Abstract
Introduction:
Diabetes mellitus has been associated with an earlier onset and increased severity of urologic diseases that often result in debilitating urologic complications. Diabetic bladder dysfunction refers to a group of bladder symptoms occurring in patients with diabetes mellitus ranging from bladder over activity to impaired bladder contractility.
Aim:
Bladder dysfunction is an under evaluated issue in women with diabetes. Aim of our study was to investigate prevalence of bladder dysfunction and its relation with other chronic complications of diabetes in women with type 2 diabetes.
Materials and Methods:
In a hospital-based cross sectional study, a cohort of women with type 2 diabetes mellitus who had lower urinary tract symptoms (LUTS) were enrolled. We used the American Urological Association Symptom Index (AUA-SI) to assess the severity of LUTS and the Indevus Urgency Severity Scale (IUSS) to assess presence of overactive bladder (OAB). Age-BMI- matched controls that did not have diabetes but had lower urinary tract symptoms were also studied and compared with women with type 2 diabetes. Urodynamic evaluation was done in willing patients.
Results:
LUTS attributable to bladder dysfunction were reported in 67% of women with type 2 diabetes after exclusion of other causes. Out of them, 36% had moderate to severe LUTS (total AUA-SI score >7). Prevalence of OAB was 53%. Urodynamic evaluation revealed presence of stress urinary incontinence in 48% patients and changes of detrusor over activity and detrusor under activity in 23% and 11% patients, respectively. Among the chronic complications of diabetes, peripheral neuropathy, nephropathy, and presence of metabolic syndrome were significantly associated with moderate to severe LUTS and OAB.
Conclusion:
Bladder dysfunction is a highly prevalent complication in women with diabetes. Chronic complications of diabetes especially neuropathy, nephropathy, and presence of metabolic syndrome are important predictors of bladder dysfunction.
Keywords: Type 2 diabetes, lower urinary tract symptoms, bladder dysfunction, diabetic cystopathy
INTRODUCTION
Diabetes mellitus has been associated with an earlier onset and increased severity of urologic diseases that often results in debilitating urologic complications. These urologic complications include bladder dysfunction and have a profound effect on the quality of life for patients with diabetes. Current understanding of bladder dysfunction reflects a progressive condition encompassing a broad spectrum of lower urinary tract symptoms (LUTS) including urinary urgency, frequency, nocturia, and incontinence. Previously, the dysfunction has been classically described as diminished bladder sensation, poor contractility, and increased post-void residual urine, termed bladder cystopathy[1] most likely represents end-stage bladder failure and is relatively uncommon.
Over 50% of men and women with diabetes have bladder dysfunction.[2,3] A number of clinical studies in men and women with diabetes have reported bladder instability or hypersensitivity as the most frequent finding, ranging from 39-61% of subjects.[2,4] Prevalence estimates of urodynamically diagnosed bladder cystopathy have ranged from 25% to 90%.[5] The wide variation reflects the lack of validated or standardized clinically significant measures used to diagnose bladder cystopathy as well as the selected referral bias.
Women with diabetes are known to be associated with higher prevalence of bladder dysfunction.[6,7] Women with obesity and diabetes were significantly more likely to have pelvic floor disorders, such as stress urinary incontinence and overactive bladder (OAB).[8] Observational studies have found that markers of more severe diabetes, including poor levels of glycemic control, are associated with an increased risk of LUTS.[9] Bladder dysfunction and LUTS are relatively under evaluated issues in women with diabetes. Aim of our study was to investigate prevalence of bladder dysfunction and its relation with other chronic complications of diabetes in women with type 2 diabetes.
MATERIALS AND METHODS
Between January and December 2012, 153 women with type 2 diabetes at the diabetes clinic of a medical college hospital were asked to participate in the study that had presence of lower urinary tract symptoms. Among them, 102 (67%) patients completed the questionnaires and were recruited in this study. Diabetic women with concurrent neurological disorders (such as stroke, Parkinsonism, spinal cord injury, and multiple sclerosis, active urinary tract infections, and previous pelvic surgery as well as evidence of pelvic organ prolapsed, bladder outlet obstruction and those on medication that could affect bladder function such as diuretics, calcium channel blockers, and narcotics) were excluded from the study. Age-BMI-matched controls who did not have diabetes but had lower urinary tract symptoms were also studied.
The parameters evaluated were age, duration of diabetes, type of diabetic therapy (diet, insulin, or hypoglycemic agents), chronic diabetic complications (retinopathy, nephropathy, and peripheral neuropathy, stroke, coronary artery disease), number of parity, and menopause status. Physical examination included blood pressure (millimeters of mercury), weight (kilograms), height (meters), and waist circumference (centimeters). Body mass index was calculated with body weight divided by the square of body height (kilograms per square meter). The following laboratory data were obtained: fasting blood sugar, glycosylated hemoglobin (HbA1c), total and high-density lipoprotein (HDL) cholesterol, triglycerides, creatinine, and urinalysis. Retinopathy was assessed by ophthalmoscopy after pupil dilation with a mydriatic agent. Peripheral neuropathy was assessed by questioning patients about symptoms of paresthesia, as well as measuring the sensory threshold (vibratory, thermal, and touch) on the feet. Patients were considered to have nephropathy if patients’ urine-albumin creatinine ratio was ≥1.5 mg/mmol and/or albuminuria in 24-h collection was ≥20 mg on two occasions. The study protocol was approved by the Institutional Ethics Committee. All participants gave their written informed consent.
Evaluation of LUTS and OAB
All patients were interviewed during the first visit of the study using a structured questionnaire containing the American Urological Association Symptom Index (AUA-SI)[10] to evaluate the severity of LUTS and the Indevus Urgency Severity Scale (IUSS)[11] to assess presence of OAB. The AUA-SI contains 7 LUTS including 3 storage symptoms (frequency, urgency, and nocturia) and 4 voiding symptoms (incomplete emptying, weak stream, abdominal straining, and intermittency). Each symptom is graded from 0 (not at all) to 5 (almost always) according to the frequency of symptom. Scores from these individual symptoms are aggregated to form total symptom score ranged from 0 to 35, which was further divided into storage symptom score (frequency + nocturia + urgency) and voiding symptom score (incomplete emptying + weak stream + intermittency + straining). The presence of OAB was defined according to the patient's complaint of urgency with or without urge incontinence as the core symptom, according to the International Continence Society recommendations. The urgency degree was classified by the IUSS. All women were asked to rate their urgency severity before toilet voiding on the following scale: 0 (none), no urgency; 1 (mild), awareness of urgency but easily tolerated; 2 (moderate), enough urgency discomfort that interferes with or shortens usual activity or tasks; and 3 (severe), extreme urgency discomfort that abruptly stops all activity or tasks. Women with an IUSS score of 2 or greater were considered to have significant OAB. We also evaluated the urinary continence status among our cohort as well. Stress incontinence was considered to be loss of “control of your urine when you laugh, cough, or during physical activities,” whereas urgent incontinence was defined as loss of “control of your urine because you feel urgent to urinate but cannot reach the bathroom in time.”
Assessment by uroflowmetry and post-void residual volume
Each patient was instructed to take 500 ml water and void to completion over a standard rotating disc flow meter (Dantec, Glostrup, Denmark) when a normal desire to void initiated. Post-Void Residual (PVR) urine volume was measured by suprapubic ultrasound immediately after voiding. The essential requirement for satisfactory urine flow rate was a minimum voided volume of 150 ml.
Urodynamic observations were based according to the following criteria (1) normal bladder function if uroflowmetry, post-void residual urine, bladder sensation, and voiding cystometry are within normal limits, (2) detrusor over activity - occurrence of involuntary contractions of the detrusor in filling phase during cystometry, (3) urinary stress incontinence - involuntary loss of urine during cystometry, which was associated with increases in abdominal pressure in the absence of detrusor muscle contraction, (4) underactive detrusor - reduced contraction of detrusor, resulting in prolonged or failure to empty bladder completely in prescribed time and high residual volume.
Filling cystometry (Medtronic Duet®; Medtronic, Minneapolis, MN, USA) was performed at an infusion rate of 10 mL/min. Bladder sensations during filling cystometry were recorded. All anti-cholinergic medications were stopped at least 2 weeks before study, and all patients who underwent urodynamic evaluation had confirmed negative urinalysis findings prior the procedure.
Definition of metabolic syndrome
We defined a woman with metabolic syndrome (MS) as the simultaneous presence of 3 or more of these risk factors: Waist circumference (≥80 cm based on a ethnicity-specific value for Chinese or South Asian population), systolic blood pressure (≥130 mmHg or diastolic blood pressure ≥ 85 mm Hg or treatment of previously diagnosed hypertension), fasting blood sugar (≥100 mg/dl or previously diagnosed type 2 diabetes, serum triglyceride level ≥150 mg/dl or specific treatment for this lipid abnormality), and HDL cholesterol (≤50 mg/dl or specific treatment for this lipid abnormality) according to National Cholesterol Education Program's Third Adult Treatment Panel.[12]
Statistical analysis
Statistical analyses were performed using statistics software SPSS 15.0 (SPSS Inc., Chicago, IL). Data were expressed as mean with standard deviation for continuous variables or as n with percentage for categorical variables. All statistical assessments were considered the significance level α =0.05. Logistic regression analysis was used to estimate the odds of moderate/severe LUTS and presence of OAB in association with duration of diabetes, glycemic control, and other diabetes complications after adjusting the confounding effect of age. Statistical tests of the regression estimates were based on the χ2 and 95% CIs were based on the Wald's test.
RESULTS
The characteristics of women with type 2 diabetes and age- and BMI-matched controls have been shown in Table 1. They did not have any significant difference in BMI, menopausal status, and parity. Table 2 is showing diabetic profile including duration of diabetes, fasting blood sugar level, HbA1c level, prevalence of diabetic complications, and insulin therapy. In the diabetes cohort, 56% of patients had metabolic syndrome.
Table 1.
Characteristics of women with type 2 diabetes and age- and BMI-matched controls
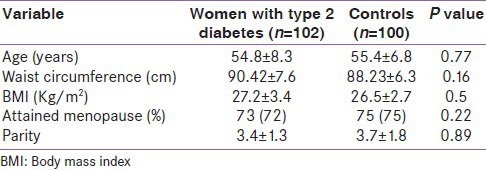
Table 2.
Clinical profile of women with type 2 diabetes

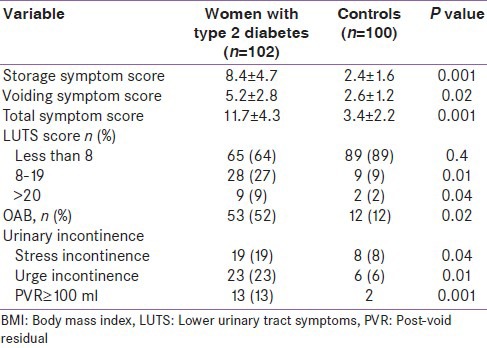
In the present study, 102/153 (67%) women had LUTS attributable to bladder dysfunction secondary to diabetes. Out of them, 36% of women with type 2 diabetes were considered to have moderate to severe LUTS (total AUA-SI score >7) as compared to 11% in control group. The storage and voiding symptom scores were significantly higher in women with diabetes than controls. The prevalence of OAB, as defined by an IUSS score of 2 or greater, was 53% in women with diabetes, which was significantly higher than in age- and BMI-matched controls. The overall prevalence of stress urinary incontinence and urge urinary incontinence in diabetic women was 19% and 23%, respectively. Uroflowmetry study revealed mean peak flow rate 14.2 ± 7.3 ml/sec in women with diabetes, while PVR ≥ 100 ml was present in 13% of these patients [Table 3].
Table 3.
LUTS in women with type 2 diabetes and age- and BMI-matched controls
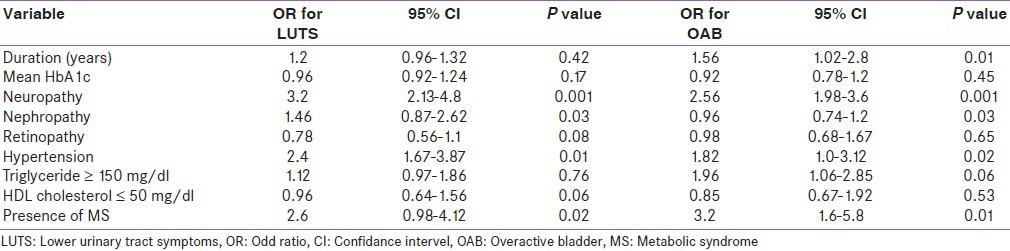
Logistic regression analysis was done to find the odds for moderate/severe LUTS and presence of OAB in relation to duration of diabetes, glycemic control, and other diabetes complications after adjusting the confounding effect of age [Table 4]. Among the chronic complications of diabetes, peripheral neuropathy (OR 3.2, 95% CI 2.13-4.8, P = 0.001) and nephropathy (OR 1.46, 95% CI 0.87-2.62, P = 0.03) were significantly associated with moderate LUTS. Age-adjusted odds ratio for OAB were also examined in multivariate logistic regression models. Duration of diabetes (OR 1.56, 95% CI 1.02-2.8, P = 0.01) was an independent predictor of OAB in addition to neuropathy and nephropathy. Presence of metabolic syndrome had significant association for both moderate LUTS and OAB, but among its components, only hypertension was found to be associated. In present study, glycemic control had no impact on LUTS or OAB.
Table 4.
Logistic regression analysis for LUTS in women with type 2 diabetes
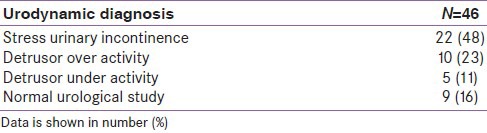
Out of 46 patients, who were evaluated for urodynamic diagnosis [Table 5], 22 (48%) had evidence of stress urinary incontinence while 10 (23%) and 5 (11%) had changes of detrusor overactivity and detrusor under activity, respectively. None of the patient from control group could be subjected for urodynamic study because of their unwillingness.
Table 5.
Urodynamic diagnosis in women with type 2 diabetes
DISCUSSION
LUTS are common in middle-aged women and have far-reaching physical, psychological, social, and economic implications. These symptoms are mostly attributed to pelvic floor disorders, uterine prolapse, pelvic surgery, and neurological disorders. Our study is first to address this important issue in women with type 2 diabetes in our population.
Diabetic cystopathy (DC) and bladder dysfunction is common in women with type 2 diabetes, may have broad spectrum of clinical presentations. These patients may be asymptomatic, or have a wide variety of voiding complaints from overactive bladder and urge incontinence to decreased bladder sensation and overflow incontinence.[13] It has received far less attention despite having a significant impact on quality of life and with significant individual health risks. Usually, by the time urologists are consulted, it has reached to an advanced stage.
We conducted a cross-sectional study on a cohort of women with type 2 diabetes treated regularly at diabetic clinic. We used the American Urological Association Symptom Index (AUA-SI) to evaluate the severity of LUTS and correlated them with clinical profiles. We also used the Indevus Urgency Severity Scale (IUSS) to assess presence of OAB and the possible association of various complications of diabetes with OAB. We found 36% of women with type 2 diabetes had moderate to severe LUTS (total AUA-SI score >7) as compared to 9% in control group. The storage and voiding symptom scores were significantly higher in women with diabetes than in controls.
AUA-SI has been validated for female lower urinary tract symptoms[14] and has now evolved from an instrument specifically designed for benign prostatic hyperplasia to one applied to LUTS in general in men and even in women. Although OAB symptoms (urgency, frequency, and nocturia) can be measured with storage symptom part of the AUA-SI questionnaire, we incorporated another validated instrument, IUSS, into the questionnaire to highlight the significance of urgency. In the present study, OAB symptom defined by IUSS was more prevalent in diabetic women and presence of MS had significant association with OAB and moderate to severe LUTS. Recent epidemiological surveys[15] have similarly demonstrated a significant association between metabolic syndrome and LUTS. Tai et al.[16] also suggested that MS may especially influence LUTS and OAB in diabetic women, probably by compounding the effect of peripheral neuropathy.
A study by Lee et al.[5] compared the voiding behavior of 194 women with type 2 diabetes with that in 162 control women, using a lower urinary tract symptoms questionnaire and uroflowmetry. They found that diabetic women had significantly higher nocturia scores, weaker urinary streams, reduced voided volumes, and lower maximal flow rates. Residual urine volume occurred in a significantly higher proportion of diabetic subjects (13.9 vs. 1.8% of controls). Similarly, Ho et al.[17] analyzed the urodynamic findings of 94 diabetic patients with a variety of lower urinary tract symptoms. Overactive bladder was seen in 36.2% of diabetics with a higher percentage of increased bladder sensation and detrusor over activity, lower peak flow rate, greater post-void residue volume, and lower bladder voiding efficiency.
Kaplan et al.[18] reported that more than half (∼55%) of diabetic patients have detrusor hyperreflexia, while another 23% have reduced detrusor contractility and a further 10% demonstrate detrusor areflexia with the remaining 11% showing indeterminate findings.
Whether cystopathy is secondary to diabetic chronic complications alone or compounded by co-existing co-morbidities is still a matter of debate. Among the chronic complications of diabetes, peripheral neuropathy and nephropathy were significantly associated with moderate LUTS. Until recently, limited studies had investigated the risk factors for DC. It has been attributed primarily to peripheral neuropathy, involving autonomic and somatic nerves.[19] Recent evidence also suggests that diabetes alters the function of detrusor muscle and urothelium as well.[20] Bansal et al.[21] concluded that evidence of neuropathy can moderately predict the presence of cystopathy. Micro vascular complications associated with diabetes might damage the innervations of the bladder, alter detrusor muscle function, or cause urothelial dysfunction.
Daneshgari et al.[22] presented the “temporal theory of diabetic bladder dysfunction,” which proposes that hyperglycemia-induced polyuria plays a major pathophysiological role during the early stages of diabetes polyuria, causing compensatory bladder hypertrophy and associated myogenic and neurogenic alterations. This stage is compatible with findings of a hyperactive bladder during urodynamic evaluation when patients present with bladder storage concerns (urgency or urge incontinence). With time and accumulation of toxic metabolites, decompensation of bladder tissue and function ensues, resulting in the classical signs and symptoms of diabetic cystopathy (hypocontractile detrusor or atonic bladder) in patients with urinary voiding problems.
Diabetes has been identified as an important independent risk factor for urinary incontinence that leads to significant distress and poorer quality of life. Recent epidemiological evidence strongly suggests that diabetes independently increases risk of urinary incontinence in women and it is 50% to 200% more common in women with type 2 diabetes than in those with normal glucose levels.[23] Look AHEAD study[24] also concluded that urinary incontinence is highly prevalent and far exceeds prevalence of other diabetes complications.
In our study, hypertension was found to be significantly associated with both moderate LUTS and OAB. Kim reported that hypertension is a risk factor for vascular diseases, and men with risk factors for vascular diseases were more likely to have LUTS than men without vascular risk factors.[25] Rohr man also found higher odds for LUTS in men with a history of hypertension.[15] It has been stated that factors activating the sympathetic nervous system increase LUTS; atherosclerosis-induced chronic bladder ischemia may produce significant changes in bladder structure and function, leading to non-compliance and over activity, the link between MS and DC may be secondary to diabetic macro vascular morbidities.
We suggest that diabetic women with more MS components complaining of LUTS warrant more extensive evaluations and more aggressive treatments. Further work is needed to determine whether improved control of some modifiable factors, such as glucose, cholesterol, waist circumference, body weight, and blood pressure, would eliminate or reduce the extent of LUTS and OAB in this population. A recent study analyzed the results of the Diabetes Prevention Program study and concluded that life style modification consisting of a 5-10% weight reduction substantially lowered symptoms of incontinence.[26]
New research initiatives should be taken up to including understanding of possible basic disease mechanisms and evidence for supporting the accurate diagnosis and treatment of diabetic cystopathy. The limitations of present study are its small sample size and cross-sectional design. We could not perform extensive urodynamic study due to technical constraints and unwillingness of our patients. A longitudinal study is required to observe whether worsening of LUTS is a harbinger of cardiovascular events in women with diabetes.
CONCLUSION
Bladder dysfunction is a highly prevalent complication in women with diabetes. They are more susceptible to develop lower urinary tract symptoms. Chronic complications of diabetes, especially neuropathy, nephropathy, and presence of metabolic syndrome, are important predictors of bladder dysfunction.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Frimodt-Moller C. Diabetic cystopathy: Epidemiology and related disorders. Ann Intern Med. 1980;92:318–32. doi: 10.7326/0003-4819-92-2-318. [DOI] [PubMed] [Google Scholar]
- 2.Goldman HB, Appell RA. Voiding dysfunction in women with diabetes mellitus. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:130–3. doi: 10.1007/s001920050032. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan SA, Te AE, Blaivas JG. Urodynamic findings in patients with diabetic cystopathy. J Urol. 1995;153:342–4. doi: 10.1097/00005392-199502000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Menendez V, Cofan F, Talbot-Wright R, Ricart MJ, Gutierrez R, Carretero P. Urodynamic evaluation in simultaneous insulin-dependent diabetes mellitus and end stage renal disease. J Urol. 1996;155:2001–4. [PubMed] [Google Scholar]
- 5.Lee WC, Wu HP, Tai TY, Liu SP, Chen J, Yu HJ. Effects of diabetes on female voiding behavior. J Urol. 2004;172:989–92. doi: 10.1097/01.ju.0000136255.83054.0c. [DOI] [PubMed] [Google Scholar]
- 6.Kebapci N, Yenilmez A, Efe B, Entok E, Demirustu C. Bladder dysfunction in type 2 diabetic patients. Neurourol Urodyn. 2007;26:814–9. doi: 10.1002/nau.20422. [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi C, Sakakibara R, Uchiyama T, Yamamoto T, Ito T, Liu Z, et al. Overactive bladder in diabetes: A peripheral or central mechanism? Neurourol Urodyn. 2007;26:807–13. doi: 10.1002/nau.20404. [DOI] [PubMed] [Google Scholar]
- 8.Lawrence JM, Lukacz ES, Liu IL, Nager CW, Luber KM. Pelvic floor disorders, diabetes, and obesity in women: Findings from the Kaiser Permanente Continence Associated Risk Epidemiology Study. Diabetes Care. 2007;30:2536–41. doi: 10.2337/dc07-0262. [DOI] [PubMed] [Google Scholar]
- 9.Hammarsten J, Hogstedt B. Hyperinsulinaemia as a risk factor for developing benign prostatic hyperplasia. Eur Urol. 2001;39:151–8. doi: 10.1159/000052430. [DOI] [PubMed] [Google Scholar]
- 10.Barry MJ, Fowler FJ, Jr, O’Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549–57. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 11.Nixon A, Colman S, Sabounjian L, Sandage B, Schwiderski UE, Staskin DR, et al. A validated patient reported measure of urinary urgency severity in overactive bladder for use in clinical trials. J Urol. 2005;174:604–7. doi: 10.1097/01.ju.0000165461.38088.7b. [DOI] [PubMed] [Google Scholar]
- 12.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults 2001 Executive Summary of The Third Report of The National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 13.Yu HJ, Lee WC, Liu SP, Tai TY, Wu HP, Chen J. Unrecognized voiding difficulty in female type 2 diabetic patients in the diabetes clinic: A prospective case-control study. Diabetes Care. 2004;27:988–9. doi: 10.2337/diacare.27.4.988. [DOI] [PubMed] [Google Scholar]
- 14.Okamura K, Nojiri Y, Osuga Y, Tange C. Psychometric analysis of international prostate symptom score for female lower urinary tract symptoms. Urology. 2009;73:1199–202. doi: 10.1016/j.urology.2009.01.054. [DOI] [PubMed] [Google Scholar]
- 15.Rohrmann S, Smit E, Giovannucci E, Platz EA. Association between markers of the metabolic syndrome and lower urinary tract symptoms in the Third National Health and Nutrition Examination Survey (NHANES III) Int J Obes. 2005;29:310–6. doi: 10.1038/sj.ijo.0802881. [DOI] [PubMed] [Google Scholar]
- 16.Tai HC, Chung SD, Ho CH, Tai TY, Yang WS, Tseng CH, et al. Metabolicsyndrome components worsen lower urinary tract symptoms in women with type 2 diabetes. J Clin Endocrinol Metab. 2010;95:1143–50. doi: 10.1210/jc.2009-1492. [DOI] [PubMed] [Google Scholar]
- 17.Ho CH, Tai HC, Yu HJ. Urodynamic findings in female diabetic patients with and without overactive bladder symptoms. Neurourol Urodyn. 2010;29:424–7. doi: 10.1002/nau.20727. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan SA, Te AE, Blaivas JG. Urodynamic findings in patients with diabetic cystopathy. J Urol. 1995;153:342–4. doi: 10.1097/00005392-199502000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Ueda T, Yoshimura N, Yoshida O. Diabetic cystopathy: Relationship to autonomic neuropathy detected by sympathetic skin response. J Urol. 1997;157:580–4. doi: 10.1016/s0022-5347(01)65209-1. [DOI] [PubMed] [Google Scholar]
- 20.Yoshimura N, Chancellor MB, Andersson KE, Christ GJ. Recent advances in understanding the biology of diabetes-associated bladder complications and novel therapy. BJU Int. 2005;95:733–8. doi: 10.1111/j.1464-410X.2005.05392.x. [DOI] [PubMed] [Google Scholar]
- 21.Bansal R, Agarwal MM, Modi M, Mandal AK, Singh SK. Urodynamic profile of diabetic patients with lower urinary tract symptoms: Association of diabetic cystopathy with autonomic and peripheral neuropathy. Urology. 2011;77:699–705. doi: 10.1016/j.urology.2010.04.062. [DOI] [PubMed] [Google Scholar]
- 22.Daneshgari F, Liu G, Birder L, Hanna-Mitchell AT, Chacko S. Diabetic bladder dysfunction: Current translational knowledge. J Urol. 2009;182:S18–26. doi: 10.1016/j.juro.2009.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lifford KL, Curhan GC, Hu FB, Barbieri RL, Grodstein F. Type 2 diabetes mellitus and risk of developing urinary incontinence. J Am Geriatr Soc. 2005;53:185–7. doi: 10.1111/j.1532-5415.2005.53565.x. [DOI] [PubMed] [Google Scholar]
- 24.Phelan S, Kanaya AM, Subak LL, Hogan PE, Espeland MA, Wing RR, et al. Action for Health in Diabetes (Look AHEAD) Research Group. Prevalence and risk factors for urinary incontinence in overweight and obese diabeticwomen: Action for health in diabetes (look ahead) study. Diabetes Care. 2009;32:1391–7. doi: 10.2337/dc09-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim S, Jeong JY, Choi YJ, Kim DH, Lee WK, Lee SH, et al. Association between Lower urinary tract symptoms and vascular risk factors in aging men, The Hallym Aging Study. Korean J Urol. 2010;51:477–82. doi: 10.4111/kju.2010.51.7.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown JS, Wing R, Barrett-Connor E, Nyberg LM, Kusek JW, Orchard TJ Diabetes Prevention Program Research Group. Lifestyle intervention is associated with lower prevalence of urinary incontinence: The Diabetes Prevention Program. Diabetes Care. 2006;29:385–90. doi: 10.2337/diacare.29.02.06.dc05-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]


