Abstract
Introduction:
Osteoporosis represents the second most common cause of endocrinopathy in patients with beta thalassemia major (BTM). Some drugs proved effective to reduce vertebral and non-vertebral fracture risk. Denosumab is a fully human monoclonal antibody to the receptor activator of nuclear factor kappa B ligand (RANKL), a member of the tumor necrosis factor receptor superfamily essential for osteoclastogenesis. The efficacy and safety of denosumab in BTM-induced osteoporosis has not been tested.
Objective:
To evaluate the efficacy and safety of anti-RANKL on the biochemical and radiological parameters of bone mineralization in patients with BTM-induced osteoporosis.
Design:
The study population was selected using the random sampling method from the patient's database of our thalassemia clinic. Transfusion-dependent BTM patients above 18 years with no history of treatment with bisphosphonates were randomly selected. Bone mineral density (BMD) of the lumbar spine (LS) and right femoral neck (FN) were measured by dual energy X-ray absorption (DEXA) scan using a calibrated method. Independent factors likely to be associated with low bone mass were determined and included in the analysis to ascertain possible associations.
Patients and Methods:
We studied 30 patients with BTM-induced osteoporosis as per World Health Organization criteria (T Score of less than − 1.0 being defined as osteopenic and a T Score of less than − 2.5 being referred as osteoporotic). 19 males and 11 females aged between 18 and 32 years, with full pubertal development (Tanner's stage 5) at the time of the study. Their mean serum ferritin concentration was 3557 ng ± 1488 ng/ml. Every patient underwent DEXA scan as a baseline and after 12 months of denosumab therapy. Biochemical evaluation including serum concentrations of creatinine, Na, K, calcium, phosphorus, parathormone, bone specific alkaline phosphatase and type 1 collagen carboxy telopetide (ICCT) using enzyme-linked immunosorbent assay (Nordic Bioscience Diagnostics A/S) was done at baseline, after a month and then every 3 months for 12 months after starting denosumab. 60 mg of denosumab was administered subcutaneously twice yearly for a year. The mean BMD T Scores at baseline were −2.7 at the LS and −2.1 at the FN.
Results:
Denosumab therapy for a year was associated with a significant increase in BMD of 9.2% (95% confidence interval [CI], 8.2-10.1) at the LS and 6.0% (95% CI, 5.2-6.7) at the FN. Denosumab treatment decreased serum ICCT levels by 56% at 1 month and normalized them in all patients at 1 year. Significant correlations were found between BMD T Score before and 1 year after denosumab in LS (r = 0.752, P < 0.001) and FN (r = 0.758 P < 0.001), respectively. The most common side effects were pain in the back and extremities (12%) and nausea (10%). Asymptomatic hypocalcaemia occurred in two patients.
Conclusion:
Denosumab therapy for a year significantly increased BMD density at LS and FN of patients with BTM and was associated with a rapid and sustained reduction in ICCT levels. Further studies are required to confirm long-term effects of this therapy.
Keywords: Bone alkaline phosphatase, bone mineral density, denosumab, osteoporosis, thalassemia, type 1 collagen carboxy telopetide
INTRODUCTION
Despite the significant improvements in the therapeutic management of beta thalassemia major (BTM) over the past few decades, osteoporosis is still a common finding, even in optimally treated patients.[1,2,3,4,5,6] The relationships between bone mineral densities (BMD) and several clinical characteristics or hematological markers have been described. Chronic anemia, bone marrow expansion due to ineffective erythropoiesis, iron toxicity, calcium and zinc deficiencies, low vitamin D levels and endocrine complications have been suggested to contribute to the etiology of bone diseases in BTM. Nevertheless, the complex etiological mechanisms of this heterogeneous osteopathy still remains incompletely clarified.[7,8,9,10,11,12]
A complex mechanism controls bone remodeling in human. This mechanism includes the receptor activator of nuclear factor kappa B ligand (RANKL), its natural receptor (RANK) and osteoprotegerin (OPG).[3,13] The RANK/RANKL pathway is a key that promotes osteoclast formation and activation and prolongs osteoclast survival. OPG acts as a decoy receptor for RANKL and prevents its interaction with RANK thereby inhibiting osteoclast formation, function and survival. Alteration of the RANK/RANKL/OPG system in favor of increased osteoclastic activity and enhanced osteoblastic dysfunction is proposed as an important mechanism in the etiology of osteoporosis in BTM.[13,14]
Hypogonadism, a common finding in BTM, is associated with enhanced RANKL activity.[15] The sex steroid hormones, androgens and estrogens, via their respective nuclear receptors, regulate BMD in humans and mice. Testosterone is likely to have direct and indirect inhibitory effects on human osteoclast formation and bone resorption.[16]
Animal model and cell culture studies suggest a direct inhibitory effect of androgens on the OPG/RANKL cytokines system. In human osteoblastic cells, testosterone and 5-dihydrotestosterone mediate an androgen receptor-induced specific inhibition of OPG messenger ribonucleic acid (mRNA) expression.[16,17,18] Androgens have also been shown to block RANKL-induced osteoclastic formation[16,19] while RANKL expression was found to be up-regulated in osteoblastic cells from androgen receptor-deficient mice.[20] The effect of oestradiol (E2) on osteoclast precursors and osteoclasts seems to be mediated by osteoblastic cells. Inhibitory effect of E2 is associated with the stimulated secretion of OPG by osteoblasts.[16]
Insulin-like growth factor-I (IGF-I) deficiency is common in children and adults with BTM. Data suggest that IGF-I may act as a coupling factor in bone remodeling by activating both bone formation and bone resorption; the latter effect appears to be mediated through the OPG/RANKL system in bone. Gorny et al.[21] found that IGF-I increased OPG expression in the same cell line.
In BTM, blunting of parathyroid hormone (PTH) response to hypocalcemia and hypoparathyroidism are a significant complication.[22] PTH significantly up regulates RANKL mRNA in primary bone marrow stromal osteoblasts, inhibits OPG gene expression at all stages of osteoblast differentiation; and exposure to PTH is associated with increased osteoclastogenesis. It appears that the osteoclastogenic activity of PTH occurs primarily by suppression of OPG gene expression in early osteoblasts and elevation of RANKL gene expression in mature osteoblasts.[23]
In turn RANKL inhibitors and more specifically a human monoclonal antibody against RANKL, denosumab, have been developed to treat a variety of bone disorders, including osteoporosis and skeletal complications of malignancy.[24] The efficacy of RANKL inhibition to suppress bone turnover and improve bone mass, microstructure and strength has been demonstrated in a variety of animal models. In OPG transgenic rats for instance, long-term suppression of osteoclasts led to an increase in bone mass and volume, associated with increased bone strength versus their wild-type littermates. In ovariectomized (OVX) mice expressing humanized RANKL mice, denosumab was superior to alendronate on improving bone mass, trabecular and cortical bone microarchitecture. Similarly, in OVX cynomologus monkeys, denosumab significantly increased BMD and reduced cortical porosity. Furthermore, in this model switching from alendronate to denosumab after 6 months led to greater reductions in BTMs and further improvement in bone mass and microarchitecture.[25]
Previous studies have focused on the characteristics of thalassemic patients with osteoporosis and their response to therapy with bisphosphonate.[10] Because RANK-RANKL and OPG play a major role in bone-resorption and seem to be the principal implicated mechanism for the development of osteoporosis in BTM, we conducted this prospective study to evaluate the effects of anti-RANKL denosumab on TM-induced osteoporosis.
PATIENTS AND METHODS
This study investigated 30 young adults with BTM, aged 20.7 ± 2.88 years, with full pubertal development (Tanner's stage 5). They were regularly transfused since early childhood and underwent chelation therapy using desferrioxamine which was replaced by deferasirox for the last 4-5 years. At the time of the study, their mean serum ferritin levels was 3557 ± 1488 ng/ml.
The Ethical Committee of Hamad Medical Center has approved the protocol of the study and informed consent was obtained from all patients included in the study.
Patients with any renal or hepatic impairment, hypothyroidism, hypocalcaemia, hypoparathyroidism or diabetes were excluded from the study.
Every patient underwent dual energy X-ray absorption scan at baseline and after 12 months of denosumab therapy. Osteoporosis as per World Health Organization criteria (T Score of less than -1.0 being defined as osteopenic and a T Score of less than -2.5 being referred as osteoporotic).[26]
All patients were evaluated biochemically by checking their liver, renal function and electrolytes including serum calcium, phosphorus, electrolytes were measured at baseline and afterwards montly or every 2 months. Bone specific alkaline phosphatase (ALP) and type 1 collagen carboxy telopeptide (ICCT) concentrations, using enzyme-linked immunosorbent assay (Nordic Bioscience Diagnostics A/S) were assessed at baseline and at 1, 3, 6 and 12 months after starting therapy.
Circulating follicle stimulating hormone, luteinizing hormone, IGF-I, total testosterone (T) in males and PTH concentrations were measured at baseline and repeated every 6 months using radio-immunometric assays.
60 mg of denosumab was administered subcutaneously every 6 month for a year.
The results represent the mean ± standard deviation. The statistical significances were analyzed using paired Student's t-test. Significant difference after versus before treatment was determined at P < 0.05. Linear regression equation was used to investigate correlations between variables.
RESULTS
Before treatment, the mean BMD T scores for patients with TM were -2.7 at the lumbar spine (LS) and −2.1 at the right femoral neck (FN) [Tables 1 and 2].
Table 1.
Biochemical and bone mineral density data in our thalassemia major patients before and after treatment with denosumab
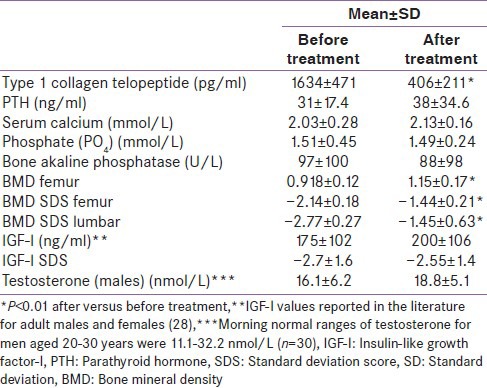
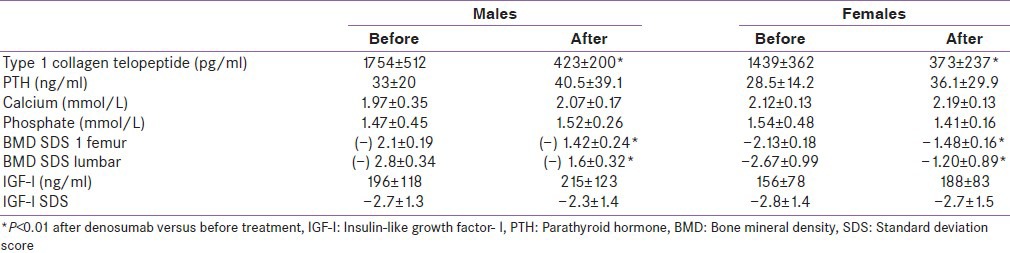
Table 2.
Biochemical and bone mineral density data in males and females thalassemia major patients before and after treatment with denosumab
Denosumab therapy for a year resulted in a significant increase in BMD of 9.2% (95% confidence interval [CI], 8.2-10.1) at the LS and 6.0% (95% CI, 5.2-6.7) at the FN.
Denosumab treatment decreased serum ICCT levels by 56% at 1 month and normalized them in all patients at 1 year. CCT1 decreased significantly after treatment [Table 1].
Serum T concentration was below the normal range for age in 9 out of 19 BTM patients (morning normal ranges of testosterone for men aged 20-30 years in our lab are: 11.1-32.2 nmol/L [n = 30]).
Their IGF-I and IGF-IStandard deviation score (SDS) were significantly decreased before and after treatment with denosumab compared to age and sex-matched published standards.[27]
Significant correlations were found between BMD T score before and 1 year after denosumab in LS (r = 0.752, P < 0.001) and FN (r = 0.758, P < 0.001), respectively.
Pain in the extremities and back was reported by 4/30 patients, gastrointestinal symptoms (nausea and abdominal pain) by 3/30 patients and hypocalcemia (1.7 and 1.8 mmol/L) in two patients but did not necessitate discontinuation of therapy.
DISCUSSION
Osteopenia and osteoporosis are still prevalent in patients with BTM (40-80%) despite significant improvement in the iron-chelation therapy.[1,2,3,6] Throughout childhood, BMD normally rises at a steady rate until the age of about 12 years and then there is sudden acceleration of bone mineral accretion, which coincides with the onset of puberty and the pubertal growth spurt. In patients with thalassemia, BMD which is already low in childhood decreases further during and after puberty, especially in patients with absent or delayed puberty.[28] Bielinski et al. have proved that thalassemic adolescents who failed to progress normally through puberty also failed to preserve adequate bone mineralization and achievement of peak bone mass.[29] Other studies have shown suboptimal bone accrual, regardless normal or induced puberty.[14,30]
The pathogenesis of osteoporosis in BTM is complex and still not clearly known. Important factors implicated in reduction of bone mass in BTM include: Delayed puberty, GH and IGF-I deficiency, parathyroid gland dysfunction, diabetes, hypothyroidism, ineffective hemopoiesis with progressive marrow expansion, direct iron toxicity on osteoblasts, as well as “toxic effect” of chelating therapy have been indicated as possible etiological factors.[2,3,4,5,6,7,8,9,10,11]
It appears that progressive “aging” of the bone starts even in childhood by the gradual development of an imbalance between augmented osteoclastic resorption and insufficient osteoblastic bone formation. In our BMT patients with markedly decreased BMD, the significantly high ICCT concentrations before treatment points out to the significant osteoclastic activity rather than decreased osteoblastic activity in the pathogenesis of osteoporosis in these patients.[31]
Members of the tumor necrosis factor receptor super-family, RANKL, RANK and OPG, play a central role in bone remodeling via conjunction with various cytokines and calciotropic hormones.[32] Several studies have recently proven that the ratio of RANKL/OPG is increased in patients with BTM and low BMD. Alterations in the RANK/RANKL/OPG system in favor of osteoclasts are characteristic in thalassemia due to complicated not clearly delineated mechanisms involving chronic anemia, iron toxicity and endocrine complications. In our study and others’, patients with BTM have elevated markers of bone resorption, such elevated markers reveal increased osteoclastic activity and enhanced osteoblastic dysfunction.[33,34,35,36,37]
Osteoclasts express IGF- I receptors and IGF- I has direct effects on their function.[37] GH and IGF- I stimulate the production of OPG and its accumulation in the bone matrix. In our BTM patients, with markedly low IGF-I level and increased ICCT concentrations, it is suggested that decreased OPG production with increased RANKL/OPG ratio can induce more osteoclastic activity. However, there was no significant correlation between IGF-I level and BMD or ICCT in our patients.
In addition, in our thalassemic patients T secretion was lower than normal range in 9/19. Decreased sex steroids act by increasing the expression of RANKL by osteoblastic cells.[31,38] This can contribute to increased osteoclastic activity in our thalassemic patients with low T. Other authors reported a negative correlation between the RANKL/OPG ratio and free T in male thalassemia patients and between E2 in female thalassemia patients. This speculates an important role of sex steroids on the RANKL/OPG system and on bone.[39] Association between hypogonadotrophic hypogonadism and osteoporosis in adult patients with TM has been reported in the past.[40,41] In addition, primary gonadal failure may also present due to iron deposition on the testes and ovaries.[42,43] However, in our patients there was no correlation between serum T level and BMD.
In our BTM patients, denosumab therapy for a year, through blocking the RANKL, significantly decreased ICCT (a bone resorption marker) levels by 56% at 1 month and normalized them in all patients after 1 year of therapy. This was associated with a significant increase in BMD T score both at the LS (9, 2%) and FN (6%).
In support of our findings, in postmenopausal women with low bone mass, randomized clinical trials have demonstrated that denosumab (60 mg s.c., every 6 months) inhibits bone resorption markedly and reversibly and improves BMD.[44]
In addition, patients treated with bisphosphonates for osteoporosis may discontinue or require a switch to other therapies. Transition to denosumab produced greater increases in BMD at all measured skeletal sites and a greater reduction in bone turnover than did continued alendronate with a similar safety profile in both groups.[45]
Pain in the extremities and back was reported by 4/30, gastrointestinal symptoms (nausea and abdominal pain) by 3/30 and asymptomatic hypocalcemia in 2/30 and hypocalcemia (serum calcium 1.7 and 1.8 mmol/L) in 2/30 patients but did not necessitate discontinuation of therapy in any of them. In other studies, active treatment was well tolerated (compliance rate was >80% after 3 years of therapy) and overall serious adverse events were balanced versus placebo, including cancer and infections.[24]
In our patients serum, ferritin was significantly high denoting inadequate chelation. Iron overload inhibits indices of osteoblast differentiation, such as the expression of type 1 collagen (mRNA and protein), the activity of ALP and the deposition of calcium by osteoblasts. Iron excess limits HedgeHog Interacting Protein Like-2 (HHIPL-2) gene expression and decreases osteoblastic activity in human MG-63 cells.[46] In postmenopausal females and middle aged males highest ferritin quartile category showed significantly faster bone loss in the total femur and femur neck in both genders. In addition, in thalassemic mice trabecular bone resorption was markedly enhanced as indicated by increases in the osteoclast surface and increased eroded surface. Therefore, toxic effect of excess iron on bone cells may contribute to the low BMD in our thalassemic patients by inhibiting osteoblast proliferation and differentiation.[46,47,48,49]
CONCLUSION
Osteoporosis in TM represents a unique clinical entity because of multifactorial etiology and of complex mechanism, which need to be clarified. In this study denosumab therapy for a year significantly increased BMD at LS and FN of patients with BTM and was associated with a rapid and sustained reduction in bone turnover markers. Further studies are required to confirm long-term effects of this therapy.
Footnotes
Source of Support: Hamad Medical Center
Conflict of Interest: None declared
REFERENCES
- 1.Baldini M, Forti S, Marcon A, Ulivieri FM, Orsatti A, Tampieri B, et al. Endocrine and bone disease in appropriately treated adult patients with beta-thalassemia major. Ann Hematol. 2010;89:1207–13. doi: 10.1007/s00277-010-1007-0. [DOI] [PubMed] [Google Scholar]
- 2.Scacchi M, Danesi L, Cattaneo A, Valassi E, Pecori Giraldi F, Argento C, et al. Bone demineralization in adult thalassaemic patients: Contribution of GH and IGF-I at different skeletal sites. Clin Endocrinol (Oxf) 2008;69:202–7. doi: 10.1111/j.1365-2265.2008.03191.x. [DOI] [PubMed] [Google Scholar]
- 3.Kyriakou A, Savva SC, Savvides I, Pangalou E, Ioannou YS, Christou S, et al. Gender differences in the prevalence and severity of bone disease in thalassaemia. Pediatr Endocrinol Rev. 2008;6(Suppl 1):116–22. [PubMed] [Google Scholar]
- 4.Voskaridou E, Terpos E. Pathogenesis and management of osteoporosis in thalassemia. Pediatr Endocrinol Rev. 2008;6(Suppl 1):86–93. [PubMed] [Google Scholar]
- 5.Pietrapertosa AC, Minenna G, Colella SM, Santeramo TM, Renni R, D’Amore M. Osteoprotegerin and RANKL in the pathogenesis of osteoporosis in patients with thalassaemia major. Panminerva Med. 2009;51:17–23. [PubMed] [Google Scholar]
- 6.Mahachoklertwattana P, Pootrakul P, Chuansumrit A, Choubtum L, Sriphrapradang A, Sirisriro R, et al. Association between bone mineral density and erythropoiesis in Thai children and adolescents with thalassemia syndromes. J Bone Miner Metab. 2006;24:146–52. doi: 10.1007/s00774-005-0661-0. [DOI] [PubMed] [Google Scholar]
- 7.Aslan I, Canatan D, Balta N, Kacar G, Dorak C, Ozsancak A, et al. Bone mineral density in thalassemia major patients from antalya, Turkey. Int J Endocrinol 2012. 2012:573298. doi: 10.1155/2012/573298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pincelli AI, Masera N, Tavecchia L, Perotti M, Perra S, Mariani R, et al. GH deficiency in adult B-thalassemia major patients and its relationship with IGF-1 production. Pediatr Endocrinol Rev. 2011;8(Suppl 2):284–9. [PubMed] [Google Scholar]
- 9.Pirinççioğlu AG, Akpolat V, Köksal O, Haspolat K, Söker M. Bone mineral density in children with beta-thalassemia major in Diyarbakir. Bone. 2011;49:819–23. doi: 10.1016/j.bone.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Skordis N, Ioannou YS, Kyriakou A, Savva SC, Efstathiou E, Savvides I, et al. Effect of bisphosphonate treatment on bone mineral density in patients with thalassaemia major. Pediatr Endocrinol Rev. 2008;6(Suppl 1):144–8. [PubMed] [Google Scholar]
- 11.Soliman AT, El Banna N, Abdel Fattah M, ElZalabani MM, Ansari BM. Bone mineral density in prepubertal children with beta-thalassemia: Correlation with growth and hormonal data. Metabolism. 1998;47:541–8. doi: 10.1016/s0026-0495(98)90237-2. [DOI] [PubMed] [Google Scholar]
- 12.Mahachoklertwattana P, Chuansumrit A, Sirisriro R, Choubtum L, Sriphrapradang A, Rajatanavin R. Bone mineral density, biochemical and hormonal profiles in suboptimally treated children and adolescents with beta-thalassaemia disease. Clin Endocrinol (Oxf) 2003;58:273–9. doi: 10.1046/j.1365-2265.2003.01707.x. [DOI] [PubMed] [Google Scholar]
- 13.Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473:139–46. doi: 10.1016/j.abb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kearns AE, Khosla S, Kostenuik PJ. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr Rev. 2008;29:155–92. doi: 10.1210/er.2007-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pepene CE, Crişan N, Coman I. Elevated serum receptor activator of nuclear factor kappa B ligand and osteoprotegerin levels in late-onset male hypogonadism. Clin Invest Med. 2011;34:E232. doi: 10.25011/cim.v34i4.15365. [DOI] [PubMed] [Google Scholar]
- 16.Michael H, Härkönen PL, Väänänen HK, Hentunen TA. Estrogen and testosterone use different cellular pathways to inhibit osteoclastogenesis and bone resorption. J Bone Miner Res. 2005;20:2224–32. doi: 10.1359/JBMR.050803. [DOI] [PubMed] [Google Scholar]
- 17.Hofbauer LC, Hicok KC, Chen D, Khosla S. Regulation of osteoprotegerin production by androgens and anti-androgens in human osteoblastic lineage cells. Eur J Endocrinol. 2002;147:269–73. doi: 10.1530/eje.0.1470269. [DOI] [PubMed] [Google Scholar]
- 18.Grundt A, Grafe IA, Liegibel U, Sommer U, Nawroth P, Kasperk C. Direct effects of osteoprotegerin on human bone cell metabolism. Biochem Biophys Res Commun. 2009;389:550–5. doi: 10.1016/j.bbrc.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 19.Huber DM, Bendixen AC, Pathrose P, Srivastava S, Dienger KM, Shevde NK, et al. Androgens suppress osteoclast formation induced by RANKL and macrophage-colony stimulating factor. Endocrinology. 2001;142:3800–8. doi: 10.1210/endo.142.9.8402. [DOI] [PubMed] [Google Scholar]
- 20.Kawano H, Sato T, Yamada T, Matsumoto T, Sekine K, Watanabe T, et al. Suppressive function of androgen receptor in bone resorption. Proc Natl Acad Sci U S A. 2003;100:9416–21. doi: 10.1073/pnas.1533500100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorny G, Shaw A, Oursler MJ. IL-6, LIF, and TNF-alpha regulation of GM-CSF inhibition of osteoclastogenesis in vitro. Exp Cell Res. 2004;294:149–58. doi: 10.1016/j.yexcr.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Gertner JM, Broadus AE, Anast CS, Grey M, Pearson H, Genel M. Impaired parathyroid response to induced hypocalcemia in thalassemia major. J Pediatr. 1979;95:210–3. doi: 10.1016/s0022-3476(79)80653-8. [DOI] [PubMed] [Google Scholar]
- 23.Huang JC, Sakata T, Pfleger LL, Bencsik M, Halloran BP, Bikle DD, et al. PTH differentially regulates expression of RANKL and OPG. J Bone Miner Res. 2004;19:235–44. doi: 10.1359/JBMR.0301226. [DOI] [PubMed] [Google Scholar]
- 24.Lewiecki EM, Bilezikian JP. Denosumab for the treatment of osteoporosis and cancer-related conditions. Clin Pharmacol Ther. 2012;91:123–33. doi: 10.1038/clpt.2011.268. [DOI] [PubMed] [Google Scholar]
- 25.Dempster DW, Lambing CL, Kostenuik PJ, Grauer A. Role of RANK ligand and denosumab, a targeted RANK ligand inhibitor, in bone health and osteoporosis: A review of preclinical and clinical data. Clin Ther. 2012;34:521–36. doi: 10.1016/j.clinthera.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. Geneva, Switzerland: World Health Organization; 1994. Assessment of Fracture Risk and its Application to Screening for Postmenopausal Osteoporosis. WHO Technical Report Series 843. [PubMed] [Google Scholar]
- 27.Friedrich N, Alte D, Völzke H, Spilcke-Liss E, Lüdemann J, Lerch MM, et al. Reference ranges of serum IGF-1 and IGFBP-3 levels in a general adult population: Results of the study of health in Pomerania (SHIP) Growth Horm IGF Res. 2008;18:228–37. doi: 10.1016/j.ghir.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Filosa A, Di Maio S, Vocca S, Saviano A, Esposito G, Pagano L. Longitudinal monitoring of bone mineral density in thalassemic patients. Genetic structure and osteoporosis. Acta Paediatr. 1997;86:342–6. doi: 10.1111/j.1651-2227.1997.tb09019.x. [DOI] [PubMed] [Google Scholar]
- 29.Bielinski BK, Darbyshire PJ, Mathers L, Crabtree NJ, Kirk JM, Stirling HF, et al. Impact of disordered puberty on bone density in beta-thalassaemia major. Br J Haematol. 2003;120:353–8. doi: 10.1046/j.1365-2141.2003.04066.x. [DOI] [PubMed] [Google Scholar]
- 30.Benigno V, Bertelloni S, Baroncelli GI, Bertacca L, Di Peri S, Cuccia L, et al. Effects of thalassemia major on bone mineral density in late adolescence. J Pediatr Endocrinol Metab. 2003;16(Suppl 2):337–42. [PubMed] [Google Scholar]
- 31.Toumba M, Skordis N. Osteoporosis syndrome in thalassaemia major: An overview. J Osteoporos 2010. 2010:537673. doi: 10.4061/2010/537673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vega D, Maalouf NM, Sakhaee K. CLINICAL Review #: The role of receptor activator of nuclear factor. kappaB (RANK)/RANK ligand/osteoprotegerin: Clinical implications. J Clin Endocrinol Metab. 2007;92:4514–21. doi: 10.1210/jc.2007-0646. [DOI] [PubMed] [Google Scholar]
- 33.Salama OS, Al-Tonbary YA, Shahin RA, Eldeen OA. Unbalanced bone turnover in children with beta-thalassemia. Hematology. 2006;11:197–202. doi: 10.1080/10245330600702851. [DOI] [PubMed] [Google Scholar]
- 34.Voskaridou E, Kyrtsonis MC, Terpos E, Skordili M, Theodoropoulos I, Bergele A, et al. Bone resorption is increased in young adults with thalassaemia major. Br J Haematol. 2001;112:36–41. doi: 10.1046/j.1365-2141.2001.02549.x. [DOI] [PubMed] [Google Scholar]
- 35.Lasco A, Morabito N, Gaudio A, Buemi M, Wasniewska M, Frisina N. Effects of hormonal replacement therapy on bone metabolism in young adults with beta-thalassemia major. Osteoporos Int. 2001;12:570–5. doi: 10.1007/s001980170079. [DOI] [PubMed] [Google Scholar]
- 36.Morabito N, Gaudio A, Lasco A, Atteritano M, Pizzoleo MA, Cincotta M, et al. Osteoprotegerin and RANKL in the pathogenesis of thalassemia-induced osteoporosis: New pieces of the puzzle. J Bone Miner Res. 2004;19:722–7. doi: 10.1359/JBMR.040113. [DOI] [PubMed] [Google Scholar]
- 37.Mochizuki H, Hakeda Y, Wakatsuki N, Usui N, Akashi S, Sato T, et al. Insulin-like growth factor-I supports formation and activation of osteoclasts. Endocrinology. 1992;131:1075–80. doi: 10.1210/endo.131.3.1505451. [DOI] [PubMed] [Google Scholar]
- 38.Manolagas SC, Kousteni S, Jilka RL. Sex steroids and bone. Recent Prog Horm Res. 2002;57:385–409. doi: 10.1210/rp.57.1.385. [DOI] [PubMed] [Google Scholar]
- 39.Voskaridou E, Terpos E. New insights into the pathophysiology and management of osteoporosis in patients with beta thalassaemia. Br J Haematol. 2004;127:127–39. doi: 10.1111/j.1365-2141.2004.05143.x. [DOI] [PubMed] [Google Scholar]
- 40.Jensen CE, Tuck SM, Agnew JE, Koneru S, Morris RW, Yardumian A, et al. High prevalence of low bone mass in thalassaemia major. Br J Haematol. 1998;103:911–5. doi: 10.1046/j.1365-2141.1998.01108.x. [DOI] [PubMed] [Google Scholar]
- 41.Skordis N, Michaelidou M, Savva SC, Ioannou Y, Rousounides A, Kleanthous M, et al. The impact of genotype on endocrine complications in thalassaemia major. Eur J Haematol. 2006;77:150–6. doi: 10.1111/j.1600-0609.2006.00681.x. [DOI] [PubMed] [Google Scholar]
- 42.De Sanctis V, Perera D, Katz M, Fortini M, Gamberini MR. Spermatozoal DNA damage in patients with B thalassaemia syndromes. Pediatr Endocrinol Rev. 2008;6(Suppl 1):185–9. [PubMed] [Google Scholar]
- 43.Berkovitch M, Bistritzer T, Milone SD, Perlman K, Kucharczyk W, Olivieri NF. Iron deposition in the anterior pituitary in homozygous beta-thalassemia: MRI evaluation and correlation with gonadal function. J Pediatr Endocrinol Metab. 2000;13:179–84. doi: 10.1515/jpem.2000.13.2.179. [DOI] [PubMed] [Google Scholar]
- 44.Moen MD, Keam SJ. Denosumab: A review of its use in the treatment of postmenopausal osteoporosis. Drugs Aging. 2011;28:63–82. doi: 10.2165/11203300-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 45.Kendler DL, Roux C, Benhamou CL, Brown JP, Lillestol M, Siddhanti S, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res. 2010;25:72–81. doi: 10.1359/jbmr.090716. [DOI] [PubMed] [Google Scholar]
- 46.Doyard M, Fatih N, Monnier A, Island ML, Aubry M, Leroyer P, et al. Iron excess limits HHIPL-2 gene expression and decreases osteoblastic activity in human MG-63 cells. Osteoporos Int. 2012;23:2435–45. doi: 10.1007/s00198-011-1871-z. [DOI] [PubMed] [Google Scholar]
- 47.Yamasaki K, Hagiwara H. Excess iron inhibits osteoblast metabolism. Toxicol Lett. 2009;191:211–5. doi: 10.1016/j.toxlet.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 48.Kim BJ, Ahn SH, Bae SJ, Kim EH, Lee SH, Kim HK, et al. Iron overload accelerates bone loss in healthy postmenopausal women and middle-aged men: A 3-year retrospective longitudinal study. J Bone Miner Res. 2012;27:2279–90. doi: 10.1002/jbmr.1692. [DOI] [PubMed] [Google Scholar]
- 49.Thongchote K, Svasti S, Sa-ardrit M, Krishnamra N, Fucharoen S, Charoenphandhu N. Impaired bone formation and osteopenia in heterozygous β(IVSII-654) knockin thalassemic mice. Histochem Cell Biol. 2011;136:47–56. doi: 10.1007/s00418-011-0823-1. [DOI] [PubMed] [Google Scholar]


