Abstract
Infection is common after stroke and is independently associated with a worse outcome. The predisposition to infection following stroke is in part related to a sympathetically mediated suppression of the peripheral immune response. The teleological explanation for this immune dysfunction is that it serves to prevent autoimmune responses to brain antigens. We believe that the systemic immune response in patients who develop infection, however, thwarts this seemingly protective response and predisposes to central nervous system autoimmunity. These autoimmune responses may mediate, at least in part, the worse outcome associated with post-stroke infection.
Keywords: autoimmune, infection, MBP, stroke, Th1, Treg
Infection is common after stroke and associated with worse stroke outcome. How infection confers a worse outcome is not clear. Our research suggests that the inflammatory response associated with systemic infection predisposes to the development of Th1-type autoimmune responses to CNS antigens exposed to circulating lymphocytes by virtue of stroke-induced breakdown of the blood–brain barrier (BBB) and ischemic injury to the brain. In addition, strategies to prevent development of these Th1-type responses are associated with improved outcome. We thus propose that the link between post-stroke infection and worse clinical outcome is the development of CNS autoimmune responses, which are provoked by infection.
Infection and stroke outcome
A link between infection and the risk of stroke has been appreciated for years (Grau et al. 2010). Recent data suggest that in addition to increasing the risk of stroke, infections occurring within a week of stroke onset increase early morbidity and mortality (Grabska et al. 2011). As suggested by clinical observations, animals with chronic systemic infections experience greater ischemic brain injury (Denes et al. 2010). Most patients who develop stroke, however, are infection free at the time of symptom onset. Given that infection is common after stroke, however, the effects of post-stroke infection on stroke outcome must also be considered. Depending on the patient population studied, published post-stroke infection rates can vary dramatically. In a systematic review of publications, post-stroke infection was estimated to occur in approximately 30% of patients (Westendorp et al. 2011). The most common infections are pneumonias (PNAs) and urinary tract infections (UTIs), each occurring in about 10% of patients with stroke; for patients admitted to the intensive care unit, the rates of infection are much higher (45% of patients with any infection, 28% with PNA, and 20% with UTI) (Westendorp et al. 2011).
In a prospective randomized controlled trial of a novel neuroprotective agent, 158/1455 (11%) of patients developed PNA in the first week, and 146/1455 (10%) developed UTI. For patients alive at day 7, those who had PNA were nearly two times more likely to die and three to four times more likely to have a poor outcome at 3 months, even after controlling for baseline prognostic variables like stroke severity, age, gender, and medical comorbidities. UTIs, on the other hand, were not associated with increased mortality, but did increase the likelihood (two to threefold) of poor outcome at 3 months (Aslanyan et al. 2004). Multiple other studies also suggest that infection is an independent risk factor for poor outcome after stroke (Tanzi et al. 2011; Grabska et al. 2011; Hong et al. 2008; Vermeij et al. 2009). Almost without exception, these studies suggest that PNA is more detrimental than UTI (Westendorp et al. 2011). Why PNA confers worse long-term outcome than UTI is unclear, but it seems likely that the systemic immune response associated with PNA is a more robust than that associated with UTI.
Few studies address infection in animal models of stroke. Given the incidence of post-stroke infection in the clinical setting, it would seem reasonable to track infection as an outcome in animal models of stroke; documentation of the incidence and severity of infection in animals receiving immunomodulatory therapies for the treatment of stroke is especially important. In at least some settings, post-stroke infection in animals is common and frequently fatal; prophylactic treatment with antibiotics prevents these infections and decreases mortality (Meisel et al. 2004). Data also show that the risk of infection is dependent on the strain of animal, suggesting that these strain differences could be capitalized upon to better understand the genesis of post-stroke infection (Schulte-Herbruggen et al. 2006).
Stroke-related immunodepression
Following experimental stroke, there is a profound dysfunction of the immune system that appears to be sympathetically mediated(Prass et al. 2003). This dysfunction is in part related to stroke-induced lymphopenia(Prass et al. 2003; Liesz et al. 2009a). In addition, lymphocytes from animals undergoing middle cerebral artery occlusion (MCAO) appear to have a defect in Th1-type immune responses, as demonstrated by impaired secretion of tumor necrosis factor (TNF)-α upon stimulation with lipopolysaccharide (LPS) and interferon (IFN)-γ upon stimulation with concanavalin A (Prass et al. 2003). A more recent study suggests that activation of the sympathetic nervous system following experimental stroke also impairs the response of hepatic invariant natural killer T cells predisposing to infection (Wong et al. 2011). Based on accumulated data, it seems likely that there are redundant pathways that lead to post-stroke immunodepression, primarily driven by the sympathetic response, but also with contributions from the endocrine system (Prass et al. 2003).
In patients, the risk of infection appears to be directly related to stroke severity/infarct volume (Chamorro et al. 2006; Hug et al. 2009; Haeusler et al. 2008; Tanzi et al. 2011). Several studies show an association between a variety of biomarkers, such as interleukin (IL)-10 and catecholamines, and post-stroke infection (Chamorro et al. 2006, 2007; Harms et al. 2011; Klehmet et al. 2009). As stroke induces a sympathetic response and a rise in a number of different cytokines, it is difficult to know whether these blood-born substances contribute to infection risk or merely serve as biomarkers for patients with severe stroke. Most of the studies reporting a link between biomarkers and infection risk do not systematically exclude patients who are already infected or control for stroke severity. In our study of 112 patients with ischemic stroke, however, we found that elevated plasma IL-1 receptor antagonist (IL-1ra) concentrations in infection-free patients were associated with an increased risk of later infection, even after controlling for stroke severity using the National Institutes of Health Stroke Scale (NIHSS) score (Tanzi et al. 2011).
Lymphopenia is common after stroke, and the degree of lymphopenia correlates with stroke severity (Czlonkowska et al. 1979; Vogelgesang et al. 2008; Urra et al. 2009b; Hug et al. 2009; Haeusler et al. 2012). Whether surviving lymphocytes function normally, however, is less clear. Some studies suggest that lymphocyte function is impaired, as detected by decreased proliferation of mitogens and decreased ex vivo secretion of pro-inflammatory cytokines (Rogers et al. 1998; Hug et al. 2009; Haeusler et al. 2012, 2008; Urra et al. 2009b). Other studies show that despite decreased lymphocyte numbers following stroke, their function is intact (Ferrarese et al. 1999; Vogelgesang et al. 2010; Tanzi et al. 2011). Table 1 shows data from our clinical study of patients with ischemic stroke and the correlation between stroke severity (based on NIHSS score or infarct volume), lymphocyte numbers, and lymphocyte function (as detected by the number of cells secreting IFN-γ in response to phytohemagglutinin). These data confirm the decrease in lymphocyte numbers associated with severe stroke, but do not show an alteration in their capacity to respond to the mitogen phytohemagglutinin. Whether these lymphocytes respond normally to typical infectious pathogens is unknown.
Table 1.
Correlation between stroke severity, lymphocyte numbers, and lymphocyte function
| Day 1 n = 25 | Day 3 n = 90 | Day 7 n = 89 | Day 30 n = 86 | |
|---|---|---|---|---|
| Correlation with NIHSS score* | ||||
| Lymphocytes | −0.656 p < 0.001 | −0.331 p = 0.001 | −0.227 p = 0.033 | −0.046 NS |
| Lymphocyte response to PHA | −0.157 NS | 0.075 NS | −0.125 NS | 0.064 NS |
|
| ||||
| Correlation with infarct volume† | ||||
| Lymphocytes | −0.564 p = 0.003 | −0.365 p < 0.001 | −0.252 p = 0.024 | −0.123 NS |
| Lymphocyte response to PHA | −0.211 NS | −0.058 NS | −0.072 NS | 0.058 NS |
Data are presented as *Spearman’s rho or †Pearson’s r.
Lymphocyte function was determined by the number of cells secreting interferon-γ in response to stimulation with PHA (as determined by enzyme-linked immunosorbent spot (ELISPOT) assay. NIHSS, National Institutes of Health Stroke Scale; PHA, phytohemagglutinin; NS, not significant (p > 0.200).
In addition to impaired lymphocyte responses following stroke, many studies demonstrate ‘deactivation’ of monocytes with loss of human leukocyte antigen-DR (Haeusler et al. 2008, Zhang et al. 2009; Urra et al. 2009a). At the same time, there are reports of increased expression of toll-like receptor (TLR) 4 on monocytes (Urra et al. 2009a). The number of monocytes in circulation following stroke, however, is elevated (Chamorro et al. 2006; Urra et al. 2009a; Hug et al. 2009). In our cohort of ischemic stroke patients, the number of monocytes was clearly related to the NIHSS score (ρ = 0.634, p < 0.001 at 72 h). Given this relationship to stroke severity, it might be expected that the number of monocytes was higher among patients who later developed an infection and that the strength of the association would be attenuated (but not lost) after controlling for stroke severity (Table 2). Other investigators have shown a similar relationship between monocyte numbers and the risk of post-stroke infection (Chamorro et al. 2006; Urra et al. 2009a). It is important to remember, however, that not all monocytes are the same, and the phenotype matters (Urra et al. 2009c).
Table 2.
The number of monocytes* in patients with and without infection
| Infection by day 15: | All patients
|
Patients with infection at 72 h eliminated (n = 7)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Yes n = 26 | No n = 77 | Uncontrolled
|
Controlled for NIHSS
|
Yes n = 19 | No n = 77 | Uncontrolled
|
Controlled for NIHSS
|
|
| p | p | p | p | |||||
| Monocytes* (thou/microL) | 0.88 (0.58, 1.13) | 0.65 (0.46, 0.83) | 0.012 | NS | 1.04 (0.65, 1.20) | 0.65 (0.46, 0.83) | <0.001 | 0.063 |
| Predictive value | Uncontrolled
|
Controlled for NIHSS
|
Uncontrolled
|
Controlled for NIHSS
|
||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Monocytes* (per thou/microL) | 10.14 (2.09–49.26) | 0.004 | 2.12 (0.30–15.16) | NS | 34.35 (4.86–242.74) | <0.001 | 8.81 (0.68–98.12) | p = 0.097 |
The number is higher among patients destined for infection in the first 15 days after stroke onset, and the difference is even more marked after eliminating patients with infection in the first 3 days after stroke onset. The difference in monocytes numbers is attenuated by controlling for stroke severity. Similarly, the odds for infection rises dramatically with every 1000 monocytes per microliter of blood and is most notable in those patients who are infection free at the time the blood was drawn.
Data are presented as median (interquartile range) and statistics are by Mann–Whitney U test.
Highest value by 72 h after stroke onset. thou/microL, thousand per microliter; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio; CI, confidence interval; NS, not significant (p > 0.200).
Consistent with the idea of immunodepression following stroke, some recent studies suggest that serum TNF-α is decreased after stroke (Vogelgesang et al. 2010; Urra et al. 2009a). Most studies, however, show a dramatic increase in plasma TNF-α and other pro-inflammatory cytokines following stroke that correlates with stroke severity (Zaremba and Losy 2001; Montaner et al. 2003; Intiso et al. 2004; Mazzotta et al. 2004; Sotgiu et al. 2006; Tuttolomondo et al. 2009; Tanzi et al. 2011; Offner et al. 2006). If lymphocytes and monocytes are not the source of this TNF-α, it is not clear what the source is. Changes in plasma IFN-γ following stroke are rarely noted, but when they are reported, they are often noted to be elevated (Urra et al. 2009a). We were unable to detect plasma IFN-γ in most of our ischemic stroke patient cohort.
Relative immunologic privilege of the brain
It has long been appreciated that the immune response within the brain differs from that in other organs. Part of this difference is related to the presence of the BBB, which limits the transit of leukocytes, as well as large molecules like immunoglobulins, into the brain. Just as important as the BBB, however, is the fact that the normal brain does not readily support the development of an immune response (Bailey et al. 2006). For instance, microglia, the resident antigen-presenting cells (APCs) in the brain, express no or very low levels of the major histocompatibility complex (MHC) II molecule, which is necessary for T-cell recognition of the antigen. In addition, the costimulatory molecules needed for activation of T cells during antigen presentation are generally absent (Dangond et al. 1997; Becker et al. 2005). Finally, the transforming growth factor (TGF)-β1, a potent immunomodulatory cytokine, is present in the resting brain and rapidly up-regulated following ischemia (Ali et al. 2001; Krupinski et al. 1996). The presence of TGF-β1 thus serves to inhibit the development of inflammatory immune responses.
Following stroke, there is at least a transient compromise in the BBB that allows cells of the immune system entrance to the brain parenchyma (Chen et al. 2009). Indeed, multiple studies show that there is an early influx of neutrophils followed by a later influx of lymphocytes. Lymphocytes can be found within the ischemic territory as early as a day or so after stroke onset and become increasingly numerous over the ensuing days (Schroeter et al. 1994; Jander et al. 1995). Within the brain, microglia become activated following stroke and are able to express MHC II, although the degree of expression is somewhat limited in comparison with other cell types (Felger et al. 2010). For instance, dendritic cells from the peripheral circulation infiltrate the infarct core within days after MCAO, while brain-resident dendritic cells (distinct from microglia) can be found at the periphery of the infarct (Felger et al. 2010). These dendritic cells are seen in close apposition to infiltrating lymphocytes, respond to IFN-γ, and are efficient at driving antigen-specific T-cell responses (Felger et al. 2010; Gottfried-Blackmore et al. 2009).
Disruption of the BBB following stroke also allows for CNS antigens to ‘leak’ into the peripheral circulation early after the onset of ischemia. The concentration of these antigens parallels the severity of the stroke and reflects cell death (Jauch et al. 2006; Herrmann et al. 2000). It is thus possible that these antigens can be processed and presented to the immune system in peripheral organs such as the spleen and lymph nodes. In fact, animal studies demonstrate the presentation of CNS antigens to lymphocytes in the cervical lymph nodes within hours of stroke onset, and a recent clinical study found evidence of CNS antigen presentation in the tonsils of patients by within a few days after stroke onset (van Zwam et al. 2009; Planas et al. 2012).
Bystander activation, the nature of the immune response, and autoimmune diseases
T-lymphocyte activation to an antigen generally requires that the antigen be processed by an APC and presented to the lymphocyte in the context of MHC II. In general, this means that the T cell must traffic to the site of antigen presentation; following stroke, antigen presentation can occur in both the brain and in the periphery. Previous studies, including our own, have demonstrated that inhibition of very late antigen-4 can improve stroke outcome in the days following MCAO (Becker et al. 2001; Relton et al. 2001; Liesz et al. 2011b). Inhibition of lymphocyte trafficking into brain by other methods, including antagonizing sphingosine-1-phosphate (S1P) receptors, has yielded mixed results, but is largely found to be beneficial as well (Liesz et al. 2011a; Wei et al. 2011; Hasegawa et al. 2010; Czech et al. 2009).
All of these studies evaluate short-term outcomes following experimental stroke, however, and therefore do not address the adaptive immune response to CNS antigens, which takes time to develop. In addition, these studies address the effects of therapies which non-selectively block the trafficking of lymphocytes, including natural killer cells and other cells that act in an antigen-non-specific manner. For lymphocytes to become activated to a specific antigen, that antigen must be recognized by appropriate T-cell receptor, and the lymphocyte must receive adequate costimulation from the APC. The nature of the immune response that develops from the interaction between the APC and the lymphocyte depends upon the microenvironment at the site of antigen presentation. (Zygmunt & Veldhoen 2011) Continued or repeated antigen exposure then leads to expansion of the lymphocyte population, a process that takes days to weeks to occur, based on demonstrations by models of experimental allergic encephalomyelitis where disease does not appear until at least day 10 after active immunization (Mannie et al. 2009).
Following stroke, the sympathetically mediated immune dysfunction tends to be associated with a reduced efficacy of circulating costimulatory cells and loss of human leukocyte antigen-DR (Hug et al. 2011; Urra et al. 2009a; Hug et al. 2009). As mentioned above, the general milieu of the brain does not support the development of inflammatory immune responses (Romo-Gonzalez et al. 2012; Suter et al. 2003). Several studies show an increase in the number of Tregs (Fig. 1) following stroke (Urra et al. 2009b; Yan et al. 2012). In experimental studies, depletion of Tregs is associated with increased inflammation, increased infarct volume at 1 week (but not at 2 days) and worse functional outcome from experimental stroke (Liesz et al. 2009b). In another study, however, depletion of Tregs prior to MCAO did not appear to affect infarct volume at 4 days (Ren et al. 2011). The role of Tregs in these studies was evaluated through depletion of Tregs by administration of anti-CD25 antibodies, meaning that all Treg cells, regardless of their antigen specificity, were depleted. Because the development of endogenous antigen-specific Treg cells takes time, it seems very unlikely that the Treg cells depleted in these studies recognized CNS antigens. Following experimental stroke, we found that the predominant immune response to myelin basic protein (MBP) after MCAO was that of a Treg response, but this response took many days to develop (Becker et al. 2005). Table 3 shows the change in the number of cells secreting TGF-β1, a Treg cytokine, in an antigen-specific fashion in our clinical study. At day 4 after stroke, there were trends for more cells to secret TGF-β1 in response to stimulation with glial fibrillary acidic protein and tetanus toxin in comparison to ‘healthy’ controls. By day 180 after stroke, the TGF-β1 response to neuron-specific enolase (NSE) and proteolipid protein were much more robust among patients with stroke, with trends toward enhanced TGF-β1 responses to other antigens as well. Furthermore, these responses tend to become more robust over time, a finding which is especially true for NSE. Finally, among patients who experienced an early infection (first 15 days), the TGF-β1 response to NSE, S100, and proteolipid protein was significantly greater (p < 0.05) at 180 days than in patients without early infection.
Fig. 1.
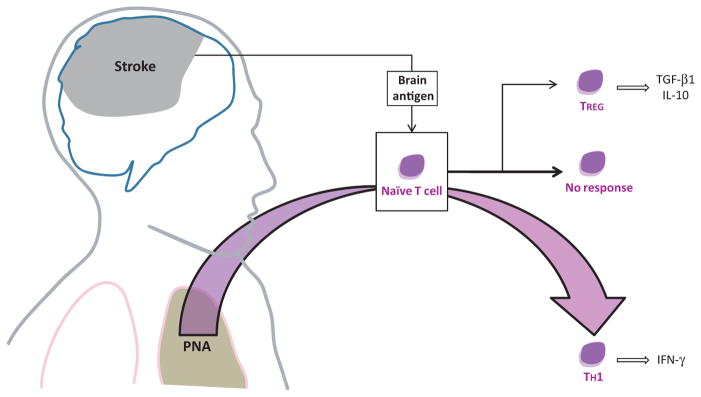
The usual lymphocyte responses to brain antigens following stroke are either no response or a TREG response. In the setting of a systemic infection, however, the immune system is activated and can predispose to the development of TH1 cells that are potentially detrimental. PNA, pneumonia; TGF, transforming growth factor; IL, interleukin; IFN, interferon.
Table 3.
Relative increase in the number of cells secreting TGF-β1 in response to stimulation with different CNS antigens and TT in patients with stroke and in ‘healthy controls’
| Controls n = 39 Median (IQR) |
Day 4
|
Day 180
|
Change over time p | |||
|---|---|---|---|---|---|---|
| Median (IQR) | p | Median (IQR) | p | |||
| MBP | 1.09 (0.90, 1.43) | 1.17 (0.88, 1.69) | NS | 1.53 (0.84, 2.66) | 0.091 | 0.086 |
| NSE | 1.20 (0.90, 1.20) | 1.41 (0.94, 2.19) | NS | 2.19 (1.20, 5.20) | < 0.001 | 0.003 |
| S100 | 1.22 (0.79, 1.78) | 1.39 (1.00, 1.98) | NS | 1.76 (0.85, 3.60) | 0.068 | 0.198 |
| PLP | 1.28 (1.03, 1.61) | 1.42 (0.92, 2.57) | NS | 1.93 (0.98, 4.32) | 0.033 | 0.185 |
| GFAP | 1.26 (0.92, 1.77) | 1.64 (0.92, 2.52) | 0.069 | 1.60 (0.99, 4.17) | 0.073 | NS |
| TT | 1.24 (0.87, 1.78) | 1.53 (1.02, 3.05) | 0.059 | 1.66 (0.97, 2.95) | 0.079 | NS |
Number of cells secreting transforming growth factor (TGF)-β1 determined by enzyme-linked immunosorbent spot (ELISPOT) assay. IQR, interquartile range; MBP, myelin basic protein; NSE, neuron-specific enolase; PLP, proteolipid protein; GFAP, glial fibrillary acidic protein; TT, tetanus toxin; significant (p < 0.05) are in bold; NS, not significant (p > 0.200).
The teleological explanation for induction of a Treg-type response is that it limits the possibility of developing detrimental autoimmune responses to brain in the setting of BBB compromise. Given the propensity for infection following stroke, however, we argue that this seemingly protective ‘immunodepressive’ response can be thwarted. Specifically, infections (PNA in particular) can induce systemic inflammation, activate APCs/dendritic cells through the TLR pathway, and alter the milieu of the brain and lymphoid organs, all of which serve to optimize the conditions for lymphocyte activation. For example, activation of TLRs through agents like LPS induces expression of MHC II and costimulatory molecules on dendritic cells and microglia (Bauer et al. 2009; Olson and Miller 2004; Raymond and Wilkie 2005). Systemic infection is also associated with an increase in circulating pro-inflammatory cytokines like TNF-α and IFN-γ (de Werra et al. 1997; Tamayo et al. 2011). The infection-induced changes in the immune system lead to a scenario whereby lymphocytes may become activated to self-antigens by virtue of a phenomenon referred to as ‘bystander activation’. Bystander activation is invoked as the trigger for a number of autoimmune diseases and explains how inflammation associated with an innate immune response (or an immune response to an unrelated antigen) can trigger a response to other antigens within the vicinity (Sfriso et al. 2010).
Autoimmunity: the link between infection and outcome?
Given the breakdown in the BBB following stroke and the potential for presentation of CNS antigens to the immune system, we assessed animals for the development of CNS autoimmune responses following stroke. In a model of severe stroke, we found that Th1-type immune responses to MBP were rare, but that the immune response could be pushed to a Th1-type response by systemic administration of LPS at the time of MCAO (Becker et al. 2005). Animals that develop a Th1-type response to MBP, in particular, have worse long-term outcome from stroke (Becker et al. 2005; Gee et al. 2008, 2009; Zierath et al. 2010). LPS is a component of the Gram-negative bacterial cell wall and a potent agonist of TLR4. Infections with Gram-negative pathogens thus have the ability to activate APCs/dendritic cells through TLR4 and create an environment more likely to result in lymphocyte activation. Increased expression of both TLR4 and TLR2 are seen after stroke and are linked to worse outcome and an increase in pro-inflammatory cytokines (Urra et al. 2009c; Yang et al. 2008; Brea et al. 2011).
On the basis of these observations, we considered the possibility that our experimental model might help explain the link between post-stroke infection and poor outcome in humans—specifically, infection may predispose to the development of deleterious autoimmune responses to brain antigens. In our clinical study, we found that PNAs, but not less serious infections, were associated with a higher proportion of patients developing a Th1 response to MBP (Becker et al. 2011). Furthermore, the immune response to MBP was an independent predictor of poor outcome. These findings appear to validate our assumptions, but need to be replicated in a larger study. The finding that PNA is more likely to be associated with the development of autoimmune responses to brain than other less severe infections (like UTIs) is consistent, however, with the observation that the long-term clinical consequences of PNA are more severe than that of UTI (Aslanyan et al. 2004; Westendorp et al. 2011).
Summary
On the basis of a number of different clinical observations regarding post-stroke infection, we considered the possibility that infections could be deleterious through activation of the immune response and predisposing toward CNS autoimmunity. Both animal and human studies seem to support this hypothesis. To date we have focused our research on the immune responses to a small number of potential CNS antigens. Years ago it was found that patients with stroke had evidence of a cellular immune response to MBP, but these studies were done in the context of studying multiple sclerosis (Youngchaiyud et al. 1974; Wang et al. 1992; Kallen et al. 1977). More recent studies have shown that autoantibodies to a variety of CNS antigens can be found after stroke (Dambinova et al. 2003; Bornstein et al. 2001). It is likely that immune responses to a multitude of different CNS antigens occur following brain injury, and that we are merely constrained in our ability to screen for these responses. The cellular and humoral responses to brain are clearly epiphenomena of injury; some of these responses may be of no consequence, but others could contribute to worse short-term as well as long-term disability. Whether autoimmune responses to brain following stroke can be prevented through prevention of infection or modulation of the post-ischemic immune response remains to be seen.
Acknowledgments
This study was funded by NINDS 5R01NS056457.
Abbreviations used
- APCs
antigen-presenting cells
- BBB
blood–brain barrier
- IFN-γ
interferon-γ
- MBP
myelin basic protein
- MCAO
middle cerebral artery occlusion
- MHC
major histocompatibility complex
- NIHSS
National Institutes of Health Stroke Scale
- NSE
neuron-specific enolase
- PNAs
pneumonias
- TGF-β1
transforming growth factor-β1
- TLR
toll-like receptor
- TNF
tumor necrosis factor
- UTIs
urinary tract infections
Footnotes
Conflicts of interest
The author has declared no potential conflicts of interest.
References
- Ali C, Docagne F, Nicole O, et al. Increased expression of transforming growth factor-beta after cerebral ischemia in the baboon: an endogenous marker of neuronal stress? J Cereb Blood Flow Metab. 2001;21:820–827. doi: 10.1097/00004647-200107000-00007. [DOI] [PubMed] [Google Scholar]
- Aslanyan S, Weir CJ, Diener HC, Kaste M, Lees KR. Pneumonia and urinary tract infection after acute ischaemic stroke: a tertiary analysis of the GAIN International trial. Eur J Neurol. 2004;11:49–53. doi: 10.1046/j.1468-1331.2003.00749.x. [DOI] [PubMed] [Google Scholar]
- Bailey SL, Carpentier PA, McMahon EJ, Begolka WS, Miller SD. Innate and adaptive immune responses of the central nervous system. Crit Rev Immunol. 2006;26:149–188. doi: 10.1615/critrevimmunol.v26.i2.40. [DOI] [PubMed] [Google Scholar]
- Bauer S, Muller T, Hamm S. Pattern recognition by Toll-like receptors. Adv Exp Med Biol. 2009;653:15–34. doi: 10.1007/978-1-4419-0901-5_2. [DOI] [PubMed] [Google Scholar]
- Becker K, Kindrick D, Relton J, Harlan J, Winn R. Antibody to the alpha4 integrin decreases infarct size in transient focal cerebral ischemia in rats. Stroke. 2001;32:206–211. doi: 10.1161/01.str.32.1.206. [DOI] [PubMed] [Google Scholar]
- Becker KJ, Kindrick DL, Lester MP, Shea C, Ye ZC. Sensitization to brain antigens after stroke is augmented by lipopolysaccharide. J Cereb Blood Flow Metab. 2005;25:1634–1644. doi: 10.1038/sj.jcbfm.9600160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker KJ, Kalil AJ, Tanzi P, et al. Autoimmune Responses to the Brain After Stroke Are Associated With Worse Outcome. Stroke. 2011;42:2763–2769. doi: 10.1161/STROKEAHA.111.619593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein NM, Aronovich B, Korczyn AD, Shavit S, Michaelson DM, Chapman J. Antibodies to brain antigens following stroke. Neurology. 2001;56:529–530. doi: 10.1212/wnl.56.4.529. [DOI] [PubMed] [Google Scholar]
- Brea D, Blanco M, Ramos-Cabrer P, Moldes O, Arias S, Perez-Mato M, Leira R, Sobrino T, Castillo J. Toll-like receptors 2 and 4 in ischemic stroke: outcome and therapeutic values. J Cereb Blood Flow Metab. 2011;31:1424–1431. doi: 10.1038/jcbfm.2010.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro A, Amaro S, Vargas M, Obach V, Cervera A, Torres F, Planas AM. Interleukin 10, monocytes and increased risk of early infection in ischaemic stroke. J Neurol Neurosurg Psychiatry. 2006;77:1279–1281. doi: 10.1136/jnnp.2006.100800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro A, Amaro S, Vargas M, Obach V, Cervera A, Gomez-Choco M, Torres F, Planas AM. Catecholamines, infection, and death in acute ischemic stroke. J Neurol Sci. 2007;252:29–35. doi: 10.1016/j.jns.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Chen B, Friedman B, Cheng Q, Tsai P, Schim E, Kleinfeld D, Lyden PD. Severe blood-brain barrier disruption and surrounding tissue injury. Stroke. 2009;40:e666–674. doi: 10.1161/STROKEAHA.109.551341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech B, Pfeilschifter W, Mazaheri-Omrani N, Strobel MA, Kahles T, Neumann-Haefelin T, Rami A, Huwiler A, Pfeilschifter J. The immunomodulatory sphingosine 1-phosphate analog FTY720 reduces lesion size and improves neurological outcome in a mouse model of cerebral ischemia. Biochem Biophys Res Commun. 2009;389:251–256. doi: 10.1016/j.bbrc.2009.08.142. [DOI] [PubMed] [Google Scholar]
- Czlonkowska A, Cyrta B, Korlak J. Immunological observations on patients with acute cerebral vascular disease. J Neurol Sci. 1979;43:455–464. doi: 10.1016/0022-510x(79)90024-8. [DOI] [PubMed] [Google Scholar]
- Dambinova SA, Khounteev GA, Izykenova GA, Zavolokov IG, Ilyukhina AY, Skoromets AA. Blood test detecting autoantibodies to N-methyl-D-aspartate neuroreceptors for evaluation of patients with transient ischemic attack and stroke. Clin Chem. 2003;49:1752–1762. doi: 10.1373/49.10.1752. [DOI] [PubMed] [Google Scholar]
- Dangond F, Windhagen A, Groves CJ, Hafler DA. Constitutive expression of costimulatory molecules by human microglia and its relevance to CNS autoimmunity. J Neuroimmunol. 1997;76:132–138. doi: 10.1016/s0165-5728(97)00043-x. [DOI] [PubMed] [Google Scholar]
- Denes A, Humphreys N, Lane TE, Grencis R, Rothwell N. Chronic systemic infection exacerbates ischemic brain damage via a CCL5 (regulated on activation, normal T-cell expressed and secreted)-mediated proinflammatory response in mice. J Neurosci. 2010;30:10086–10095. doi: 10.1523/JNEUROSCI.1227-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Abe T, Kaunzner UW, Gottfried-Blackmore A, Gal-Toth J, McEwen BS, Iadecola C, Bulloch K. Brain dendritic cells in ischemic stroke: time course, activation state, and origin. Brain Behav Immun. 2010;24:724–737. doi: 10.1016/j.bbi.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarese C, Mascarucci P, Zoia C, Cavarretta R, Frigo M, Begni B, Sarinella F, Frattola L, De Simoni MG. Increased cytokine release from peripheral blood cells after acute stroke. J Cereb Blood Flow Metab. 1999;19:1004–1009. doi: 10.1097/00004647-199909000-00008. [DOI] [PubMed] [Google Scholar]
- Gee JM, Kalil A, Thullbery M, Becker KJ. Induction of immunologic tolerance to myelin basic protein prevents central nervous system autoimmunity and improves outcome after stroke. Stroke. 2008;39:1575–1582. doi: 10.1161/STROKEAHA.107.501486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee JM, Zierath D, Hadwin J, Savos A, Kalil A, Thullbery M, Becker KJ. Long term immunologic consequences of experimental stroke and mucosal tolerance. Exp Transl Stroke Med. 2009;1:3. doi: 10.1186/2040-7378-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried-Blackmore A, Kaunzner UW, Idoyaga J, Felger JC, McEwen BS, Bulloch K. Acute in vivo exposure to interferon-gamma enables resident brain dendritic cells to become effective antigen presenting cells. Proc Natl Acad Sci USA. 2009;106:20918–20923. doi: 10.1073/pnas.0911509106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabska K, Gromadzka G, Czlonkowska A. Infections and ischemic stroke outcome. Neurol Res Int. 2011;2011:691348. doi: 10.1155/2011/691348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau AJ, Urbanek C, Palm F. Common infections and the risk of stroke. Nat Rev Neurol. 2010;6:681–694. doi: 10.1038/nrneurol.2010.163. [DOI] [PubMed] [Google Scholar]
- Haeusler KG, Schmidt WU, Fohring F, et al. Cellular immunodepression preceding infectious complications after acute ischemic stroke in humans. Cerebrovasc Dis. 2008;25:50–58. doi: 10.1159/000111499. [DOI] [PubMed] [Google Scholar]
- Haeusler KG, Schmidt WU, Foehring F, et al. Immune responses after acute ischemic stroke or myocardial infarction. Int J Cardiol. 2012;155:372–377. doi: 10.1016/j.ijcard.2010.10.053. [DOI] [PubMed] [Google Scholar]
- Harms H, Reimnitz P, Bohner G, Werich T, Klingebiel R, Meisel C, Meisel A. Influence of stroke localization on autonomic activation, immunodepression, and post-stroke infection. Cerebrovasc Dis. 2011;32:552–560. doi: 10.1159/000331922. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Suzuki H, Sozen T, Rolland W, Zhang JH. Activation of sphingosine 1-phosphate receptor-1 by FTY720 is neuroprotective after ischemic stroke in rats. Stroke. 2010;41:368–374. doi: 10.1161/STROKEAHA.109.568899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M, Vos P, Wunderlich MT, de Bruijn CH, Lamers KJ. Release of glial tissue-specific proteins after acute stroke: a comparative analysis of serum concentrations of protein S-100B and glial fibrillary acidic protein. Stroke. 2000;31:2670–2677. doi: 10.1161/01.str.31.11.2670. [DOI] [PubMed] [Google Scholar]
- Hong KS, Kang DW, Koo JS, Yu KH, Han MK, Cho YJ, Park JM, Bae HJ, Lee BC. Impact of neurological and medical complications on 3-month outcomes in acute ischaemic stroke. Eur J Neurol. 2008;15:1324–1331. doi: 10.1111/j.1468-1331.2008.02310.x. [DOI] [PubMed] [Google Scholar]
- Hug A, Dalpke A, Wieczorek N, Giese T, Lorenz A, Auffarth G, Liesz A, Veltkamp R. Infarct volume is a major determiner of post-stroke immune cell function and susceptibility to infection. Stroke. 2009;40:3226–3232. doi: 10.1161/STROKEAHA.109.557967. [DOI] [PubMed] [Google Scholar]
- Hug A, Liesz A, Muerle B, Zhou W, Ehrenheim J, Lorenz A, Dalpke A, Veltkamp R. Reduced efficacy of circulating costimulatory cells after focal cerebral ischemia. Stroke. 2011;42:3580–3586. doi: 10.1161/STROKEAHA.111.620948. [DOI] [PubMed] [Google Scholar]
- Intiso D, Zarrelli MM, Lagioia G, Di Rienzo F, Checchia De Ambrosio C, Simone P, Tonali P, Cioffi Dagger RP. Tumor necrosis factor alpha serum levels and inflammatory response in acute ischemic stroke patients. Neurol Sci. 2004;24:390–396. doi: 10.1007/s10072-003-0194-z. [DOI] [PubMed] [Google Scholar]
- Jander S, Kraemer M, Schroeter M, Witte OW, Stoll G. Lymphocytic infiltration and expression of intercellular adhesion molecule-1 in photochemically induced ischemia of the rat cortex. J Cereb Blood Flow Metab. 1995;15:42–51. doi: 10.1038/jcbfm.1995.5. [DOI] [PubMed] [Google Scholar]
- Jauch EC, Lindsell C, Broderick J, Fagan SC, Tilley BC, Levine SR. Association of serial biochemical markers with acute ischemic stroke: the National Institute of Neurological Disorders and Stroke recombinant tissue plasminogen activator Stroke Study. Stroke. 2006;37:2508–2513. doi: 10.1161/01.STR.0000242290.01174.9e. [DOI] [PubMed] [Google Scholar]
- Kallen B, Nilsson O, Thelin C. Effect of encephalitogenic protein on migration in agarose of leukoytes from patients with multiple sclerosis. A longitudinal study of patients with relapsing multiple sclerosis or with cerebral infarction. Acta Neurol Scand. 1977;55:47–56. [PubMed] [Google Scholar]
- Klehmet J, Harms H, Richter M, Prass K, Volk HD, Dirnagl U, Meisel A, Meisel C. Stroke-induced immunodepression and post-stroke infections: lessons from the preventive antibacterial therapy in stroke trial. Neuroscience. 2009;158:1184–1193. doi: 10.1016/j.neuroscience.2008.07.044. [DOI] [PubMed] [Google Scholar]
- Krupinski J, Kumar P, Kumar S, Kaluza J. Increased expression of TGF-beta 1 in brain tissue after ischemic stroke in humans. Stroke. 1996;27:852–857. doi: 10.1161/01.str.27.5.852. [DOI] [PubMed] [Google Scholar]
- Liesz A, Hagmann S, Zschoche C, et al. The spectrum of systemic immune alterations after murine focal ischemia: immunodepression versus immunomodulation. Stroke. 2009a;40:2849–2858. doi: 10.1161/STROKEAHA.109.549618. [DOI] [PubMed] [Google Scholar]
- Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, Giese T, Veltkamp R. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009b;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- Liesz A, Sun L, Zhou W, Schwarting S, Mracsko E, Zorn M, Bauer H, Sommer C, Veltkamp R. FTY720 reduces post-ischemic brain lymphocyte influx but does not improve outcome in permanent murine cerebral ischemia. PLoS ONE. 2011a;6:e21312. doi: 10.1371/journal.pone.0021312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesz A, Zhou W, Mracsko E, et al. Inhibition of lymphocyte trafficking shields the brain against deleterious neuroinflammation after stroke. Brain. 2011b;134:704–720. doi: 10.1093/brain/awr008. [DOI] [PubMed] [Google Scholar]
- Mannie M, Swanborg RH, Stepaniak JA. Experimental autoimmune encephalomyelitis in the rat. Curr Protoc Immunol. 2009;Chapter 15(Unit 15.2):15.2.1–15.2.15. doi: 10.1002/0471142735.im1502s85. Suppl 85. [DOI] [PubMed] [Google Scholar]
- Mazzotta G, Sarchielli P, Caso V, Paciaroni M, Floridi A, Floridi A, Gallai V. Different cytokine levels in thrombolysis patients as predictors for clinical outcome. Eur J Neurol. 2004;11:377–381. doi: 10.1111/j.1468-1331.2004.00798.x. [DOI] [PubMed] [Google Scholar]
- Meisel C, Prass K, Braun J, et al. Preventive antibacterial treatment improves the general medical and neurological outcome in a mouse model of stroke. Stroke. 2004;35:2–6. doi: 10.1161/01.STR.0000109041.89959.4C. [DOI] [PubMed] [Google Scholar]
- Montaner J, Rovira A, Molina CA, Arenillas JF, Ribo M, Chacon P, Monasterio J, Alvarez-Sabin J. Plasmatic level of neuroinflammatory markers predict the extent of diffusion-weighted image lesions in hyperacute stroke. J Cereb Blood Flow Metab. 2003;23:1403–1407. doi: 10.1097/01.WCB.0000100044.07481.97. [DOI] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- Planas AM, Gomez-Choco M, Urra X, Gorina R, Caballero M, Chamorro A. Brain-derived antigens in lymphoid tissue of patients with acute stroke. J Immunol. 2012;188:2156–2163. doi: 10.4049/jimmunol.1102289. [DOI] [PubMed] [Google Scholar]
- Prass K, Meisel C, Hoflich C, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198:725–736. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CR, Wilkie BN. Toll-like receptor, MHC II, B7 and cytokine expression by porcine monocytes and monocyte-derived dendritic cells in response to microbial pathogen-associated molecular patterns. Vet Immunol Immunopathol. 2005;107:235–247. doi: 10.1016/j.vetimm.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Relton JK, Sloan KE, Frew EM, Whalley ET, Adams SP, Lobb RR. Inhibition of alpha4 integrin protects against transient focal cerebral ischemia in normotensive and hypertensive rats. Stroke. 2001;32:199–205. doi: 10.1161/01.str.32.1.199. [DOI] [PubMed] [Google Scholar]
- Ren X, Akiyoshi K, Vandenbark AA, Hurn PD, Offner H. CD4+FoxP3+ regulatory T-cells in cerebral ischemic stroke. Metab Brain Dis. 2011;26:87–90. doi: 10.1007/s11011-010-9226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SL, Coe CL, Karaszewski JW. Immune consequences of stroke and cerebral palsy in adults. J Neuroimmunol. 1998;91:113–120. doi: 10.1016/s0165-5728(98)00157-x. [DOI] [PubMed] [Google Scholar]
- Romo-Gonzalez T, Chavarria A, Perez HJ. Central nervous system: a modified immune surveillance circuit? Brain Behav Immun. 2012;26:823–829. doi: 10.1016/j.bbi.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Schroeter M, Jander S, Witte OW, Stoll G. Local immune responses in the rat cerebral cortex after middle cerebral artery occlusion. J Neuroimmunol. 1994;55:195–203. doi: 10.1016/0165-5728(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Schulte-Herbruggen O, Klehmet J, Quarcoo D, Meisel C, Meisel A. Mouse strains differ in their susceptibility to poststroke infections. Neuro Immuno Modulation. 2006;13:13–18. doi: 10.1159/000092109. [DOI] [PubMed] [Google Scholar]
- Sfriso P, Ghirardello A, Botsios C, Tonon M, Zen M, Bassi N, Bassetto F, Doria A. Infections and autoimmunity: the multifaceted relationship. J Leukoc Biol. 2010;87:385–395. doi: 10.1189/jlb.0709517. [DOI] [PubMed] [Google Scholar]
- Sotgiu S, Zanda B, Marchetti B, et al. Inflammatory biomarkers in blood of patients with acute brain ischemia. Eur J Neurol. 2006;13:505–513. doi: 10.1111/j.1468-1331.2006.01280.x. [DOI] [PubMed] [Google Scholar]
- Suter T, Biollaz G, Gatto D, Bernasconi L, Herren T, Reith W, Fontana A. The brain as an immune privileged site: dendritic cells of the central nervous system inhibit T cell activation. Eur J Immunol. 2003;33:2998–3006. doi: 10.1002/eji.200323611. [DOI] [PubMed] [Google Scholar]
- Tamayo E, Fernandez A, Almansa R, et al. Pro- and anti-inflammatory responses are regulated simultaneously from the first moments of septic shock. Eur Cytokine Netw. 2011;22:82–87. doi: 10.1684/ecn.2011.0281. [DOI] [PubMed] [Google Scholar]
- Tanzi P, Cain K, Kalil A, et al. Post-stroke infection: a role for IL-1ra? Neurocrit Care. 2011;14:244–252. doi: 10.1007/s12028-010-9490-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttolomondo A, Di Sciacca R, Di Raimondo D, et al. Plasma levels of inflammatory and thrombotic/fibrinolytic markers in acute ischemic strokes: relationship with TOAST subtype, outcome and infarct site. J Neuroimmunol. 2009;215:84–89. doi: 10.1016/j.jneuroim.2009.06.019. [DOI] [PubMed] [Google Scholar]
- Urra X, Cervera A, Obach V, Climent N, Planas AM, Chamorro A. Monocytes are major players in the prognosis and risk of infection after acute stroke. Stroke. 2009a;40:1262–1268. doi: 10.1161/STROKEAHA.108.532085. [DOI] [PubMed] [Google Scholar]
- Urra X, Cervera A, Villamor N, Planas AM, Chamorro A. Harms and benefits of lymphocyte subpopulations in patients with acute stroke. Neuroscience. 2009b;158:1174–1183. doi: 10.1016/j.neuroscience.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Urra X, Villamor N, Amaro S, Gomez-Choco M, Obach V, Oleaga L, Planas AM, Chamorro A. Monocyte subtypes predict clinical course and prognosis in human stroke. J Cereb Blood Flow Metab. 2009c;29:994–1002. doi: 10.1038/jcbfm.2009.25. [DOI] [PubMed] [Google Scholar]
- Vermeij FH, Scholte op Reimer WJ, de Man P, van Oostenbrugge RJ, Franke CL, de Jong G, de Kort PL, Dippel DW. Stroke-associated infection is an independent risk factor for poor outcome after acute ischemic stroke: data from the Netherlands Stroke Survey. Cerebrovasc Dis. 2009;27:465–471. doi: 10.1159/000210093. [DOI] [PubMed] [Google Scholar]
- Vogelgesang A, Grunwald U, Langner S, Jack R, Broker BM, Kessler C, Dressel A. Analysis of lymphocyte subsets in patients with stroke and their influence on infection after stroke. Stroke. 2008;39:237–241. doi: 10.1161/STROKEAHA.107.493635. [DOI] [PubMed] [Google Scholar]
- Vogelgesang A, May VE, Grunwald U, Bakkeboe M, Langner S, Wallaschofski H, Kessler C, Broker BM, Dressel A. Functional status of peripheral blood T-cells in ischemic stroke patients. PLoS ONE. 2010;5:e8718. doi: 10.1371/journal.pone.0008718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WZ, Olsson T, Kostulas V, Hojeberg B, Ekre HP, Link H. Myelin antigen reactive T cells in cerebrovascular diseases. Clin Exp Immunol. 1992;88:157–162. doi: 10.1111/j.1365-2249.1992.tb03056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Yemisci M, Kim HH, et al. Fingolimod provides long-term protection in rodent models of cerebral ischemia. Ann Neurol. 2011;69:119–129. doi: 10.1002/ana.22186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Werra I, Jaccard C, Corradin SB, et al. Cytokines, nitrite/nitrate, soluble tumor necrosis factor receptors, and procalcitonin concentrations: comparisons in patients with septic shock, cardiogenic shock, and bacterial pneumonia. Crit Care Med. 1997;25:607–613. doi: 10.1097/00003246-199704000-00009. [DOI] [PubMed] [Google Scholar]
- Westendorp WF, Nederkoorn PJ, Vermeij JD, Dijkgraaf MG, van de Beek D. Post-stroke infection: a systematic review and meta-analysis. BMC Neurol. 2011;11:110. doi: 10.1186/1471-2377-11-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CH, Jenne CN, Lee WY, Leger C, Kubes P. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science. 2011;334:101–105. doi: 10.1126/science.1210301. [DOI] [PubMed] [Google Scholar]
- Yan J, Read SJ, Henderson RD, Hull R, O’Sullivan JD, McCombe PA, Greer JM. Frequency and function of regulatory T cells after ischaemic stroke in humans. J Neuroimmunol. 2012;243:89–94. doi: 10.1016/j.jneuroim.2011.12.019. [DOI] [PubMed] [Google Scholar]
- Yang QW, Li JC, Lu FL, Wen AQ, Xiang J, Zhang LL, Huang ZY, Wang JZ. Upregulated expression of toll-like receptor 4 in monocytes correlates with severity of acute cerebral infarction. J Cereb Blood Flow Metab. 2008;28:1588–1596. doi: 10.1038/jcbfm.2008.50. [DOI] [PubMed] [Google Scholar]
- Youngchaiyud U, Coates AS, Whittingham S, Mackay IR. Cellular-immune response to myelin protein: absence in multiple sclerosis and presence in cerebrovascular accidents. Aust N Z J Med. 1974;4:535–538. doi: 10.1111/j.1445-5994.1974.tb03233.x. [DOI] [PubMed] [Google Scholar]
- Zaremba J, Losy J. Early TNF-alpha levels correlate with ischaemic stroke severity. Acta Neurol Scand. 2001;104:288–295. doi: 10.1034/j.1600-0404.2001.00053.x. [DOI] [PubMed] [Google Scholar]
- Zhang DP, Yan FL, Xu HQ, Zhu YX, Yin Y, Lu HQ. A decrease of human leucocyte antigen-DR expression on monocytes in peripheral blood predicts stroke-associated infection in critically-ill patients with acute stroke. Eur J Neurol. 2009;16:498–505. doi: 10.1111/j.1468-1331.2008.02512.x. [DOI] [PubMed] [Google Scholar]
- Zierath D, Thullbery M, Hadwin J, Gee JM, Savos A, Kalil A, Becker KJ. CNS immune responses following experimental stroke. Neurocrit Care. 2010;12:274–284. doi: 10.1007/s12028-009-9270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zwam M, Huizinga R, Melief MJ, et al. Brain antigens in functionally distinct antigen-presenting cell populations in cervical lymph nodes in MS and EAE. J Mol Med. 2009;87:273–286. doi: 10.1007/s00109-008-0421-4. [DOI] [PubMed] [Google Scholar]
- Zygmunt B, Veldhoen M. T helper cell differentiation more than just cytokines. Adv Immunol. 2011;109:159–196. doi: 10.1016/B978-0-12-387664-5.00005-4. [DOI] [PubMed] [Google Scholar]


