Abstract
Niemann–Pick type C (NPC) disease is an autosomal recessive neurovisceral lipid and storage disorder characterized by abnormal sequestration of unesterified cholesterol within the late endosomal/lysosomal compartment of all cells in the body. This disease primarily affects children and is characterized by hepatic and pulmonary dysfunction, neurodegeneration and death at an early age. Currently, there is no effective treatment for NPC disease. It was recently discovered that 2-hydroxypropyl-β-cyclodextrin (2HPBCD), when administered systemically to a murine model of either NPC1 or NPC2 disease, significantly reduced lysosomal cholesterol accumulation in almost every organ, delayed the progression of neurodegeneration and significantly prolonged lifespan by allowing trapped cholesterol within the late endosome/lysosome to be released. When 2HPBCD was administered directly into the CNS of Npc1−/− mice, neurodegeneration was completely prevented. This review will explore the pathophysiology of NPC disease and the use of 2HPBCD as a possible therapeutic modality.
Keywords: cholesterol, cyclodextrin, lysosomal storage disease, neurodegeneration, Niemann–Pick type C, Purkinje cell
Cholesterol is essential for life. It is critical in the building and maintenance of mammalian cell membranes and is the precursor of many important organic molecules, such as steroid hormones and bile acids. Given this importance, the acquisition, transport and metabolism of cholesterol in biological systems has evolved into a highly coordinated and regulated process. However, when there is a perturbation in the regulation and/or metabolism of cholesterol, this can lead to a variety of disease processes, ranging from common entities such as atherosclerosis to rare genetic disorders such as familial hypercholesterolemia, sitosterolemia, Smith–Lemli–Opitz syndrome and Niemann–Pick type C (NPC) disease.
NPC disease
NPC disease is an autosomal recessive neurovisceral lipid storage and trafficking disorder characterized by the progressive accumulation of unesterified cholesterol within the late endosome/lysosome (LE/LY) of all cells. NPC disease belongs to a group of approximately 50 lysosomal storage diseases that arise when there is an inherited mutation inactivating one or more of the critical enzymes or transporters that function within the LE/LY. NPC disease primarily affects children and has a prevalence of approximately 1 in 150,000 live births. It is characterized by neurodegeneration, hepatic and pulmonary disease and, ultimately, death at a young age [1–10]. This disorder is due to a loss of function mutation in genes encoding either the NPC1 [11] or NPC2 [12] proteins that are essential for cholesterol transport out of the LE/LY. This results in a disruption of sterol transport and metabolism. The ensuing sequestration and buildup of unesterified cholesterol, as well as other lipids, within the LE/LY, ultimately leads to cell death and organ damage [13–17].
Cellular acquisition of cholesterol
Cellular cholesterol pools are derived from two main sources: uptake of cholesterol carried on plasma lipoproteins through the clathrin-coated pit pathway and/or de novo cholesterol synthesis from the precursor, acetyl-CoA. The majority of newly synthesized cholesterol is incorporated into the plasma membranes, while lipoprotein cholesterol has to be processed within the LE/LY prior to being utilized by the cell. However, through pulse-chase experiments, it has been shown that after being incorporated into plasma membranes, newly synthesized cholesterol is eventually destined to reach the LE/LY [18].
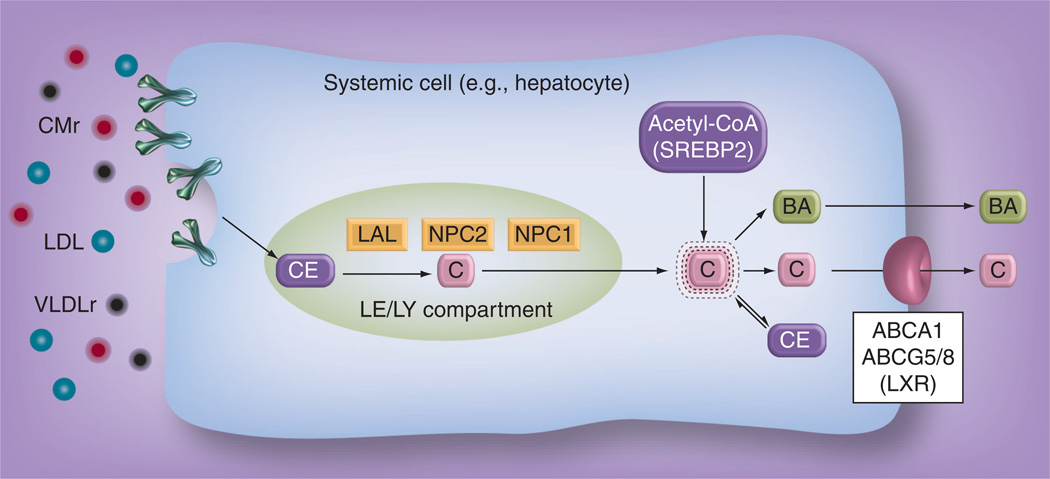
Uptake of cholesterol carried on lipoproteins occurs through two mechanisms: receptor-mediated and bulk-phase endocytosis (Figure 1). Plasma lipoproteins such as LDL, chylomicron remnants and VLDL remnants predominantly carrying cholesteryl ester (CE), diffuse from the vascular space across the endothelial barrier into the pericellular fluid. The lipoproteins are internalized into the cell through endocytic vesicles via the clathrin-coated pit pathway by either binding to a LDL receptor on the cell surface (receptor-mediated endocytosis), or through bulk water uptake (bulk-phase endocytosis) [19]. The lipoprotein-containing endosome fuses with other vesicular organelles containing acid hydrolases and eventually gives rise to the LE/LY. The LE/LY becomes acidified which results in the activation of lysosomal acid lipase. ysosomal acid lipase hydrolyzes the CE within the lipoprotein particles into unesterified cholesterol. The two lysosomal proteins, NPC1 and NPC2, then aid in the transport of unesterified cholesterol out of the LE/LY into the putative metabolically active cholesterol pool within the cytosol [20,21].
Figure 1. Cholesterol acquisition and regulation in a systemic cell.
Lipoproteins, such as CMr, LDL and VLDLr, carrying CEs are internalized from the pericellular fluid into the LE/LY through receptor-mediated and/or bulk-phase endocytosis. LAL then hydrolyzes the CEs into unesterified C. C is then exported out of the LE/LY by first binding to NPC2, which then delivers it to NPC1. NPC1 then allows the C to be transported into the metabolically active C pool located in the cytosol. The metabolically C pool is maintained at constant levels through the actions of either SREBP2, which activates the genes responsible for endogenous de novo C synthesis using the precursor acetyl-CoA; or LXR, a nuclear hormone receptor that regulates the genes involved in C efflux such as ABCA1 and ABCG5/8, and BA synthesis. C can also be esterified into CE and stored as nontoxic lipid droplets during times of excess C.
BA: Bile acid; C: Cholesterol; CE: Cholesteryl ester; CMr: Chylomicron remnant; LAL: Lysosomal acid lipase; LE/LY: Late endosome/lysosome; LXR: Liver X receptor; VLDLr: VLDL remnant.
The metabolically active cholesterol pool is tightly regulated by sensing mechanisms located within the cytosol, since an excess or deficiency of cholesterol can result in cellular dysfunction and death. Two systems are involved in regulating the size of the metabolically active cholesterol pool. The first system is controlled by SREBP2, located in the endoplasmic reticulum, which regulates the genes involved in de novo cholesterol synthesis and LDL receptor activity on the cell surface [22]. The second system is the liver X receptor (LXR), a nuclear hormone receptor that activates genes involved in cholesterol efflux, such as ABCA1 and ABCG5/8, during periods of cholesterol excess [23]. LXR also regulates key enzymes that control the biosynthetic pathway of bile acids, one of the mechanisms the body uses to eliminate cholesterol.
When the metabolically active cholesterol pool becomes expanded, feedback inhibition of SREBP2 occurs, turning off de novo cholesterol synthesis and decreasing LDL receptor activity on the cell surface [24], thereby minimizing cholesterol uptake. Cholesterol efflux pathways are also activated through the action of LXR. Excess cholesterol can also be esterified in the endoplasmic reticulum by ACAT1 and/or 2 and stored as nontoxic lipid droplets within the cytosol.
The opposite series of events occurs when the metabolically active cholesterol pool decreases. SREBP2 pathways are activated, turning on cholesterol synthesis and increasing cell surface LDL receptor activity, cholesterol efflux pathways are downregulated and CE formation is diminished in order to restore the metabolic cholesterol pool to normal levels.
Function of NPC1 & NPC2 in cholesterol transport
NPC1 is a 1278-amino acid protein located within the limiting membrane of the LE/LY. It is encoded by the NPC1 gene on chromosome 18. It spans the limiting membrane 13 times, has three large luminal loops and contains a sterol-sensing domain [25] that bears sequence homology to the sterol-sensing domain found in other proteins involved in cholesterol regulation, such as HMG-CoA reductase [26], the rate-limiting enzyme in cholesterol synthesis, and Scap [27], which transports SREBP from the endoplasmic reticulum to the golgi to activate the cholesterol synthesis pathway. It has been demonstrated that NPC1 binds cholesterol and is believed to function as a cholesterol transporter [20,21,28].
NPC2 is a smaller, 130-amino acid, non-membrane-bound, soluble protein located within the lumen of the LE/LY that has also been shown to bind cholesterol with high affinity [21,29–32]. It is encoded by the NPC2 gene located on chromosome 14.
NPC1 and NPC2 work in concert to facilitate the transport of unesterified cholesterol across the limiting membrane and out of the LE/LY. Once lipoprotein-derived CEs are delivered to the LE/LY and hydrolyzed byysosomal acid lipase, cholesterol is first bound by NPC2 [33] with the isooctyl side chain of cholesterol buried within the binding pocket and the 3β-hydroxyl group exposed [21,34]. NPC2 then interacts with the second luminal domain of NPC1 [33] and hands-off the cholesterol molecule to NPC1, where it is bound in an orientation opposite to that of NPC2, with the 3β-hydroxyl group buried within the binding pocket and the isooctyl side chain exposed [21,35,36]. NPC1 is then able to transport cholesterol out of the LE/LY into the cytosolic compartment of the cell. However, the exact mechanism remains unknown.
When there is a loss-of-function mutation in either NPC1 or NPC2, the transport of cholesterol out of the LE/LY becomes disrupted, resulting in the accumulation of cholesterol within the LE/LY, giving rise to NPC disease. Mutations in NPC1 account for 95% of all cases of NPC, while mutations in NPC2 account for the remaining 5% [2].
Despite the accumulation of excessive amounts of cholesterol within the LE/LY, NPC disease cells actually perceive a cholesterol deficiency as a result of the impaired trafficking of cholesterol out of the LE/LY to the cholesterol-sensing mechanisms located in the cytosolic compartment [37,38]. NPC disease cells respond to this perceived cholesterol deficiency by activating SREBP2, which increases the uptake and synthesis of cholesterol, downregulating LXR-controlled cholesterol efflux genes and decreasing CE formation [37,39].
Animal models of NPC disease
The pioneering work by Pentchev et al. [40,41] and Morris et al. [42–44] in the discovery and initial characterization of the murine model of NPC disease led to two landmark discoveries in 1997 that greatly advanced the field of NPC research. The first was the identification of the NPC1 gene by Carstea et al. [11], and second was the discovery of this gene defect in the murine model of NPC disease by Loftus et al. [45].
The Npc1−/− mouse model harbors a spontaneous mutation in the Npc1 gene, resulting in a truncated, nonfunctioning NPC1 protein that leads to progressive cholesterol accumulation within the LE/LY of all cells. This in turn is associated with the infiltration of lipid-laden macrophages into many organs, leading to parenchymal cell death [19]. As a result, these mice develop neurodegeneration, liver and lung dysfunction, hepatosplenomegaly and die prematurely, as is the case in human NPC disease.
The Npc1−/− mouse sequesters approximately 67 mg/kg bodyweight (bw) of excess cholesterol daily within the LE/LY of all cells; by contrast to a normal mouse, which has a constant whole-animal cholesterol pool of 2200 mg/kg bw throughout its entire lifespan, the cholesterol pool of the Npc1−/− mouse more than doubles to 5700 mg/kg bw from birth to 7 weeks of age [46]. This 3500 mg/kg bw of excess cholesterol is composed almost entirely of unesterified cholesterol. In virtually every organ, despite this large accumulation of cholesterol within the LE/LY, the perceived cholesterol shortage results in elevated rates of cholesterol synthesis, suppression of LXR-mediated cholesterol efflux and decreased formation of esterified cholesterol [47–49].
Unlike the other organs, the cholesterol levels and cholesterol synthesis rates in the whole brain and CNS of Npc1−/− mice are typically slightly decreased when compared with their unaffected counterparts, even though there is unequivocal evidence of cholesterol accumulation within the LE/LY of the individual neurons. This occurs because, as neurodegeneration progresses, there is a simultaneous loss of myelin cholesterol during demyelination, which offsets and masks the cellular buildup of cholesterol within the neurons themselves [50,51]. Also, as neurons die and the population of cells decreases, cholesterol synthesis rates within the whole brain also decrease.
Since the discovery of the Npc1−/− mouse model with the naturally occurring null mutation, other murine models of NPC1 disease have been developed. One such model involved knock-in mutations that resulted in key amino acid substitutions of the N-terminal domain of the NPC1 protein, which abolished cholesterol binding and reproduced the phenotype of the complete NPC1-deficient mouse [52]. Another group introduced a mutation into the mouse Npc1 gene that was highly similar to a commonly occurring human mutation. This resulted in slower disease progression compared with the other murine models and could serve as a good model for the late-onset, more slowly progressing forms of NPC disease that are often seen in humans [53].
In 2000, Naureckiene et al. discovered the NPC2 gene [54] and in 2004, Sleat et al. developed the murine model of NPC2 disease [12]. The Npc2−/− mouse, unlike the Npc1−/− mouse model which has no NPC1 activity, is a hypomorph that expresses up to 4% residual NPC2 protein in specific tissues. This mouse model shares many of the perturbations in lipid metabolism seen in the Npc1−/− mouse; however, the clinical presentation is less severe as reflected by less hepatoxicity, decreased neurodegeneration and a longer lifespan, which could be attributed to the residual activity of NPC2 [55] or possibly that mutations in Npc2 are less severe.
In addition to the murine models of NPC disease, there is also a feline model [56]. The cat model of NPC arises from a naturally occurring missense mutation in the Npc1 gene, which is different from the common human mutation, that results in the same biochemical abnormalities in cholesterol metabolism as seen in the other animal models [57]. These cats demonstrate many of the clinical and neuropathological features seen in the human form of NPC [57–59].
Attempted treatments of NPC disease
The severity of organ dysfunction in NPC disease is proportional to the amount of cholesterol that is sequestered in the particular tissue [19,47,60]. Therefore, the majority of the attempted therapies for NPC disease focus on decreasing the degree of cholesterol sequestration and accumulation in order to ameliorate or halt disease progression. Currently, there is no approved, effective therapy for NPC disease. Attempts have been made to try to relieve cholesterol and lipid accumulation in NPC disease by targeting various steps in the metabolic and transport pathways of sterols.
One of the first therapeutic interventions attempted was to reduce the amount of cholesterol being taken into cells through receptor-mediated and bulk-phase endocytosis, thereby decreasing the amount of cholesterol destined for the LE/LY. This was achieved through the use of cholesterol-restricted diets, cholesterol-lowering agents and drugs such as ezetemibe that effectively block intestinal cholesterol absorption [47,61–63]. These modalities showed some benefit in reducing cholesterol accumulation in organs that utilize lipoprotein-bound cholesterol, such as the liver and spleen. However, the CNS showed no improvement since, unlike other organs, plasma lipoproteins are not a cholesterol source for the CNS owing to the impenetrability of the blood–brain barrier to lipoproteins. Therefore, neurodegeneration was not prevented and lifespan was not extended in the Npc1−/− mouse model by diminishing cellular cholesterol uptake using these methods.
Another strategy involved deleting the LDL receptor in Npc1−/− mice to disrupt receptor-mediated endocytosis; however, the cholesterol content in the organs of these mice did not improve and in some cases worsened owing to increased uptake of cholesterol through the bulk-phase endocytic pathway [19,63].
Npc1−/− mice were also treated with a synthetic ligand for the nuclear hormone receptor LXR to enhance the efflux of cholesterol out of cells. Treatment with a LXR agonist increased cholesterol excretion from the brains of Npc1−/− mice, which resulted in a moderate improvement in neuroinflammation; however, the improvement in survival was only modest [60].
Another therapy that showed some promise was imatinib. Imatinib is an inhibitor of the proapoptotic gene, c-Abl, which was found to be activated in the Purkinje cells of Npc1−/− mice and believed to be contributing to neurodegeneration. When Npc1−/− mice were treated with imatinib, there was a reduction in Purkinje cell death and improved neurological symptoms. However, despite the improvements, lifespan was only extended by 12% [64].
The use of curcumin has also been explored in the treatment of NPC disease. Curcumin is the active ingredient in the spice turmeric. In one report, when curcumin was orally administered to Npc1−/− mice at a dose of 150 mg/kg/day starting on the day of weaning, their lifespan was extended by 35% compared with the untreated group [65]. It is believed that curcumin exerts its beneficial effects by ameliorating the altered intracellular calcium homeostasis seen in NPC1 disease cells [65]. Whether curcumin will become an effective treatment for NPC disease is still under debate, since another group reported only minimal-to-moderate improvements in lifespan (1.5–18%) with curcumin treatment [66].
Based on the observation that humans and mice with NPC accumulate glycosphingo-lipids, such as gangliosides, along with cholesterol within cells of the CNS and fibroblasts [14,16,67], attempts were made to relieve glycosphingolipid accumulation to try to alleviate the neurodegeneration associated with NPC disease. Mouse models of NPC1 were treated with the drug miglustat, an inhibitor of a key enzyme involved in the synthetic pathway of glycosphingolipids, which had originally been developed to treat the glycosphingolipid lysosomal storage disorder Gaucher’s disease type 1. The treated Npc1−/− animals demonstrated a reduction in glycosphingolipid accumulation within their brains, had delayed onset of neurological dysfunction, and a 25% increase in lifespan [68]. Given that glycosphingolipids were found to have no effect on cellular cholesterol pools or metabolism in Npc1−/− mice [69], the mechanism by which a reduction in glycosphingolipids imparts a therapeutic effect on NPC, a disease of abnormal cholesterol storage, is unknown. However, some believe that a reduction in glycosphingolipids, which is secondary to the abnormal cholesterol sequestration, can help decrease the congestion within lysosomes of NPC disease cells and facilitate cholesterol efflux from the lysosome. Based on the results of these animal studies, human clinical trials in both children and adults were undertaken to test the efficacy of miglustat in treating NPC disease. In these studies, the neurological improvements in the treated subjects showed favorability towards miglustat; however, many of the improvements did not reach statistical significance [70–72].
In 2004, it was reported by Griffin et al. that, due to the cholesterol transport defect, Npc1−/− mice had disrupted production of neurosteroids and decreased levels of the neurosteroid allopregnanolone, which is involved in neuronal growth, survival and differentiation [73]. They demonstrated that, when a single injection of allopregnanolone dissolved in the carrier 2-hydroxypropyl-β-cyclodextrin (2HPBCD) was administered to 7-day-old Npc1−/− mice, neurodegeneration was significantly delayed and more impressively, the lifespan of the Npc1−/− mice was nearly doubled from 67 to 124 days [73]. These compelling results were independently confirmed by other laboratories [74,75]. It was also shown that administering multiple injections of allopregnanolone dissolved in the carrier 2HPBCD resulted in even greater beneficial effects than the single injection given at 7 days of age [75]. However, it was later discovered that all the beneficial effects attributed to allopregnanolone in extending lifespan and delaying neurodegeneration in the Npc1−/− mouse were actually all due to the carrier 2HPBCD [48,76]. When allopregnanolone was administered without 2HPBCD, the beneficial effects were not seen [77]. Up until that point, 2HPBCD was thought to be an inert drug vehicle and not a potential treatment for NPC disease. This idea was based on an earlier study, in a small number of Npc1−/− mice, which reported that, despite improvements in hepatic cholesterol levels, there was minimal to no improvement in delaying the onset of neurological symptoms in Npc1−/− mice with 2HPBCD treatment [78].
Cyclodextrin
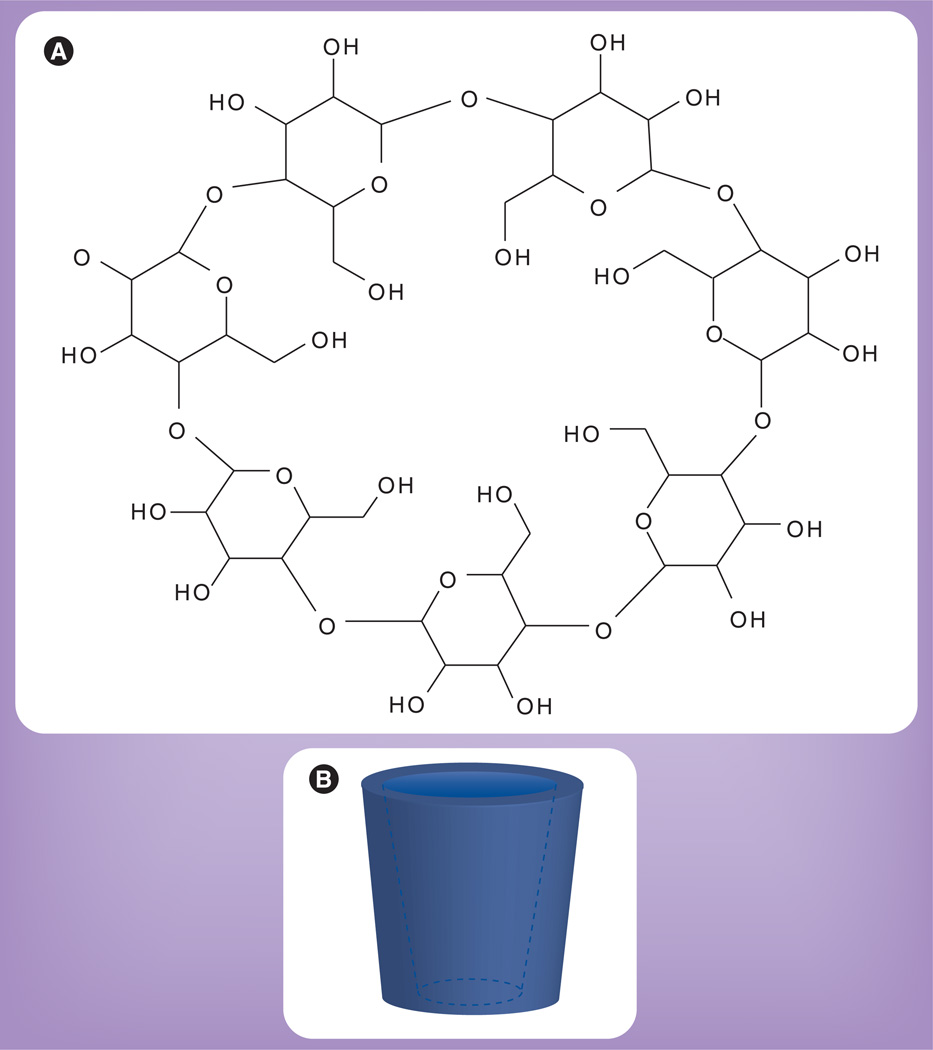
Cyclodextrins are naturally occurring cyclic oligosaccharides derived from the enzymatic conversion of starch. They are composed of a varying number of linked glucopyranose units that form a hollow cone-like toroid structure consisting of a hydrophobic cavity and a hydrophilic exterior (Figure 2). The number of glucopyranose units determines the cavity size and nomenclature of the cyclodextrin, with the most common consisting of six, seven or eight glucopyranose units and named α-, β- and γ-cyclodextrin, respectively. The unique structure of cyclodextrins allows them to form water-soluble complexes with otherwise insoluble hydrophobic compounds [79]. This property of cyclodextrins has led to their application as delivery vehicles to improve solubility, stability and bioavailability of many drugs [80]. Early toxicity studies with natural cyclodextrins revealed that, when administered parenterally, they caused nephrotoxicity and hemolysis [81,82]. In order to improve the safety profile, the parent cyclodextrins were chemically modified to create synthetic derivatives, such as 2HPBCD, which is one of the most common and least toxic derivatives [83].
Figure 2. Chemical structure of cyclodextrin.
(A) Chemical representation of β-cyclodextrin, which is composed of seven glucopyranose units. (B) 3D representation of the toroid structure of cyclodextrin, which consists of a hydrophilic exterior and hydrophobic interior.
2HPBCD is known to bind cholesterol [84,85] and has been routinely used to modulate the cellular cholesterol content in cell culture systems. By simply modifying the cyclodextrin:cholesterol molar ratio, the cellular cholesterol content can be manipulated, ranging from cholesterol depletion to enrichment [86]. At high concentrations (10–100 mM), 2HPBCD serves as a cholesterol sink and can extract and trap cholesterol. However, at low concentrations (<1 mM), 2HPBCD can also act as a cholesterol shuttle, transporting cholesterol between membranes [87,88].
Treatment of NPC disease with 2HPBCD
When Npc1−/− mice were given a single injection of 2HPBCD (4000 mg/kg) at 7 days of age, their lifespan was extended by nearly 50%. In addition to increased longevity, neurodegeneration was delayed but not completely prevented, as shown by a decrease in Purkinje cell loss within the cerebellum, one of the hallmark findings of NPC disease [48,76]. This was an indication that 2HPBCD was able to cross the blood–brain barrier and reach the brain in these infant mice, possibly due to the incomplete closure of the blood–brain barrier during this early period of development. 6 weeks after the administration of a single dose of 2HPBCD, there was a significant reduction in the amount of cholesterol sequestered within the livers of the treated Npc1−/− mice along with an improvement in liver dysfunction with near normalization of plasma aspartate aminotransferase and alanine aminotransferase levels. Additionally, the whole-animal cholesterol pool, while still elevated, was reduced from approximately 5000 to 4100 mg/kg bw in the treated animals [48].
When 2HPBCD was administered to 7-dayold Npc1−/− mice and then repeated serially there were even greater benefits, such as doubling of lifespan, decreased neuronal cholesterol and glycosphingolipid storage, normalization of cholesterol content in nearly every organ and a reduction of whole-animal cholesterol pools to essentially normal levels [52,77,89]. However, despite these improvements, the neurodegeneration, while delayed, was not completely prevented, probably due to the inability of 2HPBCD to effectively cross the completely formed blood–brain barrier in these older mice (Figure 3C). Interestingly, despite the beneficial effects seen in all the other organs, including the brain and liver, the lungs of the Npc1−/− mice did not seem to respond to 2HPBCD treatment and showed progressive macrophage infiltration and worsening pulmonary function [49,89,90]. This lack of pulmonary response warrants further investigation.
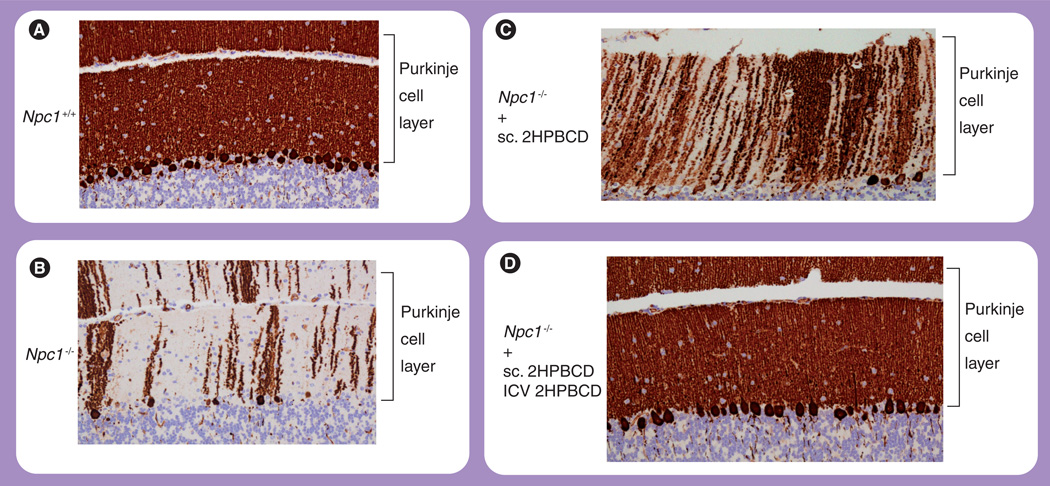
Figure 3. Cerebellar histology.
Representative histologic sections of the cerebellum stained with calbindin. (A) A 49-day-old Npc1+/+ wild-type mouse. (B) A 49-day-old Npc1−/− mouse with no treatment, demonstrating significant loss of Purkinje cells. (C) A 49-day-old Npc1−/− mouse treated with weekly injections of 2HPBCD starting at 7 days of age, demonstrating partial preservation of Purkinje cells. (D) A 49-day-old Npc1−/− mouse treated with continuous ICV infusion of 2HPBCD for 4 weeks starting at 21 days of age, with concomitant weekly injections of 2HPBCD starting at 7 days of age, demonstrating complete preservation of Purkinje cells.
2HPBCD: 2-hydroxypropyl-β-cyclodextrin; ICV: Intracerebroventricular; sc.: Subcutaneous.
When treatment of Npc1−/− mice with 2HPBCD was initiated after 7 days of age, the beneficial effects in extending lifespan were diminished [73,77], and were entirely lost when initiation of treatment was delayed until 49 days of age, despite improvements in cholesterol burden and hepatic function [49]. These mice succumbed to neurodegeneration that was not able to be halted, given 2HPBCD’s inability to traverse the mature blood–brain barrier.
Treatment of the NPC1−/− feline model with 2HPBCD also resulted in many of the beneficial effects seen in the mouse model, such as delayed neurodegeneration and amelioration of liver disease. However, 2HPBCD treatment in both normal and NPC1−/− cats raised the hearing threshold, suggesting possible damage to the peripheral auditory pathway [58]. Whether this phenomenon is species specific and only occurs in cats or extends to other species including mice and humans remains to be answered.
Mechanism of action of 2HPBCD
2HPBCD relieves the cholesterol burden in Npc1−/− mice by allowing the abnormally sequestered cholesterol to be released from the LE/LY into the cytosol, where it is normally then metabolized. After administration, 2HPBCD is apparently internalized by cells through bulkphase endocytosis into the LE/LY [91]. Through a mechanism yet unknown, 2HPBCD then allows the sequestered cholesterol to be released from the LE/LY into the cytosol. Given the difficulty of directly quantifying cholesterol efflux from the LE/LY into the metabolically active cholesterol pool within the cytosol, indirect measures of this efflux were utilized. These included changes in cholesterol synthesis rates, CE formation and activation of LXR target genes that are responsible for cholesterol transport out of the cell. Within 24 h of administration of 2HPBCD to Npc1−/− mice, cholesterol synthesis in virtually every organ, except the lung, was suppressed, which corresponded with downregulation of SREBP2 and its target genes (HMG-CoA reductase, HMG-CoA synthase and the the LDL receptor). In addition, ACAT-mediated CE formation was increased up to 14-fold, there was a corresponding reduction in unesterified cholesterol and LXR-controlled cholesterol efflux genes were activated [48]. All of these measures indicated that the cells were responding to an expansion of the metabolically active cholesterol pool. These changes were seen in Npc1−/− mice regardless of whether 2HPBCD was administered at 7 or 49 days of age [48,49]. A portion of the excess cholesterol was also converted into bile acids and excreted from the body [49]. This ability of 2HPBCD to overcome the cholesterol transport block and release the trapped cholesterol from the LE/LY into the cytosol, where it is then esterified, was also demonstrated in cultured human NPC1 cells [92]. When 2HPBCD was administered to normal mice, no changes were detected in any of the parameters of cholesterol metabolism, which indicated that 2HPBCD, by itself, does not alter cholesterol homeostasis in vivo but requires a large sequestered pool of cholesterol within the LE/LY [48,49].
Based on the ability of 2HPBCD to extract cholesterol from cells in tissue culture, it has been argued that 2HPBCD does not relieve the cholesterol accumulation in NPC1 cells by allowing the sequestered cholesterol to be released from the LE/LY. Instead, it has been contended that 2HPBCD acts by solubilizing and extracting cholesterol from the plasma membrane of NPC1 cells, with the resultant 2HPBCD–cholesterol complex then cleared by the kidneys through the urine. If this were the mechanism by which 2HPBCD functioned, it would result in cellular cholesterol depletion and the cells should respond accordingly by increasing cholesterol synthesis, decreasing CE formation and suppressing LXR-targeted cholesterol efflux, which is opposite of what was actually seen. In addition, if 2HPBCD was extracting cholesterol from cell membranes, a change in the parameters of cholesterol metabolism should also be observed in normal mice, which was not seen. Also, no significant amount of cholesterol was found in the urine of Npc1−/− mice treated with 2HPBCD [49]. To further disprove this notion, another cyclodextrin derivative, sulfobutyl ether-7-β-cyclodextrin (SBE7-β-CD), which is able to bind but not solubilize and extract cholesterol owing to its highly charged nature [93], was administered to Npc1−/− mice and was shown to be just as effective as 2HPBCD in overcoming the cholesterol transport defect in the Npc1−/− animals [55].
In addition to relieving the cholesterol block in mice missing the NPC1 protein, 2HPBCD was also effective in overcoming the cholesterol transport defect in the NPC2 hypomorphic mouse model, as indicated by suppression of cholesterol synthesis 24 h after administration [55]. Additionally, when Npc2−/− mice were treated chronically with 2HPBCD, their lifespan was also significantly prolonged [77]. When 2HPBCD was given to mice missing both NPC1 and NPC2, which share the same phenotype as the Npc1−/− mice, 2HPBCD was equally effective in releasing the trapped cholesterol from the LE/LY [55]. In vitro models have also demonstrated that 2HPBCD was able to effectively transfer cholesterol between membranes in the absence of the LE/LY-specific lipid, lysobisphosphatidic acid (LBPA) [88]. The presence of LBPA in membranes is necessary for NPC2 to effectively transfer cholesterol to and from membranes, probably through direct protein–membrane interactions, and a NPC phenotype results when cells are treated with antibodies against LBPA. [32,94]. These findings indicate that 2HPBCD is working in a fashion that is independent of the functions of both the NPC1 and NPC2 proteins and does not require the presence of LBPA.
Pharmacokinetics of 2HPBCD
2HPBCD is cleared by glomerular filtration in the kidneys and excreted unchanged into urine [95]. When 2HPBCD was administered subcutaneously to adult 49-day-old mice with fully matured renal function at a dose of 4000 mg/kg, the time it took for the clearance of half the administered dose of 2HPBCD from the body (t1/2) was approximately 1.6 h [96]. By 6 h nearly 90% of the administered dose was cleared from the body and by 24 h the 2HPBCD was essentially undetectable [49]. By contrast, when the same dose of 2HPBCD was given to 7-dayold mice, only 40% of the administered dose was cleared after 6 h, which is probably due to immature renal function in these very young mice. Given this slower clearance of 2HPBCD in the 7-day-old mice, the plasma concentration was nearly three-times higher compared with the 49-day-old mice; 5.12 (3.67 mM) versus 1.87 mg/ml (1.36 mM), and the exposure time of cells in the body to 2HPBCD was six-times greater in the 7-day-old mice compared with the mature animals. This increase in exposure to 2HPBCD in the 7-day-old animals may be one of the factors that explain why the beneficial effects seen with 2HPBCD are greater when treatment is initiated at an earlier age. However, despite the difference in clearance, the plasma concentrations of 2HPBCD ranged from 1–3 mM, a concentration range in which 2HPBCD has been shown to function as a shuttle, transporting cholesterol between cell membranes in in vitro models. The presence or absence of NPC1 did not affect the rates of 2HPBCD clearance from the body [49].
The effective dose (ED50) of 2HPBCD (given subcutaneously) leading to a 50% suppression of cholesterol synthesis, the most sensitive indirect measure of the release of the sequestered cholesterol from the LE/LY, was found to be approximately 230 mg/kg bw in mature Npc1−/− mice in all organs except the lungs, kidney and brain. The lungs were totally unresponsive to 2HPBCD. The kidneys were highly responsive to 2HPBCD with an ED50 of 31 mg/kg bw, probably reflecting the active endocytosis of 2HPBCD in the proximal tubules and the concentrating of 2HPBCD within the kidneys due to glomerular filtration and renal clearance. The brain was much less responsive to the systemic administration of 2HPBCD with an ED50 of 132,000 mg/kg bw, an indication that systemically administered 2HPBCD was not able to effectively cross the tight junctions of the capillaries that make up the blood–brain barrier in these older mice [55,78]. The inability of 2HPBCD to effectively reach the CNS was probably the reason that neurodegeneration continued to progress despite systemic treatment. Similar ED50 values were observed in the NPC2 mouse [55].
Direct CNS delivery of 2HPBCD
In order to overcome the blood–brain barrier and deliver 2HPBCD directly into the CNS of Npc1−/− mice, intracerebroventricular (ICV) injection of 2HPBCD was carried out. When 2HPBCD was administered directly into the brains of Npc1−/− mice, the ED50 value dropped from 132,000 mg/kg bw with systemic administration to 0.5 mg/kg bw, demonstrating that the brain was extremely responsive to ICV administration of 2HPBCD. In addition to the suppression of cholesterol synthesis, 24 h after ICV delivery of 2HPBCD, a doubling of CE formation in the brain and increased expression of LXR target genes was observed, indicating that 2HPBCD acutely overcame the block in cholesterol transport from the LE/LY of cells in the brains of Npc1−/− mice. Significant improvements in markers of neuroinflammation were also observed [96].
The brain ED50 value of 0.5 mg/kg bw corresponded to a 2HPBCD concentration of 0.1 mM within the CNS, which is within the concentration range where 2HPBCD acts as a cholesterol shuttle [87,88]. When primary cultures of neurons and glial cells from Npc1−/− mice were treated with 2HPBCD at the same concentration of 0.1 mM, the trapped cholesterol within the LE/LY was able to be mobilized as indicated by a reduction in filipin staining, suppression of cholesterol synthesis, and increased CE formation, again demonstrating 2HPBCD’s ability to overcome the cholesterol transport defect [97], as seen in the animal models. However, when the cultured brain cells were exposed to 2HPBCD at a concentration tenfold greater (1.0 mM), instead of releasing the trapped cholesterol from the LE/LY, cholesterol was actually extracted from the plasma membrane, which resulted in the elevation of cholesterol synthesis rates and decreased cholesterol esterification. Neurotoxicity and cell death occurred when the concentration of 2HPBCD was increased to 10 mM [97] as a result of severe cellular cholesterol depletion. Therefore, it appears that the beneficial effects of 2HPBCD in allowing the release of trapped cholesterol from the LE/LY occur at concentrations where 2HPBCD acts as a cholesterol shuttle (<1 mM), whereas higher concentrations can result in cellular cholesterol depletion and toxicity.
2HPBCD is cleared much more slowly from the CNS after ICV administration than it is from the body after systemic administration. The t1/2 of 2HPBCD clearance from the CNS was approximately 6.5 h by contrast to 1.6 h with systemic administration [96]. However, despite the slower clearance, given that repeated injections of 2HPBCD directly into the brains of Npc1−/− mice would be technically difficult, osmotic pumps were utilized to deliver a constant infusion of 2HPBCD into the CNS via a canula implanted into the left ventricle.
When Npc1−/− mice were treated with a continuous 4-week ICV infusion of 2HPBCD (0.46 mg/day) into the CNS via an osmotic pump implanted at 21 days of age, along with concurrent weekly systemic injections of 2HPBCD starting at 7 days of age, there was complete prevention of neurodegeneration with 100% preservation of Purkinje cells at 49 days of age (Figure 3D). The brain histology of the treated Npc1−/− mice was indistinguishable from that of the wild-type controls (Figure 3A). This is in stark contrast to the untreated Npc1−/− mice, which had significant neurodegeneration and only 25% survival of Purkinje cells (Figure 3B) [89,96]. Whether continuous, long-term ICV infusions of 2HPBCD can fully prevent neurodegeneration and further prolong the lifespan of Npc1−/− mice has yet to be determined.
Conclusion
Cyclodextrins represent an exciting possibility in the development of a therapy for individuals with NPC disease, for which no effective treatment is currently available. Based upon the data obtained from the animal studies demonstrating the beneficial effects of 2HPBCD, along with the fact that 2HPBCD is relatively nontoxic and has been given to humans, 2HPBCD may prove to be useful in the treatment of human NPC disease in ameliorating liver dysfunction, as well as slowing or preventing neurodegeneration. The treatment of children with NPC disease will probably require both systemic administration as well as direct delivery into the CNS to bypass the blood–brain barrier. However, before human clinical trials to test the efficacy of 2HPBCD are initiated, one critical question must be answered; whether or not 2HPBCD can actually reverse the cholesterol transport defect when given to humans as it does in the NPC mouse.
Future perspective
The discovery of 2HPBCD for the treatment of NPC disease in animal models has laid the foundation for its application in the treatment of children with this genetic disorder. The US FDA has recently approved the use of 2HPBCD for compassionate treatment of a small number of children with NPC disease. Formalized clinical trials to test the efficacy and safety of 2HPBCD are currently being designed and should be underway in the near future.
Despite all the advances that have been made, the mechanism through which cyclodextrins overcome the cholesterol transport block in NPC disease remains unknown. By exploring its mechanism of action, further insight may be gained into the cellular transport of cholesterol out of the LE/LY. This could lead to the identification of new proteins and cellular pathways that may potentially be targets for future therapies of NPC disease, as well as other lysosomal storage disorders.
Executive summary.
Niemann–Pick type C disease
-
▪
Niemann–Pick type C (NPC) disease is an autosomal recessive lysosomal storage disorder characterized by the abnormal accumulation of cholesterol within the late endosome/lysosome (LE/LY) of all cells, which results in cell death and organ dysfunction.
Cellular acquisition of cholesterol
-
▪
Cells acquire cholesterol through de novo synthesis and/or uptake of lipoproteins via receptor-mediated or bulk-phase endocytosis.
-
▪
Cellular cholesterol pools are tightly regulated, since an excess or deficiency of cholesterol can be detrimental to cells.
Functions of NPC1 & NPC2 in cholesterol transport
-
▪
NPC1 and NPC2 work in concert to facilitate the transport of cholesterol out of the LE/LY.
-
▪
Mutations in either NPC1 or NPC2 disrupt the normal trafficking of cholesterol out of the LE/LY leading to the accumulation of cholesterol within the LE/LY, the hallmark feature of NPC disease.
Animal models of NPC disease
-
▪
Murine and feline models of NPC disease share many of the same features as those seen in the human disorder and have greatly advanced the understanding of the pathogenesis of NPC disease, which has led to the development of potential therapies.
Attempted treatments of NPC disease
-
▪
Attempted treatments have involved: decreasing cholesterol levels in the LE/LY, glycosphingolipid reduction, preventing apoptosis and neurosteroid replacement.
-
▪
Most of these therapies had limited success except for neurosteroid replacement with allopregnanolone dissolved in the carrier 2-hydroxypropyl-β-cyclodextrin (2HPBCD), which resulted in a dramatic increase in the lifespan of patients and delayed neurodegeneration, which was later attributed to the carrier 2HPBCD and not the neurosteroid.
Cyclodextrin
-
▪
Cyclodextrin is a natural derivative of starch, composed of linked glucopyranose units forming a cone-like structure with a hydrophilic exterior and a hydrophobic interior, which is able to form water-soluble complexes with hydrophobic molecules.
-
▪
Cyclodextrins have been modified to form derivatives such as 2HPBCD that are relatively nontoxic for use in humans and animals.
-
▪
High concentrations of 2HPBCD can extract cholesterol from cell membranes, while at lower concentrations 2HPBCD can act as a cholesterol shuttle.
Treatment of NPC disease with 2HPBCD
-
▪
A single subcutaneous administration of 2HPBCD to Npc1−/− mice at 7 days of age results in significant prolongation of lifespan, delayed neurodegeneration and lowering of whole-body cholesterol.
-
▪
Repeated subcutaneous administration of 2HPBCD in Npc1−/− or Npc2−/− mice results in further prolongation of life, delayed neurodegeneration and normalization of cholesterol levels in almost every organ system except for the lung.
Mechanism of action of 2HPBCD
-
▪
2HPBCD is internalized by cells into the LE/LY through bulk-phase endocytosis, where it then functions to allow the release of trapped cholesterol from the LE/LY of NPC disease cells into the cytosol to be normally metabolized.
-
▪
2HPBCD works in a manner that is independent of the functions of both NPC1, NPC2 and lysobisphosphatidic acid; however, the exact mechanism remains unknown.
Pharmacokinetics of 2HPBCD
-
▪
2HPBCD is rapidly cleared by the kidneys and eliminated from the body within 24 h after administration in both normal and Npc1−/− mice.
-
▪
Most organs of Npc1−/− mice are responsive to systemic administration of 2HPBCD (as indicated by the effective dose), with the exception of the lung, which does not respond, and the brain, owing to the inability of 2HPBCD to cross the blood–brain barrier.
Direct CNS delivery of 2HPBCD
-
▪
Weekly systemic treatment with 2HPBCD along with continuous infusion of 2HPBCD into the CNS via intracerebroventricular injection, thereby bypassing the blood–brain barrier, relieves the block in cholesterol transport within the cells of the brain and completely prevents neurodegeneration in Npc1−/− mice.
Acknowledgments
B Liu acknowledges the Ara Parseghian Medical Research Foundation and US Public Health Service grants R01 HL-09610 and T32 DK-07745 for funding and support.
Footnotes
Financial & competing interests disclosure
The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Vanier MT, Wenger DA, Comly ME, Rousson R, Brady RO, Pentchev PG. Niemann–Pick disease group C: clinical variability and diagnosis based on defective cholesterol esterification. A collaborative study on 70 patients. Clin. Genet. 1988;33(5):331–348. doi: 10.1111/j.1399-0004.1988.tb03460.x. [DOI] [PubMed] [Google Scholar]
- 2.Vanier MT. Niemann–Pick disease type C. Orphanet. J. Rare Dis. 2010;5:16. doi: 10.1186/1750-1172-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yerushalmi B, Sokol RJ, Narkewicz MR, Smith D, Ashmead JW, Wenger DA. Niemann-pick disease type C in neonatal cholestasis at a North American center. J. Pediatr. Gastroenterol. Nutr. 2002;35(1):44–50. doi: 10.1097/00005176-200207000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Garver WS, Francis GA, Jelinek D, et al. The National Niemann–Pick C1 disease database: report of clinical features and health problems. Am. J. Med. Genet. A. 2007;143A(11):1204–1211. doi: 10.1002/ajmg.a.31735. [DOI] [PubMed] [Google Scholar]
- 5.Kelly DA, Portmann B, Mowat AP, Sherlock S, Lake BD. Niemann–Pick disease type C: diagnosis and outcome in children, with particular reference to liver disease. J. Pediatr. 1993;123(2):242–247. doi: 10.1016/s0022-3476(05)81695-6. [DOI] [PubMed] [Google Scholar]
- 6.Kovesi TA, Lee J, Shuckett B, Clarke JT, Callahon JW, Phillips MJ. Pulmonary infiltration in Niemann–Pick disease type C. J. Inherit. Metab. Dis. 1996;19(6):792–793. doi: 10.1007/BF01799175. [DOI] [PubMed] [Google Scholar]
- 7.Schofer O, Mischo B, Puschel W, Harzer K, Vanier MT. Early-lethal pulmonary form of Niemann–Pick type C disease belonging to a second, rare genetic complementation group. Eur. J. Pediatr. 1998;157(1):45–49. doi: 10.1007/s004310050764. [DOI] [PubMed] [Google Scholar]
- 8.Nicholson AG, Florio R, Hansell DM, et al. Pulmonary involvement by Niemann–Pick disease. A report of six cases. Histopathology. 2006;48(5):596–603. doi: 10.1111/j.1365-2559.2006.02355.x. [DOI] [PubMed] [Google Scholar]
- 9.Guillemot N, Troadec C, De Villemeur TB, Clement A, Fauroux B. Lung disease in Niemann–Pick disease. Pediatr. Pulmonol. 2007;42(12):1207–1214. doi: 10.1002/ppul.20725. [DOI] [PubMed] [Google Scholar]
- 10.Bjurulf B, Spetalen S, Erichsen A, Vanier MT, Strom EH, Stromme P. Niemann–Pick disease type C2 presenting as fatal pulmonary alveolar lipoproteinosis: morphological findings in lung and nervous tissue. Med. Sci. Monit. 2008;14(8):CS71–CS75. [PubMed] [Google Scholar]
- 11. Carstea ED, Morris JA, Coleman KG, et al. Niemann–Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 1997;277(5323):228–231. doi: 10.1126/science.277.5323.228. ▪ Discovery of the NPC1 gene.
- 12.Sleat DE, Wiseman JA, El-Banna M, et al. Genetic evidence for nonredundant functional cooperativity between NPC1 and NPC2 in lipid transport. Proc. Natl Acad. Sci. USA. 2004;101(16):5886–5891. doi: 10.1073/pnas.0308456101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie C, Turley SD, Pentchev PG, Dietschy JM. Cholesterol balance and metabolism in mice with loss of function of Niemann–Pick C protein. Am. J. Physiol. 1999;276(2 Pt 1):e336–e344. doi: 10.1152/ajpendo.1999.276.2.E336. [DOI] [PubMed] [Google Scholar]
- 14.Gondre-Lewis MC, McGlynn R, Walkley SU. Cholesterol accumulation in NPC1-deficient neurons is ganglioside dependent. Curr. Biol. 2003;13(15):1324–1329. doi: 10.1016/s0960-9822(03)00531-1. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Wu YP, Wada R, et al. Alleviation of neuronal ganglioside storage does not improve the clinical course of the Niemann–Pick C disease mouse. Hum. Mol. Genet. 2000;9(7):1087–1092. doi: 10.1093/hmg/9.7.1087. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe Y, Akaboshi S, Ishida G, et al. Increased levels of GM2 ganglioside in fibroblasts from a patient with juvenile Niemann–Pick disease type C. Brain Dev. 1998;20(2):95–97. doi: 10.1016/s0387-7604(97)00113-7. [DOI] [PubMed] [Google Scholar]
- 17.Xie C, Turley SD, Dietschy JM. Centripetal cholesterol flow from the extrahepatic organs through the liver is normal in mice with mutated Niemann–Pick type C protein (NPC1) J. Lipid Res. 2000;41(8):1278–1289. [PubMed] [Google Scholar]
- 18.Cruz JC, Chang TY. Fate of endogenously synthesized cholesterol in Niemann–Pick type C1 cells. J. Biol. Chem. 2000;275(52):41309–41316. doi: 10.1074/jbc.M008272200. [DOI] [PubMed] [Google Scholar]
- 19.Liu B, Xie C, Richardson JA, Turley SD, Dietschy JM. Receptor-mediated and bulk-phase endocytosis cause macrophage and cholesterol accumulation in Niemann–Pick C disease. J. Lipid Res. 2007;48(8):1710–1723. doi: 10.1194/jlr.M700125-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Infante RE, Abi-Mosleh L, Radhakrishnan A, Dale JD, Brown MS, Goldstein JL. Purified NPC1 protein. I. inding of cholesterol and oxysterols to a 1278-amino acid membrane protein. J. Biol. Chem. 2008;283(2):1052–1063. doi: 10.1074/jbc.M707943200. [DOI] [PubMed] [Google Scholar]
- 21.Infante RE, Radhakrishnan A, Abi-Mosleh L, et al. Purified NPC1 protein: II. Localization of sterol binding to a 240-amino acid soluble luminal loop. J. Biol. Chem. 2008;283(2):1064–1075. doi: 10.1074/jbc.M707944200. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein JL, Debose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124(1):35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 23.Repa JJ, Mangelsdorf DJ. Nuclear receptor regulation of cholesterol and bile acid metabolism. Curr. Opin Biotechnol. 1999;10(6):557–563. doi: 10.1016/s0958-1669(99)00031-2. [DOI] [PubMed] [Google Scholar]
- 24.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 2002;109(9):1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies JP, Ioannou YA. Topological analysis of Niemann–Pick C1 protein reveals that the membrane orientation of the putative sterol-sensing domain is identical to those of 3-hydroxy-3-methylglutaryl-CoA reductase and sterol regulatory element binding protein cleavage-activating protein. J. Biol. Chem. 2000;275(32):24367–24374. doi: 10.1074/jbc.M002184200. [DOI] [PubMed] [Google Scholar]
- 26.Hua X, Sakai J, Brown MS, Goldstein JL. Regulated cleavage of sterol regulatory element binding proteins requires sequences on both sides of the endoplasmic reticulum membrane. J. Biol. Chem. 1996;271(17):10379–10384. doi: 10.1074/jbc.271.17.10379. [DOI] [PubMed] [Google Scholar]
- 27.Nohturfft A, Brown MS, Goldstein JL. Sterols regulate processing of carbohydrate chains of wild-type SREBP cleavage-activating protein (SCAP), but not sterol-resistant mutants Y298C or D443N. Proc. Natl Acad. Sci. USA. 1998;95(22):12848–12853. doi: 10.1073/pnas.95.22.12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohgami N, Ko DC, Thomas M, Scott MP, Chang CC, Chang TY. Binding between the Niemann–Pick C1 protein and a photoactivatable cholesterol analog requires a functional sterol-sensing domain. Proc. Natl Acad. Sci. USA. 2004;101(34):12473–12478. doi: 10.1073/pnas.0405255101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okamura N, Kiuchi S, Tamba M, et al. A porcine homolog of the major secretory protein of human epididymis, HE1, specifically binds cholesterol. Biochim. Biophys. Acta. 1999;1438(3):377–387. doi: 10.1016/s1388-1981(99)00070-0. [DOI] [PubMed] [Google Scholar]
- 30.Ko DC, Binkley J, Sidow A, Scott MP. The integrity of a cholesterol-binding pocket in Niemann–Pick C2 protein is necessary to control lysosome cholesterol levels. Proc. Natl Acad. Sci. USA. 2003;100(5):2518–2525. doi: 10.1073/pnas.0530027100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedland N, Liou HL, Lobel P, Stock AM. Structure of a cholesterol-binding protein deficient in Niemann–Pick type C2 disease. Proc. Natl Acad. Sci. USA. 2003;100(5):2512–2517. doi: 10.1073/pnas.0437840100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheruku SR, Xu Z, Dutia R, Lobel P, Storch J. Mechanism of cholesterol transfer from the Niemann–Pick type C2 protein to model membranes supports a role in lysosomal cholesterol transport. J. Biol. Chem. 2006;281(42):31594–31604. doi: 10.1074/jbc.M602765200. [DOI] [PubMed] [Google Scholar]
- 33.Deffieu MS, Pfeffer SR. Niemann–Pick type C1 function requires lumenal domain residues that mediate cholesterol-dependent NPC2 binding. Proc. Natl Acad. Sci. USA. 2011;108(47):18932–18936. doi: 10.1073/pnas.1110439108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu S, Benoff B, Liou HL, Lobel P, Stock AM. Structural basis of sterol binding by NPC2, a lysosomal protein deficient in Niemann–Pick type C2 disease. J. Biol. Chem. 2007;282(32):23525–23531. doi: 10.1074/jbc.M703848200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Infante RE, Wang ML, Radhakrishnan A, Kwon HJ, Brown MS, Goldstein JL. NPC2 facilitates bidirectional transfer of cholesterol between NPC1 and lipid bilayers, a step in cholesterol egress from lysosomes. Proc. Natl Acad. Sci. USA. 2008;105(40):15287–15292. doi: 10.1073/pnas.0807328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kwon HJ, Abi-Mosleh L, Wang ML, et al. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell. 2009;137(7):1213–1224. doi: 10.1016/j.cell.2009.03.049. ▪ Summation of the elegant studies performed by this laboratory on the functions of NPC1 and NPC2.
- 37.Liscum L, Ruggiero RM, Faust JR. The intracellular transport of low density lipoprotein-derived cholesterol is defective in Niemann–Pick type C fibroblasts. J. Cell Biol. 1989;108(5):1625–1636. doi: 10.1083/jcb.108.5.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wojtanik KM, Liscum L. The transport of low density lipoprotein-derived cholesterol to the plasma membrane is defective in NPC1 cells. J. Biol. Chem. 2003;278(17):14850–14856. doi: 10.1074/jbc.M300488200. [DOI] [PubMed] [Google Scholar]
- 39.Pentchev PG, Comly ME, Kruth HS, et al. A defect in cholesterol esterification in Niemann–Pick disease (type C) patients. Proc. Natl Acad. Sci. USA. 1985;82(23):8247–8251. doi: 10.1073/pnas.82.23.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pentchev PG, Gal AE, Boothe AD, Fouks J, Omodeo-Sale F, Brady RO. A lysosomal storage disorder in mice characterized by the accumulation of several sphingolipids. Birth Defects Orig. Artic. Ser. 1980;16(1):225–230. [PubMed] [Google Scholar]
- 41.Pentchev PG, Gal AE, Booth AD, et al. A lysosomal storage disorder in mice characterized by a dual deficiency of sphingomyelinase and glucocerebrosidase. Biochim. Biophys. Acta. 1980;619(3):669–679. doi: 10.1016/0005-2760(80)90116-2. [DOI] [PubMed] [Google Scholar]
- 42.Morris MD, Bhuvaneswaran C, Shio H, Fowler S. Lysosome lipid storage disorder in NCTR-BALB/c mice. I Description of the disease and genetics. Am. J. Pathol. 1982;108(2):140–149. [PMC free article] [PubMed] [Google Scholar]
- 43.Shio H, Fowler S, Bhuvaneswaran C, Morris MD. Lysosome lipid storage disorder in NCTR-BALB/c mice. II. Morphologic and cytochemical studies. Am. J. Pathol. 1982;108(2):150–159. [PMC free article] [PubMed] [Google Scholar]
- 44.Bhuvaneswaran C, Morris MD, Shio H, Fowler S. Lysosome lipid storage disorder in NCTR-BALB/c mice. III. Isolation and analysis of storage inclusions from liver. Am. J. Pathol. 1982;108(2):160–170. [PMC free article] [PubMed] [Google Scholar]
- 45.Loftus SK, Morris JA, Carstea ED, et al. Murine model of Niemann–Pick C disease: mutation in a cholesterol homeostasis gene. Science. 1997;277(5323):232–235. doi: 10.1126/science.277.5323.232. [DOI] [PubMed] [Google Scholar]
- 46.Xie C, Turley SD, Dietschy JM. Cholesterol accumulation in tissues of the Niemann–Pick type C mouse is determined by the rate of lipoprotein-cholesterol uptake through the coated-pit pathway in each organ. Proc. Natl Acad. Sci. USA. 1999;96(21):11992–11997. doi: 10.1073/pnas.96.21.11992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beltroy EP, Richardson JA, Horton JD, Turley SD, Dietschy JM. Cholesterol accumulation and liver cell death in mice with Niemann–Pick type C disease. Hepatology. 2005;42(4):886–893. doi: 10.1002/hep.20868. [DOI] [PubMed] [Google Scholar]
- 48. Liu B, Turley SD, Burns DK, Miller AM, Repa JJ, Dietschy JM. Reversal of defective lysosomal transport in NPC disease ameliorates liver dysfunction and neurodegeneration in the npc1 −/− mouse. Proc. Natl Acad. Sci. USA. 2009;106(7):2377–2382. doi: 10.1073/pnas.0810895106. ▪▪ First paper reporting the ability of cyclodextrin to reverse the cholesterol transport defect in Niemann–Pick type C disease.
- 49.Liu B, Ramirez CM, Miller AM, Repa JJ, Turley SD, Dietschy JM. Cyclodextrin overcomes the transport defect in nearly every organ of NPC1 mice leading to excretion of sequestered cholesterol as bile acid. J. Lipid Res. 2010;51(5):933–944. doi: 10.1194/jlr.M000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xie C, Burns DK, Turley SD, Dietschy JM. Cholesterol is sequestered in the brains of mice with Niemann–Pick type C disease but turnover is increased. J. Neuropathol. Exp. Neurol. 2000;59(12):1106–1117. doi: 10.1093/jnen/59.12.1106. [DOI] [PubMed] [Google Scholar]
- 51.Sawamura N, Gong JS, Garver WS, et al. Site-specific phosphorylation of tau accompanied by activation of mitogen-activated protein kinase (MAPK) in brains of Niemann–Pick type C mice. J. Biol. Chem. 2001;276(13):10314–10319. doi: 10.1074/jbc.M009733200. [DOI] [PubMed] [Google Scholar]
- 52.Xie X, Brown MS, Shelton JM, Richardson JA, Goldstein JL, Liang G. Amino acid substitution in NPC1 that abolishes cholesterol binding reproduces phenotype of complete NPC1 deficiency in mice. Proc. Natl Acad. Sci. USA. 2011;108(37):15330–15335. doi: 10.1073/pnas.1112751108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maue RA, Burgess RW, Wang B, et al. A novel mouse model of Niemann–Pick type C disease carrying a D1005G-Npc1 mutation comparable to commonly observed human mutations. Hum. Mol. Genet. 2012;21(4):730–750. doi: 10.1093/hmg/ddr505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Naureckiene S, Sleat DE, Lackland H, et al. Identification of HE1 as the second gene of Niemann–Pick C disease. Science. 2000;290(5500):2298–2301. doi: 10.1126/science.290.5500.2298. [DOI] [PubMed] [Google Scholar]
- 55.Ramirez CM, Liu B, Aqul A, et al. Quantitative role of LAL, NPC2, and NPC1 in lysosomal cholesterol processing defined by genetic and pharmacological manipulations. J. Lipid Res. 2011;52(4):688–698. doi: 10.1194/jlr.M013789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lowenthal AC, Cummings JF, Wenger DA, Thrall MA, Wood PA, De Lahunta A. Feline sphingolipidosis resembling Niemann–Pick disease type C. Acta Neuropathol. 1990;81(2):189–197. doi: 10.1007/BF00334507. [DOI] [PubMed] [Google Scholar]
- 57.Brown DE, Thrall MA, Walkley SU, et al. Feline Niemann–Pick disease type C. Am. J. Pathol. 1994;144(6):1412–1415. [PMC free article] [PubMed] [Google Scholar]
- 58.Ward S, O’Donnell P, Fernandez S, Vite CH. 2-hydroxypropyl-beta-cyclodextrin raises hearing threshold in normal cats and in cats with Niemann–Pick type C disease. Pediatr. Res. 2010;68(1):52–56. doi: 10.1203/PDR.0b013e3181df4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vite CH, Ding W, Bryan C, et al. Clinical, electrophysiological, and serum biochemical measures of progressive neurological and hepatic dysfunction in feline Niemann–Pick type C disease. Pediatr. Res. 2008;64(5):544–549. doi: 10.1203/PDR.0b013e318184d2ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Repa JJ, Li H, Frank-Cannon TC, et al. Liver X receptor activation enhances cholesterol loss from the brain, decreases neuroinflammation, and increases survival of the NPC1 mouse. J. Neurosci. 2007;27(52):14470–14480. doi: 10.1523/JNEUROSCI.4823-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patterson MC, Di Bisceglie AM, Higgins JJ, et al. The effect of cholesterol-lowering agents on hepatic and plasma cholesterol in Niemann–Pick disease type C. Neurology. 1993;43(1):61–64. doi: 10.1212/wnl.43.1_part_1.61. [DOI] [PubMed] [Google Scholar]
- 62.Somers KL, Brown DE, Fulton R, et al. Effects of dietary cholesterol restriction in a feline model of Niemann–Pick type C disease. J. Inherit. Metab. Dis. 2001;24(4):427–436. doi: 10.1023/a:1010588112003. [DOI] [PubMed] [Google Scholar]
- 63.Erickson RP, Garver WS, Camargo F, Hossain GS, Heidenreich RA. Pharmacological and genetic modifications of somatic cholesterol do not substantially alter the course of CNS disease in Niemann–Pick C mice. J. Inherit. Metab. Dis. 2000;23(1):54–62. doi: 10.1023/a:1005650930330. [DOI] [PubMed] [Google Scholar]
- 64.Alvarez AR, Klein A, Castro J, et al. Imatinib therapy blocks cerebellar apoptosis and improves neurological symptoms in a mouse model of Niemann–Pick type C disease. FASEB J. 2008;22(10):3617–3627. doi: 10.1096/fj.07-102715. [DOI] [PubMed] [Google Scholar]
- 65.Lloyd-Evans E, Morgan AJ, He X, et al. Niemann–Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat. Med. 2008;14(11):1247–1255. doi: 10.1038/nm.1876. [DOI] [PubMed] [Google Scholar]
- 66.Borbon IA, Hillman Z, Duran E, Jr, Kiela PR, Frautschy SA, Erickson RP. Lack of efficacy of curcumin on neurodegeneration in the mouse model of Niemann–Pick C1. Pharmacol. Biochem. Behav. 2012;101(1):125–131. doi: 10.1016/j.pbb.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vanier MT. Lipid changes in Niemann–Pick disease type C brain: personal experience and review of the literature. Neurochem. Res. 1999;24(4):481–489. doi: 10.1023/a:1022575511354. [DOI] [PubMed] [Google Scholar]
- 68.Zervas M, Somers KL, Thrall MA, Walkley SU. Critical role for glycosphingolipids in Niemann–Pick disease type C. Curr. Biol. 2001;11(16):1283–1287. doi: 10.1016/s0960-9822(01)00396-7. [DOI] [PubMed] [Google Scholar]
- 69.Li H, Turley SD, Liu B, Repa JJ, Dietschy JM. GM2/GD2 and GM3 gangliosides have no effect on cellular cholesterol pools or turnover in normal or NPC1 mice. J. Lipid Res. 2008;49(8):1816–1828. doi: 10.1194/jlr.M800180-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patterson MC, Vecchio D, Prady H, Abel L, Wraith JE. Miglustat for treatment of Niemann–Pick C disease: a randomised controlled study. Lancet Neurol. 2007;6(9):765–772. doi: 10.1016/S1474-4422(07)70194-1. [DOI] [PubMed] [Google Scholar]
- 71.Wraith JE, Vecchio D, Jacklin E, et al. Miglustat in adult and juvenile patients with Niemann–Pick disease type C: long-term data from a clinical trial. Mol. Genet. Metab. 2010;99(4):351–357. doi: 10.1016/j.ymgme.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 72.Pineda M, Wraith JE, Mengel E, et al. Miglustat in patients with Niemann–Pick disease Type C (NP-C): a multicenter observational retrospective cohort study. Mol. Genet. Metab. 2009;98(3):243–249. doi: 10.1016/j.ymgme.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 73.Griffin LD, Gong W, Verot L, Mellon SH. Niemann–Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat. Med. 2004;10(7):704–711. doi: 10.1038/nm1073. [DOI] [PubMed] [Google Scholar]
- 74.Langmade SJ, Gale SE, Frolov A, et al. Pregnane X receptor (PXR) activation: a mechanism for neuroprotection in a mouse model of Niemann–Pick C disease. Proc. Natl Acad. Sci. USA. 2006;103(37):13807–13812. doi: 10.1073/pnas.0606218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ahmad I, Lope-Piedrafita S, Bi X, et al. Allopregnanolone treatment, both as a single injection or repetitively, delays demyelination and enhances survival of Niemann–Pick C mice. J. Neurosci. Res. 2005;82(6):811–821. doi: 10.1002/jnr.20685. [DOI] [PubMed] [Google Scholar]
- 76.Liu B, Li H, Repa JJ, Turley SD, Dietschy JM. Genetic variations and treatments that affect the lifespan of the NPC1 mouse. J. Lipid Res. 2008;49(3):663–669. doi: 10.1194/jlr.M700525-JLR200. [DOI] [PubMed] [Google Scholar]
- 77. Davidson CD, Ali NF, Micsenyi MC, et al. Chronic cyclodextrin treatment of murine Niemann–Pick C disease ameliorates neuronal cholesterol and glycosphingolipid storage and disease progression. PLoS ONE. 2009;4(9):e6951. doi: 10.1371/journal.pone.0006951. ▪ First report of the benefits of serial administration of cyclodextrin in Npc1 and Npc2 mice.
- 78.Camargo F, Erickson RP, Garver WS, et al. Cyclodextrins in the treatment of a mouse model of Niemann–Pick C disease. Life Sci. 2001;70(2):131–142. doi: 10.1016/s0024-3205(01)01384-4. [DOI] [PubMed] [Google Scholar]
- 79.Thompson DO. Cyclodextrins – enabling excipients: their present and future use in pharmaceuticals. Crit. Rev. Ther. Drug Carrier Syst. 1997;14(1):1–104. [PubMed] [Google Scholar]
- 80.Pitha J, Irie T, Sklar PB, Nye JS. Drug solubilizers to aid pharmacologists: amorphous cyclodextrin derivatives. Life Sci. 1988;43(6):493–502. doi: 10.1016/0024-3205(88)90150-6. [DOI] [PubMed] [Google Scholar]
- 81.Frank DW, Gray JE, Weaver RN. Cyclodextrin nephrosis in the rat. Am. J. Pathol. 1976;83(2):367–382. [PMC free article] [PubMed] [Google Scholar]
- 82.Irie T, Otagiri M, Sunada M, et al. Cyclodextrin-induced hemolysis and shape changes of human erythrocytes in vitro. J. Pharmacobiodyn. 1982;5(9):741–744. doi: 10.1248/bpb1978.5.741. [DOI] [PubMed] [Google Scholar]
- 83.Gould S, Scott RC. 2-Hydroxypropyl-beta-cyclodextrin (HP-beta-CD): a toxicology review. Food Chem. Toxicol. 2005;43(10):1451–1459. doi: 10.1016/j.fct.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 84.Irie T, Fukunaga K, Pitha J. Hydroxypropylcyclodextrins in parenteral use. I: Lipid dissolution and effects on lipid transfers in vitro. J. Pharm. Sci. 1992;81(6):521–523. doi: 10.1002/jps.2600810609. [DOI] [PubMed] [Google Scholar]
- 85.Ohtani Y, Irie T, Uekama K, Fukunaga K, Pitha J. Differential effects of alpha-, beta- and gamma-cyclodextrins on human erythrocytes. Eur. J. Biochem. 1989;186(1–2):17–22. doi: 10.1111/j.1432-1033.1989.tb15171.x. [DOI] [PubMed] [Google Scholar]
- 86.Christian AE, Haynes MP, Phillips MC, Rothblat GH. Use of cyclodextrins for manipulating cellular cholesterol content. J. Lipid Res. 1997;38(11):2264–2272. [PubMed] [Google Scholar]
- 87.Atger VM, de la Llera Moya M, Stoudt GW, Rodrigueza WV, Phillips MC, Rothblat GH. Cyclodextrins as catalysts for the removal of cholesterol from macrophage foam cells. J. Clin. Invest. 1997;99(4):773–780. doi: 10.1172/JCI119223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McCauliff LA, Xu Z, Storch J. Sterol transfer between cyclodextrin and membranes: similar but not identical mechanism to NPC2-mediated cholesterol transfer. Biochemistry. 2011;50(34):7341–7349. doi: 10.1021/bi200574f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ramirez CM, Liu B, Taylor AM, et al. Weekly cyclodextrin administration normalizes cholesterol metabolism in nearly every organ of the niemann-pick type C1 mouse and markedly prolongs life. Pediatr. Res. 2010;68(4):309–315. doi: 10.1203/PDR.0b013e3181ee4dd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Muralidhar A, Borbon IA, Esharif DM, et al. Pulmonary function and pathology in hydroxypropyl-beta-cyclodextin-treated and untreated Npc1−/− mice. Mol. Genet. Metab. 2011;103(2):142–147. doi: 10.1016/j.ymgme.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rosenbaum AI, Zhang G, Warren JD, Maxfield FR. Endocytosis of betacyclodextrins is responsible for cholesterol reduction in Niemann–Pick type C mutant cells. Proc. Natl Acad. Sci. USA. 2010;107(12):5477–5482. doi: 10.1073/pnas.0914309107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Abi-Mosleh L, Infante RE, Radhakrishnan A, Goldstein JL, Brown MS. Cyclodextrin overcomes deficient lysosome-to-endoplasmic reticulum transport of cholesterol in Niemann–Pick type C cells. Proc. Natl Acad. Sci. USA. 2009;106(46):19316–19321. doi: 10.1073/pnas.0910916106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rajewski RA, Traiger G, Bresnahan J, Jaberaboansari P, Stella VJ, Thompson DO. Preliminary safety evaluation of parenterally administered sulfoalkyl ether beta-cyclodextrin derivatives. J. Pharm. Sci. 1995;84(8):927–932. doi: 10.1002/jps.2600840805. [DOI] [PubMed] [Google Scholar]
- 94.Xu Z, Farver W, Kodukula S, Storch J. Regulation of sterol transport between membranes and NPC2. Biochemistry. 2008;47(42):11134–11143. doi: 10.1021/bi801328u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Frijlink HW, Visser J, Hefting NR, Oosting R, Meijer DK, Lerk CF. The pharmacokinetics of beta-cyclodextrin and hydroxypropyl-beta-cyclodextrin in the rat. Pharm. Res. 1990;7(12):1248–1252. doi: 10.1023/a:1015929720063. [DOI] [PubMed] [Google Scholar]
- 96. Aqul A, Liu B, Ramirez CM, et al. Unesterified cholesterol accumulation in late endosomes/lysosomes causes neurodegeneration and is prevented by driving cholesterol export from this compartment. J. Neurosci. 2011;31(25):9404–9413. doi: 10.1523/JNEUROSCI.1317-11.2011. ▪ ▪ Complete prevention of neurodegeneration with delivery of cyclodextrin directly into the CNS of Npc1 mice.
- 97.Peake KB, Vance JE. Normalization of cholesterol homeostasis by 2-hydroxypropylbeta- cyclodextrin in neurons and glia from Niemann–Pick C1-deficient mice. J. Biol. Chem. 2012;287(12):9290–9298. doi: 10.1074/jbc.M111.326405. [DOI] [PMC free article] [PubMed] [Google Scholar]