Abstract
Background:
Vitamin D has important actions on glucose metabolism. These include improved insulin exocytosis, direct stimulation of insulin receptor, improved uptake of glucose by peripheral tissues, improving insulin resistance. It has got various pleiotropic effects like suppression of cell mediated immunity, regulation of cell proliferation, stimulation of neurotropic factors such as nerve growth factor, Glial cell line-derived neurotrophic factor, neurotropin, suppression of RAAS, reduction of albuminuria, immunomodulatory effects, and anti-inflammatory effects. Thus, vitamin D is implicated in many ways in the pathogenesis of retinopathy, neuropathy and nephropathy.
Objectives:
To study the correlation of vitamin D levels with microvascular complications in type 2 diabetes.
Materials and Methods:
Cross-sectional case-control study of 18 patients (18-70 years), who met the American Diabetes Association 2011 criteria for type 2 diabetes, was conducted. Age and sex matched healthy controls were taken. Subjects were evaluated for the presence of microvascular complications by clinical evaluation, urine examination, fundus examination, nerve conduction studies, and various biochemical tests. 25-OH cholecalciferol levels were done for each. Cut off level for vitamin D deficiency was 20 ng/ml.
Results:
Mean vitamin D was lower in type 2 diabetics than healthy subjects (19.046 vs. 27.186 ng/ml). Prevalence of vitamin D deficiency and insufficiency was found to significantly higher in diabetics when compared to healthy subjects (P = 0.0001). Vitamin D deficiency was found to be significantly associated with neuropathy (χ2 = 5.39, df = 1, P = 0.020), retinopathy, (χ2 = 6.6, df = 1, P = 0.010) and nephropathy (χ2 = 10. 52, df = 1, P = 0.001). Lower levels of vitamin D were found to be associated with increasing prevalence of combinations of microvascular complications namely neuropathy with retinopathy (P = 0.036), neuropathy with nephropathy (P = 0.029), retinopathy with nephropathy (P = 0.022) and neuropathy with retinopathy with nephropathy (P = 0.0001).
Keywords: Diabetes, nephropathy, neuropathy, retinopathy, vitamin D
INTRODUCTION
Vitamin D has important actions on glucose metabolism. These include improved insulin exocytosis, direct stimulation of insulin receptor, improved uptake of glucose by peripheral tissues, improving insulin resistance.[1,2,3] It has got various pleiotropic effects such as suppression of cell mediated immunity, regulation of cell proliferation, stimulation of neurotropic factors like nerve growth factor, Glial cell line-derived neurotrophic factor, neurotropin, suppression of RAAS, reduction of albuminuria, immunomodulatory, anti-inflammatory and antiangiogenic effects.[4,5,6,7,8,9,10,11,12] Thus vitamin D is implicated in many ways in the pathogenesis of diabetic retinopathy, neuropathy and nephropathy.
This study was carried out to study vitamin D levels in type 2 diabetics and evaluate the association of vitamin D levels with microvascular complications in type 2 diabetes.
MATERIALS AND METHODS
The present study is an observational single centre case-control study. Ethical committee clearance was duly taken. Cases comprised of persons with type 2 diabetes aged 18-70 years with or without microvascular complications who were not receiving vitamin D or calcium supplementation. The control group comprised of age, sex and socioeconomically matched normal healthy volunteers. Informed consent was taken from all the samples included in the study.
Subjects with type 1 diabetes mellitus, glycosylated hemoglobin (HbA1c) ≥7.5%, vitamin D intake greater than 1000 IU/day, serum calcium <8 or >11 mg/dL, creatinine >1.5 mg/dL, white blood cell <2,000 or >15,000/mm3, urine albumin to creatinine ratio >150 were excluded. Patients having disorders that change the metabolism of vitamin D, significant cardiac, hepatic, renal and oncologic disease, use of medications known to affect serum phosphate levels, calcitonin, calcitriol, growth hormone, anticonvulsants, hormone replacement therapy, steroids, testosterone or vitamin A (>20,000 units/day) were also excluded. Those having sun exposure less than 3 h/week were also excluded. The screening was done in each case to assess the associated microvascular complications, which include complete physical examination, microfilament test, nerve conduction velocity, detailed fundus examination, ultrasonography of the abdomen and other biochemical investigations. fasting plasma glucose, 2 h postprandial blood sugar, HbA1c, serum vitamin D levels (25-OH vitamin D), calcium, phosphorus, urea, creatinine, liver function test and lipid profile, complete blood count, thyroid stimulating hormone, urine routine microscopy, urine microalbumin by creatinine ratio, electrocardiogram and chest X-ray were done in all subjects under study. Vitamin D deficiency was defined as levels <20 ng/ml and insufficiency 20-29 ng/ml in accordance to WHO definition.[13] Diabetic nephropathy was defined by spot urine albumin by creatinine ratio of >30. Since, vitamin D levels are affected in later stages of chronic kidney disease thus diabetics with urine albumin by creatinine ratio >150 were excluded. Vitamin D levels were done from a single laboratory using same lab assay. Chi-square test, Student's t-test and ANOVA test were used to study the statistical significance of data obtained.
RESULTS
A total of 158 cases were studied. 130 age and sex matched healthy volunteers served as controls. The mean age of cases under study was 52.85 ± 8.26 years compared to 51.87 ± 6.43 years of controls. 60.12% were males, whereas 39.88% were females in the case group while in the control group, 61.25% were males, whereas 38.75% were females. The mean duration of diabetes in the cases studied was 5.34 ± 3.09 years. It was 3.84 ± 2.7 years in cases having neuropathy alone and 3.88 ± 2.7 years and 3.88 ± 1.9 years respectively in cases having nephropathy and retinopathy alone. In cases having two microvascular complications the mean duration of diabetes was 4.3 ± 1.34 years (neuropathy with retinopathy), 4.63 ± 2.9 years (neuropathy with nephropathy) and 5.33 ± 3.24 years (retinopathy with nephropathy). Vitamin D deficiency is now a worldwide recognized problem. Most of the natural foods are deficient in vitamin D and fortification is also inadequate.[17] In the cases having all the three of neuropathy, retinopathy and nephropathy the mean age was 8 ± 2.84 years.
The mean vitamin D level was 19.046 ± 6.614 ng/ml in diabetics, while it was 27.186 ± 9.361 ng/ml in the control group. Out of the total 158 cases (59.49%) had vitamin D <20 ng/ml and only 10 (6.33%) had above 30 ng/ml. On the contrary in the control group, 45 had vitamin D level <20 ng/ml and 45 (34.61%) had levels more than 30 ng/ml. There was a significant difference in levels of vitamin D in diabetics and non-diabetics (t = 8.624, df = 286, P = 0.0001). The mean vitamin D was found to be 19.43 ± 6.418 in males and 18.56 ± 5.916 in females in diabetics, and the difference between two genders was found to be insignificant (P = 0.5651). Similarly, no significant difference was found in the vitamin D levels in males and females in the control group as well.
Vitamin D deficiency (<20 ng/ml) was present in 59.49% of the cases and 34.61% of controls. Only 6.33% cases had vitamin D level >30 ng/ml, while 35.39% of controls were found to be have sufficient (>30 ng/ml) levels of vitamin D. Overall vitamin D was found to be inadequate (vitamin D deficiency with insufficiency, or in other words levels <30 ng/ml) in 93.67% of cases and 64.61% of non-diabetics (controls). Prevalence of vitamin D deficiency and insufficiency was found to significantly higher in diabetics as compared to healthy subjects (χ2 = 36.61, df = 1, P = 0.0001). Overall vitamin D deficiency was found to be more prevalent in diabetics (χ2 = 16.7, df = 1, P = 0.0001).
Out of the 158 type 2 diabetics studied 41 (25.95%) had no microvascular complication. Single microvascular complications (retinopathy or neuropathy or nephropathy) was present in 29.11% of cases, while the combination of two was present in 25.32% and all three in 19.62% of cases.
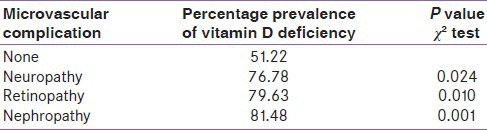
In total, 56 cases had neuropathy (either singly or in combination with neuropathy or retinopathy). Out of these, 43 were vitamin D deficient. Similarly, 43 out of 54 having retinopathy and 44 out 54 having nephropathy were found to be having vitamin D deficiency. The percentage prevalence of vitamin D deficiency in various groups is given in Table 1. Vitamin D deficiency was found to be significantly associated with neuropathy (χ2 = 5.39, df = 1, P = 0.020), retinopathy, (χ2 = 6.6, df = 1, P = 0.010) and nephropathy (χ2 = 10.5, df = 1, P = 0.001).
Table 1.
Prevalence of vitamin D deficiency in patients with microvascular complications
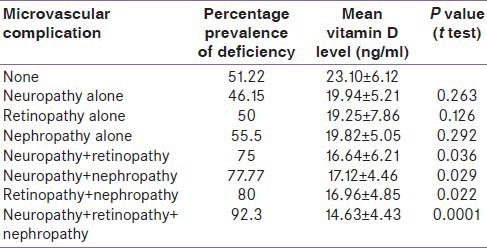
On evaluating various combinations of microvascualar complications, it was found that in cases having only one microvascular complication 46.15%, 52.38%, and 55.5% of those having neuropathy, retinopathy, and nephropathy respectively were found deficient for vitamin D. In cases having more than one microvascular complication, the percentage of vitamin D deficiency further rose to 75% (neuropathy with retinopathy), 77.77% (neuropathy with nephropathy) and 80% (retinopathy with nephropathy). In cases who had all the three of neuropathy, retinopathy and nephropathy vitamin D deficiency was92.3%. Among type 2 diabetics without any microvascular complication 51.22% were deficient in vitamin D.
The prevalence of vitamin D deficiency and mean vitamin D levels in various combinations of microvascular complications is given in Table 2. With an increasing number of complications the mean vitamin D levels were found to fall while the prevalence of vitamin D deficiency increased accordingly. The decline in vitamin D levels between the groups was found to be significant (P = 0.001 ANOVA). Further when individual groups were tested for the difference in vitamin D levels against the group having no microvasclar complication it was found that difference in levels of vitamin D in neuropathy, nephropathy and retinopathy as a single occurrence was insignificant (P = 0.263, 0.126, and 0.292 respectively). On the other hand, the difference was found to be significant in cases of neuropathy with retinopathy (P = 0.036), neuropathy with nephropathy (P = 0.029), retinopathy with nephropathy (P = 0.022) and neuropathy with retinopathy with nephropathy (P = 0.0001). Thus, declining levels of vitamin D levels were significantly related to the presence of combinations of microvascular complications.
Table 2.
Mean vitamin D levels in various microvascular complications
DISCUSSION
Low vitamin D levels have been demonstrated to predict the development of microvascular complications in diabetes.
Scragg et al. and Suzuki et al. in their observational study in type 2 diabetes mellitus (T2DM) subjects concluded that mean vitamin D level concentration in men were significantly higher than women.[14,15] However in our study, 59.15% of males and 60.12% of females in the case group and 33.27% of males and 35.46% of females in the control group were found to be deficient in vitamin D. No significant difference was found in vitamin D levels between the two genders in the case as well as a control group. Various studies have found an inverse relation between vitamin D levels and diabetes prevalence. Pittas et al. found that among women higher levels of vitamin D were associated with a lower risk for T2DM.[16] Liu et al. reported that individuals in the highest tertile of vitamin D levels have a 40% lower incidence of T2DM.[18] Gagnon et al. showed that during the 5-year follow-up of subjects, who developed diabetes had lower vitamin D levels compared with those who remained free of diabetes.[19] Pietschmann et al. and Isaia et al. showed in their respective studies that an association exists between low circulating concentrations of vitamin D and the prevalence of diabetes and impaired glucose tolerance.[20,21]
In this study, it was found that individuals with type 2 diabetes were having lower vitamin D than non-diabetics. The mean vitamin D was 19.046 ng/ml in diabetics, while it was 27.186 ng/ml in non-diabetics. The difference was significant (P = 0.0001). Vitamin D deficiency was significantly higher in cases (59.49%) as compared to controls (34.61%) (P = 0.0001). Overall prevalence of inadequacy of vitamin D (vitamin D deficiency and insufficiency, or in other words levels <30 ng/ml) was significantly higher (93.67%) in cases than in non-diabetics (64.61%) (P = 0.0001). Hence, this suggests that hypovitaminosis D is more prevalent in diabetics and the vitamin D levels are significantly lower in diabetics as compared to non-diabetics. These results match with those obtained by others like Payne et al. and Aksoy et al., who their study described that diabetic subjects had lower vitamin D levels than non-diabetic subjects.[22,23] However, Suzuki et al. in their observational study in T2DM subjects concluded that mean vitamin D level concentration in T2DM patients was not statistically different from normal. Different results may be due to smaller sample size, geographical location and the effect of weather.[15]
The prevalence of microvascular complications in our study was found to be 74.05%. Only 25.95% of diabetics were free of all microvascular complication. Single microvascular complications (retinopathy or neuropathy or nephropathy) was present in 29.11% of cases while the combination of two was present in 25.32% and all three in 19.62% of cases. Overall 76.78% of cases having neuropathy, 79.63% of cases having retinopathy and 81.48% of cases having nephropathy were deficient in vitamin D. Among diabetics having no microvascular complications, 51.22% of cases were having vitamin D deficiency. On applying Chi-square test vitamin D deficiency was found to be separately associated significantly with neuropathy (P = 0.020), retinopathy (P = 0.010) and nephropathy (P = 0.001) individually. The mean vitamin D levels were found to be 23.10 ± 6.12 (no microvascular complication), 19.94 ± 5.21 (neuropathy), 19.25 ± 7.86 (retinopathy), 19.82 ± 5.05 (nephropathy), 16.64 ± 6.21 (neuropathy with retinopathy), 17.12 ± 4.46 (neuropathy with nephropathy), 16.96 ± 4.85 (retinopathy with nephropathy) and 14.63 ± 4.43 ng/ml (neuropathy with retinopathy with nephropathy). This decrease in level of vitamin D was associated significantly with the presence of multiple microvascular complications (ANOVA, P = 0.0001).
There was a significant difference in the vitamin D levels of classes having no microvascular complication and those having multiple microvascular complications, i.e. neuropathy with retinopathy (P = 0.036), neuropathy with nephropathy (P = 0.029), retinopathy with nephropathy (P = 0.022) and neuropathy with retinopathy with nephropathy (P = 0.0001). Thus, lower vitamin D levels were found to be significantly associated with increased chances of having multiple microvascular complications.
Robinson et al. in his study found that vitamin D were significantly lower in those diabetics who had microvascular complications.[24] Aksoy et al. also showed that the mean vitamin D3 concentrations fell with increasing severity of diabetic retinopathy.[23] Payne et al. demonstrated that patients with DR were deficient in vitamin D and that diabetic subjects, especially those with proliferative diabetic retinopathy (PDR).[22] Suzuki et al. showed that the existence of PDR was significantly associated with a decrease in serum vitamin D concentrations.[15] Even in a study on type 1 diabetes, Kaur et al. found that retinopathy prevalence was higher in cases with vitamin D deficiency versus sufficiency.[25] Chaychi et al. in his study found that patients with diabetic polyneuropathy had a lower mean serum vitamin D level.[26] Soderstorm et al. demonstrated vitamin D insufficiency is associated with the adjusted composite measure of neuropathy.[27] Lee and Chen in their study on use of vitamin D as analgesic for neuropathic pain found that all patients were vitamin D insufficient and mean vitamin D level was 18 ng/ml.[28] Diaz et al. in their study found that 30.7% of adults with diabetes have nephropathy, 48.9% have vitamin D deficiency and 36.6% have vitamin D insufficiency.[29] Kim et al. in their study found that mean vitamin D level was 18.4 ± 9.8 in diabetic nephropathy and 86% of subjects were vitamin D insufficient and 46% were deficient.[30] Oh et al. found that in early stage 3 CKD mean vitamin D level was 20.4 ng/ml and 29.9% were deficient in vitamin D.[31] The results obtained in our study compare well with those obtained in above studies. Thus in conclusion, mean vitamin D levels are significantly lower in type 2 diabetics, vitamin D deficiency (<20 ng/ml) in type 2 diabetes is significantly associated with any of the individual microvascular complications, i.e. neuropathy, retinopathy, and nephropathy and type 2 diabetics with decreasing vitamin D levels have significantly increasing prevalence of combination of microvascular complications.
LIMITATIONS
The cross-sectional design of this study limits the ability to assess causality. Secondly, only one time point was recorded for the subjects in this study. There is possible selection bias as this is not a study of consecutive patients seen at our institution. Due to the small sample size results cannot be generalized.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Kadowaki S, Norman AW. Dietary vitamin D is essential for normal insulin secretion from the perfused rat pancreas. J Clin Invest. 1984;73:759–66. doi: 10.1172/JCI111269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Need AG, O’Loughlin PD, Horowitz M, Nordin BE. Relationship between fasting serum glucose, age, body mass index and serum 25 hydroxyvitamin D in postmenopausal women. Clin Endocrinol (Oxf) 2005;62:738–41. doi: 10.1111/j.1365-2265.2005.02288.x. [DOI] [PubMed] [Google Scholar]
- 3.Mitri J, Dawson-Hughes B, Hu FB, Pittas AG. Effects of vitamin D and calcium supplementation on pancreatic β cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: The Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. Am J Clin Nutr. 2011;94:486–94. doi: 10.3945/ajcn.111.011684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chabas JF, Alluin O, Rao G, Garcia S, Lavaut MN, Risso JJ, et al. Vitamin D2 potentiates axon regeneration. J Neurotrauma. 2008;25:1247–56. doi: 10.1089/neu.2008.0593. [DOI] [PubMed] [Google Scholar]
- 5.Feldman EL, Stevens MJ, Greene DA. Pathogenesis of diabetic neuropathy. Clin Neurosci. 1997;4:365–70. [PubMed] [Google Scholar]
- 6.Garcion E, Sindji L, Montero-Menei C, Andre C, Brachet P, Darcy F. Expression of inducible nitric oxide synthase during rat brain inflammation: Regulation by 1,25-dihydroxyvitamin D3. Glia. 1998;22:282–94. [PubMed] [Google Scholar]
- 7.Neveu I, Naveilhan P, Menaa C, Wion D, Brachet P, Garabédian M. Synthesis of 1,25-dihydroxyvitamin D3 by rat brain macrophages in vitro. J Neurosci Res. 1994;38:214–20. doi: 10.1002/jnr.490380212. [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Deb DK, Kong J, Ning G, Wang Y, Li G, et al. Long-term therapeutic effect of vitamin D analog doxercalciferol on diabetic nephropathy: Strong synergism with AT1 receptor antagonist. Am J Physiol Renal Physiol. 2009;297:F791–801. doi: 10.1152/ajprenal.00247.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vidotti DB, Casarini DE, Cristovam PC, Leite CA, Schor N, Boim MA. High glucose concentration stimulates intracellular renin activity and angiotensin II generation in rat mesangial cells. Am J Physiol Renal Physiol. 2004;286:F1039–45. doi: 10.1152/ajprenal.00371.2003. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Z, Yuan W, Sun L, Szeto FL, Wong KE, Li X, et al. 1, 25-Dihydroxyvitamin D3 targeting of NF-kappaB suppresses high glucose-induced MCP-1 expression in mesangial cells. Kidney Int. 2007;72:193–201. doi: 10.1038/sj.ki.5002296. [DOI] [PubMed] [Google Scholar]
- 12.Taverna MJ, Sola A, Guyot-Argenton C, Pacher N, Bruzzo F, Slama G, et al. Taq I polymorphism of the vitamin D receptor and risk of severe diabetic retinopathy. Diabetologia. 2002;45:436–42. doi: 10.1007/s00125-001-0769-2. [DOI] [PubMed] [Google Scholar]
- 13.Prevention and management of osteoporosis. World Health Organ Tech Rep Ser. 2003;921:1–164. [PubMed] [Google Scholar]
- 14.Scragg R, Sowers M, Bell C. Third National Health and Nutrition Examination Survey. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2813–8. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki A, Kotake M, Ono Y, Kato T, Oda N, Hayakawa N, et al. Hypovitaminosis D in type 2 diabetes mellitus: Association with microvascular complications and type of treatment. Endocr J. 2006;53:503–10. doi: 10.1507/endocrj.k06-001. [DOI] [PubMed] [Google Scholar]
- 16.Pittas AG, Sun Q, Manson JE, Dawson-Hughes B, Hu FB. Plasma 25-hydroxyvitamin D concentration and risk of incident type 2 diabetes in women. Diabetes Care. 2010;33:2021–3. doi: 10.2337/dc10-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holick MF, Shao Q, Liu WW, Chen TC. The vitamin D content of fortified milk and infant formula. N Engl J Med. 1992;326:1178–81. doi: 10.1056/NEJM199204303261802. [DOI] [PubMed] [Google Scholar]
- 18.Liu E, Meigs JB, Pittas AG, Economos CD, McKeown NM, Booth SL, et al. Predicted 25-hydroxyvitamin D score and incident type 2 diabetes in the Framingham Offspring Study. Am J Clin Nutr. 2010;91:1627–33. doi: 10.3945/ajcn.2009.28441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gagnon C, Lu ZX, Magliano DJ, Dunstan DW, Shaw JE, Zimmet PZ, et al. Serum 25-hydroxyvitamin D, calcium intake, and risk of type 2 diabetes after 5 years: Results from a national, population-based prospective study (the Australian Diabetes, Obesity and Lifestyle study) Diabetes Care. 2011;34:1133–8. doi: 10.2337/dc10-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pietschmann P, Schernthaner G, Woloszczuk W. Serum osteocalcin levels in diabetes mellitus: Analysis of the type of diabetes and microvascular complications. Diabetologia. 1988;31:892–5. doi: 10.1007/BF00265373. [DOI] [PubMed] [Google Scholar]
- 21.Isaia GC, Ardissone P, Di Stefano M, Ferrari D, Martina V, Porta M, et al. Bone metabolism in type 2 diabetes mellitus. Acta Diabetol. 1999;36:35–8. doi: 10.1007/s005920050142. [DOI] [PubMed] [Google Scholar]
- 22.Payne JF, Ray R, Watson DG, Delille C, Rimler E, Cleveland J. Vitamin D insufficiency in diabetic retinopathy. Endocr Pract. 2012;18:185–93. doi: 10.4158/EP11147.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aksoy H, Akçay F, Kurtul N, Baykal O, Avci B. Serum 1,25 dihydroxy vitamin D (1,25(OH) 2D3), 25 hydroxy vitamin D (25(OH) D) and parathormone levels in diabetic retinopathy. Clin Biochem. 2000;33:47–51. doi: 10.1016/s0009-9120(99)00085-5. [DOI] [PubMed] [Google Scholar]
- 24.Robinson JG, Manson JE, Larson J, Liu S, Song Y, Howard BV, et al. Lack of association between 25(OH) D levels and incident type 2 diabetes in older women. Diabetes Care. 2011;34:628–34. doi: 10.2337/dc10-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaur H, Donaghue KC, Chan AK, Benitez-Aguirre P, Hing S, Lloyd M, et al. Vitamin D deficiency is associated with retinopathy in children and adolescents with type 1 diabetes. Diabetes Care. 2011;34:1400–2. doi: 10.2337/dc11-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaychi L, Mackenzie T, Bilotta D, Lynch M, Cohen J, Comi R. Association of serum vitamin D level with diabetic polyneuropathy. Med Practise Rev Feb. 2011;2:11–5. [Google Scholar]
- 27.Soderstrom LH, Johnson SP, Diaz VA, Mainous AG., 3rd Association between vitamin D and diabetic neuropathy in a nationally representative sample: Results from 2001-2004 NHANES. Diabet Med. 2012;29:50–5. doi: 10.1111/j.1464-5491.2011.03379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee P, Chen R. Vitamin D as an analgesic for patients with type 2 diabetes and neuropathic pain. Arch Intern Med. 2008;168:771–2. doi: 10.1001/archinte.168.7.771. [DOI] [PubMed] [Google Scholar]
- 29.Diaz VA, Mainous AG, 3rd, Carek PJ, Wessell AM, Everett CJ. The association of vitamin D deficiency and insufficiency with diabetic nephropathy: Implications for health disparities. J Am Board Fam Med. 2009;22:521–7. doi: 10.3122/jabfm.2009.05.080231. [DOI] [PubMed] [Google Scholar]
- 30.Kim MJ, Frankel AH, Donaldson M, Darch SJ, Pusey CD, Hill PD, et al. Oral cholecalciferol decreases albuminuria and urinary TGF-β1 in patients with type 2 diabetic nephropathy on established renin-angiotensin-aldosterone system inhibition. Kidney Int. 2011;80:851–60. doi: 10.1038/ki.2011.224. [DOI] [PubMed] [Google Scholar]
- 31.Oh YJ, Kim M, Lee H, Lee JP, Kim H, Kim S, et al. A threshold value of estimated glomerular filtration rate that predicts changes in serum 25-hydroxyvitamin D levels: 4th Korean National Health and Nutritional Examination Survey 2008. Nephrol Dial Transplant. 2012;27:2396–403. doi: 10.1093/ndt/gfr763. [DOI] [PubMed] [Google Scholar]


