Abstract
Background:
The aim of this study is to describe the metabolic syndrome (MS) and to evaluate five diagnostic criteria of the MS with respect to their sensitivity and specificity in patients with type 2 diabetes mellitus (T2DM).
Materials and Methods:
It is a cross-sectional case control study of T2DM patients and their first degree relatives (FDRs) recruited using convenience sampling and data collected through questionnaire administered technique. Variables of interest included anthropometric indices, blood pressure, serum lipid profile, fasting blood sugar (FBS), proteinuria, and microalbuminuria. The Chi-square test was used for comparison of proportions. A P value of less than 0.05 was taken as statistically significant. Kappa statistic was used to test the degree of agreement between the diagnostic criteria.
Results:
The World Health Organization (WHO), International Diabetes Federation (IDF), revised National Cholesterol Education Program (NCEP-R), NCEP Adult Treatment Panel (ATP)-III, and American Association of Clinical Endocrinologists (AACE) criteria reported a prevalence of 87.1, 64.5, 61.3, 55.6, and 22.6%, respectively in persons with T2DM. Using the WHO criteria as a reference or gold standard, the sensitivity of the IDF, NCEP-R, NCEP ATP-III, and AACE criteria among persons with T2DM were 71.3, 67.6, 61.1, and 25.9% respectively. Using the WHO criteria as a reference or gold standard, the specificity of the IDF, NCEP-R, NCEP ATP-III, and AACE criteria among persons with T2DM were 81.3, 81.3, 81.3, and 100%, respectively. Using the WHO criteria as a reference or gold standard, the level of agreement of the IDF, NCEP-R, NCEP ATP-III, and AACE criteria with the WHO criteria among persons with T2DM (as estimated by the kappa statistics) were 0.30, 0.26, 0.21, and 0.08 respectively.
Conclusion:
The level of agreement appears to be generally poor, though the IDF criteria showed a fair level of agreement with the WHO criteria: Therefore the IDF criteria is recommended for screening of the MS in persons with T2DM because of its ease of application and its level of agreement with the WHO criteria being the best compared to the other three criteria.
Keywords: Criteria, metabolic syndrome, performance, sensitivity, specificity
INTRODUCTION
The metabolic syndrome (MS) is a cluster of cardiovascular risk factors such as diabetes mellitus (DM) and pre-diabetes, central obesity, high cholesterol, and high blood pressure.[1,2,3] It is estimated that about a quarter of the world's population has the syndrome and people with this condition are likely to have a heart attack or stroke compared with people without it.[2] The risk of developing DM is five-fold greater in people with this syndrome.[1] Clustered metabolic cardiovascular risk factors, including type 2 diabetes mellitus (T2DM), essential hypertension, obesity, dyslipidemia, and ischemic heart disease, known as the metabolic syndrome (MS), have been well-described.[4] Its prevalence rates range from 13 to 30% and 70-80% among the Caucasian nondiabetic[5,6] and diabetic[7,8] populations, respectively. The age-adjusted prevalence of the MS in an Indian urban population was 24.9%.[9] Genetically determined insulin resistance in a setting of suitable environmental factors is the pivotal pathogenic mechanism underlying the MS.[4] Lipoprotein lipase deficiency largely accounts for the lipid abnormalities,[10] while systemic hypertension is due to enhanced sympathetic activities, salt sensitivity, and increased transmembrane cation transport.[11,12] The role of tumor necrosis factor in obesity and insulin resistance has also been described.[13] Developing nations are witnessing rapid industrialization, urbanization, and increased economic prosperity. The resulting acquisition of the western lifestyle, characterized by calorie excess and physical inactivity, would provide suitable milieu for the development of the MS in genetically predisposed individuals.
The prevalence of the MS is known to vary across the world. Age and ethnicity of the populations studied are also key issues influencing the prevalence.[14] In general, the prevalence increases with age. Many studies have compared available criteria of the MS in different populations or groups. Gemalmaz et al.,[15] in a population based study compared the International Diabetes Federation (IDF) and Adult Treatment Panel (ATP) III criteria in a rural Turkish population and found a prevalence of 41.4 and 38.1%, respectively. The rate of agreement between both definitions was 91.1 ± 0.04%. Strazzullo et al.,[16] compared seven sets of criteria in an unselected sample of adult males and reported a prevalence of 8.6% for American Association of Clinical Endocrinologists (AACE) criteria, 16.4% for European Group for the Study of Insulin Resistance (EGIR) criteria, 21.1% for World Health Organization (WHO) criteria, 28.9% for ATP III (2001), and 44.5% for the IDF criteria. Eregie and Edo in their study of MS among T2DM persons in Benin City reported prevalence rates of 33.4% with the WHO criteria, 22.6% with the ATP III criteria, and 30.9% with the IDF criteria.[17]
The existence of multiple criteria for the diagnosis of the MS has resulted in many research works using different diagnostic criteria and this makes it difficult to compare such studies. The performance of some of these criteria is not well-established in T2DM individuals in our environment. Due to the multiplicity of diagnostic criteria with different cutoff points for similar components, the resultant difficulties in making appropriate comparison of studies on the MS, studies, which compare the performance of the various diagnostic tools, with a view to recommending the most appropriate for use in our environment, are urgently required.
It will be worthwhile to determine the magnitude of this syndrome in our locale using different diagnostic criteria, checking the specificity, the sensitivity, and level of agreement/correlation of these criteria. This study also attempted to identify the best criteria for use among T2DM persons in Nigeria. The multiplicity of guidelines for the diagnosis of the MS and the resultant difficulties in making appropriate comparison of studies on the MS has resulted in the need compare these guidelines. So far, there is no published local study on the sensitivity and specificity of any of these guidelines in Nigerians with T2DM.
Aims and objectives
The scope of this study is to evaluate five diagnostic criteria of the MS with respect to their sensitivity and specificity in patients with T2DM.
To determine the sensitivity, specificity, and level of agreement of the WHO criteria with that of the IDF, National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III), revised NCEP (NCEP-R), and the AACE diagnostic criteria with a view of suggesting the most suitable diagnostic criteria of the MS in persons with T2DM.
MATERIALS AND METHODS
This is a cross-sectional, case-control study carried out in the Diabetes Clinic of the University of Benin Teaching Hospital (UBTH), a 500-bed Federal Government tertiary hospital in Benin City, Edo State in the south-south geopolitical region of Nigeria. The UBTH receives referral cases from Edo State and neighboring States like Delta, Ondo, Ekiti, and Kogi States and the Federal Capital Territory, Abuja. A total of 124 subjects were recruited from the Diabetes Clinic of the UBTH using the inclusion criteria included people diagnosed as having T2DM presenting to UBTH within the last 24 months using the 1999 WHO criteria,[18] people aged 30 years and above, on treatment with oral hypoglycemic drugs plus or minus nonpharmacological therapy and not requiring insulin for survival and subjects who consented to participate in the study. The exclusion criteria included subjects diagnosed of having other types of DM, those with T2DM with age < 30 years and subjects who declined being a part of the study. For nondiabetic first degree relatives (FDRs) of persons with T2DM, 96 subjects were recruited from among the outpatient department of the UBTH (general practice clinic and consultant outpatient clinic) and staff of UBTH with the inclusion criteria been; subject must be a FDR of a diagnosed T2DM person, should be aged 30 years and above, and should not be diagnosed of having DM and subjects who consented to the study. The exclusion criteria included FDR diagnosed with DM and those that declined being a part of the study. For the control subjects: 96 control subjects were recruited from among the staff of UBTH and healthy relatives of nondiabetic patients with the inclusion criteria been nondiabetic age- and sex-matched adult with normal fasting blood sugar (FBS) less than 110 mg/dL, while the exclusion criteria included: Nondiabetics less than 30 years of age, FDR of type 2 diabetic and nondiabetics who declined being a part of the study.
Ethical approval was sought from the Ethics and Research Committee of the UBTH before the commencement of the study.
A convenience sampling technique was used to recruit 106 persons with T2DM, 106 people who are FDRs of type 2 diabetic persons and 106 controls and questionnaires were administered. The following were assessed: Anthropometric indices, blood pressure, serum lipid profile, FBS, proteinuria, and microalbuminuria.[19,20] The following terms were used in this study.
Hypertension defined as BP ≥ 140/90 mmHg
T2DM will be diagnosed in any person with DM aged 30 years and above on oral hypoglycemic drugs for control of blood sugar. FBS - 6.1-6.9 mmol/L (110-125 mg/dL) WHO NCEP-ATP III and ACCE. FBS - 5.6-6.9 mmol/L (100-125 mg/dL) NCEP-R and IDF
Obesity: a) General obesity, b) central obesity (defined by the WHO, NCEP-ATP III, IDF definition)
Dyslipidemia: Defined as presence of one or more of: (i) Hypertriglyceridemia - fasting serum triglyceride level ≥ 1.7 mmol/L (150 mg/dL) as defined by WHO, NCEP ATP III, NCEP-R, IDF, and AACE definitions. (ii) Reduced high density lipoprotein (HDL)-cholesterol: Serum HDL-cholesterol ≤ 40 mg/dL (males) or ≤50 mg/dL (females) by NCEP ATP III, NCEP-R, and IDF definitions. iii) Serum HDL-cholesterol ≤ 35 mg/dL (males) or ≤39 mg/dL (females) by WHO definition
The AACE diagnostic criteria will be met when there is overweight, dyslipidemia, impaired fasting glycemia, and hypertension all as defined by the AACE criteria plus anyone of family history of type 2 diabetes, hypertension, or CVD and sedentary lifestyle.
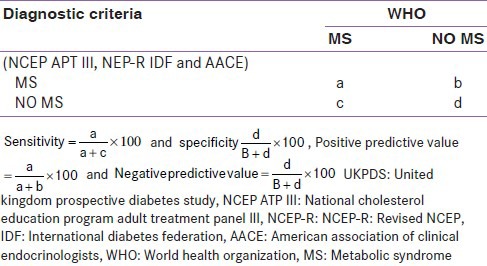
Sensitivity (%): The proportion of people with the target disorder who have a positive test. For a test to be useful in ruling out a disease, it must have a high sensitivity
Specificity (%): The proportion of people without the target disorder who have a negative test. For a test to be useful at confirming a disease, it must have a high specificity
Positive predictive value (PPV, %): This is the probability of disease among patients with a positive test
Negative predictive value (NPV, %): This is the probability of no disease among patients with a negative test.
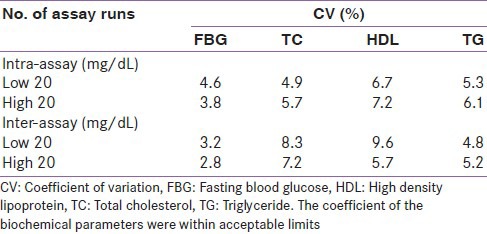
The data obtained were analyzed using the statistical software-Statistical Package for Social Sciences (SPSS) version 16. Statistical comparisons were made with Student's t-test for quantitative variables and the Chi-square test was used for comparison of proportions. A P value of less than 0.05 was taken as statistically significant. Kappa statistic was used to test the degree of agreement between the diagnostic criteria. The coefficient of variation for the biochemical assays was done in the study as shown in Table 1.
Table 1.
Coefficient of variation for the biochemical assays done in the study
RESULTS
Irrespective of the diagnostic criteria in use, more T2DM persons had the MS than persons in the control or FDR groups. Similarly, a higher number of persons in the FDR group had the MS than persons in the control or FDR group. A group by group comparison of proportions of person with MS showed a statistically significant higher proportion of persons with MS in the T2DM group (87.1%) than in the control group (13.5%) (χ2 = 1.183, degrees of freedom (df) = 1, P = 0.000) [Table 2 and Figure 1]. Using the WHO criteria as a reference standard, the performance of the other four criteria was analyzed in relation to their sensitivity, specificity, PPV and NPV. The IDF criteria had the best sensitivity (71.2%) followed closely by the NCEP-R criteria and then the IDF criteria (67.6%), however, the AACE criteria had the worse sensitivity. The specificity of the AACE criteria was 100%, while that of IDF, NCEP ATP III, and NCEP-R was 81.3% each. The PPV of the AACE criteria was superior (100%) to the others which had a PPV of over 95% each. The IDF criteria had the best NPV of the four (IDF, NCEP ATP III, NCEP-R, and AACE) criteria [Table 3]. The criteria all had a fair level of agreement with the WHO criteria except for the AACE criteria that had a slight level of agreement with the WHO criteria. See Tables 5 and 6 for the details on how the figures in Table 3 were derived. Furthermore, Figure 1 shows that irrespective of the diagnostic criteria in use, persons with T2DM have a higher prevalence of MS than both the control and the FDR groups.
Table 2.
Comparison of the prevalence of the metabolic syndrome using five
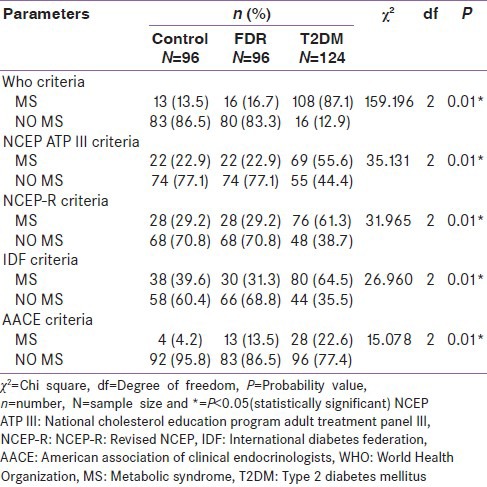
Figure 1.
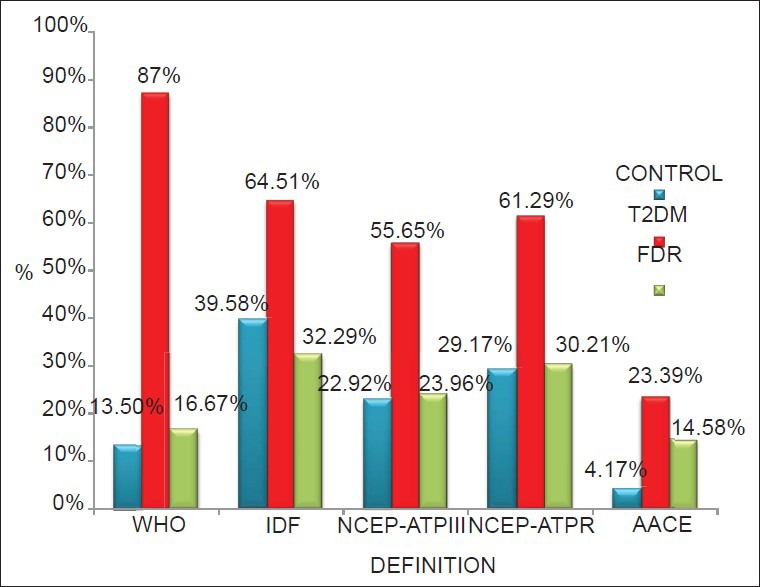
Prevalence of Metabolic syndrome in three different study groups using five guidelines
Table 3.
Comparison of the perormance and level of agreement of the other four criteria
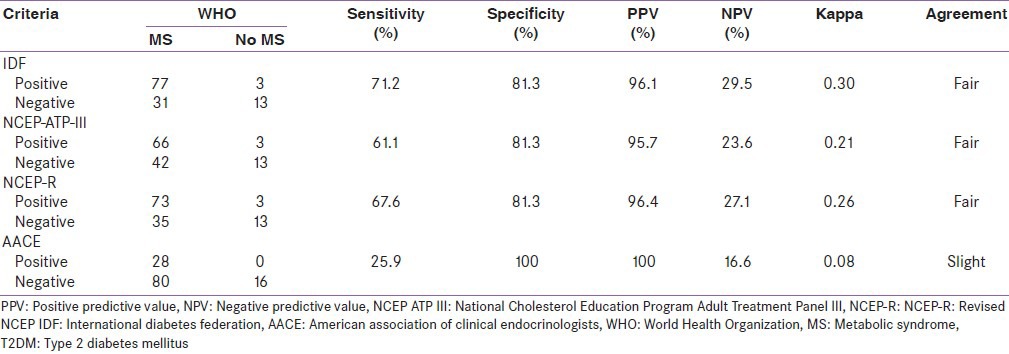
Table 5.
The key to kappa statistic
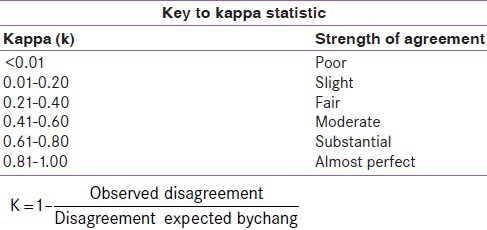
Table 6.
2×2 table comparing the diagnostic criteria and the measured UKPDS risk
DISCUSSION
Of 124 persons with T2DM who were included in this study, the prevalence of MS as defined by the WHO criteria was 87.1% [Table 4]. The sex prevalence of the MS in T2DM males was 88%, while that of females was 86.5%. Ogbera[21] reported a prevalence of MS of 86% with a sex prevalence of 86% in females and 83% in males[21] similar to the findings in this study and based on the new streamlined criteria (NCEP-R). The study by Alebiosu and Odusan[22] (which was based on the WHO criteria) reported a prevalence of 25.2% of T2DM patients. This study was, however, concluded in August, 2001. A previous study by Eregie and Edo[17] in 2003 in this locale reported a prevalence of 33.4% in persons with T2DM using the WHO criteria. The work by Adediran and Ohwovoriole[23] in 2003 reported a prevalence of 51.5% in Lagos also using modified WHO criteria in persons with T2DM. It is important to note that Adediran and Ohwovoriole[23] did not include dyslipidemia and microalbuminuria among the diagnostic criteria, and thus probably underestimated the prevalence of the MS in persons living with T2DM. The prevalence of the MS among African-Americans with T2DM was reported by Chaiken et al.,[24] to be as high as 70%.
Table 4.
Comparison of five diagnostic guidelines of the metabolic syndrome
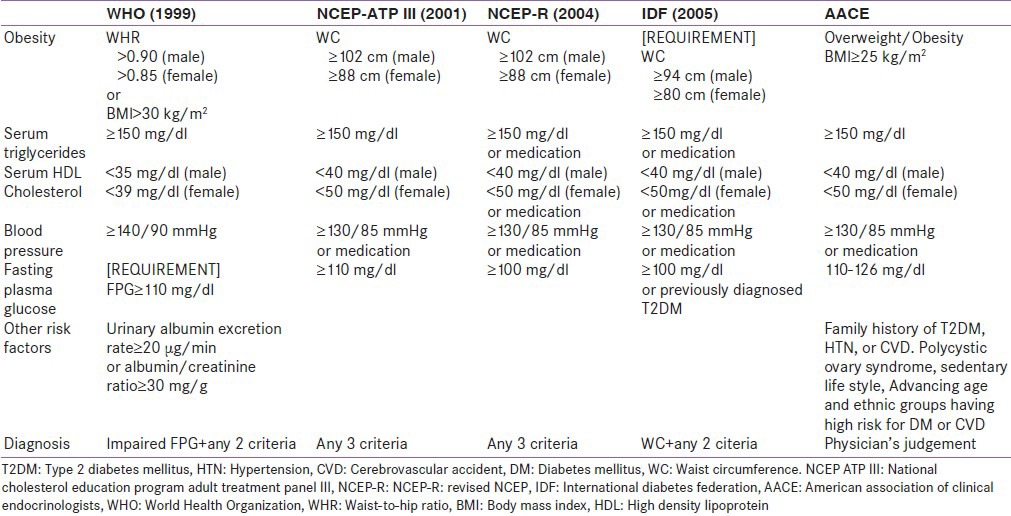
The prevalence of the MS is known to vary depending on the population being studied and the diagnostic criteria in use. In this study, WHO criteria gave a prevalence of 13.3% in the control group, 16.7% in FDR group, and 87.1% in the T2DM group. The NCEP ATP III criteria gave a prevalence of 22.9% in the control group, 22.9% in the FDR group, and 55.6% in patients with T2DM. The NCEP-R criteria gave a prevalence of 29.2% in the control group, 29.2% in the FDR group and 61.3% in the T2DM group. The IDF criteria gave a prevalence of 39.6% in the control group, 31.3% in the FDR group, and 64.5% in the T2DM group. An observed trend was that the prevalence of MS in the control and FDR groups increased with the NCEP ATP III, NCEP-R, and IDF criteria; while that of the T2DM group declined. The AACE criteria gave the least prevalence for each of the groups; 4.2% in the control group, 13.5% in the FDR group, and 22.6% in the T2DM group.
In the study by Eregie and Edo[17] which compared three criteria in a T2DM population, the WHO prevalence was 33.4%, NCEP ATP III criteria gave a prevalence of 22.6% and the IDF criteria gave a prevalence of 30.9%. A similar trend of decline was observed in this study starting from the WHO criteria to others. This tends to suggest the other criteria may underestimate the prevalence of persons with the MS or that the WHO criteria overestimates the prevalence of persons with the MS. Alshkri and Elmehdawi[25] in Libya compared the NCEP ATP III criteria and the IDF criteria in persons with T2DM and reported a prevalence of 92 and 80.8%, respectively, the IDF prevalence being higher than the NCEP ATP III just like in this study and the study by Eregie and Edo.[17]
Performance of the diagnostic criteria: Comparing the other four criteria to the WHO criteria; the sensitivity, specificity, PPV, and NPV for each of the four criteria were determined. The NCEP ATP III criteria had a sensitivity of 61.1%, specificity of 81.3%, PPV of 95.7%, and a NPV of 23.6%. The NCEP-R criteria had a sensitivity of 67.6%, specificity of 81.3%, PPV of 96.5%, and a NPV of 27.1%. The IDF criteria had a sensitivity of 71.3%, specificity of 81.3%, PPV of 96.3%, and a NPV of 29.5%. The ACCE criteria had a sensitivity of 25.9%, specificity of 100%, PPV of 100%, and a NPV of 16.6%. From these results, the AACE criteria had the best specificity (100%). The IDF criteria had the best sensitivity (71.3%) followed closely by the NCEP-R criteria. However, the AACE criteria had a poor sensitivity (25.9%) compared to the others. These have made the IDF criteria superior to the others in addition to its PPV of 96.3%. The IDF criteria therefore appears superior to the others.
The level of agreement of the WHO criteria with the NCEP ATP III, NCEP-R, and IDF criteria were found to be fair (kappa = 0.21-0.40), while that of the AACE criteria was slight (kappa = 0.083). Qiao et al.,[26] reported a poor level of agreement of the WHO, EGIR, and NCEP ATP III criteria in nondiabetic Europeans. From the parameters above, the IDF criteria may be a good alternative to the WHO criteria in the screening of T2DM persons for the MS.
CONCLUSION
Using the WHO criteria as a gold standard for the diagnosis of the MS, this study has demonstrated a good performance of the IDF criteria compared to the other three criteria. The IDF criteria are recommended for screening of the MS in persons with T2DM because of its ease of application and its level of agreement with the WHO criteria being the best compared to the other three criteria. Furthermore, the lower cutoff for hypertension will help persons with T2DM achieve and maintain their blood pressure target. Compared to the WHO criteria, the HDL cutoff of the IDF criteria (<40 mg/dL for males and <50 mg/dL for females) is higher. Knowing that the HDL cholesterol is the ‘cardiac friendly’ cholesterol, using the WHO cutoff (<35 mg/dL for males and <39 mg/dL for females) will further encourage the risk of coronary events in the T2DM persons who themselves are already at some risk by virtue of the DM they have.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Alberti KG, Zimmet P, Shaw J IDF Epidemiology Task Force Consensus Group. The Metabolic Syndrome-a new worldwide definition. Lancet. 2005;366:1059–62. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 2.The IDF consensus worldwide definition of the metabolic syndrome. [Last accessed on the 2008 Nov 6]. Available from: http://www.idf.org/webdata/docs/idf_meta_def_final.pdf .
- 3.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:460–9. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 4.Reaven GM. Role of insulin resistance in human disease. Diabetes. 1989;37:1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 5.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among U.S. adults: Finding from the 3rd national health nutrition examination survey. JAMA. 2002;287:356–9. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 6.Movakovic B, Popovic M. Occurrence of the metabolic syndrome in the population of the town of Novi Sed. Med Pregl. 2001;54:17–20. [PubMed] [Google Scholar]
- 7.Abdu-Rahim HF, Husseini A, Bjerfness F, Giacaman R, Gordon NH, Jervell J. The metabolic syndrome in the West Bank population: An urban-rural comparison. Diabetes Care. 2001;24:275–9. doi: 10.2337/diacare.24.2.275. [DOI] [PubMed] [Google Scholar]
- 8.Balkau B, Charles MA, Drivsholm T, Borch-Johnsen K, Wareham N, Yudkin JS, et al. European Group For The Study Of Insulin Resistance (EGIR). Frequency of WHO-defined metabolic syndrome in European cohort and an alternative definition of an insulin resistance syndrome. Diabetes Metab. 2002;28:364–76. [PubMed] [Google Scholar]
- 9.Gupta R, Deedwania PC, Gupta A, Rastogi S, Panwar RB, Kothari K. Prevalence of metabolic syndrome in an Indian urban population. Int J Cardiol. 2004;97:257–61. doi: 10.1016/j.ijcard.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Verges BL. Dyslipidemia in diabetes mellitus. Review of the main lipoprotein abnormalities and their consequences on the development of atherogenesis. Diabetes Metab. 1999;25:32–40. [PubMed] [Google Scholar]
- 11.Rosolova H. The sympathetic nervous system and insulin resistance. Vintr Kek. 2003;49:61–5. [PubMed] [Google Scholar]
- 12.Ginner V, Coca A, de la Sierra A. Increased insulin resistance in salt-sensitive hypertension. J Hum Hypertens. 2001;15:481–5. doi: 10.1038/sj.jhh.1001216. [DOI] [PubMed] [Google Scholar]
- 13.Hotamisligil GS. The role of TNF-alpha and TNF receptors in obesity and insulin resistance. J Intem Med. 1999;245:621–5. doi: 10.1046/j.1365-2796.1999.00490.x. [DOI] [PubMed] [Google Scholar]
- 14.Eckel RH. The metabolic syndrome. In: Fauci AS, Braunwald E, Kasper DL, et al., editors. Harrison's Principles of Internal Medicine. 17th ed. Vol. 236. United States of America: The McGraw-Hill; 2008. pp. 1509–13. [Google Scholar]
- 15.Gemalmaz A, Aydin S, Basak O. Prevalence of the Metabolic Syndrome in a Rural Turkish Population: Comparison and Concordance of Two Diagnostic Criteria. Turk J Med Sci. 2008;38:159–65. [Google Scholar]
- 16.Strazzullo P, Barbato A, Siani A, Cappuccio FP, Versiero M, Schiattarella P, et al. Diagnostic criteria for metabolic syndrome: A comparative analysis in an unselected adult male population. Metab Clin Exp. 2008;57:355–61. doi: 10.1016/j.metabol.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Eregie A, Edo A. Diagnosing the metabolic syndrome: A comparative evaluation of three Diagnostic tools. Poster presentation. 19th World Diabetes Congress, International Diabetes Federation. December 3rd-7th 2006 Cape Town, South Africa. Diabetic Med. 2006;23 [Google Scholar]
- 18.World Health Organization Consultation Group. Diagnosis and classification of diabetes mellitus WHO/NCD/NCS/99. 2nd ed. Part 1. Geneva: World Health Organization; 1999. Definition, diagnosis and classification of diabetes mellitus and its complications; pp. 1–59. [Google Scholar]
- 19.Physical Status: The use and interpretation of Anthropometry. Geneva: WHO; 1995. WHO Technical Report Series-854. [PubMed] [Google Scholar]
- 20.Trinder P. Determination of glucose in blood using the glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem. 1969;6:2. [Google Scholar]
- 21.Ogbera AO. Prevalence and gender distribution of the metabolic syndrome. Diabetol Metab Synd. 2010;2:1. doi: 10.1186/1758-5996-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alebiosu OC, Odusan BO. Metabolic syndrome in subjects with type 2 diabetes mellitus. J Natl Med Assoc. 2004;96:817–21. [PMC free article] [PubMed] [Google Scholar]
- 23.Adediran OS, Ohwovoriole AE. Paris, France: 2003. Aug 24-29, Prevalence of the metabolic syndrome among Nigerians with type 2 diabetes mellitus. Poster presentation, 18th International Diabetes Federation Congress on Diabetes Metabolism; pp. 4s30–31. [Google Scholar]
- 24.Chaiken RL, Banerji MA, Huey H, Levbovit HE. Do Blacks with NIDDM have an insulin-resistance syndrome? Diabetes. 1993;42:444–9. doi: 10.2337/diab.42.3.444. [DOI] [PubMed] [Google Scholar]
- 25.Alshkri M, Elmehdawi R. Metabolic syndrome among type 2 diabetes patients in Benghazi, Libya: A pilot study. [Last accessed on 2008 Nov 06]. Available from: www.ljm.org.ly . [DOI] [PMC free article] [PubMed]
- 26.Qiao Q, Laatikainen T, Zethelius B, Stegmayr B, et al. Comparison of different definitions of the metabolic syndrome in relation to the risk of developing stroke and coronary heart disease in Finnish and Swedish cohorts. [Last accessed on 2010 Jul 23]. Available from: htt://stroke.ahajournals.org/cgi/content/full/40/2/337 . [DOI] [PubMed]


