates. Behavioral testing was initiated after allowing the background current to stabilize (∼25–30 min).
We first determined changes in DA levels during individual CS+ and CS− trials (30 s each), using a 1-s period before cue presentation for background subtraction. Average DA concentration changes are reported for consecutive 1-s periods and for the entire cue delivery period. DA concentration by time traces for individual trials were separately analyzed to identify and characterize transient DA release events using Mini Analysis software (Synaptosoft, Decautur, GA). Increases in current that exceeded 2.5 × the root mean square of current sampled from the pre-CS period were identified as DA transients. Peak amplitudes were calculated as the difference between a peak and the local minimum occurring 0.5–3 s before that peak. Transient areas were calculated by taking the area under a peak between minima occurring before (<2 s) and after (<4 s) that peak. The first of these values was used as the transient onset to calculate the rise time from onset to peak amplitude. Custom software was used to determine peri-response times for DA transients based on peak concentration times.
Data Analysis
Linear mixed modeling was used to analyze main effects and interactions for all behavioral and voltammetric data. Models included drug group (cocaine vs saline; between-subjects) as a fixed effect and subject as a random effect. Cue type (CS+ and CS− repeated measure) was included as a fixed effect, along with the group × cue interaction term. For each dependent measure we conducted a set of three orthogonal planned contrasts to separately assess the cue effect for each group and to assess the group effect for the CS+ period. Significance testing was conducted using degrees of freedom that would apply to an analysis by conventional mixed-design ANOVA, an approach that is particularly conservative with respect to type I errors.
Peri-response (± 5 s) DA transient frequency data were normalized by dividing the total number of transients that occurred in each 1-s bin by the total number of transients occurring during the 10-s peri-press epoch. Time bin (1–10 s; repeated measure) was treated as a fixed effect, along with group and cue type. Given the apparent nonlinear (quadratic) relationship between time and DA transient frequency, we included a second-order term for the time bin effect and interaction terms involving this effect in the corresponding models.
Separate linear mixed models were used to determine the predictive relationship between each of our measures of DA signaling (average change in DA concentration during 30 s trial, transient frequency, average transient amplitude, and average transient area) across CS+ trials and the change in lever pressing (rate during CS+ period−rate during pre-CS+ period) across CS+ trials. Rat was included as a random factor to control for subject-specific variability (eg, electrode sensitivity and placement).
Mediation, or path, analysis is a useful statistical tool for assessing the nature of the relationship between three or more variables (see McGinty et al, 2013 and Buckholtz et al, 2010 for recent applications of this general approach in neuroscience). Importantly, although this approach cannot prove causality, it does provide a statistical tool with which one can evaluate the veracity of causal hypotheses. Simple mediation between a predictor variable (X) and an output variable (Y) occurs if there is a significant indirect effect of X on Y through a third variable (M) (Preacher and Hayes, 2004; Shrout and Bolger, 2002). This indirect effect is the product of two coefficients: the total effect of X on M (path a) and the direct effect of M on Y (path b). Importantly, as the mediated effect of X on Y by M increases, the direct effect of X on Y (controlling for M) necessarily decreases. This ability to account for (or explain away) at least some of the effect of X on Y is the fundamental feature of a mediating variable. We conducted a mediation analysis using linear mixed models (as described above) to test the hypothesis that the effect of cocaine exposure (X) on CS+-evoked lever pressing (Y) was mediated by the average DA concentration change during CS+trials (M). The mediated effect was tested by bootstrapping (10 000 replications) the product of the coefficients for paths a and b. Significant mediation was indicated if the bias corrected confidence interval (95% level) for the mediated effect did not contain zero.
RESULTS
To determine the impact of cocaine exposure on cue-evoked reward seeking and phasic DA signaling, we conducted a PIT test in which the CS+ and CS− were noncontingently presented while rats had the opportunity to lever press without reward. As predicted, the elevation in lever pressing during CS+ trials (relative to the pre-CS period) was greater for the cocaine-exposed group than for the unexposed group (Figure 1a; see Table 1 for a summary of fixed effects analyses). Furthermore, this heightened behavioral sensitivity to the CS+ was accompanied by augmented mesolimbic DA signaling (see Figure 2a for representative data). For instance, the mean DA concentration change during CS+ presentations was greater for cocaine-exposed rats than for unexposed rats (Figure 1b; see Figure 2b for time course), although the group × CS interaction for this measure narrowly failed to reach significance (Table 1). Cocaine preexposure also affected certain features of phasic DA signaling, as measured by our DA transient analysis. Specifically, we found that the average peak amplitude of DA transients was significantly elevated during CS+ trials (relative to CS− trials), but only in the cocaine group (Figure 1c and Table 1). We also analyzed the average area under the peak for DA transients, a measure that better reflects the duration and overall magnitude of transient DA release events than their peak amplitude. This measure of DA signaling was strongly affected by cocaine preexposure. Not only was a cue-specific difference in DA transient area only observed in the cocaine group, the area of DA transients during CS+ trials was significantly greater for cocaine-exposed rats than for unexposed rats (Figure 1d and Table 1). The frequency of DA transients tended to be generally greater on CS+ trials than on CS− trials, there being no significant interaction with cocaine preexposure (Table 1). However, planned contrasts revealed that the cue effect was only statistically significant for cocaine-exposed rats (Figure 1e).
Table 1. Summarized Results of Linear Mixed-Model Analyses for Data Presented in Figure 1.
|
DA transient |
|||||
|---|---|---|---|---|---|
| Δ Press rate | ΔDA | Amplitude | Area | Frequency | |
| Group | F1, 17=1.59 p=0.22 | F1, 17=0.28 p=0.60 | F1, 17=0.86 p=0.37 | F1, 17=1.70 p=0.21 | F1, 17=0.22 p=0.64 |
| Cue | F1, 17=21.19 p<0.0001*** | F1, 17=1.62 p=0.22 | F1, 17=11.21 p<0.01** | F1, 17=15.16 p<0.01** | F1, 17=17.22 p<0.001*** |
| Group × cue | F1, 17=6.13 p=0.024* | F1, 17=3.40 p=0.083 | F1, 17=5.29 p=0.034* | F1, 17=16.05 p<0.001*** | F1, 17=2.66 p=0.12 |
Degrees of freedom based on conventional mixed-design ANOVA. P-values represent two-tailed test. Significant planned contrasts indicated in Figure 1. See Materials and Methods for further details.
Asterisks indicate significance at *p<0.05, **p<0.01, ***p<0.001.
Figure 2.
Representative PIT trial data from saline- and cocaine-treated rats. (a) Pseudocolor plots show voltammetric data as background-subtracted current (z axis in color) across applied scan potential (Eapp; y axis) over time (x axis), for CS+ (left) and CS− (right) trials. CS onset indicated by vertical dotted line. Peak DA oxidation occurs at approximately +0.6 V. Example current (y axis) by voltage (x axis) traces are placed to the right of each plot. Below the plots are traces showing fluctuations in estimated DA concentration (nM) over time. Peak times for identified DA transients are indicated by black circles and lever press times are indicated by Xs. (b) Mean change in DA concentration (+SEM, dotted lines) over consecutive 1 s periods during CS presentations, relative to 1 s pre-CS period, averaged across trials and subjects.
Importantly, these effects of repeated cocaine exposure were not apparent in baseline lever pressing or DA signaling (see Supplementary Table S1 for means and summary of group effects). Cocaine- and saline-treated rats did not differ in their baseline rates of lever pressing during pre-CS periods (p>0.05), nor did they differ in DA transient amplitude or area (p's>0.05). Interestingly, although a group difference in the frequency of DA transients during pre-CS periods was detected, this measure was actually lower in rats preexposed to cocaine (p<0.05; see Supplementary Table S1 as well as Supplementary Figure S4 for further analysis of transient frequency data).
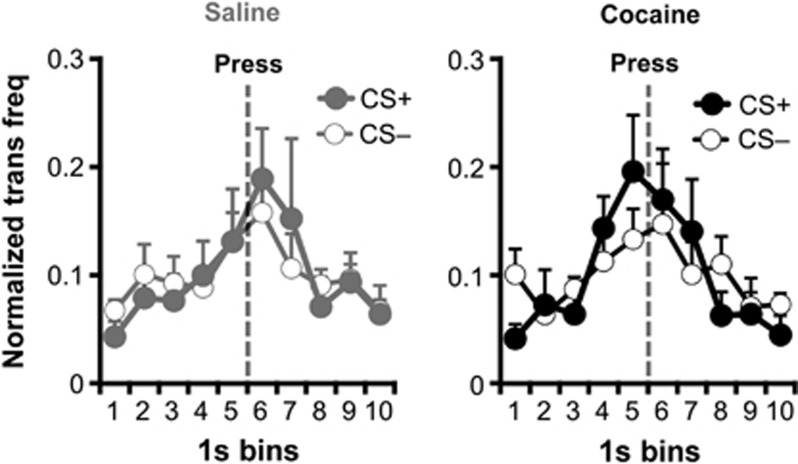
A frequency distribution analysis of DA transients surrounding individual lever presses was conducted to more fully characterize the relationship between phasic DA signaling and reward seeking. The results reveal a temporal correlation between DA transients and reward seeking, with the probability of DA transient occurrence ramping up in the period leading up to the lever press and ramping down in the postlever press period (see Figure 3 for results). Importantly, DA transients that occurred during cue periods had an average rise time of 1.42 s (SEM=0.13) for cocaine-exposed rats and 1.37 s (SEM=0.038) for unexposed rats. Thus, although press-related DA transients tended to reach their peak amplitude around the time the lever press was being executed, most of these press-related transients began before that press, during the initiation of reward seeking. Importantly, although the earlier analysis indicates that phasic DA signaling and lever pressing differed between drug exposure groups and CS trial types (CS+ vs CS−), the current analysis—which normalizes for DA transient and lever press frequency—indicates that the temporal relationship between these output variables was similar—although not identical—across groups and cue types (see Supplementary Figure S5 for examples of temporally contiguous DA transients and lever presses on CS− trials and see Supplementary Figure S6 for further analysis of the peri-response frequency distribution).
Figure 3.
Normalized frequency of DA transients (+SEM) over time for 10-s epochs surrounding individual lever press actions (indicated by dotted vertical line) during CS+ (filled) and CS− (open) trials for saline- and cocaine-treated rats. The likelihood of DA transients increased during the initiation of lever pressing and decreased in the postpress period (quadratic effect of time bin: F9, 153=44.12, p<0.001). A significant cue type × quadratic effect of time bin was also detected (F9, 153=4.50, p<0.001), indicating that the temporal correlation was more pronounced during CS+ trials, although significant quadratic time bin effects were observed for trials with the CS+ (F9, 153=28.97, p<0.001) and the CS− (F9, 153=15.27, p<0.001). No other effect or interaction was significant. See Supplementary Figure S6 for further peri-response frequency distribution analysis.
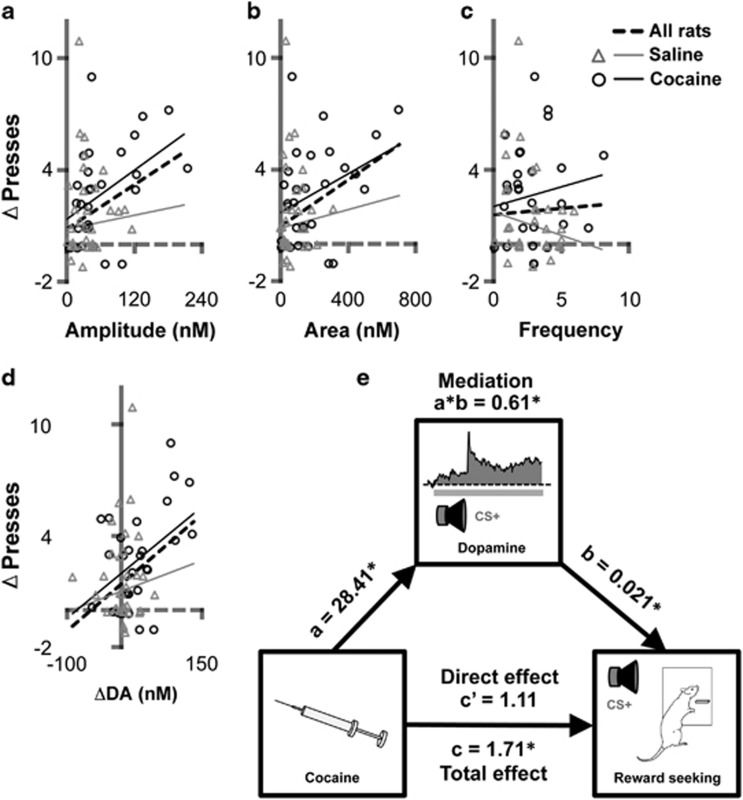
Because cocaine exposure was effective in potentiating the influence of the CS+ on lever pressing and phasic mesolimbic DA signaling, we conducted separate linear mixed-model analyses to further determine the predictive relationship between each measure of DA signaling and CS+-evoked (ie, CS+−pre-CS+) lever pressing (Table 2 and Figure 4a–d). Significant positive correlations were detected for three measures: mean DA transient amplitude (Figure 4a) and area (Figure 4b), and mean DA concentration change (Figure 4d). These relationships were apparent when the data from all rats were analyzed together. Although cocaine exposure did not significantly interact with any of these DA measures in predicting response rate (p's>0.29), it is worth noting that significant positive correlations were also observed for transient amplitude and mean DA concentration change when analyses were limited to the cocaine group (Table 2). No correlation was detected between DA transient frequency and CS+-evoked lever pressing (Figure 4c). However, we did detect a significant correlation between lever pressing and DA transient frequency when data from both trial types and pre-CS periods were included in the analysis (Supplementary Figure S7). Together with the peri-response analysis described above (Figure 3), this finding suggests that the relationship between DA transient occurrence and lever press performance was not specific to CS+ trials (see also Supplementary Figure S5).
Table 2. Regression of Lever Pressing on Individual DA Measurements During CS+ Trials (See Figure 4).
|
DA transient |
||||
|---|---|---|---|---|
| ΔDA (nM) | Amplitude (nM) | Area (nM) | Frequency | |
| All rats (19 rats) | 0.025 (0.0086) p=0.006** | 0.020 (0.0084) p=0.022* | 0.0063 (0.0025) p=0.017* | 0.071 (0.21) p=0.73 |
| Cocaine (9 rats) | 0.024 (0.0093) p=0.019* | 0.022 (0.0088) p=0.021* | 0.0052 (0.0027) p=0.072 | 0.21 (0.23) p=0.38 |
| Saline (10 rats) | 0.011 (0.018) p=0.56 | 0.0063 (0.018) p=0.73 | 0.0025 (0.0072) p=0.73 | −0.25 (0.31) p=0.43 |
Unstandardized regression coefficients (SEM) for each DA measure are derived from separate linear mixed model analyses conducted on data from all rats (top row), cocaine-treated rats (middle row), and saline-treated rats (bottom row). P-values represent two-tailed test.
Figure 4.
Individual differences in CS+-evoked lever pressing and DA signaling. Scatterplots show relationship between change in lever press performance during CS+ trials (CS+−pre-CS+ y axis) and each of four measures of DA signaling (x axis): the amplitude (a), area (b), and frequency (c) of DA transients during CS+ trials, and the average change in DA concentration (d) during those trials. Data points (jittered) are for all trials with the CS+ for rats preexposed to saline or cocaine. Lines show regression slopes generated by linear mixed-model analysis, separately plotted for each group and for all rats. See Table 2 for regression coefficients and significance values for individual analyses. (e) Summary of the mediation (path) analysis. The mediated effect of cocaine exposure on reward seeking through DA signaling is the product of two distinct paths: path a is the total effect of cocaine exposure on DA signaling and path b is the direct effect of DA signaling on reward seeking that controls for the effect of cocaine. A bootstrapped estimate of the product of paths a and b, which represents the mediated effect, was significant. Unstandardized coefficients are shown for each path (*p<0.05).
We next conducted a mediation (path) analysis to determine whether the effect of cocaine exposure on CS+-evoked reward seeking could be attributed to its effect on CS+-evoked mesolimbic DA signaling (Figure 4e). Cocaine exposure group was treated as the predictor variable (X) and CS+-evoked lever pressing as the output variable (Y). Mean change in DA concentration during CS+ trials was selected as the putative mediator variable (M) because it is an inclusive measure of DA signaling that incorporates information about transient frequency and magnitude and because the above analyses indicate that it was a reliable predictor of task performance and was sensitive to cocaine exposure (see Supplementary Table S2 for mediation analyses involving more targeted DA transient measures). Evaluating whether DA signaling is a significant mediator of cocaine's effect on CS+-evoked lever pressing involved several steps. First, we established that there was an effect to mediate. As discussed above, cocaine exposure had a significant facilitatory effect on CS+-evoked lever pressing (Path c, total effect of X on Y; c=1.71, SE=0.80, p<0.05). Second, we confirmed that cocaine exposure was associated with a significant increase in DA levels during CS+ trials (Path a, total effect of X on M; a=28.41, SE=12.01, p<0.05). We then assessed a model in which lever pressing was regressed on both cocaine exposure and DA signaling, allowing us to assess the direct effects of each variable on lever pressing while controlling for the other variable. We detected a significant direct effect of DA on lever pressing (Path b; b=0.021, SE=0.009, p<0.05), demonstrating that a relationship exists between these two variables that cannot be attributed to secondary, potentially independent, effects of cocaine. Furthermore, and consistent with mediation, we found the direct effect of cocaine exposure on lever pressing (Path c') was not significant (c'=1.11, SE=0.80, p=0.18). Thus, information about DA signaling during CS+ trials was a good predictor of reward seeking and explained a substantial component of the total effect of cocaine exposure on task performance. Analysis of the product of paths a and b confirmed that the indirect, or mediated, effect of cocaine exposure through DA signaling was significant (a × b=0.61, SE=0.35, confidence interval: 0.11 and 1.67 at p<0.05).
DISCUSSION
We used FSCV to monitor phasic DA signaling in the nucleus accumbens core of cocaine-exposed and unexposed rats during a PIT test, a relatively pure assay of cue-triggered incentive motivation. Relative to controls, cocaine-exposed rats exhibited a marked enhancement in both reward seeking and phasic DA signaling during the CS+. Furthermore, the influence of the CS+ on reward seeking was positively correlated with the magnitude of DA release, but not the frequency of DA transients, during those trials, consistent with our analyses of phasic DA signaling during PIT expression in drug-naive rats (Wassum et al, 2013). This should not be surprising given that small DA transients occur spontaneously (Wightman et al, 2007) and should, therefore, be uncorrelated with task performance. However, for conditions that support robust PIT performance (eg, cocaine-treated rats in the current study or drug-naive rats tested using an optimal PIT protocol), CS+ presentations tend to elicit large DA release events (relative to those occurring at other times) that tend to be associated with bouts of vigorous lever pressing (Wassum et al, 2013). Indeed, statistical analysis revealed that the effect of cocaine exposure on phasic DA signaling during CS+ trials accounted for a significant portion of its effect on lever pressing, consistent with the hypothesis that this behavioral effect of cocaine is mediated by mesolimbic DA activity.
Previous studies have found evidence of conditioned DA release in the nucleus accumbens as rats initiate cocaine self-administration (Phillips et al, 2003; Stuber et al, 2005; Willuhn et al, 2010). These findings clearly implicate the mesolimbic DA system in cocaine-seeking behavior. However, although the stimuli controlling behavior in such situations may engage Pavlovian incentive processes, they also have the potential to directly elicit behavior through other processes including stimulus–response (habit) learning or goal-directed action selection. This behavioral approach is therefore not suitable for selectively assessing the response-invigorating influence of noncontingent Pavlovian stimuli, a process that is considered to play a central role in drug craving and relapse (Jentsch and Taylor, 1999; O'Brien et al, 1998; Robinson and Berridge, 1993; Stewart et al, 1984). The PIT paradigm used here was specifically developed to assess this behavioral process.
We recently adapted the PIT paradigm to demonstrate the excitatory influence of a cocaine-paired CS+ on instrumental cocaine seeking (LeBlanc et al, 2012). Although that finding indicates that Pavlovian incentive motivation plays a role in instigating the pursuit of cocaine, it does not address the question of whether repeated cocaine exposure affects this process, the main topic of the current study. However, this question is not readily addressed using a cocaine-motivated PIT task because training rats to self-administer and/or anticipate cocaine necessarily involves giving them repeated cocaine exposure, which makes it difficult to vary this factor across conditions. The current study avoids this issue by assessing the effect of repeated cocaine administration on PIT using a food-motivated task. Our findings confirm previous reports showing that repeated psychostimulant exposure enhances expression of the PIT effect (LeBlanc et al, 2013a, 2013b; Saddoris et al, 2011; Wyvell and Berridge, 2001), and extends this work to show that this change in behavior is accompanied by augmented phasic DA release in the nucleus accumbens core. Consistent with this finding, studies using microdialysis, which tracks relatively slow changes in brain chemistry, have shown that animals given repeated drug exposure exhibit more prominent DA responses in the nucleus accumbens shell and amygdala to natural rewards and/or associated cues (Bassareo et al, 2013; Harmer and Phillips, 1999), indicating that chronic drug exposure can have widespread, persistent effects on reward-related DA transmission. However, this approach lacks the ability to characterize the transient DA release events associated with phasic DA cell activity. By using FSCV, a technique with much better temporal resolution, we were able to establish that repeated cocaine exposure alters several aspects of CS+-evoked phasic DA signaling in the nucleus accumbens core and that these discrete DA release events were temporally correlated with the performance of individual reward-seeking actions.
Such alterations in DA release may be the product of long-term adaptations in the mesolimbic DA system caused by repeated cocaine intake (Addy et al, 2010; Cass et al, 1993; Chen et al, 2008; Izenwasser and Cox, 1990; Kalivas and Duffy, 1990; Pettit et al, 1990). That said, drug-associated situational cues are known to alter DA system activity (Duvauchelle et al, 2000; Weitemier and Murphy, 2009) and expression of voluntary reward-seeking actions (Ostlund et al, 2010; Xie et al, 2012), and this raises the possibility that cocaine-induced learning contributed to the effects described here. However, rats in the current study were administered cocaine in the chambers used for initial training, but were retrained and tested in a distinct chamber (used for FSCV recordings) that was never directly associated with cocaine. Furthermore, we recently found a similar enhancement of PIT in rats allowed to self-administer cocaine in one context but tested in a distinct context (LeBlanc et al, 2013a). Finally, in a separate behavioral study (see Supplementary Information and Supplementary Figure S8), we found that the enhancement of PIT produced by repeated cocaine exposure was similar across contexts associated with either cocaine or saline injections. When considered together, these various findings suggest that the effect of repeated cocaine exposure on PIT performance is not strongly dependent on cocaine-induced context conditioning.
Although these results advance our understanding of how experience with cocaine can lead to persistent alterations in behavior, it is important to more fully characterize the role of this mechanism in the addiction process. Basic research on Pavlovian incentive motivation indicates that cues have the ability to trigger reward seeking even when those actions have undesirable consequences (Balleine and Ostlund, 2007; Holland, 2004; Rescorla, 1994). Repeated drug exposure potentiates this influence, potentially contributing to compulsive drug seeking and relapse. The complexity of drug addiction, however, suggests that it is the product of not one but a multitude of interacting processes, including the loss of response inhibition and goal-directed control, together with an overreliance on stimulus-bound habits (Belin et al, 2013; Jentsch and Taylor, 1999; Ostlund and Balleine, 2008b). Understanding how these processes are mediated by the brain and interact to produce pathological drug seeking is a major goal for future research.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
We thank Drs Mimi Liljeholm and Niall Murphy for valuable comments and discussions, and Dr Scott Ng-Evans for technical assistance. This work was supported by research grant DA029035 to SBO, DA09359 and DA05010 to NTM, and DA035443 to KMW.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Addy NA, Daberkow DP, Ford JN, Garris PA, Wightman RM. Sensitization of rapid dopamine signaling in the nucleus accumbens core and shell after repeated cocaine in rats. J Neurophysiol. 2010;104:922–931. doi: 10.1152/jn.00413.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Ostlund SB. Still at the choice-point - action selection and initiation in instrumental conditioning. Ann N Y Acad Sci. 2007;1104:147–171. doi: 10.1196/annals.1390.006. [DOI] [PubMed] [Google Scholar]
- Bassareo V, Cucca F, Cadoni C, Musio P, Di Chiara G. Differential influence of morphine sensitization on accumbens shell and core dopamine responses to morphine- and food-conditioned stimuli. Psychopharmacology (Berl) 2013;225:697–706. doi: 10.1007/s00213-012-2856-0. [DOI] [PubMed] [Google Scholar]
- Belin D, Belin-Rauscent A, Murray JE, Everitt BJ. Addiction: failure of control over maladaptive incentive habits. Curr Opin Neurobiol. 2013. [DOI] [PubMed]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, et al. Dopaminergic network differences in human impulsivity. Science. 2010;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoni C, Solinas M, Di Chiara G. Psychostimulant sensitization: differential changes in accumbal shell and core dopamine. Eur J Pharmacol. 2000;388:69–76. doi: 10.1016/s0014-2999(99)00824-9. [DOI] [PubMed] [Google Scholar]
- Cass WA, Gerhardt GA, Gillespie K, Curella P, Mayfield RD, Zahniser NR. Reduced clearance of exogenous dopamine in rat nucleus accumbens, but not in dorsal striatum, following cocaine challenge in rats withdrawn from repeated cocaine administration. J Neurochem. 1993;61:273–283. doi: 10.1111/j.1471-4159.1993.tb03565.x. [DOI] [PubMed] [Google Scholar]
- Chen BT, Bowers MS, Martin M, Hopf FW, Guillory AM, Carelli RM, et al. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron. 2008;59:288–297. doi: 10.1016/j.neuron.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A, Balleine B. Motivational control of goal-directed action. Anim Learn Behav. 1994;22:1–18. [Google Scholar]
- Dickinson A, Smith J, Mirenowicz J. Dissociation of Pavlovian and instrumental incentive learning under dopamine antagonists. Behav Neurosci. 2000;114:468–483. doi: 10.1037//0735-7044.114.3.468. [DOI] [PubMed] [Google Scholar]
- Duvauchelle CL, Ikegami A, Asami S, Robens J, Kressin K, Castaneda E. Effects of cocaine context on NAcc dopamine and behavioral activity after repeated intravenous cocaine administration. Brain Res. 2000;862:49–58. doi: 10.1016/s0006-8993(00)02091-6. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, et al. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer CJ, Phillips GD. Enhanced dopamine efflux in the amygdala by a predictive, but not a non-predictive, stimulus: facilitation by prior repeated D-amphetamine. Neuroscience. 1999;90:119–130. doi: 10.1016/s0306-4522(98)00464-3. [DOI] [PubMed] [Google Scholar]
- Holland PC. Relations between Pavlovian-instrumental transfer and reinforcer devaluation. J Exp Psychol Anim Behav Process. 2004;30:104–117. doi: 10.1037/0097-7403.30.2.104. [DOI] [PubMed] [Google Scholar]
- Izenwasser S, Cox BM. Daily cocaine treatment produces a persistent reduction of [3H]dopamine uptake in vitro in rat nucleus accumbens but not in striatum. Brain Res. 1990;531:338–341. doi: 10.1016/0006-8993(90)90797-f. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Effect of acute and daily cocaine treatment on extracellular dopamine in the nucleus accumbens. Synapse. 1990;5:48–58. doi: 10.1002/syn.890050104. [DOI] [PubMed] [Google Scholar]
- LeBlanc KH, Maidment NT, Ostlund SB.2013Impact of repeated intravenous cocaine administration on incentive motivation depends on mode of drug delivery Addiction Biologydoi: 10.1111/adb.12063 [DOI] [PMC free article] [PubMed]
- LeBlanc KH, Maidment NT, Ostlund SB. Repeated cocaine exposure facilitates the expression of incentive motivation and induces habitual control in rats. PLoS One. 2013;8:e61355. doi: 10.1371/journal.pone.0061355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc KH, Ostlund SB, Maidment NT. Pavlovian-to-instrumental transfer in cocaine seeking rats. Behav Neurosci. 2012;126:681–689. doi: 10.1037/a0029534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lex A, Hauber W. Dopamine D1 and D2 receptors in the nucleus accumbens core and shell mediate Pavlovian-instrumental transfer. Learn Mem. 2008;15:483–491. doi: 10.1101/lm.978708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty VB, Lardeux S, Taha SA, Kim JJ, Nicola SM. Invigoration of reward seeking by cue and proximity encoding in the nucleus accumbens. Neuron. 2013;78:910–922. doi: 10.1016/j.neuron.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez IA, Williams MT, Bhavsar A, Lu AP, Bizon JL, Setlow B. Long-lasting sensitization of reward-directed behavior by amphetamine. Behav Brain Res. 2009;201:74–79. doi: 10.1016/j.bbr.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM. The flexible approach hypothesis: unification of effort and cue-responding hypotheses for the role of nucleus accumbens dopamine in the activation of reward-seeking behavior. J Neurosci. 2010;30:16585–16600. doi: 10.1523/JNEUROSCI.3958-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocjar C, Panksepp J. Chronic intermittent amphetamine pretreatment enhances future appetitive behavior for drug- and natural-reward: interaction with environmental variables. Behav Brain Res. 2002;128:189–203. doi: 10.1016/s0166-4328(01)00321-7. [DOI] [PubMed] [Google Scholar]
- Nordquist RE, Voorn P, de Mooij-van Malsen JG, Joosten RN, Pennartz CM, Vanderschuren LJ. Augmented reinforcer value and accelerated habit formation after repeated amphetamine treatment. Eur Neuropsychopharmacol. 2007;17:532–540. doi: 10.1016/j.euroneuro.2006.12.005. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion. J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. The disunity of Pavlovian and instrumental values. Behav Brain Sci. 2008;31:456–457. [Google Scholar]
- Ostlund SB, Balleine BW. On habits and addiction: an associative analysis of compulsive drug seeking. Drug Discov Today Dis Models. 2008;5:235–245. doi: 10.1016/j.ddmod.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Maidment NT. Dopamine receptor blockade attenuates the general incentive motivational effects of noncontingently delivered rewards and reward-paired cues without affecting their ability to bias action selection. Neuropsychopharmacology. 2012;37:508–519. doi: 10.1038/npp.2011.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Maidment NT, Balleine BW.2010Alcohol-paired contextual cues produce an immediate and selective loss of goal-directed action in rats Front Integr Neurosci 4doi: 10.3389/fnint.2010.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit HO, Pan HT, Parsons LH, Justice JB., Jr. Extracellular concentrations of cocaine and dopamine are enhanced during chronic cocaine administration. J Neurochem. 1990;55:798–804. doi: 10.1111/j.1471-4159.1990.tb04562.x. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Transfer of instrumental control mediated by a devalued outcome. Anim Learn Behav. 1994;22:27–33. [Google Scholar]
- Rescorla RA, Solomon RL. Two-process learning theory: relationships between Pavlovian conditioning and instrumental learning. Psychol Rev. 1967;74:151–182. doi: 10.1037/h0024475. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Saddoris MP, Stamatakis A, Carelli RM. Neural correlates of Pavlovian-to-instrumental transfer in the nucleus accumbens shell are selectively potentiated following cocaine self-administration. Eur J Neurosci. 2011;33:2274–2287. doi: 10.1111/j.1460-9568.2011.07683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Yager LM, Robinson TE. Cue-evoked cocaine ‘craving': role of dopamine in the accumbens core. J Neurosci. 2013;33:13989–14000. doi: 10.1523/JNEUROSCI.0450-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Methods. 2002;7:422–445. [PubMed] [Google Scholar]
- Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev. 1984;91:251–268. [PubMed] [Google Scholar]
- Stuber GD, Roitman MF, Phillips PE, Carelli RM, Wightman RM. Rapid dopamine signaling in the nucleus accumbens during contingent and noncontingent cocaine administration. Neuropsychopharmacology. 2005;30:853–863. doi: 10.1038/sj.npp.1300619. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Jentsch JD. Repeated intermittent administration of psychomotor stimulant drugs alters the acquisition of Pavlovian approach behavior in rats: differential effects of cocaine, d-amphetamine and 3,4- methylenedioxymethamphetamine (‘Ecstasy') Biol Psychiatry. 2001;50:137–143. doi: 10.1016/s0006-3223(01)01106-4. [DOI] [PubMed] [Google Scholar]
- Wassum KM, Ostlund SB, Balleine BW, Maidment NT. Differential dependence of Pavlovian incentive motivation and instrumental incentive learning processes on dopamine signaling. Learn Mem. 2011;18:475–483. doi: 10.1101/lm.2229311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Ostlund SB, Loewinger GC, Maidment NT. Phasic mesolimbic dopamine release tracks reward seeking during expression of Pavlovian-to-instrumental transfer. Biol Psychiatry. 2013;73:747–755. doi: 10.1016/j.biopsych.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitemier AZ, Murphy NP. Accumbal dopamine and serotonin activity throughout acquisition and expression of place conditioning: correlative relationships with preference and aversion. Eur J Neurosci. 2009;29:1015–1026. doi: 10.1111/j.1460-9568.2009.06652.x. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Heien ML, Wassum KM, Sombers LA, Aragona BJ, Khan AS, et al. Dopamine release is heterogeneous within microenvironments of the rat nucleus accumbens. Eur J Neurosci. 2007;26:2046–2054. doi: 10.1111/j.1460-9568.2007.05772.x. [DOI] [PubMed] [Google Scholar]
- Willuhn I, Wanat MJ, Clark JJ, Phillips PE. Dopamine signaling in the nucleus accumbens of animals self-administering drugs of abuse. Curr Top Behav Neurosci. 2010;3:29–71. doi: 10.1007/7854_2009_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Incentive sensitization by previous amphetamine exposure: increased cue-triggered ‘wanting' for sucrose reward. J Neurosci. 2001;21:7831–7840. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Arguello AA, Reittinger AM, Wells AM, Fuchs RA. Role of nicotinic acetylcholine receptors in the effects of cocaine-paired contextual stimuli on impulsive decision making in rats. Psychopharmacology (Berl) 2012;223:271–279. doi: 10.1007/s00213-012-2715-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.