Abstract
Type 2 diabetes mellitus (DM) and cancer are common diseases that are frequently diagnosed in the same individual. An association between the two conditions has long been postulated. Here, we review the epidemiological evidence for increased risk of cancer, decreased cancer survival, and decreased rates of cancer screening in diabetic patients. The risk for several cancers, including cancers of the pancreas, liver, colorectum, breast, urinary tract, and endometrium, is increased in patients with DM. In a pooled risk analysis weighting published meta-analytic relative risk (RR) for individual cancer by differences in their incidence rates, we found a population RR of 0.97 (95 % CI, 0.75–1.25) in men and 1.29 (95 % CI, 1.16–1.44) in women. All meta-analyses showed an increased relative risk for cancer in diabetic men, except studies of prostate cancer, in which a protective effect was observed. The relationship between diabetes and cancer appears to be complex, and at present, a clear temporal relationship between the two conditions cannot be defined. DM also impacts negatively on cancer-related survival outcomes and cancer screening rates. The overwhelming evidence for lower cancer screening rates, increased incidence of certain cancers, and poorer prognosis after cancer diagnosis in diabetic patients dictates a need for improved cancer care in diabetic individuals through improved screening measures, development of risk assessment tools, and consideration of cancer prevention strategies in diabetic patients. Part two of this review focuses on the biological and pharmacological mechanisms that may account for the association between DM and cancer.
Keywords: Diabetes, Cancer, Review, Meta-analysis
Introduction
Type 2 diabetes mellitus (DM) and cancer are common conditions that are frequently diagnosed in the same individual. A link between the two conditions has been postulated for almost 80 years, but only in the past decade has significant epidemiological evidence been amassed to suggest that this link might be causal [1]. Furthermore, because of the high frequency of diagnosis, even minor reciprocal influences between DM and cancer may have a major impact on the incidence and outcomes of disease [2].
There is strong evidence to suggest that cancer incidence is increased in patients with DM. Epidemiological studies clearly indicate that the risk for developing cancer in diabetic patients is not cancer specific. The risk for several types of cancer including cancers of the pancreas, liver, breast, colorectum, urinary tract, and female reproductive organs has been demonstrated to be increased in diabetic patients [3–82]. However, several confounding factors make it difficult to accurately assess cancer risk in diabetic patients including DM duration, varying metabolic profiles, and the possible presence of shared cancer-promoting factors. In addition to an increased risk for cancer incidence, considerable evidence is now in the literature to suggest that patients with DM have a lower rate of cancer screening and cancer survival than patients without DM. In the first part of this review, we highlight the epidemiological evidence for increased cancer risk, discuss conflicting evidence regarding the relationship between diabetes duration and cancer incidence, describe in depth the evidence for decreased cancer survival and increased cancer mortality in diabetic patients with a focus on the differences between these two measures of cancer prognosis, and discuss shortcomings in cancer screening/surveillance in diabetic individuals and the potential barriers to screening that need to be overcome.
Cancer risk
Evidence for increased cancer risk
That diabetes is a risk factor for cancer is supported by ample evidence showing increased frequency of several cancers in diabetic patients, notably pancreatic, hepatic, colorectal, breast, urinary tract, and endometrial cancer [3–82]. An exception is prostate cancer, for which diabetes appears protective [83, 84]. Studies demonstrating this association have been conducted across several different countries, including Italy [9, 26, 42], Sweden [3], Japan [35, 37, 40, 41, 61, 65], Scotland [66], the USA [7, 13, 14, 17, 20, 28, 36, 47, 48, 50, 55, 78–80], the UK [81], Turkey [11], China [8, 39], and New Zealand [24]. Information sources used to ascertain DM and cancer diagnoses in these international studies are quite diverse and include self-report in surveys and questionnaires; hospital discharge and admission records; well-defined long-term national longitudinal cohorts; documentation of fasting or postprandial glucose tests; medical records; and for cancer diagnosis, use of histological evaluation and cancer registry or death registry [3–80]. The majority of studies related to the relationship between DM and cancer are case–control and retrospective studies, with a few longitudinal cohort studies [3–80]. Most studies included both men and women and reported the relative risk (RR) of cancer following duration of DM for greater than 1 year. The RR varies with many factors including duration of diabetes, sex, family history, type of diabetic medication, duration of medication use, and specific cancer type. These and other potential confounders were controlled for in most studies. Several meta-analyses have demonstrated a link between diabetes and different types of cancer and are summarized in Table 1. A few cancers, however, have a slightly different relationship with diabetes as discussed below.
Table 1.
Summary of meta-analyses of the risk for different cancers (by site) in patients with diabetes
| Cancer type | First author, # study design | Cancer RR (95 % CI) | Cancer RR (95 % CI)—male | Cancer RR (95 % CI)—female |
|---|---|---|---|---|
| Pancreas | Huxley [34] | 1.73 (1.59–1.88) | 1.57 (1.3–1.89) | |
| Overall | 1.82 (1.66–1.89) | |||
| 17 Case–control | 1.94 (1.53–2.46) | |||
| 19 Cohort | 1.73 (1.59–1.88) | |||
| Everhart [19] | N/A | N/A | ||
| Overall | 2.1 (1.6–2.8) | |||
| 11 Case–control | 1.8 (1.1–2.7) | |||
| 9 Cohort | 2.6 (1.6–4.1) | |||
| Breast | Larsson [45] | N/A | ||
| Overall | 1.20 (1.12–1.28) | 1.20 (1.12–1.28) | ||
| 5 Case–control | 1.18 (1.05–1.32) | 1.18 (1.05–1.32) | ||
| 15 Cohort | 1.20 (1.11–1.30) | 1.20 (1.11–1.30) | ||
| Wolf [82] | N/A | |||
| 4 Case–control | 1.13 (0.99–1.28) | 1.13 (0.99–1.28) | ||
| 6 Cohort | 1.25 (1.19–1.3) | 1.25 (1.19–1.3) | ||
| Prostate | Bonovas [83] | N/A | ||
| Overall | 0.91 (0.86–0.96) | 0.91 (0.86–0.96) | ||
| 5 Case–control | 0.9 (0.7–1.2) | 0.9 (0.7–1.2) | ||
| 9 Cohort | 0.9 (0.9–1.0) | 0.9 (0.9–1.0) | ||
| Kasper [84] | N/A | |||
| Overall | 0.84 (0.76–0.93) | 0.84 (0.76–0.93) | ||
| 9 Case–control | 0.89 (0.72–1.11) | 0.89 (0.72–1.11) | ||
| 10 Cohort | 0.81 (0.71–0.92) | 0.81 (0.71–0.92) | ||
| Liver | Wang [73] | 1.15 (0.84–1.59) | 1.66 (1.14–2.41) | |
| 25 Cohort | 2.01 (1.61–2.51) | |||
| El-Serag [18] | N/A | N/A | ||
| Overall | 2.5 (1.8–2.9) | |||
| 13 Case–control | 2.5 (1.5–3.1) | |||
| 12 Cohort | 2.5 (1.9–3.2) | |||
| Noto [65] | N/A | N/A | ||
| 7 Unspecified | OR 3.64 (2.61–5.07) | |||
| Biliary tract/gallbladder | Ren [68] | |||
| Overall | 1.43 (1.18–1.72) | 1.31 (1.17–1.47) [biliary] | 1.29 (1.13–1.46) [biliary] | |
| 8 Case–control | 1.50 (0.72–3.13) | 1.26 (0.99–1.62) [gallbladder] | 1.37 (1.16–1.61) [gallbladder] | |
| 13 Cohort | 1.42 (1.16–1.74) | |||
| Bladder | Larsson [53] | N/A | N/A | |
| Overall | 1.24 (1.08–1.42) | |||
| 7 Case–control | 1.37 (1.04–1.80) | |||
| 3 Cohort | 1.43 (1.18–1.74) | |||
| Endometrium | Friberg [22] | N/A | ||
| Overall | 2.10 (1.75–2.53) | 2.10 (1.75–2.53) | ||
| 13 Case–control | 2.22 (1.80–2.74) | 2.22 (1.80–2.74) | ||
| 3 Cohort | 1.62 (1.21–2.16) | 1.62 (1.21–2.16) | ||
| Colorectal | Larsson [44] | 1.29 (1.15–1.44) | 1.33 (1.23–1.44) | |
| Overall | 1.30 (1.2–1.4) | |||
| 6 Case–control | 1.36 (1.23–1.5) | |||
| 9 Cohort | 1.29 (1.16–1.43) | |||
| Non-Hodgkin lymphoma | Mitri [63] | 0.98 (0.79–1.22) | 1.38 (1.06–1.8) | |
| Overall | 1.19 (1.04–1.35) | |||
| 5 Cohort | 1.41 (1.07–1.88) | |||
| 11 Case–control | 1.12 (0.95–1.31) | |||
| Chao [12] | 1.15 (0.84–1.59) | 1.6 (1.15–2.22) | ||
| Overall | 1.3 (1.1–1.5) | |||
| 10 Case–control | 1.2 (1.0–1.4) | |||
| 3 Cohort | 1.8 (1.3–2.5) | |||
| All cancer | Noto [65] | 1.14 (1.06–1.23) | 1.18 (1.08–1.28) | |
| Overall | 1.10 (1.04–1.17) | |||
| 12 Cohort | ||||
| 1 Case–control |
N/A not available
The association between DM and pancreatic cancer demonstrates a pattern that is different from that of other cancers. Several studies suggest a bimodal increase in the incidence of pancreatic cancer in diabetic patients because DM may be both an early manifestation of pancreatic cancer and a risk factor for the development of pancreatic cancer after a longer duration of DM. The combined RR from a meta-analysis of pancreatic cancer in individuals with DM was 1.82 (95 % CI, 1.66–1.89) [34]. The RR for shorter duration of DM (<4 years) was 2.1 compared to 1.5 for DM of longer duration (>5 years) [34]. The relationship between pancreatic cancer and DM may be complicated further by other factors such as family history of DM [8], smoking [8], and insulin use [48], which act synergistically to increase the risk for developing pancreatic cancer.
Unlike most other cancers, which show increased occurrence in patients with DM, a decreased incidence of prostate cancer is observed in diabetic patients, implying a protective effect. Two separate meta-analyses found similar RRs of 0.91 (95 % CI, 0.86–0.96) [83] and 0.84 (95 % CI, 0.76–0.93) [84] for developing prostate cancer in patients with DM compared to non-diabetic patients. Circulating levels of testosterone are lower in diabetic than non-diabetic men [85], and testosterone has been associated with an elevated risk of prostate cancer [86]. Therefore, it has been suggested that the lower risk of prostate cancer in diabetic patients may be due to lower levels of testosterone [83].
In various studies examining the relationship between cancer and DM, the highest risks have been demonstrated for hepatocellular cancer [18, 65, 73] and pancreatic cancer [19, 34]. Both the liver and pancreas are involved in glucose homeostasis and are exposed to high insulin concentrations. The pancreas is where insulin is produced and the portal circulation exposes hepatic cells to high insulin concentrations. In one meta-analysis, 18 of 25 cohort studies showed that DM was associated with an increased incidence of hepatocellular cancer (HCC) with a RR of 2.01 (95 % CI, 1.61–2.51) compared to individuals without DM [73]. The higher incidence of hyperlipidemia and consequent higher risk of non-alcoholic steatohepatitis in diabetic patients has been suggested to be a link to the increased risk of liver cancer in individuals with DM [73].
Less common solid malignancies, skin cancer, and hematologic malignancies are not as well studied. A large study from Veterans Affairs hospitals assessed the risk of various cancer among a large cohort (n = 4,501,578) of black and white US military veterans [7]. The cancer risk among men with DM (n = 594,815) was compared to the risk among men without DM (n = 3,906,763). This large study reported RR for several less common cancers, and indeed, individuals with DM, compared to those without DM, were at increased risk for several of these cancers, including biliary tract cancer (RR = 1.41, 95 % CI, 1.22–1.62), kidney cancer (RR = 1.09, 95 % CI, 1.03–1.16), leukemia (RR = 1.14, 95 % CI, 1.08–1.21), and melanoma (RR = 1.13, 95 % CI, 1.03–1.24). Interestingly, some other cancers showed a decreased risk in diabetic patients apart from prostate cancer (RR = 0.89, 95 % CI = 0.87–0.91), including brain (RR = 0.91, 95 % CI = 0.82–0.99), buccal cavity (RR = 0.85, 95 % CI = 0.82–0.89), lung (RR = 0.79, 95 % CI = 0.77–0.80), esophageal (RR = 0.77, 95 % CI = 0.72–0.82), and laryngeal (RR = 0.76, 95 % CI = 0.71–0.80) cancer [7].
Population-level relative risk of cancer with diabetes
Since different cancers may demonstrate different risk profiles with DM, the overall burden of risk on the population may differ from that reported for individual cancers. To determine this, a pooled analysis of the meta-analytic risks reported for the association between DM and cancer was performed on the 14 studies listed in Table 2. Meta-analyses included were those that addressed the topic of diabetes-related risk in a specific kind of cancer. A search for articles was performed by two investigators (AAO, JME) using the MEDLINE database with an end search date of 31 June 2011, using the search terms “diabetes,” “cancer,” and “meta-analysis.” References of relevant articles were checked for additional articles. The quality of studies that were included in the pooled risk analysis was determined using the Oxman and Guyatt index of scientific quality [87]. Studies were determined to have a high score, indicating overall good methodological quality. Pooled analysis was performed using the MetaXL software, version 1.0 (2011, EpiGear International Pty Ltd, Australia) that allows use of population (and quality) weights to pool studies for determining the population-level impact of the individual risks of several cancers in men and women (Figs. 1, 2). Population weights were based on the US cancer incidence of the specific cancer included in the model [88]. Cancers with low incidence are down-weighted when relative risks are averaged for the pooled analysis. Thus, the pooled RR over meta-analyses of single cancers reflects population cancer risk expected from DM. Pooling cancers into a single group without reference to their widely varying incidence, as done in some meta-analyses, would be incorrect, as it ignores the fact that global cancer risk related to DM is expected to be influenced by both site-specific DM-related RRs as well as the incidence of that cancer in the population.
Table 2.
Meta-analyses used in pooling risk associated with specific cancers
| Cancer Site | Study ID | First author |
|---|---|---|
| Pancreas | Pancreatic1 | Huxley [34] |
| Pancreatic2 | Everhart [19] | |
| Breast | Breast1 | Larrson [45] |
| Breast2 | Wolf [82] | |
| Prostate | Prostate1 | Kasper [84] |
| Prostate2 | Bonovas [83] | |
| Liver | Hepatocellular1 | El-Serag [18] |
| Hepatocellular2 | Wang [73] | |
| Bladder | Bladder | Larsson [53] |
| Endometrium | Endometrial | Friberg [22] |
| Colon | CRC | Larsson [44] |
| Non-Hodgkin lymphoma | NHL1 | Mitri [63] |
| NHL2 | Chao [12] | |
| Biliary tract | Biliary | Ren [68] |
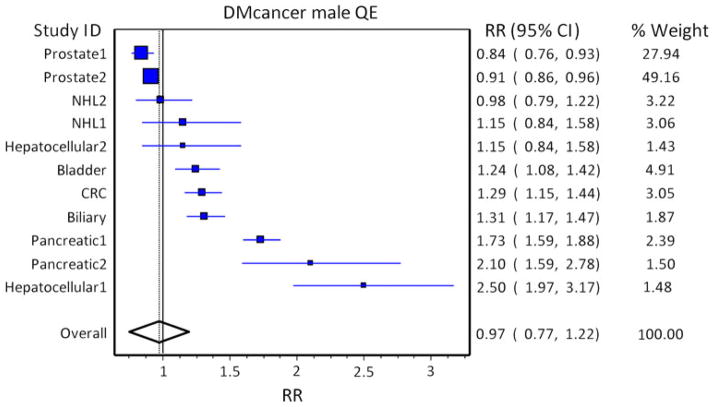
Fig. 1.
Pooled risk analysis of cancer in diabetic males. Pooled risk of cancer in the population with diabetes using population weights and quality weights in diabetic males. Studies are arranged in increasing order of the effect size (indicated by box size), and population weights are based on US cancer incidence of the specific cancer. The solid line at 1 indicates a relative risk (RR) of 1.0, and the solid line through the diamond represents the overall RR, with the diamond representing the 95 % CI
Fig. 2.
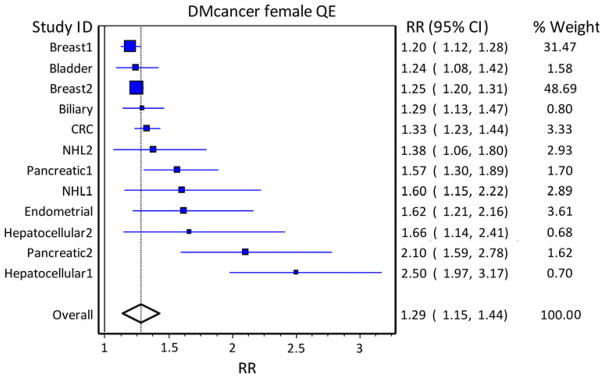
Pooled risk analysis of cancer in diabetic females. Pooled risk of cancer in the population with diabetes using population weights and quality weights in diabetic females. Studies are arranged in increasing order of the effect size (indicated by box size), and population weights are based on US cancer incidence of the specific cancer. The solid line at 1 indicates a relative risk (RR) of 1.0, and the solid line through the diamond represents the overall RR, with the diamond representing the 95 % CI
In men, the population RR of cancer in DM versus non-DM subjects was 0.97 (95 % CI, 0.75–1.22). All cancer studies included showed an increased RR of cancer in men with DM, except for the two studies of prostate cancer, for which DM appears to play a protective role as discussed. In women, the population RR for cancer was 1.29 (95 % CI, 1.16–1.44). It is clear that from a population perspective, prostate cancer protection is sufficient to offset excess population RR from other cancers in men, and the gender difference in population RR for cancer is due to prostate cancer, which makes up the bulk of cancers in men, but is not increased by DM. Therefore, overall, there is no increased population burden of cancer in diabetic men, but there is an increased burden on specific cancers. Pooled risk analysis of various cancer types is limited by previous publication of meta-analyses on only certain types of cancer, but is informative and demonstrates a clear increase in the incidence of most cancer types in subjects with DM and the large effect of prostate cancer on incidence in men.
Diabetes duration, hyperglycemia, and cancer risk
Type 2 DM is a progressive disease characterized by genetic susceptibility, development of insulin resistance, decline in β-cell function, impaired glucose tolerance over time, and eventually, the appearance of clinical type 2 DM. During this trajectory of diabetes development, individuals develop progressive hyperglycemia and increasing dependence on medication used to regulate blood glucose levels. With decline in β-cell function, most individuals with diabetes eventually require insulin therapy [2, 89] resulting in prolonged exposure to endogenous insulin during the pre-diabetes and insulin resistance phase, as well as subsequent exposure to exogenous insulin when the pancreas fails to produce insulin later in the course of the disease.
Unlike hyperglycemia, which defines the clinical onset of diabetes and is progressive and present in varying severity, hyperinsulinemia, oxidative stress, and chronic inflammation are present for a considerable length of time before DM onset and continue in a self-perpetuating loop over the course of diabetes. While it is difficult to separate these latter effects from that of diabetes and hyperglycemia per se, the pattern of latency between diabetes and cancer may be suggestive of causality. A short latency period or no consistent pattern of duration would favor shared risk factors, such as hyperinsulinemia, being the link between diabetes and cancer. On the other hand, a long latency period or a consistent pattern of diabetes duration and cancer would suggest diabetes by itself, as defined by hyperglycemia, to be a risk factor for cancer development. Several studies have investigated the latency period between clinical diabetes and cancer. Hassan et al. [29] reported an increased risk of hepatocellular cancer (HCC) after diagnosis of DM. In their study, 87 % of patients with HCC had DM prior to diagnosis of HCC, with an overall adjusted odds ratio (AOR) of 4.4 (95 % CI, 3.0–6.3). Patients with DM of 2–5 years duration were compared to those with longer duration of DM. Estimated AORs for those with a DM duration of 6–10 years and those with a DM duration >10 years were 1.8 (95 % CI, 0.8–4.1) and 2.2 (95 % CI, 1.2–4.8), respectively [29]. Hazard ratios (HRs) for longer duration were thus similar to shorter duration of diabetes without any clear trend.
Another observed temporal pattern of cancer development post-DM diagnosis is characterized by a short period of unchanged risk followed by an increased risk and eventually a decline in risk. Flood et al. [21] evaluated women enrolled in a large cohort study for development of DM and subsequent colorectal cancer. During the first 4 years after DM diagnosis, colorectal cancer risk was essentially the same compared to those who had never been diagnosed with DM [21]. Women who had DM for 4–8 years had an increased RR for colorectal cancer incidence (RR = 2.36, 95 % CI, 0.96–5.79). With longer duration of DM, a decline in risk was noted, eventually achieving a plateau where no difference in risk was discernible in patients with or without a diagnosis of DM. A similar pattern was observed by Michels et al. [90] related to duration of DM and development of breast cancer. The HR for breast cancer was similar in those with increasing duration up to 15 years and was even lower for longer durations. However, while women with DM for >20 years had a lower HR for breast cancer than non-diabetic women, there were few cases in this group [90].
The association of pancreatic cancer with DM duration appears to be more complex, with studies indicating an overall increased risk followed by a decline in risk. DM may be a cause of pancreatic cancer, but also a consequence of pancreatic cancer. Pancreatic cancer can destroy islet cells, leading to diabetes. In fact, adult sudden onset diabetes is an indicator to pursue evaluation for a pancreatic malignancy [48]. If DM is in fact an early manifestation of pancreatic cancer, the latency period would be short. If DM is an etiologic factor for pancreatic cancer, the relationship between DM and cancer incidence would follow the same pattern described above.
Several studies have illustrated a pattern of reverse causality between DM and pancreatic cancer. Ben et al. [8] reported a higher incidence of pancreatic cancer in new-onset DM of <2-year duration (AOR 4.43, 95 % CI, 3.44–5.72) compared to controls without DM. A moderate increase in risk of pancreatic cancer was observed among cases with long-standing diabetes of >2-year duration (AOR 2.11, 95 % CI, 1.51–2.94) [8]. Wang et al. [48] also reported early high risk followed by decreasing risk over time with risk of pancreatic cancer varying with duration of DM: for DM duration 1–4 years, OR 2.4 (95 % CI, 1.4–4.0); DM duration 5–9 years, OR 2.0 (95 % CI, 1.2–3.4); and DM duration ≥10 years, OR 0.86 (95 % CI, 0.52–1.4). Results reported by Johnson et al. [91] are in agreement with pancreatic cancer incidence, higher in early post-diagnosis of DM. They report an HR for pancreatic cancer of 3.71 at 3 months to 1 year, 2.94 at 1–2 years, 1.78 at 2–3 years, and 1.65 at 3–10 years. Other investigators have observed a similar clustering in pancreatic cancer incidence within 2 years of new-onset DM and have suggested close surveillance for pancreatic cancer for at least 2 years in patients with new-onset diabetes [44, 46]. The relationship between pancreatic cancer and DM may be complicated further by other factors such as family history of DM [8], smoking [8], and insulin use [48], which act synergistically to increase risk.
The relationship between DM duration and prostate cancer is indicative of an extended period of unchanged risk post-DM diagnosis, followed by a declining risk over time. DM is associated with a reduced risk of prostate cancer (OR 0.27–0.96) [92–95], with little change in magnitude with increasing duration of DM [95]. Pierce et al. [93] demonstrated that the median time from DM diagnosis to this protective effect is approximately 6 years. Consistent with this finding, Tavani et al. [94] reported no significant association between DM and prostate cancer within the first 5 years of DM diagnosis (OR = 1.07, 95 % CI, 0.68–1.66). However, after 5 years, the risk of prostate cancer declined with ORs of 0.96 and 0.78 for a diagnosis of diabetes 5–9 years and ≥10 years prior, respectively [94]. Johnson et al. [91] reported an initial increase in prostate cancer incidence in the first 3 months following diagnosis of DM (HR = 1.98, 95 % CI, 1.57–2.48), and following this initial peak, a protective effect similar to that observed by others was noted (HR = 0.81, 95 % CI, 0.76–0.87). Reduced testosterone levels in men with DM may be part of the explanation for the observed decrease in prostate cancer incidence. There is some weak evidence to suggest that the inverse association between prostate cancer and DM is greater for well- versus poorly-differentiated cancers [95].
It is clear from these studies that there are many complex factors associated with DM and with cancer development, making it difficult to distinguish a defined temporal relationship between duration of diabetes and cancer. Diabetes treatment and duration of such treatment complicate this relationship even further. Nevertheless, we may overall conclude that duration of diabetes is not linked to eventual cancer emergence by strong epidemiologic evidence, and thus at present, the hypothesis of a shared risk factor at diabetes initiation for the two diseases seems favored. Therefore, diabetes may not be expected to mediate cancer occurrence directly through associated hyperglycemia or its associated complications, and better glycemic control would not be expected to decrease cancer risk. Indeed, in a recent meta-analysis of three trials reporting cancer incidence [96–98] for the study arms with 357 events in 47,974 person-years with improved glycemic control and 380 events in 45,009 person-years in the control arms, the pooled risk ratio for cancer incidence for participants with improved glycemic control was 0.91 (95 % CI, 0.79–1.05; I2 = 0 %) [99]. It was concluded that data from large randomized controlled trials of intensified glycemic control suggest that cancer risk is not reduced by improving glycemic control in type 2 diabetes [99]. We conclude, therefore, that it is possible that shared risk factors present at the time of diabetes emergence promote the onset of the oncogenic milieu. Prospective epidemiological evidence is required to verify this hypothesis.
Cancer prognosis
A pre-existing diagnosis of DM negatively impacts cancer-related outcomes. Reasons for this association have been suggested to be the result of complex, multi-factorial processes, but no theories have been substantiated. It is important to recognize this association in order to emphasize cancer prevention, cancer screening, careful treatment decisions, and subsequent long-term surveillance in patients with DM. Several studies of both cancer survival and cancer mortality have been performed, and the distinction between the two types of studies is particularly important for this discussion. Survival analyses compare the differential intervals to death in groups of cancer patients with and without DM. Cancer mortality, however, compares the rate of cancer-specific death in diabetic and non-diabetic patients and is influenced by both cancer incidence and survival, and a change in incidence is likely to effect a change in mortality.
Survival in cancer patients with DM has been investigated either over a short time period, usually 30 days postoperatively, or over the long-term, and several of these studies have been further analyzed by meta-analysis (Table 3). In analyses of short-term survival, four studies showed decreased postoperative survival in diabetic patients undergoing emergency surgery for colon cancer [100, 101], hepatectomy for metastatic colorectal cancer [102], or planned resection for colorectal cancer [103]. The largest study of colorectal cancer (CRC) and DM by Davila et al. [103] evaluated 30-day survival in 32,621 patients with and without DM from the Veteran’s Affairs Database and showed a significant decrease in survival following surgical resection for CRC in patients with DM (HR 1.19, 95 % CI, 1.04–1.36). However, another similar study found no significant difference in 30-day survival between CRC patients with and without DM, although overall 5-year survival in patients treated with curative intent was decreased in patients with DM [104]. With regard to other gastrointestinal cancers, decreased 30-day survival has also been reported for diabetic patients with cancer of the esophagus or gastroesophageal junction [105–109]. After resection of pancreatic adenocarcinoma, DM did not influence postoperative outcomes, including 60-day survival, with the exception of fistula development [110]. Studies investigating short-term survival following lung cancer surgery have reported mixed results. With the exception of one report of decreased risk of 30-day postoperative survival in lung cancer patients undergoing lesser resections [111], there was generally no significant association between DM and 30-day survival following pneumonectomy [111–114].
Table 3.
Summary of meta-analyses of survival in cancer patients with diabetes
Risk estimates for long-term, all-cause survival in cancer patients with and without DM have also been evaluated, and several meta-analyses have been done to summarize the findings (Table 3). One meta-analysis assessed the effect of DM on long-term, all-cause survival, as it relates to all cancers. Barone et al. [115] evaluated 23 studies and found that DM was associated with decreased survival (HR = 1.41, 95 % CI, 1.28–1.55). In a subgroup analysis, DM was associated with decreased long-term survival in patients with endometrial, breast, and colorectal cancer [115], consistent with the findings described above [104]. Similarly, in meta-analyses of survival in patients with breast or prostate cancer, DM was associated with decreased survival (Table 3) [116, 117]. It is interesting to note that despite the observed protective effect of DM on the risk of prostate cancer, patients who do get prostate cancer and have DM have a shorter survival than those without DM [117].
Although not a meta-analysis, another large study from the Asia–Pacific region also examined the influence of raised blood glucose and DM on cancer survival [118]. Thirty-six Asian and Australasian cohort studies provided 367,361 participants with 6 % diabetic patients at baseline. With a median follow-up of 4.0 years, there were 5,992 deaths due to cancer. Participants with diabetes had decreased all-cause cancer survival compared to those without (HR = 1.23, 95 % CI, 1.12–1.35). DM was also associated with decreased survival due to cancer of the liver (HR = 1.51, 95 % CI, 1.19–1.91), pancreas (HR = 1.78, 95 % CI, 1.20–2.65), and the colorectum (HR = 1.32, 95 % CI, 0.98–1.78) [118].
Several other important studies report association between DM and cancer mortality. An increased cancer mortality rate was found in diabetic patients independent of body mass index (BMI) or obesity in 1145 patients enrolled in the Zwolle Outpatient Diabetes project Integrating Available Care (ZODIAC) study with a standardized mortality rate (SMR) in diabetic patients of 1.38 (95 % CI, 1.07–1.75) [119]. There are also several reports of meta-analyses of cancer mortality in patients with and without DM (Table 4). Noto et al. [120] performed a meta-analysis consisting of 257,222 subjects with DM from 12 cohort studies and found DM to be associated with an increased risk of mortality (RR = 1.16, 95 % CI, 1.03–1.30). Similarly, meta-analyses have been performed for breast, colon, and hepatocellular cancer mortality, and all found an increased risk of mortality in patients with DM (Table 4) [44, 45, 73]. Mortality studies are more difficult to interpret because mortality depends on both incidence and survival. As shown in Table 4, all four meta-analyses also found an increase in the overall incidence of cancer associated with DM, which is likely to influence the increased risk of cancer mortality observed in these studies. In these meta-analyses, there is little difference in the strength of association between DM and cancer incidence and DM and cancer mortality, making it difficult to draw conclusions, since increased cancer incidence may be the cause of the increased mortality. However, consideration of survival and mortality analyses together makes a strong case for an association between DM and cancer prognosis, especially for breast [45, 115, 116], prostate [117], colorectal [44, 100–103, 115, 121], and endometrial [115] cancer.
Table 4.
Summary of meta-analyses of cancer-specific mortality risk in patients with diabetes
| Cancer site (number of studies) | First author | RR (95 % CI) of cancer | RR (95 % CI) of death (cause specific) |
|---|---|---|---|
| All cancer types (12) | Noto [120] | 1.10 (1.04–1.17) | 1.16 (1.03–1.30) |
| Breast (5) | Larsson [45] | 1.20 (1.12–1.28) | 1.24 (0.95–1.62) |
| Colon (6) | Larsson [44] | 1.30 (1.20–1.40) | 1.26 (1.05–1.50) |
| Hepatocellular (3) | Wang [73] | 2.01 (1.61–2.51) | 1.56 (1.30–1.87) |
Glycemic status and cancer prognosis
While the mechanisms behind the effects of DM on cancer prognosis are not clear, it is likely that the effect of the hyperglycemia of DM on other outcomes related to survival may be implicated. Indeed, a large data set from 17 European population-based or occupational cohorts involved in the DECODE study, which was composed of 26,460 men and 18,195 women aged 25–90 years, suggests that glycemic status is important in the relationship between diabetes and cancer survival [121]. After a follow-up period of 5.9–36.8 years with adjustment for cohort, age, BMI, total cholesterol, blood pressure, and smoking status, cancer-specific survival was decreased for those with abnormal glycemic status. Compared with normal glucose tolerance, the multivariable-adjusted HRs (95 % CI) for all cancer death increased with worsening glucose tolerance reaching 1.13 (1.00–1.28) for pre-diabetes, 1.27 (1.02–1.57) for previously undiagnosed diabetes, and 1.71 (1.35–2.17) for known diabetes in men, and the results were similar in women [121]. This analysis showed a significant decrease in survival for cancers of the stomach, colorectum, and liver in men with pre-diabetes and DM, as well as decreased survival for cancers of the liver and pancreas in women with DM. This sort of relationship was also demonstrated by the Emerging Risk Factors Collaboration [122] who examined the use of fasting glucose as a biomarker for cancer survival. After adjustment for age, sex, smoking, and BMI, the HR for cancer-specific death in patients with elevated fasting glucose was 1.25 (95 % CI, 1.19–1.31). DM was associated with decreased survival for cancers of the liver, pancreas, ovary, colorectum, lung, bladder, and breast. The HR was reduced after adjustment for glycemia measures, but remained unchanged following adjustment for blood pressure, lipid levels, inflammation, or renal markers. Fasting glucose levels >100 mg/dl were associated with decreased survival [122]. In contrast to survival studies, mortality studies do not appear to show this trend. This is demonstrated by four trials that have reported cancer mortality for intensive glycemic control (222 events in 53,892 person-years) and standard control (155 events in 38,743 person-years) arms [123–126]. The SRR for cancer mortality in a meta-analysis was reported by Johnson and Bowker [99] to be 1.00 (95 % CI, 0.81–1.24). Additionally, in the three studies that reported cancer incidence, there was no difference between study arms (RR = 0.91, 95 % CI, 0.79–1.05). As mentioned above, hyperglycemia per se does not seem to impact on cancer risk, and while it does seem to impact on cancer survival, the interaction between risk and survival may not be of sufficient magnitude to alter mortality.
It should be mentioned that glycemic influences on survival may simply be a proxy for factors that are not directly linked to the level of control, but rather to adverse associates of uncontrolled diabetes per se. Such factors include diabetics being less health conscious, chemotherapy not working optimally in the diabetic state [127], and uncontrolled DM being associated with more advanced-stage breast cancer at presentation or with altered regimens for breast cancer treatment and increased toxicity from chemotherapy [116]. In prostate cancer patients, an association has been reported between DM and radiation therapy, complication rates, recurrence, and treatment failure [117]. These may be pathways through which survival decreases with DM, despite the protective effect of DM on the occurrence of prostate cancer. Thus, patients who do get prostate cancer and have DM are at a greater risk of mortality than those without DM. Other factors associated with the diabetic state per se include more non-cancer deaths, later stage at cancer diagnosis, less cancer treatment, and more co-morbidity [115, 128]. There could also be added effects of the diabetic environment creating the potential for greater tumor cell proliferation and the influence of other DM-related co-morbidities on clinical decisions. Also, there is a poorer response to cancer treatment in patients with pre-existing DM (e.g., increased risk of infection and intra-operative complications), presentation with a more advanced stage cancer due to suboptimal cancer screening of people with pre-existing DM (see next section), and inappropriate management of DM during cancer treatment [115].
We conclude that, regardless of the mechanism or explanation responsible for this association, decreased cancer survival and increased cancer mortality in diabetic patients seem well described. Therefore, it is important to emphasize cancer screening and prevention in people with a pre-existing DM diagnosis and to optimize both DM and cancer care after cancer diagnosis.
Cancer screening
As discussed extensively above, patients with DM have an increased risk of developing certain types of cancer, including cancer of the breast, liver, pancreas, endometrium, colorectum, and bladder, and have a worse prognosis after cancer diagnosis. Screening/surveillance to detect cancer at early stages is important for patients to have the best possible prognosis. In 2010, the American Diabetes Association and the American Cancer Society (ACS) presented a consensus report suggesting that cancer incidence is associated with DM and that a healthy diet, physical activity, and weight management could reduce risk and improve outcomes of DM and some forms of cancer [129]. The report also encouraged appropriate cancer screening for patients with DM [129]. However, specific DM-related cancer screening recommendations have not been made. ACS Guidelines for Cancer Screening address breast, cervical, prostate, endometrial, and colorectal cancer [130]. The ACS does not provide specific guidelines for screening for other cancers and does not specifically address screening differences for patients with DM.
Despite the large body of evidence linking diabetes and cancer, cancer screening rates are still reported to be lower for patients with DM. Several studies have demonstrated this to be the case in the United States. A case–control study of 424 women aged 50–75 years from the Midwest reported that from August 1997 to January 2000, women with DM had lower mammogram rates compared to controls with an OR of 0.63 (78.1 % vs. 84.9 %, p = 0.002), even after adjustment for insurance status and race (OR 0.70, p = 0.27) [131]. In another study, screening patterns in women with self-reported DM living in 12 different states were evaluated using the Predisposing, Reinforcing, and Enabling Constructs in Educational Diagnosis and Evaluation (PRECEDE) model, with data from the Centers for Disease Control Behavioral Risk Factor Surveillance System [132]. There was no significant association found between a diagnosis of DM and mammography screening rates; however, there was a significant association between screening rates for cervical cancer and DM diagnosis, with 78 % of women with DM being screened for cervical cancer compared to 86 % of non-diabetic women (p < 0.01) [132]. Another study using data obtained from a telephone survey as part of the Behavioral Risk Factor Surveillance System reported that women with DM had a lower adjusted prevalence of cervical cancer screening (74 % vs. 79 %, p < 0.05), with an AOR of 0.73 (95 % CI, 0.66–0.81), although a higher adjusted prevalence was reported for colorectal cancer screening (63 % vs. 60 %, p < 0.05), with an AOR of 1.14 (95 % CI, 1.04–1.24) [133]. A prospective study in Pennsylvania compared characteristics of elderly women who did or did not have a screening mammogram and Pap during the first 2 years that these services were a Medicare Part B benefit (1991–1992) [134]. Of women still alive after 3 years (n = 2,175), 44.6 % had a mammogram within the study time period and 14.6 % had a Pap test. Women who were younger, more educated, had supplemental insurance, did not require assistance with activities of daily living, and did not have DM or arthritis were more likely to have mammograms. Younger, college-educated, and non-widowed women were more likely to have Pap smears [134]. Another study of elderly women took advantage of the Surveillance, Epidemiology, and End Results (SEER) Program’s Medicare files to evaluate cancer screening and found that women with DM were less likely to have mammogram (OR = 0.83, 95 % CI 0.78–0.88) or colorectal cancer screening (OR = 0.79, 95 % CI, 0.70–0.88) [135]. Women who were seen by their obstetrician/gynecologist had the highest rates of all three services evaluated in this study (mammogram, colorectal cancer screening, and bone density) [135].
Lower cancer screening rates in diabetic patients are not only reported for the US population but also globally. In a study of 675 women with DM and 5,772 women without DM, women with DM had lower rates of participation in the following tests: clinical breast exam, Pap test, self-breast exam, skin check, mammography, or breast ultrasound. Women without DM performed screening at the recommended interval more frequently [136]. A study in Spain involving women with and without DM asked women whether they had a diagnosis of DM and whether they had a mammogram done within 2 years prior or had a Pap test done in the 3 years prior [137]. Women with DM were less likely to receive mammography compared to those without DM (57.9 % vs. 61.9 %; OR = 0.84, 95 % CI, 0.72–0.97; p ≤ 0.05) or have a Pap smear (61.5 % vs. 65.6 %; OR = 0.82, 95 % CI, 0.66–0.98; p ≤ 0.05). Higher education level was a positive predictor for both tests [137]. In Canada, health databases were accessed for data from years 1999 to 2002 in a retrospective cohort study of women aged 50–67 years to compare mammogram rates between women who had DM for a minimum of 2 years (n = 69,168) to women without DM (n = 663,519). Diabetic women were older, had more physician visits, and were more often from a lower-income neighborhood. Despite the greater number of physician visits, the OR for women with DM of having a mammogram during the study time period was 0.68 (95 % CI, 0.67–0.70; p < 0.001) [138].
While cancer screening is clearly lower in diabetic patients, the reasons for this are not clear. The effects of demographic characteristics such as education, ethnicity, and socioeconomic status appear to influence the effects of DM on cancer screening, although this is not extensively described in the literature. A study published in 2001 based on data from the North Carolina Behavioral Risk Factor Surveillance System analyzed colorectal cancer screening rates in diabetic and non-diabetic patients [139]. While there was no difference overall between patients with and without DM, ethnic minorities and people with lower education and income who were also diabetic were less likely to undergo screening for colorectal cancer than their non-diabetic counterparts [139]. A comprehensive study of preventative care in women with DM found that women with DM were less likely to have a Pap test than those without and that the diabetic women least likely to access preventative care were at the extremes of the life cycle, poor, and less educated [140].
In patients with DM, overall cancer screening rates are lower, the incidence of certain cancers is higher, and prognosis is poorer after cancer diagnosis. Recognition of the lower screening rates and reasons thereof provides an opportunity to explore ways to improve cancer screening in diabetic patients. Mechanisms for improved screening may include increased availability and affordability of testing, better education of patients and healthcare providers, adjustment of screening guidelines for diabetic patients, and targeting of certain groups of people with DM at high risk of lower screening or for increased cancer development. Development of a tool to simultaneously assess the risk of DM and cancer may be quite valuable in this respect. It is important for screening guidelines to take into account patients at higher risk due to DM diagnosis and to measure whether those adjustments make a difference in the care of diabetic patients. Additionally, continued investigation into screening measures for other cancers thought to be related to DM is needed. Finally, there should be consideration for increased prevention strategies and the use of chemoprotective agents (e.g., metformin) for cancer in diabetic individuals.
Conclusion
Overwhelming evidence suggests that diabetic patients are at increased risk of several cancers, including cancers of the pancreas, liver, colorectum, breast, urinary tract, and endometrium [30–82]. An interesting exception to this phenomenon is prostate cancer, where DM seems to be protective [83, 84]. When meta-analytic risk was averaged across various types of cancer incorporating their site-specific incidence, there was an increased risk of cancer observed in women, but not in men. In men, all studies of cancer risk in diabetic patients indicated increased risk, except for those of prostate cancer, which has a relatively high incidence and accounts for the lack of population RR change between diabetic and non-diabetic men. As the link between DM and cancer incidence appears to be different for different cancers, it is important for future studies to focus on rarer cancers and to limit analyses of “all cancers,” as generalizations may allow for an association between one type of cancer and DM to be diluted by a cancer with no association with DM, as demonstrated in our population risk analysis for cancer in men (Fig. 1). The development of cancer in the context of DM is complex. No clear temporal relationship between DM and cancer has been defined, and at present, data suggest that the relationship between the two conditions may be related to shared risk factors, as opposed to a causal relationship. Prospective studies designed to answer the questions regarding DM duration and cancer incidence are needed to better understand causality.
In addition to increased cancer risk, diabetic patients also have a poorer prognosis following cancer diagnosis. This association is most effectively demonstrated by studies that focus on survival in cancer patients with and without DM. Studies of cancer mortality are more difficult to interpret due to the well-demonstrated increased incidence of cancer in diabetic patients. More detailed, prospective analyses that examine cancer prognosis in the context of DM are needed in order to advance our understanding of the effects of DM on cancer outcomes and to determine the factors involved in this association, as they may be therapeutic targets. Several reasons for decreased survival in diabetic patients following cancer diagnosis have been hypothesized, including increased tumor cell proliferation in an environment of hyperinsulinemia and hyperglycemia, differences in cancer treatment, influence of DM-related co-morbidities on treatment decisions, poorer response to cancer treatments in patients with DM, presentation with advanced stage cancer due to suboptimal screening, and inappropriate management of DM during cancer treatment [115]. Hyperglycemia, which defines the diabetic condition, is an especially attractive target. However, recent studies suggesting that controlling the hyperglycemia characteristic of DM does not decrease the risk of cancer compared to diabetic individuals with poor glycemic control, indicate that exploration of other possibilities is important [96–99]. Whether glycemic control in DM impacts on cancer prognosis remains to be determined.
Decreased cancer screening rates in diabetic patients are perplexing as diabetic individuals tend to have more interaction with the healthcare system than non-diabetic individuals [138]. Future investigation into the barriers to screening in patients with DM is an important step toward improving screening. Additionally, development of screening measures for cancers thought to be related to DM may help to reduce the excess burden of cancer seen in the diabetic population. Recognition of the increased cancer risk, suboptimal cancer screening, and reduced survival after cancer diagnosis in diabetic patients provides an opportunity for better-quality cancer care in these patients through improved screening and the development of risk assessment tools and risk prevention strategies for cancer and diabetes.
Contributor Information
Adedayo A. Onitilo, Email: onitilo.adedayo@marshfieldclinic.org, Department of Hematology/Oncology, Marshfield Clinic Weston Center, 3501 Cranberry Boulevard, Weston, WI 54476, USA. Marshfield Clinic Research Foundation, Marshfield, WI, USA. School of Population Health, University of Queensland, Brisbane, Australia
Jessica M. Engel, Department of Hematology/Oncology, Marshfield Clinic Cancer Care, Stevens Point, WI, USA
Ingrid Glurich, Marshfield Clinic Research Foundation, Marshfield, WI, USA.
Rachel V. Stankowski, Marshfield Clinic Research Foundation, Marshfield, WI, USA
Gail M. Williams, School of Population Health, University of Queensland, Brisbane, Australia
Suhail A. Doi, School of Population Health, University of Queensland, Brisbane, Australia
References
- 1.Chowdhury TA. Diabetes and cancer. QJM. 2010;103(12):905–915. doi: 10.1093/qjmed/hcq149. [DOI] [PubMed] [Google Scholar]
- 2.Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009;16(4):1103–1123. doi: 10.1677/ERC-09-0087. [DOI] [PubMed] [Google Scholar]
- 3.Adami HO, McLaughlin J, Ekbom A, et al. Cancer risk in patients with diabetes mellitus. Cancer Causes Control. 1991;2(5):307–314. doi: 10.1007/BF00051670. [DOI] [PubMed] [Google Scholar]
- 4.Adami HO, Chow WH, Nyren O, et al. Excess risk of primary liver cancer in patients with diabetes mellitus. J Natl Cancer Inst. 1996;88(20):1472–1477. doi: 10.1093/jnci/88.20.1472. [DOI] [PubMed] [Google Scholar]
- 5.Alokail MS, Al-Daghri NM, Al-Attas OS, Hussain T. Combined effects of obesity and type 2 diabetes contribute to increased breast cancer risk in premenopausal women. Cardiovasc Diabetol. 2009;8:33. doi: 10.1186/1475-2840-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson KE, Anderson E, Mink PJ, et al. Diabetes and endometrial cancer in the Iowa women’s health study. Cancer Epidemiol Biomarkers Prev. 2001;10(6):611–616. [PubMed] [Google Scholar]
- 7.Atchison EA, Gridley G, Carreon JD, Leitzmann MF, McGlynn KA. Risk of cancer in a large cohort of U.S. veterans with diabetes. Int J Cancer. 2011;128(3):635–643. doi: 10.1002/ijc.25362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ben Q, Cai Q, Li Z, et al. The relationship between new-onset diabetes mellitus and pancreatic cancer risk: a case-control study. Eur J Cancer. 2011;47(2):248–254. doi: 10.1016/j.ejca.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Bonelli L, Aste H, Bovo P, et al. Exocrine pancreatic cancer, cigarette smoking, and diabetes mellitus: a case-control study in northern Italy. Pancreas. 2003;27(2):143–149. doi: 10.1097/00006676-200308000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Campbell PT, Deka A, Jacobs EJ, et al. Prospective study reveals associations between colorectal cancer and type 2 diabetes mellitus or insulin use in men. Gastroenterology. 2010;139(4):1138–1146. doi: 10.1053/j.gastro.2010.06.072. [DOI] [PubMed] [Google Scholar]
- 11.Cetin M, Colak R, Bayram F, Altinbas M, Unal A, Kelestimur F. High prevalence of diabetes in patients with pancreatic cancer in central Anatolia, Turkey. Diabetes Res Clin Pract. 2002;58(2):97–100. doi: 10.1016/s0168-8227(02)00130-4. [DOI] [PubMed] [Google Scholar]
- 12.Chao C, Page JH. Type 2 diabetes mellitus and risk of non-Hodgkin lymphoma: a systematic review and meta-analysis. Am J Epidemiol. 2008;168(5):471–480. doi: 10.1093/aje/kwn160. [DOI] [PubMed] [Google Scholar]
- 13.Chari ST, Leibson CL, Rabe KG, Ransom J, de Andrade M, Petersen GM. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology. 2005;129(2):504–511. doi: 10.1053/j.gastro.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chari ST, Leibson CL, Rabe KG, et al. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology. 2008;134(1):95–101. doi: 10.1053/j.gastro.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen CQ, Fang LK, Cai SR, et al. Effects of diabetes mellitus on prognosis of the patients with colorectal cancer undergoing resection: a cohort study with 945 patients. Chin Med J (Engl) 2010;123(21):3084–3088. [PubMed] [Google Scholar]
- 16.Chow WH, Gridley G, Nyren O, et al. Risk of pancreatic cancer following diabetes mellitus: a nationwide cohort study in Sweden. J Natl Cancer Inst. 1995;87(12):930–931. doi: 10.1093/jnci/87.12.930. [DOI] [PubMed] [Google Scholar]
- 17.DeMeo MT. Pancreatic cancer and sugar diabetes. Nutr Rev. 2001;59(4):112–115. doi: 10.1111/j.1753-4887.2001.tb06997.x. [DOI] [PubMed] [Google Scholar]
- 18.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4(3):369–380. doi: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Everhart J, Wright D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. JAMA. 1995;273(20):1605–1609. [PubMed] [Google Scholar]
- 20.Fisher WE. Diabetes: risk factor for the development of pancreatic cancer or manifestation of the disease? World J Surg. 2001;25(4):503–508. doi: 10.1007/s002680020344. [DOI] [PubMed] [Google Scholar]
- 21.Flood A, Strayer L, Schairer C, Schatzkin A. Diabetes and risk of incident colorectal cancer in a prospective cohort of women. Cancer Causes Control. 2010;21(8):1277–1284. doi: 10.1007/s10552-010-9555-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friberg E, Orsini N, Mantzoros CS, Wolk A. Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia. 2007;50(7):1365–1374. doi: 10.1007/s00125-007-0681-5. [DOI] [PubMed] [Google Scholar]
- 23.Friberg E, Mantzoros CS, Wolk A. Diabetes and risk of endometrial cancer: a population-based prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2007;16(2):276–280. doi: 10.1158/1055-9965.EPI-06-0751. [DOI] [PubMed] [Google Scholar]
- 24.Frye JN, Inder WJ, Dobbs BR, Frizelle FA. Pancreatic cancer and diabetes: is there a relationship? A case-controlled study. Aust N Z J Surg. 2000;70(10):722–724. doi: 10.1046/j.1440-1622.2000.01940.x. [DOI] [PubMed] [Google Scholar]
- 25.Fujino Y, Mizoue T, Tokui N, Yoshimura T. Prospective study of diabetes mellitus and liver cancer in Japan. Diabetes Metab Res Rev. 2001;17(5):374–379. doi: 10.1002/dmrr.214. [DOI] [PubMed] [Google Scholar]
- 26.Gullo L, Pezzilli R, Morselli-Labate AM. Diabetes and the risk of pancreatic cancer. N Engl J Med. 1994;331(2):81–84. doi: 10.1056/NEJM199407143310203. [DOI] [PubMed] [Google Scholar]
- 27.Gullo L, Pezzilli R. Diabetes and pancreatic cancer. Pancreas. 2004;28(4):451–452. doi: 10.1097/00006676-200405000-00018. [DOI] [PubMed] [Google Scholar]
- 28.Gupta S, Vittinghoff E, Bertenthal D, et al. New-onset diabetes and pancreatic cancer. Clin Gastroenterol Hepatol. 2006;4(11):1366–1372. doi: 10.1016/j.cgh.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 29.Hassan MM, Curley SA, Li D, et al. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer. 2010;116(8):1938–1946. doi: 10.1002/cncr.24982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He J, Stram DO, Kolonel LN, Henderson BE, Le Marchand L, Haiman CA. The association of diabetes with colorectal cancer risk: the multiethnic cohort. Br J Cancer. 2010;103(1):120–126. doi: 10.1038/sj.bjc.6605721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hemminki K, Li X, Sundquist J, Sundquist K. Risk of cancer following hospitalization for type 2 diabetes. Oncologist. 2010;15(6):548–555. doi: 10.1634/theoncologist.2009-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hjalgrim H, Frisch M, Ekbom A, Kyvik KO, Melbye M, Green A. Cancer and diabetes–a follow-up study of two population-based cohorts of diabetic patients. J Intern Med. 1997;241(6):471–475. doi: 10.1111/j.1365-2796.1997.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 33.Hu FB, Manson JE, Liu S, et al. Prospective study of adult onset diabetes mellitus (type 2) and risk of colorectal cancer in women. J Natl Cancer Inst. 1999;91(6):542–547. doi: 10.1093/jnci/91.6.542. [DOI] [PubMed] [Google Scholar]
- 34.Huxley R, Ansary-Moghaddam A, de González AB, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92(11):2076–2083. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inoue M, Iwasaki M, Otani T, Sasazuki S, Noda M, Tsugane S. Diabetes mellitus and the risk of cancer: results from a large-scale population-based cohort study in Japan. Arch Intern Med. 2006;166(17):1871–1877. doi: 10.1001/archinte.166.17.1871. [DOI] [PubMed] [Google Scholar]
- 36.Jamal MM, Yoon EJ, Vega KJ, Hashemzadeh M, Chang KJ. Diabetes mellitus as a risk factor for gastrointestinal cancer among American veterans. World J Gastroenterol. 2009;15(42):5274–5278. doi: 10.3748/wjg.15.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khan M, Mori M, Fujino Y, et al. Site-specific cancer risk due to diabetes mellitus history: evidence from the Japan collaborative cohort (JACC) study. Asian Pac J Cancer Prev. 2006;7(2):253–259. [PubMed] [Google Scholar]
- 38.Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Preliminary communication: glycated hemoglobin, diabetes, and incident colorectal cancer in men and women: a prospective analysis from the European prospective investigation into cancer-Norfolk study. Cancer Epidemiol Biomarkers Prev. 2004;13(6):915–919. [PubMed] [Google Scholar]
- 39.Kuang TT, Jin dY, Wang DS, et al. Clinical epidemiological analysis of the relationship between pancreatic cancer and diabetes mellitus: data from a single institution in China. J Dig Dis. 2009;10(1):26–29. doi: 10.1111/j.1751-2980.2008.00359.x. [DOI] [PubMed] [Google Scholar]
- 40.Kuriki K, Tokudome S, Tajima K. Association between type II diabetes and colon cancer among Japanese with reference to changes in food intake. Asian Pac J Cancer Prev. 2004;5(1):28–35. [PubMed] [Google Scholar]
- 41.Kuriki K, Hirose K, Tajima K. Diabetes and cancer risk for all and specific sites among Japanese men and women. Eur J Cancer Prev. 2007;16(1):83–89. doi: 10.1097/01.cej.0000228404.37858.40. [DOI] [PubMed] [Google Scholar]
- 42.La Vecchia C, Negri E, Franceschi S, D’Avanzo B, Boyle P. A case-control study of diabetes mellitus and cancer risk. Br J Cancer. 1994;70(5):950–953. doi: 10.1038/bjc.1994.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.La Vecchia C, Negri E, Decarli A, Franceschi S. Diabetes mellitus and the risk of primary liver cancer. Int J Cancer. 1997;73(2):204–207. doi: 10.1002/(sici)1097-0215(19971009)73:2<204::aid-ijc7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 44.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97(22):1679–1687. doi: 10.1093/jnci/dji375. [DOI] [PubMed] [Google Scholar]
- 45.Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer. 2007;121(4):856–862. doi: 10.1002/ijc.22717. [DOI] [PubMed] [Google Scholar]
- 46.Pannala R, Leirness JB, Bamlet WR, Basu A, Petersen GM, Chari ST. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology. 2008;134(4):81–987. doi: 10.1053/j.gastro.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vinikoor LC, Long MD, Keku TO, Martin CF, Galanko JA, Sandler RS. The association between diabetes, insulin use, and colorectal cancer among Whites and African Americans. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1239–1242. doi: 10.1158/1055-9965.EPI-08-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang F, Gupta S, Holly EA. Diabetes mellitus and pancreatic cancer in a population-based case-control study in the San Francisco Bay Area. California Cancer Epidemiol Bio-markers Prev. 2006;15(8):1458–1463. doi: 10.1158/1055-9965.EPI-06-0188. [DOI] [PubMed] [Google Scholar]
- 49.Will JC, Galuska DA, Vinicor F, Calle EE. Colorectal cancer: another complication of diabetes mellitus? Am J Epidemiol. 1998;147(9):816–825. doi: 10.1093/oxfordjournals.aje.a009534. [DOI] [PubMed] [Google Scholar]
- 50.Yalniz M, Pour PM. Diabetes mellitus: a risk factor for pancreatic cancer? Langenbecks Arch Surg. 2005;390(1):66–72. doi: 10.1007/s00423-004-0469-8. [DOI] [PubMed] [Google Scholar]
- 51.La Vecchia C, Negri E, Decarli A, Franceschi S. Diabetes mellitus and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 1997;6(12):1007–1010. [PubMed] [Google Scholar]
- 52.Larsson SC, Permert J, Hakansson N, Naslund I, Bergkvist L, Wolk A. Overall obesity, abdominal adiposity, diabetes and cigarette smoking in relation to the risk of pancreatic cancer in two Swedish population-based cohorts. Br J Cancer. 2005;93(11):1310–1315. doi: 10.1038/sj.bjc.6602868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Larsson SC, Orsini N, Brismar K, Wolk A. Diabetes mellitus and risk of bladder cancer: a meta-analysis. Diabetologia. 2006;49(12):2819–2823. doi: 10.1007/s00125-006-0468-0. [DOI] [PubMed] [Google Scholar]
- 54.Li CI, Daling JR, Tang MT, Malone KE. Relationship between diabetes and risk of second primary contralateral breast cancer. Breast Cancer Res Treat. 2011;125(2):545–551. doi: 10.1007/s10549-010-1035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li D, Tang H, Hassan MM, Holly EA, Bracci PM, Silverman DT. Diabetes and risk of pancreatic cancer: a pooled analysis of three large case-control studies. Cancer Causes Control. 2011;22(2):189–197. doi: 10.1007/s10552-010-9686-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Limburg PJ, Anderson KE, Johnson TW, et al. Diabetes mellitus and subsite-specific colorectal cancer risks in the Iowa women’s health study. Cancer Epidemiol Biomarkers Prev. 2005;14(1):133–137. [PubMed] [Google Scholar]
- 57.Limburg PJ, Vierkant RA, Fredericksen ZS, et al. Clinically confirmed type 2 diabetes mellitus and colorectal cancer risk: a population-based, retrospective cohort study. Am J Gastroenterol. 2006;101(8):1872–1879. doi: 10.1111/j.1572-0241.2006.00725.x. [DOI] [PubMed] [Google Scholar]
- 58.Lindblad P, Chow WH, Chan J, et al. The role of diabetes mellitus in the aetiology of renal cell cancer. Diabetologia. 1999;42(1):107–112. doi: 10.1007/s001250051122. [DOI] [PubMed] [Google Scholar]
- 59.Lindemann K, Vatten LJ, Ellstrom-Engh M, Eskild A. Body mass, diabetes and smoking, and endometrial cancer risk: a follow-up study. Br J Cancer. 2008;98(9):1582–1585. doi: 10.1038/sj.bjc.6604313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lipscombe LL, Goodwin PJ, Zinman B, McLaughlin JR, Hux JE. Diabetes mellitus and breast cancer: a retrospective population-based cohort study. Breast Cancer Res Treat. 2006;98(3):349–356. doi: 10.1007/s10549-006-9172-5. [DOI] [PubMed] [Google Scholar]
- 61.Luo J, Iwasaki M, Inoue M, et al. Body mass index, physical activity and the risk of pancreatic cancer in relation to smoking status and history of diabetes: a large-scale population-based cohort study in Japan–the JPHC study. Cancer Causes Control. 2007;18(6):603–612. doi: 10.1007/s10552-007-9002-z. [DOI] [PubMed] [Google Scholar]
- 62.Mackenzie T, Zens MS, Ferrara A, Schned A, Karagas MR. Diabetes and risk of bladder cancer: evidence from a case-control study in New England. Cancer. 2011;117(7):1552–1556. doi: 10.1002/cncr.25641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mitri J, Castillo J, Pittas AG. Diabetes and risk of Non-Hodgkin’s lymphoma: a meta-analysis of observational studies. Diabetes Care. 2008;31(12):2391–2397. doi: 10.2337/dc08-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ng Y, Husain I, Waterfall N. Diabetes mellitus and bladder cancer–an epidemiological relationship? Pathol Oncol Res. 2003;9(1):30–31. doi: 10.1007/BF03033711. [DOI] [PubMed] [Google Scholar]
- 65.Noto H, Osame K, Sasazuki T, Noda M. Substantially increased risk of cancer in patients with diabetes mellitus: a systematic review and meta-analysis of epidemiologic evidence in Japan. J Diabetes Complications. 2010;24(5):345–353. doi: 10.1016/j.jdiacomp.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 66.Ogunleye AA, Ogston SA, Morris AD, Evans JM. A cohort study of the risk of cancer associated with type 2 diabetes. Br J Cancer. 2009;101(7):1199–1201. doi: 10.1038/sj.bjc.6605240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parazzini F, La Vecchia C, Negri E, et al. Diabetes and endometrial cancer: an Italian case-control study. Int J Cancer. 1999;81(4):539–542. doi: 10.1002/(sici)1097-0215(19990517)81:4<539::aid-ijc6>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 68.Ren HB, Yu T, Liu C, Li YQ. Diabetes mellitus and increased risk of biliary tract cancer: systematic review and meta-analysis. Cancer Causes Control. 2011;22(6):837–847. doi: 10.1007/s10552-011-9754-3. [DOI] [PubMed] [Google Scholar]
- 69.Ren X, Zhang X, Zhang X, et al. Type 2 diabetes mellitus associated with increased risk for colorectal cancer: evidence from an international ecological study and population-based risk analysis in China. Public Health. 2009;123(8):540–544. doi: 10.1016/j.puhe.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 70.Salazar-Martinez E, Lazcano-Ponce EC, Lira-Lira GG, et al. Case-control study of diabetes, obesity, physical activity and risk of endometrial cancer among Mexican women. Cancer Causes Control. 2000;11(8):707–711. doi: 10.1023/a:1008913619107. [DOI] [PubMed] [Google Scholar]
- 71.Saltzman BS, Doherty JA, Hill DA, et al. Diabetes and endometrial cancer: an evaluation of the modifying effects of other known risk factors. Am J Epidemiol. 2008;167(5):607–614. doi: 10.1093/aje/kwm333. [DOI] [PubMed] [Google Scholar]
- 72.Shu X, Ji J, Li X, Sundquist J, Sundquist K, Hemminki K. Cancer risk among patients hospitalized for type 1 diabetes mellitus: a population-based cohort study in Sweden. Diabet Med. 2010;27(7):791–797. doi: 10.1111/j.1464-5491.2010.03011.x. [DOI] [PubMed] [Google Scholar]
- 73.Wang C, Wang X, Gong G, et al. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: A systematic review and meta-analysis of cohort studies. Int J Cancer. 2012;130(7):1639–1648. doi: 10.1002/ijc.26165. [DOI] [PubMed] [Google Scholar]
- 74.Washio M, Mori M, Khan M, et al. Diabetes mellitus and kidney cancer risk: the results of Japan collaborative cohort study for evaluation of cancer risk (JACC study) Int J Urol. 2007;14(5):393–397. doi: 10.1111/j.1442-2042.2007.01744.x. [DOI] [PubMed] [Google Scholar]
- 75.Weiderpass E, Gridley G, Persson I, Nyren O, Ekbom A, Adami HO. Risk of endometrial and breast cancer in patients with diabetes mellitus. Int J Cancer. 1997;71(3):360–363. doi: 10.1002/(sici)1097-0215(19970502)71:3<360::aid-ijc9>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 76.Wu AH, Yu MC, Tseng CC, Stanczyk FZ, Pike MC. Diabetes and risk of breast cancer in Asian-American women. Carcinogenesis. 2007;28(7):1561–1566. doi: 10.1093/carcin/bgm081. [DOI] [PubMed] [Google Scholar]
- 77.Yang YX, Hennessy S, Lewis JD. Type 2 diabetes mellitus and the risk of colorectal cancer. Clin Gastroenterol Hepatol. 2005;3(6):587–594. doi: 10.1016/s1542-3565(05)00152-7. [DOI] [PubMed] [Google Scholar]
- 78.Pannala R, Basu A, Petersen GM, Chari ST. New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol. 2009;10(1):88–95. doi: 10.1016/S1470-2045(08)70337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Polednak AP. Prevalence and predictors of comorbid diabetes among newly diagnosed Hispanic cancer patients in Connecticut. Cancer Detect Prev. 2007;31(6):453–456. doi: 10.1016/j.cdp.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 80.Silverman DT, Schiffman M, Everhart J, et al. Diabetes mellitus, other medical conditions and familial history of cancer as risk factors for pancreatic cancer. Br J Cancer. 1999;80(11):1830–1837. doi: 10.1038/sj.bjc.6690607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Swerdlow AJ, Laing SP, Qiao Z, et al. Cancer incidence and mortality in patients with insulin-treated diabetes: a UK cohort study. Br J Cancer. 2005;92(11):2070–2075. doi: 10.1038/sj.bjc.6602611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wolf I, Sadetzki S, Catane R, Karasik A, Kaufman B. Diabetes mellitus and breast cancer. Lancet Oncol. 2005;6(2):103–111. doi: 10.1016/S1470-2045(05)01736-5. [DOI] [PubMed] [Google Scholar]
- 83.Bonovas S, Filioussi K, Tsantes A. Diabetes mellitus and risk of prostate cancer: a meta-analysis. Diabetologia. 2004;47(6):1071–1078. doi: 10.1007/s00125-004-1415-6. [DOI] [PubMed] [Google Scholar]
- 84.Kasper JS, Giovannucci E. A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2056–2062. doi: 10.1158/1055-9965.EPI-06-0410. [DOI] [PubMed] [Google Scholar]
- 85.Haffner SM, Shaten J, Stem MP, Smith GD, Kuller L. Low levels of sex hormone-binding globulin and testosterone predict the development of non-insulin-dependent diabetes mellitus in men. MRFIT Research Group. Multiple risk factor intervention trial. Am J Epidemiol. 1996;143(9):889–897. doi: 10.1093/oxfordjournals.aje.a008832. [DOI] [PubMed] [Google Scholar]
- 86.Gann PH, Hennekens CH, Ma J, Longcope C, Stampfer MJ. Prospective study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst. 1996;88(16):1118–1126. doi: 10.1093/jnci/88.16.1118. [DOI] [PubMed] [Google Scholar]
- 87.Oxman AD, Guyatt GH. Validation of an index of the quality of review articles. J Clin Epidemiol. 1991;44(11):1271–1278. doi: 10.1016/0895-4356(91)90160-b. [DOI] [PubMed] [Google Scholar]
- 88.Centers for Disease Control and Prevention National Program of Cancer Registries 2011, United States Cancer Statistics. Cancer types grouped by race and ethnicity. 2007 Available at: http://www.cdc.gov/cancer/prostate/statistics/race.htm.
- 89.Cannata D, Fierz Y, Vijayakumar A, LeRoith D. Type 2 diabetes and cancer: what is the connection? Mt Sinai J Med. 2010;77(2):197–213. doi: 10.1002/msj.20167. [DOI] [PubMed] [Google Scholar]
- 90.Michels KB, Solomon CG, Hu FB, et al. Type 2 diabetes and subsequent incidence of breast cancer in the nurses’ health study. Diabetes Care. 2003;26(6):1752–1758. doi: 10.2337/diacare.26.6.1752. [DOI] [PubMed] [Google Scholar]
- 91.Johnson JA, Bowker SL, Richardson K, Marra CA. Time-varying incidence of cancer after the onset of type 2 diabetes: evidence of potential detection bias. Diabetologia. 2011;54(9):2263–2271. doi: 10.1007/s00125-011-2242-1. [DOI] [PubMed] [Google Scholar]
- 92.Baradaran N, Ahmadi H, Salem S, et al. The protective effect of diabetes mellitus against prostate cancer: role of sex hormones. Prostate. 2009;69(16):1744–1750. doi: 10.1002/pros.21023. [DOI] [PubMed] [Google Scholar]
- 93.Pierce BL, Plymate S, Ostrander EA, Stanford JL. Diabetes mellitus and prostate cancer risk. Prostate. 2008;68(10):1126–1132. doi: 10.1002/pros.20777. [DOI] [PubMed] [Google Scholar]
- 94.Tavani A, Gallus S, Bosetti C, et al. Diabetes and the risk of prostate cancer. Eur J Cancer Prev. 2002;11(2):125–128. doi: 10.1097/00008469-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 95.Turner EL, Lane JA, Donovan JL, et al. Association of diabetes mellitus with prostate cancer: nested case-control study (Prostate testing for cancer and treatment study) Int J Cancer. 2011;128(2):440–446. doi: 10.1002/ijc.25360. [DOI] [PubMed] [Google Scholar]
- 96.Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366(9493):1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 97.Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 98.Home PD, Pocock SJ, Beck-Nielsen H, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373(9681):2125–2135. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- 99.Johnson JA, Bowker SL. Intensive glycaemic control and cancer risk in type 2 diabetes: a meta-analysis of major trials. Diabetologia. 2011;54(1):25–31. doi: 10.1007/s00125-010-1933-3. [DOI] [PubMed] [Google Scholar]
- 100.Tsugawa K, Koyanagi N, Hashizume M, et al. Therapeutic strategy of emergency surgery for colon cancer in 71 patients over 70 years of age in Japan. Hepatogastroenterology. 2002;49(44):393–398. [PubMed] [Google Scholar]
- 101.Koperna T, Kisser M, Schulz F. Emergency surgery for colon cancer in the aged. Arch Surg. 1997;132(9):1032–1037. doi: 10.1001/archsurg.1997.01430330098018. [DOI] [PubMed] [Google Scholar]
- 102.Little SA, Jarnagin WR, DeMatteo RP, Blumgart LH, Fong Y. Diabetes is associated with increased perioperative mortality but equivalent long-term outcome after hepatic resection for colorectal cancer. J Gastrointest Surg. 2002;6(1):88–94. doi: 10.1016/s1091-255x(01)00019-1. [DOI] [PubMed] [Google Scholar]
- 103.Davila JA, Rabeneck L, Berger DH, El-Serag HB. Postoperative 30-day mortality following surgical resection for colorectal cancer in veterans: changes in the right direction. Dig Dis Sci. 2005;50(9):1722–1728. doi: 10.1007/s10620-005-2925-x. [DOI] [PubMed] [Google Scholar]
- 104.Jullumstro E, Kollind M, Lydersen S, Edna TH. Diabetes mellitus and outcomes of colorectal cancer. Acta Oncol. 2009;48(3):361–367. doi: 10.1080/02841860802637765. [DOI] [PubMed] [Google Scholar]
- 105.Abunasra H, Lewis S, Beggs L, Duffy J, Beggs D, Morgan E. Predictors of operative death after oesophagectomy for carcinoma. Br J Surg. 2005;92(8):1029–1033. doi: 10.1002/bjs.5049. [DOI] [PubMed] [Google Scholar]
- 106.Bartels H, Stein HJ, Siewert JR. Preoperative risk analysis and postoperative mortality of oesophagectomy for resectable oesophageal cancer. Br J Surg. 1998;85(6):840–844. doi: 10.1046/j.1365-2168.1998.00663.x. [DOI] [PubMed] [Google Scholar]
- 107.Karl RC, Schreiber R, Boulware D, Baker S, Coppola D. Factors affecting morbidity, mortality, and survival in patients undergoing Ivor Lewis esophagogastrectomy. Ann Surg. 2000;231(5):635–643. doi: 10.1097/00000658-200005000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wright CD, Kucharczuk JC, O’Brien SM, Grab JD, Allen MS. Predictors of major morbidity and mortality after esophagectomy for esophageal cancer: a society of thoracic surgeons general thoracic surgery database risk adjustment model. J Thorac Cardiovasc Surg. 2009;137(3):587–596. doi: 10.1016/j.jtcvs.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 109.Zhang GH, Fujita H, Yamana H, Kakegawa T. A prediction of hospital mortality after surgical treatment for esophageal cancer. Surg Today. 1994;24(2):122–127. doi: 10.1007/BF02473392. [DOI] [PubMed] [Google Scholar]
- 110.Chu CK, Mazo AE, Sarmiento JM, et al. Impact of diabetes mellitus on perioperative outcomes after resection for pancreatic adenocarcinoma. J Am Coll Surg. 2010;210(4):463–473. doi: 10.1016/j.jamcollsurg.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 111.Duque JL, Ramos G, Castrodeza J, et al. Early complications in surgical treatment of lung cancer: a prospective, multicenter study. Grupo Cooperativo de Carcinoma Broncogénico de la Sociedad Española de Neumología y Cirugía Torácica. Ann Thorac Surg. 1997;63(4):944–950. doi: 10.1016/s0003-4975(97)00051-9. [DOI] [PubMed] [Google Scholar]
- 112.Au J, el-Oakley R, Cameron EW. Pneumonectomy for bronchogenic carcinoma in the elderly. Eur J Cardiothorac Surg. 1994;8(5):247–250. doi: 10.1016/1010-7940(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 113.Dominguez-Ventura A, Allen MS, Cassivi SD, Nichols FC, III, Deschamps C, Pairolero PC. Lung cancer in octogenarians: factors affecting morbidity and mortality after pulmonary resection. Ann Thorac Surg. 2006;82(4):1175–1179. doi: 10.1016/j.athoracsur.2006.04.052. [DOI] [PubMed] [Google Scholar]
- 114.Romano PS, Mark DH. Patient and hospital characteristics related to in-hospital mortality after lung cancer resection. Chest. 1992;101(5):1332–1337. doi: 10.1378/chest.101.5.1332. [DOI] [PubMed] [Google Scholar]
- 115.Barone BB, Yeh HC, Snyder CF, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008;300(23):2754–2764. doi: 10.1001/jama.2008.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Peairs KS, Barone BB, Snyder CF, et al. Diabetes mellitus and breast cancer outcomes: a systematic review and meta-analysis. J Clin Oncol. 2011;29(1):40–46. doi: 10.1200/JCO.2009.27.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Snyder CF, Stein KB, Barone BB, et al. Does pre-existing diabetes affect prostate cancer prognosis? A systematic review. Prostate Cancer Prostatic Dis. 2010;13(1):58–64. doi: 10.1038/pcan.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lam EK, Batty GD, Huxley RR, et al. Associations of diabetes mellitus with site-specific cancer mortality in the Asia-Pacific region. Ann Oncol. 2011;22(3):730–738. doi: 10.1093/annonc/mdq405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Landman GW, Ubink-Veltmaat LJ, Kleefstra N, Kollen BJ, Bilo HJ. Increased cancer mortality in type 2 diabetes (ZODIAC-3) Anticancer Res. 2008;28(2B):1373–1375. [PubMed] [Google Scholar]
- 120.Noto H, Tsujimoto T, Sasazuki T, et al. Significantly increased risk of cancer in patients with diabetes mellitus: a systematic review and meta-analysis. Endocr Pract. 2011;17(4):616–628. doi: 10.4158/EP10357.RA. [DOI] [PubMed] [Google Scholar]
- 121.Zhou XH, Qiao Q, Zethelius B, et al. Diabetes, prediabetes and cancer mortality. Diabetologia. 2010;53(9):1867–1876. doi: 10.1007/s00125-010-1796-7. [DOI] [PubMed] [Google Scholar]
- 122.Seshasai SR, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UPDOS 33) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 124.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):854–865. [PubMed] [Google Scholar]
- 125.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 126.Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jiralerspong S, Palla SL, Giordano SH, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol. 2009;27(20):3297–3302. doi: 10.1200/JCO.2009.19.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Martin JH, Coory MD, Valery PC, Green AC. Association of diabetes with survival among cohorts of Indigenous and non-Indigenous Australians with cancer. Cancer Causes Control. 2009;20(3):355–360. doi: 10.1007/s10552-008-9249-z. [DOI] [PubMed] [Google Scholar]
- 129.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33(7):1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.American Cancer Society. Guidelines for the Early Detection of Cancer. 2011 Available at: http://www.cancer.org/Healthy/FindCancerEarly/CancerScreeningGuidelines/american-cancer-society-guidelines-for-the-early-detection-of-cancer.
- 131.Beckman TJ, Cuddihy RM, Scheitel SM, Naessens JM, Killian JM, Pankratz VS. Screening mammogram utilization in women with diabetes. Diabetes Care. 2001;24(12):2049–2053. doi: 10.2337/diacare.24.12.2049. [DOI] [PubMed] [Google Scholar]
- 132.Marshall JG, Cowell JM, Campbell ES, McNaughton DB. Regional variations in cancer screening rates found in women with diabetes. Nurs Res. 2010;59(1):34–41. doi: 10.1097/NNR.0b013e3181c3bd07. [DOI] [PubMed] [Google Scholar]
- 133.Zhao G, Ford ES, Ahluwalia IB, Li C, Mokdad AH. Prevalence and trends of receipt of cancer screenings among US women with diagnosed diabetes. J Gen Intern Med. 2009;24(2):270–275. doi: 10.1007/s11606-008-0858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ives DG, Lave JR, Traven ND, Schulz R, Kuller LH. Mammography and pap smear use by older rural women. Public Health Rep. 1996;111(3):244–250. [PMC free article] [PubMed] [Google Scholar]
- 135.McBean AM, Yu X. The underuse of screening services among elderly women with diabetes. Diabetes Care. 2007;30(6):1466–1472. doi: 10.2337/dc06-2233. [DOI] [PubMed] [Google Scholar]
- 136.Karathanasi I, Kamposioras K, Cortinovis I, et al. Moving ahead in diabetics’ cancer screening; food for thought from the Hellenic experience. Eur J Cancer Care (Engl) 2009;18(3):255–263. doi: 10.1111/j.1365-2354.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- 137.Jimenez-Garcia R, Hernandez-Barrera V, Carrasco-Garrido P, Gil A. Prevalence and predictors of breast and cervical cancer screening among Spanish women with diabetes. Diabetes Care. 2009;32(8):1470–1472. doi: 10.2337/dc09-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lipscombe LL, Hux JE, Booth GL. Reduced screening mammography among women with diabetes. Arch Intern Med. 2005;165(18):2090–2095. doi: 10.1001/archinte.165.18.2090. [DOI] [PubMed] [Google Scholar]
- 139.Bell RA, Shelton BJ, Paskett ED. Colorectal cancer screening in North Carolina: associations with diabetes mellitus and demographic and health characteristics. Prev Med. 2001;32(2):163–167. doi: 10.1006/pmed.2000.0785. [DOI] [PubMed] [Google Scholar]
- 140.Owens MD, Beckles GL, Ho KK, Gorrell P, Brady J, Kaftarian JS. Women with diagnosed diabetes across the life stages: underuse of recommended preventive care services. J Womens Health (Larchmt) 2008;17(9):1415–1423. doi: 10.1089/jwh.2008.1125. [DOI] [PubMed] [Google Scholar]