Abstract
Objective To systematically review the evidence (and quality) for distraction and hypnosis for needle-related pain and distress in children and adolescents. To explore the effects of distraction characteristics (e.g., adult involvement, type of distracter), child age, and study risk of bias on treatment efficacy. Methods 26 distraction and 7 hypnosis trials were included and self-report, observer-report, and behavioral pain intensity and distress examined. Distraction studies were coded for 4 intervention characteristics, and all studies coded for child age and study risk of bias. Results Findings showed strong support for distraction and hypnosis for reducing pain and distress from needle procedures. The quality of available evidence was low, however. Characteristics of distraction interventions, child age, and study risk of bias showed some influence on treatment efficacy. Conclusions Distraction and hypnosis are efficacious in reducing needle-related pain and distress in children. The quality of trials in this area needs to be improved.
Keywords: distraction, distress, empirically supported treatments, hypnosis, needles, pain, randomized controlled trials
Introduction
Despite being a very common experience for both healthy and medically ill children (American Academy of Pediatrics, 2013; Stevens et al., 2011), needle procedures are highly feared by many children (Hart & Bossert, 1994; Taddio et al., 2012) and are often accompanied by significant pain and distress for children and parents alike. Effective management of pain and distress during needle procedures is critical for children, as poorly managed experiences can lead to increased pain and distress at subsequent needle procedures, the development of needle phobia, and avoidance of preventative, diagnostic, or necessary medical intervention (Chen, Zeltzer, Craske, & Katz, 2000; Taddio et al., 2010, 2012).
In addition to pharmacological and physiological strategies, psychological interventions are a vital part of a three-pronged approach for managing needle procedures, such as childhood vaccinations (Taddio et al., 2010). An earlier narrative review published in the Journal of Pediatric Psychology supported cognitive behavioral therapy as a “well-established treatment” for procedure-related pain in children (Powers, 1999); however, an abundance of new research has been published and reviewed since that time. This includes several iterations of a Cochrane systematic review and meta-analysis examining the effects of various psychological interventions for managing needle-related pain and distress in children aged 2–19 years (Uman, Chambers, McGrath, & Kisely, 2006, 2008). The most recent Cochrane Review, completed in 2013, revealed strong support for the efficacy of distraction and hypnosis for reducing pain and/or distress during needle procedures (Uman et al., 2013). Interestingly, these were also the two interventions with the greatest number of trials to date. At that time, limited or no evidence was available to support the use of other psychological interventions, such as providing procedural information/preparation to the child, combined cognitive-behavioral techniques, or suggestions to the child that an intervention would reduce pain or distress.
Despite the growing body of evidence examining distraction and hypnosis for needle-related pain and distress, several areas in need of additional inquiry were outlined in the recent Cochrane Review to effectively guide future research and clinical practice. Specifically, the review noted the wide variability in types of distraction-based interventions, the potential influence of child age on intervention efficacy, and potential relationships between risk of bias in study design and estimates of treatment efficacy (Uman et al., 2013).
Type of Distraction Intervention
To date, distraction has been the most extensively studied and empirically supported psychological intervention for needle-related pain and distress in children. Indeed, 19 of 39 studies included in the most recent Cochrane Review, and 12 of 18 new studies identified since the previous Cochrane Review, tested the effects of distraction alone (Uman et al., 2013), suggesting an increase in the number of trials over time. Studies employing distraction use a heterogeneous assortment of strategies with little to no empirical evidence examining whether differences between distraction interventions influence their efficacy. The Cochrane Review argued that the field will benefit most from studies directly comparing the efficacy of different types of distraction techniques (e.g., Bellieni et al., 2006). However, with the overall efficacy of distraction firmly established (Uman et al., 2013), additional analyses identifying the characteristics of effective distraction interventions are warranted.
Although few studies assess the fidelity with which a distraction intervention is delivered (McCarthy et al., 2010) or the degree of actual child engagement with the distracter (MacLaren & Cohen, 2005), the effective element of distraction is believed to be its ability to capture the child’s cognitive resources, in addition to other possible physiological and behavioral mechanisms, making it difficult to attend to the needle procedure (DeMore & Cohen, 2005). Researchers have distinguished “active/interactive” from “passive” distraction, with the former designed to engage more of the child’s senses and require manipulation of the environment and/or problem-solving (e.g. playing a video game versus watching television; Dahlquist et al., 2007; Koller & Goldman, 2012). Although interactive forms of distraction have been hypothesized to more effectively distract children through heightened engagement, previous studies for acute procedural and experimental pain and distress have shown mixed results (Dahlquist et al., 2010; Klassen, Liang, Tjosvold, Klassen, & Hartling, 2008; MacLaren & Cohen, 2005; Nilsson, Finnstrom, & Kokinsky, 2008). Use of novel high-tech devices, such as virtual reality helmets or robots, is one way in which researchers have strived to capture and maintain children’s attention during needle procedures (Beran, Ramirez-Serrano, Vanderkooi, & Kuhn, 2013; Gold, Kim, Kant, Joseph, & Rizzo, 2006); however, these devices may be cost prohibitive in some contexts and it is unclear whether the additional costs are justified by increased treatment efficacy.
It may also be relevant to consider the degree of adult involvement in the distraction intervention (i.e., parent or nurse). Some children may be more effectively able to distract themselves (e.g., children who are older or less distressed; Chambers, Taddio, Uman, & McMurtry, 2009) and parental presence alone has been shown to influence children’s pain experiences (Vervoort et al., 2008). For example, children who were distracted by television while undergoing a venipuncture reported less pain than those distracted by their mothers (Bellieni et al., 2006). Additionally, the impact of child choice is also deserving of consideration (DeMore & Cohen, 2005; Koller & Goldman, 2012; Sander Wint, Eshelman, Steele, & Guzzetta, 2002), as studies have noted differences in children’s preferences of distracters, which may differentially maximize treatment efficacy during a given procedure (Sinha, Christopher, Fenn, & Reeves, 2006; Windich-Biermeier, Sjoberg, Dale, Eshelman, & Guzzetta, 2007).
Age Effects
A limitation of the research to date is that intervention efficacy has not been examined by age (Uman et al., 2013). There is ample justification for the consideration of child age in the efficacy of psychological interventions for procedural pain given the significant developmental changes that occur throughout early childhood to late adolescence. Although not synonymous, age is the easiest and most commonly applied proxy for child development. Previous research has highlighted the relevance of development to pain experiences, with younger children reporting greater pain intensity and unpleasantness following a venipuncture, irrespective of the treatment received (Goodenough et al., 1997, 1999). Consideration of age-groups in pediatric randomized controlled trials has been advocated for by Standards for Research (StaR) in Child Health, an international initiative geared to enhance reliability and relevance of clinical trials in children (Williams et al., 2012).
While the recent Cochrane Review showed strong efficacy of hypnosis and distraction for needle-related procedural pain and distress in children aged 2–19 years as a group (Uman et al., 2013), closer analyses may reveal a differential impact for children of various ages when considered separately (Kleiber & Harper, 1999; Richardson, Smith, McCall, & Pilkington, 2006). Indeed, several trials from the recent Cochrane Review reported a significant impact of age on intervention efficacy overall (e.g., Kuttner, 1988; Sinha et al., 2006) or for specific outcomes of distress and not pain (Fanurik, Koh, & Schmitz, 2000), although others trials did not (e.g., Tak & van Bon, 2006). This suggests the importance of a meta-analytic approach for examining age effects for outcomes of pain and distress following intervention for needle procedures to provide clarity about the nature of these relationships. Psychological interventions differ in their use of cognitive and/or behavioral techniques and directive support given to children by parents or other adults. Given that younger children tend to use more behavioral coping strategies and rely more heavily on parents, whereas older children and adolescents use more cognitive strategies and are more independent (Skinner & Zimmer-Gembeck, 2007), differential efficacy may be seen across development for interventions that rely more or less on each of these approaches. A better understanding of potential age effects on treatment efficacy can be used to most effectively match children with interventions most likely to be beneficial for them (Uman et al., 2010).
Quality of Trials and Risk of Bias
The importance of high-quality trials has been increasingly recognized through the development of guidelines directing study design and reporting (e.g., CONSORT statement; Altman et al., 2001) and plays a role in the assessed quality of evidence when combined across studies (e.g., Grading of Recommendations Assessment, Development, and Evaluation [GRADE]; Guyatt et al., 2011). A previous review judged the majority of trials examining psychological interventions for needle-related pain and distress for children to be of poor to low quality based on information available in publications, with some improvement in trials conducted more recently (Uman et al., 2010). The Cochrane Collaboration recently developed a risk of bias tool for randomized trials, judging risk of bias in areas of selection, performance, detection, attrition, reporting, and other (Higgins et al., 2011). Despite the suggested improvement in trial quality, the recent Cochrane Review revealed that all studies examining psychological interventions for needle-related pain and distress showed the majority of bias domains at high or unclear risk (Uman et al., 2013). Assessment of risk of bias in study design and reporting is critical, as trials with unclear or high risk of bias in one or more of these domains have been associated with exaggerated treatment effects (Hartling et al., 2012; Savovic et al., 2012). Consideration of risk of bias in study design is important for assessing the validity of evidence supporting efficacy of various psychological interventions for needle-related pain and distress in children and adolescents.
Aims of the Review
The current systematic review provides an in-depth examination of the evidence for distraction and hypnosis as psychological interventions for needle-related procedural pain and distress in children and adolescents. It guides application of existing evidence to future research and clinical practice by (1) reviewing the evidence for the use of distraction and hypnosis overall; (2) investigating the influence of characteristics of distraction interventions on treatment efficacy (i.e., adult versus no adult involvement, no/low- versus high-technology distracter[s], interactive versus passive, child choice versus no child choice of distracter[s]); (3) assessing the potential impact of child age on treatment efficacy for distraction and hypnosis; and (4) assessing the impact of assessed risk of bias in study design on treatment efficacy for distraction and hypnosis.
Methods
A more detailed description of study selection and identification, data collection, synthesis of results, and analyses is available in the recently published Cochrane Review (Uman et al., 2013).
Study Eligibility Criteria
Consistent with the recent Cochrane Review (Uman et al., 2013), eligible studies were (1) randomized controlled trials with at least five participants per study arm; (2) included children aged 2–19 years of age undergoing a needle-related procedure; (3) published in a peer-reviewed journal; (4) included at least one study arm examining a psychological intervention with a comparator control arm; and (5) assessed pain and/or distress (i.e., anxiety, stress, or fear) using a reliable and valid measure during or following the needle procedure. Study exclusion criteria were (1) quasi-randomization procedures or lack of true random assignment; (2) inclusion of children with diagnosed needle phobias; (3) needle procedures during or following surgery; or (4) unavailability of data necessary for pooling (e.g., means, standard deviations, corresponding sample cell sizes) in a publication, after attempts to contact study authors, or necessary data could not be calculated from other data reported in publication.
Given the focus of the current review, additional inclusion criteria were (1) Randomized controlled trials (RCTs) examining the effects of hypnosis or any type of distraction (defined in the same manner Uman et al., 2006) alone or in combination with one other psychological intervention; and (2) published in English. Consistent with the Cochrane Review (Uman et al., 2006), hypnosis was defined as dissociation from painful experience and distress via hypnotic induction, suggestions, and imagined fantasy, similar to but more involved than imagery. Given the overlap between imagery and hypnosis we relied on author definitions to distinguish between the two. Distraction included either cognitive techniques, designed to shift attention away from procedure-related pain or specific counter activities (e.g., counting, listening to music, non-procedure-related talk) or behavioral techniques, designed to shift attention away from procedure-related pain or specific counter activities (e.g., videotapes, games, interactive books).
Information Sources
The following six electronic databases were searched for relevant studies: Cochrane Central Register of Controlled Trials (CENTRAL), Medline, PsycINFO, Excerpta Medica dataBASE (EMBASE), Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Web of Science (ISI Web of Knowledge). Database searches were conducted on four occasions (February 2005, December 2010, March 2012, and March 2013) for previous Cochrane Reviews (Uman et al., 2006, 2013) and include from inception of respective databases up to March 20, 2013. Posts were also made in 2005 and 2012 to relevant listservs for any additional studies, including Pain in Child Health, Pediatric Pain, American Psychological Association's (APA) Society of Pediatric Psychology Division 54, and APA’s Health Psychology Division 38.
Search
Search strategies were developed in consultation with a reference librarian and the Cochrane Pain, Palliative and Supportive Care Group. Complete database search strategies for CENTRAL, Medline, PsycINFO, EMBASE, CINAHL, and IBI Web of Knowledge are available in the Cochrane Review (Uman et al., 2013). For example, MeSH terms for needles and pain, hypnosis, age-related MeSH terms as well distress and forms of distraction, and various common needle procedures were included.
Study Selection
Two review authors independently screened all titles and abstracts from database searches and those identified from listservs for study eligibility. Full articles were obtained and reviewed for all abstracts identified as relevant or potentially relevant to confirm study eligibility. Reasons for exclusions were documented for all full articles that were obtained. Any discrepancies about study selection were resolved by a third review author.
Data Collection Process
Data collection was performed using forms developed by review authors. For each included study, review authors recorded information about study design, participant demographics and diagnoses (where applicable), type of needle procedure, details about intervention and control arms, measures and data for relevant outcome variables, study setting and location, and other pertinent details for describing studies. Extracted means, standard deviations, and sample sizes for relevant outcome variables were double checked by a trained research assistant.
Several attempts were made to contact study authors via email when data necessary for pooling was not available in publications and/or when greater detail was needed from study authors to classify studies for planned subanalyses (i.e., additional information about distraction intervention). If authors did not respond, attempts were made to calculate missing data using other measures of variation reported in publications (e.g., calculating standard deviations from standard errors, confidence intervals, etc.) as per recommended statistical formulae (Cochrane Handbook, 2011). When authors could not be contacted, did not respond, or when data were not available to calculate outcomes, studies or individual outcomes with missing data were excluded from the review.
Data Items
The following outcome variables were collected and considered for meta-analysis:
Pain Intensity
Child or Adolescent Self-Report. Could include variations of visual analogue scales (VAS), numeric rating scales (NRS), verbal rating scales (VRS), or faces scales (e.g., Faces Pain Scale-Revised; Hicks, von Baeyer, Spafford, van Korlaar, & Goodenough, 2001). No scale is considered appropriate for uniform use across development (Stinson, Kavanagh, Yamada, Gill, & Stevens, 2006; Tomlinson, von Baeyer, Stinson, & Sung, 2010) and, as such, variations in which self-report measures were used was expected.
Observer Report. Observer report could include any of the self-report measures above as completed by a parent, caregiver, nurse, physician, or other health professional that was present. Observer report is less desirable than self-report as various factors influence observers’ estimates of child pain, often leading to over- or underestimation as compared with child self-report (Chambers, Corkum, & Rusak, 2008; Goubert, Vervoort, Cano, & Crombez, 2009); however, they are an important source of information, particularly for younger children or when self-report is not possible.
Behavioral Rating Scale. Could include any behavioral measure of pain, typically completed by trained researchers or health professionals (e.g., the Children’s Hospital of Eastern Ontario Pain Scales; McGrath et al., 1985, or the Faces Legs Activity Cry Consolability Scale; Merkel, Voepel-Lewis, Shayevitz, & Malviya, 1997).
Distress
Child or Adolescent Self-Report. Could include variations of VAS, NRS, VRS, or faces scales used to assess distress (i.e., fear and/or anxiety).
Observer Report. Observer report could include any of the self-report measures above as completed by a parent, caregiver, nurse, physician, or other health professional that was present.
Behavioral Rating Scale. Could include any behavioral measures of distress, typically completed by trained researchers or health professionals (e.g., the Observational Scale of Behavioral Distress; Jay, Ozolins, & Elliott, 1983, or variation of the Child-Adult Medical Procedure Interaction Scale; Blount et al., 1989; Blount, Sturges, & Powers, 1990; Blount, Bunke, Cohen, & Forbes, 2001).
Risk of Bias Within and Across Studies
Risk of bias was assessed for all included studies using the Cochrane Risk of Bias tool (Higgins et al., 2011). This tool assesses risk of bias in seven areas, including adequate sequence generation, allocation concealment, blinding of participants and study personnel, blinding of outcome assessment, incomplete outcome data addressed, free of selective reporting, and other bias. Blinding of outcome assessment, incomplete outcome data addressed, and free of selective reporting were only considered for relevant outcomes of pain and distress included in this review. Ratings for all studies were made independently by two review authors with any discrepancies resolved through consensus.
Summary Measures
Standardized mean differences (SMDs) using a random-effects model and 95% confidence intervals were calculated using RevMan 5.2 software. This approach was taken owing to the differing measures used across studies to assess outcomes of pain and distress and the continuous nature of all outcome variables, as well as the considerable methodological heterogeneity across studies in intervention, sample characteristics, and needle procedures (Cochrane Handbook, 2011; Guyatt et al., 2013). A negative SMD with both confidence intervals falling in the negative range reveals a significant effect in favor of the intervention. Analyses were conducted separately for each outcome variable for both distraction and hypnosis interventions. A minimum of two studies per comparison group was considered necessary for conducting planned meta-analyses.
Quality of Evidence
The quality of evidence for distraction and hypnosis was rated using the GRADE system as applied to continuous outcomes (Guyatt et al., 2011, 2013). GRADE ratings were assessed separately for all outcomes with data from more than one study for both distraction and hypnosis. As per GRADE recommendations (Cochrane Handbook, 2011; Guyatt et al., 2011), the quality of evidence is considered in five areas: limitations in the design and implementation, indirectness of evidence, unexplained heterogeneity or inconsistency of results, imprecision of results, and high probability of publication bias. Given that all included studies were randomized controlled trials, the quality of evidence began as high and was downgraded given available information relevant to each of those five areas. Criteria for downgrading evidence in each area was established a priori by three review authors based on GRADE recommendations (Cochrane Handbook, 2011; Guyatt et al., 2011) and applied through consensus of four review authors (see footnotes in Tables I and II). Data were analyzed using RevMan 5.2 and GRADEprofiler software.
Table I.
Study Characteristics
| Study, year | Sample size (n) | Age range | Needle procedure | Included intervention(s) | Included pain intensity and distress outcomes |
|---|---|---|---|---|---|
| Distraction | |||||
| Balan, 2009 | nt = 50; nc = 50 | 5–12 years | Venepuncture | Music via headphones | Self-reported pain |
| Observer-reported pain | |||||
| Bellieni, 2006 | nt = 46; nc = 23 | 7–12 years | Venepuncture |
|
Self-reported pain |
| Observer-reported pain | |||||
| Blount, 1992 | nt = 30; nc = 30 | 3–7 years | Immunization | Distraction + coping skills training (parent) | Behavioral measure of distress |
| Caprilli, 2007 | nt = 54; nc = 54 | 4–13 years | Venepuncture | Live musicians | Self-reported pain |
| Behavioral measure of distress | |||||
| Cassidy, 2002 | nt = 29; nc = 33 | 5-year olds | Immunization (DPTP) | TV musical cartoon | Self-reported pain |
| Behavioral measure of pain | |||||
| Cavendar, 2004 | nt = 20; nc = 23 | 4–11 years | Venepuncture or IV insertion | Distraction + parent positioning | Self-reported pain |
| Self-reported distress | |||||
| Observer-reported distress | |||||
| Behavioral measure of distress | |||||
| Fanurik, 2000 | nt = 80; nc = 80 | 2–16 years | IV insertion | Age appropriate distracters (e.g., bubbles, books, music) | Self-reported pain |
| Fowler-Kerry, 1987 | nt = 120; nc = 80 | 4.5–6.5 years | Immunization |
|
Self-reported pain |
| Gold, 2006 | nt = 10; nc = 10 | 8–12 years | IV insertion | Virtual reality | Self-reported pain |
| Gonzalez, 1993 | nt = 14; nc = 14 | 3–7 years | Immunization | Mother-led verbal distraction | Self-reported pain |
| Behavioral measure of distress | |||||
| Gupta, 2006 | nt = 25; nc = 25 | 6–12 years | Venepuncture | Child squeezing rubber ball | Self-reported pain |
| Inal, 2012 | nt = 61; nc = 62 | 6–12 years | Venepuncture | “Flippits®” distraction cards | Self-reported pain |
| Observer-reported pain | |||||
| Observer-reported distress | |||||
| Jeffs, 2007 | nt = 19; nc = 8 | 11–17 years | Allergy testing (injection) |
|
Self-reported pain |
| Kleiber, 2001 | nt = 22; nc = 22 | 4–7 years | IV insertion | Parent coaching + distraction | Self-reported pain |
| Observer-reported distress | |||||
| Behavioral measure of distress | |||||
| Kristjansdottir, 2011 | nt = 78; nc = 39 | 13–15 years | Immunization (polio) |
|
Self-reported pain |
| Kuttner, 1988 | nt = 17; nc = 8 | 3–6 years | Bone marrow aspiration | Books, bubbles, toys | Behavioral measure of distress |
| McCarthy, 2010 | nt = 249; nc = 293 | 4–10 years | IV insertion | Parent distraction coaching training (information + distracters) | Self-reported pain |
| Observer-reported distress | |||||
| Behavioral measure of distress | |||||
| Nguyen, 2010 | nt = 20; nc = 20 | 7–12 years | Lumbar puncture | Music via headphones | Self-reported pain |
| Self-reported distress | |||||
| Noguchi, 2006 | nt = 42; nc = 20 | 4–6 years | Immunization |
|
Self-reported pain |
| Observer-reported pain | |||||
| Behavioral measure of distress | |||||
| Press, 2003 | nt = 48; nc = 46 | 6–16 years | Venepuncture | Music via headphones and asked a question | Self-reported pain |
| Observer-reported pain | |||||
| Sander Wint, 2002 | nt = 17; nc = 13 | 10–19 years | Lumbar puncture | Virtual reality distraction | Self-reported pain |
| Sinha, 2006 | nt = 120; nc = 120 | 6–18 years | Sutures (laceration repair) | Music, video, games, cartoon (or books, bubbles) | Self-reported pain |
| Self-reported distress | |||||
| Observer-reported distress | |||||
| Tak, 2006 | nt = 20; nc = 20 | 3–12 years | Venepuncture |
|
Self-reported pain |
| Behavioral measure of distress | |||||
| Vessey, 1994 | nt = 50; nc = 50 | 3–12 years | Venepuuncture | Visual distraction (kaleidoscope) | Self-reported pain |
| Behavioral measure of pain | |||||
| Wang, 2008 | nt = 100; nc = 100 | 8–9 years | Venepuncture | Cartoon videos | Self-reported pain |
| Windich-Biermeier, 2007 | nt = 22; nc = 28 | 5–18 years | Venepuncture or Venous port access | Parent coaching + distraction (book, bubbles, video game, virtual reality) | Self-reported pain |
| Self-reported distress | |||||
| Hypnosis | |||||
| Huet, 2011 | nt = 14; nc = 15 | 5–12 years | Local dental anesthetic | Hypnosis (three-step Ericksonian procedure via hypnotherapist) | Behavioral measure of pain |
| Katz, 1987 | nt = 18; nc = 18 | 6–11 years | Bone marrow aspiration | Training in hypnosis and self-hypnosis from psychologist | Self-reported pain |
| Self-reported distress | |||||
| Behavioral measure of distress | |||||
| Kuttner, 1988 | nt = 17; nc = 8 | 3–6 years | Bone marrow aspiration | Therapist taught hypnotic suggestion using the child’s favorite story | Behavioral measure of distress |
| Liossi, 1999 | nt = 20; nc = 10 | 5–15 years | Bone marrow aspiration | Hypnosis through visual imagery and analgesic suggestion, relaxation techniques | Self-reported pain |
| Self-reported distress | |||||
| Behavioral measure of distress | |||||
| Liossi, 2003 | nt = 40; nc = 20 | 6–16 years | Lumbar puncture |
|
Self-reported pain |
| Self-reported distress | |||||
| Behavioral measure of distress | |||||
| Liossi, 2006 | nt = 15; nc = 30 | 6–16 years | Lumbar puncture |
|
Self-reported pain |
| Self-reported distress | |||||
| Behavioral measure of distress | |||||
| Liossi, 2009 | nt = 15; nc = 15 | 6–16 years | Venepuncture |
|
Self-reported pain |
| Self-reported distress | |||||
| Behavioral measure of distress | |||||
Note. nt = number of children in the treatment condition; nc = number of children in the control condition.
Table II.
GRADE Summary of Findings for Distraction for Needle-Related Procedural Pain and Distress in Children and Adolescents
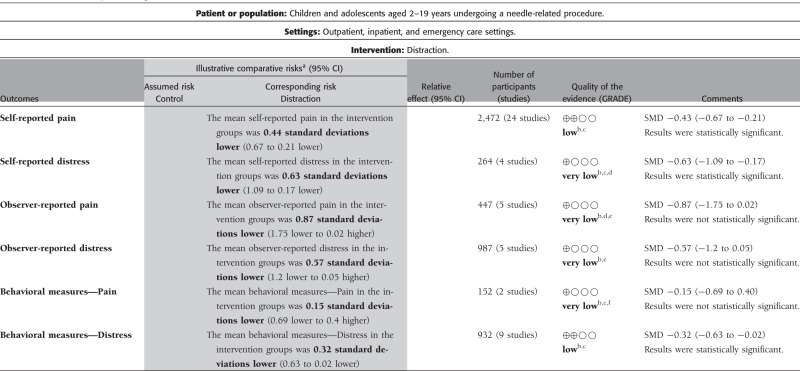 |
Note. GRADE Working Group grades of evidence:
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
aThe basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI = Confidence interval.
bThe majority of trials had unclear/high risk of bias in allocation concealment and selective reporting of outcomes.
cModerate heterogeneity (I-squared) > 45%.
dAnalysis based on <400 participants per group.
eConsiderable heterogeneity (I-squared) > 90%.
fAnalysis based on <100 participants per group.
Synthesis of Results
Several studies included multiple control or comparison conditions. Consistent with the recent Cochrane Review (Uman et al., 2013), the control condition that most isolated the unique contribution of the intervention was selected for comparison. For example, if a study compared (1) no intervention control; (2) EMLA only; and (3) EMLA plus distraction, then the group receiving EMLA only was selected as the comparison group to best examine the unique effects of distraction. One study included two interventions using the same distraction techniques (i.e., distraction only and distraction plus suggestion; Fowler-Kerry & Lander, 1987). Given the design of the study, two comparison groups were available that appropriately isolated the unique effects of the distraction intervention (i.e., no treatment control and suggestion only). Outcomes for both distraction interventions and outcomes for both comparison groups were respectively pooled together using recommended statistical formulae for all analyses (Cochrane Handbook, 2011). Another study examined two different distraction interventions (Bellieni et al., 2006). In this case, outcomes for both distraction interventions were pooled together only for analyses examining the overall efficacy of distraction and for planned subanalyses examining the influence of age and risk on treatment effects, but entered separately in subanalyses focused on distraction subtypes, as they differed in relevant ways (i.e., degree of adult involvement).
It was also possible for individual studies to include multiple measures for the same outcome, such as using a visual analogue scale to examine both parent and nurse observer-reported pain or multiple behavioral measures for the same construct. Consistent with the recent Cochrane Review (Uman et al., 2013), multiple measurements for the same outcome were pooled using recommended statistical formulae for combining means and standard deviations (Cochrane Handbook, 2011). Furthermore, some studies assessed outcomes at multiple time points, such as assessing pain and/or distress during and at several occasions following the needle procedure. It was determined a priori that the measurement occurring during the procedure was prioritized for inclusion in analyses; however, in instances when it was not assessed, the first possible measurement occurring postprocedure was included. Preprocedure assessments of distress and/or anticipated pain were not examined as distraction or hypnosis interventions are not uniformly designed to address those outcomes.
Heterogeneity using the chi-square test and the I2 statistic was calculated for all analyses using RevMan 5.2 software. The chi-square test indicates heterogeneity across studies, which is often present in meta-analyses given the level of clinical and methodological diversity across included studies (Higgins et al., 2011), and is particularly relevant for the current review given the variety of distraction interventions. The I2 statistic provides a measure of inconsistency in estimate of treatment effects across studies and is useful for indicating the impact of heterogeneity on meta-analytic findings (Higgins et al., 2011).
Planned Subanalyses
A chi-square test for subgroup differences was conducted for all subanalyses using RevMan 5.2 software. Given the considerable heterogeneity in sample characteristics, treatment setting, and needle procedures, random effects models were used for the analysis within each subgroup. An I2 statistic assessing heterogeneity was also computed for each subanalysis (Cochrane Handbook, 2011).
Distraction Subtypes
Based on previous research (Bellieni et al., 2006; Beran et al., 2013; Nilsson et al., 2008; Sinha et al., 2006), the following characteristics of distraction interventions were identified for subanalyses: adult involvement, use of no/low versus high technology, active versus passive nature of intervention, and child choice of distracter. Included distraction studies were categorized for all four subanalyses using operational definitions developed by review authors and based on previous research. When studies could fall under multiple categories (e.g., when both low- and high-technology distracters were used), studies were categorized based on what the majority of participants received as reported in publications or through contact with study authors. Studies were coded independently by two review authors with discrepancies resolved by a third review author. Authors were contacted by e-mail for more information when sufficient detail was not available in publications to accurately categorize studies.
Adult Involvement Versus no Adult Involvement. This domain considered the involvement of an adult in the distraction intervention. Adults could include a parent, nurse, physician, etc. Adult involvement was considered present when a specific supportive or trained role was articulated for the adult within the intervention.
No/Low Technology Versus High Technology. This domain considered the type of materials used in the distraction intervention. Books, bubbles, balls, kaleidoscopes were considered low technology. Music/musicians, CDs, headphones, virtual reality, or television were considered high technology. If multiple distracters were reported, studies were coded based on the type of distracter that was used by the majority of children (when available).
Interactive Versus Passive. This domain considered the degree of involvement warranted by the child. Distraction was considered interactive when the child was required to interact with technology or respond to questions posed by other individuals.
Child Choice Versus No Child Choice. This domain considered the child’s role in the distraction intervention. Child choice was considered to be any choice made solely by the child, such as choosing a distracter, type of music, etc. It was not considered child choice if an adult made the choice. Unless explicitly stated, it was assumed that there was no child choice.
Age Effects
All included studies were categorized into one of three age-groups as per categories outlined by the Standards for Research (StaR) in Child Health (Williams et al., 2012): Early Childhood (2–5 years old), Middle Childhood (6–11 years old), or Adolescence (12–19 years old). Studies were categorized based on reported overall mean age of study sample. When mean age was not reported, studies were categorized based on median age (when reported) or into the category within which the majority of the sample age range fell (e.g., study including 3–6 year olds categorized as Early Childhood). However, if studies reported outcomes separated by age-group, then each age-group was categorized and considered separately for age subanalyses. These approaches were taken so as not to unnecessarily exclude studies from age subanalyses.
Risk of Bias Subanalyses
As per previous empirical investigations examining the impact of study risk of bias on estimates of treatment effects, three risk of bias domains were considered for subanalyses: blinding (of participants and study personnel, and of outcome assessment), random sequence generation, and allocation concealment (Savovic et al., 2012). However, given that blinding is challenging (and often impossible) to achieve in studies examining psychological interventions, a decision was made to exclude blinding from planned subanalyses. Therefore, risk of bias subanalyses focused on adequacy of random sequence generation and allocation concealment. Consistent with previous investigations (Savovic et al., 2012), studies rated as low risk of bias in their respective domains were considered to represent adequate random sequence generation or allocation concealment. Studies rated as unclear or high risk of bias were considered to reflect inadequate random sequence generation or allocation concealment.
Results
Study Selection
A total of 2,865 abstracts were identified and reviewed, of which 2,698 were excluded for not meeting eligibility criteria. An additional 123 records were excluded after full-text articles were assessed for eligibility, leaving a possible 44 studies eligible for inclusion. Of these, 12 did not provide the data necessary, in publications or via author contact, for data pooling. This left a total of 32 studies to be included in the meta-analysis. See Figure 1 for the PRIMSA flowchart.
Figure 1.
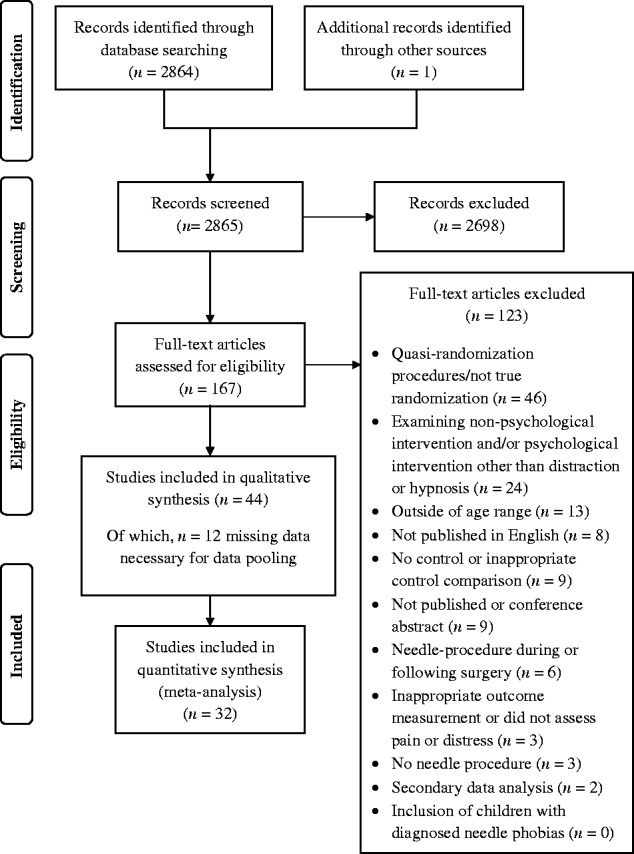
PRISMA flowchart.
Study Characteristics
Characteristics for all included distraction and hypnosis studies are available in Table I and in the Supplementary Tables 7–38.
Distraction
A total of 37 studies examined the effects of distraction on needle-related procedural pain and/or distress; however, only 26 studies provided necessary data and are included in the meta-analysis (See Table I).
Hypnosis
A total of nine studies examined the effects of hypnosis on needle-related procedural pain and/or distress; however, only seven studies provided necessary data and were included in the meta-analysis (See Table I).
Risk of Bias Within and Across Studies
Risk of bias ratings and relevant rationale for each included study are available in Supplementary Tables 39–70. Summary of risk of bias ratings are available in Figures 2 and 3, respectively.
Figure 2.
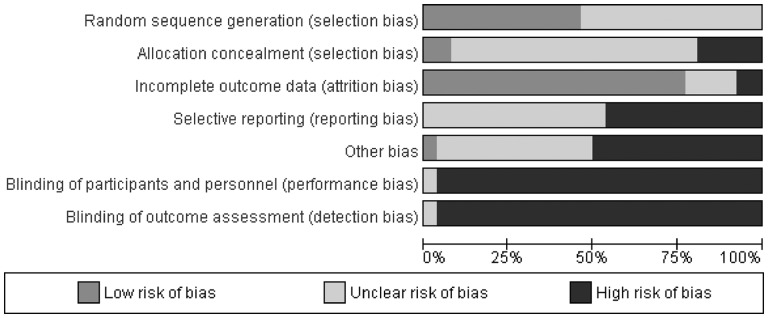
Summary of risk of bias across all included distraction studies.
Figure 3.
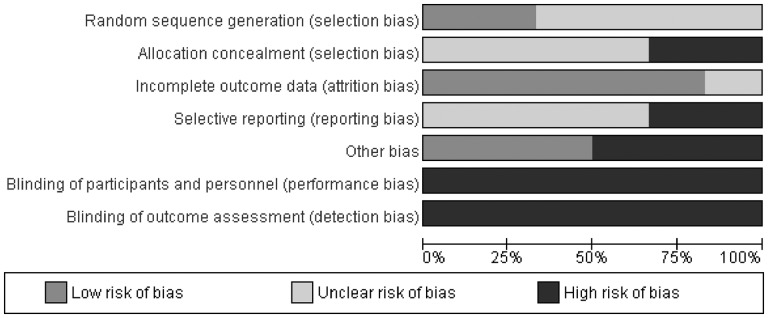
Summary of risk of bias across all included hypnosis studies.
Distraction
Incomplete outcome data addressed was the domain with the least risk of bias overall with 20 studies (77%) rated as low, 4 (15%) as unclear, and 2 (8%) as high risk of bias. All but one study (96%) had high risk of bias for blinding of participants and study personnel, as well as blinding of outcome assessment. For random sequence generation, all studies were rated as low (n = 12; 46%) or unclear (n = 14; 54%) risk of bias. The majority of studies (n = 19; 73%) were rated as unclear risk of bias for allocation concealment, with fewer rated as high (n = 5; 19%) or low (n = 2; 8%) risk of bias in that domain. Greater than half of the studies were rated as unclear risk of bias for selective reporting (n = 14; 54%), with all others rated as high risk of bias (n = 12; 46%). Most studies were rated as high (n = 13; 50%) or unclear (n = 12; 46%) risk of other bias, with only one study rated as low (4%).
Hypnosis
Similarly, incomplete outcome data addressed was the domain with the least risk of bias overall with five studies (83%) rated as low risk of bias and only one study (17%) as unclear. Blinding of participants and personnel and blinding of outcome assessment had the poorest risk of bias across studies with all studies rated as high. For random sequence generation, four studies (67%) were rated as unclear and two studies (33%) as low. The majority of studies (n = 4; 67%) were rated as unclear risk of bias for random sequence generation, allocation concealment, and selective reporting. The remaining studies were rated as low risk of bias in random sequence generation (n = 2; 33%), high risk of bias for allocation concealment (n = 2; 33%), and high risk of bias for selective reporting (n = 2; 33%). The three most recent studies (50%) were rated as low for risk of other bias, with the three earlier studies rated as high risk of other bias.
Results of Individual Studies and Synthesis of Results
Distraction
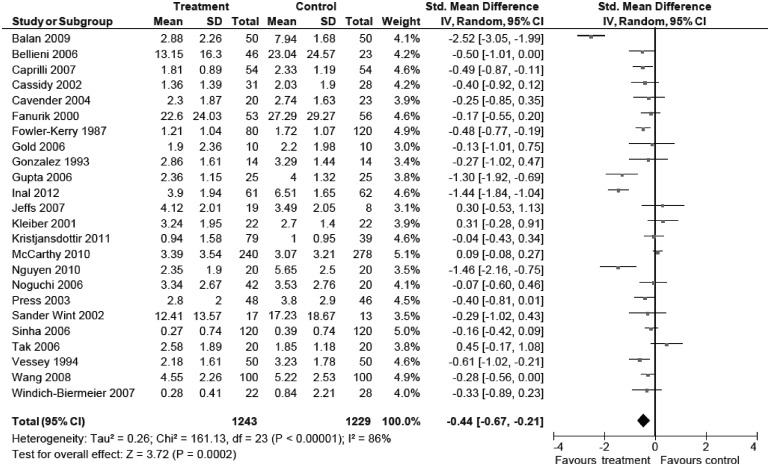
See Figure 4 for the summary of intervention effect estimates and confidence intervals, as well as the forest plot of included studies examining the efficacy of distraction on self-reported pain. Summaries and forest plots for remaining pain and distress outcomes for distraction are available in Supplementary Figures 5–9.
Figure 4.
Forest plot of studies examining distraction for self-reported pain.
Pain Intensity. Twenty-four studies including 2,473 participants (nt = 1,243) revealed a significant effect of distraction on self-reported pain (SMD = −0.44 [−0.67, −0.21], Z = 3.72, p < .01, I2 = 86%). Analysis of the five studies examining the effects of distraction with 447 participants (nt = 247) on observer-reported pain was not significant (SMD = −0.87 [−1.75, 0.02], Z = 1.92, p = .05, I2 = 94%). Two studies examining distraction on behavioral measures of pain for 152 participants (nt = 77) was also not significant (SMD = −0.15 [−0.69, 0.40], Z = 0.53, p = .59, I2 = 62%).
Distress. Four studies including 264 participants (nt = 130) revealed a significant effect of distraction on self-reported distress (SMD = −0.63 [−1.09, −0.17], Z = 2.70, p < .01, I2 = 66%). Five studies on observer-reported distress of distraction including 987 participants (nt = 470) was not significant (SMD = −0.57 [−1.20, 0.05], Z = 1.79, p = .07, I2 = 94%). Nine studies including 932 participants (nt = 454) revealed a significant effect of distraction on behavioral measures of distress (SMD = −0.32 [−0.63, −0.02], Z = 2.06, p < .05, I2 = 71%).
Hypnosis
See Supplementary Figures 10–12 for summary of intervention effect estimates and confidence intervals, as well as forest plots of included studies assessing the overall efficacy of hypnosis on pain and distress.
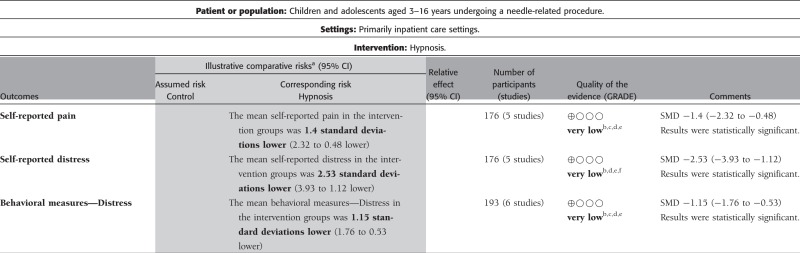
Pain Intensity. Five studies including 176 participants (nt = 97) revealed a significant effect of hypnosis on self-reported pain (SMD = −1.40 [−2.32, −0.48], Z = 2.97, p < .01, I2 = 85%). Although several studies examined the effect of hypnosis on observer-reported and behavioral measures of pain, no studies assessing observer-reported pain and only one study assessing behavioral measure of pain (Huet et al., 2011) provided data necessary for inclusion in meta-analysis. Therefore, no conclusions could be drawn.
Distress. Five studies including 176 participants (nt = 97) revealed a significant effect of hypnosis on self-reported distress (SMD = −2.53 [−3.93, −1.12], Z = 3.53, p < .01, I2 = 91%). Six studies including 193 participants (nt = 106) revealed a significant effect of hypnosis on behavioral measures of distress (SMD = −1.15 [−1.76, −0.53], Z = 3.66, p < .01, I2 = 71%). Although multiple studies examined the effects of hypnosis on observer-reported distress, only one study included data necessary for inclusion in meta-analysis (Katz et al., 1987); as such, no conclusions could be drawn.
Quality of Evidence Summary
Distraction
The GRADE ratings for distraction are presented in Table II. The quality of evidence was low for the outcomes of self-reported pain and behavioral measures of distress. Thus, further research is likely to have an important impact on our confidence in the estimate of these effects and is likely to change the estimates for these two outcomes. The quality of evidence was very low for all other outcomes, including self-reported distress, observer-reported pain and distress, and behavioral measures of pain. Given this, we are very uncertain of the estimate of effects for these outcomes.
Hypnosis
The GRADE ratings for hypnosis are presented in Table III. The quality of evidence was very low for all meta-analyzed outcomes, including self-reported pain and distress, and behavioral measures of distress. Thus, we are very uncertain of the estimate of the effects for hypnosis.
Table III.
GRADE Summary of Findings for Hypnosis for Needle-Related Procedural Pain and Distress in Children and Adolescents
 |
Note. GRADE Working Group grades of evidence:
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
aThe basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI = Confidence interval.
bThe majority of trials had unclear/high risk of bias in allocation concealment and selective reporting of outcomes.
cModerate heterogeneity (I-squared) >45%.
dVast majority of trials from one expert group.
eAnalysis based on <100 participants per group.
fConsiderable heterogeneity (I-squared) >90%.
Planned Subanalyses
Distraction Subtypes
See Table IV for a summary of intervention effect estimates, confidence intervals, and test of subgroup differences for distraction subanalyses. Forest plots are available in Supplementary Figures 13–27. For the two studies comparing two different distraction interventions to a control group (Bellieni et al., 2006; Jeffs, 2007), the distraction interventions were categorized and considered separately in the distraction subanalyses. Furthermore, given that only two studies examined the effect of distraction on behavioral measures of pain, this outcome was not included in subanalyses.
Table IV.
Summary of Distraction Subanalyses
| Outcomes | Number of interventions |
SMD [95% CI] |
χ2 | p | I2 (%) | ||
|---|---|---|---|---|---|---|---|
| Adult involvement | No adult involvement | Adult involvement | No adult involvement | ||||
| Pain: self-report | 11 (nt = 607; nc = 645) | 15 (nt = 636; nc = 586) | −0.36 [−0.66, −0.05] | −0.50 [−0.84, −0.15] | 0.35 | .55 | 0 |
| Pain: observer report | 3 (nt = 132; nc = 130) | 3 (nt = 115; nc = 93) | −0.40 [−1.40, 0.59] | −1.14 [−2.50, 0.22] | 0.73 | .39 | 0 |
| Pain: behavioral measures | 1 (nt = 50; nc = 50) | 1 (nt = 27; nc = 25) | – | – | – | – | – |
| Distress: self-report | 2 (nt = 42; nc = 51) | 2 (nt = 88; nc = 83) | −0.24 [−0.65, 0.17] | −1.00 [−1.71, −0.29] | 3.31 | .07 | 70 |
| Distress: observer report | 4 (nt = 350; nc = 397) | 1 (nt = 120; nc = 120) | – | – | – | – | – |
| Distress: behavioral | 6 (nt = 384; nc = 430) | 3 (nt = 70; nc = 48) | −0.47 [−0.86, −0.08] | 0.02 [−0.51, 0.56] | 2.10 | .15 | 52 |
| No/low tech | High tech | No/low tech | High tech | ||||
|---|---|---|---|---|---|---|---|
| Pain: self-report | 7 (nt = 215; nc = 208) | 18 (nt = 788; nc = 745) | −0.57 [−1.06, −0.08] | −0.42 [−0.68, −0.16] | 0.28 | .60 | 0 |
| Pain: observer report | 2 (nt = 84; nc = 84) | 4 (nt = 163; nc = 139) | −0.64 [−2.05, 0.76] | −0.83 [−1.56, 0.30] | 0.04 | .84 | 0 |
| Pain: behavioral measures | 1 (nt = 50; nc = 50) | 1 (nt = 27; nc = 25) | – | – | – | – | – |
| Distress: self-report | 1 (nt = 20; nc = 23) | 3 (nt = 110; nc = 111) | – | – | – | – | – |
| Distress: observer report | 3 (nt = 103; nc = 107) | 1 (nt = 120; nc = 120) | – | – | – | – | – |
| Distress: behavioral | 5 (nt = 94; nc = 97) | 3 (nt = 116; nc = 94) | −0.34 [−0.88, 0.20] | −0.43 [−0.85,−0.01] | 0.07 | .79 | 0 |
| Interactive | Passive | Interactive | Passive | ||||
|---|---|---|---|---|---|---|---|
| Pain: self-report | 8 (nt = 307; nc = 293) | 18 (nt = 936; nc = 938) | −0.50 [−0.83, −0.18] | −0.41 [−0.69, −0.12] | 0.19 | .66 | 0 |
| Pain: observer report | 3 (nt = 151; nc = 127) | 3 (nt = 96; nc = 96) | −0.55 [−1.45, 0.34] | −0.99 [−2.55, 0.58] | 0.22 | .64 | 0 |
| Pain: behavioral measures | 1 (nt = 50; nc = 50) | 1 (nt = 27; nc = 25) | – | – | – | – | – |
| Distress: self-report | 2 (nt = 42; nc = 51) | 2 (nt = 88; nc = 83) | −0.24 [−0.65, 0.17] | −1.00 [−1.71, −0.29] | 3.31 | .07 | 70 |
| Distress: observer report | 2 (nt = 81; nc = 85) | 3 (nt = 389; nc = 432) | −1.35 [−2.60, −0.11] | −0.14 [−0.40, 0.13] | 3.55 | .06 | 72 |
| Distress: behavioral | 5 (nt = 154; nc = 135) | 4 (nt = 300; nc = 343) | −0.41 [−0.88, 0.06] | −0.07 [−0.23, 0.09] | 1.81 | .18 | 45 |
| Child choice | No child choice | Child choice | No child choice | ||||
|---|---|---|---|---|---|---|---|
| Pain: self-report | 9 (nt = 455; nc = 412) | 17 (nt = 798; nc = 819) | −0.22 [−0.45, 0.00] | −0.54 [−0.87, −0.20] | 2.37 | .12 | 58 |
| Pain: observer report | 0 | 6 (nt = 247; nc = 223) | – | – | – | – | – |
| Pain: behavioral measures | 0 | 2 (nt = 77; nc = 75) | – | – | – | – | – |
| Distress: self-report | 4 (nt = 130; nc = 134) | 0 | – | – | – | – | – |
| Distress: observer report | 3 (nt = 162; nc = 165) | 2 (nt = 308; nc = 352) | −0.29 [−0.71, 0.13] | −1.01 [−2.87, 0.86] | 0.53 | .47 | 0 |
| Distress: behavioral | 2 (nt = 42; nc = 42) | 7 (nt = 412; nc = 433) | −0.21 [−0.63, 0.21] | −0.35 [−0.73, 0.04] | 0.22 | .64 | 0 |
Note. Bolded values indicate results significantly in favor of treatment efficacy.
SMD = standard mean difference; CI = confidence intervals; χ2 = chi-square test of subgroup differences; I2 = measure of heterogeneity; nt = number of participants in study treatment groups; nc = number of participants in study control groups.
Adult Involvement Versus No Adult Involvement. Of the 26 distraction interventions examining self-reported pain, 11 (42%) had adult involvement. Studies including adult involvement (SMD = −0.36 [−0.66, −0.05], Z = 2.28, p < .05, I2 = 83%), as well as those without adult involvement (SMD = −0.50 [−0.84, −0.15], Z = 2.84, p < .01, I2 = 86%), showed a significant effect of distraction on self-reported pain. The test of subgroup differences did not reveal significant differences (χ2 = 0.35, p = .55, I2 = 0%). Of the six distraction interventions examining observer-reported pain, three (50%) had adult involvement. Studies including adult involvement did not reveal a significant effect (SMD = −0.40 [−1.40, 0.59], Z = 0.79, p = .43, I2 = 93%), and neither did those without adult involvement (SMD = −1.14 [−2.50, 0.22], Z = 1.64, p = .10, I2 = 95%). The test of subgroup differences showed no significant difference between distraction interventions with or without adult involvement on observer-reported pain (χ2 = 0.73, p = .39, I2 = 0%).
Of the four distraction interventions examining self-reported distress, two (50%) were identified as having adult involvement. Studies without adult involvement showed a significant effect (SMD = −1.00 [−1.71, −0.29], Z = 2.77, p < .01, I2 = 70%), whereas studies including adult involvement did not reveal a significant effect (SMD = −0.24 [−.65, 0.17], Z = 1.16, p = .25, I2 = 0%). The test of subgroup differences between distraction interventions with or without adult involvement on self-reported distress was not significant (χ2 = 3.31, p = .07, I2 = 70%). Of the nine distraction interventions using behavioral measures of distress, six (67%) were identified as having adult involvement. Studies including adult involvement showed a significant effect (SMD = −0.47 [−0.86, −0.08], Z = 2.34, p < .05, I2 = 78%), whereas studies without adult involvement did not reveal a significant effect (SMD = 0.02 [−0.51, 0.56], Z = 0.09, p = .93, I2 = 45%) of distraction on behavioral measures of distress. The test of subgroup differences did not reveal significant differences between distraction interventions (χ2 = 2.10, p = .15, I2 = 52%). No conclusions could be drawn about the impact of adult involvement on observer-reported distress, as the comparison included only a single study in the no adult involvement category.
No/Low Technology Versus High Technology. Despite contact with study authors, one study (McCarthy et al., 2010) could not be clearly categorized, as both types of distracters were used by participating children; therefore, it was excluded from this category.
Of the 25 distraction interventions examining self-reported pain, 7 (28%) were identified as using no/low-technology distracters. Studies using no/low technology (SMD = −0.57 [−1.06, −0.08], Z = 2.26, p < .05, I2 = 82%), as well as those using high technology (SMD = −0.42 [−0.68, −0.16], Z = 3.14, p < .01, I2 = 82%), showed a significant effect of distraction on self-reported pain. The test of subgroup differences did not reveal significant differences between distraction interventions (χ2 = 0.28, p = .60, I2 = 0%). Of the six distraction interventions examining observer-reported pain, two (33%) used no/low-technology distracters. Neither studies using no/low technology (SMD = −0.64 [−2.05, 0.76], Z = 0.90, p = .37, I2 = 94%) nor studies using high technology (SMD = −0.83 [−1.97, 0.30], Z = 1.44, p = .15, I2 = 95%) revealed a significant effect. The test of subgroup differences was not significant on observer-reported pain (χ2 = 0.04, p = .84, I2 = 0%).
Of the eight distraction interventions using behavioral measures of distress, five (63%) were identified as using no or low-technology distracters. Studies using high technology showed a significant effect (SMD = −0.43 [−0.85, −0.01], Z = 2.01, p < .05, I2 = 51%), whereas studies using no/low technology did not (SMD = −0.34 [−0.88, 0.20], Z = 1.23, p = .22, I2 = 68%).However, the test of subgroup differences did not reveal significant difference between groups on behavioral measures of distress (χ2 = 0.07, p = .79, I2 = 0%). No conclusions could be drawn about the impact of technology on self- or observer-reported distress, as only single studies were categorized in the no/low-technology and high-technology groups for the respective analyses.
Interactive Versus Passive. Of the 26 distraction interventions examining self-reported pain, 8 (31%) were identified as interactive. Interactive distraction studies (SMD = −0.50 [−0.83, −0.18], Z = 3.01, p < .01, I2 = 72%), as well as passive distraction studies (SMD = −0.41 [−0.69, −0.12], Z = 2.81, p < .01, I2 = 86%), showed a significant effect on self-reported pain. The test of subgroup differences did not reveal significant differences (χ2 = 0.19, p = .66, I2 = 0%). Of the six distraction interventions examining observer-reported pain, three (50%) were identified as interactive. Neither interactive (SMD = −0.55 [−1.45, 0.34], Z = 1.21, p = .23, I2 = 92%) nor passive distraction studies (SMD = −0.99 [−2.55, 0.58], Z = 1.23, p = .22, I2 = 96%) revealed a significant effect. The test of subgroup differences was not significant (χ2 = 0.22, p = .64, I2 = 0%).
Of the four distraction interventions examining self-reported distress, two (50%) were identified as interactive. Passive distraction studies showed a significant effect (SMD = −1.00 [−1.71, −0.29], Z = 2.77, p < .01, I2 = 70%), whereas interactive distraction studies did not reveal a significant effect (SMD = −.24 [−0.65, 0.17], Z = 1.16, p = .25, I2 = 0%). The test of subgroup differences between interactive and passive distraction interventions was not significant (χ2 = 3.31, p = .07, I2 = 70%). Of the five distraction interventions examining observer-reported distress, two (40%) were identified as interactive. Interactive distraction studies showed a significant effect (SMD = −1.35 [−2.60, −0.11], Z = 2.14, p < .05, I2 = 91%), whereas passive distraction studies did not reveal a significant effect (SMD = −0.14 [−0.40, 0.13], Z = 1.01, p = .31, I2 = 60%). The test of subgroup differences was not significant (χ2 = 3.55, p = .06, I2 = 72%). Of the nine distraction interventions using behavioral measures of distress, five (56%) were identified as interactive. Neither interactive distraction studies (SMD = −0.41 [−0.88, 0.06], Z = 1.69, p = .09, I2 = 71%) nor passive interaction studies (SMD = −0.07 [−0.23, 0.09], Z = 0.83, p = .41, I2 = 1%) revealed a significant effect. The test of subgroup differences was not significant (χ2 = 1.81, p = .18, I2 = 45%).
Child Choice Versus No Child Choice. Of the 26 distraction interventions examining self-reported pain, 9 (35%) included some child choice. Studies not including child choice (SMD = −0.54 [−0.87, −0.20], Z = 3.16, p < .01, I2 = 89%) showed a significant effect, whereas studies including child choice (SMD = −0.22 [−0.45, 0.00], Z = 1.97, p = .05, I2 = 53%) did not. The test of subgroup differences did not reveal a significant difference (χ2 = 2.37, p = .12, I2 = 58%). No conclusions could be drawn about the impact of child choice on observer-reported pain, as no studies included child choice.
Of the five distraction interventions examining observer-reported distress, three (60%) included child choice. Neither studies including child choice (SMD = −0.29 [−0.71, 0.13], Z = 1.37, p = .17, I2 = 57%) nor studies without child choice (SMD = −1.01 [−2.87, 0.86], Z = 1.06, p = .29, I2 = 98%) were significant. The test of subgroup differences did not reveal significant difference (χ2 = 0.53, p = .47, I2 = 0%). Of the nine distraction interventions using behavioral measures of distress, two (22%) were identified as having child choice. Studies without child choice were not significant (SMD = −0.35 [−0.73, 0.04], Z = 1.76, p = .08, I2 = 78%), whereas studies with child choice did not reveal a significant effect (SMD = −0.21 [−0.63, 0.21], Z = 0.98, p = .33, I2 = 0%). The test of subgroup differences was not significant (χ2 = 0.22, p = .64, I2 = 0%). No conclusions could be drawn about the impact of child choice on self-reported distress, as all studies included child choice.
Age Effects
Distraction. In the study by Fanurik and colleagues (2000), data for self-reported pain was presented separately for children in three age-groups (5–8, 9–12, and 13–16 year olds). As such, these age-groups were categorized and considered separately in the subsequent age subanalyses; the younger two age-groups were categorized as Middle Childhood and the oldest age-group was Adolescence. Given that only two studies examined the effect of distraction on behavioral measures of pain, this outcome was not analyzed. See Table V for a summary of intervention effect estimates, confidence intervals, and test of subgroup differences for age subanalyses. Forest plots are available in Supplementary Figures 28–29.
Table V.
Summary of Age Subanalyses
| Outcomes | Number of interventions |
SMD [95% CI] |
χ2 | p | I2 (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Early childhood | Middle childhood | Adolescence | Early childhood | Middle childhood | Adolescence | ||||
| Distraction | |||||||||
| Pain: self-report | 5 (nt = 189;nc = 204) | 17 (nt = 920;nc = 946) | 4 (nt = 134;nc = 79) | −0.24 [−0.52, 0.05] | −0.57 [−0.88, −0.27] | −0.03 [−0.31, 0.25] | 6.56 | .04 | 70 |
| Pain: observer report | 1 (nt = 42; nc = 20) | 4 (nt = 205;nc = 180) | 0 | – | – | – | – | – | – |
| Pain: behavioral | 1 (nt = 27; nc = 25) | 1 (nt = 50; nc = 50) | 0 | – | – | – | – | – | – |
| Distress: self-report | 0 | 4 (nt = 130;nc = 134) | 0 | – | – | – | – | – | – |
| Distress: observer report | 1 (nt = 22; nc = 22) | 4 (nt = 448;nc = 495) | 0 | – | – | – | – | – | – |
| Distress: behavioral | 5 (nt = 116;nc = 94) | 4 (nt = 338;nc = 384) | 0 | −0.31 [−0.85, 0.22] | −0.31 [−0.71, 0.10] | – | 0.00 | .99 | 0 |
| Hypnosis | |||||||||
| Pain: self-report | 0 | 5 (nt = 97; nc = 79) | 0 | – | – | – | – | – | – |
| Pain: observer report | 0 | 0 | 0 | – | – | – | – | – | – |
| Pain: behavioral | 0 | 1 (nt = 14; nc = 15) | 0 | – | – | – | – | – | – |
| Distress: self-report | 0 | 5 (nt = 97; nc = 79) | 0 | – | – | – | – | – | – |
| Distress: observer report | 0 | 1 (nt = 17; nc = 19) | 0 | – | – | – | – | – | – |
| Distress: behavioral | 1 (nt = 9; nc = 9) | 5 (nt = 97; nc = 79) | 0 | – | – | – | – | – | – |
Note. Bolded values indicate results significantly in favor of treatment efficacy.
SMD = standard mean difference; CI = confidence intervals; χ2 = chi-square test of subgroup differences; I2 = measure of heterogeneity; nt = number of participants in study treatment groups; nc = number of participants in study control groups.
Of the 26 distraction interventions examining self-reported pain, 5 (19%) were categorized as Early Childhood, 17 (65%) as Middle Childhood, and 4 (15%) as Adolescence. Analyses revealed a significant impact in Middle Childhood (SMD = −0.57 [−0.88, −0.27], Z = 3.64, p < .01, I2 = 89%), but not in Early Childhood (SMD = −0.24 [−0.52, 0.05], Z = 1.64, p = .10, I2 = 38%) or Adolescence (SMD = −0.03 [−0.31, 0.25], Z = 0.21, p = .84, I2 = 0%). The test of subgroup differences revealed a significant difference depending on age-group (χ2 = 6.56, p < .05, I2 = 70%). Of the nine distraction interventions using behavioral measures of distress, five (56%) were categorized as Early Childhood and four (44%) as Middle Childhood; no studies were categorized as Adolescence. No significant effect was observed in Early (SMD = −0.31 [−0.85, 0.22], Z = 1.15, p = .25, I2 = 69%) or Middle Childhood (SMD = −0.31 [−0.71, 0.10], Z = 1.49, p = .14, I2 = 75%). The test of subgroup differences did not reveal significant differences (χ2 = 0.00, p = .99, I2 = 0%). No conclusions could be drawn about the impact of age on observer-reported pain, self-reported distress, and observer-reported distress given that all or all but one study was categorized as Middle Childhood.
Hypnosis. Of the seven studies examining hypnosis, six (86%) were categorized as Middle Childhood and only one (14%) as Early Childhood. Given this lack of variability, subanalyses were not conducted.
Risk of Bias Subanalyses
See Table VI for a summary of intervention effect estimates, confidence intervals, and test of subgroup differences for risk of bias subanalyses. Forest plots are available in Supplementary Figures 30–37.
Table VI.
Summary of Risk of Bias Subanalyses
| Outcomes | Number of Interventions |
SMD [95% CI] |
χ2 | p | I2 (%) | ||
|---|---|---|---|---|---|---|---|
| Adequate random sequence generation | Inadequate random sequence generation | Adequate random sequence generation | Inadequate random sequence generation | ||||
| Distraction | |||||||
| Pain: self-report | 12 (nt = 545; nc = 456) | 12 (nt = 698; nc = 773) | −0.63 [−1.04, −0.22] | −0.23 [−0.45, −0.02] | 2.83 | .09 | 65 |
| Pain: observer report | 4 (nt = 199; nc = 154) | 1 (nt = 48; nc = 46) | – | – | – | – | – |
| Pain: behavioral | 2 (nt = 77; nc = 75) | 0 | – | – | – | – | – |
| Distress: self-report | 2 (nt = 42; nc = 51) | 2 (nt = 88; nc = 83) | −0.24 [−0.65, 0.17] | −1.00 [−1.71, −0.29] | 3.31 | .07 | 70 |
| Distress: observer report | 2 (nt = 81; nc = 85) | 3 (nt = 389; nc = 432) | −1.35 [−2.60, −0.11] | −0.14 [−0.40, 0.13] | 3.55 | .06 | 72 |
| Distress: behavioral | 2 (nt = 62; nc = 43) | 7 (nt = 392; nc = 435) | −0.26 [−0.66, 0.14] | −0.33 [−0.73, 0.06] | 0.07 | .79 | 0 |
| Hypnosis | |||||||
| Pain: self-report | 2 (nt = 30; nc = 30) | 3 (nt = 67; nc = 49) | −1.32 [−1.88, −0.75] | −1.50 [−3.19, 0.20] | 0.04 | .84 | 0 |
| Pain: observer report | 0 | 0 | – | – | – | – | – |
| Pain: behavioral | 1 (nt = 14; nc = 15) | 0 | – | – | – | – | – |
| Distress: self-report | 2 (nt = 30; nc = 30) | 3 (nt = 67; nc = 49) | −3.11 [−4.51, −1.70] | −2.15 [−4.13, −0.16] | 0.60 | .44 | 0 |
| Distress: observer report | 1 (nt = 17; nc = 19) | 0 | – | – | – | – | – |
| Distress: behavioral | 2 (nt = 30; nc = 30) | 4 (nt = 76; nc = 57) | −1.73 [−2.33, −1.12] | −0.86 [−1.64, −0.09] | 2.96 | .09 | 66 |
| Adequate Allocation Concealment | Inadequate Allocation Concealment | Adequate Allocation Concealment | Inadequate Allocation Concealment | ||||
|---|---|---|---|---|---|---|---|
| Distraction | |||||||
| Pain: self-report | 2 (nt = 51; nc = 48) | 22 (nt = 1192; nc = 1181) | −0.90 [−1.93, 0.13] | −0.40 [−0.64, −0.16] | 0.85 | .36 | 0 |
| Pain: observer report | 0 | 5 (nt = 247; nc = 200) | – | – | – | – | – |
| Pain: behavioral | 1 (nt = 27; nc = 25) | 1 (nt = 50; nc = 50) | – | – | – | – | – |
| Distress: self-report | 1 (nt = 20; nc = 20) | 3 (nt = 110; nc = 114) | – | – | – | – | – |
| Distress: observer report | 0 | 5 (nt = 470; nc = 517) | – | – | – | – | – |
| Distress: behavioral | 0 | 9 (nt = 454; nc = 478) | – | – | – | – | – |
| Hypnosis | |||||||
| Pain: self-report | 0 | 5 (nt = 97; nc = 79) | – | – | – | – | – |
| Pain: observer report | 0 | 0 | – | – | – | – | – |
| Pain: behavioral | 0 | 1 (nt = 14; nc = 15) | – | – | – | – | – |
| Distress: self-report | 0 | 5 (nt = 97; nc = 79) | – | – | – | – | – |
| Distress: observer report | 1 (nt = 17; nc = 19) | 0 | – | – | – | – | – |
| Distress: behavioral | 0 | 6 (nt = 106; nc = 87) | – | – | – | – | – |
Note. Bolded values indicate results significantly in favor of treatment efficacy.
SMD = standard mean difference; CI = confidence intervals; χ2 = chi-square test of subgroup differences; I2 = measure of heterogeneity; nt = number of participants in study treatment groups; nc = number of participants in study control groups.
Distraction. Studies reporting both adequate (SMD = −0.63 [−1.04, −0.22], Z = 3.04, p < .01, I2 = 89%) and inadequate (SMD = −0.23 [−0.45, −0.02], Z = 2.09, p < .05, I2 = 70%) sequence generation revealed a significant impact of distraction on self-reported pain. The test of subgroup differences was not significant (χ2 = 2.83, p = .09, I2 = 65%). Studies reporting adequate allocation concealment were not significant (SMD = −0.90 [−1.93, 0.13], Z = 1.71, p = .09, I2 = 82%), whereas studies categorized as inadequate allocation concealment indicated a significant effect (SMD = −0.40 [−0.64, −0.16], Z = 3.29, p < .01, I2 = 86%). The test of subgroup differences was not significant (χ2 = 0.85, p = .36, I2 = 0%). For observer-reported pain, only one distraction study was categorized as reporting inadequate sequence generation and all five studies were categorized as inadequate allocation concealment; therefore, no subanalyses were conducted. Given that only two studies examined the effect of distraction on behavioral measures of pain, this outcome was not considered for risk of bias subanalyses.
Studies reporting adequate sequence generation were not significant (SMD = −0.24 [−0.65, 0.17], Z = 1.16, p = .25, I2 = 0%), whereas studies reporting inadequate sequence generation did reveal a significant effect (SMD = −1.00 [−1.71, −0.29], Z = 2.77, p < .01, I2 = 70%) of distraction on self-reported distress. The test of subgroup differences for the adequacy of allocation concealment on self-reported distress was not significant (χ2 = 3.31, p = .07, I2 = 70%). No conclusions were drawn about the impact of allocation concealment on treatment efficacy for self-reported distress, as all but one study reported inadequate allocation concealment. Of the five studies examining observer-reported distress, two (40%) reported adequate sequence generation. Studies reporting adequate sequence generation showed a significant effect of distraction on observer-reported distress (SMD = −1.35 [−2.60, −0.11], Z = 2.14, p < .05, I2 = 91%), whereas studies reporting inadequate sequence generation were not significant (SMD = −0.14 [−0.40, 0.13], Z = 1.01, p = .31, I2 = 60%). The test of subgroup differences for the adequacy of sequence generation on observer-reported distress was not significant (χ2 = 3.55, p = .06, I2 = 72%). Of the nine studies examining behavioral measures of distress, two (22%) were categorized as reporting adequate sequence generation. Neither studies reporting adequate (SMD = −0.26 [−0.66, 0.14], Z = 1.26, p = .21, I2 = 0%) or inadequate (SMD = −0.33 [−0.73, 0.06], Z = 1.66, p = .10, I2 = 78%) sequence generation showed a significant effect, and the test of subgroup differences was not significant (χ2 = 0.07, p = .79, I2 = 0%). No studies assessing observer-reported or behavioral measures of distress were categorized as adequate allocation concealment, and therefore no subanalyses were conducted for these outcomes.
Hypnosis. Studies reporting adequate sequence generation revealed a significant effect (SMD = −1.32 [−1.88, −0.75], Z = 4.55, p < .01, I2 = 0%) of hypnosis on self-reported pain, whereas studies reporting inadequate sequence generation were not significant (SMD = −1.50 [−3.19, 0.20], Z = 1.73, p = .08, I2 = 92%). The test of subgroup differences did not reveal significant differences between studies (χ2 = 0.04, p = .84, I2 = 0%). Studies reporting both adequate and inadequate sequence generation revealed significant effects of hypnosis on self-reported distress (SMD = −3.11 [−4.51, −1.70], Z = 4.34, p < .01, I2 = 68% versus SMD = −2.15 [−4.13, −0.16], Z = 2.12, p < .05, I2 = 93%) and behavioral measures of distress (SMD = −1.73 [−2.33, −1.12], Z = 5.59, p < .01, I2 = 0% versus SMD = −0.86 [−1.64, −0.09], Z = 2.19, p < .05, I2 = 74%, respectively). The tests of subgroup differences did not reveal significant differences between studies with or without adequate sequence generation for self-reported distress (χ2 = 0.60, p = .44, I2 = 0%), or for behavioral measures of distress (χ2 = 2.96, p = .09, I2 = 66%).
Given that all hypnosis studies were categorized as reporting inadequate allocation concealment, no subanalyses examining the effects of allocation concealment on efficacy of hypnosis were conducted.
Discussion
The goal of this systematic review was to provide a detailed examination of the evidence for distraction and hypnosis. These were the two psychological interventions identified as having strong empirical support for reducing pain and distress for children undergoing needle procedures in a recent Cochrane Review (Uman et al., 2013). Specifically, this review and meta-analysis was the first to systematically examine a number of areas previously proposed to impact treatment efficacy, including characteristics of distraction interventions, child age, and risk of bias in study design.
Summary of Findings
Distraction and Subanalyses
Twenty-six randomized controlled trials examining the effects of distraction in 2,548 children aged 2–19 years met inclusion criteria and provided data necessary for pooling. Meta-analysis revealed that distraction led to significant reductions in children’s self-reported pain and distress, and behavioral measures of distress, as well as reductions (not reaching statistical significance) in observer-reported pain and distress during needle-related procedures. There was no evidence currently to support the efficacy of distraction for behavioral measures of pain.
As is consistent with previous systematic reviews (DeMore & Cohen, 2005; Kleiber & Harper, 1999; Malloy & Milling, 2010), distraction is a highly used and investigated psychological intervention for managing pain and distress during a variety of medical procedures, including needles, across a range of health care settings. Earlier reviews more strongly emphasized reductions in distress and provided evidence for the reduction of pain using behavioral measures (DeMore & Cohen, 2005; Kleiber & Harper, 1999). The current review offers stronger evidence for the efficacy of distraction for self-reported pain and less for behavioral measures of pain, likely due to the more stringent inclusion criteria and meta-analytic approach, as well as the increase in studies assessing self-reported pain over time. Furthermore, the current review extends the extant evidence and available support beyond childhood and into adolescence.
Distraction Subtypes. After providing strong evidence for the use of distraction overall, the recent Cochrane Review (Uman et al., 2013) advocated strongly for the field to begin dismantling the effective components of distraction interventions. Based on the previously suggested relevance of four characteristics known to vary across distraction interventions (Bellieni et al., 2006; Gold et al., 2006; Nilsson et al., 2008; Sinha et al., 2006), all distraction interventions were categorized based on their degree of adult involvement, the use of no/low versus high-tech distracters, the interactive versus passive nature of the distraction, and the availability of child choice in distracters. Although analyses revealed no statistically significant subgroup differences for intervention efficacy based on distraction subtypes, a number of comparisons suggest areas for further research to determine whether real differences are found. Specifically, interventions without adult involvement or using passive distraction were marginally more efficacious for reducing self-reported distress than those with adult involvement or interactive. Furthermore, interactive distraction interventions were marginally more efficacious for reducing observer-reported distress. Given that these findings are not statistically significant, they should be interpreted cautiously. However, greater effect sizes have previously been noted for pain and distress with distraction interventions requiring overt behavioral responses from the child, engaging multiple sensory modalities, or lacking child choice (DeMore & Cohen, 2005). This supports the recommendation that future studies directly compare the efficacy of different types of distraction techniques and assess the actual degree of child engagement with the distracter (MacLaren & Cohen, 2005) and intervention fidelity (McCarthy et al., 2010) on treatment efficacy. It should be noted that the efficacy of distraction for reducing self-reported pain appears particularly robust irrespective of variability across distraction interventions.
Age Effects. This review reveals the application of distraction across a broad age range of children and adolescents, of which only a few studies appear to tailor distraction strategies to child developmental level (e.g., Sinha et al., 2006). By applying the Standards for Research (StaR) in Child Health guidelines for age subanalyses (Williams et al., 2012), all included studies were categorized based on sample mean age into Early Childhood (2–5 year olds), Middle Childhood (6–11 year olds), and Adolescence (12–19 year olds) and compared across pain and distress outcomes. A significant subgroup difference was observed for self-reported pain, with studies reporting a sample mean age in Middle Childhood showing a significant effect of distraction on self-reported pain. Conversely, there was no evidence supporting distraction for self-reported pain for studies reporting a sample mean age in Early Childhood or Adolescence. Unfortunately, behavioral measures of distress was the only other outcome that could be examined given the limited number of studies with a mean age in Early Childhood or Adolescence for other outcomes. The vast majority of evidence supporting the efficacy of distraction for needle-related pain and distress is from studies with children with a mean age between 6 and 11 years, with limited examination or evidence specifically available for younger children (≤5 years) or adolescents (≥12 years). Although there may be face value to tailoring distraction strategies based on child age, studies that use different distracters with different age-groups or do not examine age through planned group comparisons ultimately offer limited benefit for identifying which distracters are most effective for particular children. Future studies should explore potential interactions between child age and distraction characteristics on intervention efficacy.
Hypnosis and Subanalyses
Seven randomized controlled trials examining the effects of hypnosis in 222 children aged 3–16 years met inclusion criteria and provided data necessary for data pooling. Meta-analysis revealed that hypnosis led to significant reductions in children’s self-reported pain and distress, and behavioral measures of distress during needle-related procedures. No conclusions could be drawn about observer-reported pain and distress or behavioral measures of pain due to a lack of studies examining those outcomes. Previous reviews have similarly supported the efficacy of hypnosis for reducing children’s pain and distress during needle procedures, as well as for a broader range of medical procedures (Accardi & Milling, 2009; Richardson et al., 2006). This review enhances our confidence in such conclusions given its meta-analytic approach and more rigorous inclusion criteria.
Age Effects. Although previous research suggests that child age may be particularly relevant for hypnosis efficacy (Kuttner, 1988), we were unable to conduct planned age subanalyses for hypnosis, as all but one study reported a sample mean age in Middle Childhood (between 6 and 11 years), thereby precluding statistical comparisons. Thus, although studies included children from early childhood (≤5 years) through to adolescence (≥12 years), we are unable to draw conclusions about the specific efficacy of hypnosis in those age-groups. We strongly encourage researchers to plan for age-group comparisons in future studies (Williams et al., 2012), as it remains unclear at what age hypnosis may be particularly effective.
Level of hypnotic suggestibility has been repeatedly linked to the efficacy of hypnosis for procedural pain and distress (Richardson et al., 2006). Normative changes in hypnotic suggestibility are noted across development and are believed to peak between 8 and 12 years of age (Accardi & Milling, 2009), suggesting that children of this age may be most likely to benefit from hypnosis. However, other research suggests that younger children are particularly well-suited for hypnosis given its imaginative requirements (Kuttner, 1988). Hypnosis may continue to demonstrate efficacy when examined specifically in adolescents given strong evidence for its efficacy in reducing pain in adulthood (Montgomery, DuHamel, & Redd, 2000). Child age may also interact with particular characteristics of hypnosis interventions to influence treatment efficacy, such as self-hypnosis (Liossi & Hatira, 2003), the focus on analgesic suggestions (direct) versus more general suggestion (indirect; Richardson et al., 2006), or the application of manualized treatments across wide age ranges (Liossi et al., 2006) versus individual tailoring of the hypnotic suggestion to children’s own interests and developmental level (Kuttner, 1988).
Quality of the Evidence
Despite the promising support for distraction and hypnosis for reducing needle-related pain and distress in children, closer consideration suggests concerns with the quality of available evidence on which these conclusions are based. As the first application of the GRADE system to this line of research (Guyatt et al., 2011, 2013), the quality of evidence for distraction and hypnosis was rated as low to very low across all pain and distress outcomes. This implies that further research is likely to impact the estimate of efficacy of distraction for self-reported pain and behavioral measures of distress, and suggests uncertainty about the true effects of distraction for other pain and distress outcomes and for hypnosis. This is, in part, reflected by the considerable heterogeneity observed in several analyses.
This disappointing current state of affairs should not be surprising given the limitations in study design that have been repeatedly identified in previous reviews of distraction and hypnosis alike (Accardi & Milling, 2009; DeMore & Cohen, 2005; Kleiber & Harper, 1999; Richardson et al., 2006; Uman et al., 2006, 2008, 2013). Despite identifying and selecting the highest quality of evidence available for inclusion in this review, a lack of quality in study design, small samples, and generally poor reporting led to the evidence being downgraded. The majority of studies with unclear or high risk of bias across several domains, particularly allocation concealment, selective reporting, and random sequence generation is concerning. Furthermore, many studies include small numbers of participants or chose only one or two outcomes to assess primarily self-reported pain intensity. As such, the quality of evidence was also often downgraded owing to small numbers of participants available for analyses. While 32 studies were included in the meta-analysis, an additional 12 eligible studies were unfortunately excluded, as they did not provide necessary data for pooling in published reports or via contact with study authors. These are areas in which researchers can directly and easily improve the quality of evidence they produce. Blinding of participants and personnel, as well as outcome assessment, are also problematic for risk of bias; however, blinding is admittedly difficult and sometimes impossible given the nature of psychological interventions. Of particular relevance for hypnosis are the majority of trials having been conducted by one expert group. This weakens overall confidence in the evidence for this intervention given the potential for limited generalizability.
Risk of Bias Subanalyses
Subanalyses examining the effect of adequate random sequence generation and allocation concealment (i.e., low risk of bias) on intervention efficacy revealed no significant findings, although a number of group differences not reaching statistical significance were observed. In particular, studies reporting adequate random sequence generation were marginally more efficacious for reducing self-reported pain and observer-reported distress with distraction, and reducing behavioral measures of distress with hypnosis. This is a positive observation, as it suggests overall that more rigorous studies are supporting the efficacy of distraction and hypnosis, decreasing the likelihood that poorly designed studies are driving overestimates of treatment effects as have been noted in reviews from other areas (Hamm et al., 2010; Hartling et al., 2012; Savovic et al., 2012). Distraction studies reporting inadequate random sequence generation were marginally more efficacious for self-reported distress. Subgroup analyses revealed no significant differences based on adequacy of allocation concealment in estimating efficacy of distraction studies in reducing self-reported pain. Owing to the overwhelming categorization of all but two distraction studies and one hypnosis study as reporting inadequate allocation concealment, this was the only outcome for which the impact of allocation concealment on treatment efficacy could be estimated.
Limitations
While this review and meta-analysis is the first to empirically examine the impact of distraction subtypes, child age, or risk of bias in study design on the efficacy of psychological interventions for needle procedures, there are inherent limitations to its approach. Subgroup analyses are observational by nature and are not based on randomized comparisons. As such, conclusions based on subgroup findings need to be viewed as tentative. Although the meta-analytic approach increases the validity of this review’s findings and critically addresses the repeated criticism of small sample sizes in studies in this area (Richardson et al., 2006), we caution readers given the noted methodological and statistical variability seen between studies and in a number of conducted analyses. Our use of random effects analyses to address heterogeneity implies that findings are estimates of average treatment effects with variability expected based on specific intervention or study design (Cochrane Handbook, 2011). To conduct planned subanalyses of distraction characteristics and age, distraction interventions were categorized based on the distracter(s) received by the majority of children, and studies were categorized based on the sample mean age (followed by other available age information). This approach was taken in an effort to maximize inclusion of studies and children in the analyses, although we acknowledge that it inherently misclassifies a portion of children. The subanalyses conducted in this review offer insight into previously unexamined areas, and the identified limitations suggest areas for future randomized controlled trials specifically designed to address these comparisons.
Clinical Implications and Recommendations
The review reiterates the strong support both statistically and clinically for use of distraction and hypnosis to manage needle-related pain and distress in children and adolescents; however, the quality of evidence on which that support is based is generally poor. These interventions were selected for the current review given that they were the only psychological interventions with evidence supporting their efficacy as revealed in a recent Cochrane Review in this area (Uman et al., 2013). Specifically, this review includes studies supporting the efficacy of distraction during a variety of routine and more invasive needle procedures across a range of outpatient, inpatient, community, and emergency care settings. The majority of evidence for hypnosis is drawn from studies in pediatric oncology involving more invasive needle procedures, such as lumbar punctures and bone marrow aspirations. The broad age range of children included in distraction and hypnosis studies suggests the wide applicability of these interventions across development; however, clinicians should be aware that less or limited evidence is available for their specific application in younger children (≤5 years) and adolescents (≥12 years). Furthermore, that most of these interventions are not tailored based on child age, despite the potential relevance of differences in hypnotic suggestibility (Accardi & Milling, 2009) or coping preferences (Skinner & Zimmer-Gembeck, 2007) across development.
The robustness of distraction for reducing self-reported pain intensity, irrespective of variations in distraction interventions, suggests that clinicians should feel confident using any number of available distracter(s) to manage children’s perceived pain during needle procedures. This is promising for the clinical utility of distraction, given that clinicians can use distraction interventions as appropriate for their setting, availability, resources, and expertise. This may be particularly relevant given the trend toward high-tech and costly distracters (e.g., Beran et al., 2013), which may not be accessible to all clinicians. However, additional research is needed to determine whether or not the efficacy of distraction remains despite intervention heterogeneity across other pain and distress outcomes and across child development. For example, distraction does not always happen in isolation. Some studies included in this review use other interventions alongside distraction (e.g., topical anesthetics or preparation/information with distraction; see Uman et al., 2006, for a broader discussion of combined interventions). As has been noted elsewhere (DeMore & Cohen, 2005), time-efficiency and ease of use are also critical for clinical utility. This may be particularly relevant when selecting between psychological interventions given the significant variability in by whom and for how long distraction is delivered, as well as the potential need for additional training for clinicians and children to effectively benefit from hypnosis.
Conclusions
Over the past 10–15 years, the field has made several leaps forward in the amount of evidence supporting the efficacy of distraction and hypnosis for needle-related pain and distress in children and adolescents (Kleiber & Harper, 1999; Wild & Espie, 2004). With the efficacy of these interventions established, we recommend that the field turn its efforts toward determining the best match of treatment and/or characteristics of treatments for individual children given their age, their previous experiences, the health care setting, and the given needle procedure. Critically, this can only be achieved by increasing the quality of the evidence produced. Researchers conducting trials in this area should draw from available guidelines and standards for designing pediatric trials generally (StaR Child Health) and specifically in pediatric pain regarding recruitment and consent (Caldwell et al., 2012), adequacy of sample size (van der Tweel et al., 2012), minimization of risk of bias (Hartling et al., 2012), considerations of child age (Williams et al., 2012), and selection, measurement, and reporting of outcomes (Sinha et al., 2012), including PedIMMPACT (McGrath et al., 2008) and CONSORT (Altman et al., 2001). It would be unfortunate for future reviews in this area to continue reiterating the same limitations that have plagued the field for years.
Supplementary Data
Supplementary data can be found at: http://www.jpepsy.oxfordjournals.org/
Acknowledgments
The authors thank Leah Wofsy for her research assistance, and the Cochrane Pain, Palliative, and Supportive Care team for their support with previous related Cochrane Reviews.
Funding
The Cochrane Review, which this work extends, was funded in part by a grant from the IWK Health Centre awarded to L.S.U. K.A.B. is a Vanier Canada Graduate Scholar funded by the Canadian Institutes of Health Research (CIHR). M.N. is supported by a Post-PhD Fellowship from CIHR. K.A.B. and M.N. are trainee members of Pain in Child Health: a strategic training initiative through CIHR. C.T.C. is a Canada Research Chair and her research is funded by CIHR and the Canada Foundation for Innovation.
Conflicts of interest: None declared.
References
- Accardi M C, Milling L S. The effectiveness of hypnosis for reducing procedure-related pain in children and adolescents: A comprehensive methodological review. Journal of Behavioral Medicine. 2009;32:328–339. doi: 10.1007/s10865-009-9207-6. [DOI] [PubMed] [Google Scholar]
- Altman D G, Schulz K F, Moher D, Egger M, Davidoff F, Elbourne D, Gøtzsche P C, Lang T CONSORT GROUP (Consolidated Standards of Reporting Trials) The revised CONSORT statement for reporting randomized trials: Explanation and elaboration. Annals of Internal Medicine. 2001;134:663–694. doi: 10.7326/0003-4819-134-8-200104170-00012. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics. American academy of pediatrics. 2013 Retrieved from www.aap.org. Retrieved October 8, 2013. [Google Scholar]
- Balan R, Bavdekar S B, Jadhav S. Can Indian classical instrumental music reduce pain felt during venepuncture? Indian Journal of Pediatrics. 2009;76:469–473. doi: 10.1007/s12098-009-0089-y. [DOI] [PubMed] [Google Scholar]
- Bellieni C V, Cordelli D M, Raffaelli M, Ricci B, Morgese G, Buonocore G. Analgesic effect of watching TV during venipuncture. Archives of Disease in Childhood. 2006;91:1015–1017. doi: 10.1136/adc.2006.097246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran T N, Ramirez-Serrano A, Vanderkooi O G, Kuhn S. Reducing children's pain and distress towards flu vaccinations: A novel and effective application of humanoid robotics. Vaccine. 2013;31:2772–2777. doi: 10.1016/j.vaccine.2013.03.056. [DOI] [PubMed] [Google Scholar]
- Blount R L, Bachanas P J, Powers S W, Cotter M C, Franklin A, Chaplin W, et al. Training children to cope and parents to coach them during routine immunizations: Effects of child, parent and staff behaviors. Behavior Therapy. 1992;23:689–705. [Google Scholar]
- Blount R L, Bunke V, Cohen L L, Forbes C J. The child-adult medical procedure interaction scale-short form (CAMPIS-SF): Validation of a rating scale for children's and adults' behaviors during painful medical procedures. Journal of Pain and Symptom Management. 2001;22:591–599. doi: 10.1016/s0885-3924(01)00303-7. [DOI] [PubMed] [Google Scholar]
- Blount R L, Corbin S M, Sturges J W, Wolfe V V, Prater J M, James L D. The relationship between adult's behavior and child coping and distress during BMA LP procedures: A sequential analysis. Behavior Therapy. 1989;20:585–601. [Google Scholar]
- Blount R L, Sturges J W, Powers S W. Analysis of child and adult behavioral variations by phase of medical procedure. Behavior Therapy. 1990;21:33–48. [Google Scholar]
- Caldwell P H, Dans L, de Vries M C, Newman Ba Hons J, Sammons H, Spriggs M, Bioeth M, Tambe P, Van't Hoff W, Woolfall K, Young B, Offringa M, StaR Child Health Group Standard 1: Consent and recruitment. Pediatrics. 2012;129:S118–S123. doi: 10.1542/peds.2012-0055D. [DOI] [PubMed] [Google Scholar]
- Caprilli S, Anastasi F, Grotto R P, Scollo Abeti M, Messeri A. Interactive music as a treatment for pain and stress in children during venipuncture: A randomized prospective study. Journal of Developmental and Behavioral Pediatrics. 2007;28:399–403. doi: 10.1097/DBP.0b013e31811ff8a7. [DOI] [PubMed] [Google Scholar]
- Cassidy K, Reid G J, McGrath P J, Finley G A, Smith D J, Morley C, Szudek E A, Morton B. Watch needle, watch TV: Audiovisual distraction in preschool immunization. Pain Medicine. 2002;3:108. doi: 10.1046/j.1526-4637.2002.02027.x. [DOI] [PubMed] [Google Scholar]
- Cavender K, Goff M D, Hollon E C, Guzzetta C E. Parents' positioning and distracting children during venipuncture: Effects on children's pain, fear, and distress. Journal of Holistic Nursing. 2004;22:32–56. doi: 10.1177/0898010104263306. [DOI] [PubMed] [Google Scholar]
- Chambers C T, Corkum P, Rusak B. Commentary: The importance of sleep in pediatric chronic pain - a wake-up call for pediatric psychologists. Journal of Pediatric Psychology. 2008;33:333–334. doi: 10.1093/jpepsy/jsn001. [DOI] [PubMed] [Google Scholar]
- Chambers C T, Taddio A, Uman L S, McMurtry C M. Psychological interventions for reducing pain and distress during routine childhood immunizations: A systematic review. Clinical Therapeutics. 2009;31:S77–S103. doi: 10.1016/j.clinthera.2009.07.023. [DOI] [PubMed] [Google Scholar]
- Chen E, Zeltzer L K, Craske M G, Katz E R. Children's memories for painful cancer treatment procedures: Implications for distress. Child Development. 2000;71:933–947. doi: 10.1111/1467-8624.00200. [DOI] [PubMed] [Google Scholar]
- Cochrane Handbook (2011). In J.P. T. Higgins & S. Green (Eds.), Cochrane handbook for systematic reviews of interventions (5.1.0 ed.). The Cochrane Collaboration. Retrieved from www.cochrane-handbook.org.
- Dahlquist L M, McKenna K D, Jones K K, Dillinger L, Weiss K E, Ackerman C S. Active and passive distraction using a head-mounted display helmet: Effects on cold pressor pain in children. Health Psychology. 2007;26:794–801. doi: 10.1037/0278-6133.26.6.794. [DOI] [PubMed] [Google Scholar]
- Dahlquist L M, Weiss K E, Law E F, Sil S, Herbert L J, Horn S B, Wohlheiter K, Ackerman C S. Effects of videogame distraction and a virtual reality type head-mounted display helmet on cold pressor pain in young elementary school-aged children. Journal of Pediatric Psychology. 2010;35:617–625. doi: 10.1093/jpepsy/jsp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMore M, Cohen L L. Distraction for pediatric immunization pain: A critical review. Journal of Clinical Psychology in Medical Settings. 2005;12:281–291. [Google Scholar]
- Fanurik D, Koh J L, Schmitz M L. Distraction techniques combined with EMLA: Effects on IV insertion pain and distress in children. Children's Health Care. 2000;29:87–101. [Google Scholar]
- Fowler-Kerry S, Lander J R. Management of injection pain in children. Pain. 1987;30:169–175. doi: 10.1016/0304-3959(87)91072-4. [DOI] [PubMed] [Google Scholar]
- Gold J I, Kim S H, Kant A J, Joseph M H, Rizzo A S. Effectiveness of virtual reality for pediatric pain distraction during i.v. placement. Cyberpsychology and Behavior. 2006;9:207–212. doi: 10.1089/cpb.2006.9.207. [DOI] [PubMed] [Google Scholar]
- Gonzalez J C, Routh D K, Armstrong F D. Effects of maternal distraction versus reassurance on children's reactions to injections. Journal of Pediatric Psychology. 1993;18:593–604. doi: 10.1093/jpepsy/18.5.593. [DOI] [PubMed] [Google Scholar]
- Goodenough B, Kampel L, Champion G D, Laubreaux L, Nicholas M K, Ziegler J B, McInerney M. An investigation of the placebo effect and age-related factors in the report of needle pain from venipuncture in children. Pain. 1997;72:383–391. doi: 10.1016/s0304-3959(97)00062-6. [DOI] [PubMed] [Google Scholar]
- Goodenough B, Thomas W, Champion G D, Perrott D, Taplin J E, von Baeyer C L, Ziegler J B. Unravelling age effects and sex differences in needle pain: Ratings of sensory intensity and unpleasantness of venipuncture pain by children and their parents. Pain. 1999;80:179–190. doi: 10.1016/s0304-3959(98)00201-2. [DOI] [PubMed] [Google Scholar]
- Goubert L, Vervoort T, Cano A, Crombez G. Catastrophizing about their children's pain is related to higher parent-child congruency in pain ratings: An experimental investigation. European Journal of Pain. 2009;13:196–201. doi: 10.1016/j.ejpain.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Gupta D, Agarwal A, Dhiraaj S, Tandon M, Kumar M, Singh R S, Singh P K, Singh U. An evaluation of efficacy of balloon inflation on venous cannulation pain in children: A prospective, randomized, controlled study. Anesthesia and Analgesia. 2006;102:1372–1375. doi: 10.1213/01.ane.0000205741.82299.d6. [DOI] [PubMed] [Google Scholar]
- Guyatt G, Oxman A D, Akl E A, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, Jaeschke R, Rind D, Meerpohl J, Dahm P, Schünemann H J. GRADE guidelines: 1. introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- Guyatt G, Thorlund K, Oxman A D, Walter S D, Patrick D, Furukawa T A, Johnston B C, Karanicolas P, Akl E A, Vist G, Kunz R, Brozek J, Kupper L L, Martin S L, Meerpohl J J, Alonso-Coello P, Christensen R, Schunemann H J. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles-continuous outcomes. Journal of Clinical Epidemiology. 2013;66:173–183. doi: 10.1016/j.jclinepi.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Hamm M P, Hartling L, Milne A, Tjosvold L, Vandermeer B, Thomson D, Curtis S, Klassen T P. A descriptive analysis of a representative sample of pediatric randomized controlled trials published in 2007. BMC Pediatrics. 2010;10 doi: 10.1186/1471-2431-10-96. 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart D, Bossert E. Self-reported fears of hospitalized school-age children. Journal of Pediatric Nursing. 1994;9:83–90. [PubMed] [Google Scholar]
- Hartling L, Hamm M, Klassen T, Chan A W, Meremikwu M, Moyer V, Scott S, Moher D, Offringa M, StaR Child Health Group Standard 2: Containing risk of bias. Pediatrics. 2012;129:S124–S131. doi: 10.1542/peds.2012-0055E. [DOI] [PubMed] [Google Scholar]
- Hicks C L, von Baeyer C L, Spafford P A, van Korlaar I, Goodenough B. The faces pain scale - revised: Toward a common metric in pediatric pain measurement. Pain. 2001;93:173–183. doi: 10.1016/S0304-3959(01)00314-1. [DOI] [PubMed] [Google Scholar]
- Higgins J P, Altman D G, Gotzsche P C, Juni P, Moher D, Oxman AD, Savovic J, Schulz K F, Weeks L, Sterne J A, Cochrane Bias Methods Group; Cochrane Statistical Methods Group The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet A, Lucas-Polomeni M M, Robert J C, Sixou J L, Wodey E. Hypnosis and dental anesthesia in children: A prospective controlled study. The International Journal of Clinical and Experimental Hypnosis. 2011;59:424–440. doi: 10.1080/00207144.2011.594740. [DOI] [PubMed] [Google Scholar]
- Inal S, Kelleci M. Distracting children during blood draw: Looking through distraction cards is effective in pain relief of children during blood draw. International Journal of Nursing Practice. 2012;18:210–219. doi: 10.1111/j.1440-172X.2012.02016.x. [DOI] [PubMed] [Google Scholar]
- Jay S M, Ozolins M, Elliott C H. Assessment of children's distress during painful medical procedures. Health Psychology. 1983;2:133–147. [Google Scholar]
- Jeffs D A. A pilot study of distraction for adolescents during allergy testing. Journal for Specialists in Pediatric Nursing. 2007;12:170–185. doi: 10.1111/j.1744-6155.2007.00110.x. [DOI] [PubMed] [Google Scholar]
- Katz E R, Kellerman J, Ellenberg L. Hypnosis in the reduction of acute pain and distress in children with cancer. Journal of Pediatric Psychology. 1987;12:379–394. doi: 10.1093/jpepsy/12.3.379. [DOI] [PubMed] [Google Scholar]
- Klassen J A, Liang Y, Tjosvold L, Klassen T P, Hartling L. Music for pain and anxiety in children undergoing medical procedures: A systematic review of randomized controlled trials. Ambulatory Pediatrics. 2008;8:117–128. doi: 10.1016/j.ambp.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Kleiber C, Craft-Rosenberg M, Harper D C. Parents as distraction coaches during IV insertion: A randomized study. Journal of Pain and Symptom Management. 2001;22:851–861. doi: 10.1016/s0885-3924(01)00316-5. [DOI] [PubMed] [Google Scholar]
- Kleiber C, Harper D C. Effects of distraction on children's pain and distress during medical procedures: A meta-analysis. Nursing Research. 1999;48:44–49. doi: 10.1097/00006199-199901000-00007. [DOI] [PubMed] [Google Scholar]
- Koller D, Goldman R D. Distraction techniques for children undergoing procedures: A critical review of pediatric research. Journal of Pediatric Nursing. 2012;27:652–681. doi: 10.1016/j.pedn.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Kristjansdottir O, Kristjansdottir G. Randomized clinical trial of musical distraction with and without headphones for adolescents' immunization pain. Scandinavian Journal of Caring Sciences. 2011;25:19–26. doi: 10.1111/j.1471-6712.2010.00784.x. [DOI] [PubMed] [Google Scholar]
- Kuttner L. Favorite stories: A hypnotic pain-reduction technique for children in acute pain. American Journal of Clinical Hypnosis. 1988;30:289–295. doi: 10.1080/00029157.1988.10402752. [DOI] [PubMed] [Google Scholar]
- Liossi C, Hatira P. Clinical hypnosis versus cognitive behavioral training for pain management with pediatric cancer patients undergoing bone marrow aspirations. The International Journal of Clinical and Experimental Hypnosis. 1999;47:104–116. doi: 10.1080/00207149908410025. [DOI] [PubMed] [Google Scholar]
- Liossi C, Hatira P. Clinical hypnosis in the alleviation of procedure-related pain in pediatric oncology patients. The International Journal of Clinical and Experimental Hypnosis. 2003;51:4–28. doi: 10.1076/iceh.51.1.4.14064. [DOI] [PubMed] [Google Scholar]
- Liossi C, White P, Hatira P. Randomized clinical trial of local anesthetic versus a combination of local anesthetic with self-hypnosis in the management of pediatric procedure-related pain. Health Psychology. 2006;25:307–315. doi: 10.1037/0278-6133.25.3.307. [DOI] [PubMed] [Google Scholar]
- Liossi C, White P, Hatira P. A randomized clinical trial of a brief hypnosis intervention to control venepuncture-related pain of paediatric cancer patients. Pain. 2009;142:255–263. doi: 10.1016/j.pain.2009.01.017. [DOI] [PubMed] [Google Scholar]
- MacLaren J E, Cohen L. A comparison of distraction strategies for venipuncture distress in children. Journal of Pediatric Psychology. 2005;30:387–396. doi: 10.1093/jpepsy/jsi062. [DOI] [PubMed] [Google Scholar]
- Malloy K M, Milling L S. The effectiveness of virtual reality distraction for pain reduction: A systematic review. Clinical Psychology Review. 2010;30:1011–1018. doi: 10.1016/j.cpr.2010.07.001. [DOI] [PubMed] [Google Scholar]
- McCarthy A M, Kleiber C, Hanrahan K, Zimmerman M B, Westhus N, Allen S. Impact of parent-provided distraction on child responses to an IV insertion. Children's Health Care. 2010;39:125–141. doi: 10.1080/02739611003679915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath P J, Johnson G, Goodman J T, Schillinger J, Dunn J, Chapman J. CHEOPS: A behavioral scale for rating postoperative pain in children. In: Fields H L, Dubner R, Cervero F, editors. Advances in pain research and therapy. New York, NY: Raven Press; 1985. pp. 395–402. [Google Scholar]
- McGrath P J, Walco G A, Turk D C, Dworkin R H, Brown M T, Davidson K, Eccleston C, Finley G A, Goldschneider K, Haverkos L, Hertz S H, Ljungman G, Palermo T, Rappaport B A, Rhodes T, Schechter N, Scott J, Sethna N, Svensson O K, Stinson J, von Baeyer C L, Walker L, Weisman S, White R E, Zajicek A, Zeltzer L, PedIMMPACT Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. The Journal of Pain. 2008;9:771–783. doi: 10.1016/j.jpain.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Merkel S I, Voepel-Lewis T, Shayevitz J R, Malviya S. The FLACC: A behavioral scale for scoring postoperative pain in young children. Pediatric Nursing. 1997;23:293–297. [PubMed] [Google Scholar]
- Montgomery G H, DuHamel K N, Redd W H. A meta-analysis of hypnotically induced analgesia: How effective is hypnosis? The International Journal of Clinical and Experimental Hypnosis. 2000;48:138–153. doi: 10.1080/00207140008410045. [DOI] [PubMed] [Google Scholar]
- Nguyen T N, Nilsson S, Hellstrom A L, Bengtson A. Music therapy to reduce pain and anxiety in children with cancer undergoing lumbar puncture: A randomized clinical trial. Journal of Pediatric Oncology Nursing. 2010;27:146–155. doi: 10.1177/1043454209355983. [DOI] [PubMed] [Google Scholar]
- Nilsson S, Finnstrom B, Kokinsky E. The FLACC behavioral scale for procedural pain assessment in children aged 5-16 years. Paediatric Anaesthesia. 2008;18:767–774. doi: 10.1111/j.1460-9592.2008.02655.x. [DOI] [PubMed] [Google Scholar]
- Noguchi L K. The effect of music versus nonmusic on behavioral signs of distress and self-report of pain in pediatric injection patients. Journal of Music Therapy. 2006;43:16–38. doi: 10.1093/jmt/43.1.16. [DOI] [PubMed] [Google Scholar]
- Powers S W. Empirically supported treatments in pediatric psychology: Procedure-related pain. Journal of Pediatric Psychology. 1999;24:131–145. doi: 10.1093/jpepsy/24.2.131. [DOI] [PubMed] [Google Scholar]
- Press J, Gidron Y, Maimon M, Gonen A, Goldman V, Buskila D. Effects of active distraction on pain of children undergoing venipuncture: Who benefits from it? The Pain Clinic. 2003;15:261–269. [Google Scholar]
- Richardson J, Smith J E, McCall G, Pilkington K. Hypnosis for procedure-related pain and distress in pediatric cancer patients: A systematic review of effectiveness and methodology related to hypnosis interventions. Journal of Pain and Symptom Management. 2006;31:70–84. doi: 10.1016/j.jpainsymman.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Sander Wint S, Eshelman D, Steele J, Guzzetta C E. Effects of distraction using virtual reality glasses during lumbar punctures in adolescents with cancer. Oncology Nursing Forum. 2002;29:E8–E15. doi: 10.1188/02.ONF.E8-E15. [DOI] [PubMed] [Google Scholar]
- Savovic J, Jones H, Altman D, Harris R, Juni P, Pildal J, Als-Nielsen B, Balk E, Gluud C, Gluud L, Ioannidis J, Schulz K, Beynon R, Welton N, Wood L, Moher D, Deeks J, Sterne J. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: Combined analysis of meta-epidemiological studies. Health Technology Assessment. 2012;16:1–82. doi: 10.3310/hta16350. [DOI] [PubMed] [Google Scholar]
- Sinha I P, Altman D G, Beresford M W, Boers M, Clarke M, Craig J, Alberighi O D, Fernandes R M, Hartling L, Johnston B C, Lux A, Plint A, Tugwell P, Turner M, van der Lee J H, Offringa M, Williamson P R, Smyth R L, StaR Child Health Group Standard 5: Selection, measurement, and reporting of outcomes in clinical trials in children. Pediatrics. 2012;129:S146–S152. doi: 10.1542/peds.2012-0055H. [DOI] [PubMed] [Google Scholar]
- Sinha M, Christopher N C, Fenn R, Reeves L. Evaluation of nonpharmacologic methods of pain and anxiety management for laceration repair in the pediatric emergency department. Pediatrics. 2006;117:1162–1168. doi: 10.1542/peds.2005-1100. [DOI] [PubMed] [Google Scholar]
- Skinner E A, Zimmer-Gembeck M J. The development of coping. Annual Review of Psychology. 2007;58:119–144. doi: 10.1146/annurev.psych.58.110405.085705. [DOI] [PubMed] [Google Scholar]
- Stevens B J, Abbott L K, Yamada J, Harrison D, Stinson J, Taddio A, Barwick M, Latimer M, Scott S D, Rashotte J, Campbell F, Finley G A, CIHR Team in Children's Pain Epidemiology and management of painful procedures in children in Canadian hospitals. Canadian Medical Association Journal. 2011;183:E403–E410. doi: 10.1503/cmaj.101341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson J N, Kavanagh T, Yamada J, Gill N, Stevens B. Systematic review of the psychometric properties, interpretability and feasibility of self-report pain intensity measures for use in clinical trials in children and adolescents. Pain. 2006;125:143–157. doi: 10.1016/j.pain.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Taddio A, Appleton M, Bortolussi R, Chambers C, Dubey V, Halperin S, Hanrahan A, Ipp M, Lockett D, MacDonald N, Midmer D, Mousmanis P, Palda V, Pielak K, Riddell R P, Rieder M, Scott J, Shah V. Reducing the pain of childhood vaccination: An evidence-based clinical practice guideline. Canadian Medical Association Journal. 2010;182:E843–E855. doi: 10.1503/cmaj.101720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddio A, Ipp M, Thivakaran S, Jamal A, Parikh C, Smart S, Sovran J, Stephens D, Katz J. Survey of the prevalence of immunization non-compliance due to needle fears in children and adults. Vaccine. 2012;30:4807–4812. doi: 10.1016/j.vaccine.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Tak J H, van Bon W H. Pain- and distress-reducing interventions for venipuncture in children. Child: Care, Health and Development. 2006;32:257–268. doi: 10.1111/j.1365-2214.2006.00578.x. [DOI] [PubMed] [Google Scholar]
- Tomlinson D, von Baeyer C L, Stinson J N, Sung L. A systematic review of faces scales for the self-report of pain intensity in children. Pediatrics. 2010;126:e1168–e1198. doi: 10.1542/peds.2010-1609. [DOI] [PubMed] [Google Scholar]
- Uman L S, Birnie K A, Noel M, Parker J A, Chambers C T, McGrath P J, Kisely S. Psychological interventions for needle-related procedural pain and distress in children and adolescents. The Cochrane Database of Systematic Reviews. 2013;10:1–137. doi: 10.1002/14651858.CD005179.pub3. [DOI] [PubMed] [Google Scholar]
- Uman L S, Chambers C T, McGrath P J, Kisely S. Psychological interventions for needle-related procedural pain and distress in children and adolescents (review) The Cochrane Database of Systematic Reviews. 2006;4:1–72. doi: 10.1002/14651858.CD005179.pub2. [DOI] [PubMed] [Google Scholar]
- Uman L S, Chambers C T, McGrath P J, Kisely S. A systematic review of randomized controlled trials examining psychological interventions for needle-related procedural pain and distress in children and adolescents: An abbreviated Cochrane review. Journal of Pediatric Psychology. 2008;33:842–854. doi: 10.1093/jpepsy/jsn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uman L S, Chambers C T, McGrath P J, Kisely S, Matthews D, Hayton K. Assessing the quality of randomized controlled trials examining psychological interventions for pediatric procedural pain: Recommendations for quality improvement. Journal of Pediatric Psychology. 2010;35:693–703. doi: 10.1093/jpepsy/jsp104. [DOI] [PubMed] [Google Scholar]
- van der Tweel I, Askie L, Vandermeer B, Ellenberg S, Fernandes R M, Saloojee H, Bassler D, Altman D G, Offringa M, van der Lee J H, StaR Child Health Group Standard 4: Determining adequate sample sizes. Pediatrics. 2012;129:S138–S145. doi: 10.1542/peds.2012-0055G. [DOI] [PubMed] [Google Scholar]
- Vervoort T, Craig K D, Goubert L, Dehoorne J, Joos R, Matthys D, Buysse A, Crombez G. Expressive dimensions of pain catastrophizing: A comparitive analysis of school children and children with clinical pain. Pain. 2008;134:59–68. doi: 10.1016/j.pain.2007.03.038. [DOI] [PubMed] [Google Scholar]
- Vessey J A, Carlson K L, McGill J. Use of distraction with children during an acute pain experience. Nursing Research. 1994;43:369–372. [PubMed] [Google Scholar]
- Wang Z X, Sun L H, Chen A P. The efficacy of non-pharmacological methods of pain management in school-age children receiving venepuncture in a paediatric department: A randomized controlled trial of audiovisual distraction and routine psychological intervention. Swiss Medical Weekly. 2008;138:579–584. doi: 10.4414/smw.2008.12224. [DOI] [PubMed] [Google Scholar]
- Wild M R, Espie C A. The efficacy of hypnosis in the reduction of procedural pain and distress in pediatric oncology: A systematic review. Journal of Developmental and Behavioral Pediatrics. 2004;25:207–213. doi: 10.1097/00004703-200406000-00010. [DOI] [PubMed] [Google Scholar]
- Williams K, Thomson D, Seto I, Contopoulos-Ioannidis D G, Ioannidis J P, Curtis S, Constantin E, Batmanabane G, Hartling L, Klassen T, for the StaR Child Health Group Standard 6: Age groups for pediatric trials. Pediatrics. 2012;129:S153–S160. doi: 10.1542/peds.2012-0055I. [DOI] [PubMed] [Google Scholar]
- Windich-Biermeier A, Sjoberg I, Dale J C, Eshelman D, Guzzetta C E. Effects of distraction on pain, fear, and distress during venous port access and venipuncture in children and adolescents with cancer. Journal of Pediatric Oncology Nursing. 2007;24:8–19. doi: 10.1177/1043454206296018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.