Abstract
Background:
Tuberculosis (TB) and diabetes mellitus (DM) are two diseases that are individually relatively common and of immense public health significance globally. There is a growing awareness on a global scale on the possible relationship between TB and DM. Nigeria is a country with a high burden of TB and an increasing incidence of DM. We set out to determine the frequency of occurrence of undiagnosed DM in TB patients.
Materials and Methods:
This was an observational study that was carried out in TB patients recruited from 56 DOT centers in Lagos, Nigeria. The main objective of the study was to determine the disease burden of DM in patients with TB by comparing the frequency of occurrence of DM in TB to the occurrence of DM in people without TB. Screening was carried out by staff-nurses and community health workers-of these DOT facilities who all had capacity building on the detection of DM at the start of the project.
Results:
Of 4000 TB patients, a total of 480 (12.3%) had DM. Of the pool of DM patients, newly diagnosed cases of DM were 310 (8%) in number and previously known persons with DM were 170 (4.3%). The newly diagnosed cases of DM made up 64% of the cases of DM. In the study population without TB, a total of 112 (5.6%) had DM. The number of newly diagnosed cases of DM were 40 in number and these made up 2% of this study group. The number of persons who were already known to have DM was 72 and these made up 3.6% of the study population. New cases of DM made up 44% of the total number of cases of DM detected in persons without TB.
Conclusion:
The detection rates of DM in patients with TB are higher than in persons without TB. Given the fact that DM may negatively impact TB treatment, we suggest that routine screening be carried out for TB in persons with DM.
Keywords: Diabetes mellitus, Nigeria, screening, tuberculosis
INTRODUCTION
Tuberculosis (TB) is a communicable disease caused by any of the several species of Mycobacterium referred to as tubercle bacillus. Nigeria has a high TB burden and ranks 10th among the 22 high-burden TB countries in the world.[1] Diabetes mellitus (DM) is a chronic metabolic disease that is assuming epidemic proportions and the global increase is poised to occur more in developing countries like Nigeria. There is increasing robust data being churned out on the occurrence of DM in persons with TB. It is interesting to note that a lot of these reports are from Asian countries,[2,3] where the documented prevalence of TB and DM is high.
TB may be a potential risk factor for the development of DM and a high prevalence of DM has been documented in patients with TB.[4,5,6] DM on the contrary is a well-documented risk factor for the development of TB. DM was shown in a report on weighted major risk factors for TB from 22 high burden countries to carry a relative risk of 3.1[7] for the development of TB. Globally, the diabetes pandemic is driven by an increase in the adult population, increased longevity, and changes in behavior associated with rapidly increasing urbanization and development. An overlooked understudied potential risk factor for the development of DM is TB and in a bid to create global awareness and sensitization of the possible occurrence of this deadly duo, the World Diabetes Foundation (WDF) is sponsoring research on these diseases. Detection of DM in TB in Nigeria is a WDF sponsored project carried out in Lagos state and one of the key objectives is the empowering of healthcare personnel who treat people with TB and also to build capacity in this regard toward the detection of DM in TB patients.
The occurrence of both DM and TB may impact adversely on each other with resultant poor glycaemic control of DM and poor response to anti-TB treatment. Some reports on DM in TB[8] note that persons with DM and TB tend to have more symptoms than TB patients without DM. Some data[9,10] also indicate that patients with DM who have TB present a higher bacillary load in sputum and may have higher rates of multidrug resistant, which is often associated with treatment failure.
In their preliminary report on the detection of DM in TB from Nigeria, Olayinka et al.,[11] in their study of 350 patients with TB discovered that the prevalence of DM was 5.7% and half of these were newly detected,
The main objective of the study was to document the disease burden of DM in patients with TB by determining the frequency of occurrence of DM in TB. We also aimed to compare the occurrence of DM in patients with TB to that of people without TB in a bid to determine if persons with TB tended to have a higher DM burden compared with persons without TB.
MATERIALS AND METHODS
Study setting and population
This was an observational cross-sectional study carried out on Lagos state which is located in the South western region of Nigeria and has a population of 20 million people. The total number of healthcare facilities that offer TB care is 206 in number and is spread within primary, secondary, tertiary, and private healthcare facilities. The private health facilities are five in number, tertiary facilities are two, secondary healthcare facilities 42, and the others are located within 157 primary healthcare facilities. Nigeria is ranked 4th among the 22 worst affected countries in the world and the 1st in Africa. In Nigeria, Lagos state carries 8.4% of Nigeria's TB burden and consistently has been responsible for about 11% of the cases of TB registered in Nigeria. The state program is implementing the internationally recommended strategy for TB control called the new Stop TB Strategy and this program has been in place since 2003.
Protocol for screening for DM in persons with TB
All healthcare facilities rendering TB care included in this report were provided with glucometers and test strips. The staff of these centers had earlier been pooled together in groups and had didactic lectures and practical sessions on the detection/screening for DM. This was done to ensure continuity in the screening exercise even after the lifetime of the research work. Attending staff (usually nurses and community healthcare officers) were instructed to perform DM screening for patients known to have TB or those with newly diagnosed TB. All positive results were vetted by medical doctors. The diagnosis of pulmonary TB (PTB) was made when of any two of the following were present-positive sputum smear by microscopic examination of Ziehl-Neelsen stained sputum slides for acid-fast bacilli, chest radiographs with suggestive features of TB and clinical symptoms and signs of TB.[12] DM screening was done using capillary blood samples with glucose meters that read plasma equivalents of glucose. Prescreening, TB patients were asked if they had previously been diagnosed with DM. For those with no known history of DM, screening was carried out in the morning, in a fasted state. All suspicious/abnormal glucose results were repeated on another date still in a fasted state to confirm the diagnosis of DM. Measurement of fasting plasma glucose concentration was performed in participants using capillary blood with glucose meters that provide plasma equivalent readings. (The Finetest Auto-coding™, Infopia Co., Ltd. Korea). The diagnosis of DM was made based on the 1999 World Health Organization (WHO) guidelines (fasting plasma glucose levels ≥126 mg/dL or 7.1 mmol/L are in the diagnostic range for DM).
The inclusion criteria were age ≥12 years diagnosed and on treatment for TB. Exclusion criteria included pregnancy women, TB treatment naive patients, and those who did not give consent.
Hypertension is defined by the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure as a blood pressure of ≥140/90 mm Hg.[13]
Data on histories of TB, family history of DM, and other information pertaining to their TB status was captured via interviewer-administered questionnaires. Anthropometric indices which included body mass indices and waist circumference measurements were documented.
Protocol for screening DM in persons without TB
Persons without past/present histories of management of TB were recruited for screening for DM during DM camps. These camps were held in designated public facilities in the Lagos metropolis. Persons who did not have histories of present or past treatment of TB were included in the study population.
Capillary samples were used and fasting and random glucose levels were determined as deemed applicable. The number of persons who were previously known to have DM was noted. Those with no known diagnosis of DM underwent a random blood glucose test or fasting plasma glucose test based on a capillary blood sample with a glucometer. The diagnosis of DM was made using the WHO criteria, (Fasting blood glucose (FBG) > 7.1 mmol/L or ≥126 mg/dL and casual plasma glucose levels (>11.1 mmol/L or ≥200 mg/dL) indicating DM.[14]
Statistical analyses were carried using SPSS package version 17. The proportions of persons with TB who had DM and proportions of persons without DM were determined. Clinical parameters were compared within the TB study group between TB patients with DM and TB patients without DM. Test statistics used included Student's t-test and Chi-square. A P ≤ 0.05 was statistically significant.
Ethical approval for this study was obtained from the Lagos State Ministry of Health and parental/guardian consent for study participants aged less than 16 years of age.
RESULTS
Of the 4000 TB patients (median age 33 years), 2383 (60%) were males and for the 1,687 persons without TB 1000 (median age 34 years) 1000 (59%) were males.
The mean age of persons without TB was higher than that of persons with TB, but there was no statistically significant difference noted (36.2 ± 13.1 vs. 35.6 ± 13.2, P = 0.2).
Diabetes in TB patients
In the TB patients, a total of 480 (12%) had DM. Of the cases of DM, newly diagnosed cases of DM were 310 (8%) in number and previously known persons with DM were 170 (4.3%). The newly diagnosed cases of DM made up 64% of the cases of DM.
Diabetes in persons without TB
This group was made up of 1000 males and 687 females. Their ages ranged from 12 to 85 years. The mean waist circumference was 71.2 (25.2) cm and ranged from 22 to 89 cm.
A total of 94 persons making up of 5.6% did not have any form of literacy. A small number of persons without TB 48 (2.9%) were known to have a history of hypertension.
The proportion of persons in group who had DM was 5.6%. The number of newly diagnosed cases of DM was 40 in number and made up 2% of this group of people. The number of persons who were already known to have DM was 72 and this made up 3.6% of the study population. New cases of DM made up 44% of the total number of cases of DM detected in persons without TB.
Clinical features of persons with TB and DM
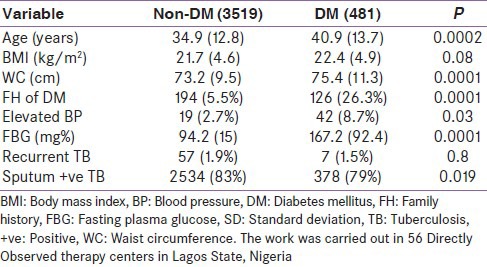
We compared clinical parameters between persons who had DM and TB and persons with TB but no DM. Mean age and anthropometric indices were higher in the group with DM. These and other results are shown in Table 1.
Table 1.
Comparison of the clinical characteristics of with and without diabetes mellitus
DISCUSSION
Current research focus is, however, on the occurrence of DM in TB.
Our results show that newly diagnosed cases of DM were detected in 8% of persons with TB and this accounted for two-thirds of the cases of DM in this group of patients. In the study group without TB which was age-matched for the group with TB, the proportion of persons with newly diagnosed DM was significantly lower. Our results are pointers to the potential increased risk of occurrence of DM in persons with TB.
The possible mechanisms that have been postulated for the diabetogenic effects of TB and also as DM being a putative risk factor for the development of TB include the following DM is associated with reduced cellular immunitya reduced T-helper1 (Th1) cytokine response level, tumor necrosis factor alpha production, and interleukin (IL)-1 beta and IL-6 production is seen among people with concomitant diabetes and TB as compared with nondiabetic individuals.[15,16,17] The decrease in Tlymphocyte number and function is primarily responsible for the susceptibility of diabetics to TB. On the contrary, TB patients may be predisposed to developing DM possibly as a result of decreased hepatic gluconeogenesis, increased hepatic glycogenolysis, and the hyperglycaemic effects of the anti-TB drug, isoniazid.
Our documented prevalence rate of 8% of newly diagnosed DM in TB is comparable to that of an Indian Report-6.5%[18] but was higher than that of a report from China-2.9%[19] Data from Tanzanian[20] indicate a high prevalence rate-16.5% of the occurrence of DM in TB. One factor which may be responsible for this scenario may be the screening technique employed-the standard 75 g 2 h oral glucose tolerance test. It is known that fasting plasma glucose will fail to diagnose 30% of previously undiagnosed DM and the Oral glucose tolerance test (OGTT) has a higher specificity and sensitivity in diagnosing DM.
In our report, smear positive TB was noted in comparable proportions between persons with TB and DM and those with TB and no DM.
Of concern is the appropriate timing for screening of DM in TB patients. This is because TB may induce infection-related temporary hyperglycaemia which may resolve at the end of TB treatment. We screened patients who were already on treatment for TB and did not have a particular time for testing the patients.
Although evaluation for risk factors for DM was not an objective of this report; we, however, compared clinical parameters within the TB cohort. Our results indicated that older age, family history of DM, higher mean anthropometric indices, and higher proportions of persons with hypertension were characteristic features of the group of TB patients that had DM. These clinical parameters are the usual risk factors for persons with type 2 DM.
DM and TB may present with similar symptoms which include lethargy, fatigue, and weight loss. Patients with TB who are thought to be poorly responsive to anti-TB medications may actually have DM as a comorbidity contributing to the aforestated scenario. Some of the staff of these centers attested to the fact that some people who were being treated for TB recurrence actually had DM which was hitherto undiagnosed.
The strength of this report is that the screening for DM at the TB centers was carried out by the staff (nurses and community health workers) of the DOT centers. The DOT centers in Lagos are usually manned by the aforestated cadre of staff under the supervision of few medical doctors. The successful capacity building for DM detection engendered by this project will hopefully promote synergy in TB and DM detection in our practice. There has been some research on bidirectional screening on DM/TB and the yield when screening for TB in DM as compared with when screening for DM in TB is small. Screening for DM is cheap, easy to carry out, and healthcare personnel with basic health education can be empowered to facilitate the detection of DM.
CONCLUSION
We have shown that the burden of DM in TB patients is high and that it is feasible to carry out screening for DM as a routine test for persons with TB.
LIMITATIONS
One of the limitations of this report is that other than waist circumference dimensions, we did not measure anthropometric indices and did not have robust clinical data of the group of persons without TB.
Footnotes
Source of Support: World Diabetes Foundation
Conflict of Interest: None declared
REFERENCES
- 1.World Health Organization. Geneva, Switzerland: WHO; 2012. Global tuberculosis report 2012. WHO/HTM/TB/2012.6. [Google Scholar]
- 2.Viswanathan V, Kumpatla S, Aravindalochanan V, Rajan R, Chinnasamy C, Srinivasan R, et al. Prevalence of diabetes and pre-diabetes and associated risk factors among tuberculosis patients in India. PLOS One. 2012;7:e41367. doi: 10.1371/journal.pone.0041367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta S, Shenoy VP, Bairly I, Srinivasa H, Mukhopadhyay C. Diabetes mellitus and HIV as co-morbidities in tuberculosis patients of rural South India. J Infect Public Health. 2011;4:140–4. doi: 10.1016/j.jiph.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Nichols GP. Diabetes among young tuberculous patients; a review of the association of the two diseases. Am Rev Tuberc. 1957;76:1016–30. doi: 10.1164/artpd.1957.76.6.1016. [DOI] [PubMed] [Google Scholar]
- 5.Mugusi F, Swai AB, Alberti K, Melarty DG. Increased prevalence of diabetes mellitus in patients with pulmonary tuberculosis in Tanzania. Tubercle. 1990;71:271–6. doi: 10.1016/0041-3879(90)90040-f. [DOI] [PubMed] [Google Scholar]
- 6.Jeon CY, Harries AD, Baker MA, Hart JE, Kapur A, Lönnroth K, et al. Bi-directional screening for tuberculosis and diabetes: A systematic review. Trop Med Int Health. 2010;15:1300–14. doi: 10.1111/j.1365-3156.2010.02632.x. [DOI] [PubMed] [Google Scholar]
- 7.Maurice J. WHO framework targets tuberculosis-diabetes link. Lancet. 2011;378:1209–10. doi: 10.1016/s0140-6736(11)61527-4. [DOI] [PubMed] [Google Scholar]
- 8.Restrepo BI, Fisher-Hock SP, Crespo JG, Whitney E, Perez A, Smith B, et al. Nuevo Santander Tuberculosis Trackers. Type 2 diabetes and tuberculosis in a dynamic bi-national border population. Epidemiol Infect. 2007;135:483–91. doi: 10.1017/S0950268806006935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singla R, Khan N, Al-Sharif N, Ai-Sayegh MO, Shaikh MA, Osman MM. Influence of diabetes on manifestations and treatment outcome of pulmonary TB patients. Int J Tuberc Lung Dis. 2006;10:74–9. [PubMed] [Google Scholar]
- 10.Guler M, Unsal E, Dursun B, Aydln O, Capan N. Factors influencing sputum smear and culture conversion time among patients with new case pulmonary tuberculosis. Int J Clin Pract. 2007;61:231–5. doi: 10.1111/j.1742-1241.2006.01131.x. [DOI] [PubMed] [Google Scholar]
- 11.Olayinka AO, Anthonia O, Yetunde K. Prevalence of diabetes mellitus in persons with tuberculosis in a tertiary health centre in Lagos, Nigeria. Indian J Endocrinol Metab. 2013;17:486–9. doi: 10.4103/2230-8210.111646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Geneva, Switzerland: World Health Organization; Treatment of tuberculosis: Guidelines for national program. WHO/CDS/TB/2003.313. [Google Scholar]
- 13.Martin J. Hypertension Guidelines: Revisiting the JNC 7 Recommendations. J Lanc Gen Hospital. 2008;3:3. [Google Scholar]
- 14.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–97. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 15.Geerlings SE, Hopelman AI. Immune dysfunction in patients with diabetes mellitus (DM) FEMS Immunol Med Microbiol. 1999;26:259–65. doi: 10.1111/j.1574-695X.1999.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 16.Tsukaguchi K, Yoneda T, Yoshikawa M, Tokuyama T, Fu A, Tomoda K, et al. Case study of interleukin-1 beta, tumor necrosis factor alpha and interleukin-6 production by peripheral blood monocytes in patients with diabetes mellitus complicated by pulmonary tuberculosis. Kekkaku. 1992;67:755–60. [PubMed] [Google Scholar]
- 17.Sidibe EH, Sankale M. Diabetes and pulmonary tuberculosis: Epidemiology, pathophysiology and symptomatology. J Fr Stud Res Health. 2007;17:29–32. [Google Scholar]
- 18.Dave P, Shah A, Chauhan M, Kumar AM, Harries AD, Malhotra S, et al. Screening patients with tuberculosis for diabetes mellitus in Gujarat, India. Public Health Action. 2013;3 doi: 10.5588/pha.13.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Lin Y, Mi F, Tan S, Liang B, Guo C, et al. Screening of patients with tuberculosis for diabetes mellitus in China. Trop Med Int Health. 2012;17:1294–301. doi: 10.1111/j.1365-3156.2012.03068.x. [DOI] [PubMed] [Google Scholar]
- 20.Ruslami R, Aarnoutse RE, Alisjahbana B, van der Ven AJ, Van Crevel R. Implications of the global increase of diabetes for tuberculosis control and patient care. Trop Med Int Health. 2010;15:1289–99. doi: 10.1111/j.1365-3156.2010.02625.x. [DOI] [PubMed] [Google Scholar]


