Abstract
Objective:
Recent studies have shown that early pregnancy hypothyroxinemia (lower free thyroxin [FT4] and normal thyroid stimulating hormone [TSH] concentration) has deleterious effects on neuro-intellectual development of children. This study was designed to know its incidence in local pregnant women.
Materials and Methods:
Urinary iodine (UI) and serum thyroid related hormone (FT4, free triiodothyronine [FT3], and TSH) were determined in 254 pregnant women during the first trimester. UI and thyroid related hormones were determined by colorimetric (Sandell-Kolthoff) and radioimmunoassay method respectively.
Results:
Most of the pregnant women (n = 202; 79.5%) were iodine deficient (ID; UI <100 μg/L) and only 52 (20.5%) women were taking sufficient iodine (IS; UI ≥ 100 μg/L). Mean levels of FT4, FT3, and TSH were 13.0 ± 2.8 pmol/L, 3.8 ± 1.1 pmol/L and 1.2 ± 1.1 mIU/L, respectively. Maternal FT4 levels were significantly correlated with UI (r = 0.36; P < 0.001). Mean FT4 level in IS women was significantly (P < 0.05) higher than ID women. However, mean FT3 and TSH levels were not significantly different in both groups. FT4 reference range in IS pregnant women was 10.2-19.4 pmol/L. Hypothyroxinemia (FT4 <10.2 pmol/L and TSH <2.5 mIU/L) was diagnosed in 30 (11.8%) pregnant women. Its incidence was almost entirely confined to ID pregnant women with an odd ratio of 8.5 (95% confidence interval: 1.1-64.3).
Conclusion:
About 12% pregnant women residing in urban areas of Pakistan are hypothyroxinemic because of low iodine intake.
Keywords: Iodine, pregnancy, thyroid hormone, thyroid stimulating hormone
INTRODUCTION
Maternal thyroid hormones (TH; T4: 3,5,3’,5’- tetraiodothyronine/thyroxine; T3: 3,5,3’-triiodothyronine) production is increased up to 50% during pregnancy.[1,2] It is because of the marked increase in circulating levels of thyroxin binding globulin (TBG), the direct stimulation of the thyroid gland by elevated levels of human chorionic gonadotropin and increased enzymatic activity of Type III monodeiodinase.[2] These changes are physiological adaptations aimed at increased synthesis of TH particularly during the first half of pregnancy. A number of maternal factors particularly restricted availability of iodine and thyroid autoimmunity hinder the accomplishment of this task.[1,2,3] The consequent maternal TH deficiency is manifested as hypothyroxinemia or transient hypothyroidism during pregnancy.[2,3]
The term hypothyroxinemia refers to low production of serum free thyroxin (FT4) without concordant increase in thyroid stimulating hormone (TSH).[2,3,4] Women living in areas of iodine deficiency may develop hypothyroxinemia during the very 1st weeks of pregnancy.[2,3] Nevertheless, this condition is still not accepted as a separate thyroid disease entity and there is controversy about absolute value of serum T4 level that will define hypothyroxinemia in pregnant women.[4,5] Non-pregnant reference range of FT4 is not useful for this purpose and FT4 normal range calculated specifically in pregnant women taking sufficient iodine[6] or 10th percentile level of FT4 is employed to identify hypothyroxinemia in pregnant women.[5,7]
The clinical importance of early pregnancy maternal hypothyroxinemia in neuro-intellectual development of the fetus has recently been recognized.[2,5,6] This is because of the fact that THs play an important role during pregnancy in the development of the brain and neurological network of the fetus by regulating the expression of specific genes.[3,8] Before the onset of fetal thyroid gland activity around mid-gestation, the embryonic brain can only obtain TH from maternal sources that crosses the placenta and reaches the fetus.[2,3,8] In early pregnancy hypothyroxinemia, decrease in the availability of maternal FT4 may result in adverse effects on the timely sequence of developmental events in the human fetus. This early damage in normal brain formation may affect subsequent intellectual and neuropsychological development of the fetus and is a major reason of decreased mental development of non-cretin children residing in iodine deficient areas.[2,3,9]
Pakistan is considered one of the iodine deficient countries in the region.[10] Deficiency of iodine in pregnant women and its effect on the outcome of pregnancy is one of the prevailing problems of Pakistan, which is so far neglected. This paper is a part of a large cohort study[11,12] conducted to know the incidence of maternal iodine deficiency and thyroid autoimmunity and their effects on maternal and neonatal thyroid function in local pregnant women. We have already described the existence of the mild to moderate iodine deficiency in these pregnant women.[11] In this study, we have determined relation of iodine deficiency and early pregnancy hypothyroxinemia. Moreover, some potential deleterious aspects of neuro-intellectual development of children associated with gestational hypothyroxinemia are also highlighted.
MATERIALS AND METHODS
Pregnant women during early pregnancy were recruited consecutively at the outpatient Department of Obstetrics and Gynecology Government Mian Munshi Hospital, Lahore. Details of the recruitment procedure and exclusion criteria are described elsewhere.[11,12] The Institutional Review Board at University of the Punjab, Lahore approved the study. Both urine and blood samples were collected from each selected woman for determination of urinary iodine (UI) and serum thyroid related hormones (FT4, free triiodothyronine [FT3], and TSH) respectively. UI concentration was determined at the Institute of Chemistry, University of the Punjab Lahore by Sandell-Kolthoff reaction (modified) as recommended by World Health Organization (WHO).[13] The detail of urine sample collection, UI determination and iodine intake status of these women is provided in a previous study.[12] Each blood sample was collected in a disposable syringe and allowed to coagulate at room temperature. Collected blood samples were immediately transferred to the laboratory and sera were separated by centrifugation (2000g) for 5 min at room temperature with a centrifuge (Model NF 1215, Nuve, Turkey). Determination of free THs (FT4 and FT3) was carried out by using radioimmunoassay (RIA) and TSH was estimated by immunoradiometric assay (IRMA) at RIA laboratories, Centre for Nuclear Medicine, Mayo Hospital Lahore by using commercial kits of Immunotech Inc. (Beckman Coulter, Czech Republic and France). Both FT4 and FT3 were determined by competitive RIA, while concentration of TSH was determined by a “sandwich type” IRMA utilizing antibody labeled with 125I. Measurement of radioactivity, fitting of the standard curve and analysis of samples was carried out using a computerized gamma counter (Cap-RIA 16, CAPINTEC; Inc. PA, USA). All assays were carried out in duplicate. Assay reliability was determined by the use of commercially derived control sera of low and high concentrations, which were included in every run. Interassay and intraassay precisions as indicated by percentage of coefficients of variation (%CV) were: FT4 (3.8% and 4.9%), FT3 (3.3% and 7.8%) and TSH (4.4% and 5.9%). The term hypothyroxinemia indicated pregnant women with low serum FT4 and normal TSH concentrations.[5,7] Serum TSH levels below 2.5 mIU/L were considered normal in accordance to recent recommendations.[14] Low serum FT4 level was determined in comparison to FT4 normal range (mean ± 2 standard deviation [SD]) calculated specifically in those pregnant women taking sufficient iodine (UI > 100 μg/L).[6]
Results are presented as means (±SD), median or otherwise specified. Data were analyzed using Statistical Package for Social Sciences (SPSS) version 17.0 (SPSS Inc. 2010, Mapinfo Corp. New York, USA). Group mean of thyroid related hormones were compared by Student's t-test. Chi-square test was used to compare frequencies among different groups. Correlations were carried out using Pearson's correlation. All statistical tests were considered statistically significant whenever P < 0.05.
RESULTS
The study sample consisted of 254 pregnant women. Their ages ranged from 17 to 40 years (mean ± SD, 24.2 ± 4.9). Mean gestational age of pregnant sample was 9.9 ± 2.0 weeks (range: 5-14 weeks) with more than 80% of the pregnancies enrolled before 12th week of gestation. They were comprised of 84 (33.1%) primigravida and 170 multigravida women who had 1-9 pregnancies (average, 4.2) and 0-7 alive children (average, 2.4). The average parity of the study sample was 3.5 ± 2.3.
The distribution of UI and iodine intake status of pregnant women according to WHO criteria is shown in Table 1. Only 52 (20.5%) women were taking sufficient iodine (UI > 100 μg/L) while rest of the women (n = 202; 79.5%) had UI excretion below 100 μg/L and hence were iodine deficient.[12] Mean levels of FT4 was 13.0 ± 2.8 pmol/L (range: 7.3-25.8 pmol/L), that of FT3 was 3.8 ± 1.1 pmol/L (range: 2.1-8.8 pmol/L) and TSH was 1.2 ± 1.1 mIU/L (range: 0.02-8.4 mIU/L). Pearson's correlation coefficient (r) between maternal UI and thyroid related hormone showed that only FT4 was positively correlated with UI at significant level (r = 0.361; P < 0.001), while positive correlation of FT3 (r = 0.112) and TSH (r = 0.001) were not only weak, but also non-significant (P = 0.075 and 0.983, respectively). However, both FT4 and FT3 levels were negatively correlated to TSH at significant level (r = −0.206, P = 0.001 and r = −0.328, P < 0.0001, respectively).
Table 1.
Iodine intake status of pregnant women based on UI excretion
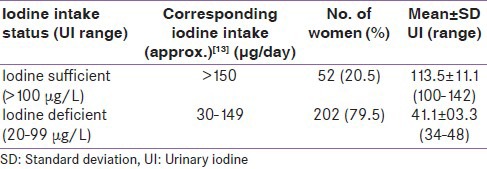
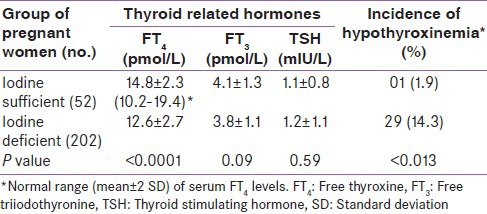
Table 2 shows the mean values of thyroid related hormones in iodine sufficient and iodine deficient pregnant women. Mean FT4 concentration in iodine sufficient women was significantly (P < 0.0001) higher than iodine deficient women. However, mean FT3 and TSH levels were not significantly different in both groups of pregnant women (P = 0.06 and 0.95 respectively). As indicated above, hypothyroxinemic pregnant women have normal TSH and lower FT4 concentration as compared to FT4 reference range computed from data of pregnant women taking sufficient iodine (UI >100 μg/L) at comparable gestational age. In this study 52 pregnant women were taking sufficient iodine. The serum FT4 reference range in these pregnant women was 10.2-19.4 pmol/L [Table 2]. Our data revealed that overall 34 pregnant women had FT4 concentration below the lower limit of this range. Among them 30 women (11.8% of total sample) had TSH levels within normal range and thus were hypothyroxinemic. The FT4 concentration in hypothyroxinemic women ranged from 7.6 to 10.1 pmol/L. Comparison of inter-group incidence of hypothyroxinemia [Table 2] showed that its incidence was significantly higher in iodine deficient than iodine sufficient group of pregnant women (14.3% vs. 1.9%; χ2 = 26.1, P < 0.0001). The odd ratio for hypothyroxinemia was 8.5 (95% confidence interval: 1.1-64.3) in iodine deficient pregnant women.
Table 2.
Mean levels of thyroid function tests and incidence of hypothyroxinemia in pregnant women taking sufficient and deficient dietary iodine
DISCUSSION
In this study, incidence of early pregnancy hypothyroxinemia was determined in local pregnant women. In iodine deficient area like ours the main cause of maternal hypothyroxinemia is low iodine intake, which is best reflected by low UI excretion.[13] According to our UI data most of the pregnant women included in this study had iodine intake below the recommended value.[2,13] This is in accordance to the finding that dietary content of iodine in typical Pakistani diet is 40 μg/day, which is 3.8 times lower than recommended for adult subject (150 μg/day).[15] Iodine intake at such a level during pregnancy often consequent upon reduced synthesis of maternal T4, TBG desaturation by T4, critical decrease in FT4 levels and consequently hypothyroxinemia.[2,3] In regions where iodine intake is sufficient, as is the case of USA, incidence of early pregnancy hypothyroxinemia is reported 1.3%,[16] while in iodine deficient areas more than one-third of the pregnant women are affected.[17,18] In the present study, hypothyroxinemia was found in 12% of the pregnant women and was mostly confined to iodine deficient women who as a group had incidence up to 14%. This frequency was comparatively lower as compared to other studies carried out in areas with low iodine intake levels.[6,7] The lower limit of FT4 reference range calculated in our iodine sufficient pregnant women (10.2 pmol/L) was much lower than that of Vermiglio et al. (2004) or 10th percentile level of FT4 reported by Pop et al. (2003) (13.1 pmol/L and 12.1 pmol/L, respectively).[6,7] Applying these thresholds to our data will increase the percentage of hypothyroxinemic women than reported in this study. The results of this and other studies[17,18] support the notion that early pregnancy hypothyroxinemia is more frequent than primary thyroid failure among pregnant women residing iodine deficient areas.[2,9]
Maternal T4 has been detected in the first trimester coelomic fluid as early as 6th week of gestation.[8,19] The main active TH in fetal brain is T3, derived in large part from Type-II 5’- deiodination of maternal T4.[8] Maternal T3 can’t cross the placenta due to high activity of Type-III deiodinase, which rapidly convert it into inactive triiodothyronine (rT3).[2,3,8] Thus maternal T4 available to the embryonic brain is likely to be the only substrate for the generation of the intracellular T3 that occupies cerebral nuclear receptors.[8,19] Early pregnancy maternal hypothyroxinemia is an underlying cause of decreased mental development and irreversible neurological damages described in children born in iodine deficient areas.[17,20] These neurological alterations range over a broad spectrum of neurobehavioral impairments, ranging from subtle IQ deficit to attention deficit and hyperactivity disorder.[6,7,17,21,22] The affected children are difficult to diagnose as they are not clinically (or even subclinically) hypothyroid, although their brain is likely to be selectively T3-deficient.[9]
This is probably the first study to report frequency of early pregnancy hypothyroxinemia in Pakistan. Its potential detrimental effects on neuropsychological development of children[21,23] require urgent determination as it is directly linked to human resources development. A limitation of this study is that we have not excluded pregnant women with autoimmune thyroiditis (TPO-Ab positive) from our data. The reason is that we mainly intend to highlight the role of low iodine intake as the main cause of hypothyroxinemia through this study. Moreover, doing so would have reduced the number of pregnant women taking sufficient iodine that is already fewer than should be.
At present universal screening of pregnant women for thyroid dysfunction is not recommended except aggressive targeted case-finding.[24] Such recommendations are based mainly on research studies conducted in iodine sufficient areas where main cause of maternal hypothyroxinemia is autoimmune thyroiditis. In developing countries like Pakistan pregnant women taking moderately low iodine also constitute the high risk group whose early detection and follow-up can enhance the efficacy of case finding. Recent studies have shown that reduction in incidence of maternal hypothyroxinemia can be achieved only if the iodine deficiency is corrected well before pregnancy.[17] Thus, keeping in view the role of iodine deficiency as a cause of brain damage media and medical community should strengthen the ongoing iodine supplementation program sponsored by Government of Pakistan.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Soldin OP, Tractenberg RE, Hollowell JG, Jonklaas J, Janicic N, Soldin SJ. Trimester-specific changes in maternal thyroid hormone, thyrotropin, and thyroglobulin concentrations during gestation: Trends and associations across trimesters in iodine sufficiency. Thyroid. 2004;14:1084–90. doi: 10.1089/thy.2004.14.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glinoer D. Clinical and biological consequences of iodine deficiency during pregnancy. Endocr Dev. 2007;10:62–85. doi: 10.1159/000106820. [DOI] [PubMed] [Google Scholar]
- 3.de Escobar GM, Obregón MJ, del Rey FE. Iodine deficiency and brain development in the first half of pregnancy. Public Health Nutr. 2007;10:1554–70. doi: 10.1017/S1368980007360928. [DOI] [PubMed] [Google Scholar]
- 4.Moleti M, Trimarchi F, Vermiglio F. Doubts and concerns about isolated maternal hypothyroxinemia. J Thyroid Res 2011. 2011:463029. doi: 10.4061/2011/463029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pop VJ, Vulsma T. Maternal hypothyroxinaemia during (early) gestation. Lancet. 2005;365:1604–6. doi: 10.1016/S0140-6736(05)66489-6. [DOI] [PubMed] [Google Scholar]
- 6.Vermiglio F, Lo Presti VP, Moleti M, Sidoti M, Tortorella G, Scaffidi G, et al. Attention deficit and hyperactivity disorders in the offspring of mothers exposed to mild-moderate iodine deficiency: A possible novel iodine deficiency disorder in developed countries. J Clin Endocrinol Metab. 2004;89:6054–60. doi: 10.1210/jc.2004-0571. [DOI] [PubMed] [Google Scholar]
- 7.Pop VJ, Brouwers EP, Vader HL, Vulsma T, van Baar AL, de Vijlder JJ. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: A 3-year follow-up study. Clin Endocrinol (Oxf) 2003;59:282–8. doi: 10.1046/j.1365-2265.2003.01822.x. [DOI] [PubMed] [Google Scholar]
- 8.Bernal J, Guadaño-Ferraz A, Morte B. Perspectives in the study of thyroid hormone action on brain development and function. Thyroid. 2003;13:1005–12. doi: 10.1089/105072503770867174. [DOI] [PubMed] [Google Scholar]
- 9.de Escobar M, del Rey FE, Obregon MJ. Maternal hypothyroxinemia and neurodevelopment: To screen or not to screen; to treat or not to treat. Hot Thyroidology. 2002. http://www.hotthyroidology.com; Online ISSN: 2075-2202 . No. 2.
- 10.Azizi F, Mehran L. Experiences in the prevention, control and elimination of iodine deficiency disorders: A regional perspective. East Mediterr Health J. 2004;10:761–70. [PubMed] [Google Scholar]
- 11.Elahi S, Hussain Z. A Longitudinal study of changes in thyroid related hormones among pregnant women residing in an iodine deficient urban area. ISRN Endocrinol 2013. 2013:234031. doi: 10.1155/2013/234031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elahi S, Rizvi NB, Nagra SA. Iodine deficiency in pregnant women of Lahore. J Pak Med Assoc. 2009;59:741–3. [PubMed] [Google Scholar]
- 13.World Health Organization. A Guide for Program Managers. 3rd ed. Geneva: World Health Organization; 2007. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination. [Google Scholar]
- 14.De Groot L, Abalovich M, Alexander EK, Amino N, Barbour L, Cobin RH, et al. Management of thyroid dysfunction during pregnancy and postpartum: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:2543–65. doi: 10.1210/jc.2011-2803. [DOI] [PubMed] [Google Scholar]
- 15.Akhter P, ur-Rehman K, Orfi SD, Ahmad N. Assessment of iodine levels in the Pakistani diet. Nutrition. 2004;20:783–7. doi: 10.1016/j.nut.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 16.Casey BM, Dashe JS, Spong CY, McIntire DD, Leveno KJ, Cunningham GF. Perinatal significance of isolated maternal hypothyroxinemia identified in the first half of pregnancy. Obstet Gynecol. 2007;109:1129–35. doi: 10.1097/01.AOG.0000262054.03531.24. [DOI] [PubMed] [Google Scholar]
- 17.Berbel P, Mestre JL, Santamaría A, Palazón I, Franco A, Graells M, et al. Delayed neurobehavioral development in children born to pregnant women with mild hypothyroxinemia during the first month of gestation: The importance of early iodine supplementation. Thyroid. 2009;19:511–9. doi: 10.1089/thy.2008.0341. [DOI] [PubMed] [Google Scholar]
- 18.Suárez-Rodríguez M, Azcona-San Julián C, Alzina de Aguilar V. Hypothyroxinemia during pregnancy: The effect on neurodevelopment in the child. Int J Dev Neurosci. 2012;30:435–8. doi: 10.1016/j.ijdevneu.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Contempré B, Jauniaux E, Calvo R, Jurkovic D, Campbell S, de Escobar GM. Detection of thyroid hormones in human embryonic cavities during the first trimester of pregnancy. J Clin Endocrinol Metab. 1993;77:1719–22. doi: 10.1210/jcem.77.6.8263162. [DOI] [PubMed] [Google Scholar]
- 20.Vitti P, Aghini-Lombardi F, Chiovato L, Ferretti G, Pinchera A. Neuropsychological assessment in humans living in mild to moderate iodine deficiency. In: de Vijlder J, Morreale de Escobar G, editors. The Thyroid and the Brain. Vol. 1. Stuttgart, Germany: Schattauer Verlag; 2003. [Google Scholar]
- 21.Li Y, Shan Z, Teng W, Yu X, Li Y, Fan C, et al. Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25-30 months. Clin Endocrinol (Oxf) 2010;72:825–9. doi: 10.1111/j.1365-2265.2009.03743.x. [DOI] [PubMed] [Google Scholar]
- 22.Henrichs J, Bongers-Schokking JJ, Schenk JJ, Ghassabian A, Schmidt HG, Visser TJ, et al. Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: The generation R study. J Clin Endocrinol Metab. 2010;95:4227–34. doi: 10.1210/jc.2010-0415. [DOI] [PubMed] [Google Scholar]
- 23.Elahi S. Thyroid Hormones in Normal Pregnant Women of Pakistan. Saarbrucken Germany: LAMMBERT Academic Publishing; 2012. Role of gestational iodine deficiency and thyroid autoimmunity in maternal and neonatal complications; pp. 83–6. [Google Scholar]
- 24.Abalovich M, Amino N, Barbour LA, Cobin RH, De Groot LJ, Glinoer D, et al. Management of thyroid dysfunction during pregnancy and postpartum: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2007;92:S1–47. doi: 10.1210/jc.2007-0141. [DOI] [PubMed] [Google Scholar]


