Abstract
The study of collagen IV has benefited greatly from the seminal work conducted by Arthur Veis and colleagues over three decades ago. Through a series of electron microscopy studies focused on lens basement membrane, an appreciation was gained for the distinct network-forming properties of collagen IV. Veis correctly suggested that network assembly is a phenomenon of the non-collagenous termini of the molecule. This review seeks to document how the field advanced following these seminal conclusions, including recent discoveries regarding the molecular reinforcement of networks that support Veis’ conclusions.
Keywords: Arthur Veis, LBM, sulfilimine bonds, electron microscopy, collagen networks, NC1 domain
Introduction
Collagen IV sits at the nexus of biomechanics, protein chemistry, and cell biology, offering tantalizing promise as a key to unlocking secrets of extracellular matrix biology. Similar to other matured disciplines, the field of collagen IV research has faced its own share of inherent technical challenges, including navigating the insolubility and massive size of the protein of interest. Historically, lens capsule has provided a convenient source of collagen IV due its relatively thick basement membrane and ease of isolation. As the biochemical properties of collagen IV has become progressively characterized, lens basement membrane (LBM) has repeatedly proved its merit alongside other well-known tissues sources including placenta and glomerulus.
The influence of collagen IV on cellular function may be inferred by noting the positioning of basement membranes underneath epithelial layers, and empiric evidence now supports the role of collagen IV in tissue stabilization [1,2]. Indeed, basement membrane disturbances due to genetic mutations with collagen IV alleles are the cause of Alport’s Syndrome [3] as well as some forms of cataracts [4,5] and stroke [6]. Moreover, collagen IV houses the target epitopes of Goodpasture’s autoimmune disease with immunogenicity being dependent on the conformation of the expressed protein [3,7]. Recent discoveries are now beginning to shed light on the molecular basis by which collagen IV supports the structural maintenance of tissues, namely in the covalent reinforcement of networks via sulfilimine cross-links [2].
It would thus appear that the field of collagen IV might be approaching an inflection point whereby deep biochemical understanding finds fresh appreciation within the context of cellular biology. In this regard, the distinctive qualities of LBM continue to place this unique matrix at a vanguard position within the field. Yet to pause the forward direction, great perspective is gained by first reflecting on the seminal work contributed by Dr. Arthur Veis towards understanding the collagenous scaffold of LBM. Through rigorous biochemical examinations and beautifully conducted electron microscopy, Veis and colleagues identified the critical structural differentiators of collagen IV networks that remain pillars in the field today.
Setting a Course: Arthur Veis and Colleagues
The Veis laboratory published a series of works more than three decades ago on the structural characteristics of lens capsule collagen [8–13]. From these works, one can greatly appreciate the distinctiveness by which collagen IV networks are structurally differentiated from D-periodic collagen fibrils. Electron micrographs of pepsin-solubilized lens basement membrane showed collagen IV as possessing unique cross-striations [8], and subsequent images revealed “dumbbell-shaped” molecules forming lattice-like networks of collagen [9].
Differences between the superstructure of collagen Types I and IV ultimately pointed Veis & colleague towards exploring the non-collagenous termini of collagen IV as a potential mechanistic explanation. Rigorous examination of pepsin-digested LBM led the Veis team to conclude that the non-collagenous end region of collagen IV is a critical determinant between the formation of networks and D-periodic fibrils [10]. These thoughts betray remarkable predictive insight into collagen IV network assembly as recent discoveries now point to covalent reinforcement of the C-terminal quaternary structure being a critical feature of collagen IV networks, influencing the overall structural integrity and essential functionality of basement membranes [2].
Discovering Networks
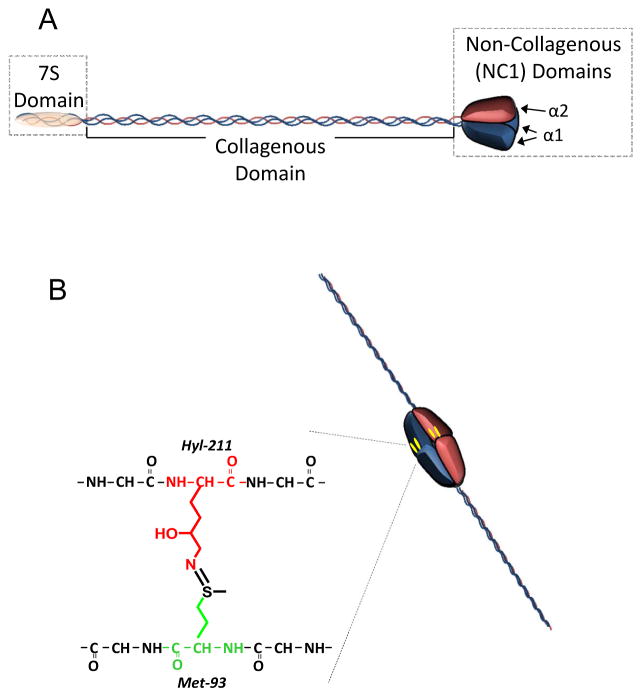
Since these publications, type IV collagen has been found to be composed of six homologous α chains (α1–6) that associate into heterotrimeric collagen IV protomers with each comprising a specific chain combination of α112, α345, or α556 [14]. Though differing in composition, all protomers share structural similarities of a lengthy triple-helical collagenous domain with the triplet peptide sequence of Gly-X-Y [15], and ends in a globular C-terminal region termed the non-collagenous 1 (NC1) domain (Figure 1). The N-termini is composed of a helical domain termed the 7S domain, named for its sedimentation coefficient [16], which is also involved in protomer-protomer interactions. Assembly of the heterotrimer initiates within the NC1 domains [17,18], then followed by the left-handed helical winding of the collagenous domain to establish the full-length protomer molecule.
Figure 1. Schematic of collagen IV protomeric structure.
(A) Domain structure of collagen IV protomer showing N-terminal 7S domain, triple helical collagenous domain, and C-terminal NC1 domain. (B) Adjoining protomers form a hexameric complex from constituent NC1 domains, which is covalently reinforced by sulfilimine cross-links.
Adjoining NC1 trimers connect via head-to-head interactions to form a hexameric quaternary structure from the six constituent α chain NC1 domains. Electrostatic interactions are present internally along the trimer-trimer interface, and it is likely that ionic bonds between the two trimers are central to the process of hexamer assembly [19]. Analysis of tissue isolates revealed additional covalent sulfilimine bonding (-S=N-) connecting methionine-93 and hydroxylysine-211 residues from opposing trimers [20]. Outside the NC1 hexamers, collagen IV networks assemble through extensive protomer-protomer interactions along the length of the heterotrimer. The N-terminal 7S domains of four independent protomers are covalently bound together into a dodecamer of α chains, thus allowing the protomer to be covalently secured at either termini [21]. Noncovalent interactions form rapidly along the collagenous domains among protomers to complete the mesh-like structure [22].
The mechanical functions of collagen IV in LBM are perhaps better understood from the standpoint of the α345 networks of collagen IV. Notably, LBM was a direct participant in the discovery of the collagen IV α3 chain [23], though the α345 network is expressed at low levels in this particular matrix. Mutational damage within a single chain of the α345 collagen IV protomer causes a loss of the entire α345 network, resulting in a spectrum of complications that corresponds to the normal biodistribution of the network and includes anterior lenticonus of the lens [3]. The α345 network is similar to the more widespread α112 network by containing sulfilimine cross-links [7] yet differs structurally by assuming a more interconnected and cross-linked superstructure [24], likely conferring enhanced tensile strength to the basement membrane. The loss of this network is associated with destabilization of the matrices where the α345 network is typically found, as evidenced by elevated 3-hydroxyproline in the urine of Alport’s patients suggesting degradation of the glomerular basement [25] as well as by increased strain in the lenses of Alport’s mice as measured by increase in lens thickness under osmostic stress [26]. Thus, the structure and functions of collagen IV networks may be influenced by the composition of the network.
Distinctiveness of LBM
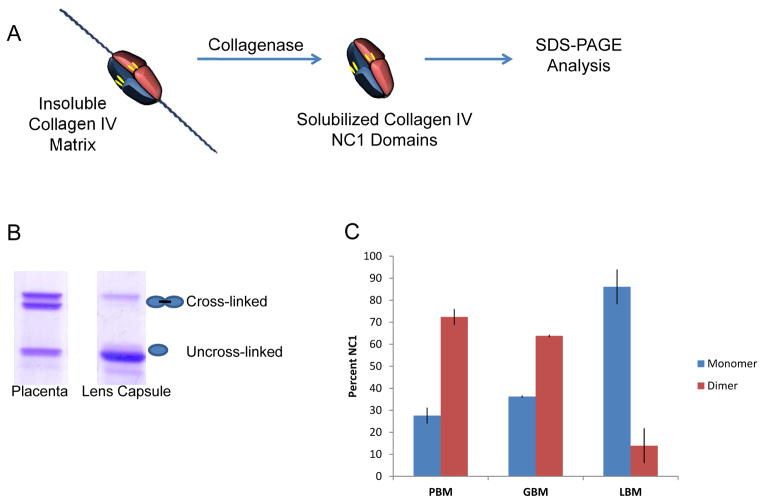
Covalent cross-linking of collagen IV NC1 domains via sulfilmine bonds creates a dimerized NC1 molecule that can be resolved from the monomeric subunits by SDS-PAGE. Indeed, densitometric analysis of dimer and monomer banding on SDS-PAGE provides a means of estimating the relative amount of sulfilimine cross-linked versus uncross-slinked NC1 domains, following their isolation from matrix via bacterial collagenase treatment. Notably, the percentage of crosslinked dimers is not constant throughout an organism but is rather dependent on the particular tissue source. Placental and glomerular basement membranes contain high amounts of sulfimine bonding while NC1 hexamers isolated from lens capsule contain minor amounts of cross-linking (Figure 2).
Figure 2. LBM is distinguished among basement membranes by its low amount of sulfilimine cross-linking.
(A) Schematic depicting the solubilization of NC1 domains from collagen IV matrices via collagenase treatment. (B) Non-reducing SDS-PAGE analysis of NC1 isolated from placenta and lens basement membrane. Upper protein bands represent NC1 dimers containing sulfilimine cross-links, while lower protein bands represent uncrosslinked NC1 domains. (C) Densitometic analysis of SDS-PAGE gels reveal LBM as being comprised of mostly monomeric NC1 domains, which contrasts with NC1 isolated from placental and glomerular basement membranes.
Collagen IV sulfilimine bonds are revealing themselves to be critical components of collagen IV networks, holding influence over the stability of nearby tissues. Strikingly, genetic mutation of either peroxidasin or collagen IV in Caenorhabditis elegans produce similar phenotypes of embryonic lethality due to basement membrane instability [27,28]. Using the Drosophila model system, a hypomorphic mutation in the enzyme resulted in strong reduction of collagen IV sulfilimine bonds, disordered extracellular matrices, disrupted tissue architecture, and embryonic lethality [2]. Interestingly, clinical observations of mutations within the enzyme catalyst of sulfilimine bond formation, peroxidasin (PXDN), note the development of congenital cataracts and other forms of anterior segment dysgenesis [29].
LBM contains noticeably less sulfilimine cross-links than other basement membranes, yet the cause is unclear. The mechanism of collagen IV sulfilimine bond formation is likely present in LBM, as seen by PXDN expression in lens and corneal epithelia in post-natal day 60 mice [29]. Embryonic expression of the enzyme is apparently restricted to corneal epithelia in E18.5 mice [29]. Considering that halides are required for sulfilimine bond formation [2], the aqueous and vitreous humors contain similar levels of chloride as found in serum [30,31]. The direct exposure of LBM to these fluids suggests that it has adequate amounts of halide to support bond formation, despite apparently lower chloride levels within lens interior [32]. Thus, after delineating the mechanism of sulfilmine bond formations, a natural next question might address how the occurrence of sulfilimine biochemistry is controlled in vivo. The significance of this issue is elevated by studies in flies and nematodes suggesting a critical role for sulfilimine-reinforced collagen IV scaffolding in maintaining the organization of basement membranes and tissues [2,27]. As in past extracellular discoveries, LBM is here poised to play a key role in understanding the tissue biology of collagen IV.
An Interface of Basement Membranes and Cell Biology
Anatomically, LBM encases the cellular components of lens, underlying a polarized layer of simple cuboidal epithelial cells as well as lens fiber cells. Obvious features required of LBM are optical transparency as well as flexibility to permit lens focusing via accommodation, yet the latter must be tempered with a degree of rigidity to control lens shape. The capsule acts as an encasement to support the lens, and is often utilized during the surgical repair of cataracts. In the current technique of extracapsular cataract extraction, the internal components of a cloudy lens are replaced with an artificial intraocular lens, and the capsule is left largely intact so as to house the synthetic lens. Unfortunately, posterior capsule opacification (PCO) develops as a complication in approximately 20–40% of patients at 2–5 years post-surgery [33] though this rate varies with age. Also termed “secondary cataracts”, they result from the proliferation and activity of residual lens epithelial cells along the posterior capsule in a response that is similar to wound healing [33].
Collagen IV has been shown to promote adherence of rabbit lens epithelial cells in vitro [34]. Efforts to identify synthetic materials that reduce the development of PCO revealed that adhesion of the collagen IV, fibronectin, and laminin to the intraocular lens prevents cell migration and may reduce the risk of PCO [35]. Of further interest is the role of growth factors in promoting epithelial cell migration, with particular interest placed on fibroblast growth factor, transforming growth factor β, epidermal growth factor, hepatocyte growth factor, insulin-like growth factor, and interleukin 1 and 6 [33,35]. Considering the evidence from Drosophila that collagen IV networks regulate the activity of growth factors through a scaffolding-based mechanism [36], it would be interesting to see whether the networks are involved with directing lens epithelial cell migration post-operatively.
Conclusion
LBM has established itself as a valuable model for investigating collagen IV biochemistry. The attention drawn by Veis and colleagues to lens collagen IV networks has developed into a broad understanding of the structure and linkages of these networks. Moving forward, this knowledge base provides a platform for dissecting the mechanisms by which collagen IV influences cellular behavior.
On a personal note, Art Veiss has been an inspirational scientist and leader in the collagen field for over five decades We are all grateful that he continues to maintain this influential role today, as evidenced through his active participation in the 2013 Gordon Research Conference on collagen as well as his recent work on the fundamental contributions of water to biogenic mineralization processes [37]. Grateful acknowledgement is also due for his inspirational contributions to a review article that he coauthored with the Hudson laboratory on the mechanism of collagen assembly [38].
Footnotes
Declaration of Author Interests: None
References
- 1.Poschl E, Schlotzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131:1619–28. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- 2.Bhave G, Cummings CF, Vanacore RM, Kumagai-Cresse C, Ero-Tolliver IA, Rafi M, Kang JS, Pedchenko V, Fessler LI, Fessler JH, Hudson BG. Peroxidasin forms sulfilimine chemical bonds using hypohalous acids in tissue genesis. Nat Chem Biol. 2012;8:784–90. doi: 10.1038/nchembio.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG. Alport’s Syndrome, Goodpasture’s Syndrome, and Type IV Collagen. N Eng J Med. 2003;348:2534–56. doi: 10.1056/NEJMra022296. [DOI] [PubMed] [Google Scholar]
- 4.Shah S, Kumar Y, McLean B, Churchill A, Stoodley N, Rankin J, Rizzu P, van der Knaap M, Jardine P. A dominantly inherited mutation in collagen IV A1 (COL4A1) causing childhood onset stroke without porencephaly. Eur J Paediatr Neurology. 2010;14:182–7. doi: 10.1016/j.ejpn.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Colville DJ, Savige J. Alport Syndrome. A review of the ocular manifestations. Ophthalmic Genet. 1997;18:161–73. doi: 10.3109/13816819709041431. [DOI] [PubMed] [Google Scholar]
- 6.Gould DB, Phalan FC, van Mil SE, Sundberg JP, Vahedi K, Massin P, Bousser MG, Heutink P, Miner JH, Tournier-Lasserve E, John SWM. Role of COL4A1 in small-vessel disease and hemorrhagic stroke. N Eng J Med. 2006;354:1489–1496. doi: 10.1056/NEJMoa053727. [DOI] [PubMed] [Google Scholar]
- 7.Vanacore R, Pedchenko V, Bhave G, Hudson BG. Sulphilimine cross-links in Goodpasture’s disease. Clin Exp Immunol 2011. 2011;164:4–6. doi: 10.1111/j.1365-2249.2011.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz D, Veis A. Characterization of basement membrane collagen of bovine anterior lens capsule via segment-long-spacing crystallites and the specific cleavage of the collagen by pepsin. FEBS Lett. 1978;85:326–32. doi: 10.1016/0014-5793(78)80484-0. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz D, Veis A. Characterization of bovine anterior-lens-capsule basement-membrane collagen. 2. Segment-long-spacing precipitates: further evidence for large N-terminal and C-terminal extensions. Eur J Biochem. 1980;103:29–37. doi: 10.1111/j.1432-1033.1980.tb04285.x. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz D, Chin-Quee T, Veis A. Characterization of bovine anterior-lens-capsule basement-membrane collagen. 1. Pepsin susceptibility, salt precipitation and thermal gelation: a property of non-collagen component integrity. Eur J Biochem. 1980;103:21–7. doi: 10.1111/j.1432-1033.1980.tb04284.x. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz D, Veis A. Structure of bovine anterior lens capsule basement membrane collagen molecules from electron microscopy. Biopolymers. 1979;18:2363–7. doi: 10.1002/bip.1979.360180924. [DOI] [PubMed] [Google Scholar]
- 12.Uitto VJ, Schwartz D, Veis A. Degradation of basement-membrane collagen by neutral proteases from human leukocytes. Eur J Biochem. 1980;105:409–17. doi: 10.1111/j.1432-1033.1980.tb04515.x. [DOI] [PubMed] [Google Scholar]
- 13.Veis A, Schwartz D. The structure of acid-soluble basement membrane collagen from bovine anterior lens capsule: molecular parameters and thermal gelation properties. Coll Relat Res. 1981;1:269–86. doi: 10.1016/s0174-173x(81)80004-0. [DOI] [PubMed] [Google Scholar]
- 14.Khoshnoodi J, Pedchenko V, Hudson BG. Mammalian collagen IV. Microsc Res Tech. 2008;71:357–70. doi: 10.1002/jemt.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Timpl R, Bruckner P, Fietzek P. Characterization of pepsin fragments of basement membrane collagen obtained from a mouse tumor. Eur J Biochem. 1979;95:255–263. doi: 10.1111/j.1432-1033.1979.tb12961.x. [DOI] [PubMed] [Google Scholar]
- 16.Risteli J, Bachinger HP, Engel J, Furthmayr H, Timpl R. 7-S collagen: characterization of an unusual basement membrane structure. Eur J Biochem. 1980;108:239–250. doi: 10.1111/j.1432-1033.1980.tb04717.x. [DOI] [PubMed] [Google Scholar]
- 17.Boutaud A, Borza DB, Bondar O, Gunwar S, Netzer KO, Singh N, Ninomiya Y, Sado Y, Noelken ME, Hudson BG. Type IV collagen of the glomerular basement membrane. Evidence that the chain specificity of network assembly is encoded by the noncollagenous NC1 domains. J Biol Chem. 2000;275:30716–24. doi: 10.1074/jbc.M004569200. [DOI] [PubMed] [Google Scholar]
- 18.Khoshnoodi J, Sigmundsson K, Cartailler JP, Bondar O, Sundaramoorthy M, Hudson BG. Mechanism of chain selection in the assembly of collagen IV: a prominent role for the alpha2 chain. J Biol Chem. 2006;281:6058–69. doi: 10.1074/jbc.M506555200. [DOI] [PubMed] [Google Scholar]
- 19.Sundaramoorthy M, Meiyappan M, Todd P, Hudson BG. Crystal structure of NC1 domains. Structural basis for type IV collagen assembly in basement membranes. J Biol Chem. 2002;277:31142–53. doi: 10.1074/jbc.M201740200. [DOI] [PubMed] [Google Scholar]
- 20.Vanacore R, Ham AJ, Voehler M, Sanders CR, Conrads TP, Veenstra TD, Sharpless KB, Dawson PE, Hudson BG. A sulfilimine bond in collagen IV. Science. 2009;325:1230–4. doi: 10.1126/science.1176811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Timpl R, Wiedemann H, van Delden V, Kuhn K. A network model for the organization of type IV collagen molecules in basement membranes. Eur J Biochem. 1981;120:203–11. doi: 10.1111/j.1432-1033.1981.tb05690.x. [DOI] [PubMed] [Google Scholar]
- 22.Yurchenco PD, Furthmayr H. Self-assembly of basement membrane collagen. Biochemistry. 1984;23:1839–50. doi: 10.1021/bi00303a040. [DOI] [PubMed] [Google Scholar]
- 23.Butkowski RJ, Langeveld JP, Wieslander J, Hamilton J, Hudson BG. Localization of the Goodpasture epitope to a novel chain of basement membrane collagen. J Biol Chem. 1987;262:7874–7. [PubMed] [Google Scholar]
- 24.Gunwar S, Ballester F, Noelken ME, Sado Y, Ninomiya Y, Hudson BG. Glomerular basement membrane. Identification of a novel disulfide-cross-linked network of alpha3, alpha4, and alpha5 chains of type IV collagen and its implications for the pathogenesis of Alport syndrome. J Biol Chem. 1998;273:8767–75. doi: 10.1074/jbc.273.15.8767. [DOI] [PubMed] [Google Scholar]
- 25.Bartosch B, Vycudilik W, Popow C, Lubec G. Urinary 3-hydroxyproline excretion in Alport’s syndrome: a non-invasive screening test? Arch Dis Child. 1991;66:248–51. doi: 10.1136/adc.66.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gyoneva L, Segal Y, Dorfman KD, Boracas VH. Mechanical response of wild-type and Alport murine lens capsules during osmotic swelling. Exp Eye Res. 2013;113C:87–91. doi: 10.1016/j.exer.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Gotenstein JR, Swale RE, Fukuda T, Wu Z, Giurumescu CA, Goncharov A, Jin Y, Chisholm AD. The C. elegans peroxidasin PXN-2 is essential for embryonic morphogenesis and inhibits adult axon regeneration. Development. 2010;137:3603–13. doi: 10.1242/dev.049189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta MC, Graham PL, Kramer JM. Characterization of alpha1(IV) collagen mutations in Caenorhabditis elegans and the effects of alpha1 and alpha2(IV) mutations on type IV collagen distribution. J Cell Biol. 1997;137:1185–1196. doi: 10.1083/jcb.137.5.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan K, Rudkin A, Parry DA, Burdon KP, McKibbin M, Logan CV, Abdelhamed ZI, Muecke JS, Fernandez-Fuentes N, Laurie KJ, Shires M, Fogarty R, Carr IM, Poulter JA, Morgan JE, Mohamed MD, Jafri H, Raashid Y, Meng N, Piseth H, Toomes C, Casson RJ, Taylor GR, Hammerton M, Sheridan E, Johnson CA, Inglehearn CF, Craig JE, Ali M. Homozygous mutations in PXDN cause congenital cataract, corneal opacity, and developmental glaucoma. Am J Hum Genet. 2011;89:464–73. doi: 10.1016/j.ajhg.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kinsey VE. Aqueous-humor/plasma-chloride ratios in rabbits, dogs, and human beings. J Gen Physiol. 1949;32:329–38. doi: 10.1085/jgp.32.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jashnani KD, Kale SA, Rupani AB. Vitreous humor: biochemical constituents in estimation of postmortem interval. J Forensic Sci. 2010;55:1523–7. doi: 10.1111/j.1556-4029.2010.01501.x. [DOI] [PubMed] [Google Scholar]
- 32.Paterson CA, Eck BA. Chloride concentration and exchange in rabbit lens. Exp Eye Res. 1971;11:207–13. doi: 10.1016/s0014-4835(71)80024-6. [DOI] [PubMed] [Google Scholar]
- 33.Awasthi N, Guo S, Wagner BJ. Posterior capsular opacification: a problem reduced but not yet eradicated. Arch Ophthalmol. 2009;127:555–62. doi: 10.1001/archophthalmol.2009.3. [DOI] [PubMed] [Google Scholar]
- 34.Olivero DK, Furcht LT. Type IV collagen, laminin, and fibronectin promote the adhesion and migration of rabbit lens epithelial cells in vitro. Invest Ophthalmol Vis Sci. 1993;34:2825–34. [PubMed] [Google Scholar]
- 35.Raj SM, Vasavada AR, Johar SR, Vasavada VA, Vasavada VA. Post-operative capsular opacification: a review. Int J Biomed Sci. 2007;3:237–50. [PMC free article] [PubMed] [Google Scholar]
- 36.Sawala A, Sutcliffe C, Ashe HL. Multistep molecular mechanism for bone morphogenetic protein extracellular transport in the Drosophila embryo. Proc Natl Acad Sci USA. 2012;109:11222–7. doi: 10.1073/pnas.1202781109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dorvee JR, Veis A. Water in the formation of biogenic minerals: Peeling away the hydration layers. J Struct Biol. 2013 Jun 19; doi: 10.1016/j.jsb.2013.06.007. S1047-8477(13)00162-7. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khoshnoodi J, Cartailler JP, Alvares K, Veis A, Hudson BG. Molecular recognition in the assembly of collagens: terminal noncollagenous domains are key recognition modules in the formation of triple helical protomers. J Biol Chem. 2006;281:38117–21. doi: 10.1074/jbc.R600025200. [DOI] [PubMed] [Google Scholar]